Abstract
Abeliophyllum distichum is rich in polyphenols and flavonoids with various bioactivities; however, studies on enzymatic modifications to enhance its functional properties remain limited. This study investigated the effect of Viscozyme® L treatment on the secondary metabolite profile of A. distichum leaves. Phytochemical profiling using liquid chromatography–electrospray ionization tandem mass spectrometry revealed a decrease in the total number of detectable compounds, from 26 in the untreated extract to 16 in the enzyme-treated extract. Following Viscozyme® L treatment, a notable shift in metabolite composition was observed, with significant enrichment of flavonoid glycosides, pyranone derivatives, and amino acid-related metabolites. Quantitative high-performance liquid chromatography analysis showed significant reductions in glycosylated compounds such as rutin (1), acteoside (2), and isoacteoside (3), while the aglycone quercetin (4) content increased more than four-fold compared to the control. These results indicate that Viscozyme® L facilitates the deglycosylation of flavonoid glycosides into their aglycone forms. This enzymatic transformation suggests a potential strategy to enhance the bioavailability and functional value of A. distichum leaf extracts for nutraceutical and pharmaceutical applications.
1. Introduction
Abeliophyllum distichum, commonly known as white forsythia or Korean abeliophyllum, is a rare, monotypic, and endemic plant species belonging to the Oleaceae family [1]. Although native to the Korean Peninsula, it was first introduced to the horticultural world following its discovery in 1919 [2,3]. In traditional Korean folk medicine, various parts of A. distichum have long been used as remedies for ailments such as gastrointestinal disorders, inflammation [4]. Recent scientific evidence attributes these therapeutic properties to its bioactive components, notably the phenylpropanoid glycoside acteoside [3]. The mechanism for its anti-inflammatory and gastroprotective effects involves the modulation of key signaling pathways such as nuclear factor-κB and mitogen-activated protein kinases [5], which suppresses the production of pro-inflammatory mediators such as inducible nitric oxide synthase and various cytokines [6].
Several studies have identified A. distichum as an excellent source of polyphenols and flavonoids, with the leaves typically containing higher concentrations of these compounds than the stems [7,8]. One study reported polyphenol and flavonoid contents of 50.64 mg/g and 13.53 mg/g, respectively, in the leaves [4]. In addition to its anti-inflammatory effects, research has provided evidence for the antioxidant, anticancer, and anti-osteoporotic properties of this plant [9,10]. Furthermore, safety evaluations have shown that the plant is non-genotoxic, with a reported LD50 of 2000 mg/kg, indicating very low toxicity [4].
Despite its promising therapeutic potential, research on the use of enzymatic modification aimed at enhancing or altering the bioactive metabolite profile of A. distichum remains limited. Enzyme-assisted biocatalysis using commercial enzyme complexes such as Viscozyme® L (a multi-enzyme formulation containing cellulase, hemicellulase, and pectinase) is an effective method for degrading plant cell walls, thereby facilitating the release of bound phenolic compounds and altering the structural composition of secondary metabolites [11]. Studies on other medicinal plants have demonstrated that enzymatic treatment can increase the yield of extractable aglycones and enhance physiological activity [12]. The present study investigates the potential effects of Viscozyme® L treatment on the secondary metabolome of A. distichum leaves [13,14].
Using high-performance liquid chromatography (HPLC) and liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI/MS), we characterize and compare the phytochemical profiles of untreated and enzyme-treated extracts, with a particular focus on phenolic and flavonoid compounds. This study provides insights into the bioconversion potential of Viscozyme® L and its applicability in enhancing the functional properties of A. distichum leaves as a natural health-promoting material.
2. Materials and Methods
2.1. Plant Materials
The leaves of A. distichum Nakai were harvested on 20 June 2024, from Misun Farm Co., Ltd., Goesan, Republic of Korea (Figure 1).
Figure 1.
Whole plant (a), leaf (b), and leaf powders (c) of A. distichum.
