Gas Sensing for Poultry Farm Air Quality Monitoring to Enhance Welfare and Sustainability
Abstract
1. Introduction
2. Environmental Gases
2.1. Ammonia ()
2.2. Nitrous Oxide
2.3. Methane ()
2.4. Carbon Dioxide ()
2.5. Hydrogen Sulfide ()
3. Discussion
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, T.H.; Bak, S.Y.; Kim, S.; Lee, S.H.; Han, Y.J.; Yi, M. Decoration of CuO NWs Gas Sensor with ZnO NPs for Improving NO2 Sensing Characteristics. Sensors 2021, 21, 2103. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal Oxide Based Heterojunctions for Gas Sensors: A Review. Nanomaterials 2021, 11, 1026. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Viet, N.N.; Thong, L.V.; Hung, C.M.; Hoa, N.D.; Duy, N.V.; Hong, H.S.; Hieu, N.V. Comparative study on the gas-sensing performance of ZnO/SnO2 external and ZnO–SnO2 internal heterojunctions for ppb H2S and NO2 gases detection. Sens. Actuators B Chem. 2021, 334, 129606. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Ling, W.; Zhu, D.; Pu, Y. UV excited gas sensing SnO2-ZnO aerogels to ppb-level ethanol detection. Sens. Actuators B Chem. 2021, 337, 129815. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, J.; Lee, C.; Lee, S.W.; Lin, L. A comprehensive review on piezoelectric energy harvesting technology: Materials, mechanisms, and applications. Appl. Phys. Rev. 2018, 5, 041306. [Google Scholar] [CrossRef]
- Jiang, G.; Goledzinowski, M.; Comeau, F.J.E.; Zarrin, H.; Lui, G.; Lenos, J.; Veileux, A.; Liu, G.; Zhang, J.; Hemmati, S.; et al. Free-Standing Functionalized Graphene Oxide Solid Electrolytes in Electrochemical Gas Sensors. Adv. Funct. Mater. 2016, 26, 1729–1736. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Boulart, C.; Mowlem, M.C.; Connelly, D.P.; Dutasta, J.P.; German, C.R. A novel, low-cost, high performance dissolved methane sensor for aqueous environments. Opt. Express 2008, 16, 12607. [Google Scholar] [CrossRef]
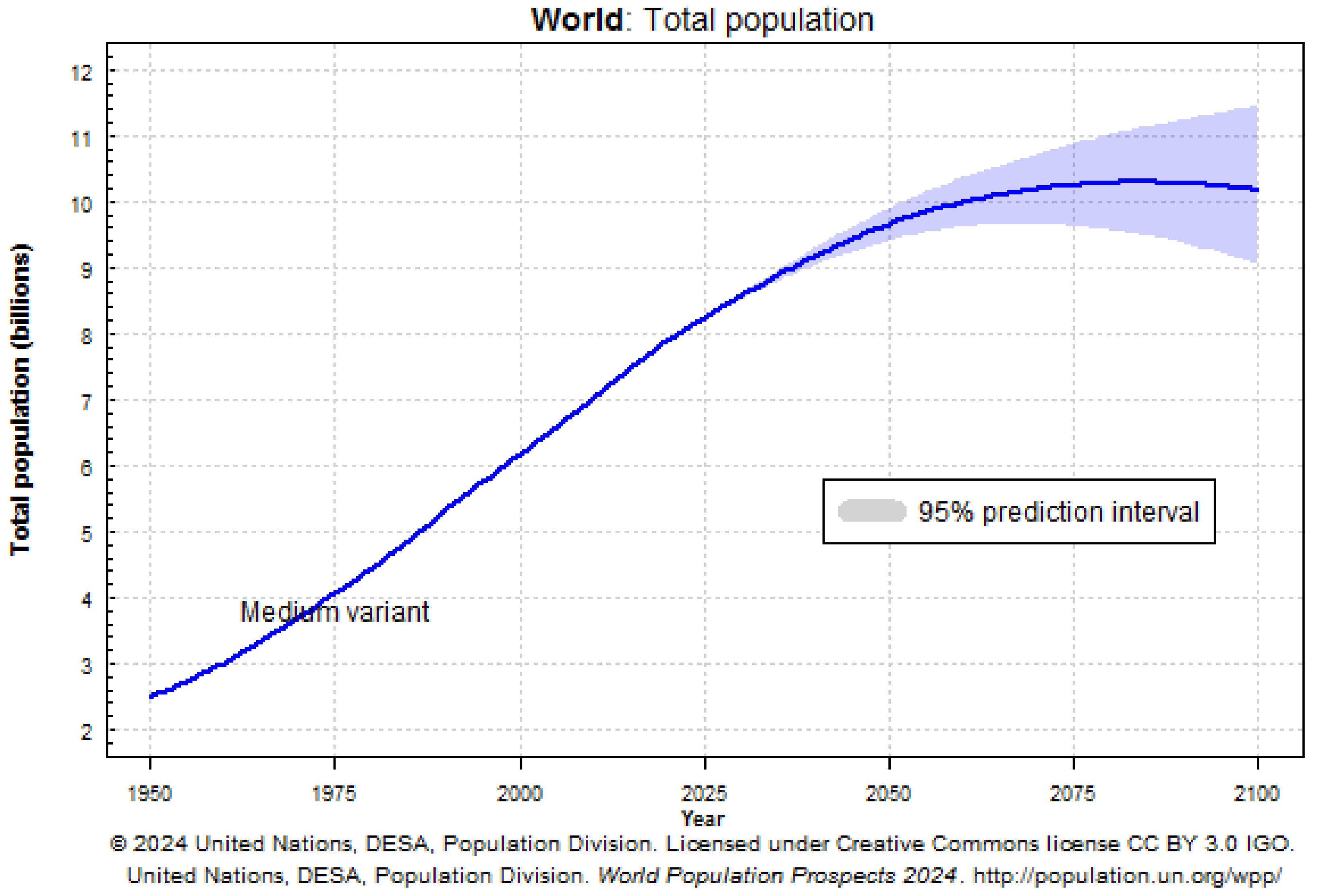
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2024. 2025. Available online: https://population.un.org/wpp/ (accessed on 3 September 2025).
