An Electrochemical Aptamer Sensor with ZIF-8 Loaded CuNPs Composites for Aflatoxin B1 Determination
Abstract
1. Introduction
2. Experiments
2.1. Reagents and Materials
2.2. Apparatus
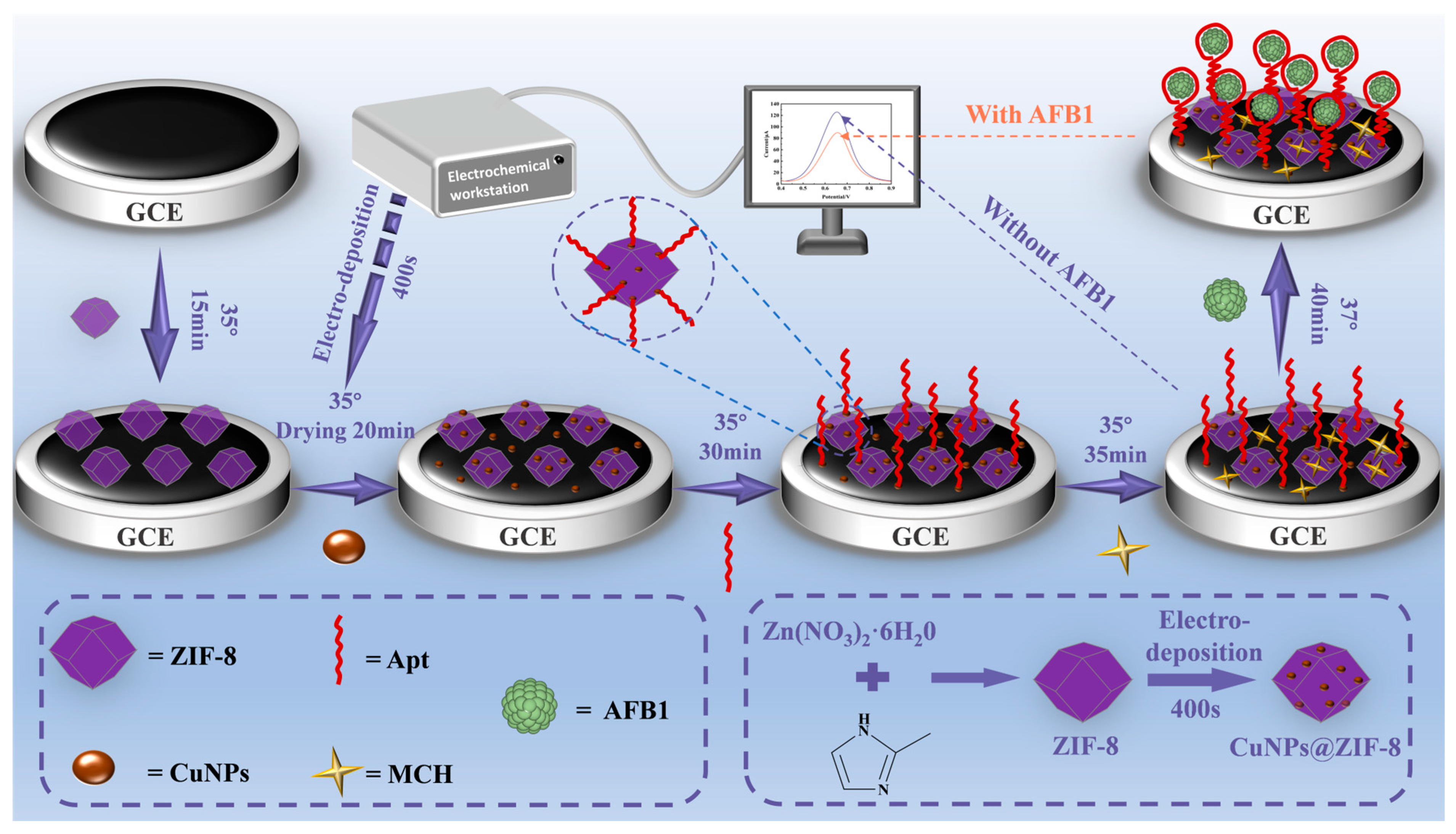
2.3. Experimental Principle
2.4. Synthesis of ZIF-8
2.5. Preparation of Electrochemical Aptamer Sensors
2.6. Electrochemical Measurements
2.7. Pre-Processing of Real Corn Samples
3. Results and Discussion
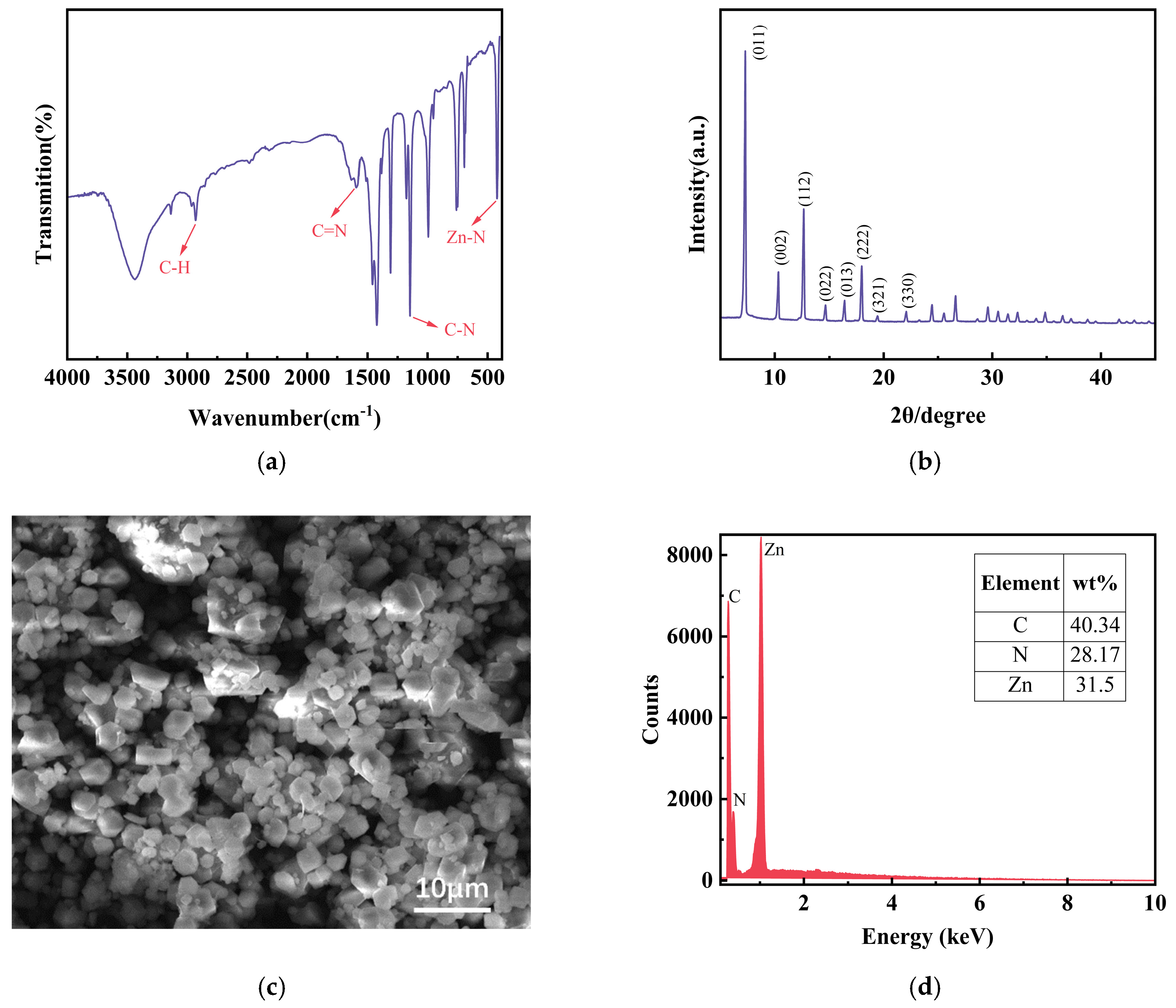
3.1. Physio-Chemical Characterization of Nanomaterials
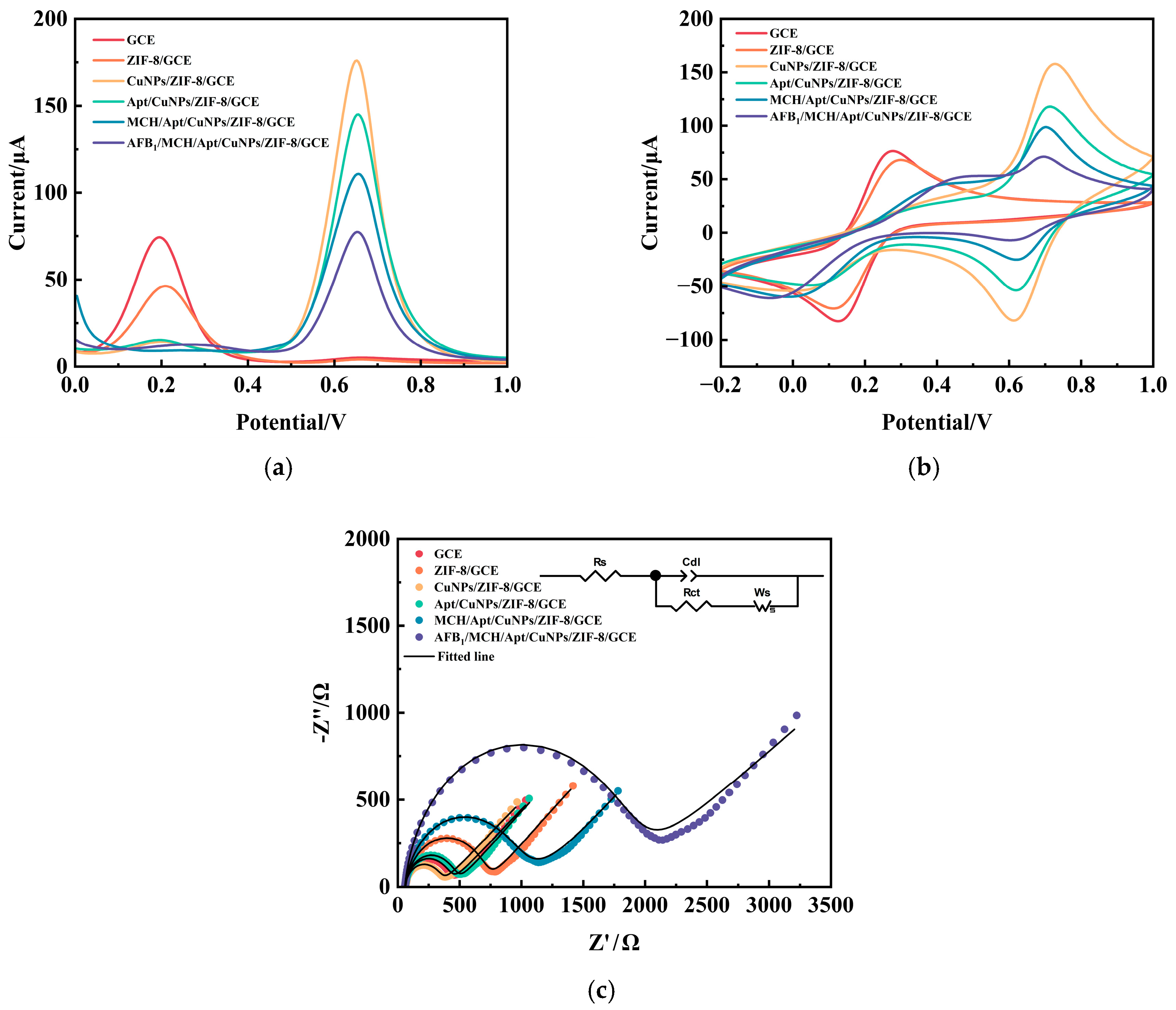
3.2. Electrochemical Characterization of AFB1 Aptamer Sensors
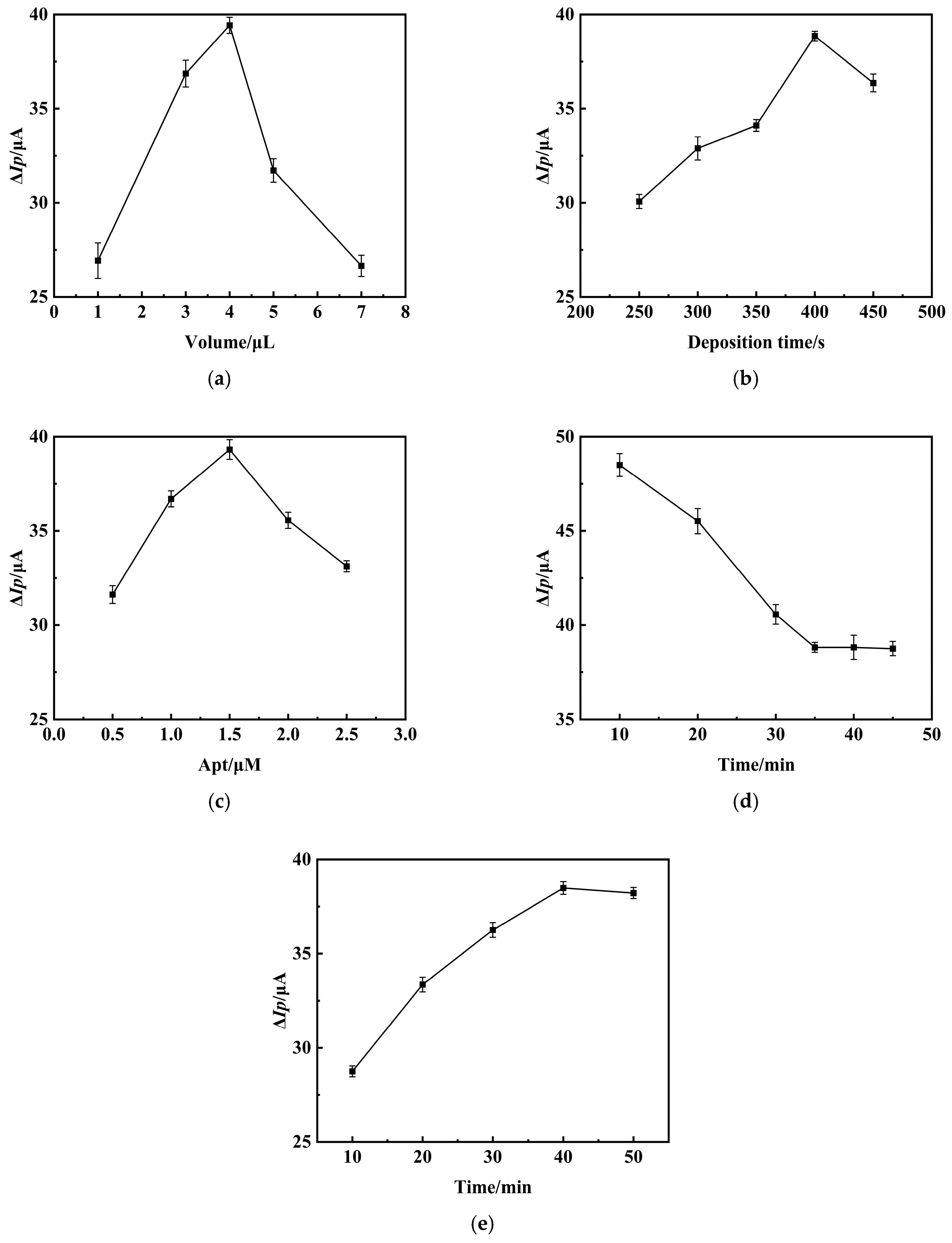
3.3. Optimization of AFB1 Aptamer Sensor Preparation Conditions
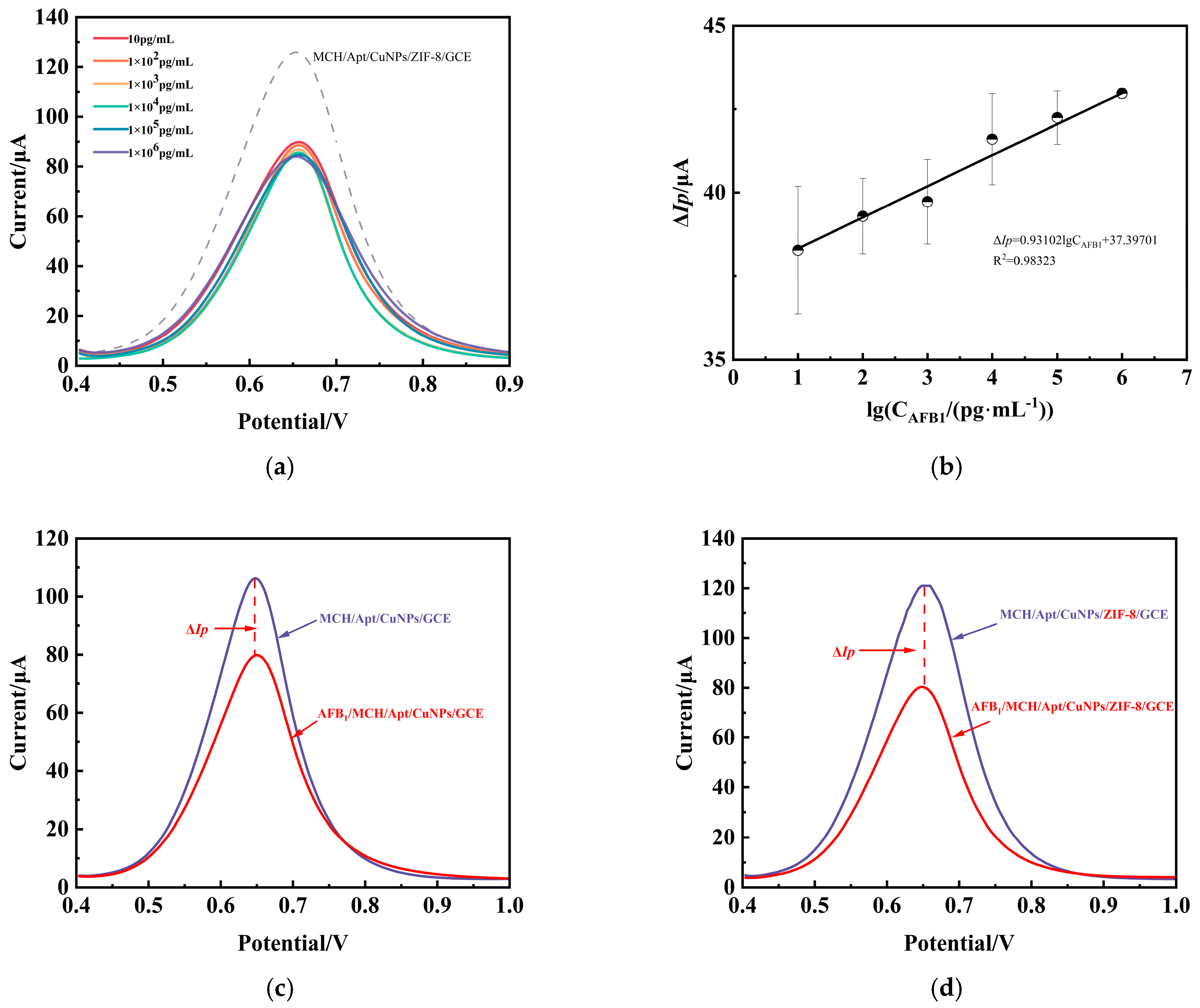
3.4. Detection of AFB1 by the Aptamer Sensor DPV
3.5. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, C.; Yang, J.; Wang, Y.; Ding, G.; Guo, L.; Qin, J. Mechanisms and transformed products of aflatoxin B1 degradation under multiple treatments: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2263–2275. [Google Scholar] [CrossRef]
- Tian, D.; Wang, J.; Zhuang, Q.; Wu, S.; Yu, Y.; Ding, K. An electrochemiluminescence biosensor based on Graphitic carbon nitride luminescence quenching for detection of AFB1. Food Chem. 2023, 404, 134183. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Wu, J.; Han, G.; Liu, Y.; Zou, P. A novel fluorescent aptasensor based on exonuclease-assisted triple recycling amplification for sensitive and label-free detection of aflatoxin B1. J. Hazard. Mater. 