Nanoparticle-Coated Optical Hydrogen Sensor for Early Gas Detection of Lithium-Ion Battery Failure
Abstract
1. Introduction
2. Materials and Methods
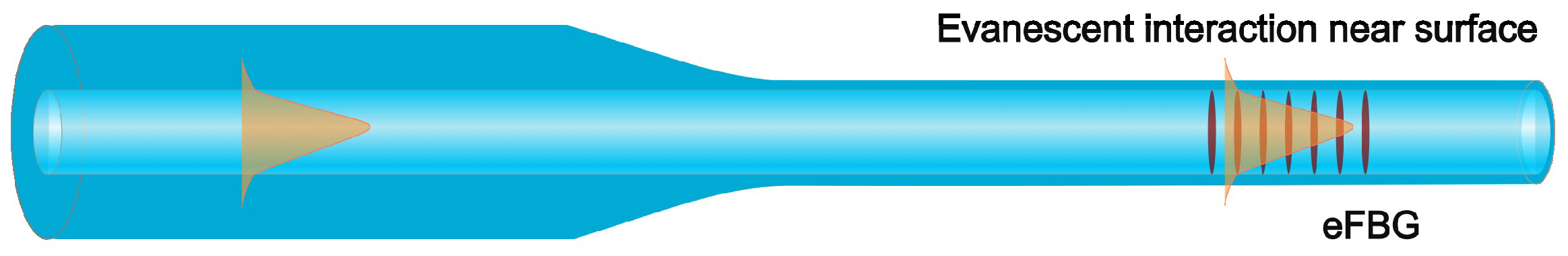
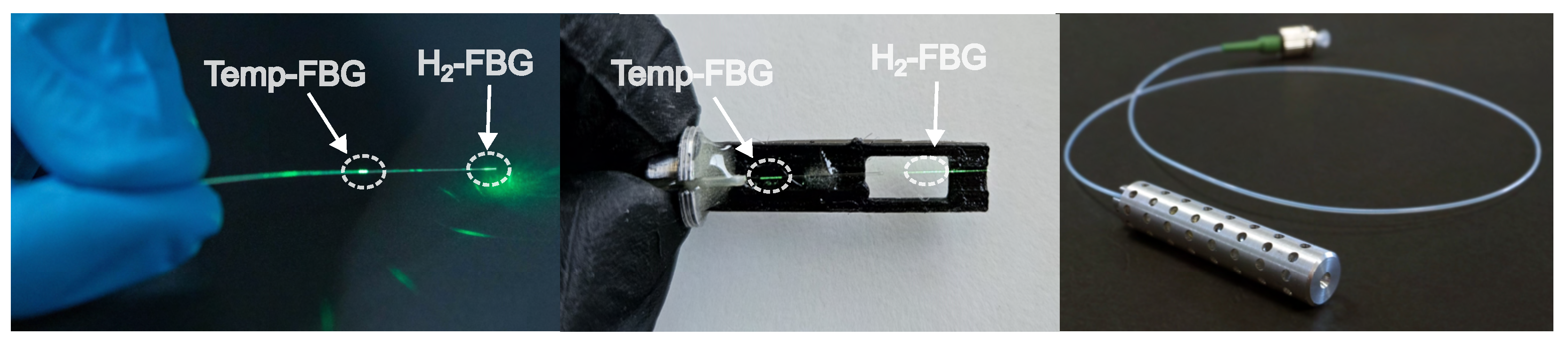
2.1. Optical Fiber Hydrogen-Detection Sensor
2.2. Device Under Test
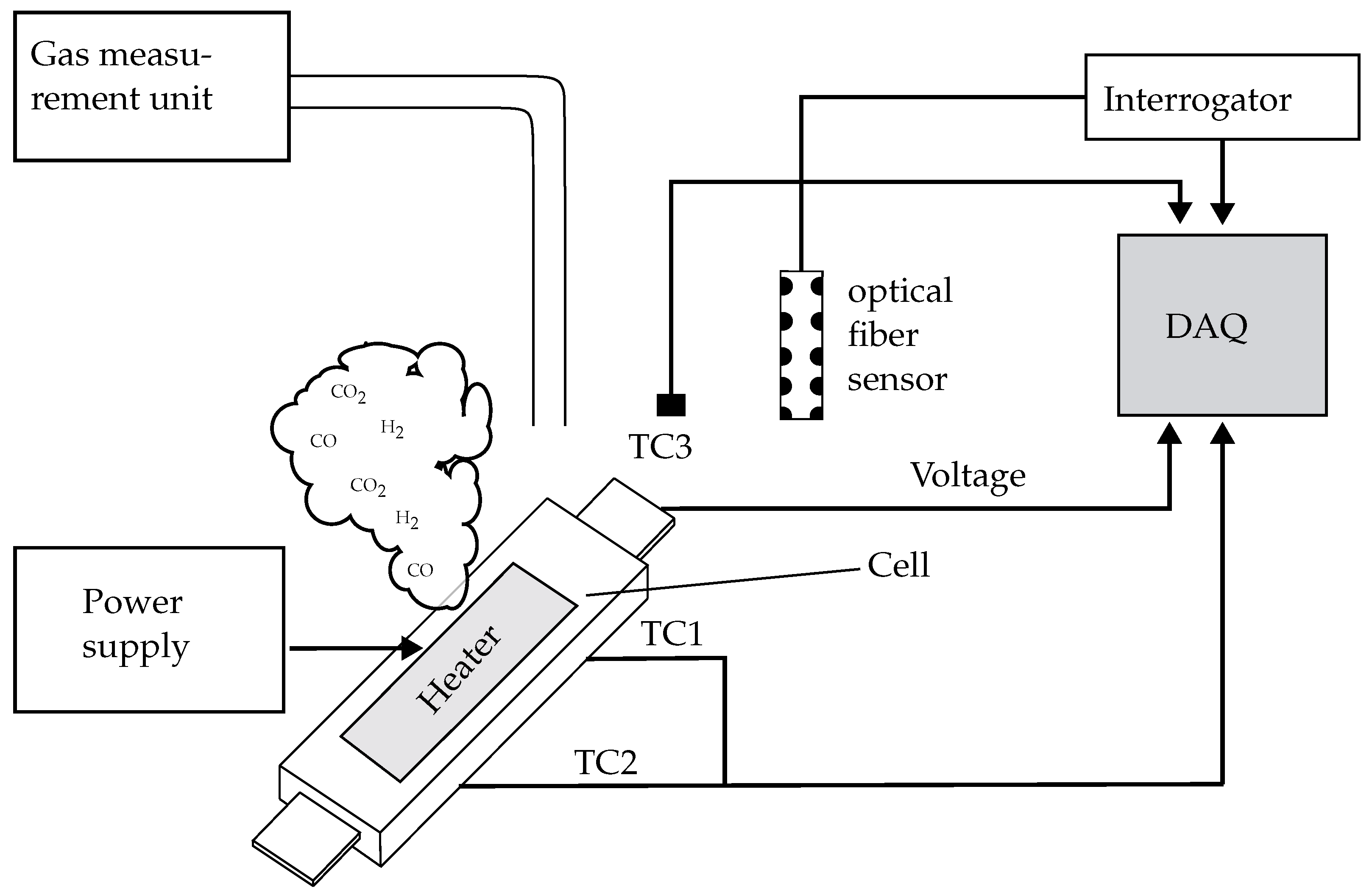
2.3. Experimental Setup and Procedure
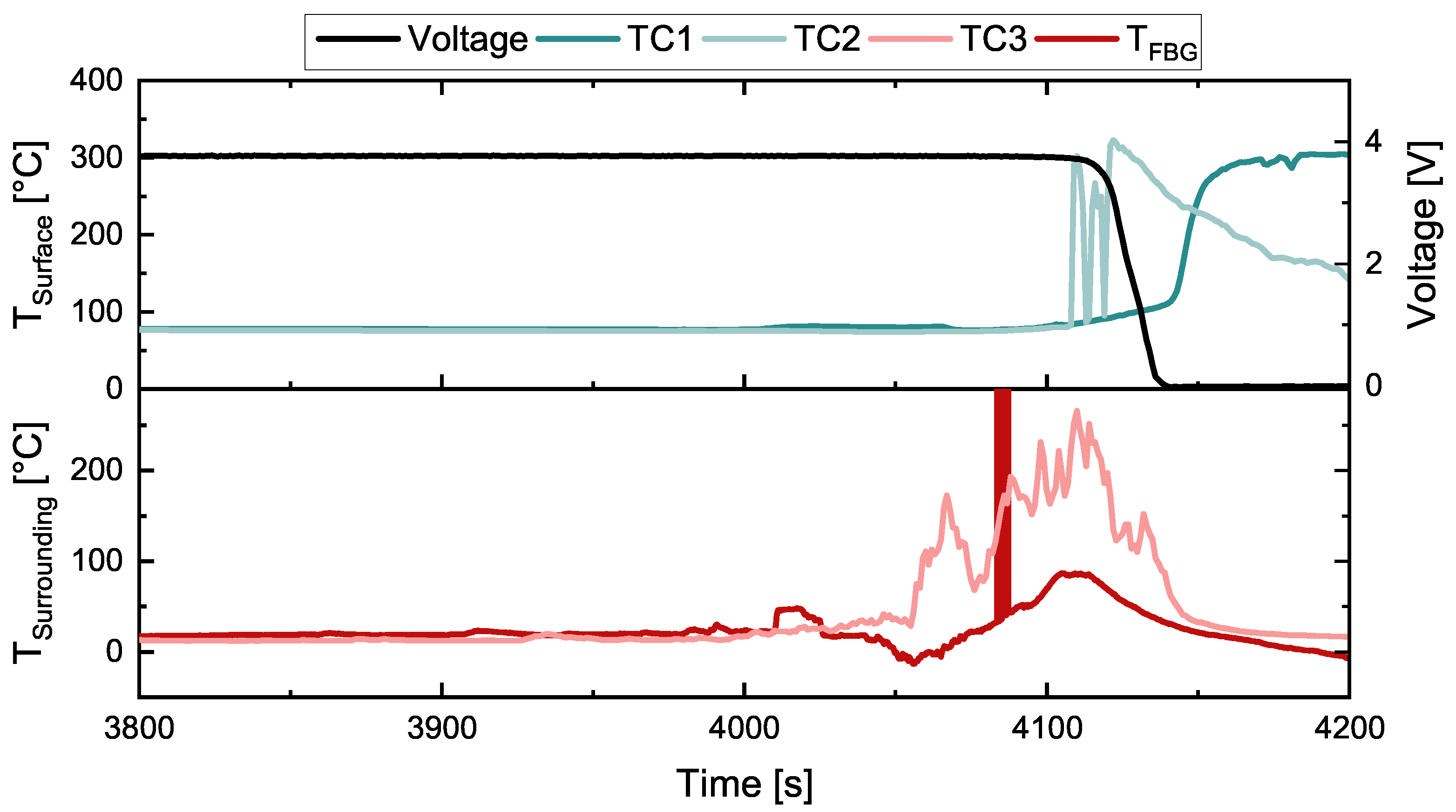
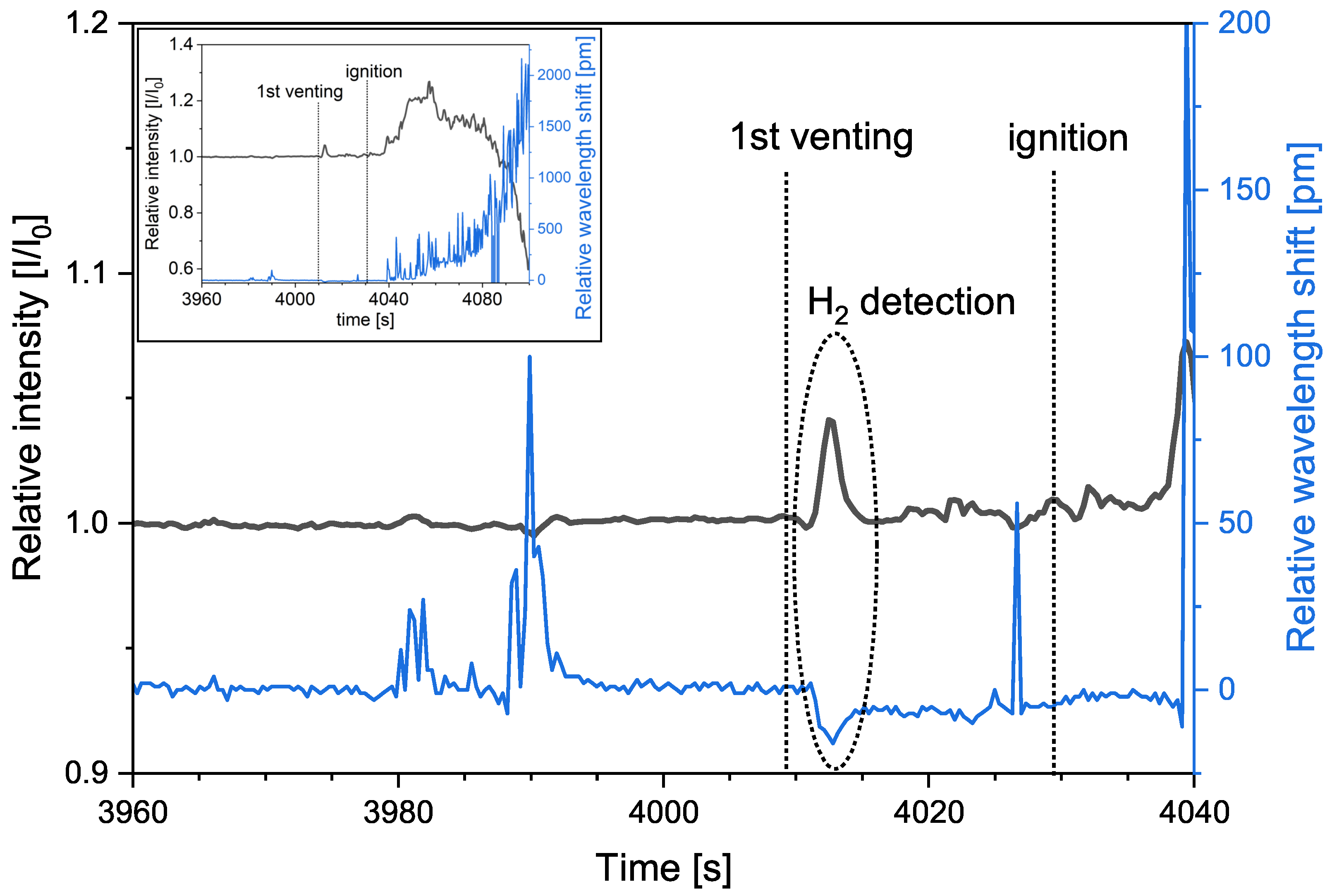
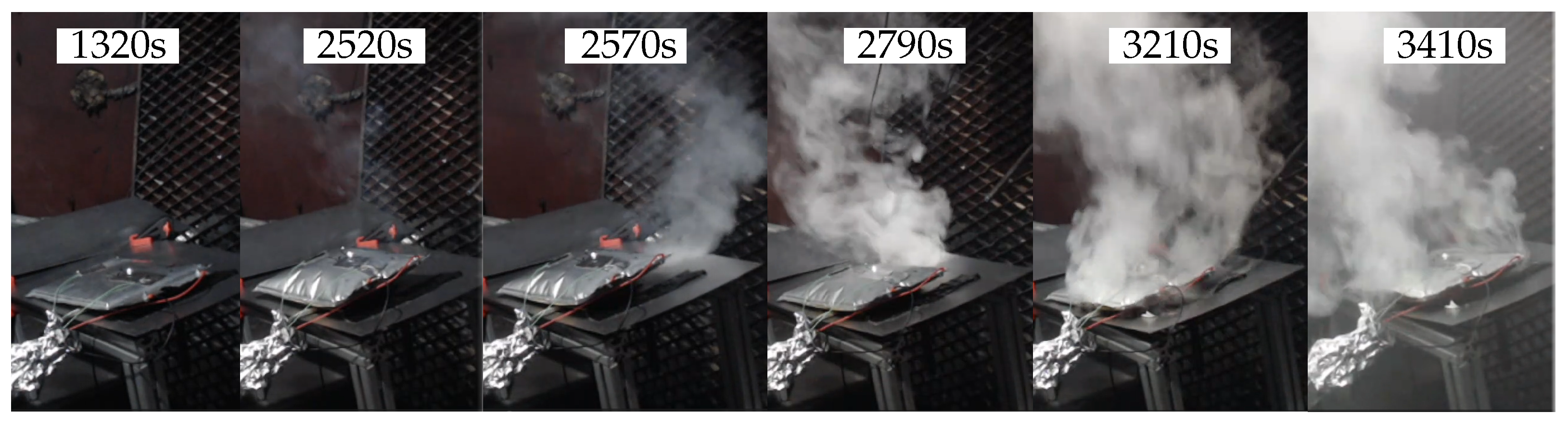
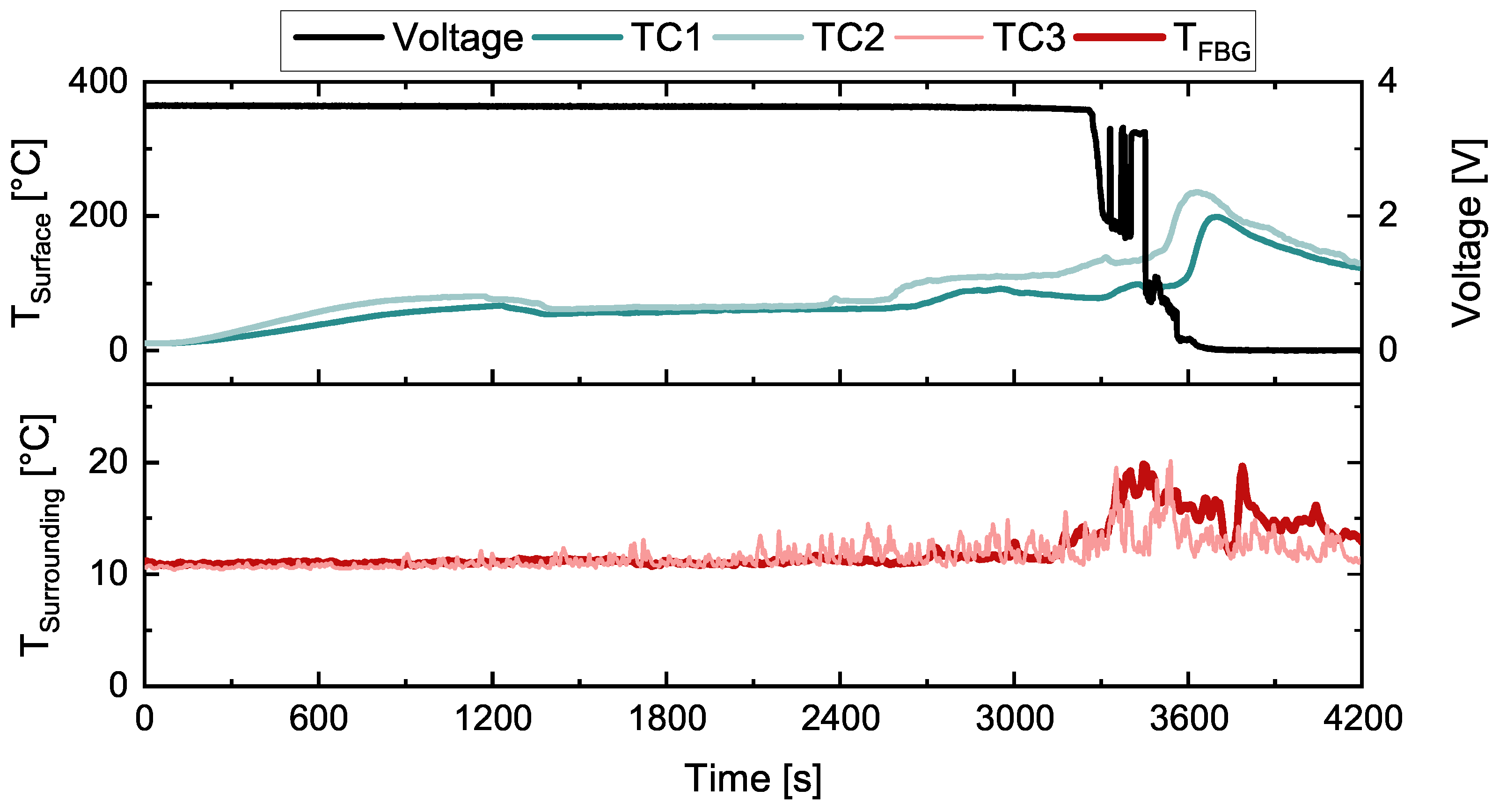
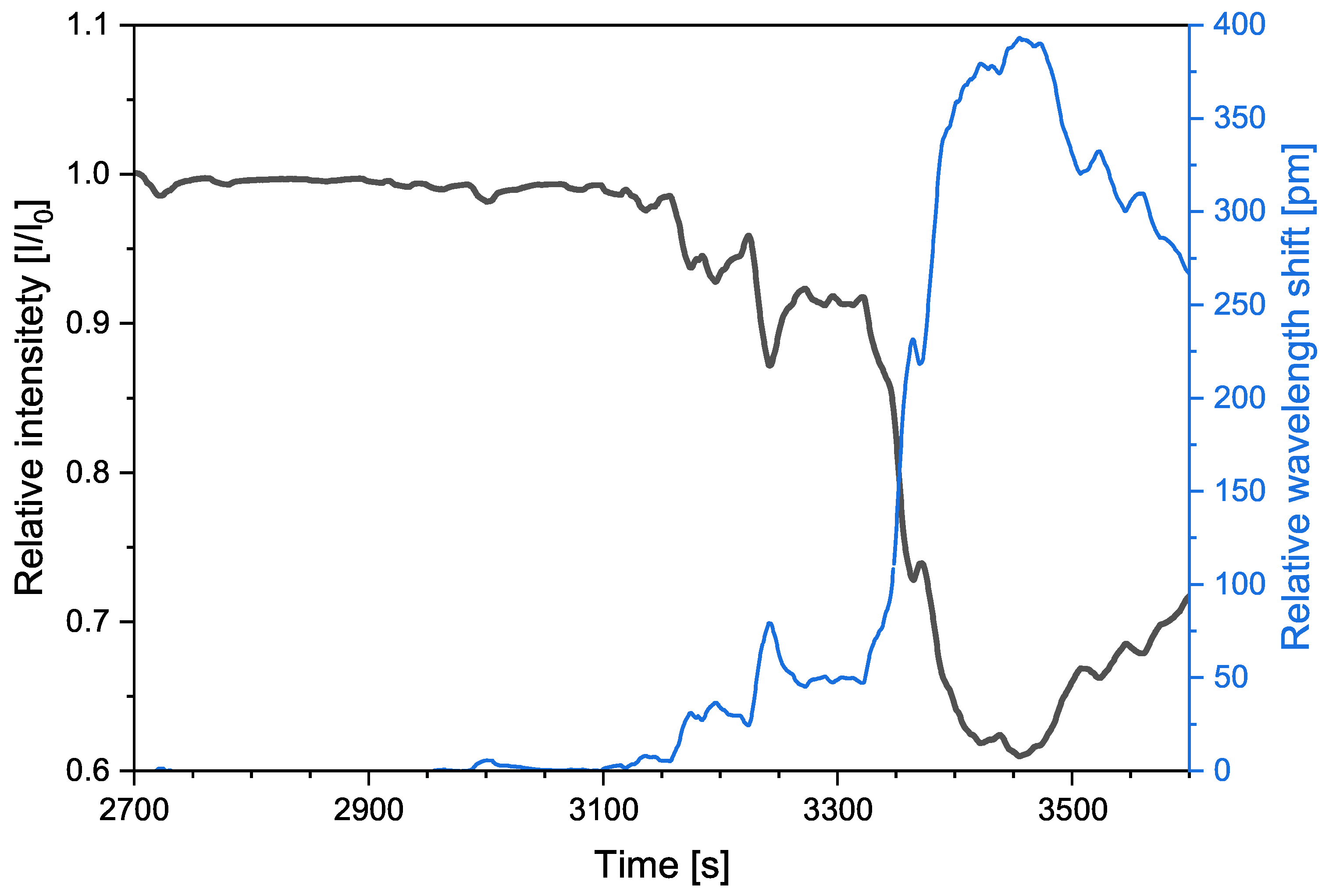
3. Results
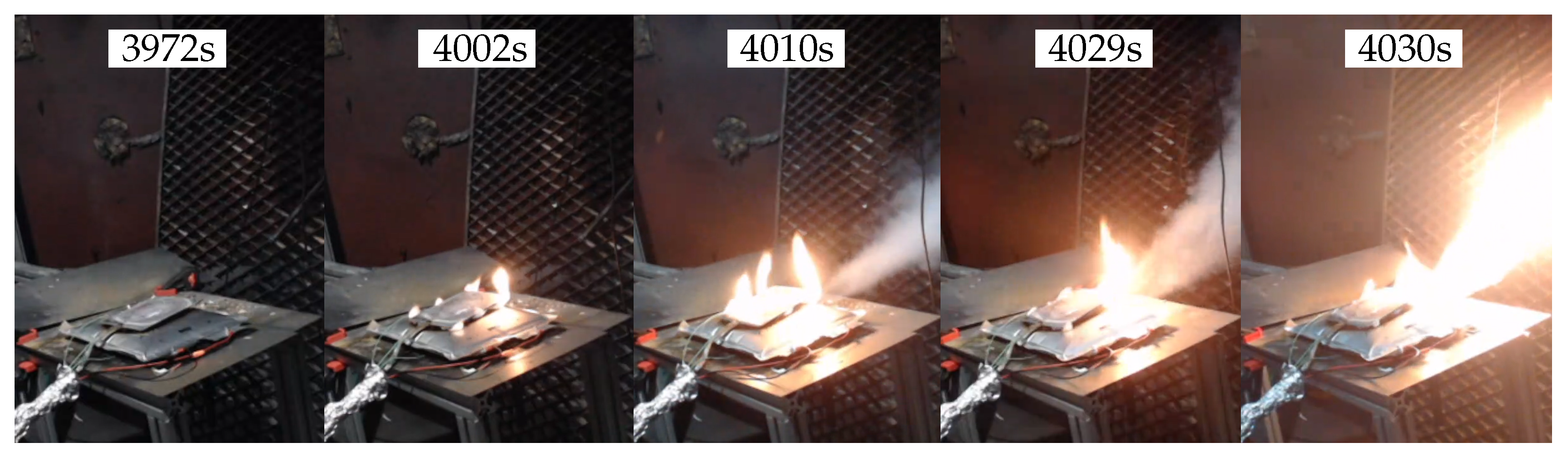
3.1. First Test
3.2. Second Test
3.3. Influence of CO on the Sensor Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | Carbon monoxide |
| eFBG | etched optical fiber with FBG |
| FBG | Fiber-Bragg grating |
| fcc | face-centered cubic |
| LIB | Lithium-ion battery |
| LSPR | localized surface plasmon resonance |
| NMC | Lithium nickel manganese cobalt oxides |
| PbP | point-by-point |
| Pd | Palladium |
| SCCM | Standard cubic centimeters per minute |
| SOC | State of charge |
| SOH | Stage of health |
| TC | Thermocouple |
| TR | Thermal runaway |
References
- Maisel, F.; Neef, C.; Marscheider-Weidemann, F.; Nissen, N.F. A forecast on future raw material demand and recycling potential of lithium-ion batteries in electric vehicles. Resour. Conserv. Recycl. 2023, 192, 106920. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Degen, F.; Winter, M.; Bendig, D.; Tübke, J. Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nat. Energy 2023, 8, 1284–1295. [Google Scholar] [CrossRef]
- Hendricks, C.; Williard, N.; Mathew, S.; Pecht, M. A failure modes, mechanisms, and effects analysis (FMMEA) of lithium-ion batteries. J. Power Sources 2015, 297, 113–120. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Rahe, C.; Li, W.; Lu, L.; He, X.; Sauer, D.U.; Ouyang, M. Internal short circuit evaluation and corresponding failure mode analysis for lithium-ion batteries. J. Energy Chem. 2021, 61, 269–280. