Fluorinated Chromium Phthalocyanine Thin Films: Characterization and Ammonia Vapor Detection
Abstract
1. Introduction
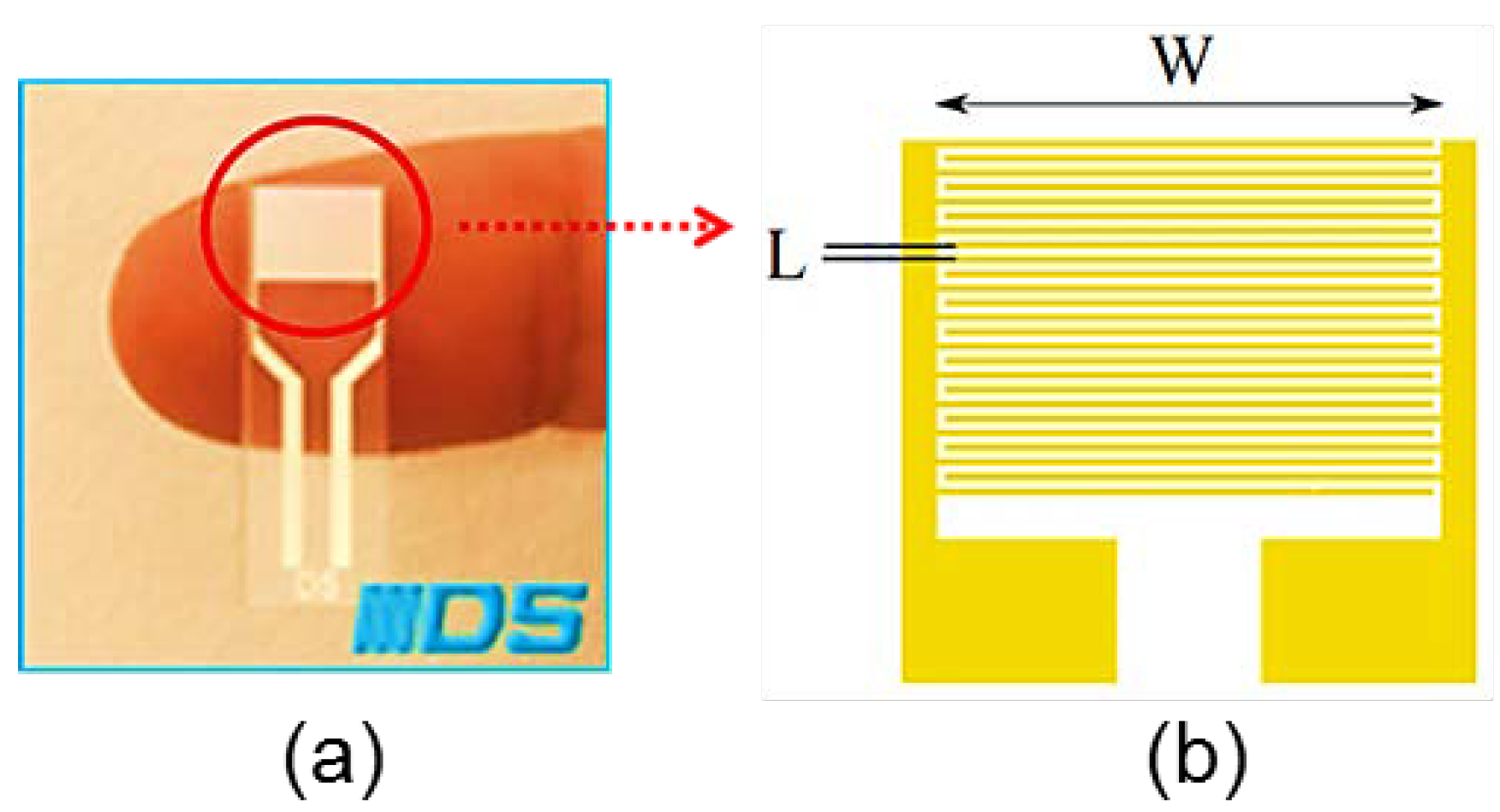
2. Materials and Methods
3. Characterization Techniques
4. Results and Discussion
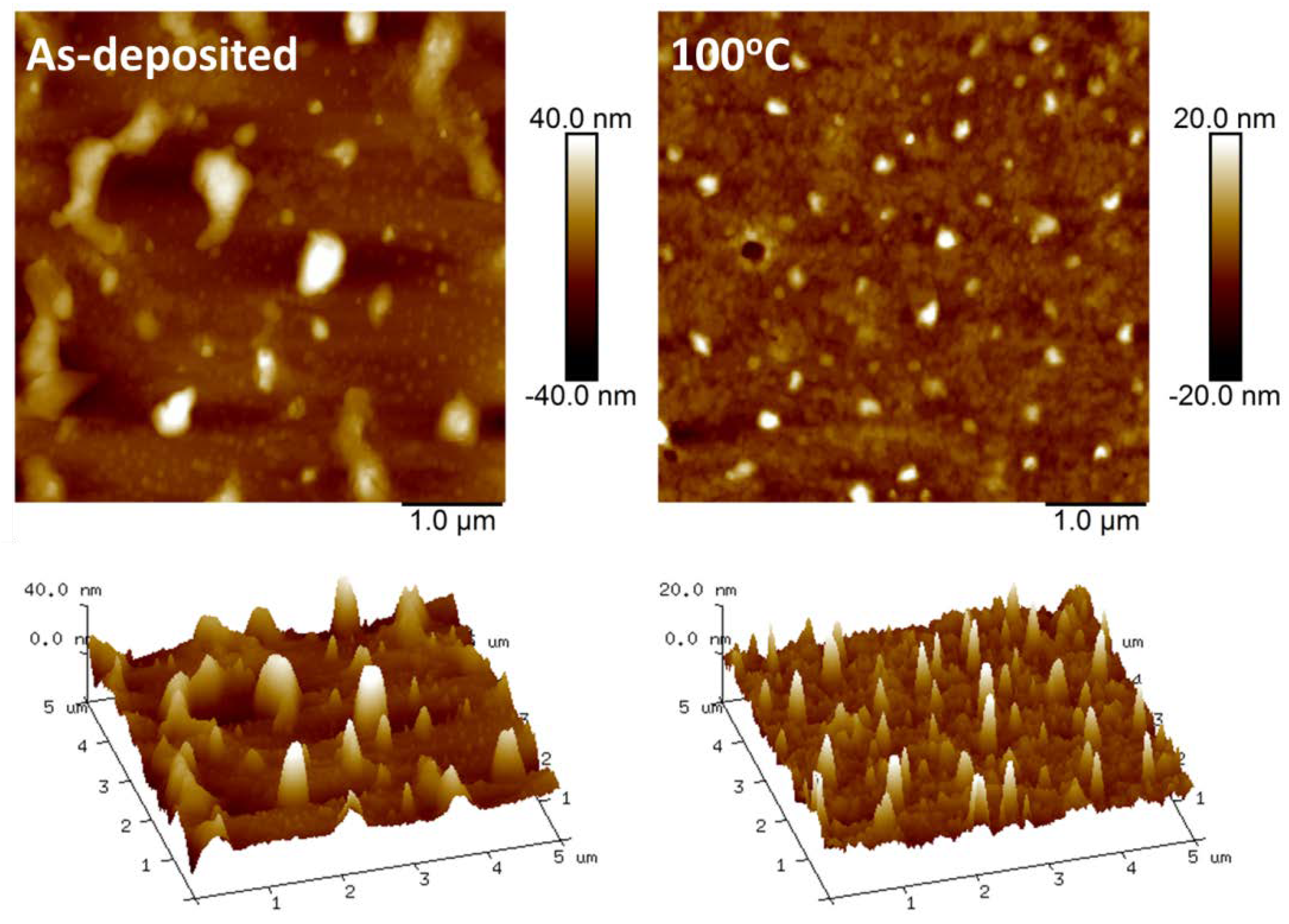
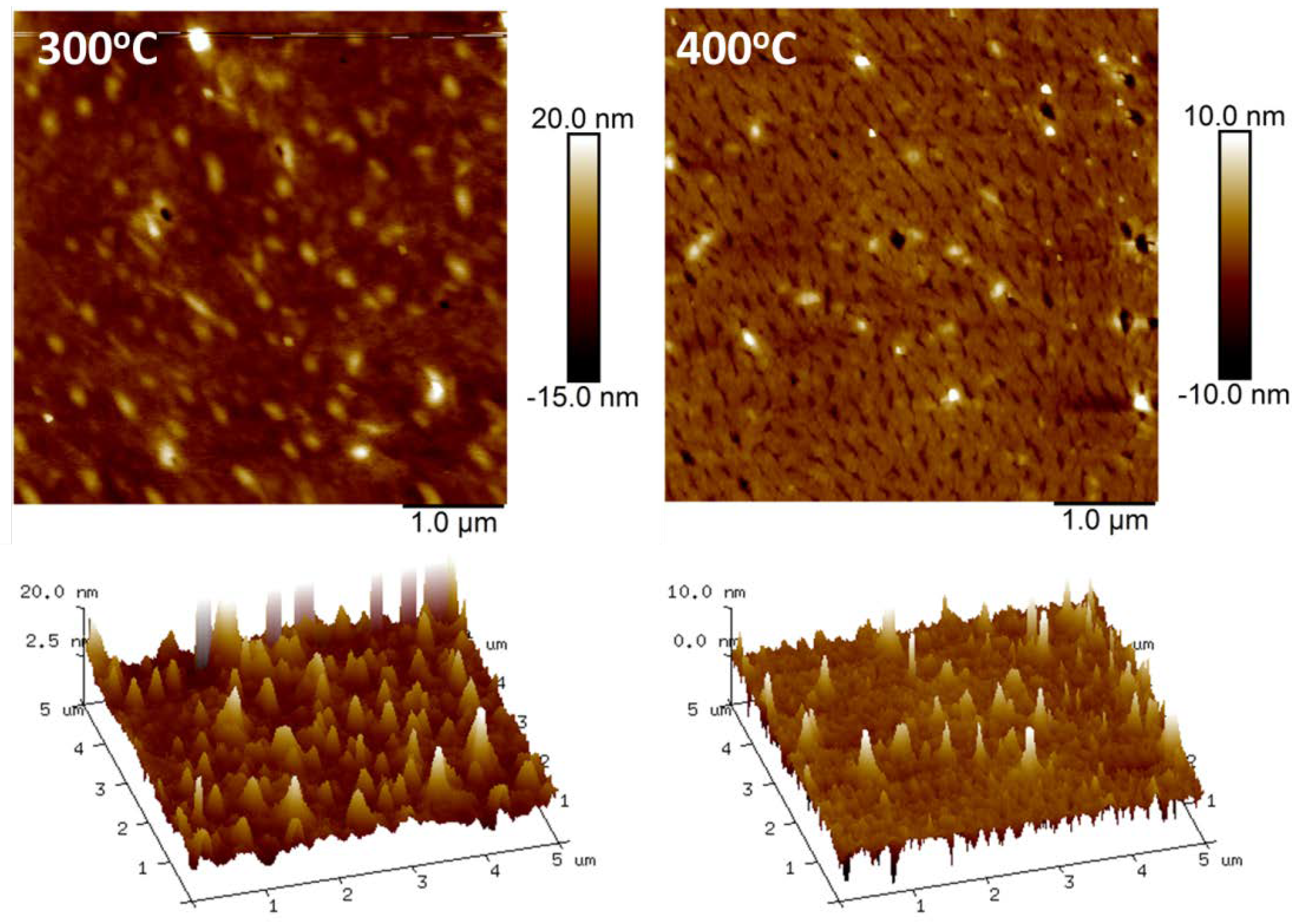
4.1. Atomic Force Microscopy (AFM)
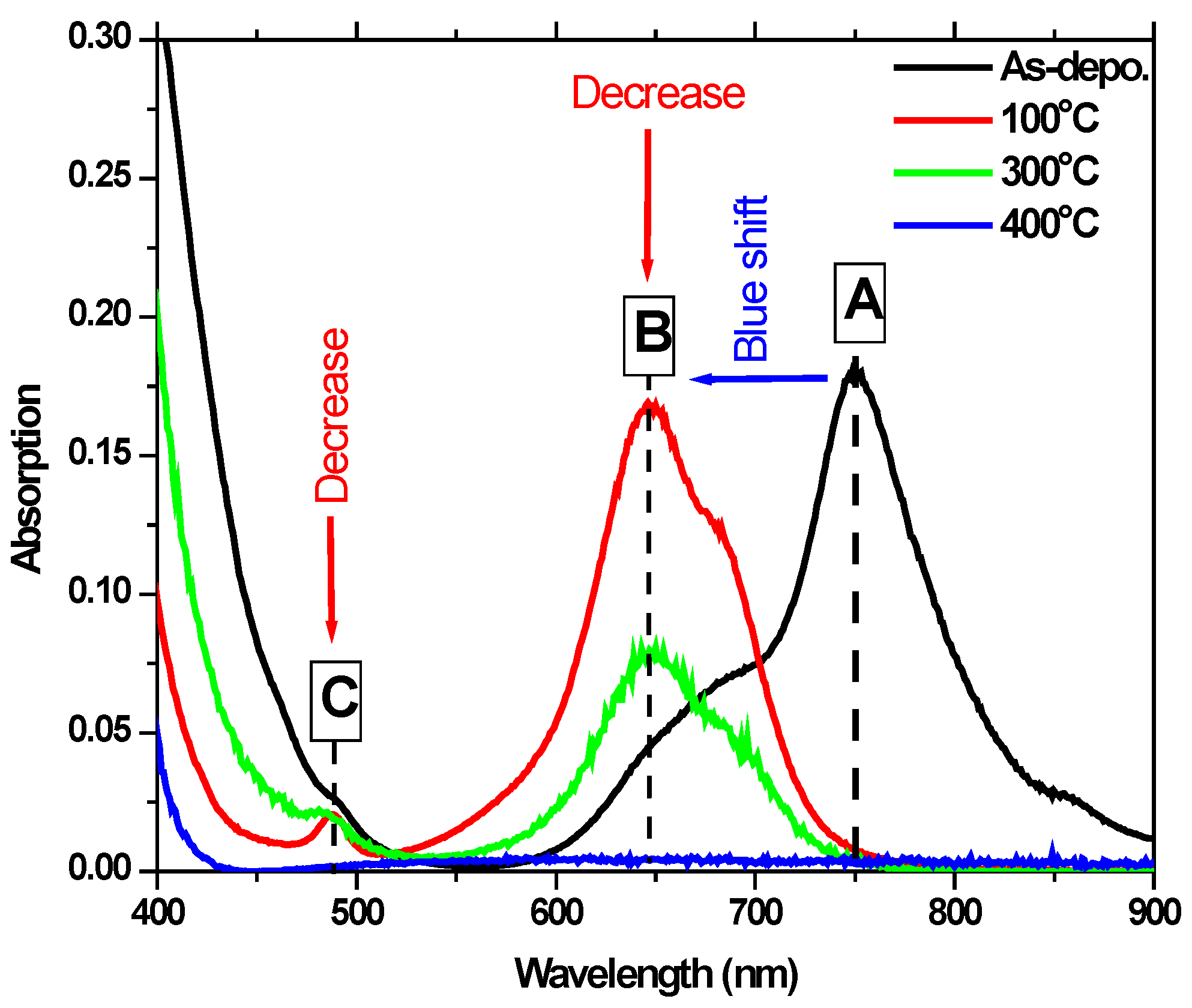
4.2. UV-Vis Absorption Spectroscopy
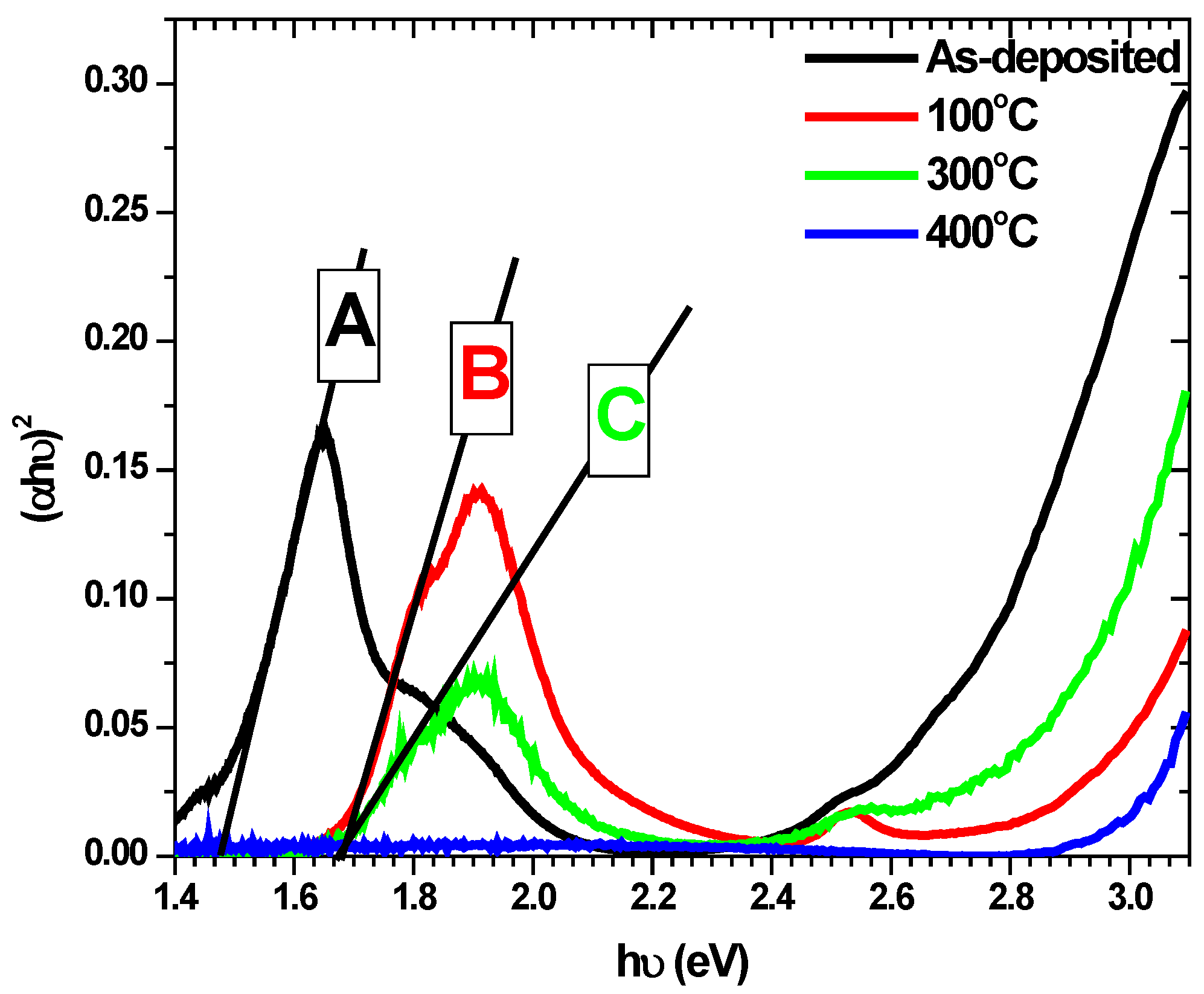
4.3. Band Gap Determination
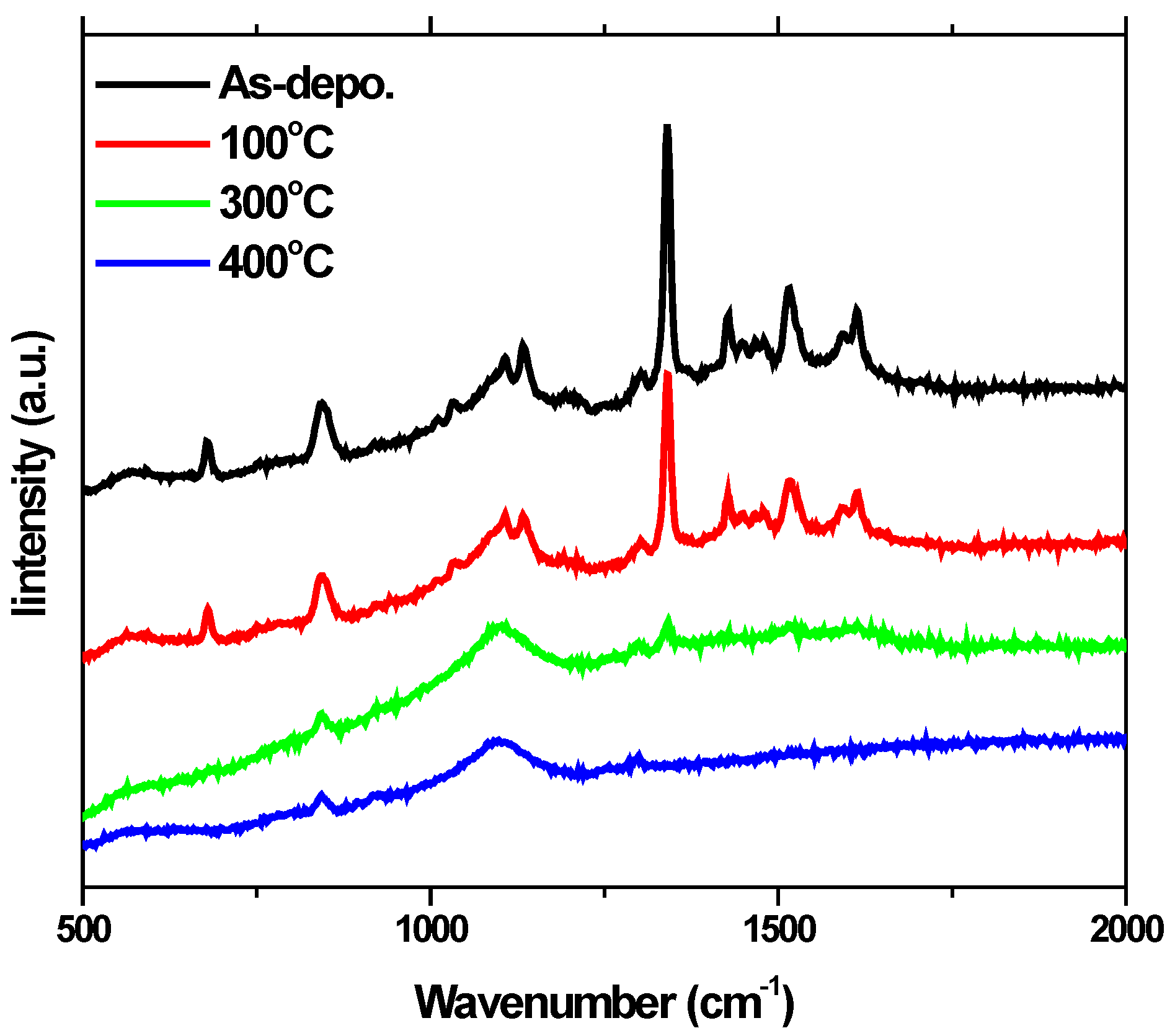
4.4. Raman Spectra
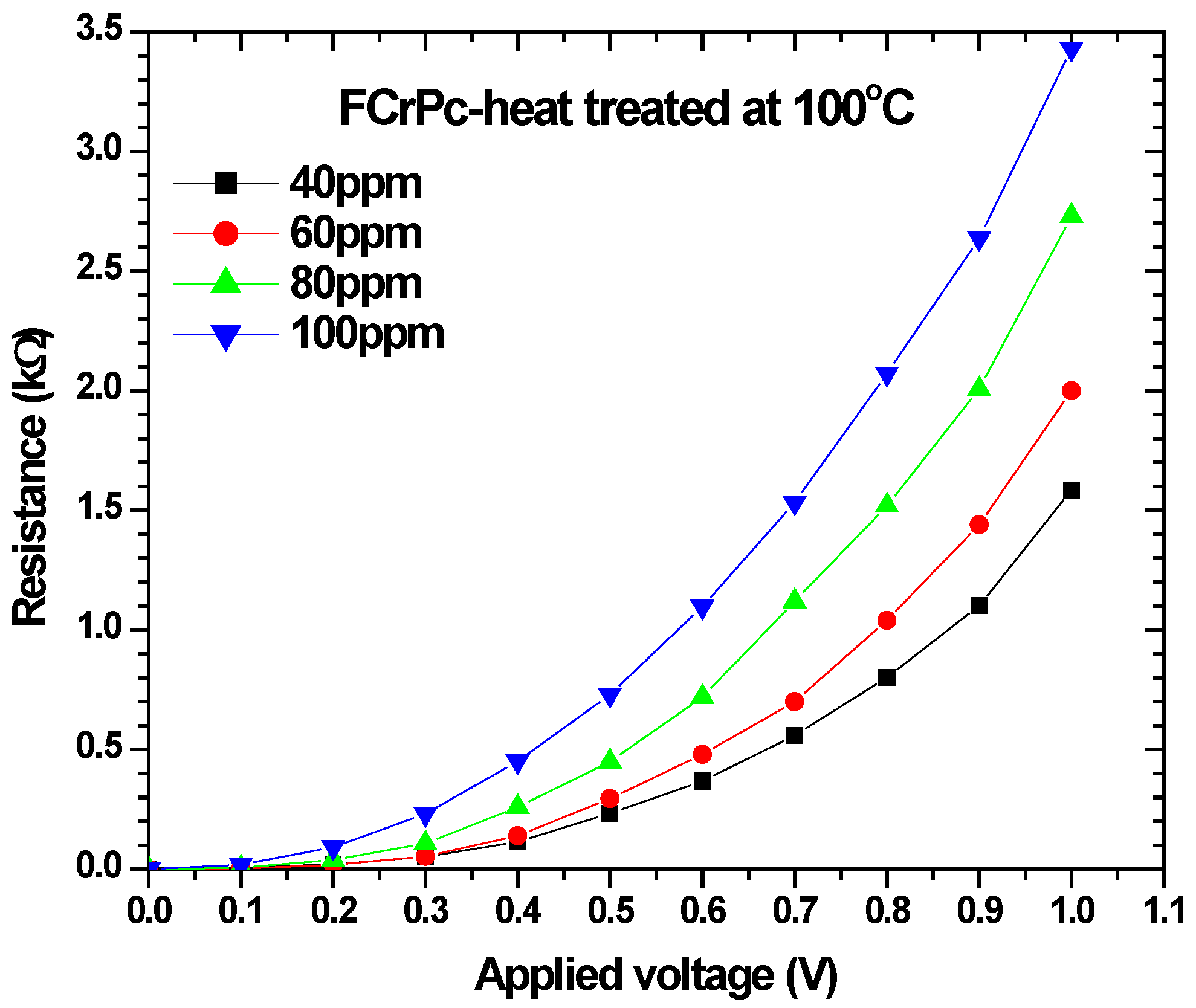
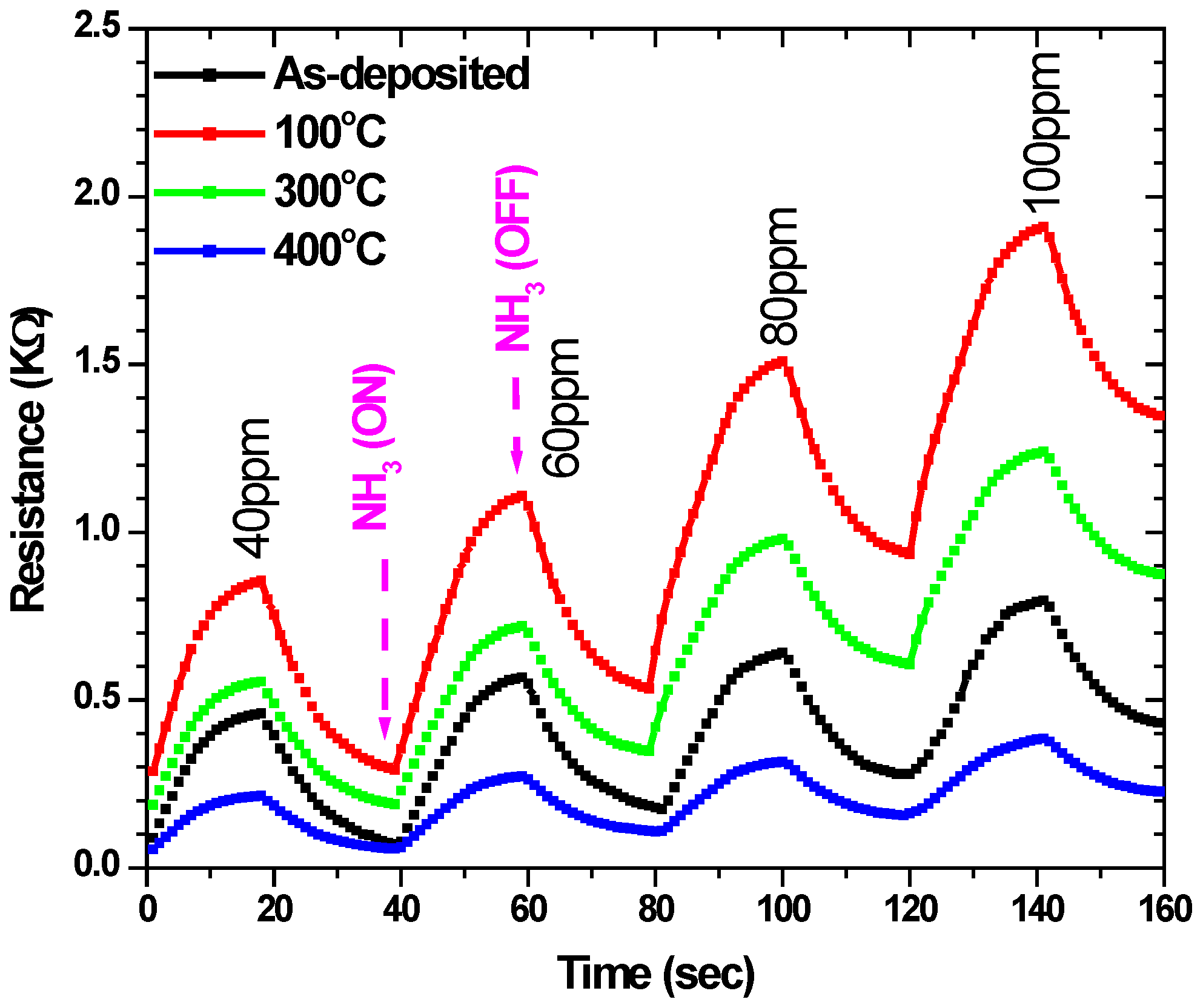
4.5. FCrPc Films Interaction with Amonia Gas
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Jatoi, A.W.; Kyohei, Y.; Kim, K.O.; Song, K.H.; Lee, J.; Zhu, C.; Tsuiki, H.; Kim, I.S. Deodorant activity of phthalocyanine complex nanofiber. Text. Res. J. 2018, 88, 630–635. [Google Scholar] [CrossRef]
- Fomo, G.; Nwaji, N.; Nyokong, T. Low symmetric metallophthalocyanine modified electrode via click chemistry for simultaneous detection of heavy metals. J. Electroanal. Chem. 2018, 813, 58–66. [Google Scholar] [CrossRef]
