Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors vQRS
Abstract
1. Introduction
2. Experimental Details
2.1. Materials for Sensors’ Selectivity Tailoring
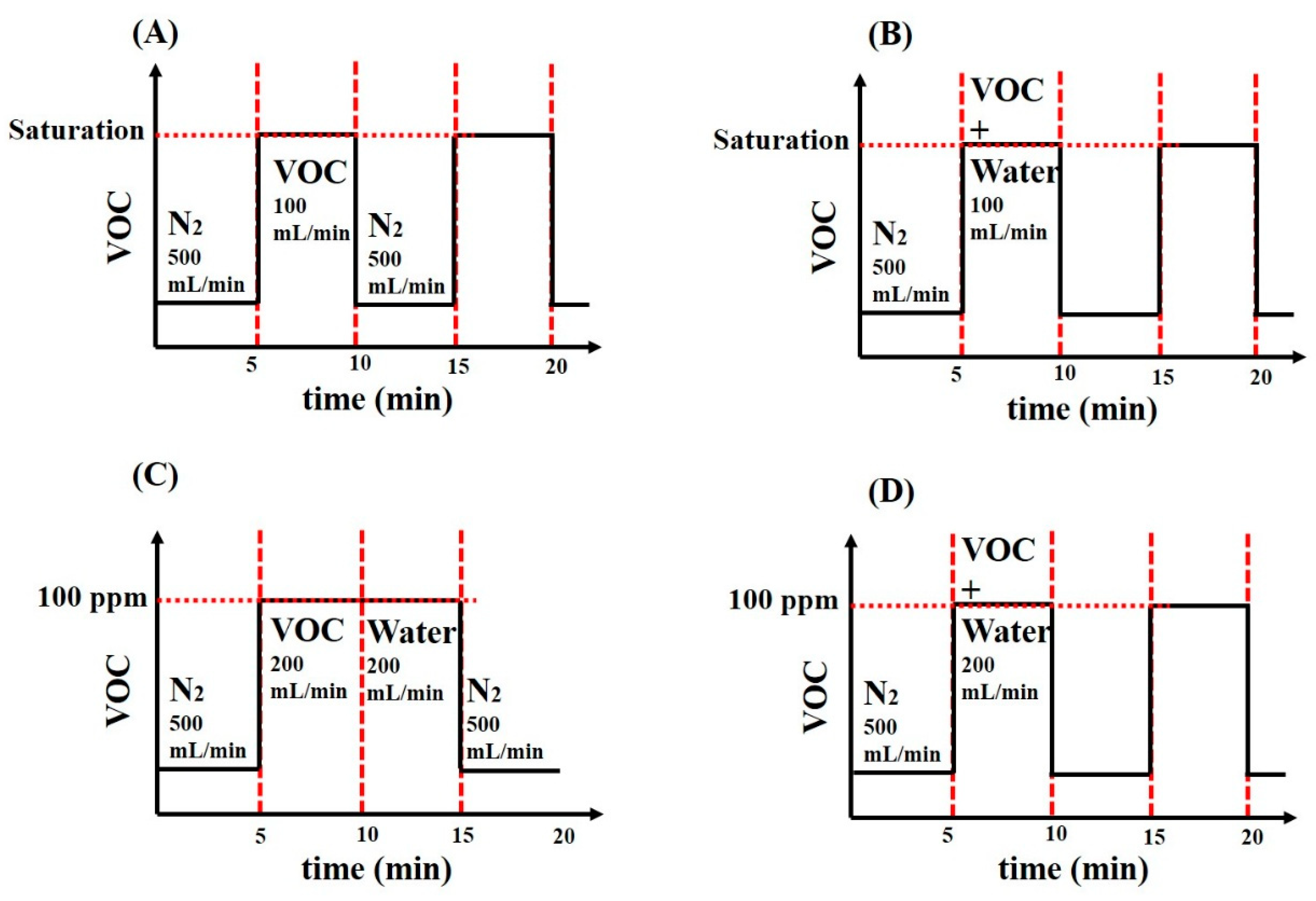
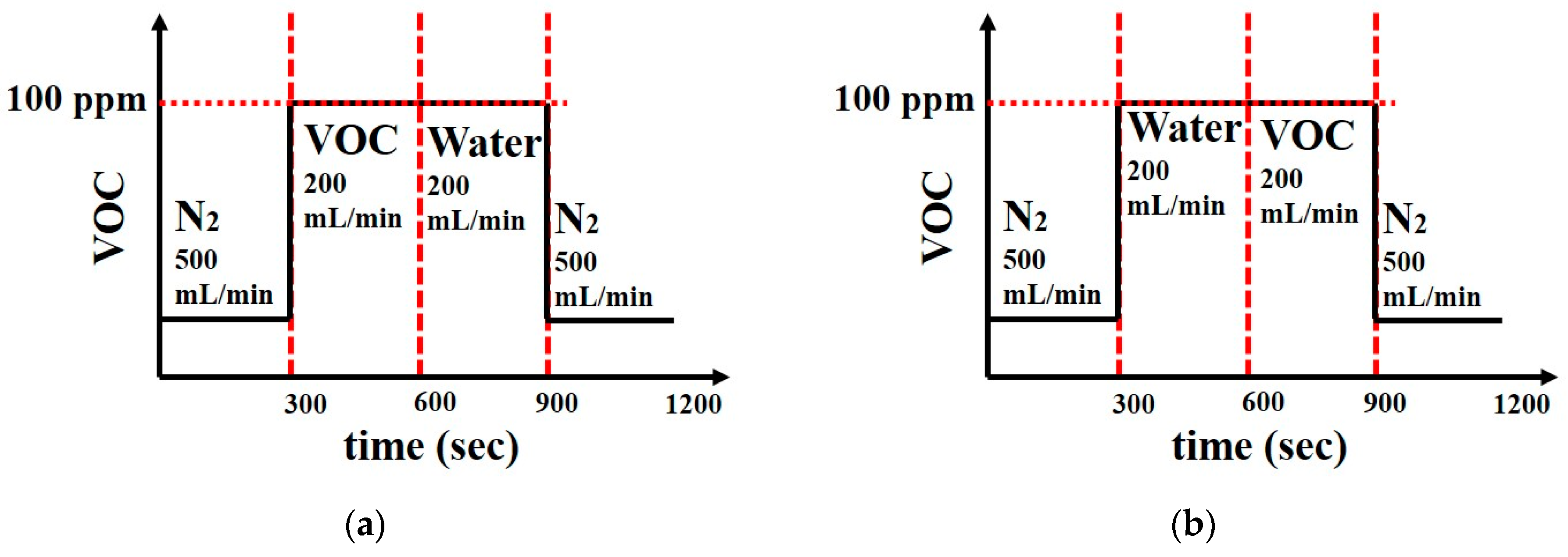
2.2. Sensors’ VOC Supply Protocols
2.3. Sensors’ Fabrication by Additive Nano-Structuring
2.4. Sensors’ Characterization by Dynamic Vapor Analysis (DVA)
3. Results and Discussion
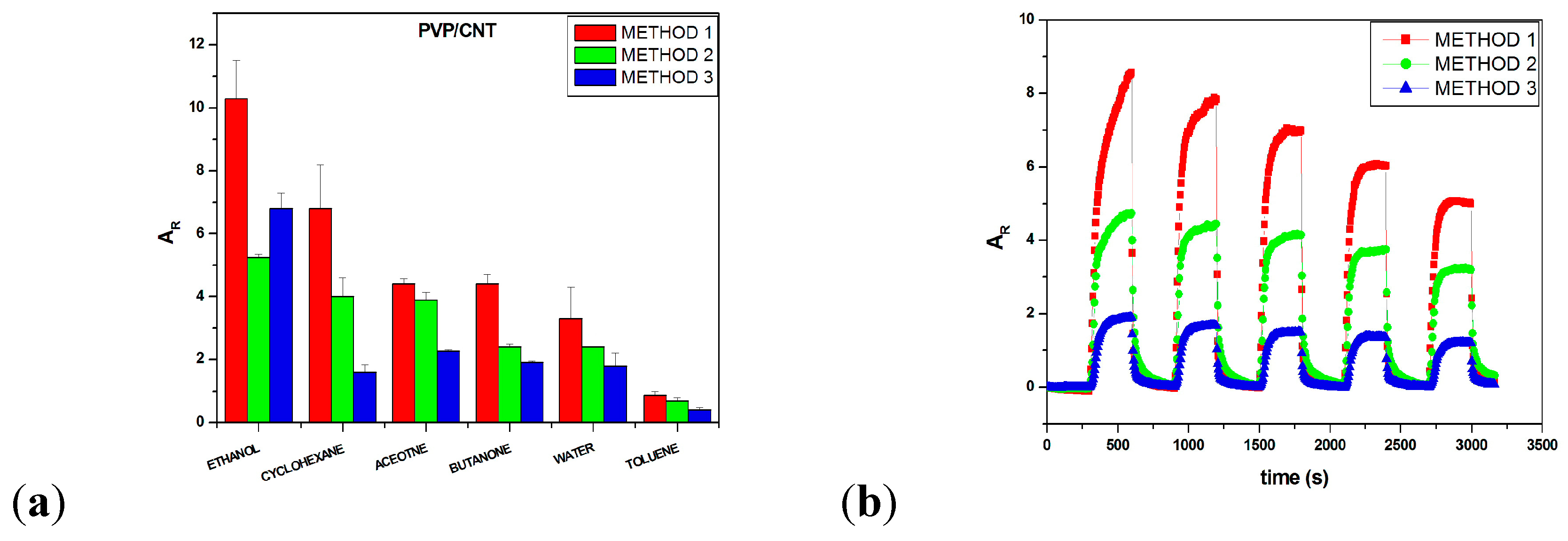
3.1. Optimization of Sensors’ Fabrication