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J.; Qin, Y.; Chen, J.; Wang, J.; Yu, X.; Zhang, B.; Zou, Y.; Hong, Y.; Li, Z.; et al. Full-Dimensional Analysis of Gaseous Products to Unlocking In Depth Thermal Runaway Mechanism of Li-Ion Batteries. Small 2024, 20, e2406110. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Fuchs, A. Comparing Different Thermal Runaway Triggers for Two Automotive Lithium-Ion Battery Cell Types. J. Electrochem. Soc. 2020, 167, 130542. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Amano, K.O.A.; Hahn, S.K.; Tschirschwitz, R.; Rappsilber, T.; Krause, U. An Experimental Investigation of Thermal Runaway and Gas Release of NMC Lithium-Ion Pouch Batteries Depending on the State of Charge Level. Batteries 2022, 8, 41. [Google Scholar] [CrossRef]
- Said, A.O.; Lee, C.; Stoliarov, S.I. Experimental investigation of cascading failure in 18650 lithium ion cell arrays: Impact of cathode chemistry. J. Power Sources 2020, 446, 227347. [Google Scholar] [CrossRef]
- Ribière, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Mao, B.; Chen, M.; Huang, Z.; Wang, Q. Experimental study on thermal runaway and fire behaviors of large format lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116949. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Willstrand, O.; Pushp, M.; Andersson, P.; Brandell, D. Impact of different Li-ion cell test conditions on thermal runaway characteristics and gas release measurements. J. Energy Storage 2023, 68, 107785. [Google Scholar] [CrossRef]
- Lammer, M.; Königseder, A.; Gluschitz, P.; Hacker, V. Influence of aging on the heat and gas emissions from commercial lithium ion cells in case of thermal failure. J. Electrochem. Sci. Eng. 2018, 8, 101–110. [Google Scholar] [CrossRef]
- Brimblecombe, P. Air Composition and Chemistry; Air Composition & Chemistry, Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Wang, X.X.; Li, Q.T.; Zhou, X.Y.; Hu, Y.M.; Guo, X. Monitoring thermal runaway of lithium-ion batteries by means of gas sensors. Sens. Actuators B Chem. 2024, 411, 135703. [Google Scholar] [CrossRef]
- Hu, D.; Huang, S.; Wen, Z.; Gu, X.; Lu, J. A review on thermal runaway warning technology for lithium-ion batteries. Renew. Sustain. Energy Rev. 2024, 206, 114882. [Google Scholar] [CrossRef]