- de Lucena, N.C.; Miyazaki, C.M.; Shimizu, F.M.; Constantino, C.J.; Ferreira, M. Layer-by-layer composite film of nickel phthalocyanine and montmorillonite clay for synergistic effect on electrochemical detection of dopamine. Appl. Surf. Sci. 2018, 436, 957–966. [Google Scholar] [CrossRef]
- Banimuslem, H.; Hassan, A.; Basova, T.; Yushina, I.; Durmuş, M.; Tuncel, S.; Esenpınar, A.A.; Gürek, A.G.; Ahsen, V. Copper phthalocyanine functionalized single-walled carbon nanotubes: Thin film deposition and sensing properties. Key Eng. Mater. 2014, 605, 461–464. [Google Scholar] [CrossRef]
- Banimuslem, H.; Hassan, A.; Basova, T.; Gülmez, A.D.; Tuncel, S.; Durmus, M.; Gürek, A.G.; Ahsen, V. Copper phthalocyanine/single walled carbon nanotubes hybrid thin films for pentachlorophenol detection. Sens. Actuators B Chem. 2014, 190, 990–998. [Google Scholar] [CrossRef]
- Basova, T.; Hassan, A.; Yuksel, F.; Gürek, A.G.; Ahsen, V. Optical detection of pentachlorophenol in water using thin films of octa-tosylamido substituted zinc phthalocyanine. Sens. Actuators B Chem. 2010, 150, 523–528. [Google Scholar] [CrossRef]