2021, 415, 125584. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Zhu, C.; Wang, M.; Liu, Y.; You, T. A ratiometry-induced successive reusable electrochemical aptasensing platform: Efficient monitoring of aflatoxin B1 in peanut. Sens. Actuators B Chem. 2021, 336, 129021. [Google Scholar] [CrossRef]
- Abd-Allah, G.A.; El-Fayoumi, R.I.; Smith, M.J.; Heckmann, R.A.; O’Neill, K.L. A comparative evaluation of aflatoxin B1 genotoxicity in fish models using the Comet assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1999, 446, 181–188. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Ghali, R.; Belouaer, I.; Hdiri, S.; Ghorbel, H.; Maaroufi, K.; Hedilli, A. Simultaneous HPLC determination of aflatoxins B1, B2, G1 and G2 in Tunisian sorghum and pistachios. J. Food Compos. Anal. 2009, 22, 751–755. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Zhai, W.; Feng, X.; Fan, X.; Chen, A.; Wang, M. A lateral flow strip based on a truncated aptamer-complementary strand for detection of type-B aflatoxins in nuts and dried figs. Toxins 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sánchez, I.; Ramírez-García, L.; Gracia-Lor, E.; Madrid-Albarran, Y. Simultaneous determination of aflatoxins B1, B2, G1 and G2 in commercial rices using immunoaffinity column clean-up and HPLC-MS/MS. Food Chem. 2022, 395, 133611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Song, S.; Sun, M.; Kuang, H.; Xu, C.; Guo, L. Colloidal gold immunochromatographic assay for the detection of total aflatoxins in cereals. Food Chem. 2025, 472, 142877. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xie, J.; Huang, Z. Rapid and simultaneous detection of aflatoxin B1, zearalenone, and T-2 toxin in medicinal and edible food using gold immunochromatographic test strip. Foods 2023, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Charernchai, S.; Chikae, M.; Phan, T.T.; Wonsawat, W.; Hirose, D.; Takamura, Y. Automated paper-based femtogram sensing device for competitive enzyme-linked immunosorbent assay of aflatoxin B1 using submicroliter samples. Anal. Chem. 2022, 94, 5099–5105. [Google Scholar] [CrossRef]
- Batrinou, A.; Houhoula, D.; Papageorgiou, E. Rapid detection of mycotoxins on foods and beverages with enzyme-linked immunosorbent assay. Qual. Assur. Saf. Crops Foods 2020, 12, 40–49. [Google Scholar] [CrossRef]
- Pereira, C.S.; Cunha, S.C.; Fernandes, J.O. Validation of an enzyme-linked immunosorbent assay (ELISA) test kit for determination of aflatoxin B1 in corn feed and comparison with liquid-chromatography tandem mass spectrometry (LC-MS/MS) method. Food Anal. Methods 2020, 13, 1806–1816. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Qian, S.; Li, H.; Fu, P.; Zhou, H.; Zheng, J. An ultrasensitive lateral flow immunoassay platform for foodborne biotoxins and pathogenic bacteria based on carbon-dots embedded mesoporous silicon nanoparticles fluorescent reporter probes. Food Chem. Chem. 2023, 399, 133970. [Google Scholar] [CrossRef]