- Abdalwareth, A.; Flachenecker, G.; Angelmahr, M.; Schade, W. Optical fiber evanescent hydrogen sensor based on palladium nanoparticles coated Bragg gratings. Sens. Actuators A Phys. 2023, 361, 114594. [Google Scholar] [CrossRef]
- Butler, M.A. Optical fiber hydrogen sensor. Appl. Phys. Lett. 1984, 45, 1007–1009. [Google Scholar] [CrossRef]
- Zeakes, J.; Murphy, K.; Elshabini-Riad, A.; Claus, R. Modified extrinsic Fabry-Perot interferometric hydrogen gas sensor. In Proceedings of the Proceedings of LEOS’94, Boston, MA, USA, 31 October–3 November 1994; IEEE: Piscataway, NJ, USA, 1994; Volume 2, pp. 235–236. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, H.W.; Tian, J.Y.; Li, R.L.; Rahman, Z.U.; Kong, Q.P. Cultural diffusion of Indo-Aryan languages into Bangladesh: A perspective from mitochondrial DNA. Mitochondrion 2018, 38, 23–30. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, C.; Xu, B.; Li, Y. Optical fiber hydrogen sensor with single Sagnac interferometer loop based on vernier effect. Sens. Actuators B Chem. 2018, 255, 3011–3016. [Google Scholar] [CrossRef]
- Butler, M.A. Micromirror optical-fiber hydrogen sensor. Sens. Actuators B Chem. 1994, 22, 155–163. [Google Scholar] [CrossRef]
- Slaman, M.; Dam, B.; Pasturel, M.; Borsa, D.; Schreuders, H.; Rector, J.; Griessen, R. Fiber optic hydrogen detectors containing Mg-based metal hydrides. Sens. Actuators B Chem. 2007, 123, 538–545. [Google Scholar] [CrossRef]
- Xu, B.; Chang, R.; Li, P.; Wang, D.N.; Zhao, C.L.; Li, J.Q.; Yang, M.; Duan, L.Z. Reflective optical fiber sensor based on light polarization modulation for hydrogen sensing. J. Opt. Soc. Am. B 2019, 36, 3471. [Google Scholar] [CrossRef]
- Sekimoto, S.; Nakagawa, H.; Okazaki, S.; Fukuda, K.; Asakura, S.; Shigemori, T.; Takahashi, S. A fiber-optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens. Actuators B Chem. 2000, 66, 142–145. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yu, X.; Cao, K.; Liao, J. Greatly etched fiber Bragg grating hydrogen sensor with Pd/Ni composite film as sensing material. Sens. Actuators B Chem. 2012, 174, 253–257. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yu, X.; Lu, H. Optical hydrogen sensor based on etched fiber Bragg grating sputtered with Pd/Ag composite film. Opt. Fiber Technol. 2013, 19, 26–30. [Google Scholar] [CrossRef]