- Diallo, A.K.; Tardy, J.; Zhang, Z.Q.; Bessueille, F.; Jaffrezic-Renault, N.; Lemiti, M. Trimethylamine biosensor based on pentacene enzymatic organic field effect transistor. Appl. Phys. Lett. 2009, 94, 263302. [Google Scholar] [CrossRef]
- Geng, B.; Zhan, F.; Jiang, H.; Xing, Z.; Fang, C. Facile production of self-assembly hierarchical dumbbell-like COOH nanostructures and their room-temperature CO-gas-sensing properties. Cryst. Growth Des. 2008, 8, 3497–3500. [Google Scholar] [CrossRef]
- Ryer, J.E. Helth effect of ammonia. Plant. Oper. Prog. 1991, 10, 228–232. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Van den Breg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Gu, C.; Yu, K.; Mrng, F.; Liu, J. A novel highly sensitive gas ionization sensor for ammonia detection. Sens. Actuators A Phys. 2009, 150, 218–223. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline-titanium dioxide nanocomposite thin film. Sens. Actuators B Chem. 2007, 125, 644–650. [Google Scholar] [CrossRef]
- Fadlallah, M.; Eckern, U.; Romero, A.; Schwingenschlogl, U. Electronic transport properties of (fluorinated) metal phthalocyanine. New J. Phys. 2016, 18, 013003. [Google Scholar] [CrossRef]
- Selva, M. Green approaches to highly selective processes: Reactions of dimethyl carbonate over both zeolites and base catalysts. Pure Appl. Chem. 2007, 79, 1855–1867. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, N.; Samanta, S.; Singh, A.; Debnath, A.K.; Chauhan, A.K.; Roy, M.; Prasad, R.; Roy, K.; Chehimi, M.M.; et al. Room temperature detection of H2S by flexible gold–cobalt phthalocyanine heterojunction thin films. Sens. Actuators B Chem. 2015, 206, 653–662. [Google Scholar] [CrossRef]
- Banimuslem, H.; Hassan, A.; Basova, T.; Esenpınar, A.A.; Tuncel, S.; Durmuş, M.; Gürek, A.G.; Ahsen, V. Dye-modified carbon nanotubes for the optical detection of amines vapours. Sens. Actuators B Chem. 2015, 207, 224–234. [Google Scholar] [CrossRef]
- Banimuslem, H.; Hassan, A.; Basova, T.; Durmus, M.; Tuncel, S.; Esenpınar, A.A.; Gürek, A.G.; Ahsen, V. Copper Phthalocyanine Functionalized Single-Walled Carbon Nanotubes: Thin Films for Optical Detection. J. Nanosci. Nanotechnol. 2015, 15, 2157–2167. [Google Scholar] [CrossRef]
