Exploring the Emotion of Disgust: Differences in Smelling and Feeling
Abstract
:1. Introduction
2. Experiment 1
2.1. Method
2.1.1. Participants and Design
2.1.2. Materials
Disgust Induction
Odors
Disgust Sensitivity Measurement
2.1.3. Procedure
2.2. Data Analyses
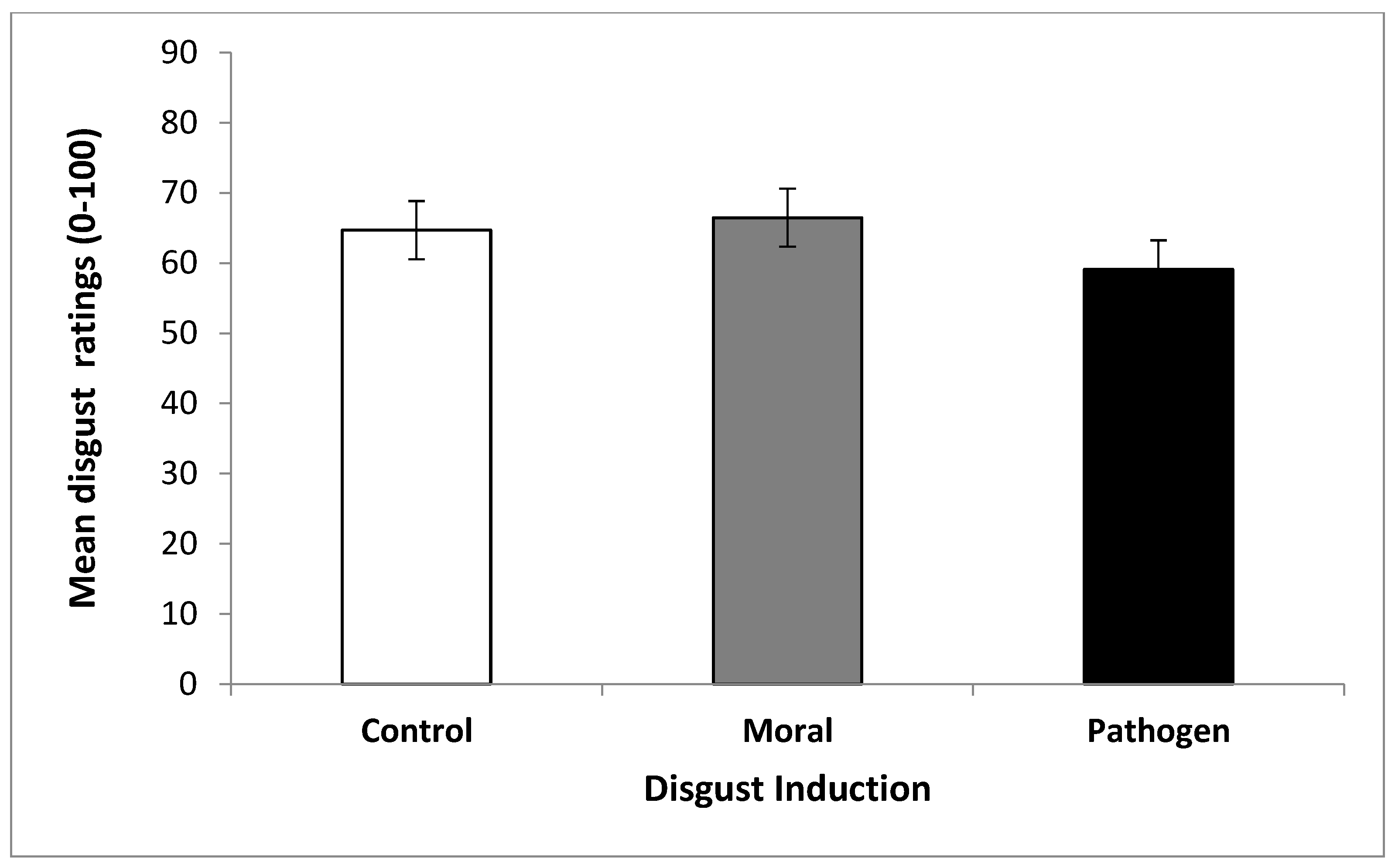
2.3. Results
2.3.1. Odor Ratings
2.3.2. Disgust Sensitivity—TDD
2.4. Discussion of Study 1
3. Experiment 2
3.1. Method
3.1.1. Participants and Design
3.1.2. Materials
Odor
Odor and Emotion Ratings
3.1.3. Procedure
3.2. Data Analyses
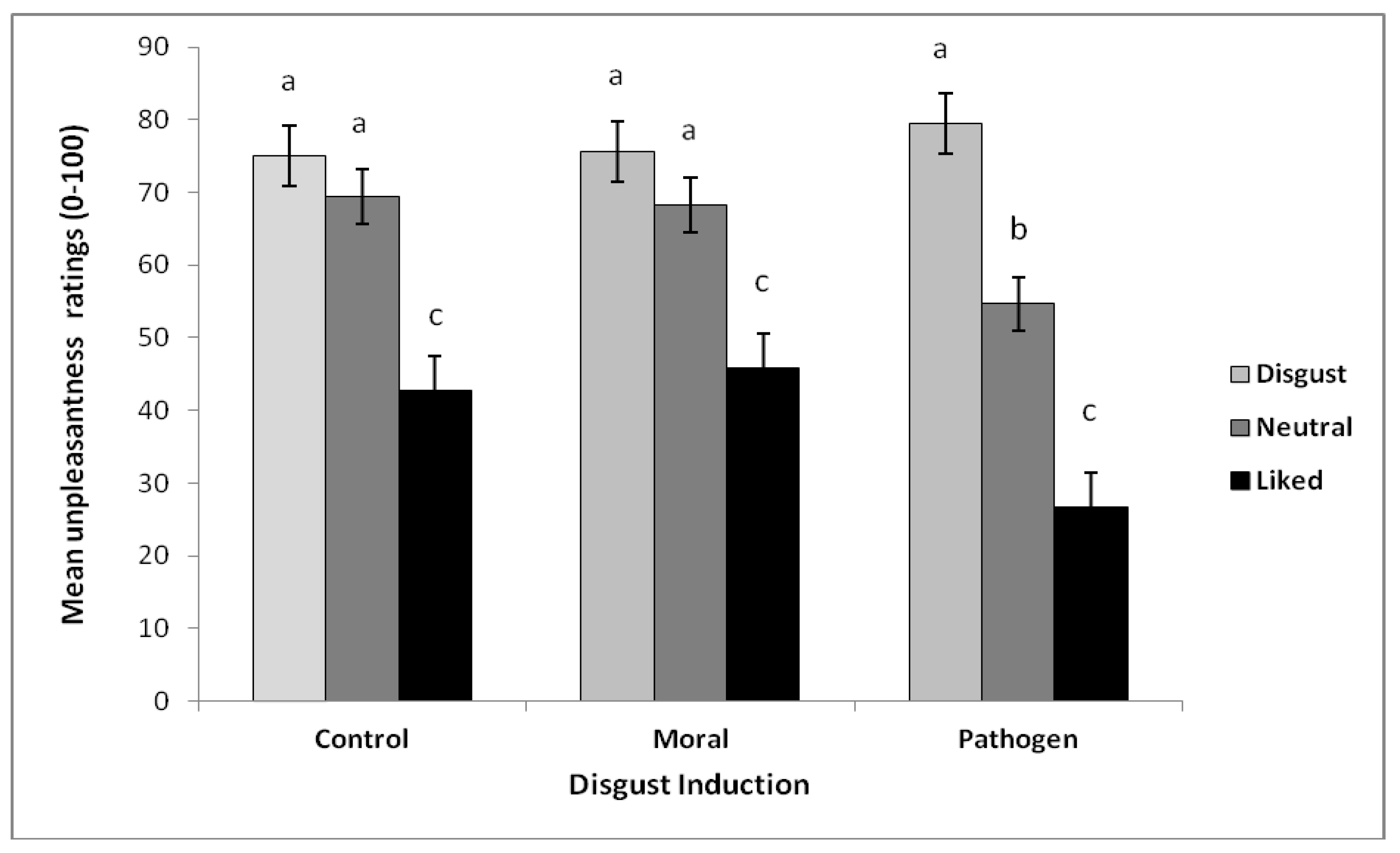
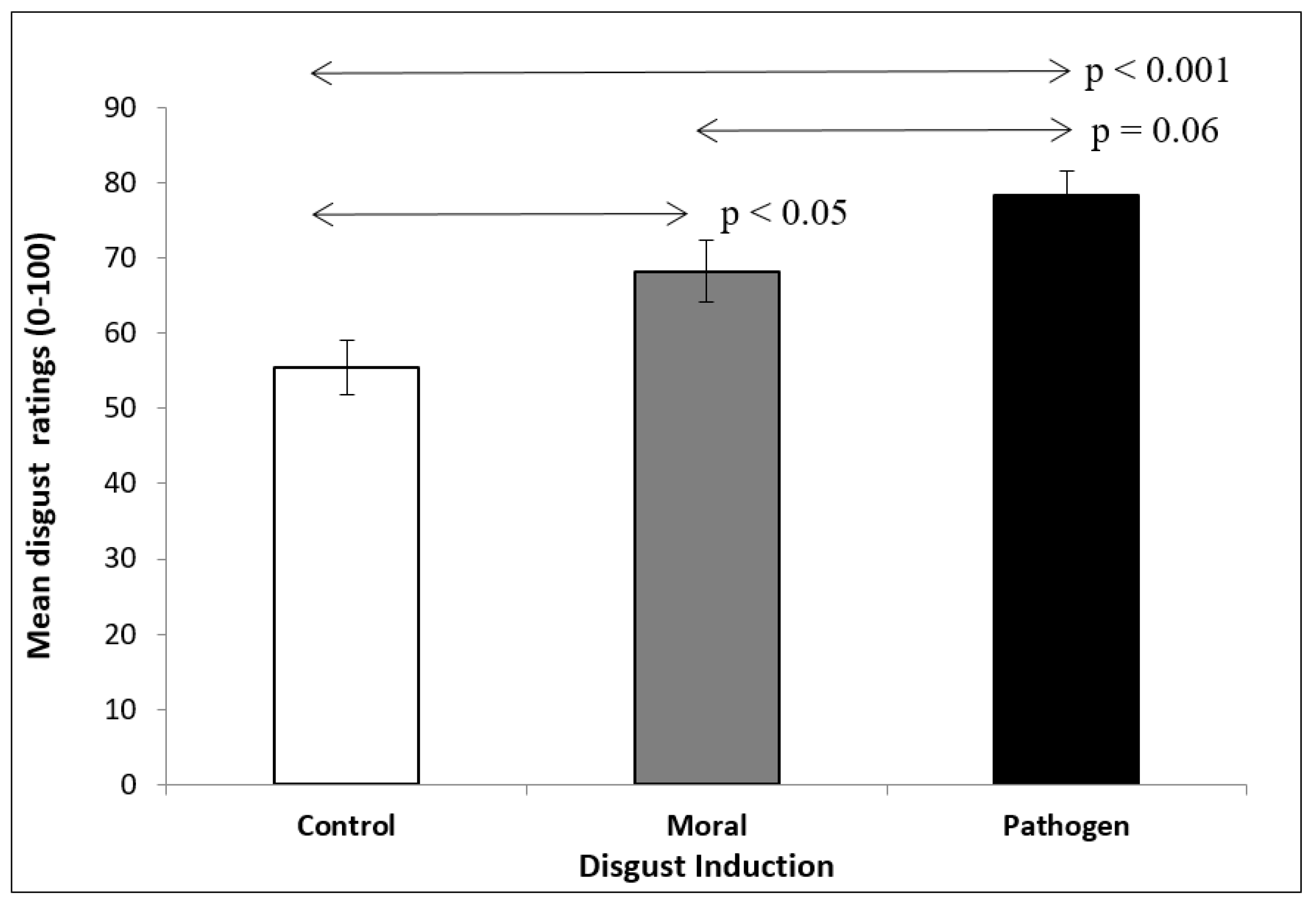
3.3. Results
3.3.1. Odor Test
3.3.2. Effects on Emotion
3.4. Disgust Sensitivity—TDD
Correlations
3.5. General Discussion
Ethical Approval
Acknowledgments
Author Contributions
Informed Consent
Conflicts of Interest
References
- Tybur, J.M.; Lieberman, D.; Griskevicius, V. Microbes, Mating, and Morality: Individual Differences in Three Functional Domains of Disgust. J. Personal. Soc. Psychol. 2009, 97, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Rozin, P.; Fallon, A.E. A perspective on disgust. Psychol. Rev. 1987, 94, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Anderson, A.K. Understanding disgust. Ann. N. Y. Acad. Sci. 2012, 1251, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Eskine, K.J.; Kacinik, N.A.; Prinz, J.J. A Bad Taste in the Mouth: Gustatory Disgust Influences Moral Judgment. Psychol. Sci. 2011, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.F.; Goodwin, G.P. Does incidental disgust amplify moral judgment? A meta-analytic review of experimental evidence. Perspect. Psychol. Sci. 2015, 10, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Herz, R. PROP Taste sensitivity is related to visceral but not moral disgust. Chemosens. Percept. 2011, 4, 72–79. [Google Scholar] [CrossRef]