2.2. Instruments and Reagents
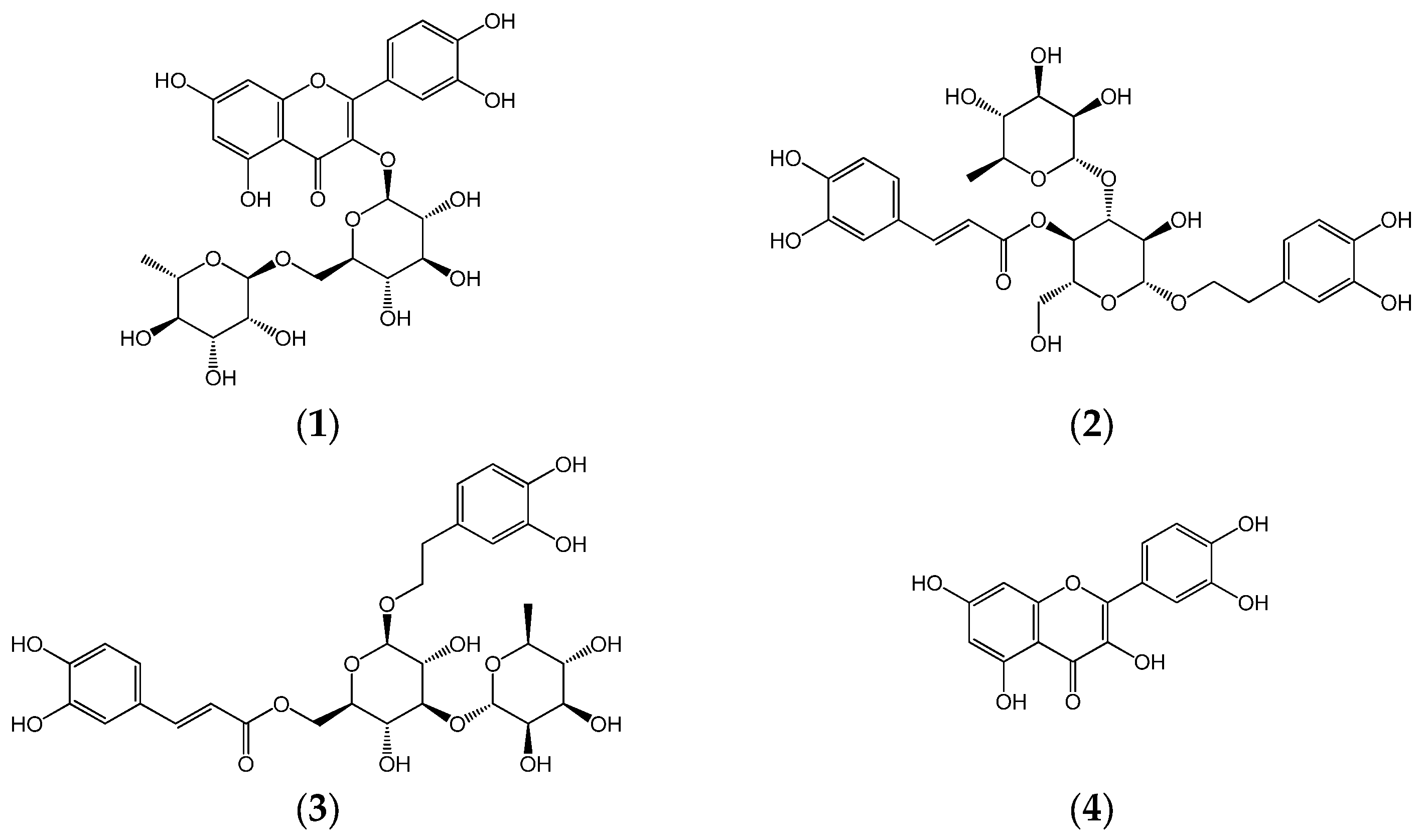
Metabolite profiling was conducted using a Thermo Vanquish Flex ultra high-performance liquid chromatography (UHPLC) system coupled with a Thermo Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA). HPLC analysis was performed using an Agilent 1260 Infinity II Quaternary Pump (Santa Clara, CA, USA) equipped with a variable wavelength detector (Agilent VWD), a pump, and an autosampler. HPLC-grade solvents, including water and methanol (MeOH), were purchased from Honeywell (Burdick and Jackson, Muskegon, MI, USA), while acetonitrile (ACN) was sourced from Scharlau (Barcelona, Spain). Trifluoroacetic acid (TFA) and formic acid were obtained from Thermo Fisher Scientific (Cleveland, OH, USA). Standard compounds—rutin (1), acteoside (2), isoacteoside (3), and quercetin (4)—were procured from the Natural Product Institute of Science and Technology (www.nist.re.kr; accessed on 7 March 2025), Anseong, Republic of Korea (Figure 2).