3.2. Effect of Water Vapor on VOC Sensing
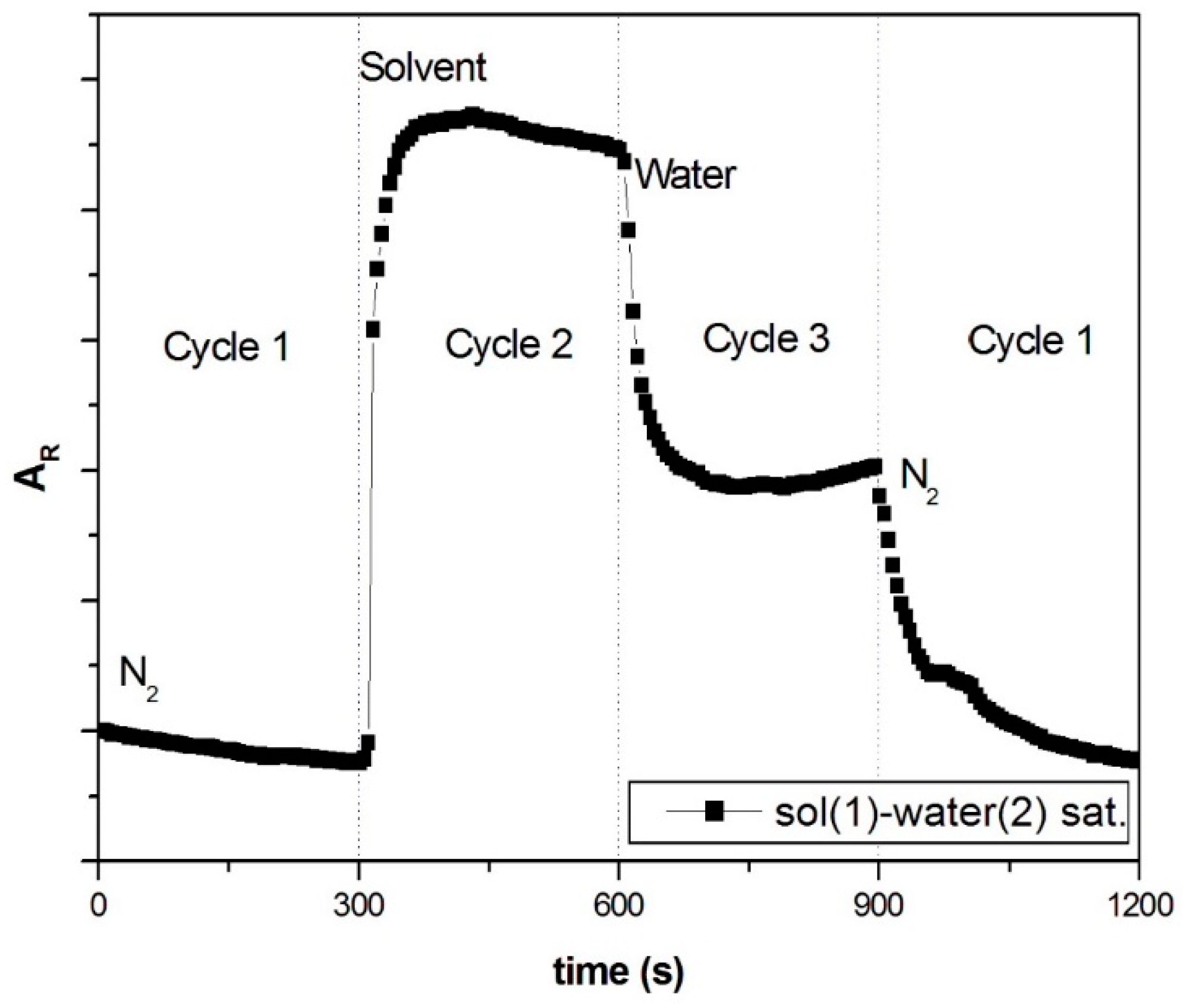
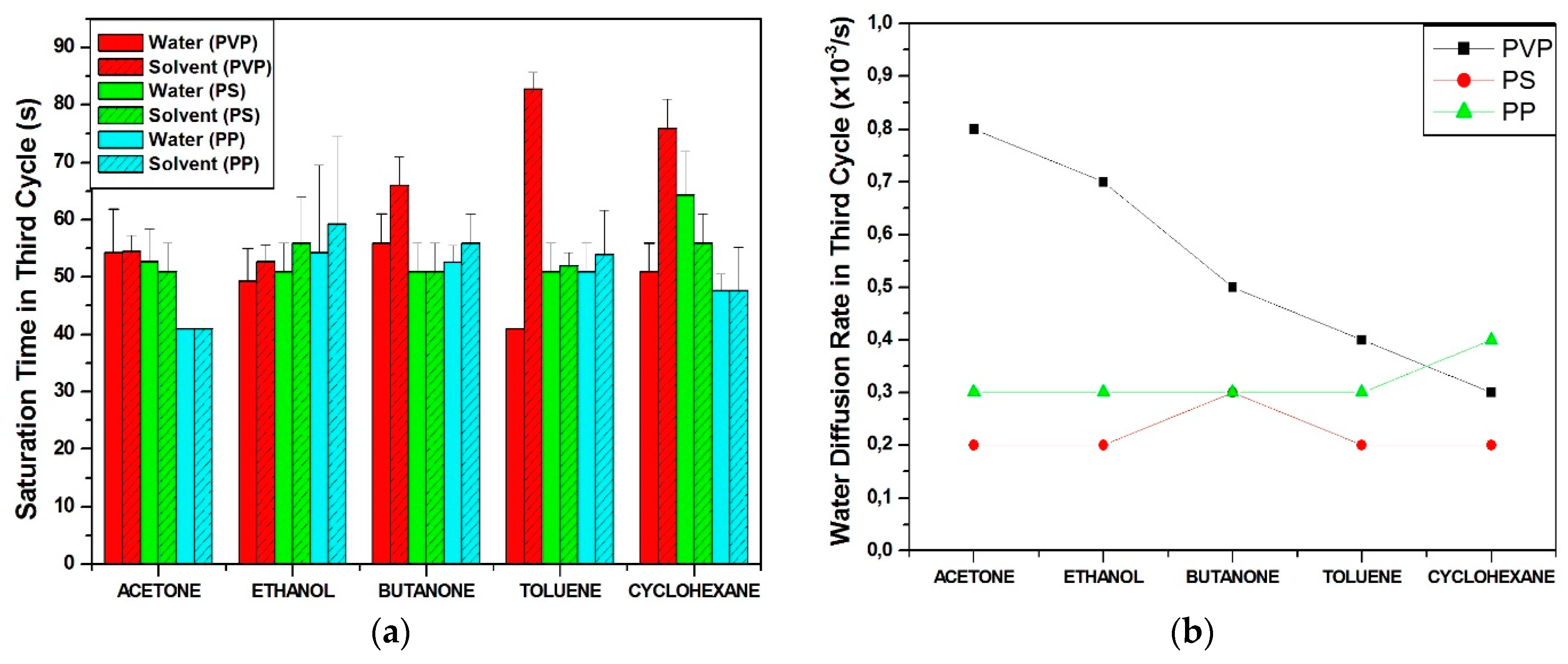
3.2.1. Sensing of VOC in Saturated Conditions
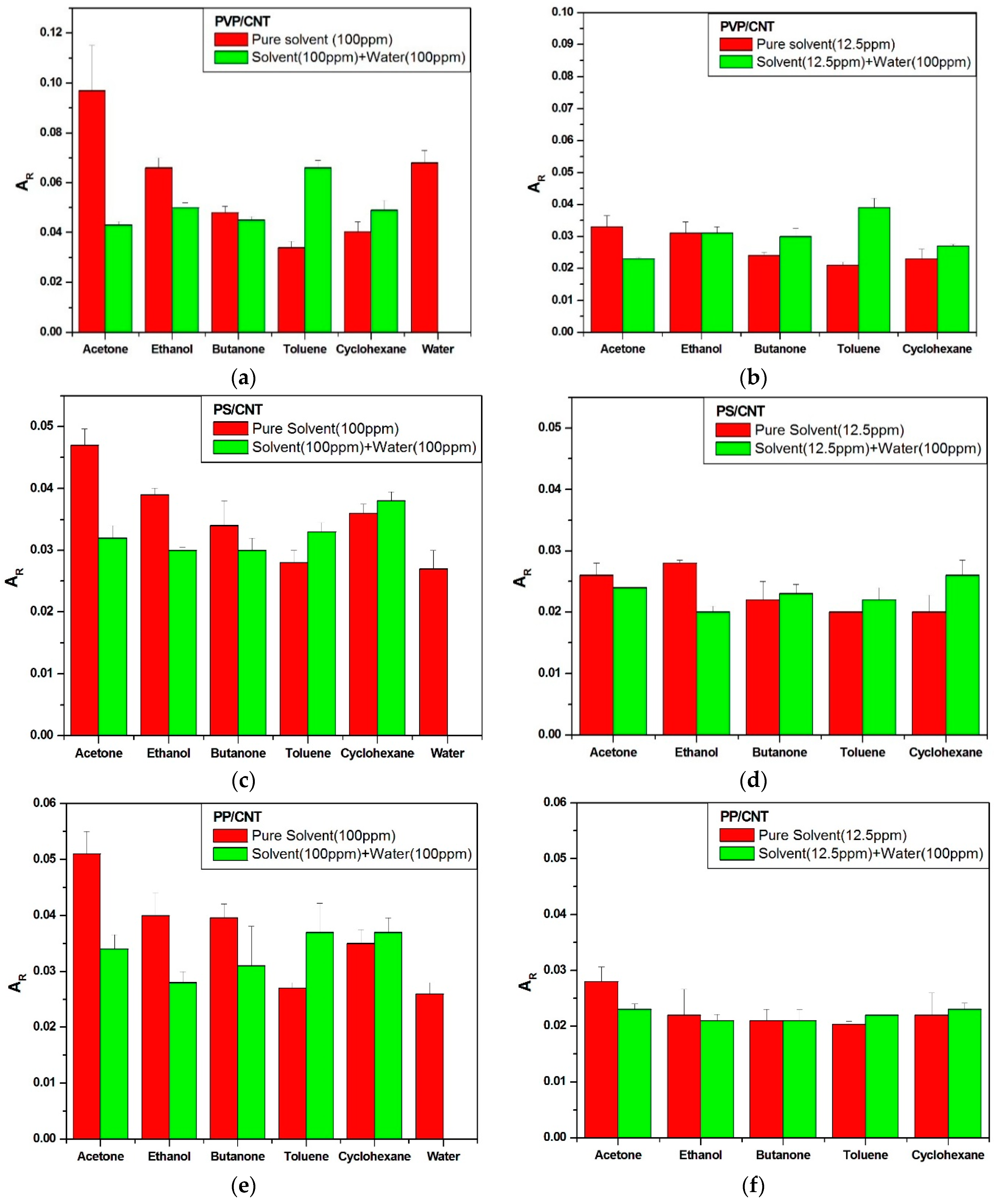
3.2.2. Effects of Water Vapors at Low VOC Concentration
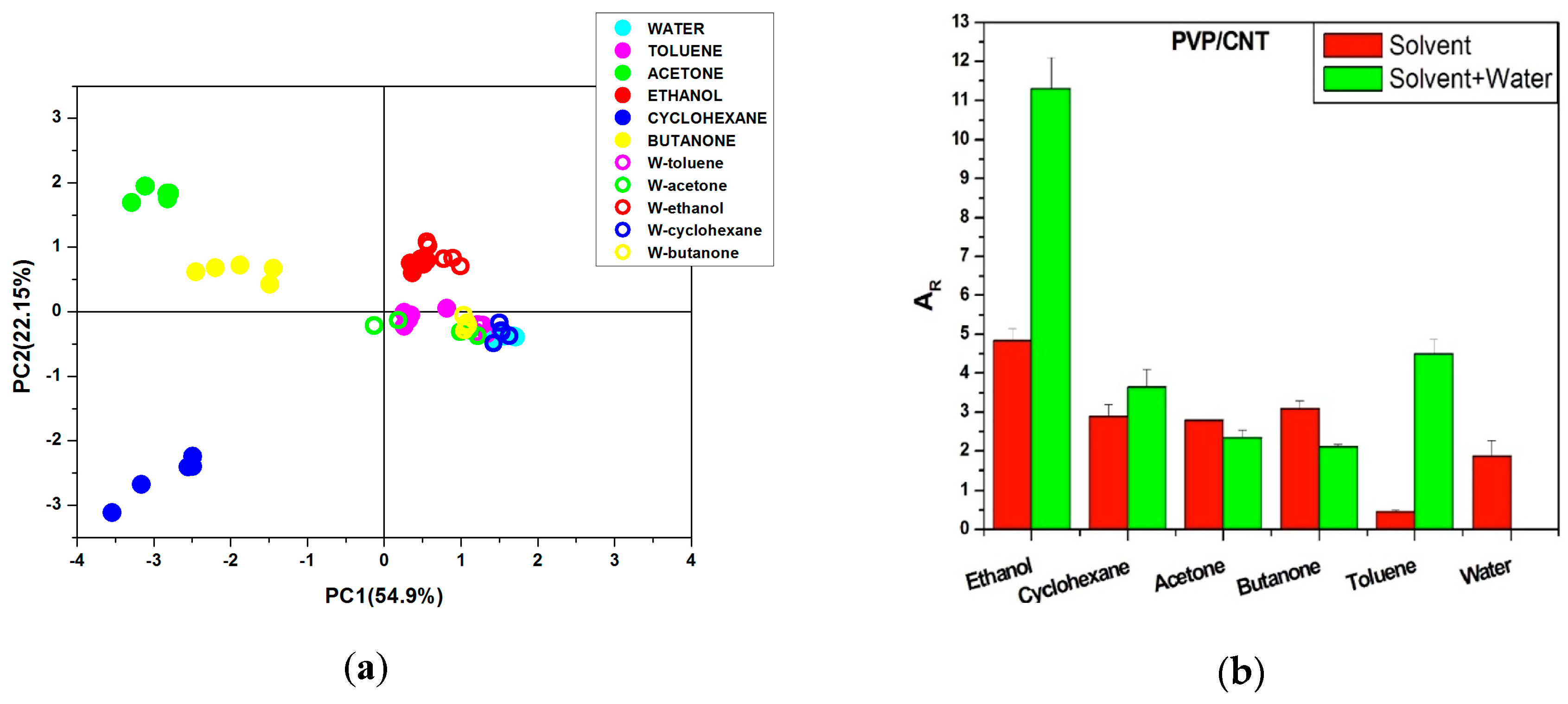
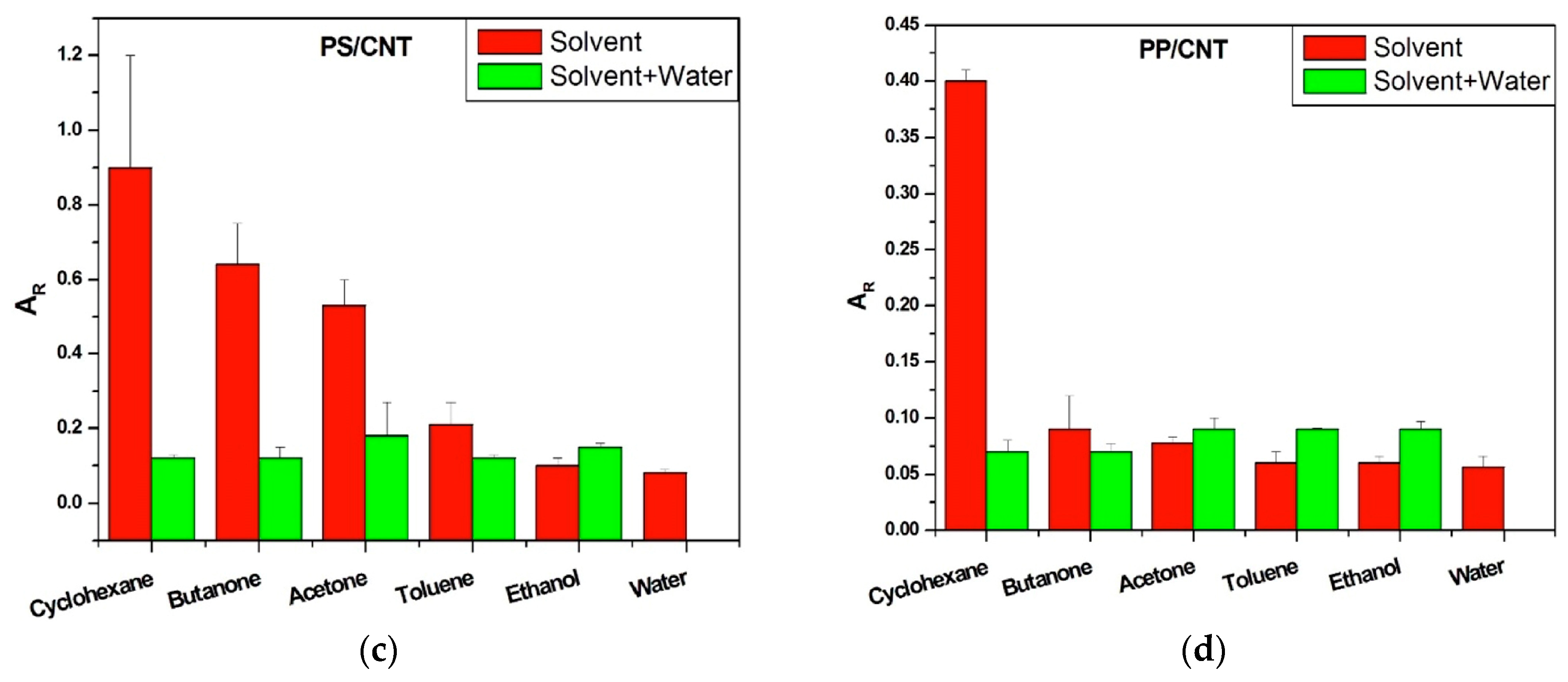
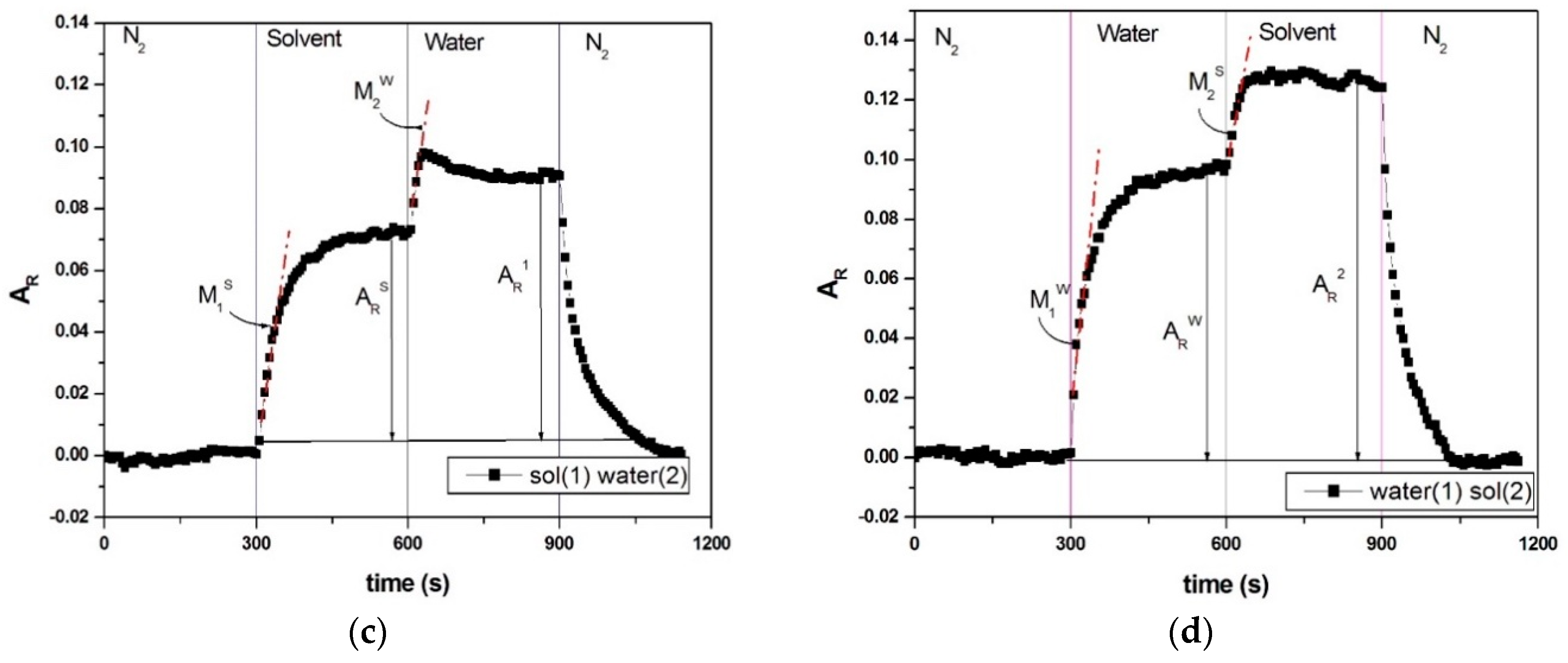
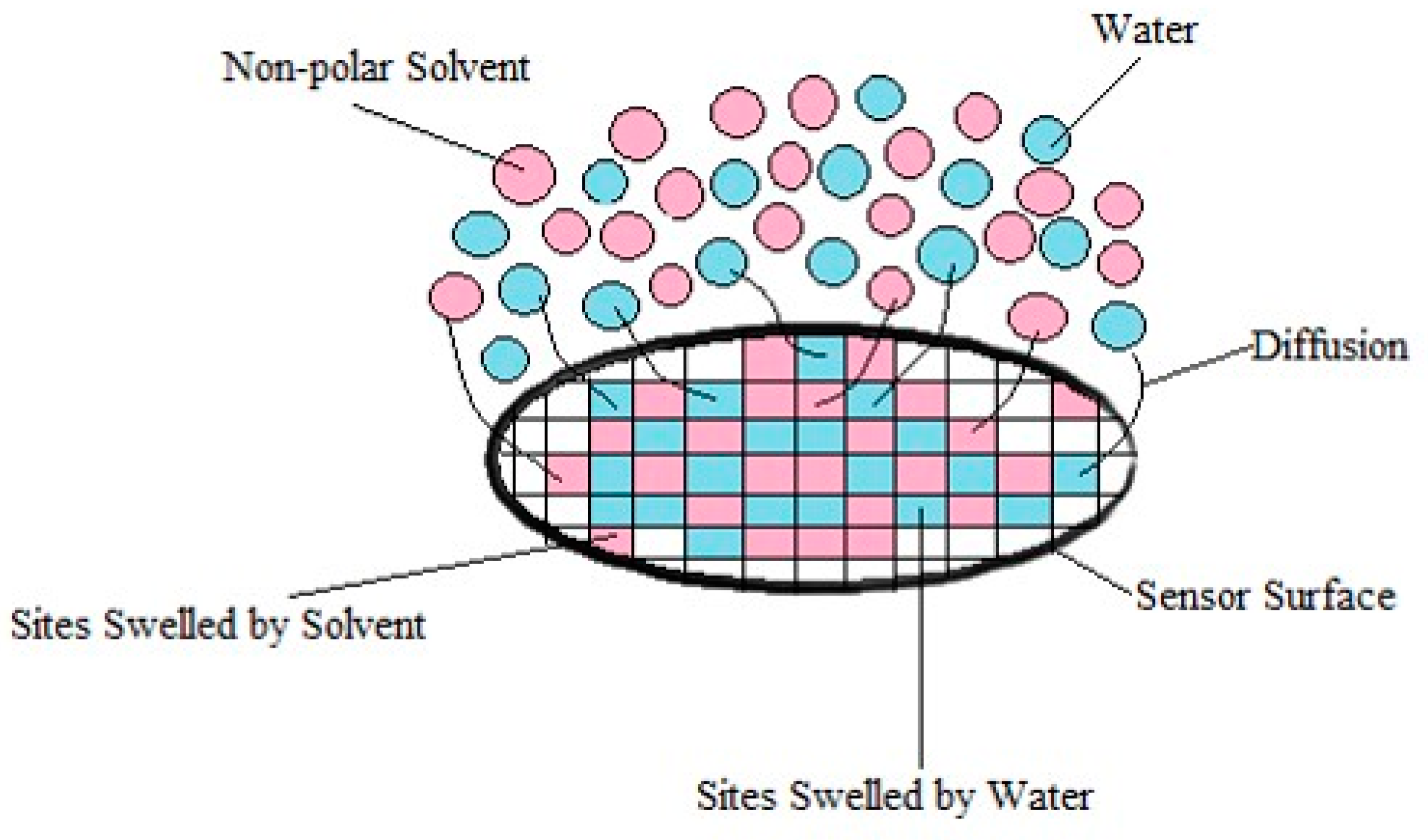
Effects of Interactions between Water and VOC Molecules
