Abstract
This study presents a highly sensitive non-enzymatic electrochemical sensor for detecting Sunset Yellow, a common food additive in beverages, based on palladium-cerium oxide composite decorated carbon black (CB). The sensing material was prepared by depositing palladium nanoparticles onto cerium oxide nanocubes, followed by the uniform dispersion of CB through sonication in a water bath. The strong metal–support interaction between palladium and cerium oxide significantly enhances catalytic activity, while the CB ensures excellent conductivity and structural support for the catalyst. Under optimized conditions, the sensor exhibits a linear response to Sunset Yellow concentrations in the range from 1 to 100 nM, with a limit of detection (LOD) of 0.056 nM. Additionally, the sensor demonstrates remarkable selectivity and stability. Practical application in real orange juice samples yielded recoveries from 99.11% and 101.34%, confirming its reliability for real-world beverage analysis.
1. Introduction
Food coloring, a type of dye added to food products, is an important component of the food industry. It compensates for color loss during high-temperature high-humidity processing and imparts a naturally vibrant appearance to food. To develop more cost-effective and stable products, manufacturers are constantly improving the solubility, stability, and other characteristics of food colorings [1,2]. However, the use of food colorings also raises safety concerns. An excessive intake of certain colorings can accumulate in the body, leading to various functional disorders such as allergies, attention deficit hyperactivity disorder, and heart disease [3,4]. Numerous countries and regions have established stringent legal regulations governing the permissible levels of food colorings in consumable products [5]. Rising health awareness and stricter food safety controls have elevated the significance of food coloring analysis and research.
Among the vast array of food colorings, azo dyes are the most widely used synthetic colorants [6], primarily due to their simple synthetic routes, controllable structural stability, and low production costs. Sunset Yellow FCF, also known as Food Yellow 3, is a common azo dye extensively used in various yellow or orange foods such as beverages, candies, pastries, and jellies, making it an indispensable colorant in the food industry [7]. Despite its widespread application, increasing research indicates that an excessive intake of Sunset Yellow may pose potential threats to human health, leading to various diseases such as kidney failure, liver damage, and immune system suppression [8]. Therefore, the effective control of Sunset Yellow usage and an emphasis on accurate determination in food is crucial, which is of great significance to safeguarding consumer health rights [9]. Currently, various methods exist for determining Sunset Yellow in food samples, including fluorescence, high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), surface-enhanced Raman scattering (SERS), colorimetry, and enzyme-linked immunosorbent assay (ELISA) [10,11,12]. However, these traditional methods generally suffer from drawbacks such as being time-consuming, complex to operate, and high cost. Moreover, they often require large sophisticated equipment and specialized operators, making it challenging to meet the need for rapid and convenient on-site detection in food production, distribution, and regulation. Therefore, developing a more straightforward, faster, more economical, and easily implementable on-site analysis technique for Sunset Yellow is crucial [13]. Compared with other analytical methods, electrochemical methods offer significant advantages such as simple operation, low cost, high sensitivity, and ease of miniaturization, and they enable rapid and real-time detection [14,15]. This makes them suitable for various applications in food safety, environmental monitoring, clinical diagnostics, and other fields [6,16,17]. Consequently, developing a Sunset Yellow sensor based on electrochemical principles has significant research and practical value.
Carbon black (CB), a highly versatile nanomaterial, is widely employed in electrochemical sensor fabrication owing to its exceptional electrical conductivity, extensive surface area, and abundant functional groups for molecular anchoring [18,19]. When combined with catalytically active materials, CB significantly enhances sensor performance, improving sensitivity and detection efficiency [20,21]. Noble metal catalysts, especially palladium group metal catalysts, have garnered considerable attention due to their excellent electron transfer properties and high catalytic activity for numerous chemical reactions [22]. Cerium oxide (CeO2), an essential rare earth oxide, exhibits unique redox properties through the Ce4+/Ce3+ redox couple [23]. Furthermore, the presence of cerium can increase the migration of lattice oxygen in CeO2-containing catalysts, thereby promoting catalytic reactions [24]. Therefore, CeO2 plays a crucial role in catalytic oxidation, oxygen storage, gas sensors, and other fields [25]. In addition, cerium can serve as an active component of catalysts and support noble metal catalysts [26]. For supported palladium catalysts, an in-depth study of the interaction between the support and palladium or palladium oxide can help reveal how the support’s properties influence its catalytic performance, thereby providing theoretical guidance for the design of efficient catalysts [27].