- Li, S.; Zhong, X.; Xu, Y.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Smartphone-based reading system integrated with phycocyanin-enhanced latex nanospheres immunoassay for on-site determination of aflatoxin B1 in foodstuffs. Food Chem. 2021, 360, 130019. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, B.; Sheng, P.; Liao, X.; Shi, L.; Fang, L.; Zhou, L.; Kong, W. Aptasensors for mycotoxins in foods: Recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2032–2073. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional nucleic acid sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, C.; Zhao, Z. Detection of Aflatoxin B1 in Wheat Based on Nucleic Aptamer Chemiluminescence Sensor. Sensors 2025, 25, 988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, C.Z.; Zhao, Z.K.; Xu, K. An Electrochemical Immunosensor Based on Chitosan–Graphene Nanosheets for Aflatoxin B1 Detection in Corn. Molecules 2024, 29, 1461. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label–free electrochemical aptasensor based on platinum nanoparticles loaded on Fe–based metal–organic frameworks. Food Chem. 2020, 310, 125820. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Hu, Q.; Lei, X.; Guo, R.; You, J.; Tian, Y.; Yang, F.; Lv, M. Covalent-metal organic frameworks: Preparation and applications. Chem. Eng. J. 2024, 483, 149217. [Google Scholar] [CrossRef]