- Horberg, E.J.; Oveis, C.; Keltner, D.; Cohen, A.B. Disgust and the moralization of purity. J. Personal. Soc. Psychol. 2009, 97, 963. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. The Expression of Emotions in Man and Animal; University of Chicago Press: Chicago, IL, USA, 1965. [Google Scholar]
- Stevenson, R.J. An Initial Evaluation of the Functions of Human Olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D. The role of the Chemical Senses in Disgust’s Disease Avoidance. Chem. Senses 2017, 42, 455–456. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Boakes, R.A.; Wilson, J.P. Counter-conditioning following human odor-taste and color-taste learning. Learn. Motiv. 2000, 31, 114–127. [Google Scholar] [CrossRef]
- Prescott, J.; Taylor, A.; Roberts, D. Psychological processes in flavour perception. In Flavor Perception; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 256–277. [Google Scholar]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Fitness, J. Moral Hypervigilance: The Influence of Disgust Sensitivity in the Moral Domain. Emotion 2008, 8, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Curtis, V.; de Barra, M.; Aunger, R. Disgust as an adaptive system for disease avoidance behaviour. Philos. Trans. R. Soc. B 2011, 366, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Oaten, M.; Stevenson, R.J.; Case, T.I. Disgust as a Disease-Avoidance Mechanism. Psychol. Bull. 2009, 135, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Hennig, J.; Possel, P.; Netter, P. Sensitivity to disgust as an indicator of neuroticism: A psychobiological approach. Personal. Individ. Differ. 