In this study, we developed a highly sensitive electrochemical sensor based on a palladium-cerium oxide/CB (Pd-CeO2/CB) composite to rapidly and efficiently detect Sunset Yellow. The Pd-CeO2/CB composite serves as a high-performance non-enzymatic sensing material, and when integrated with an electrochemical detection method, this system offers a promising solution for monitoring Sunset Yellow in food safety applications. As shown in Figure 1, to achieve efficient catalytic oxidation of Sunset Yellow, the Pd-CeO2 nanoparticles were physically mixed with CB to form a conductive matrix. The interfacial sites on the surface of the Pd-CeO2/CB composite can effectively catalyze the electrochemical oxidation of Sunset Yellow, resulting in an enhancement effect on its detection. To achieve optimal sensor performance, the electrochemical behavior of Sunset Yellow was systematically investigated using cyclic voltammetry (CV) and amperometry. Critical experimental parameters, including the pH and mass ratio of the composite materials, were carefully optimized to enhance detection sensitivity and reproducibility.
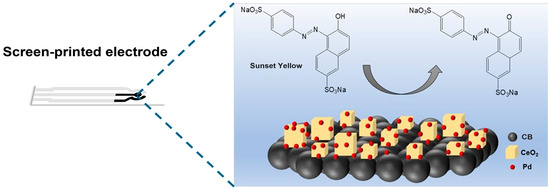
Figure 1.
Schematic of the Pd-CeO2/CB composite-based Sunset Yellow electrochemical sensor.
2. Materials and Methods
2.1. Materials
Cerium oxide, palladium, and Nafion were purchased from Merck KGaA, Darmstadt, Germany. Carbon black (Vulcan XC 72R) was obtained from Cabot Corporation, Boston, MA, USA. Sunset Yellow, benzoic acid, sodium carbonate, ascorbic acid, potassium sorbate, and acesulfame were purchased from Aladdin, Shanghai, China. All chemical reagents were of analytical grade and used directly without further purification. The orange and carbonated orange juice used in the experiments were purchased from a local supermarket.
2.2. Preparation of Pd-CeO2/CB Composite
In this work, the Pd-CeO2 nanocomposite was prepared following a previously reported method [28]. First, commercially available CeO2 powder was calcined in a single-zone tube furnace at a high temperature of 900 °C for 15 h. Subsequently, the calcined CeO2 powder and palladium powder were mixed in a predetermined mass ratio and thoroughly dispersed in an ultrasonic cleaner until the color of the mixture changed from off-white to a uniform gray-white, yielding the Pd-CeO2 composite material. Then, a 2 mg mixture of Pd-CeO2/CB composite (at 1:9, 3:7, 1:1, 7:3, or 9:1 weight ratio) was sonicated for 15 min in a solution of 100 µL ethanol and 25 µL Nafion to create a homogeneous suspension for electrode modification.
2.3. Sensor Fabrication
In this study, a screen-printed electrode (SPE) was used to fabricate an electrochemical sensor. This electrode integrates an Ag/AgCl reference electrode, a carbon counter electrode, and a carbon working electrode (1.2 mm in diameter) on a polyethylene terephthalate (PET) substrate. The material preparation process for electrode modification was as follows: First, a mixture of 2 mg Pd-CeO2/CB composite (with a weight ratio of 7:3) was dispersed in a mixed solution of 100 µL ethanol and 25 µL Nafion, and treated under ultrasonic conditions for 15 min to obtain a homogeneous suspension. Subsequently, 1 µL of the above suspension was carefully drop-cast onto the surface of the working electrode of the SPE. After natural drying, it could be used for subsequent electrochemical testing. CB/SPE, Pd/SPE, and NCs/SPE electrodes were prepared using the same method as controls.
2.4. Preparation of Sunset Yellow Standard Solutions
A series of Sunset Yellow standard solutions with different concentrations was prepared to perform quantitative detection of Sunset Yellow. Specifically, 5.2 mg of Sunset Yellow powder was dissolved in 1 mL of phosphate-buffered saline (PBS) at pH 7.4, and a 10 mM Sunset Yellow stock solution was obtained by ultrasonication. Then, the stock solution was serially diluted with PBS to prepare Sunset Yellow standard working solutions of different concentrations for electrochemical testing.