- ○
- : the maximum relative amplitude of the solvent response in a solvent/water sequence,
- ○
- : the maximum relative amplitude of the water response in a solvent/water sequence,
- ○
- : the slope of the curve of the solvent in the second cycle,
- ○
- : the slope of curve of water in third cycle.
- ○
- : the maximum relative amplitude of the water response in a water/solvent sequence,
- ○
- : the maximum relative amplitude of the solvent response in a water/solvent sequence,
- ○
- : the slope of the curve of water in second cycle,
- ○
- : the slope of the curve of the solvent in third cycle.
- ○
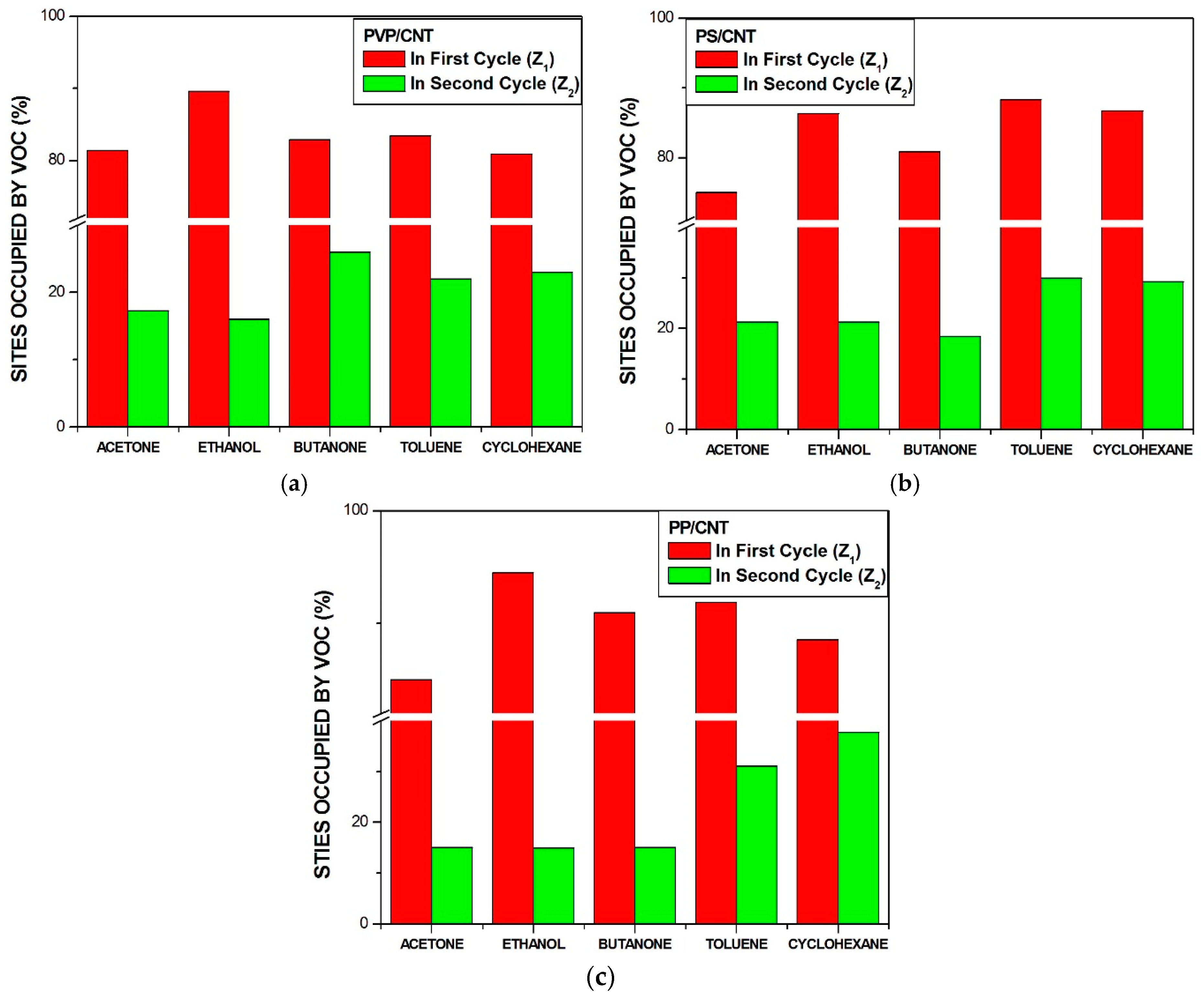
- Firstly, when polar molecules such as H2O are occupying active sites, they are hindering the absorption of newly arriving molecules in particular at the conducting junctions but depending on their affinity with the macromolecules of the CPC transducer, some sites can be freed.
- ○
- Secondly, the non-polar vapors like cyclohexane and toluene are more effective to free the active sites occupied by water at junctions, than polar VOC such as acetone and ethanol because their affinity for the polymer matrices is larger. Additionally, polar–polar interactions resulting in partial solubility of polar solvents molecules in water could prevent them from directly competing on the sites. This behavior is further confirmed in Section 3.3, and the corresponding mechanism is discussed in Section 3.4.