1996, 20, 589–596. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Maboshe, W.; Hummel, T. Habituation effects of pleasant and unpleasant odors. Int. J. Psychophysiol. 2013, 88, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Bensafi, M.; Sobel, N.; Khan, R.M. Hedonic-specific activity in piriform cortex during odor imagery mimics that during odor perception. J. Neurophysiol. 2007, 98, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Schnall, S.; Haidt, J.; Clore, G.L.; Jordan, A.H. Disgust as embodied moral judgment. Personal. Soc. Psychol. Bull. 2008, 34, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Kim, D.A.; Susskind, J.M.; Anderson, A.K. In Bad Taste: Evidence for the Oral Origins of Moral Disgust. Science 2009, 323, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.J.; Lundström, J.N.; Kimball, B.A.; Gordon, A.R.; Karshikoff, B.; Hosseini, N.; Sorjonen, K.; Olgart Höglund, C.; Solares, C.; Soop, A.; et al. The scent of disease: Human body odor contains an early chemosensory cue of sickness. Psychological Sci. 2014, 25, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Regenbogen, C.; Axelsson, J.; Lasselin, J.; Porada, D.K.; Sundelin, T.; Peter, M.G.; lsson, M.J. Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl. Acad. Sci. USA 2011, 114, 6400–6405. [Google Scholar] [CrossRef] [PubMed]