2.5. Electrochemical Detection
The electrocatalytic activity of the Pd-CeO2/CB composite material was evaluated using CV and amperometry with a CHI 660E electrochemical workstation. CV was performed with a scan rate of 50 mV/s within a potential window of 0.4 V to 0.8 V. Amperometry was conducted at a constant potential of 0.55 V for 100 s to record the change in current.
3. Results
3.1. Characterization of the Sensing Material
As shown in the TEM characterization images at different magnifications (Figure 2a,b), palladium particles with smaller particle sizes were distributed on the large-sized CeO2 particles, and the CB particles were tightly bound to each other to form agglomerates, which were stably dispersed around the perimeter of the Pd-CeO2 composites. In Figure 2c, in addition to having (111), (200), (220), (311), and (222), which are characteristic crystal faces of CeO2, the (111) and (220) faces at crystal axis angles of 40° and 68° are characteristic crystal faces of palladium, which are in agreement with the results previously reported [29,30].
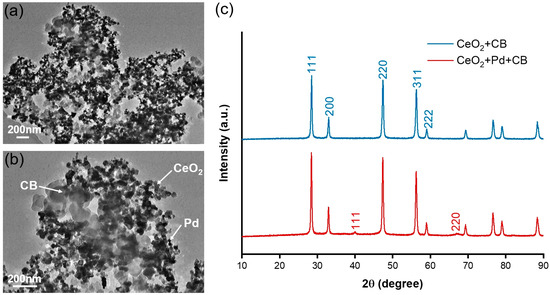
Figure 2.
(a,b) Transmission electron microscopy and (c) XRD characterization of Pd-CeO2/CB composite.
The composite’s catalytic performance was enhanced through three synergistic mechanisms: thermal treatment of CeO2 induced structural reorganization, exposing more catalytically active crystal facets [31]; palladium nanoparticle deposition created strong metal–support interactions with CeO2, significantly boosting surface oxidation reactions [32]; and the high surface area of the CB matrix provided extensive reaction interfaces. This ternary synergy collectively amplified the composite’s electrochemical activity, ensuring the later electrochemical catalysis of Sunset Yellow.
3.2. Electrocatalysis of Sunset Yellow by Pd-CeO2-CB
To verify the catalytic activity of the composite material, a comparative test was conducted on four electrode sets using CV: (1) a bare SPE (beige curve), (2) a Pd-loaded electrode (green curve), (3) a CeO2-loaded electrode (blue curve), and (4) a Pd-CeO2 composite-loaded electrode (red curve). A 10 mM Sunset Yellow solution was tested with the four electrode sets, and the results are shown in Figure 3a. The Pd-CeO2 composite material exhibited a distinct oxidation peak at 0.55 V compared with the other single materials, indicating that the composite material can effectively catalyze the oxidation reaction of Sunset Yellow, and the catalytic effect was significantly better than its individual components. Combining the Sunset Yellow oxidation mechanism shown in Figure 1 (hydroxylation to a carbonyl group) with previous research [33], the above results confirm the feasibility of using Pd-CeO2 composite material as a nano-catalytic material for the electrochemical detection of Sunset Yellow.
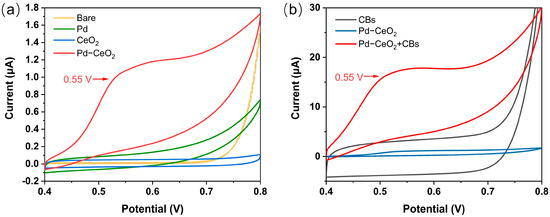
Figure 3.
(a) CV of a 10 mM Sunset Yellow solution tested with different materials; (b) CVs of CB material, PdCeO2, and PdCeO2/CB composite obtained from 10 mM Sunset Yellow solution.