- ○
- The third kind of effect concerns semi-polar vapors like butanone which adsorption depends on the nature of the macromolecules of sensors, they can behave like non-polar vapors for PVP and like polar ones for aPS and aPP.
Effects of Interactions between VOC Molecules and Sensor’s Macromolecules
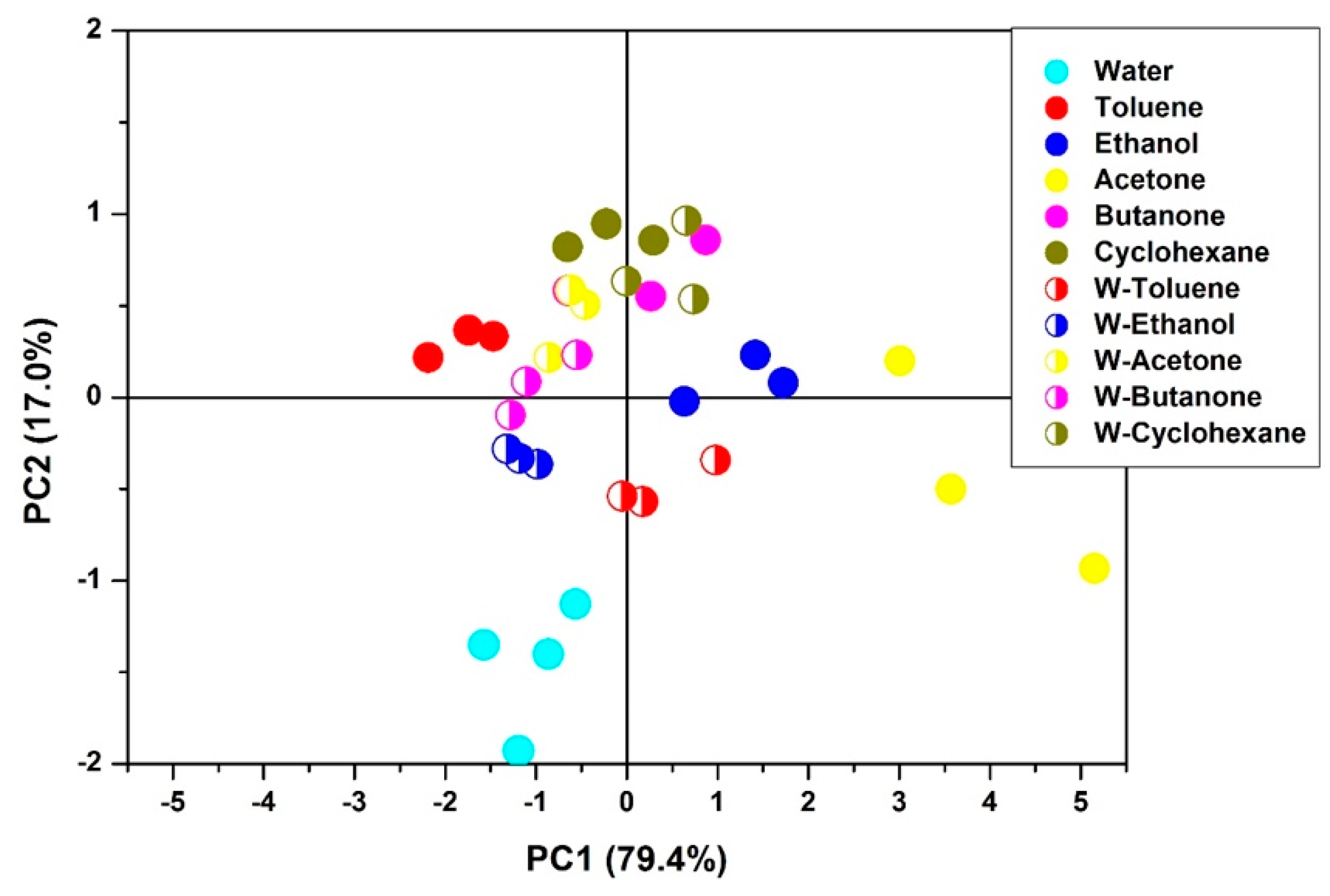
3.3. Sensing Some Tens of ppm of VOC in a Humid Environment
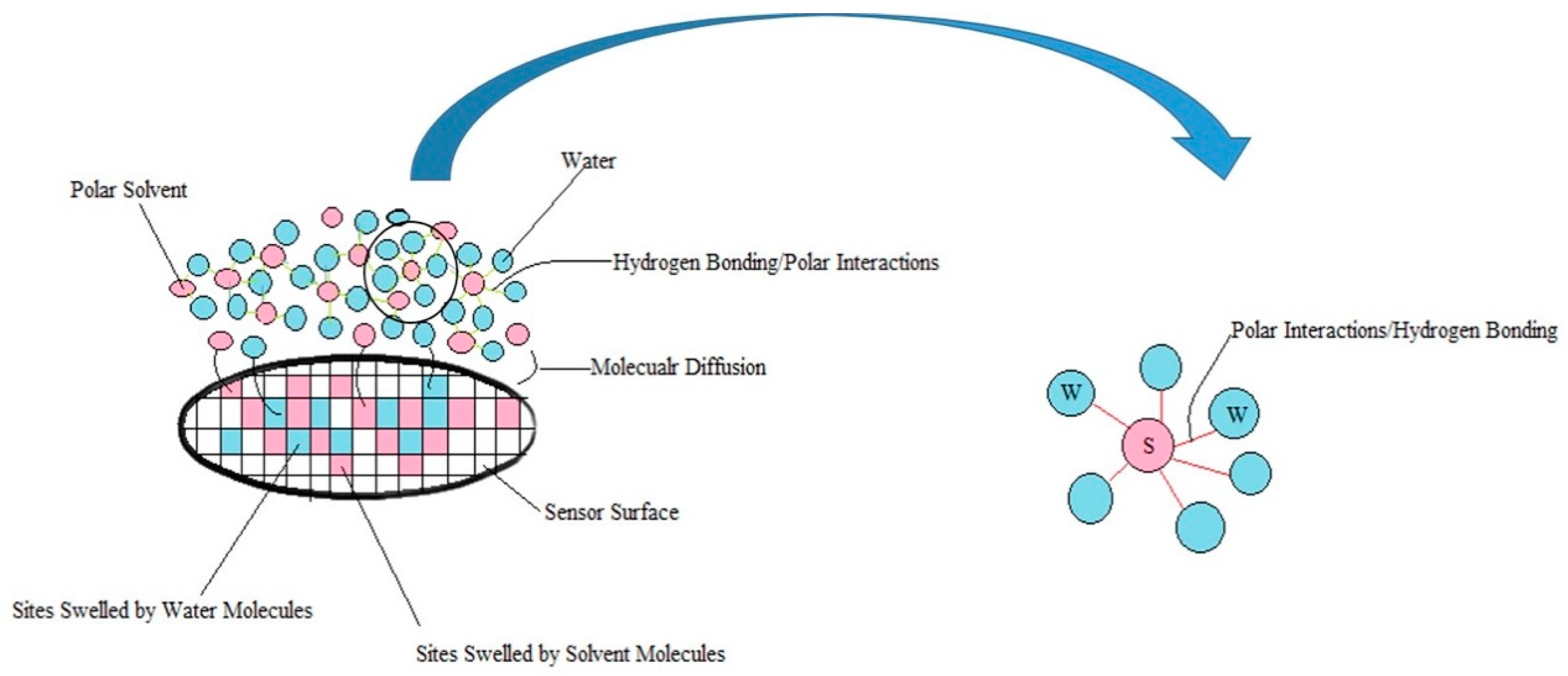
3.4. Chemo-Resistive Behavior of vQRS Exposed to VOC-H2O Mixes
4. Conclusions
- ○
- By combining with them before the adsorption on the transducer or competing with them for the adsorption on the nano-junctions. Humidity will also affect the adsorption of VOC on the polymer matrix of the transducers depending on their polarity. It is observed that the response of vQRS to polar VOC (tested with acetone and ethanol) is generally decreased by moisture whereas the opposite trend is obtained for non-polar VOC (tested with cyclohexane and toluene). For semi-polar vapors like butanone, the chemo-resistive behavior of mixes with water depends on the proportion of both vapors. These findings are suggesting that H2O by combining with analytes before their adsorption can compete or collaborate to respectively improve or degrade the detection by the vQRS.