- Seltzer, W.; Moum, S.G.; Goldhaft, T.M. A Method for the Treatment of Animal Wastes to Control Ammonia and Other Odors. Poult. Sci. 1969, 48, 1912–1918. [Google Scholar] [CrossRef]
- Bist, R.B.; Subedi, S.; Chai, L.; Yang, X. Ammonia emissions, impacts, and mitigation strategies for poultry production: A critical review. J. Environ. Manag. 2023, 328, 116919. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, X.; Yuan, W.; Li, Z.; Lu, N.; Wang, S.; Wu, Y.; Fan, S.; Hua, Z. Efficient NH3 Detection Based on MOS Sensors Coupled with Catalytic Conversion. ACS Sens. 2020, 5, 1838–1848. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Nunez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Metal Oxide Gas Sensors, a Survey of Selectivity Issues Addressed at the SENSOR Lab, Brescia (Italy). Sensors 2017, 17, 714. [Google Scholar] [CrossRef]
- Guo, S.Y.; Hou, P.X.; Zhang, F.; Liu, C.; Cheng, H.M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, A.S.; Zou, B.; Zhang, H.X.; Yan, K.L.; Lin, Y. Advances of metal–organic frameworks for gas sensing. Polyhedron 2018, 154, 83–97. [Google Scholar] [CrossRef]
- Kumbhar, M.B.; Chandak, V.S.; Kulal, P.M. Enhanced ammonia gas sensing performance at room temperature of binder-free NiO, Cu and Co-doped NiO thin films synthesized via the SILAR method. Mater. Chem. Phys. 2025, 329, 130065. [Google Scholar] [CrossRef]
- Marimuthu, G.; Priyadharsini, C.I.; Palanisamy, G.; Periyasami, G.; Lee, J.; Kim, I.; Sivaprakash, P. Flower-like nickel oxide nanostructures: Superior ammonia gas sensing and efficient dye removal behavior under UV–visible light illumination. J. Mol. Struct. 2025, 1321, 140152. [Google Scholar] [CrossRef]
- Hjiri, M.; Algessair, S.; Dhahri, R.; Albargi, H.B.; Mansour, N.B.; Assadi, A.A.; Neri, G. Ammonia gas sensors based on undoped and Ca-doped ZnO nanoparticles. RSC Adv. 2024, 14, 5001–5011. [Google Scholar] [CrossRef]
- Liu, Z.; Han, D.; Liu, L.; Li, D.; Han, X.; Chen, Y.; Liu, X.; Zhuo, K.; Cheng, Y.; Sang, S. Ultrasensitive ammonia gas sensor based on Ti3C2Tx/Ti3AlC2 planar composite at room temperature. Sens. Actuators B Chem. 2023, 378, 133149. [Google Scholar] [CrossRef]
- Himabindu, B.; Devi, N.S.M.P.L.; Nagaraju, P.; Kanth, B.R. A nanostructured Al-doped ZnO as an ultra-sensitive room-temperature ammonia gas sensor. J. Mater. Sci. Mater. Electron. 2023, 34, 1014. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C.; et al. The gas sensor utilizing polyaniline/MoS2 nanosheets/SnO2 nanotubes for the room temperature detection of ammonia. Sens. Actuators B Chem. 2021, 332, 129444. [Google Scholar] [CrossRef]
- Mi, Q.; Zhang, D.; Zhang, X.; Wang, D. Highly sensitive ammonia gas sensor based on metal-organic frameworks-derived CoSe2@nitrogen-doped amorphous carbon decorated with multi-walled carbon nanotubes. J. Alloy. Compd. 2021, 860, 158252. [Google Scholar] [CrossRef]
- Liu, I.P.; Chang, C.H.; Chou, T.C.; Lin, K.W. Ammonia sensing performance of a platinum nanoparticle-decorated tungsten trioxide gas sensor. Sens. Actuators B Chem. 2019, 291, 148–154. [Google Scholar] [CrossRef]
- Biskupski, D.; Herbig, B.; Schottner, G.; Moos, R. Nanosized titania derived from a novel sol–gel process for ammonia gas sensor applications. Sens. Actuators B Chem. 2011, 153, 329–334. [Google Scholar] [CrossRef]
- Pawar, S.G.; Chougule, M.A.; Patil, S.L.; Raut, B.T.; Godse, P.R.; Sen, S.; Patil, V.B. Room Temperature Ammonia Gas Sensor Based on Polyaniline-TiO2 Nanocomposite. IEEE Sens. J. 2011, 11, 3417–3423. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Durgadevi, E.; Navaneethan, M.; Patil, V.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.; Hayakawa, Y. Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres. J. Alloy. Compd. 2017, 721, 182–190. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B. Selective detection of ammonia using spray pyrolysis deposited pure and nickel doped ZnO thin films. Appl. Surf. Sci. 2014, 311, 405–412. [Google Scholar] [CrossRef]
- Patil, D.; Patil, L.; Patil, P. Cr2O3-activated ZnO thick film resistors for ammonia gas sensing operable at room temperature. Sens. Actuators B Chem. 2007, 126, 368–374. [Google Scholar] [CrossRef]
- Anantachaisilp, S.; Smith, S.M.; Ton-That, C.; Osotchan, T.; Moon, A.R.; Phillips, M.R. Tailoring Deep Level Surface Defects in ZnO Nanorods for High Sensitivity Ammonia Gas Sensing. J. Phys. Chem. C 2014, 118, 27150–27156. [Google Scholar] [CrossRef]
- Bahu, M.; Kumar, K.; Bahu, T. CuO-ZnO semiconductor gas sensor for ammonia at room temperature. J. Electron. Devices 2012, 14, 1137–1141. [Google Scholar]
- Bannov, A.G.; Jasek, O.; Manakhov, A.; Marik, M.; Necas, D.; Zajickova, L. High-Performance Ammonia Gas Sensors Based on Plasma Treated Carbon Nanostructures. IEEE Sens. J. 2017, 17, 1964–1970. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Kushwaha, N.; Mittal, J. Ammonia Gas Sensing Using Thin Film of MnO2 Nanofibers. IEEE Sens. J. 2016, 16, 4691–4695. [Google Scholar] [CrossRef]
- Li, C.F.; Hsu, C.Y.; Li, Y.Y. NH3 sensing properties of ZnO thin films prepared via sol–gel method. J. Alloy. Compd. 2014, 606, 27–31. [Google Scholar] [CrossRef]
- Chou, P.C.; Chen, H.I.; Liu, I.P.; Chen, C.C.; Liou, J.K.; Hsu, K.S.; Liu, W.C. On the Ammonia Gas Sensing Performance of a RF Sputtered NiO Thin-Film Sensor. IEEE Sens. J. 2015, 15, 3711–3715. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, H.I.; Hsu, C.S.; Huang, C.C.; Wu, J.S.; Chou, P.C.; Liu, W.C. ZnO-Nanorod-Based Ammonia Gas Sensors With Underlying Pt/Cr Interdigitated Electrodes. IEEE Electron Device Lett. 2012, 33, 1486–1488. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, A.K.; Nath, R.; Gupta, B.K.; Varma, G.D. Probing the highly efficient room temperature ammonia gas sensing properties of a luminescent ZnO nanowire array prepared via an AAO-assisted template route. Dalton Trans. 2014, 43, 5713–5720. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.S.; Dharmaraj, P.; Purushothaman, V.; Ramakrishnan, V.; Jeganathan, K. Point defects assisted NH3 gas sensing properties in ZnO nanostructures. Sens. Actuators B Chem. 2015, 212, 10–17. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, D.; Yang, Z.; Li, X.; Hu, N.; Yang, C.; Wei, H.; Yin, G.; He, D.; Zhang, Y. ZnO Nanowire-Reduced Graphene Oxide Hybrid Based Portable NH3 Gas Sensing Electron Device. IEEE Electron Device Lett. 2015, 36, 1376–1379. [Google Scholar] [CrossRef]
- Dalólio, F.S.; da Silva, J.N.; de Oliveira, A.C.C.; de Fátima Ferreira Tinôco, I.; Barbosa, R.C.; de Oliveira Resende, M.; Albino, L.F.T.; Coelho, S.T. Poultry litter as biomass energy: A review and future perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Pereira, J.; Fangueiro, D.; Chadwick, D.R.; Misselbrook, T.H.; Coutinho, J.; Trindade, H. Effect of cattle slurry pre-treatment by separation and addition of nitrification inhibitors on gaseous emissions and N dynamics: A laboratory study. Chemosphere 2010, 79, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Bolan, N.S.; Bhandral, R.; Hedley, C.B.; Luo, J. A review of emissions of methane, ammonia, and nitrous oxide from animal excreta deposition and farm effluent application in grazed pastures. N. Z. J. Agric. Res. 2004, 47, 513–544. [Google Scholar] [CrossRef]