- Snow, A.; Barger, W. Phthalocyanine films in chemical sensors. In Phthalocyanine Properties and Applications; Leznoff, A., Ed.; Wiley-VCH: Weinheim, Germany, 1989; pp. 341–392. [Google Scholar]
- Miyata, T.; Kawaguchi, S.; Ishii, M.; Minami, T. High sensitivity chlorine gas sensors using Cu phthalocyanine thin films. Thin Solid Films 2003, 425, 255–259. [Google Scholar] [CrossRef]
- Bohrer, F.I.; Colesniuc, C.N.; Park, J.; Schuller, I.K.; Kummel, A.C.; Trogler, W.C. Selective detection of vapor phase hydrogen peroxide with phthalocyanine chemiresistors. J. Am. Chem. Soc. 2008, 130, 3712–3723. [Google Scholar] [CrossRef]
- Evyapan, M.; Kadem, B.; Basova, T.V.; Yushina, I.V.; Hassan, A.K. Study of the sensor response of spun metal phthalocyanine films to volatile organic vapors using surface plasmon resonance. Sens. Actuators B Chem. 2016, 236, 605–613. [Google Scholar] [CrossRef]
- He, N.; Chen, Y.; Bai, J.; Wang, J.; Blau, W.; Zhu, J. Preparation and optical limiting properties of multiwalled carbon nanotubes with -conjugated metal-free phthalocyanine moieties. J. Phys. Chem. C 2009, 113, 13029–13035. [Google Scholar] [CrossRef]
- Basova, T.; Koltsov, E.; Gurek, A.; Atilla, D.; Ahsen, V.; Hassan, A. Investigation of liquid crystalline behavior of copper oktakisalkylthiophthalocyanine and its film properties. Mater. Sci. Eng. C 2008, 28, 303–308. [Google Scholar] [CrossRef]
- Basova, T.; Durmus, M.; Ahsen, V.; Hassan, A. Effect of interface on the orientation of the liquid crystalline nickel phthalocyanine films. J. Phys. Chem. C 2009, 113, 19251–19257. [Google Scholar] [CrossRef]
- Hassan, B.; Li, H.; McKeown, N. The control of molecular self-association in spin-coated films of substituted phthalocyanines. J. Mater. Chem. 2000, 10, 39–45. [Google Scholar] [CrossRef]
- Hatsusaka, K.; Ohta, K.; Yamamoto, I.; Shirai, H. Discotic liquid crystals of transition metal complexes, part 30: Spontaneous uniform homeotropic alignment of octakis(dialkoxyphenoxy)phthalocyaninatocopper(II) complexes. J. Mater. Chem. 2001, 11, 423–433. [Google Scholar] [CrossRef]