- ○
- The different experimental sequences of test shed light on the notion of “number of available active sites towards junctions’ disconnection”, for both polar and non-polar VOC, at the origin of the chemo-resistive signal. This brought elements of understanding and interpretations of non-trivial experiments of vapor sensing with VOC/H2O blends. For example, when submitting the vQRS to a VOC/H2O sequence both at saturation results in a negative contribution of H2O, whereas when the successive flows only contain 100 ppm of vapor, this contribution is positive. The order of the vapors also matters, and the comparison shows which amount of sites can be occupied by H2O or not, depending on the nature the VOC.
- ○
- A better understanding of vQRS capability and robustness in the presence of moisture is also provided by experiments showing that VOC only present at 100 ppm and 12.5 ppm can be detected among 100 pmm of H2O, especially if a PCA projection is used. Although the evidence of the preservation of vQRS discrimination ability at low VOC concentrations was provided, it was of course strongly decreased with the number of analyte molecules and the ratio of H2O molecules. Thus, any device able to minimize the amount of water in the blend of vapor composing the volatolome will improve the pattern recognition and in fine the diagnostic score.
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Gardner, J.W.; Shin, H.W.; Hines, E.L. An electronic nose system to diagnose illness. Sens. Actuators B Chem. 2000, 70, 19–24. [Google Scholar] [CrossRef]
- di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Respir. Crit. Care Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Hockstein, N.G.; Thaler, E.R.; Torigian, D.; Miller, W.T.; Deffenderfer, O.; Hanson, C.W. Diagnosis of pneumonia with an electronic nose: Correlation of vapor signature with chest computed tomography scan findings. Laryngoscope 2004, 114, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P. Sniffing out lung cancer. Nat. Nanotechnol. 2009, 4, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; van der Schee, M.P.; Massaro, T.; Schiavulli, N.; Brinkman, P.; Pinca, A.; Carratú, P.; Spanevello, A.; Resta, O.; Musti, M.; et al. An electronic nose distinguishes exhaled breath of patients with Malignant Pleural Mesothelioma from controls. Lung Cancer. 2012, 75, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Zuri, L.; Haick, H. Combined volatolomics for monitoring of human body chemistry. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Costello, B.d.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.G.; Besse, T.J.; Duane, W.C.; Evans, C.D.; DeMaster, E.G. Effect of regulating cholesterol biosynthesis on breath isoprene excretion in men. Lipids 1993, 28, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Diabetic ketoacidosis. Lancet 1995, 345, 767–772. [Google Scholar] [CrossRef]
- Cope, K.; Risby, T.; Diehl, A.M. Increased gastrointestinal ethanol production in obese mice: Implications for fatty liver disease pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.L.; Cortivo, L.A.D.; Maturo, J. Endogenous isopropanol: Forensic and biochemical implications. J. Anal. Toxicol. 1984, 8, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.R.; Grum, C.M.; Boxer, L.A.; Simon, R.H.; Ketai, L.H.; Devall, L.J. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet 1986, 327, 11–14. [Google Scholar] [CrossRef]
- Chan, H.P.; Lewis, C.; Thomas, P.S. Exhaled breath analysis: Novel approach for early detection of lung cancer. Lung Cancer 2009, 63, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Gagermeier, J.; Dilling, D.; Alex, C.; Lowery, E.; Kovacs, E.J.; Love, R.B.; Fisichella, P.M. A review of the potential applications and controversies of non-invasive testing for biomarkers of aspiration in the lung transplant population. Clin. Transplant. 2010, 24, E54–E61. [Google Scholar] [CrossRef]
- Taylor, R.; Agius, L. The biochemistry of diabetes. Biochem. J. 1988, 250, 625–640. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Cheema, T.; Greenberg, J. Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin. Chim. Acta 2004, 344, 189–194. [Google Scholar] [CrossRef]
- Davidson, G.P. The breath hydrogen test: An evaluation. J. Paediatr. Child Heal. 1988, 24, 1–2. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F.E. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Haick, H. Chemical sensors for breath gas analysis: The latest developments at the Breath Analysis Summit 2013. J. Breath Res. 2014, 8, 27103. [Google Scholar] [CrossRef] [PubMed]
- Zee, F.; Judy, J.W. Micromachined polymer-based chemical gas sensor array. Sens. Actuators B Chem. 2001, 72, 120–128. [Google Scholar] [CrossRef]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive, carbon black−polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Zilberman, Y.; Tisch, U.; Shuster, G.; Pisula, W.; Feng, X.; Müllen, K.; Haick, H. Carbon nanotube/hexa-peri-hexabenzocoronene bilayers for discrimination between nonpolar volatile organic compounds of cancer and humid atmospheres. Adv. Mater. 2010, 22, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Haick, H. Detection of nonpolar molecules by means of carrier scattering in random networks of carbon nanotubes: Toward diagnosis of diseases via breath samples. Nano Lett. 2009, 9, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Park, Y.T.; Castro, M.; Grunlan, J.C.; Feller, J.F. Fine control of carbon nanotubes-polyelectrolyte sensors sensitivity by electrostatic layer by layer assembly (eLbL) for the detection of volatile organic compounds (VOC). Talanta 2012, 88, 396–402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mills, A.; Lepre, A.; Wild, L. Breath-by-breath measurement of carbon dioxide using a plastic film optical sensor. Sens. Actuators B Chem. 1997, 39, 419–425. [Google Scholar] [CrossRef]
- Cui, D.; Cai, X.; Han, J.; He, Z.; Zhu, H.; Feng, G. Lipid LB films for room temperature ethanol gas assay. Sens. Actuators B Chem. 1997, 45, 229–232. [Google Scholar] [CrossRef]
- Boot, J.D.; de Ridder, L.; de Kam, M.L.; Calderon, C.; Mascelli, M.A.; Diamant, Z. Comparison of exhaled nitric oxide measurements between NIOX MINO® electrochemical and Ecomedics chemiluminescence analyzer. Respir. Med. 2008, 102, 1667–1671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuban, P.; Berg, J.M.; Dasgupta, P.K. Durable microfabricated high-speed humidity sensors. Anal. Chem. 2004, 76, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors? A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yoo, H.W.; Kim, J.Y.; Jung, W.B.; Jin, M.L.; Kim, J.S.; Jeon, H.J.; Jung, H.T. High-resolution p-type metal oxide semiconductor nanowire array as an ultrasensitive sensor for volatile organic compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Shehada, N.; Brönstrup, G.; Funka, K.; Christiansen, S.; Leja, M.; Haick, H. Ultrasensitive silicon nanowire for real-world gas sensing: Noninvasive diagnosis of cancer from breath volatolome. Nano Lett. 2015, 15, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Breer, H. Olfactory receptors: Molecular basis for recognition and discrimination of odors. Anal. Bioanal. Chem. 2003, 377, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Missbach, C.; Dweck, H.K.M.; Vogel, H.; Vilcinskas, A.; Stensmyr, M.C.; Hansson, B.S.; Grosse-Wilde, E. Evolution of insect olfactory receptors. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Advances in olfaction-inspired biomaterials applied to bioelectronic noses. Sens. Actuators B Chem. 2018, 257, 511–537. [Google Scholar] [CrossRef]