- Rice, J.; Caldwell, D.; Humenik, F.; Vanotti, A.; Nienaber, B. Animal Agriculture and the Environment: National Center for Manure & Animal Waste Management White Papers; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Broucek, J. Nitrous Oxide Release from Poultry and Pig Housing. Pol. J. Environ. Stud. 2018, 27, 467–479. [Google Scholar] [CrossRef]
- Ahmad, N.; Umar, A.; Kumar, R.; Alam, M. Microwave-assisted synthesis of ZnO doped CeO2 nanoparticles as potential scaffold for highly sensitive nitroaniline chemical sensor. Ceram. Int. 2016, 42, 11562–11567. [Google Scholar] [CrossRef]
- Faisal, A.D. Synthesis of ZnO comb-like nanostructures for high sensitivity H2S gas sensor fabrication at room temperature. Bull. Mater. Sci. 2017, 40, 1061–1068. [Google Scholar] [CrossRef]
- Fleischer, M.; Kornely, S.; Weh, T.; Frank, J.; Meixner, H. Selective gas detection with high-temperature operated metal oxides using catalytic filters. Sens. Actuators B Chem. 2000, 69, 205–210. [Google Scholar] [CrossRef]
- Amali, R.; Lim, H.; Ibrahim, I.; Huang, N.; Zainal, Z.; Ahmad, S. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Brown, M.D.; Schoenfisch, M.H. Catalytic selectivity of metallophthalocyanines for electrochemical nitric oxide sensing. Electrochim. Acta 2018, 273, 98–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Zhang, X.; Fang, J.; Zhao, Y. Open-path sensor based on QCL for atmospheric N2O measurement. Results Phys. 2021, 31, 104909. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Sarsembina, M.; Mentbayeva, A.; Bakenov, Z.; Soltabayev, B. CuO/TiO2 heterostructure-based sensors for conductometric NO2 and N2O gas detection at room temperature. Sens. Actuators B Chem. 2023, 397, 134635. [Google Scholar] [CrossRef]
- Rahman, M.T.; Khan, R.R.; Tian, Y.; Ibrahim, H.; Dong, L. High-Sensitivity and Room-Temperature Nitrous Oxide Sensor Using Au Nanoparticles-Decorated MoS2. IEEE Sens. J. 2023, 23, 18994–19001. [Google Scholar] [CrossRef]
- Kanazawa, E.; Sakai, G.; Shimanoe, K.; Kanmura, Y.; Teraoka, Y.; Miura, N.; Yamazoe, N. Metal oxide semiconductor N2O sensor for medical use. Sens. Actuators B Chem. 2001, 77, 72–77. [Google Scholar] [CrossRef]
- Rout, C.; Ganesh, K.; Govindaraj, A.; Rao, C. Sensors for the nitrogen oxides, NO2, NO and N2O, based on In2O3 and WO3 nanowires. Appl. Phys. A 2006, 85, 241–246. [Google Scholar] [CrossRef]
- Lančok, J.; Santoni, A.; Penza, M.; Loreti, S.; Menicucci, I.; Minarini, C.; Jelinek, M. Tin oxide thin films prepared by laser-assisted metal–organic CVD: Structural and gas sensing properties. Surf. Coatings Technol. 2005, 200, 1057–1060. [Google Scholar] [CrossRef]
- Al-Kerwi, M.S.M.; Mardenli, O.; Jasim, M.R.M.; Al-Majeed, M.A. Effects of Harmful Gases Emitted from Poultry Houses on Productive and Health Performance. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 012082. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Resources—Global Livestock Environmental Assessment Model (GLEAM). 2025. Available online: https://www.fao.org/gleam/resources/en/ (accessed on 2 September 2025).
- Beaver, R.L.; Field, W.E. Summary of Documented Fatalities in Livestock Manure Storage and Handling Facilities-1975-2004. J. Agromedicine 2007, 12, 3–23. [Google Scholar] [CrossRef]
- Riche, E.L.L.; Vanderzaag, A.; Wagner-Riddle, C.; Dunfield, K.E.; Sokolov, V.K.; Gordon, R. Do volatile solids from bedding materials increase greenhouse gas emissions for stored dairy manure? Can. J. Soil Sci. 2017, 97, 512–521. [Google Scholar] [CrossRef]
- Calvet, S.; Estellés, F.; Cambra-López, M.; Torres, A.; den Weghe, H.V. The influence of broiler activity, growth rate, and litter on carbon dioxide balances for the determination of ventilation flow rates in broiler production. Poult. Sci. 2011, 90, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Méda, B.; Hassouna, M.; Flechard, C.; Lecomte, M.; Germain, K.; Picard, S.; Cellier, P.; Robin, P. Housing emissions of NH3, N2O and CH4 and outdoor emissions of CH4 and N2O from organic broilers. In Proceedings of the XVth International Congress of the International Society for Animal Hygiene, Vienna, Austria, 3–7 July 2011; Tribun EU: Berlin, Germany, 2011; Volume 1, pp. 215–218. [Google Scholar]
- Brouček, J.; Čermák, B. Emission of Harmful Gases from Poultry Farms and Possibilities of Their Reduction. Ekológia 2015, 34, 89–100. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Mitra, P.; Mukhopadhyay, A.K. ZnO thin film as methane sensor. Bull. Pol. Acad. Sci. Tech. Sci. 2007, 55, 281–285. [Google Scholar]
- Basu, S.; Basu, P.K. Nanocrystalline Metal Oxides for Methane Sensors: Role of Noble Metals. J. Sens. 2009, 2009, 861968. [Google Scholar] [CrossRef]
- Kim, B.; Lu, Y.; Hannon, A.; Meyyappan, M.; Li, J. Low temperature Pd/SnO2 sensor for carbon monoxide detection. Sens. Actuators B Chem. 2013, 177, 770–775. [Google Scholar] [CrossRef]
- Dong, L.; Li, C.; Sanchez, N.P.; Gluszek, A.K.; Griffin, R.J.; Tittel, F.K. Compact CH4 sensor system based on a continuous-wave, low power consumption, room temperature interband cascade laser. Appl. Phys. Lett. 2016, 108, 011106. [Google Scholar] [CrossRef]
- Nagai, D.; Nishibori, M.; Itoh, T.; Kawabe, T.; Sato, K.; Shin, W. Ppm level methane detection using micro-thermoelectric gas sensors with Pd/Al2O3 combustion catalyst films. Sens. Actuators B Chem. 2015, 206, 488–494. [Google Scholar] [CrossRef]
- Mehrabadi, Z.S.; Ahmadpour, A.; Shahtahmasebi, N.; Mohagheghi, M.M.B. Synthesis and characterization of Cu doped cobalt oxide nanocrystals as methane gas sensors. Phys. Scr. 2011, 84, 015801. [Google Scholar] [CrossRef]
- Sun, X.; Li, M.; Wang, Y.; Qin, C.; Cao, J.; Wang, Y. NiO/ZnO heterojunction microspheres for methane detection at room temperature. Opt. Mater. 2024, 148, 114893. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Wang, J.; Xiang, L. Conductometric methane gas sensors based on ZnO/Pd@ZIF-8: Effect of dual filtering of ZIF-8 to increase the selectivity. Sens. Actuators B Chem. 2023, 383, 133600. [Google Scholar] [CrossRef]
- Carbone, M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251. [Google Scholar] [CrossRef]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuators B Chem. 2019, 296, 126710. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R. Characterization of nickel oxide decorated-reduced graphene oxide nanocomposite and its sensing properties toward methane gas detection. J. Mater. Sci. Mater. Electron. 2016, 27, 3723–3730. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Faglia, G.; Sberveglieri, G. Reduced graphene oxide/ZnO nanocomposite for application in chemical gas sensors. RSC Adv. 2016, 6, 34225–34232. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Xia, B. Facile fabrication of ZnO nanocrystalline-modified graphene hybrid nanocomposite toward methane gas sensing application. J. Mater. Sci. Mater. Electron. 2015, 26, 5937–5945. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sens. Actuators B Chem. 2012, 166-167, 156–164. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Meng, X.; Zhang, Z.; Cao, J. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH4. Surfaces Interfaces 2021, 24, 101110. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Wagner, T.; Bauer, M.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. X-ray absorption near-edge spectroscopy investigation of the oxidation state of Pd species in nanoporous SnO2 gas sensors for methane detection. Thin Solid Film. 2011, 520, 909–912. [Google Scholar] [CrossRef]