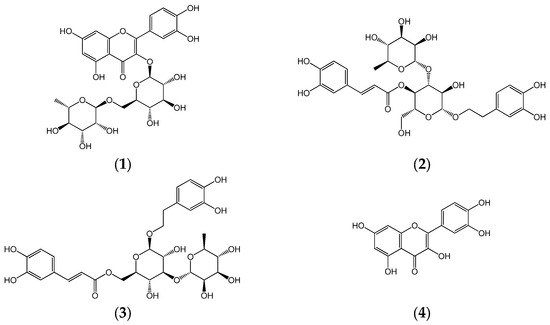
Figure 2.
Chemical structures of rutin (1), acteoside (2), isoacteoside (3), and quercetin (4).
2.3. Extraction and Preparation of Viscozyme® L-Treated A. Distichum Leaves
Immediately after harvesting on 20 June 2024, the leaves of A. distichum were dried at 38 °C for 24 h. This temperature was selected based on research indicating that drying conditions in the range of 30–40 °C are often ideal for minimizing the degradation of heat-sensitive metabolites, such as polyphenols, and preserving their chemical integrity [15]. The dried leaves were ground using a blender, and 6.7 g of powdered material was transferred to a 250 mL Erlenmeyer flask containing 40 mL of distilled water. Then, 1 mL of Viscozyme® L was added, and the mixture was incubated in a shaking water bath (BS-11, JeioTech, Seoul, Republic of Korea) at 70 °C for 6 h. A similar treatment, replacing Viscozyme® L with distilled water, has been applied as a control assay. To inactivate the enzyme, the mixture was heated at 99 °C for 5 min. Following enzymatic hydrolysis, 70% ethanol was added, and the mixture was extracted by immersion in a shaking water bath at 80 °C for 24 h. The resulting extract was filtered and stored at −20 °C until further analysis.
2.4. LC-ESI/MS Conditions
LC-ESI/MS profiling was performed using LC-Orbitrap analysis with a Thermo Q-Exactive mass spectrometer (San Jose, CA, USA) coupled with a Thermo Vanquish UHPLC system, equipped with a heated electrospray ionization source (H-ESI) operating in positive mode. The analytical column used was a Waters Cortecs T3 (2.1 × 150 mm, 1.6 µm; Milford, MA, USA), maintained at 45 °C. The injection volume was 4 µL, and the flow rate was 0.3 mL/min. The mobile phases were (A) 0.1% formic acid in water and (B) 0.1% formic acid in ACN. Gradient elution was performed as follows: 0–0.1 min 5% B; 10 min 10% B; 50–55 min 95% B; 55.1 min, returned to 5% B. Mass spectrometric detection was conducted in both positive and negative ionization modes over an m/z range of 100–1500, with a resolution of 70,000 (MS1). Spray voltage was set at 3.5 kV (positive mode) and 2.5 kV (negative mode), with a capillary temperature of 320 °C. Sheath gas, auxiliary gas, and sweep gas were set to 50, 10, and 1 arb units, respectively. ESI/MS fragmentation was performed using stepped normalized collision energies of 10, 30, and 50.
2.5. HPLC Conditions
Quantitative analysis of the chemical composition of A. distichum leaves was performed using a reverse-phase HPLC system. Chromatographic separation was achieved using a YMC Pack Pro C18 column (4.6 × 250 mm, 5 μm; Kyoto, Japan). The analysis was conducted using a gradient method with 0.1% TFA in water (solvent A) and ACN (solvent B). The gradient elution system was as follows: 0–10 min, 93% A; 15–20 min, 77% A; 30 min, 50% A; 31–35 min, 0% A; 50–60 min, 83% A. The column temperature was maintained at 30 °C. The injection volume was 10 μL, and the flow rate was set to 1.0 mL/min. Detection was carried out at a wavelength of 254 nm. The HPLC analysis was performed with reference to a previously reported method, with minor modifications [16].
2.6. Calibration Curve
A stock standard solution was prepared by dissolving four compounds in MeOH at a concentration of 1 mg/mL. The working solutions used to construct calibration curves were prepared by serial dilution of the stock solution to the desired concentrations. Leaf samples of A. distichum were dissolved in MeOH at 10 mg/mL. Both standard and sample solutions were passed through 0.20 μm polyvinylidene fluoride filters prior to analysis. Compound concentrations in the samples were quantified based on the calibration curves. The calibration function for each compound was calculated using peak area (Y) versus concentration (X, μg/10 μL) (Table 1).