- Curtis, V.; Aunger, R.; Rabie, T. Evidence that disgust evolved to protect from risk of disease. Proc. R. Soc. Lond. Ser. B 2004, 271, S131–S133. [Google Scholar] [CrossRef] [PubMed]
- Oberg, C.; Larsson, M.; Backman, L. Differential sex effects in olfactory functioning: The role of verbal processing. J. Int. Neuropsychol. Soc. 2002, 8, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Fessler, D.M.; Navarrete, C.D. Domain-specific variation in disgust sensitivity across the menstrual cycle. Evol. Hum. Behav. 2003, 24, 406–417. [Google Scholar] [CrossRef]
- Fessler, D.M.; Eng, S.J.; Navarrete, C.D. Elevated disgust sensitivity in the first trimester of pregnancy: Evidence supporting the compensatory prophylaxis hypothesis. Evol. Hum. Behav. 2005, 26, 344–351. [Google Scholar] [CrossRef]
| Group | Group Differences | |||
|---|---|---|---|---|
| Control (n = 30) | Moral (n = 30) | Pathogen (n = 30) | ||
| Age | 28.3 (1.9) | 28.3 (1.9) | 29.7 (1.8) | F < 1, NS |
| Anxiety | 8.0 (0.6) | 6.1 (0.5) | 7.6 (0.5) | F = 3.54, p = 0.03 |
| Depression | 2.6 (0.5) | 2.0 (0.3) | 2.6 (0.2) | F < 1, NS |
| Hunger | 36.8 (4.3) | 37.5 (5.1) | 44.3 (4.9) | F < 1, NS |
| Group | |||
|---|---|---|---|
| Control (n = 30) | Moral (n = 30) | Pathogen (n = 30) | |
| TDD (Moral) | 31.9 (0.8) | 30.6 (0.9) | 30.9 (1.2) |
| TDD (Sexual) | 26.4 (1.4) | 27.7 (1.3) | 24.4 (1.3) |
| TDD (Pathogen) | 30.9 (1.2) | 24.4 (1.3) | 26.9 (0.9) |
| Group | |||
|---|---|---|---|
| Control (n = 24) | Moral (n = 23) | Pathogen (n = 23) | |
| Age | 21.0 (0.5) | 20.7 (0.3) | 20.9 (0.3) |
| Odor Disgust | 55.4 (3.6) | 68.2 (4.1) | 78.2 (3.3) |
| Odor Unpleasantness | 77.0 (2.4) | 81.7 (4.6) | 90.4 (3.2) |