To investigate the improving effect of CB on the catalytic activity of the Pd-CeO2 composite, the following comparative experiments were designed: (1) CB (black curve), (2) Pd-CeO2 composite (blue curve), and (3) Pd-CeO2/CB composite (red curve) were used to modify the SPE and measure a 10 mM Sunset Yellow solution. As shown in Figure 3b, the sensor loaded with CB had a large capacitive current, but no prominent catalytic oxidation peak was observed, indicating that CB itself has no catalytic effect on Sunset Yellow, but can effectively improve the material’s conductivity. The Pd-CeO2 modified SPE without CB exhibited a weak oxidation peak at 0.55 V, with a small oxidation current value. However, the Pd-CeO2/CB-based sensor exhibited more excellent catalytic activity, and the oxidation current value was significantly enhanced (by more than tenfold) compared with the one without the addition of CB. The above results indicate that the introduction of CB not only improves the electrical conductivity of the composite material but also significantly improves its catalytic activity, thereby improving the electrochemical performance of the sensor.
3.3. Optimization of Detection Conditions for a Sunset Yellow Electrochemical Sensor
To investigate the influence of pH on the electrochemical detection of Sunset Yellow, the following experiment was performed: Phosphate buffer solutions (0.01 M) with various pH values (ranging from 1 to 7) were prepared by adjusting the pH of a 0.01 M NaH2PO4 solution with 1.0 mM HCl or 0.1 mM NaOH as needed, using a calibrated pH meter. Subsequently, the phosphate buffer solutions were mixed with a 10 mM Sunset Yellow stock solution to prepare a series of standard solutions with pH values from 1.0 to 7.0 and Sunset Yellow concentrations of 100 μM. Next, amperometry was used to test the above standard solutions and examine the effect of pH on the oxidation current of Sunset Yellow. Electrochemical optimization revealed a pH-dependent response profile (Figure 4a), where maximum current output occurred at pH 2.0. As the pH value gradually increased, the response current value decreased, and a significant decrease occurred at pH values of 4.0–5.0. This may be because the electrocatalytic oxidation of Sunset Yellow is highly dependent on proton involvement, making lower pH conditions more favorable for the reaction and resulting in a stronger electrocatalytic signal. Therefore, comprehensively considering the above factors, this study determines that the optimal pH value for Sunset Yellow detection is 2.0.
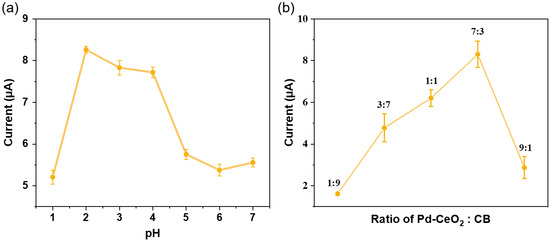
Figure 4.
(a) Effect of different pH on Sunset Yellow test; (b) effect of different Pd-CeO2–CB mass ratios on the Sunset Yellow test.
To optimize the electrochemical performance of the sensor, the optimal mass ratio of the Pd-CeO2 composite to CB was investigated. Based on the assumption that Pd-CeO2 mainly provides catalytic oxidation activity while CB mainly provides conductivity, the two materials were mixed at five mass ratios of 1:9, 3:7, 1:1, 7:3, and 9:1, and tested using amperometry after dilution to a Sunset Yellow concentration of 100 μM in a pH 2.0 phosphate buffer solution.
As shown in Figure 4b, the sensor’s oxidation current reached its maximum value when the mass ratio of Pd-CeO2 to CB was 7:3, and the oxidation current was significantly higher than other mass ratios within the error range. This result highlights a competing relationship between the number of catalytically active sites and the electrical conductivity of the sensing material. An excess of CB (e.g., 1:9 ratio) ensures high conductivity, yet the correspondingly low Pd–CeO2 content leads to a shortage of active sites, impairing overall performance. Conversely, a high Pd–CeO2 ratio (e.g., 9:1) results in an incomplete conductive network due to insufficient CB, markedly increasing internal resistance. Additionally, elevated Pd–CeO2 levels encourage agglomeration, further diminishing the availability of active sites. The 7:3 mass ratio achieves an optimal balance, maintaining high active site density and facilitating efficient electron conduction and mass transport, yielding the highest electrocatalytic performance. Therefore, in subsequent experiments, this study will use this optimal mass ratio to prepare the Sunset Yellow electrochemical sensor.