Table 1.
Calibration curve equations for compounds 1–4.
2.7. Statistical Analysis
All results are reported as mean ± standard deviation and were derived from three independent trials (n = 3). Statistical comparisons were performed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons, using GraphPad Prism software (version 8.0.2; GraphPad Software, Boston, MA, USA). Metabolite set enrichment analysis (MSEA) was conducted using the enrichment analysis module in MetaboAnalyst 6.0 [https://www.metaboanalyst.ca/ (accessed on 7 July 2025)].
3. Results
3.1. LC-ESI/MS Profiling and MSEA for Chemical Structure Classification
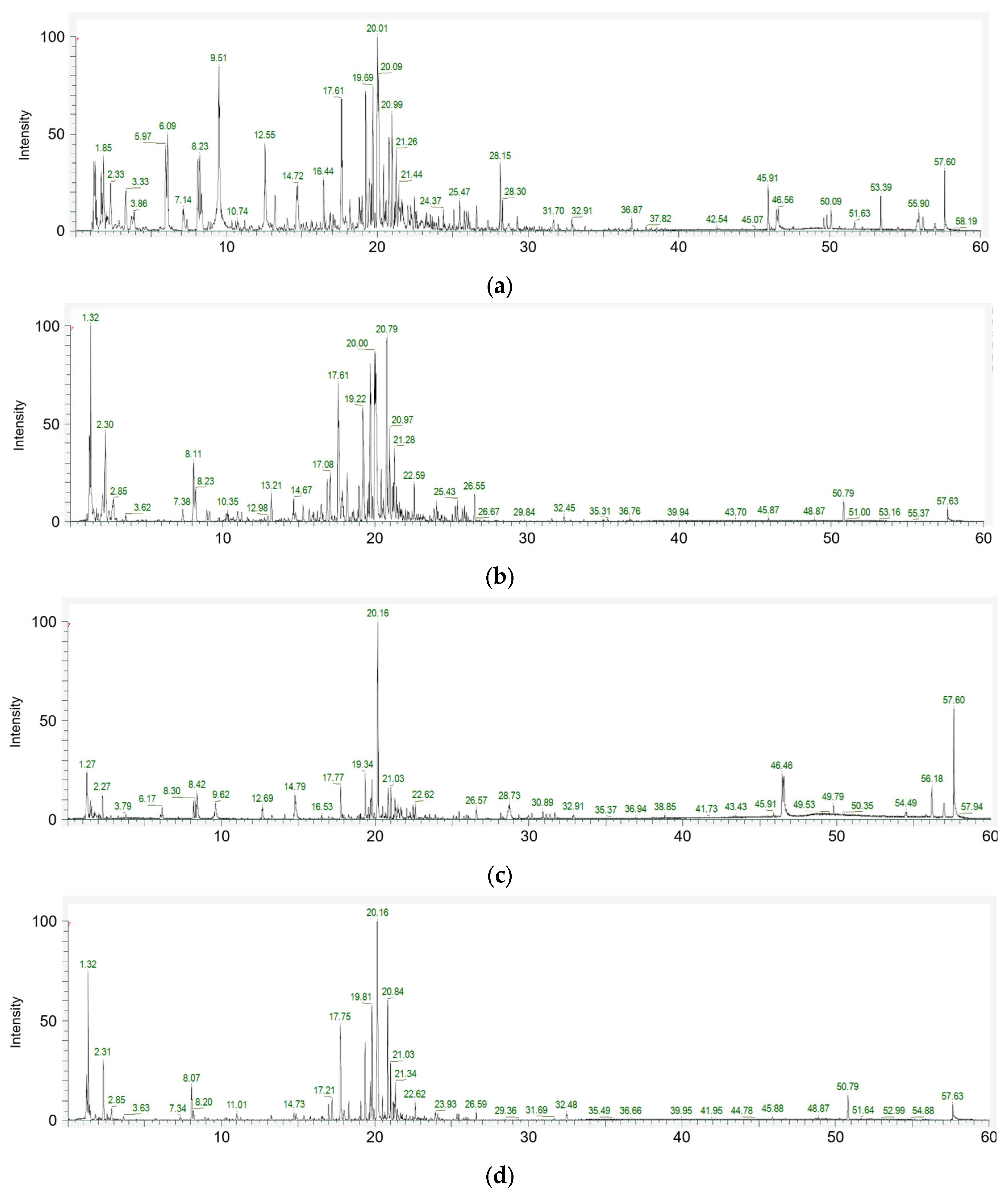
The metabolite profiles of A. distichum leaf extracts, both before and after enzyme treatment, were analyzed using LC-ESI/MS (Figure 3). In the untreated control group, a total of 26 compounds were identified. In contrast, only 16 compounds were detected in the Viscozyme® L-treated group, indicating a reduction in the overall number of compounds (Table 2 and Table 3).