- Trino, L.D.; Albano, L.G.S.; Granato, D.C.; Santana, A.G.; de Camargo, D.H.S.; Correa, C.C.; Bufon, C.C.B.; Leme, A.F.P. ZIF-8 metal–organic framework electrochemical biosensor for the detection of protein–protein interaction. Chem. Mater. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Pham, T.N.; Tien, V.M.; Ong, V.H.; Le, N.T.N.; Ho, T.N.L.; Le, H.D.T.; Hoa, N.Q.; Tran, H.V.; Xuan, D.N.; Quang, H.T.; et al. Electrochemical Kinetic Behaviors and Sensing Performance of Au@ ZIF-8 and Ag@ ZIF-8 Nanocomposites-Based Platforms Towards Ultrasensitive Detection of Chloramphenicol. J. Electrochem. Soc. 2024, 171, 077517. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Z.; Wang, X.; Suo, Z.; Liu, Y.; Wei, M. A dual-signal ratiometric electrochemical aptasensor based on Thi/Au/ZIF-8 and catalytic hairpin assembly for ultra-sensitive detection of aflatoxin B1. Microchim. Acta 2024, 191, 256. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Yan, L.; Li, N.; Shi, J.; Jiang, C. Recent developments in detection using noble metal nanoparticles. Crit. Rev. Anal. Chem. 2020, 50, 97–110. [Google Scholar] [CrossRef]
- Putri, L.A.; Prabowo, Y.D.; Dewi, D.M.M.; Mumtazah, Z.; Adila, F.P.; Fadillah, G.; Amrillah, T.; Triyana, K.; Nugroho, F.A.A.; Wasisto, H.S. Review of noble metal nanoparticle-based colorimetric sensors for food safety monitoring. ACS Appl. Nano Mater. 2024, 7, 19821–19853. [Google Scholar] [CrossRef]