3.4. Detection of Sunset Yellow by Pd-CeO2/CB Electrochemical Sensor
Building upon the optimized experimental conditions described above, this study further evaluated the sensitivity of the Sunset Yellow electrochemical sensor. Specifically, a series of Sunset Yellow standard solutions with concentrations ranging from 1 to 100 nM was prepared using a pH 2.0 phosphate buffer solution. Amperometry was then used to detect the different concentrations of Sunset Yellow at a constant potential of 0.55 V. As shown in Figure 5a, the sensor’s response current increased with the gradual rise in Sunset Yellow concentration over the 100 s test period. The sensor can still produce a clear response signal even at a Sunset Yellow concentration as low as 1 nM. This fully demonstrates that the Sunset Yellow electrochemical sensor has high sensitivity and rapid response, and can detect trace amounts of Sunset Yellow.
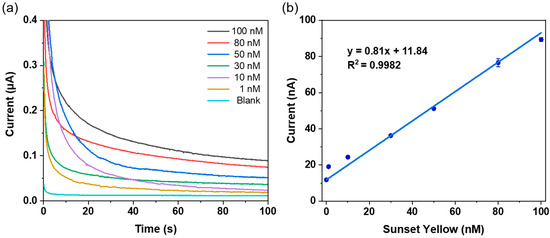
Figure 5.
(a) Amperometric curves for different concentrations of Sunset Yellow; (b) the response current shows a good linear relationship with Sunset Yellow concentration.
According to the linear curve shown in Figure 5b, the response current value increased with the increase in Sunset Yellow concentration, exhibiting a good linear relationship, with a linear regression coefficient of determination (R2) of 0.9982 and a linear regression equation of
The limit of detection (LOD) was calculated as the mean response current of the pH 2.0 phosphate buffer solution (blank) plus three times its standard deviation (SD), then a LOD of 0.056 nM can be obtained. The limit of quantification (LOQ) was calculated as the mean blank current plus ten times its standard deviation, yielding a value of 0.236 nM. As shown in Table 1, the performance of the Sunset Yellow electrochemical sensor proposed in this study was compared with that of non-enzymatic electrochemical sensors based on other nano-catalytic materials (mainly comparing the linear range and limit of detection). The results show that the sensor designed in this study, combining an SPE and the Pd-CeO2/CB composite, is easy to prepare and structurally stable, and exhibits excellent sensitivity and a low detection limit in Sunset Yellow detection. This fully demonstrates the effectiveness of this study.

Table 1.
Comparison of this sensor with other reported non-enzymatic electrochemical sensors for detecting Sunset Yellow.
3.5. Sensor Selectivity, Consistency, and Stability Studies
The interference of co-existing components in fruit juice beverages on Sunset Yellow detection was investigated to evaluate the sensor’s selectivity in complex systems. Since various fruit juice beverages often contain multiple additives (to ensure the freshness, flavor, and taste of the beverage), the testing environment for fruit juice samples is quite complex. Benzoic acid (BA), sodium carbonate (SC), ascorbic acid (AA), potassium sorbate (PS), and acesulfame were selected as interfering substances, and 100 nM of Sunset Yellow and 100 nM of the interfering components solution were prepared in a pH 2.0 phosphate buffer solution. Then, the current response of each solution was measured using amperometry at a potential of 0.55 V, and the results are shown in Figure 6.
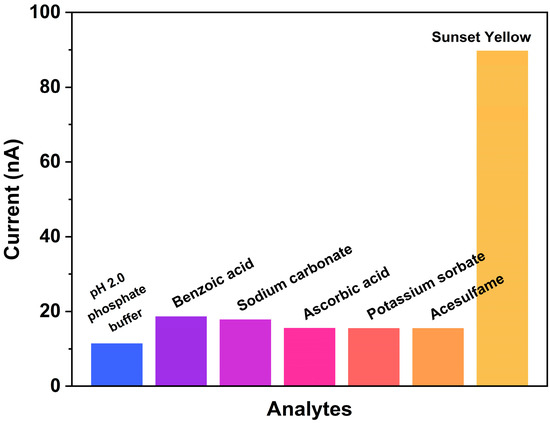
Figure 6.
The proposed sensor exhibited good selectivity for Sunset Yellow against five potential interfering components, showing high specificity in complex environments.