- Amann, A.; Poupart, G.; Telser, S.; Ledochowski, M.; Schmid, A.; Mechtcheriakov, S. Applications of breath gas analysis in medicine. Int. J. Mass Spectrom. 2004, 239, 227–233. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Piotrowicz, G.; Gębicki, J.; Namieśnik, J. Sample preparation and recent trends in volatolomics for diagnosing gastrointestinal diseases. TrAC Trends Anal. Chem. 2018, 108, 38–49. [Google Scholar] [CrossRef]
- Smith, D.; Wang, T.; Sulé-Suso, J.; Spanel, P.; el Haj, A. Quantification of acetaldehyde released by lung cancer cells in vitro using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Kischkel, S.; Sawacki, A.; Liebau, T.; Mieth, M.; Schubert, J.K. Impact of sampling procedures on the results of breath analysis. J. Breath Res. 2008, 2, 026007. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.D.; Lindstrom, A.B. Measurement of volatile organic compounds in exhaled breath as collected in evacuated electropolished canisters. J. Chromatogr. B Biomed. Sci. Appl. 1995, 665, 271–279. [Google Scholar] [CrossRef]
- Mleth, M.; Schubert, J.K.; Gröger, T.; Sabei, B.; Kischkel, S.; Fuchs, P.; Hein, D.; Zimmermann, R.; Miekisch, W. Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal. Chem. 2010, 82, 2541–2551. [Google Scholar] [CrossRef]
- Knutson, M.D.; Viteri, F.E. Concentrating breath samples using liquid nitrogen: A reliable method for the simultaneous determination of ethane and pentane. Anal. Biochem. 1996, 242, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef]
- Nag, S.; Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.F. Spray layer-by-layer assembly of POSS functionalized CNT quantum chemo-resistive sensors with tuneable selectivity and ppm resolution to VOC biomarkers. Sens. Actuators B Chem. 2016, 222, 362–373. [Google Scholar] [CrossRef]
- Linders, M.J.G.; van den Broeke, L.J.P.; Kapteijn, F.; Moulijn, J.A.; van Bokhoven, J.J.G.M. Binary adsorption equilibrium of organics and water on activated carbon. AIChE J. 2001, 47, 1885–1892. [Google Scholar] [CrossRef]
- Cal, M.P.; Rood, M.J.; Larson, S.M. Removal of VOCs from humidified gas streams using activated carbon cloth. Gas Sep. Purif. 1996, 10, 117–121. [Google Scholar] [CrossRef]
- Sullivan, P.D.; Rood, M.J.; Hay, K.J.; Qi, S. Adsorption and electrothermal desorption of hazardous organic vapors. J. Environ. Eng. 2001, 127, 217–223. [Google Scholar] [CrossRef]
- Dreisbach, B.F.; Lösch, H.W. Adsorption measurement of water/ethanol mixtures on activated carbon fiber. Chem. Eng. Technol. 2001, 24, 1001–1005. [Google Scholar] [CrossRef]
- Heinen, A.W.; Peters, J.A.; van Bekkum, H. Competitive adsorption of water and toluene on modified activated carbon supports. Appl. Catal. A Gen. 2000, 194, 193–202. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Adsorption of water and methanol on micro- and mesoporous wood-based activated carbons. Langmuir 2000, 16, 5435–5440. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Jagiełło, J.; Schwarz, J.A.; Krzyzanowski, A. Effect of surface chemistry on sorption of water and methanol on activated carbons. Langmuir 1996, 12, 6480–6486. [Google Scholar] [CrossRef]
- Taqvi, S.M.; Appel, W.S.; LeVan, M.D. Coadsorption of organic compounds and water vapor on BPL activated carbon. 4. Methanol, ethanol, propanol, butanol, and modeling. Ind. Eng. Chem. Res. 1999, 38, 240–250. [Google Scholar] [CrossRef]
- Rodríguez-Mirasol, J.; Bedia, J.; Cordero, T.; Rodríguez, J.J. Influence of water vapor on the adsorption of VOC on lignin-based activated carbons. Sep. Sci. Technol. 2005, 40, 3113–3135. [Google Scholar] [CrossRef]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.S.; Shin, B.; Lee, T.; Kim, J.S.; Lee, S.; Jun, S.C.; Park, H.H.; et al. Chemiresistive electronic nose toward detection of biomarkers in exhaled breath. ACS Appl. Mater. Interfaces. 2016, 8, 20969–20976. [Google Scholar] [CrossRef] [PubMed]
- Belmares, M.; Blanco, M.; Goddard, W.A.; Ross, R.B.; Caldwell, G.; Chou, S.H.; Pham, J.; Olofson, P.M.; Thomas, C. Hildebrand and hansen solubility parameters from molecular dynamics with applications to electronic nose polymer sensors. J. Comput. Chem. 2004, 25, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Thomson, G.W. The Antoine equation for vapor-pressure data. Chem. Rev. 1946, 38, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Kitak, T.; Dumičić, A.; Planinšek, O.; Šibanc, R.; Srčič, S. Determination of solubility parameters of ibuprofen and ibuprofen lysinate. Molecules 2015, 20, 21549–21568. [Google Scholar] [CrossRef] [PubMed]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Lu, J.; Park, B.J.; Kumar, B.; Castro, M.; Choi, H.J.; Feller, J.F. Polyaniline nanoparticle-carbon nanotube hybrid network vapour sensors with switchable chemo-electrical polarity. Nanotechnology 2010, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Feller, J.F.; Castro, M.; Lu, J. Conductive bio-Polymer nano-Composites (CPC): Chitosan-carbon nanotube transducers assembled via spray layer-by-layer for volatile organic compound sensing. Talanta 2010, 81, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Castro, M.; Feller, J.F. Controlled conductive junction gap for chitosan-carbon nanotube quantum resistive vapour sensors. J. Mater. Chem. 2012, 22, 10656–10664. [Google Scholar] [CrossRef]
- Nag, S.; Duarte, L.; Bertrand, E.; Celton, V.; Castro, M.; Choudhary, V.; Guégan, P.; Feller, J.F. Ultrasensitive QRS made by supramolecular assembly of functionalized cyclodextrins and graphene for the detection of lung cancer VOC biomarkers. J. Mater. Chem. B Biol. Med. 2014, 2, 6571–6579. [Google Scholar] [CrossRef]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef]
- Bouvrée, A.; Feller, J.F.; Castro, M.; Grohens, Y.; Rinaudo, M. Conductive Polymer nano-bioComposites (CPC): Chitosan-carbon nanoparticle a good candidate to design polar vapour sensors. Sens. Actuators B Chem. 2009, 138, 138–147. [Google Scholar] [CrossRef]
- Toryanik, A.I.; Taranenko, V.N. Molecular mobility and structure in water-acetone mixtures. J. Struct. Chem. 1988, 28, 714–719. [Google Scholar] [CrossRef]
| Name | PVP | aPS | aPP |
|---|---|---|---|
| Formula |  |  | 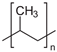 |
| Tg (°C) | 110 to 180 | 100 | −10 |
| Mn (g·mol−1) | 1.3 × 106 | 2.5 × 105 | 2.5 × 105 |
| δT (MPa1/2) | 26.28 | 22.69 | 16.77 |
| Solvent | Formula | Solubility Parameter δT (MPa1/2) [58] | % of Solubility in Water | Concentration at Saturation (ppm) [59] |
|---|---|---|---|---|
| Water | H2O | 47.8 | 100 | 2763 |
| Ethanol | C2H5OH | 26.5 | 100 | 7005 |
| Acetone | CH3COCH3 | 19.9 | 100 | 27,751 |
| Butanone | C2H5COCH3 | 19.1 | 28 | 11,366 |
| Toluene | C6H5CH3 | 18.2 | 0.05 | 3371 |
| Cyclohexane | C6H12 | 16.8 | 0.01 | 11,729 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.-F. Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors vQRS. Chemosensors 2018, 6, 64. https://doi.org/10.3390/chemosensors6040064
Sachan A, Castro M, Choudhary V, Feller J-F. Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors vQRS. Chemosensors. 2018; 6(4):64. https://doi.org/10.3390/chemosensors6040064
Chicago/Turabian StyleSachan, Abhishek, Mickaël Castro, Veena Choudhary, and Jean-Francois Feller. 2018. "Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors vQRS" Chemosensors 6, no. 4: 64. https://doi.org/10.3390/chemosensors6040064
APA StyleSachan, A., Castro, M., Choudhary, V., & Feller, J.-F. (2018). Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors vQRS. Chemosensors, 6(4), 64. https://doi.org/10.3390/chemosensors6040064