- Jeppsson, K.H. SE—Structure and Environment. J. Agric. Eng. Res. 2000, 77, 429–440. [Google Scholar] [CrossRef]
- Reece, F.; Lott, B. Effect of Carbon Dioxide on Broiler Chicken Performance. Poult. Sci. 1980, 59, 2400–2402. [Google Scholar] [CrossRef]
- Pedersen, S.; Blanes-Vidal, V.; Joergensen, H.; Chwalibog, A.; Haeussermann, A.; Heetkamp, M.; Aarnink, A. Carbon Dioxide Production in Animal Houses: A literature review. Agric. Eng. Int. CIGR J. 2008, X, 1–19. [Google Scholar]
- Cândido, M.G.L.; Xiong, Y.; Gates, R.S.; Tinôco, I.F.F.; Koelkebeck, K.W. Effects of carbon dioxide on turkey poult performance and behavior. Poult. Sci. 2018, 97, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Helbacka, N.; Casterline, J.; Smith, C. The Effect of High CO2 Atmosphere on the Laying Hen. Poult. Sci. 1963, 42, 1082–1084. [Google Scholar] [CrossRef]
- Frank, F.; Burger, R. The Effect of Carbon Dioxide Inhalation and Sodium Bicarbonate Ingestion on Egg Shell Deposition. Poult. Sci. 1965, 44, 1604–1606. [Google Scholar] [CrossRef]
- Knížatová, M.; Brouček, J.; Mihina, Š. Seasonal differences In levels of carbon dioxide and ammonia In broiler housing. Slovak J. Anim. Sci. 2010, 43, 105–112. [Google Scholar]
- Irvine, P.J.; Lunt, D.J.; Stone, E.J.; Ridgwell, A. The fate of the Greenland Ice Sheet in a geoengineered, high CO2 world. Environ. Res. Lett. 2009, 4, 045109. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Erdmann, C.A.; Apte, M.G. Mucous membrane and lower respiratory building related symptoms in relation to indoor carbon dioxide concentrations in the 100-building BASE dataset. Indoor Air 2004, 14, 127–134. [Google Scholar] [CrossRef]
- Marchi, M.; Neri, E.; Pulselli, F.M.; Bastianoni, S. CO2 recovery from wine production: Possible implications on the carbon balance at territorial level. J. CO2 Util. 2018, 28, 137–144. [Google Scholar] [CrossRef]
- Moriaux, A.L.; Vallon, R.; Cilindre, C.; Parvitte, B.; Liger-Belair, G.; Zeninari, V. Development and validation of a diode laser sensor for gas-phase CO2 monitoring above champagne and sparkling wines. Sens. Actuators B Chem. 2018, 257, 745–752. [Google Scholar] [CrossRef]
- Molina, A.; Escobar-Barrios, V.; Oliva, J. A review on hybrid and flexible CO2 gas sensors. Synth. Met. 2020, 270, 116602. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. CO2 and Greenhouse Gas Emissions. Our World in Data. 2023. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 8 September 2025).
- Grote, M.; Williams, I.; Preston, J. Direct carbon dioxide emissions from civil aircraft. Atmos. Environ. 2014, 95, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Tans, P.; Thoning, K.W. Trends in globally-averaged CO2 determined from NOAA Global Monitoring Laboratory measurements. Version: Friday, 05-Sep-2025 12:12:59 MDT. [CrossRef]
- Azuma, K.; Kagi, N.; Yanagi, U.; Osawa, H. Effects of low-level inhalation exposure to carbon dioxide in indoor environments: A short review on human health and psychomotor performance. Environ. Int. 2018, 121, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xiong, Y.; Gates, R.; Wang, Y.; Koelkebeck, K. Air temperature, carbon dioxide, and ammonia assessment inside a commercial cage layer barn with manure-drying tunnels. Poult. Sci. 2020, 99, 3885–3896. [Google Scholar] [CrossRef]
- Aguayo-López, M.; Capitán-Vallvey, L.; Fernández-Ramos, M. Optical sensor for carbon dioxide gas determination, characterization and improvements. Talanta 2014, 126, 196–201. [Google Scholar] [CrossRef]
- Ogura, K.; Shiigi, H.; Oho, T.; Tonosaki, T. A CO2 Sensor with Polymer Composites Operating at Ordinary Temperature. J. Electrochem. Soc. 2000, 147, 4351. [Google Scholar] [CrossRef]
- Deng, Y. Applications of Semiconducting Metal Oxide Gas Sensors; Springer Nature: Singapore, 2023; pp. 325–385. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Sharma, J.; Gartia, M.R. Nanomaterial-Based CO2 Sensors. Nanomaterials 2020, 10, 2251. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Bolli, E.; Bellucci, A.; Mastellone, M.; Mezzi, A.; Orlando, S.; Polini, R.; Salerno, R.; Santagata, A.; Valentini, V.; Trucchi, D.M. Engineered SnO2-based thin films for efficient CO2 gas sensing at room temperature. Appl. Surf. Sci. 2025, 683, 161795. [Google Scholar] [CrossRef]
- Rodrigues, J.; Shimpi, N.G. High Performance-Low Cost-Chemiresitive BaTiO3 Nanospheres Based CO2 Gas Sensor for Air Quality Monitoring. ChemistrySelect 2024, 9, e202401547. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Liu, Z.; Li, L.; Shi, C.; Qin, H.; Hu, J. CO2-sensing properties and mechanism of nano-SnO2 thick-film sensor. Sens. Actuators B Chem. 2016, 227, 73–84. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Chemiresistive Sensor Based on Zinc Oxide Nanoflakes for CO2 Detection. ACS Appl. Nano Mater. 2019, 2, 700–706. [Google Scholar] [CrossRef]
- Kannan, P.K.; Saraswathi, R.; Rayappan, J.B.B. CO2 gas sensing properties of DC reactive magnetron sputtered ZnO thin film. Ceram. Int. 2014, 40, 13115–13122. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; Mir, L.E.; Fazio, E.; Neri, F.; Barreca, F.; Donato, N.; Bonavita, A.; Leonardi, S.G.; Neri, G. ZnO:Ca nanopowders with enhanced CO2 sensing properties. J. Phys. D Appl. Phys. 2015, 48, 255503. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kang, H.; Choi, N.J.; Park, K.H.; Lee, H.K. A carbon dioxide gas sensor based on cobalt oxide containing barium carbonate. Sens. Actuators B Chem. 2017, 248, 987–992. [Google Scholar] [CrossRef]
- Manasa, M.V.; PS, P.R.; Sreedhar, B. High performance CO2 gas sensor based on noble metal functionalized semiconductor nanomaterials for health and environmental safety. Mater. Res. Express 2019, 6, 125041. [Google Scholar] [CrossRef]
- Hunge, Y.; Yadav, A.; Kulkarni, S.; Mathe, V. A multifunctional ZnO thin film based devices for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sens. Actuators B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Klentz, R.; Fedde, M. Hydrogen sulfide: Effects on avian respiratory control and intrapulmonary CO2 receptors. Respir. Physiol. 1978, 32, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.C.; Richert, B.T.; Sutton, A.L.; Bowers, K.A.; Herr, C.T.; Kelly, D. Effects of Dietary Manipulation on Pig Performance, Manure Composition, Hydrogen Sulfide and Ammonia Levels in Swine Buildings. Purdue University Swine Day Report. 2000. Available online: https://www.ansc.purdue.edu/swine/swineday/sday00/psd24-2000.html (accessed on 8 September 2025).