As shown in Figure 6, the current responses of the common interfering substances were significantly lower than that of Sunset Yellow. This pronounced difference in signal intensity demonstrates the sensor’s excellent selectivity toward Sunset Yellow. The high selectivity can be attributed to the specifically designed recognition interface, which exhibits good electrocatalytic activity toward Sunset Yellow molecules compared with other interferents. These results confirm the sensor’s anti-interference capability and reliability for practical applications.
To evaluate the precision and inter-batch reproducibility of the fabricated Pd-CeO2/CB SPEs, five independently prepared electrodes were used to detect a 100 nM Sunset Yellow standard solution in a pH 2.0 phosphate buffer solution. As shown in Figure 7a, the current responses of the five electrodes were highly consistent, with a calculated relative standard deviation (RSD) of 4.2%, demonstrating that the sensor fabrication method is reliable and possesses good reproducibility.
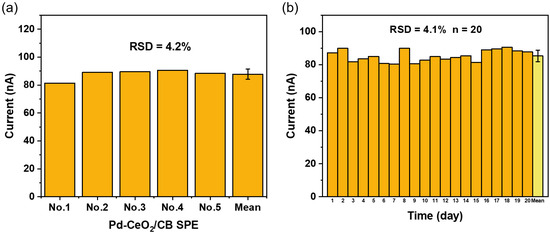
Figure 7.
(a) Reproducibility and (b) stability of the Pd-CeO2/CB sensor for the measurements of Sunset Yellow.
To verify the sensor’s stability, its long-term performance was investigated. The prepared Sunset Yellow sensors were placed in a desiccator for dark storage, and one sensor was randomly selected every day for the subsequent 20 days. A 100 nM Sunset Yellow standard solution was tested with a pH 2.0 phosphate buffer solution, and the experimental results are shown in Figure 7b. The results show that the RSD of the measured current value of the sensor was only 4.1% within 20 days, indicating that it has excellent long-term stability.
3.6. Real Beverage Sample Testing
To evaluate the application potential of this highly sensitive Sunset Yellow sensor in real samples, we selected two commercially available orange beverages labeled as “Sunset Yellow-free” as real test subjects and complex matrices for recovery experiments. The experimental procedure was as follows: First, the purchased orange juice and carbonated orange juice beverages were diluted 10-fold with a pH 2.0 phosphate buffer solution. Then, Sunset Yellow was added to the diluted samples to make their concentrations 80, 50, and 30 nM, respectively. Subsequently, the prepared Sunset Yellow electrochemical sensor was used to detect the spiked real samples using amperometry, and the measured current values were substituted into a pre-established standard curve to calculate the concentration of Sunset Yellow. Finally, the recovery rate and relative standard deviation were calculated based on the measured and spiked concentrations.
As shown in Table 2, the recovery rates of the sensor for the two real samples were in the range of 99.11% to 101.34%. This result indicates that both beverages are indeed free of Sunset Yellow, consistent with their labeling claims. It also confirms that the electrochemical sensor developed in this study exhibits excellent accuracy and reliability. The sensor not only enables effective detection of Sunset Yellow in real beverage samples but also provides a verifying method for the authenticity of “no artificial colorants” claims on food products. By helping to ensure transparency for consumers and protect public health, this technology demonstrates significant potential for applications in food safety.

Table 2.
Detection results of real beverage samples.
4. Conclusions
In summary, this study successfully developed an electrochemical microsensor with facile operation, high sensitivity, good selectivity, and satisfactory stability to rapidly detect Sunset Yellow in real beverage samples. The sensor utilizes a Pd-CeO2/CB composite material as a non-enzymatic catalyst, effectively enhancing the sensor’s performance. The CV confirmed the highly active catalytic effect of the Pd-CeO2/CB composite material on Sunset Yellow. The introduction of CB not only increased the specific surface area and improved conductivity but also enhanced the sensor’s electrochemical response to Sunset Yellow. Amperometric detection of Sunset Yellow at concentration gradients in pH 2.0 revealed a linear relationship between the response current and Sunset Yellow concentration in the range from 1 to 100 nM, with a LOD of 0.056 nM. This electrochemical microsensor exhibits excellent specific selectivity, consistency, and stability for Sunset Yellow. It was also successfully applied to detect Sunset Yellow in real beverage samples with complex matrices, fully demonstrating its application potential in rapid food safety detection.