- Tabib-Azar, M.; Sutapun, B.; Petrick, R.; Kazemi, A. Highly sensitive hydrogen sensors using palladium coated fiber optics with exposed cores and evanescent field interactions. Sens. Actuators B Chem. 1999, 56, 158–163. [Google Scholar] [CrossRef]
- Sumida, S.; Okazaki, S.; Asakura, S.; Nakagawa, H.; Murayama, H.; Hasegawa, T. Distributed hydrogen determination with fiber-optic sensor. Sens. Actuators B Chem. 2005, 108, 508–514. [Google Scholar] [CrossRef]
- Cao, R.; Wu, J.; Liang, G.; Ohodnicki, P.R.; Chen, K.P. Functionalized PdAu Alloy on Nanocones Fabricated on Optical Fibers for Hydrogen Sensing. IEEE Sens. J. 2020, 20, 1922–1927. [Google Scholar] [CrossRef]
- Lin, K.; Lu, Y.; Chen, J.; Zheng, R.; Wang, P.; Ming, H. Surface plasmon resonance hydrogen sensor based on metallic grating with high sensitivity. Opt. Express 2008, 16, 18599. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Eklund, R.; Nilsson, S.; Langhammer, C. A fiber-optic nanoplasmonic hydrogen sensor via pattern-transfer of nanofabricated PdAu alloy nanostructures. Nanoscale 2018, 10, 20533–20539. [Google Scholar] [CrossRef]
- Tobiška, P.; Hugon, O.; Trouillet, A.; Gagnaire, H. An integrated optic hydrogen sensor based on SPR on palladium. Sens. Actuators B Chem. 2001, 74, 168–172. [Google Scholar] [CrossRef]
- Perrotton, C.; Westerwaal, R.J.; Javahiraly, N.; Slaman, M.; Schreuders, H.; Dam, B.; Meyrueis, P. A reliable, sensitive and fast optical fiber hydrogen sensor based on surface plasmon resonance. Opt. Express 2013, 21, 382. [Google Scholar] [CrossRef]
- Aray, A.; Ranjbar, M.; Shokoufi, N.; Morshedi, A. Plasmonic fiber optic hydrogen sensor using oxygen defects in nanostructured molybdenum trioxide film. Opt. Lett. 2019, 44, 4773. [Google Scholar] [CrossRef]