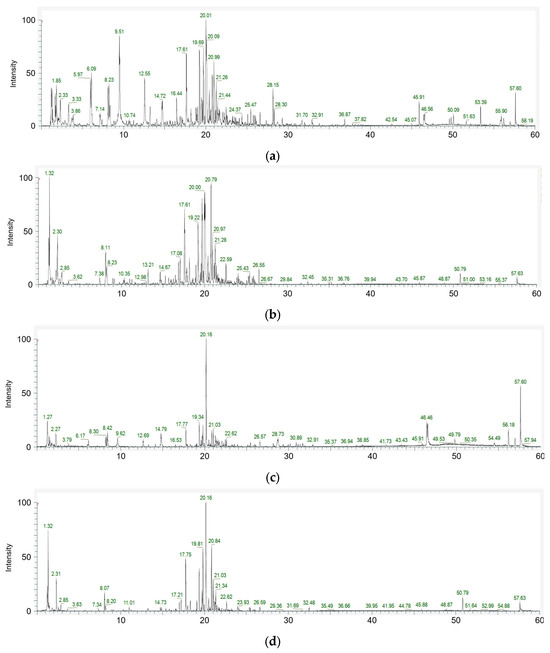
Figure 3.
Total ion chromatogram of the positive (a) and negative (b) modes of A. distichum leaf extract; positive (c) and negative (d) modes of Viscozyme® L-treated A. distichum leaf extract.

Table 2.
Profiling of compounds in A. distichum leaf extract via LC-ESI/MS analysis.

Table 3.
Profiling of compounds in Viscozyme® L-treated A. distichum leaf extract via LC-ESI/MS analysis.
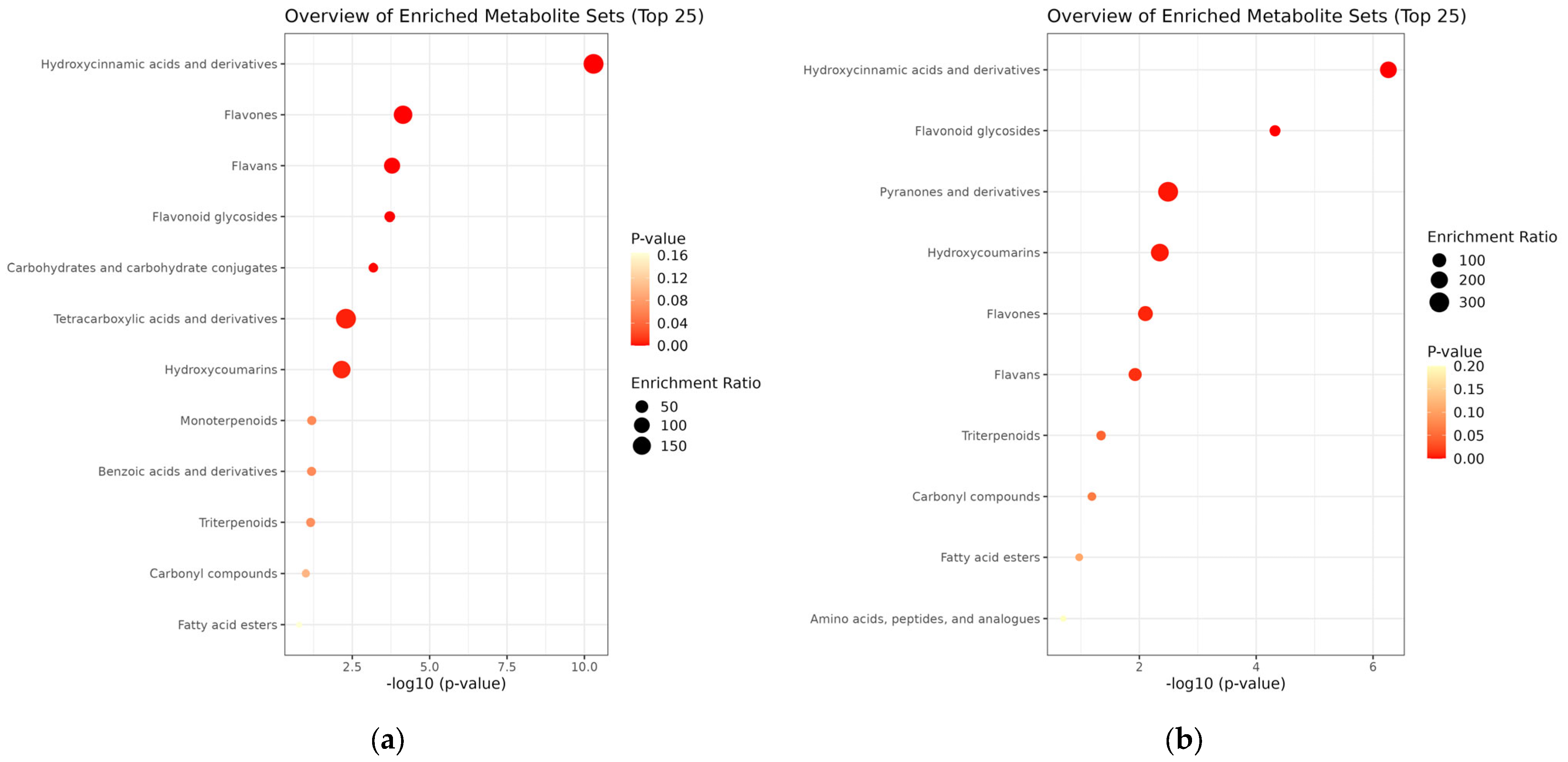
To better understand the biological significance of the metabolic changes induced by Viscozyme® L treatment in A. distichum leaves, enrichment analysis was performed using the differentially accumulated metabolites identified via LC-ESI/MS profiling. The enrichment module conducts MSEA using several libraries, comprising approximately 6300 metabolite sets. For this analysis, we selected the “chemical structures” library, which includes