- Wu-Haan, W.; Powers, W.; Angel, R.; Applegate, T. The use of distillers dried grains plus solubles as a feed ingredient on air emissions and performance from laying hens. Poult. Sci. 2010, 89, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.T.; Kim, G.; Islam, M.M.; Mun, H.S.; Bostami, A.R.; Yang, C.J. Effects of dietary chlorine dioxide on growth performance, intestinal and excreta microbiology, and odorous gas emissions from broiler excreta. J. Appl. Poult. Res. 2015, 24, 502–510. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Islam, M.M.; Mun, H.S.; Sim, H.J.; Kim, Y.J.; Yang, C.J. Effects ofBacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 2014, 93, 1963–1971. [Google Scholar] [CrossRef]
- Guarrasi, J.; Trask, C.; Kirychuk, S. A Systematic Review of Occupational Exposure to Hydrogen Sulfide in Livestock Operations. J. Agromed. 2015, 20, 225–236. [Google Scholar] [CrossRef]
- Bostami, A.; Mun, H.; Kim, D.; Yang, C.J. Evaluation of halal tallow and haram lard combinations on growth performance, immunity, cecal microbiology and noxious gas emissions in boilers. Int. J. Adv. Res. 2016, 4, 2376–2390. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [CrossRef]
- Hoang, N.V.; Hung, C.M.; Hoa, N.D.; Duy, N.V.; Park, I.; Hieu, N.V. Excellent detection of H2S gas at ppb concentrations using ZnFe2O4 nanofibers loaded with reduced graphene oxide. Sens. Actuators B Chem. 2019, 282, 876–884. [Google Scholar] [CrossRef]
- Priya, M.; Subha, P.; Aswathy, P.; Merin, K.; Jayaraj, M.; Kumar, K.R. Selective detection of hydrogen sulphide from the background of low concentration reducing gases. Mater. Chem. Phys. 2021, 260, 124038. [Google Scholar] [CrossRef]
- Guo, L.; Xie, N.; Wang, C.; Kou, X.; Ding, M.; Zhang, H.; Sun, Y.; Song, H.; Wang, Y.; Lu, G. Enhanced hydrogen sulfide sensing properties of Pt-functionalized α-Fe2O3 nanowires prepared by one-step electrospinning. Sens. Actuators B Chem. 2018, 255, 1015–1023. [Google Scholar] [CrossRef]
- Guo, M.; Wang, B.; Bian, H.; Tao, Z.; Luo, X.; Cui, Y.; Huang, J.; Tu, P. Low-temperature ppm-level H2S flexible gas sensor on the basis of Ag-modified ZnO. Mater. Sci. Semicond. Process. 2025, 185, 108944. [Google Scholar] [CrossRef]
- Khan, L.A.; Ali, S.; Ali, N.; Zhu, L.; Zulfiqar, S.; shah, S.; Hussain, S.A.; Shaik, M.R.; Khan, T.; Khan, G.; et al. Investigation of the Fe-doped SnO2 NPs with enhanced H2S gas sensing performance. Ceram. Int. 2025, 51, 6783–6792. [Google Scholar] [CrossRef]
- Roopa; Pradhan, B.K.; Mauraya, A.K.; Chatterjee, K.; Pal, P.; Muthusamy, S.K. High-sensitive and fast-responsive In2O3 thin film sensors for dual detection of NO2 and H2S gases at room temperature. Appl. Surf. Sci. 2024, 678, 161111. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wang, X.; Wang, Q.; Huang, B.; Li, Y.; Hua, X.; Liu, G.; Li, B.; Zhou, J.; et al. Binder-free CuO nanoneedle arrays based tube-type sensor for H2S gas sensing. Sens. Actuators B Chem. 2021, 326, 128993. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.; Mhlongo, G. H2S detection capabilities with fibrous-like La-doped ZnO nanostructures: A comparative study on the combined effects of La-doping and post-annealing. J. Alloys Compd. 2019, 797, 284–301. [Google Scholar] [CrossRef]
- Mousavi, S.; Kang, K.; Park, J.; Park, I. A room temperature hydrogen sulfide gas sensor based on electrospun polyaniline–polyethylene oxide nanofibers directly written on flexible substrates. RSC Adv. 2016, 6, 104131–104138. [Google Scholar] [CrossRef]