- Sutapun, B.; Tabib-Azar, M.; Kazemi, A.A. Fiber optic Bragg grating sensors for hydrogen gas sensing. In Proceedings of the Optical Engineering for Sensing and Nanotechnology (ICOSN ’99), Yokohama, Japan, 16–18 June 1999; Yamaguchi, I., Ed.; SPIE: Amsterdam, The Netherlands, 1999; Volume 3740, pp. 278–283. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Janssens, S.; Hunze, A. Palladium-Based Hydrogen Sensors Using Fiber Bragg Gratings. J. Light. Technol. 2018, 36, 850–856. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Hunze, A. Optimizing the sensitivity of palladium based hydrogen sensors. Sens. Actuators B Chem. 2018, 259, 10–19. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Hunze, A. Improving the Sensitivity of Palladium-Based Fiber Optic Hydrogen Sensors. J. Light. Technol. 2018, 36, 2166–2174. [Google Scholar] [CrossRef]
- Buric, M.; Chen, K.P.; Bhattarai, M.; Swinehart, P.R.; Maklad, M. Active Fiber Bragg Grating Hydrogen Sensors for All-Temperature Operation. IEEE Photonics Technol. Lett. 2007, 19, 255–257. [Google Scholar] [CrossRef]
- Xiang, F.; Wang, G.; Qin, Y.; Yang, S.; Zhong, X.; Dai, J.; Yang, M. Improved Performance of Fiber Bragg Hydrogen Sensors Assisted by Controllable Optical Heating System. IEEE Photonics Technol. Lett. 2017, 29, 1233–1236. [Google Scholar] [CrossRef]
- Yang, S.; Wang, G.; Xiang, F.; Qin, Y.; Dai, J.; Yang, M. Pt nanoparticles encapsulated in mesoporous tungsten oxide to enhance the repeatability of a FBG hydrogen sensor. Opt. Mater. Express 2018, 8, 1493. [Google Scholar] [CrossRef]
- Yang, M.; Qin, Y.; Ma, Y.; Wang, G.; Xiang, F.; Wang, M.; Dai, J.; Chen, Z.; Xia, J.; Zhou, L. High-sensitivity fiber optic hydrogen sensor in air by optimizing a self-referenced demodulating method. Appl. Opt. 2018, 57, 8011. [Google Scholar] [CrossRef]
- Wicke, E.; Brodowsky, H.; Züchner, H. Hydrogen in palladium and palladium alloys. In Hydrogen in Metals II; Springer: Berlin/Heidelberg, Germany, 1978; pp. 73–155. [Google Scholar] [CrossRef]
- Blackford, B.L.; Arnold, C.S.; Mulhern, P.J.; Jericho, M.H. A scanning tunneling microscope study of a palladium sphere in hydrogen gas: Expansion and surface topology. J. Appl. Phys. 1994, 76, 4054–4060. [Google Scholar] [CrossRef]