| Odor Intensity | 48.7 (4.4) | 57.8 (3.9) | 69.1 (3.1) |
| Feelings of Disgust | 44.1 (4.1) | 50.1 (4.4) | 65.7 (3.7) |
| Feelings of Anger | 12.9 (1.9) | 35.2 (3.3) | 16.1 (3.4) |
| TDD (Moral) | 29.7 (1.2) | 25.7 (1.1) | 24.7 (1.3) |
| TDD (Sexual) | 17.5 (1.6) | 21.5 (1.5) | 22.6 (1.5) |
| TDD (Pathogen) | 30.2 (1.0) | 26.0 (1.2) | 29.7 (1.0) |
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| 1. | TDD-P | 1 | 0.52 * | 0.34 | 0.46 * | 0.60 ** | 0.15 |
| 2. | Odor Disgust | 1 | 0.70 ** | 0.78 ** | 0.69 ** | 0.33 | |
| 3. | Odor Intensity | 1 | 0.46 * | 0.66 * | −0.15 | ||
| 4. | Odor Unpleasantness | 1 | 0.64 * | 0.26 | |||
| 5. | Feelings of disgust | 1 | 0.21 | ||||
| 6. | Feelings of anger | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafford, L.D.; Fleischman, D.S.; Le Her, N.; Hummel, T. Exploring the Emotion of Disgust: Differences in Smelling and Feeling. Chemosensors 2018, 6, 9. https://doi.org/10.3390/chemosensors6010009
Stafford LD, Fleischman DS, Le Her N, Hummel T. Exploring the Emotion of Disgust: Differences in Smelling and Feeling. Chemosensors. 2018; 6(1):9. https://doi.org/10.3390/chemosensors6010009
Chicago/Turabian StyleStafford, Lorenzo D., Diana S. Fleischman, Nicholas Le Her, and Thomas Hummel. 2018. "Exploring the Emotion of Disgust: Differences in Smelling and Feeling" Chemosensors 6, no. 1: 9. https://doi.org/10.3390/chemosensors6010009
APA StyleStafford, L. D., Fleischman, D. S., Le Her, N., & Hummel, T. (2018). Exploring the Emotion of Disgust: Differences in Smelling and Feeling. Chemosensors, 6(1), 9. https://doi.org/10.3390/chemosensors6010009