sub-classes (1250 sub-chemical class metabolite sets or lipid sets). The MSEA results for chemical structure classification of the key metabolites in the A. distichum leaf extracts revealed classification into 12 sub-classes in the untreated extract, and 10 sub-classes in the Viscozyme® L-treated extract (Figure 4).
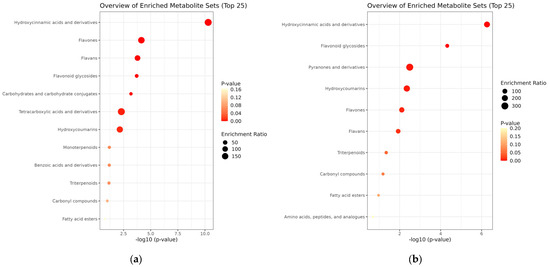
Figure 4.
MSEA-based chemical structure sub-class classification (1250 sub-chemical class metabolite or lipid sets) for A. distichum extract (a) and Viscozyme® L-treated A. distichum leaf extract (b).
3.2. HPLC Quantitative Analysis
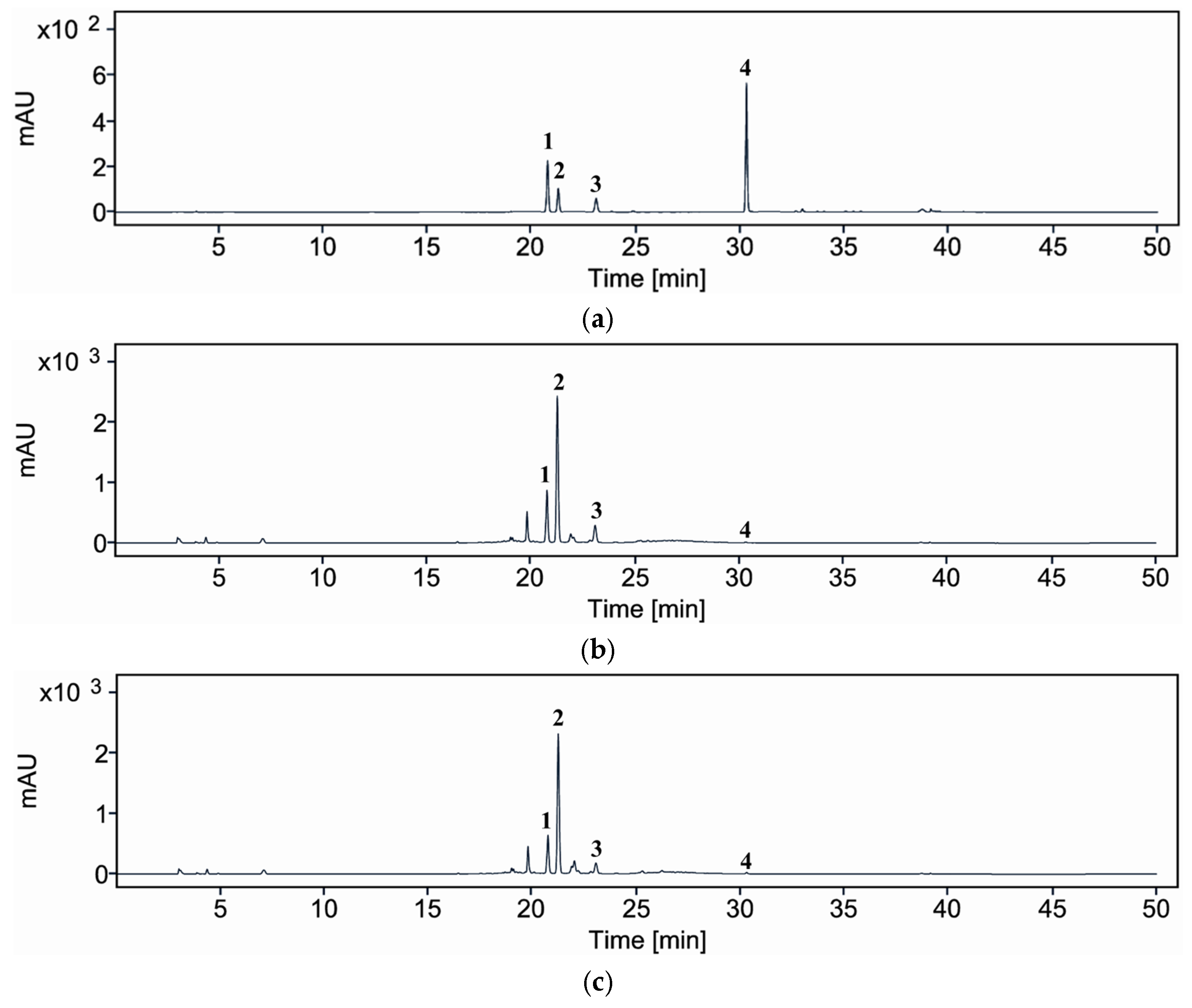
To evaluate the impact of enzymatic treatment on the phytochemical composition of A. distichum leaf extract, comparative HPLC analysis was conducted on extracts prepared with and without Viscozyme® L (Figure 5; Table 4). The HPLC results for glycosylated compounds showed that