Author Contributions
Conceptualization, X.W. and Y.Z. (Yongheng Zhu); data curation, Z.L., W.C. and X.W.; funding acquisition, P.X. and X.L.; investigation, Z.L.; methodology, X.W., P.X. and X.L.; resources, Y.Z. (Yuan Zhang) and X.L.; supervision, X.L.; validation, Z.L., W.C., Q.W. and H.L.; visualization, Z.L. and X.W.; writing—original draft, Z.L. and X.W.; writing—review and editing, P.X., Y.Z. (Yuan Zhang) and Y.Z. (Yongheng Zhu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2021YFB3200800), National Natural Science Foundation of China (62227815, 62271473, U21A20500).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to (specify the reason for the restriction).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and synthetic flavylium-based dyes: The chemistry behind the color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Dey, S.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. Adv. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, R. Artificial food dyes and attention deficit hyperactivity disorder. Nutr. Rev. 2011, 69, 385–391. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Ji, L.; Cheng, Q.; Wu, K.; Yang, X. Cu-BTC frameworks-based electrochemical sensing platform for rapid and simple determination of Sunset yellow and Tartrazine. Sens. Actuators B 2016, 231, 12–17. [Google Scholar] [CrossRef]
- Abbey, J.; Fields, B.; O’Mullane, M.; Tomaska, L.D. Food Additives: Colorants. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 459–465. [Google Scholar]
- Millichap, J.G.; Yee, M.M. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics 2012, 129, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Zhang, T.; Wang, M.; Gao, L.; Yang, Z.; Yang, Z. Rational design of ultrahigh sensitive sunset yellow sensor based on 3D hierarchical porous graphitic carbon with sub-nanopores. Food Chem. 2021, 365, 130631. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.B.; Du, L.M.; Tian, H.; Hao, C.X.; Wang, Z.F.; Wang, J.Y. A rapid shaking-based ionic liquid dispersive liquid phase microextraction for the simultaneous determination of six synthetic food colourants in soft drinks, sugar- and gelatin-based confectionery by high-performance liquid chromatography. Food Chem. 2013, 141, 182–186. [Google Scholar] [CrossRef]
- Sorouraddin, M.-H.; Rostami, A.; Saadati, M. A simple and portable multi-colour light emitting diode based photocolourimeter for the analysis of mixtures of five common food dyes. Food Chem. 2011, 127, 308–313. [Google Scholar] [CrossRef]
- Rovina, K.; Prabakaran, P.P.; Siddiquee, S.; Shaarani, S.M. Methods for the analysis of Sunset Yellow FCF (E110) in food and beverage products- a review. TrAC Trends Anal. Chem. 2016, 85, 47–56. [Google Scholar] [CrossRef]
- Kolozof, P.-A.; Florou, A.B.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. In-situ tailoring of the electrocatalytic properties of screen-printed graphite electrodes with sparked generated molybdenum nanoparticles for the simultaneous voltammetric determination of sunset yellow and tartrazine. Sens. Actuators B 2020, 304, 127268. [Google Scholar] [CrossRef]
- Karimi, F.; Demir, E.; Aydogdu, N.; Shojaei, M.; Taher, M.A.; Asrami, P.N.; Alizadeh, M.; Ghasemi, Y.; Cheraghi, S. Advancement in electrochemical strategies for quantification of Brown HT and Carmoisine (Acid Red 14) From Azo Dyestuff class. Food Chem. Toxicol. 2022, 165, 113075. [Google Scholar] [CrossRef] [PubMed]
- Dmukhailo, A.; Tvorynska, S.; Trukhym, M.; Dubenska, L. Electrochemical behavior of the synthetic food diazo dye Brilliant Black BN (E151) and the first voltammetric method for its determination. Talanta 2025, 293, 128119. [Google Scholar] [CrossRef]
- Zheng, Y.; Mao, S.; Zhu, J.; Fu, L.; Zare, N.; Karimi, F. Current status of electrochemical detection of sunset yellow based on bibliometrics. Food Chem. Toxicol. 2022, 164, 113019. [Google Scholar] [CrossRef] [PubMed]
- Georgescu State, R.; van Staden, J.F.; Staden, R.-I.S.-v. Review—Recent trends on the electrochemical sensors used for the determination of tartrazine and sunset yellow FCF from food and beverage products. J. Electrochem. Soc. 2022, 169, 017509. [Google Scholar] [CrossRef]