- Kawasaki, A.; Itoh, S.; Shima, K.; Kato, K.; Ohashi, H.; Ishikawa, T.; Yamazaki, T. Change in the crystalline structure during the phase transition of the palladium–hydrogen system. Phys. Chem. Chem. Phys. 2015, 17, 24783–24790. [Google Scholar] [CrossRef]
- Syrenova, S.; Wadell, C.; Nugroho, F.A.A.; Gschneidtner, T.A.; Diaz Fernandez, Y.A.; Nalin, G.; Świtlik, D.; Westerlund, F.; Antosiewicz, T.J.; Zhdanov, V.P.; et al. Hydride formation thermodynamics and hysteresis in individual Pd nanocrystals with different size and shape. Nat. Mater. 2015, 14, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Tew, M.W.; Miller, J.T.; van Bokhoven, J.A. Particle Size Effect of Hydride Formation and Surface Hydrogen Adsorption of Nanosized Palladium Catalysts: L3 Edge vs K Edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2009, 113, 15140–15147. [Google Scholar] [CrossRef]
- Frieske, H.; Wicke, E. Magnetic Susceptibility and Equilibrium Diagram of PdHn. Ber. Bunsenges. Phys. Chem. 1973, 77, 48–52. [Google Scholar] [CrossRef]
- Metzroth, L.J.T.; Miller, E.M.; Norman, A.G.; Yazdi, S.; Carroll, G.M. Accelerating Hydrogen Absorption and Desorption Rates in Palladium Nanocubes with an Ultrathin Surface Modification. Nano Lett. 2021, 21, 9131–9137. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Lam, B.; MacLeod, B.P.; Sherbo, R.S.; Moreno-Gonzalez, M.; Fork, D.K.; Berlinguette, C.P. Facets and vertices regulate hydrogen uptake and release in palladium nanocrystals. Nat. Mater. 2019, 18, 454–458. [Google Scholar] [CrossRef]
- Shooshtari, M. Gold-decorated vertically aligned carbon nanofibers for high-performance room-temperature ethanol sensing. Microchim. Acta 2025, 192, 517. [Google Scholar] [CrossRef]
- Burgmeier, J.; Schippers, W.; Emde, N.; Funken, P.; Schade, W. Femtosecond laser-inscribed fiber Bragg gratings for strain monitoring in power cables of offshore wind turbines. Appl. Opt. 2011, 50, 1868. [Google Scholar] [CrossRef]
- Eisner, L.; Flachenecker, G.; Schade, W. Doped silica sol layer coatings on evanescent field fiber Bragg gratings for optical detection of nitroaromate based explosives. Sens. Actuators A Phys. 2022, 343, 113687. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, Y.; Wu, Y. A comparative study on air transport safety of lithium-ion batteries with different SOCs. Appl. Therm. Eng. 2020, 179, 115679. [Google Scholar] [CrossRef]