- Ahmadpor, H.; Milani-Hosseini, S.M.R. A comparative study of different techniques for quantum dot nano-particles immobilization on glass surface for fabrication of solid phase fluorescence opto-sensors. Surf. Interfaces 2021, 23, 100907. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Li, X.; Wang, R.; Zhang, Y.; Chen, W.; Song, K.; Wang, G.; Shi, G. ZIF-8/ZIF-67 solid electrolyte ozone sensor at room temperature. Sens. Actuators A Phys. 2023, 354, 114281. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Hu, C.; Zuo, C.; Wang, P.; Chen, W.; Ao, T. Efficient removal of tetracycline by a hierarchically porous ZIF-8 metal organic framework. Environ. Res. 2021, 198, 111254. [Google Scholar] [CrossRef]
- Ahmad, U.; Ullah, S.; Rehman, A.; Najam, T.; Alarfaji, S.S.; Jamshaid, M.; Kumar, O.P.; Ullah, S.; Shahid, M.; Shah, S.S.A.; et al. ZIF-8 composites for the removal of wastewater pollutants. ChemistrySelect 2024, 9, 202401719. [Google Scholar] [CrossRef]
- Pei, Z.; Fei, P.; Zhang, A.; Guo, J.; Hao, J.; Jia, J.; Dong, H.; Shen, Q.; Wei, L.; Jia, H.; et al. Thermal oxygen sensitization modification and its visible light catalytic antibacterial performance for ZIF-8. J. Alloys Compd. 2022, 904, 164055. [Google Scholar] [CrossRef]
- Meng, D.; Gan, X.; Tian, T. An electrochemical sensing method for aflatoxin B1 detection based on Pt-coordinated titanium-based porphyrin MOF. Int. J. Electrochem. Sci. 2022, 17, 220247. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Zhu, C.; Shen, X.; Liu, Y.; You, T. Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut. J. Hazard. Mater. 2020, 387, 122001. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Simultaneous measurement of ochratoxin A and aflatoxin B1 using a duplexed-electrochemical aptasensor based on carbon nanodots decorated with gold nanoparticles and two redox probes hemin@ HKUST-1 and ferrocene@ HKUST-1. Talanta 2024, 266, 124947. [Google Scholar] [CrossRef]
- Rahmanian, H.; Malekkiani, M.; Dadmehr, M.; Es’haghi, Z.; Moshirian-Farahi, S.S. A biosensor integrating the electrochemical and fluorescence strategies for detection of aflatoxin B1 based on a dual-functionalized platform. Anal. Chim. Acta 2024, 1323, 343085. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.; Wu, T.; Zhang, X.; Ren, X.; Feng, R.; Du, Y.; Lee, J.; Liu, X.; Wei, Q. Zirconium based metal–organic frameworks with aggregation-induced electrochemiluminescence for sensitive analysis of aflatoxin B1 by signal dual-amplification strategy. Chem. Eng. J. 2024, 500, 157308. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Hong, Y.; Cai, X.; Chen, X.; Liang, H.; Xu, X.; Wang, Y.; Li, C.; Sun, D. Polycarboxyl ionic liquid functionalized Yb-MOFs nanoballs based dual-wavelength responsive photoelectrochemical aptasensor for the simultaneous determination of AFB1 and OTA. Anal. Chim. Acta 2024, 1298, 342383. [Google Scholar] [CrossRef] [PubMed]

| Sensors | Linear Range | LOD | References |
|---|---|---|---|
| AP/Ti-MOFs-Pt/GCE | 0.1–75 μg/L | 31 ng/L | [34] |
| AFB1/Fc-apt/MCH/cDNA/AuNPs/THI-rGO/GCE | 0.05–20 ng/mL | 0.016 ng/mL | [35] |
| AFB1/BSA/anti-AFB1/CS-GNs/GCE | 0.05–25 ng/mL | 0.021 ng/mL | [20] |