the total content of rutin (1), acteoside (2), and isoacteoside (3) decreased from 402.52 mg/g DW in the control extract to 350.76 mg/g DW in the Viscozyme® L-treated sample. Among these, acteoside (286.65 mg/g DW) and isoacteoside (65.66 mg/g DW) were the predominant constituents, followed by rutin (50.21 mg/g DW). Upon Viscozyme® L treatment, the total glycosylated compounds content decreased to 350.76 mg/g DW, indicating an overall reduction in the quantified metabolites. Specifically, the levels of rutin (1), acteoside (2), and isoacteoside (3) significantly decreased to 36.72, 273.66, and 40.38 mg/g DW, respectively, compared to those in the control. In contrast, the amount of aglycone quercetin (4) markedly increased to 0.29 mg/g DW in the enzyme-treated sample, showing a more than fourfold increase relative to the control (0.07 mg/g DW).
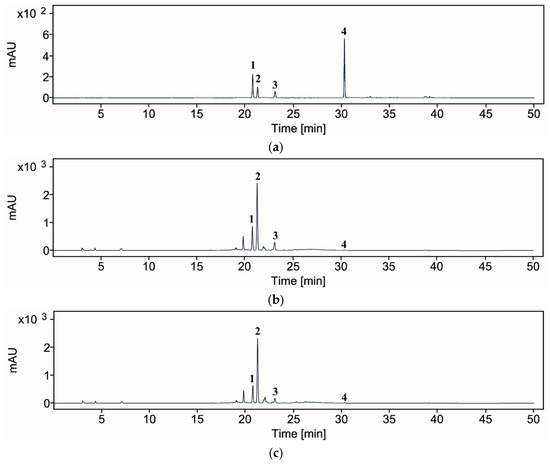
Figure 5.