- Li, H.; Duan, Q.; Zhao, C.; Huang, Z.; Wang, Q. Experimental investigation on the thermal runaway and its propagation in the large format battery module with Li(Ni1/3Co1/3Mn1/3)O2 as cathode. J. Hazard. Mater. 2019, 375, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, Y.; Duan, Q.; Qin, P.; Sun, J.; Wang, Q. Heating position effect on internal thermal runaway propagation in large-format lithium iron phosphate battery. Appl. Energy 2022, 325, 119778. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Masalkovaitė, K.; Gasper, P.; Finegan, D.P. Predicting the heat release variability of Li-ion cells under thermal runaway with few or no calorimetry data. Nat. Commun. 2024, 15, 8399. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries 2020, 6, 30. [Google Scholar] [CrossRef]
- RaviPrakash, J.; McDaniel, A.; Horn, M.; Pilione, L.; Sunal, P.; Messier, R.; McGrath, R.; Schweighardt, F. Hydrogen sensors: Role of palladium thin film morphology. Sens. Actuators B Chem. 2007, 120, 439–446. [Google Scholar] [CrossRef]
- Xu, C.; Fan, Z.; Zhang, M.; Wang, P.; Wang, H.; Jin, C.; Peng, Y.; Jiang, F.; Feng, X.; Ouyang, M. A comparative study of the venting gas of lithium-ion batteries during thermal runaway triggered by various methods. Cell Rep. Phys. Sci. 2023, 4, 101705. [Google Scholar] [CrossRef]
- Neurock, M. First-principles analysis of the hydrogenation of carbon monoxide over palladium. Top. Catal. 1999, 9, 135–152. [Google Scholar] [CrossRef]
- Ogura, S.; Okada, M.; Fukutani, K. Near-Surface Accumulation of Hydrogen and CO Blocking Effects on a Pd–Au Alloy. J. Phys. Chem. C 2013, 117, 9366–9371. [Google Scholar] [CrossRef]
- Ogura, S.; Fukutani, K. Dynamic Blocking by CO of Hydrogen Transport across Pd70Au30(110) Surfaces. J. Phys. Chem. C 2017, 121, 3373–3380. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Kadkhodazadeh, S.; Wagner, J.B.; Langhammer, C. Rationally Designed PdAuCu Ternary Alloy Nanoparticles for Intrinsically Deactivation-Resistant Ultrafast Plasmonic Hydrogen Sensing. ACS Sens. 2019, 4, 1424–1432. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Wang, J.; Wang, C.; He, X.; Cheng, Z.; Li, H.; Mei, W.; Wang, Q. Thermal runaway and gas venting behaviors of large-format prismatic sodium-ion battery. Energy Storage Mater. 2025, 77, 104197. [Google Scholar] [CrossRef]