| AFB1/Bioconj/Apts/AuNPs-CNDs/GCE | 10–10 × 104 pg/mL | 5.2 pg/mL | [36] |
| Apt/Fe3O4@AuNPs/ZIF-8/CS/GCE | 0.5–10 × 104 pg/mL | 0.32 pg/mL | [37] |
| H2-AgNPs/cDNA/MCH/H1/AuNPs/Zr-TCPB/GCE | 1–10 × 104 pg/mL | 0.79 pg/mL | [38] |
| AFB1/AuNRs- Apt/AuNPs- Apt/BSA/hDNA/Yb-MOF@AuNPs/GCE | 1–50 × 104 pg/mL | 0.40 pg/mL | [39] |
| AFB1/MCH/Apt/CuNPs/ZIF-8/GCE | 10–1.0 × 106 pg/mL | 1.13 pg/mL | This work |
| Added AFB1 (ng/mL) | Found AFB1 (ng/mL) | Recovery (%) |
|---|---|---|
| 1 | 1.013 ± 0.012 | 101.33 ± 1.206 |
| 10 | 9.666 ± 0.104 | 96.663 ± 1.037 |
| 100 | 105.720 ± 1.025 | 105.72 ± 1.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wu, C.; Zhao, Z.; Xue, R. An Electrochemical Aptamer Sensor with ZIF-8 Loaded CuNPs Composites for Aflatoxin B1 Determination. Chemosensors 2025, 13, 342. https://doi.org/10.3390/chemosensors13090342
Chen J, Wu C, Zhao Z, Xue R. An Electrochemical Aptamer Sensor with ZIF-8 Loaded CuNPs Composites for Aflatoxin B1 Determination. Chemosensors. 2025; 13(9):342. https://doi.org/10.3390/chemosensors13090342
Chicago/Turabian StyleChen, Juncheng, Caizhang Wu, Zhike Zhao, and Ruihao Xue. 2025. "An Electrochemical Aptamer Sensor with ZIF-8 Loaded CuNPs Composites for Aflatoxin B1 Determination" Chemosensors 13, no. 9: 342. https://doi.org/10.3390/chemosensors13090342
APA StyleChen, J., Wu, C., Zhao, Z., & Xue, R. (2025). An Electrochemical Aptamer Sensor with ZIF-8 Loaded CuNPs Composites for Aflatoxin B1 Determination. Chemosensors, 13(9), 342. https://doi.org/10.3390/chemosensors13090342