- Cougo, C.M.d.S.; Pezzin, S.H.; Pachekoski, W.M.; Amico, S.C. 14—Multiscale hybrid composites with carbon-based nanofillers. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin, Asiri, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 449–470. [Google Scholar]
- Cao, H.; Zhu, M.; Li, Y. Decoration of carbon nanotubes with iron oxide. J. Solid State Chem. 2006, 179, 1208–1213. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Chapter 9—Electroanalytical bioplatforms based on carbon nanostructures as new tools for diagnosis. In Nanotechnology and Biosensors; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 269–306. [Google Scholar]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.S.; Fatibello-Filho, O. Nanostructured carbon black for simultaneous sensing in biological fluids. Sens. Actuators B Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Tian, R.; Ye, L.; Zhang, J.; Rao, M.; Li, G. Platinum-group metals: Demand, supply, applications and their recycling from spent automotive catalysts. J. Environ. Chem. Eng. 2023, 11, 110237. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Sun, Y.; Li, K.; Shang, T.; Wan, Y. Recent advances and perspectives of CeO2-based catalysts: Electronic properties and applications for energy storage and conversion. Front. Chem. 2022, 10, 1089708. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Lu, G. Promotional effect of cerium on Mo–V–Te–Nb mixed oxide catalyst for ammoxidation of propane to acrylonitrile. Fuel Process. Technol. 2015, 130, 71–77. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R.; et al. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar] [CrossRef]
- Alemayehu, A.; Biesuz, M.; Javan, K.Y.; Tkach, A.; Vilarinho, P.M.; Sglavo, V.M.; Tyrpekl, V. Ultrafast high-temperature sintering of gadolinia-doped ceria. J. Eur. Ceram. Soc. 2023, 43, 4837–4843. [Google Scholar] [CrossRef]
- Sanchez, S.I.; Small, M.W.; Zuo, J.-m.; Nuzzo, R.G. Structural characterization of Pt–Pd and Pd–Pt core–shell nanoclusters at atomic resolution. J. Am. Chem. Soc. 2009, 131, 8683–8689. [Google Scholar] [CrossRef]
- Eom, N.; Messing, M.E.; Johansson, J.; Deppert, K. General trends in core–shell preferences for bimetallic nanoparticles. ACS Nano 2021, 15, 8883–8895. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Yu, H.; Davydova, E.S.; Ash, U.; Miller, H.A.; Bonville, L.; Dekel, D.R.; Maric, R. Palladium-ceria nanocatalyst for hydrogen oxidation in alkaline media: Optimization of the Pd–CeO2 interface. Nano Energy 2019, 57, 820–826. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Cao, S.; Gao, F.; Zhang, Y.; Yang, Y. One-step electrochemical approach for the preparation of graphene wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim. Acta 2012, 74, 151–157. [Google Scholar] [CrossRef]
- Li, L.; Zheng, H.; Guo, L.; Qu, L.; Yu, L. Construction of novel electrochemical sensors based on bimetallic nanoparticle functionalized graphene for determination of sunset yellow in soft drink. J. Electroanal. Chem. 2019, 833, 393–400. [Google Scholar] [CrossRef]
- Pogacean, F.; Coros, M.; Mirel, V.; Magerusan, L.; Barbu-Tudoran, L.; Vulpoi, A.; Stefan-van Staden, R.-I.; Pruneanu, S. Graphene-based materials produced by graphite electrochemical exfoliation in acidic solutions: Application to Sunset Yellow voltammetric detection. Microchem. J. 2019, 147, 112–120. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem. 2016, 190, 889–895. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H. Hydrothermal synthesis of CuFe2O4 nanoparticles for highly sensitive electrochemical detection of sunset yellow. Food Chem. Toxicol. 2022, 165, 113048. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xie, N.; Huang, W.; Chen, J.; Yu, L. Vertically Aligned manganese-doped ZnO nanorods synthesized on glassy carbon electrode for detection of colorants in soft drinks. Int. J. Electrochem. Sci. 2020, 15, 9146–9153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).