- Song, B.Y.; Zhang, X.F.; Huang, J.; Cheng, X.L.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Porous Cr2O3 Architecture Assembled by Nano-Sized Cylinders/Ellipsoids for Enhanced Sensing to Trace H2S Gas. ACS Appl. Mater. Interfaces 2022, 14, 22302–22312. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yan, L.; He, Y.; Li, H.; Liu, L.; Cheng, Y.; Du, L. The fabrication of In2O3 toruloid nanotubes and their room temperature gas sensing properties for H2S. Mater. Res. Express 2017, 4, 095022. [Google Scholar] [CrossRef]
- Nakla, W.; Wisitsora-at, A.; Tuantranont, A.; Singjai, P.; Phanichphant, S.; Liewhiran, C. H2S sensor based on SnO2 nanostructured film prepared by high current heating. Sens. Actuators B Chem. 2014, 203, 565–578. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Hung, C.M.; Toan, N.V.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. One-step fabrication of SnO2 porous nanofiber gas sensors for sub-ppm H2S detection. Sens. Actuators A Phys. 2020, 303, 111722. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Cho, H.J.; Kim, N.H.; Kim, I.D. Mesoporous SnO2 Nanotubes via Electrospinning–Etching Route: Highly Sensitive and Selective Detection of H2S Molecule. ACS Appl. Mater. Interfaces 2017, 9, 26304–26313. [Google Scholar] [CrossRef]
- He, H.; Zhao, C.; Xu, J.; Qu, K.; Jiang, Z.; Gao, Z.; Song, Y.Y. Exploiting Free-Standing p-CuO/n-TiO2 Nanochannels as a Flexible Gas Sensor with High Sensitivity for H2S at Room Temperature. ACS Sens. 2021, 6, 3387–3397. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, K.; Li, Q.; Zhu, Z.Q.; Wan, Q. Room-temperature high-sensitivity H2S gas sensor based on dendritic ZnO nanostructures with macroscale in appearance. J. Appl. Phys. 2008, 103, 104305. [Google Scholar] [CrossRef]
- Abdullah, A.N.; Kamarudin, K.; Kamarudin, L.M.; Adom, A.H.; Mamduh, S.M.; Mohd Juffry, Z.H.; Bennetts, V.H. Correction model for metal oxide sensor drift caused by ambient temperature and humidity. Sensors 2022, 22, 3301. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Tipparaju, V.V.; Tsow, F.; Xian, X. Mitigation of humidity interference in colorimetric sensing of gases. ACS Sens. 2020, 6, 303–320. [Google Scholar] [CrossRef]
- Prudenza, S.; Rubio, A.P.; Bax, C.; Marzocchi, M.; Casadio, M.; Capelli, L. Preliminary Study for the Implementation of a Software Method for Humidity Compensation in E-noses for Outdoor Applications. Chem. Eng. Trans. 2022, 95, 175–180. [Google Scholar] [CrossRef]
- Xu, P.; Song, K.; Xia, X.; Chen, Y.; Wang, Q.; Wei, G. Temperature and Humidity Compensation for MOS Gas Sensor Based on Random Forests. In Proceedings of the Intelligent Computing, Networked Control, and Their Engineering Applications; Yue, D., Peng, C., Du, D., Zhang, T., Zheng, M., Han, Q., Eds.; Springer: Singapore, 2017; pp. 135–145. [Google Scholar]
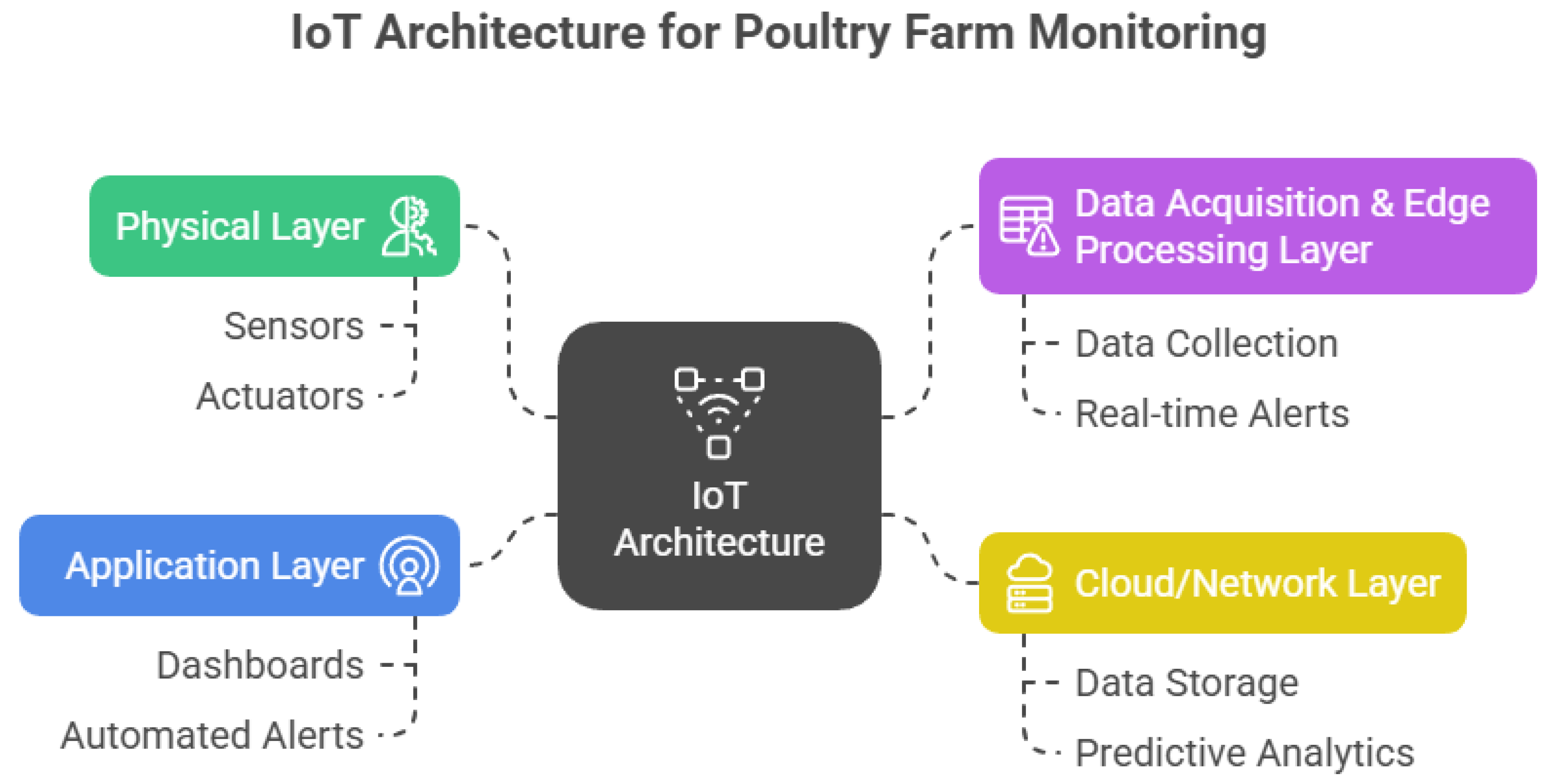
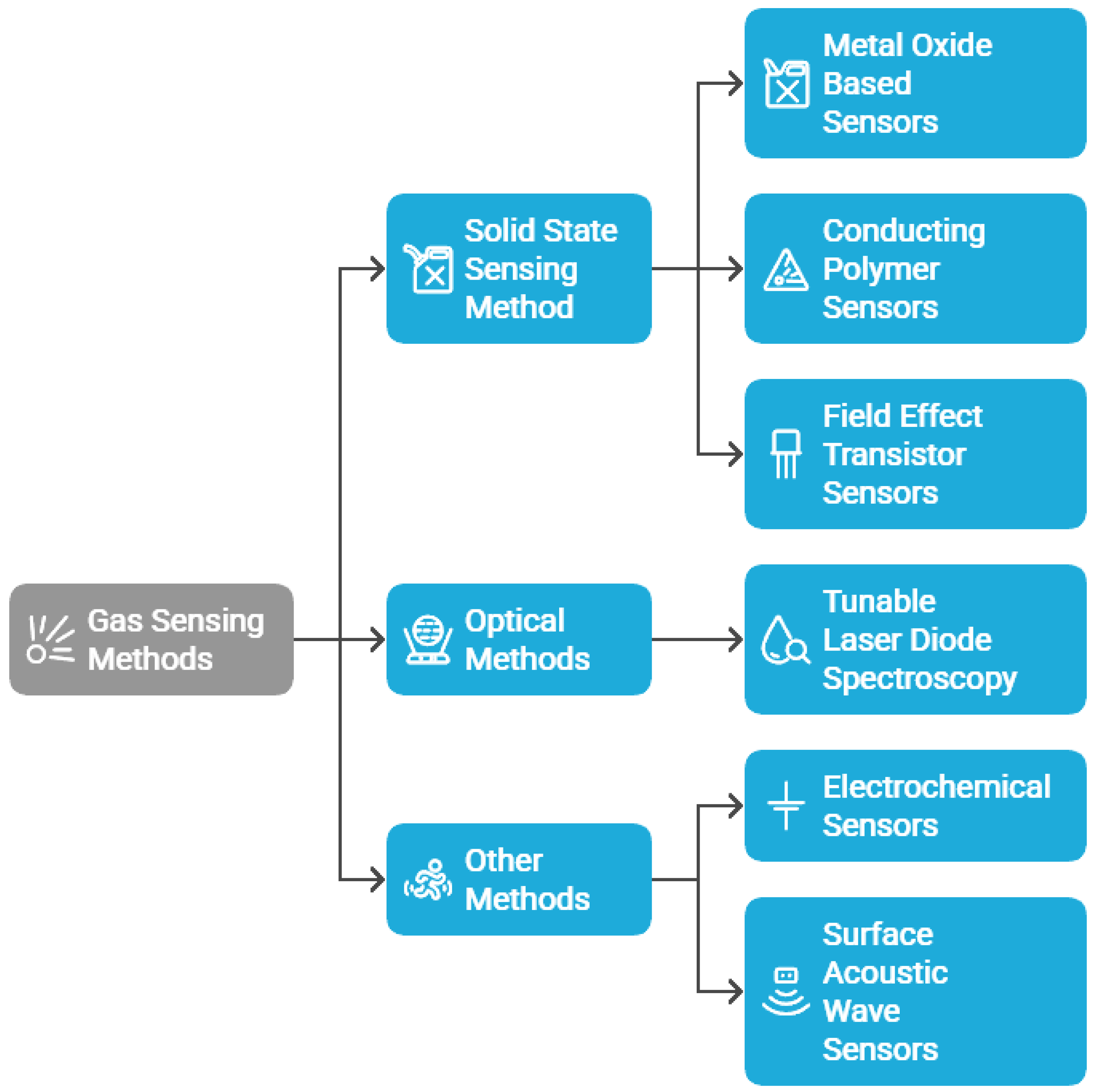

| Feature/System | Monitored Parameters | Sensors Used | Actuators | Communication Protocol | Field-Tested | Advanced Features | Ref. |
|---|---|---|---|---|---|---|---|
| WiMoCoSPH | Temperature, relative humidity, CO2, NH3 | HDC1080, CCS811, MQ-135 | Fans, heaters, sprinklers, curtains | Wi-Fi (HTTP) | Yes | Distributed architecture, web dashboard, curtain control | [11] |
| Smart Poultry Farm | Temperature, humidity, NH3, water level | DHT11, MQ6, ultrasonic sensor | cooling fan, heater, sprayer, water motor | Wi-Fi (Blynk cloud/App) | Yes | Automated water refill, lighting strategy | [12] |
| Smart Sensors and AI-based PLM | T, H, NH3, weight, behavior | DHT22, MQ-135, HX711, camera | Actuators for environmental control | MQTT | Yes | AI for health prediction, image processing for behavior | [13] |
| Low-Cost IoT-based IS | T, H, weight, feed consumption, mortality | SHT20, JARM ESP32 board | Fans, heating lamps | LoRa/Wi-Fi | Yes | Controlled experiment on weight gain, low-cost design | [14] |
| Materials | Method | Op.Temp (°C) | Concentration | Response (Ra/Rg, Rg/Ra, ) | Year | Res/RecTime (s) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|