- Huang, L.; Lu, T.; Xu, G.; Zhang, X.; Jiang, Z.; Zhang, Z.; Wang, Y.; Han, P.; Cui, G.; Chen, L. Thermal runaway routes of large-format lithium-sulfur pouch cell batteries. Joule 2022, 6, 906–922. [Google Scholar] [CrossRef]


| Cathode | Gas Vol. | CO | Other | Source | ||
|---|---|---|---|---|---|---|
| (l ) | Concentration in % | |||||
| NMC | 1.96 | 22 | 28 | 37 | 13 | [13] |
| NMC | 2.02 | 21 (±5) | 27 (±4) | 24 (±5) | 28 | [14] |
| NMC | 3 | 18 (±3) | 20 (±4) | 38 (±4) | 24 | [15] |
| NMC | - | 8 (±8) | - | - | 92 | [16] |
| NMC | - | 11 | 31 | 24 | 34 | [17] |
| Test | Cathode | Capacity | Cell Total | SOC | Format |
|---|---|---|---|---|---|
| #1 | NMC | 39.6 Ah | 1 | 40% | pouch |
| #2 | NMC | 40.1 Ah | 1 | 40% | pouch |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropkowski, L.; Abdalwareth, A.; Brüdigam, C.; Angelmahr, M.; Schade, W. Nanoparticle-Coated Optical Hydrogen Sensor for Early Gas Detection of Lithium-Ion Battery Failure. Chemosensors 2025, 13, 348. https://doi.org/10.3390/chemosensors13090348
Kropkowski L, Abdalwareth A, Brüdigam C, Angelmahr M, Schade W. Nanoparticle-Coated Optical Hydrogen Sensor for Early Gas Detection of Lithium-Ion Battery Failure. Chemosensors. 2025; 13(9):348. https://doi.org/10.3390/chemosensors13090348
Chicago/Turabian StyleKropkowski, Leonard, Ahmad Abdalwareth, Christoff Brüdigam, Martin Angelmahr, and Wolfgang Schade. 2025. "Nanoparticle-Coated Optical Hydrogen Sensor for Early Gas Detection of Lithium-Ion Battery Failure" Chemosensors 13, no. 9: 348. https://doi.org/10.3390/chemosensors13090348
APA StyleKropkowski, L., Abdalwareth, A., Brüdigam, C., Angelmahr, M., & Schade, W. (2025). Nanoparticle-Coated Optical Hydrogen Sensor for Early Gas Detection of Lithium-Ion Battery Failure. Chemosensors, 13(9), 348. https://doi.org/10.3390/chemosensors13090348