- Basova, T.; Gurek, A.; Ahsen, V. Investigation of liquid-crystalline behavior of nickel octakisalkylthiophtahlocyanines and orientation of their films. Mater. Sci. Eng. 2002, 22, 99–104. [Google Scholar] [CrossRef]
- Jeon, J.W.; Jeon, D.W.; Sahoo, T.; Kim, M.; Baek, J.H.; Hoffman, J.L.; Kim, N.S.; Lee, I.H. Effect of annealing temperature on optical band-gap of amorphous indium zinc oxide film. J. Alloys Compd. 2011, 509, 10062–10065. [Google Scholar] [CrossRef]
- Kadem, B.; Hassan, A.; Cranton, W. Efficient P3HT: PCBM bulk heterojunction organic solar cells; effect of post deposition thermal treatment. J. Mater. Sci. Mater. Electron. 2016, 27, 7038–7048. [Google Scholar] [CrossRef]
- Jain, A.; Sagar, P.; Mehra, R.M. Band gap widening and narrowing in moderately and heavily doped n-ZnO films. Solid-State Electron. 2006, 50, 1420–1424. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous optical absorption limit in InSb. Phys. Rev. 1954, 93, 632. [Google Scholar] [CrossRef]
- Basova, T.V.; Kolesov, B.A.; Gürek, A.G.; Ahsen, V. Raman polarization study of the film orientation of liquid crystalline NiPc. Thin Solid Films 2001, 385, 246–251. [Google Scholar] [CrossRef]
- Srivastava, R.; Lal, P.; Dwivedi, R. Sensing mechanism in tin oxide-based thick film gas sensors. Sens. Actuators B 1994, 21, 213–218. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banimuslem, H.A.; Kadem, B.Y. Fluorinated Chromium Phthalocyanine Thin Films: Characterization and Ammonia Vapor Detection. Chemosensors 2018, 6, 63. https://doi.org/10.3390/chemosensors6040063
Banimuslem HA, Kadem BY. Fluorinated Chromium Phthalocyanine Thin Films: Characterization and Ammonia Vapor Detection. Chemosensors. 2018; 6(4):63. https://doi.org/10.3390/chemosensors6040063
Chicago/Turabian StyleBanimuslem, Hikmat Adnan, and Burak Y. Kadem. 2018. "Fluorinated Chromium Phthalocyanine Thin Films: Characterization and Ammonia Vapor Detection" Chemosensors 6, no. 4: 63. https://doi.org/10.3390/chemosensors6040063
APA StyleBanimuslem, H. A., & Kadem, B. Y. (2018). Fluorinated Chromium Phthalocyanine Thin Films: Characterization and Ammonia Vapor Detection. Chemosensors, 6(4), 63. https://doi.org/10.3390/chemosensors6040063