| Co-doped NiO | SILAR method | 27 | 50 ppm | – | 2025 | 20/16 | Thin film | [19] |
| NiO | Solvothermal | 27 | 100 ppm | – | 2025 | 40/44 | Nanoflower | [20] |
| Ca-doped ZnO | Sol-gel | 300 | 4000 ppm | 33 for 4000 ppm | 2024 | 5/221 | – | [21] |
| Ti2C-Tx/Ti3AlC2 | Drop coating | 27 | 50 ppb | 1.2 | 2023 | – | – | [22] |
| Al-doped ZnO | Co-precipitation method | 27 | 1 ppm | – | 2023 | 26/18 | Nanoflowers | [23] |
| PANI/MoS2/SnO2 | Hydrothermal + in situ polymerization | 27 | 100 ppm–200 ppb | 10.9% for 100 ppm | 2021 | – | – | [24] |
| CoSe2@NC/MWCNTs | Reaction | 27 | 10 ppm | – | 2021 | 4/27 | – | [25] |
| Pt-decorated WO3 | RF sputtering | 250 | 1–1000 ppm | 3.37 for 1 ppm | 2019 | 26 | – | [26] |
| TiO2 | Sol-gel + Hydrothermal | 350 | 43 ppm | – | 2011 | – | – | [27] |
| PANI-TiO2 | Spin Coating | 27 | 20–100 ppm | 72% for 20 ppm | 2011 | – | – | [28] |
| Mn-doped ZnO | Hydrothermal method | 150 | 20–100 ppm | 28.584 | 2017 | 4/10 | Wurtzite | [29] |
| Ni-doped ZnO | Spray pyrolysis | 27 | 25–1000 ppm | 2.52 for 100 ppm | 2014 | – | Thin film | [30] |
| Cr2O3–ZnO | Dipping technique | 27 | 100 ppm | 1.37 | 2007 | 25/75 | Thin film | [31] |
| ZnO nanorods | Hydrothermal method | 27 | 100 ppm | 22.8 | 2014 | – | Thin film | [32] |
| ZnO | Dipping technique | 27 | 100 ppm | 13 | 2012 | – | Thin film | [33] |
| Carbon nanotubes | Plasma-enhanced CVD | 27 | 100 ppm | 25 | 2017 | – | – | [34] |
| MnO2 | Chemical route | 27 | 100 ppm | 20 | 2016 | 70/85 | Thin film | [35] |
| ZnO | Sol-gel method | 150 | 50–600 ppm | 57 for 600 ppm | 2014 | 160/660 | Thin film | [36] |
| ZnO | RF sputtering | 250 | 1000 ppm | 289 for 1000 ppm | 2015 | 31/78 | Thin film | [37] |
| ZnO nanorods | Hydrothermal growth | 300 | 10–1000 ppm | 80.6 for 1000 ppm | 2012 | – | Nanorods | [38] |
| ZnO nanowires | AAO template method | 50 ppm | 28/29 | 2014 | – | Nanowires | [39] | |
| ZnO Nanostructures | RF sputtering | 26 | 25 ppm | 49/19 | 2015 | – | Nanorods | [40] |
| ZnO | Carbothermal + Hummer’s method | 26 | 0.1 ppm | 7.2 | 2015 | 50/200 | – | [41] |
| Materials | Method | Op. Temp (°C) | Concentration | Response (Ra/Rg, Rg/Ra, ) | Year | Res/RecTime (s) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|
| CuO:TiO2 | GLAD | 27 | 5 ppm | – | 2023 | 36/50 | Nanorod | [53] |
| MoS2–Au NPs | Facile solution-mixing | 30 ppm | 7.6 | 2023 | 406/516 | Nanoparticles | [54] | |
| SnO2 | Hydrolysis method | 500 | 300 ppm | 90 | 2001 | 20/60 | – | [55] |
| InO3 | Thermal evaporation | 150 | 10 ppm | 60 | 2006 | 20/20 | Nanowires | [56] |
| WO3 | Solvothermal method | – | 10 ppm | 20–25 | 2006 | 10/60 | Nanowires | [56] |
| SnO2 | L-MOCVD | 210 | 100 ppm | 11.5 | 2005 | – | Thin film | [57] |
| Emission Rate | Unit | Context | Ref. |
|---|---|---|---|
| 0.44 | mg/h per bird | Summer | [62] |
| 1.87 | mg/h per bird | Winter | [62] |
| 13 | mg/day | Daily average | [63] |
| 82.63 | mg/kg·h | Mean body mass of 1.92 kg | [64] |
| Materials | Method | Op. Temp (°C) | Concentration | Response (Ra/Rg, Rg/Ra, ) | Year | Res/RecTime (s) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|
| NiO/ZnO | Hydrothermal method | 5000 | 8.6 | 2024 | 32/182 | Nanospheres | [72] | |
| ZnO-Pd@ZIF-8 | Self-templated method | 210 | 100 | 20.6 | 2023 | 9.9/3.3 | Nanorods | [73] |
| CQDs@NiO | Hydrothermal method | 150 | 30 | 77 | 2020 | 10/14 | – | [74] |
| Pt-doped SnO2 | Hydrothermal method | 100 | 500 | 1.98 | 2019 | – | Flower-like | [75] |
| NiO/SnO2 | Hydrothermal method | 250 | 1000 | 15 | 2016 | 18/20 | – | [76] |
| RGO/ZnO | Electrochemical anodization | 250 | 500 | 1.67 | 2016 | – | – | [77] |
| ZnO/SnO2 | Hydrothermal method | 190 | 500 | 8 | 2015 | – | Nanorods | [78] |
| Pd/Al2O3 | Colloidal method | 400 | 1 | – | – | – | – | [70] |
| SnO2–NiO | DC sputtering | 400 | 200 | 127 | 2014 | – | Thin films | [79] |
| SnO2–Pd | RF sputtering | 220 | 200 | 97.2 | 2012 | – | Thin film | [80] |
| Ag-doped ZnO | Solvothermal method | 200 | 5000 | 20.15 | 2021 | 118/119 | Flower-like microsphere | [81] |
| ZnO | PE-CVD | 300 | 500 | 1.70 | 2010 | 60/120–180 | – | [82] |
| Pd-doped SnO2 | Chemical method | 600 | 6500 | 21 | 2011 | – | – | [83] |
| Materials | Method | Op. Temp (°C) | Concentration | Response (Ra/Rg, Rg/Ra, ) | Year | Res/RecTime (s) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|
| SnO2 | Electron beam evaporation | 20 | 5000 | – | 2025 | 01/120 | Thin film | [107] |
| BaTiO3 | Co-precipitation method | 27 | 50 | – | 2024 | 23/20 | Nanospheres | [108] |
| SnO2 | Co-precipitation | 240 | 20,000 | 1.185 | 2016 | 31/47 | Nanoparticles | [109] |
| ZnO | Precipitation | 250 | 1000 | 0.125 | 2019 | 9/9 | Nanoflakes | [110] |
| ZnO | DC sputtering | 300 | 500–10,000 | 1.13 | 2014 | 20/20 for 1000 ppm | Thin film | [111] |
| Ca-doped ZnO | Sol-gel | 450 | 0–10,000 | 1.13 | 2015 | 10/10 | Nanoparticles | [112] |
| Ba-doped Co3O4 | Solvothermal method | 200 | 500 | – | 2017 | 227/245 | Hexagonal | [113] |
| Pd@MoO3/NiO | Hydrothermal | 150 | 1000 | 96.1 | 2019 | 30/20 | Nanoparticles | [114] |
| ZnSnO3 | Hydrothermal | 400 | 4.65 | – | 73/79 | Nano powders | [5] | |
| ZnO | Spray pyrolysis | 350 | 400 | 2.86 | – | 75/108 | Thin film | [115] |
| Materials | Method | Op. Temp (°C) | Concentration | Response (Ra/Rg, Rg/Ra, ) | Year | Res/RecTime (s) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ag/ZnO | Hydrothermal | 120 | 1 ppm | – | 2025 | 190/120 | Nanoparticles | [127] |
| Fe-doped SnO2 | Co-precipitation | 275 | 100 ppm | 92 | 2025 | – | Nanoparticles | [128] |
| In2O3 | Thermal oxidation | 27 | 5 ppm | – | 2024 | 36/18 | Thin film | [129] |
| CuO | Magnetron Sputtering | 150 | 10 ppm | 76.5 | 2021 | 92/196 | Needle array | [130] |
| La-doped ZnO | Electrospinning | 175 | 90 ppm | 6485 | 2019 | 53.7/20.7 | Nanofibers | [131] |
| PANI–PEO | Electrospinning | 26 | 1 ppm | 5 | 2016 | 120/250 | Nanofibers | [132] |
| Cr2O3 | Air calcination (scallion roots) | 170 | 100 ppm | 42.81 | 2022 | 73/192 | Nanosized cylinders, ellipsoids | [133] |
| In2O3 | Electrospinning | 25 | 50 ppm | 320.14 | 2017 | 45/12 | Nanotubes | [134] |
| SnO2 | HCH | 150 | 10 ppm | 25 | 2014 | 0.5/3 | Nanowires | [135] |
| SnO2 | Electrospinning | 350 | 1 ppm | 15.2 | 2014 | 15/230 | Porous nanofibers | [136] |
| SnO2 | Electrospinning | 300 | 5 ppm | 154.8 | 2017 | 99.5/111 | Nanotubes | [137] |
| P–CuOx–TiO2 | Electrochemical anodization | 26 | 100 ppm | 1.88 | 2021 | 41/92 | Nanochannels | [138] |
| ZnO | CVD method | 27 | 4 ppm | 6 | 2017 | 22/540 | Comb-like | [48] |
| ZnO | CVD | 30 | 100 ppm | 17.3 | 2008 | 20/50 | ZnO dendrites | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, I.e.; Comini, E. Gas Sensing for Poultry Farm Air Quality Monitoring to Enhance Welfare and Sustainability. Chemosensors 2025, 13, 347. https://doi.org/10.3390/chemosensors13090347
Abbas Ie, Comini E. Gas Sensing for Poultry Farm Air Quality Monitoring to Enhance Welfare and Sustainability. Chemosensors. 2025; 13(9):347. https://doi.org/10.3390/chemosensors13090347
Chicago/Turabian StyleAbbas, Ibn e, and Elisabetta Comini. 2025. "Gas Sensing for Poultry Farm Air Quality Monitoring to Enhance Welfare and Sustainability" Chemosensors 13, no. 9: 347. https://doi.org/10.3390/chemosensors13090347
APA StyleAbbas, I. e., & Comini, E. (2025). Gas Sensing for Poultry Farm Air Quality Monitoring to Enhance Welfare and Sustainability. Chemosensors, 13(9), 347. https://doi.org/10.3390/chemosensors13090347