HPLC/UV chromatograms of compounds 1–4 (a), A. distichum leaf extract (control) (b), and Viscozyme® L-treated A. distichum leaf extract (c); rutin (1), acteoside (2), isoacteoside (3), and quercetin (4).

Table 4.
Content of compounds 1–4 in the samples.
4. Discussion
A. distichum, a traditional medicinal herb, has been actively investigated for its diverse biological activities, including anti-diabetic and anti-inflammatory effects [17]. It is well recognized that enzymatic biotransformation plays a critical role in activating numerous prodrugs [18,19] and in enhancing the bioactivity of natural products across various applications [20]. In previous studies, cell wall-degrading enzymes such as Pectinex® Ultra SP-L and Viscozyme® L have been reported to improve polyphenol recovery and enhance radical-scavenging activity in plant extracts [21]. Similarly, in the case of mulberry leaves, enzymatic biotransformation has been shown to increase tyrosinase inhibitory activity in leaf extracts [22]. These findings collectively underscore the potential of enzyme-assisted processing as an effective strategy for modulating and enhancing the functional properties of herbal extracts.
In the initial stage of this study, enzymatic biotransformation of A. distichum leaf extract was performed using the hydrolytic enzyme complex Viscozyme® L, followed by metabolite profiling of the transformed samples through LC-ESI/MS and targeted HPLC analysis. A key observation in this study was the reduction in the total number of detectable metabolites following Viscozyme® L treatment, from 26 in the control to 16. While this might initially suggest a loss of chemical diversity, the quantitative analysis indicates this is not a net loss of bioactivity but rather a targeted biotransformation that enhances the extract’s functional value. This process is driven by the selective enzymatic conversion of glycosides into their corresponding aglycones. Specifically, the hydrolysis of glycosidic bonds led to a significant decrease in compounds like rutin (1) and acteoside (2), which was accompanied by a more than four-fold increase in the highly bioactive aglycone, quercetin (4). This conversion is crucial, as aglycones are well-documented to be more readily absorbed in the gastrointestinal tract, thus possessing greater bioavailability and often higher pharmacological potency than their glycoside precursors [23,24,25]. Therefore, this enzymatic treatment should be interpreted not as a degradation of active compounds, but as a strategic modulation designed to enhance the overall therapeutic potential of the A. distichum extract.
To systematically interpret the complex metabolite profiles and identify biologically relevant patterns, MSEA was conducted using LC-ESI/MS data. The results revealed distinct differences in metabolite enrichment patterns between the untreated and Viscozyme® L-treated A. distichum extracts. MSEA of the key metabolites in the A. distichum extracts showed that metabolites in the untreated extract were distributed across 12 sub-classes, while those in the Viscozyme® L-treated extract were distributed across 10 sub-classes (Figure 4). In the untreated extract, metabolites belonging to the flavonoid class—particularly flavans, flavones, and flavonoid glycosides—along with hydroxycinnamic acid and coumarin derivatives, were significantly enriched. This profile aligns with the well-documented secondary metabolite composition of A. distichum, which is known to be rich in polyphenolic compounds [26]. Interestingly, following Viscozyme® L treatment, a notable shift in metabolite composition was observed. Specifically, flavonoid glycosides, pyranone derivatives, and amino acid-related metabolites showed significant enrichment. These findings suggest that enzymatic hydrolysis facilitated the cleavage of glycosidic bonds, thereby generating a broader range of low-molecular-weight metabolites. In particular, the marked increase in flavonoid aglycone enrichment implies that enzymatic treatment may enhance the bioavailability of glycosylated flavonoids by producing more accessible or soluble forms. This chemical profile shift underscores the potential of enzymatic biotransformation to modulate the functional properties of A. distichum leaf extracts.
In addition to qualitative metabolite profiling by LC-ESI/MS, targeted quantitative analysis was conducted using HPLC to compare the concentrations of representative glycosylated and aglycone compounds. Four major compounds were selected to assess the changes in glycoside and aglycone content between untreated and Viscozyme® L-treated A. distichum leaf extracts. This complementary approach enabled a more precise evaluation of the enzymatic effects on chemical composition, particularly the conversion of glycosides into their corresponding aglycones. Notably, a seemingly paradoxical phenomenon was observed: although the overall number of detected compound types decreased, the content of a specific bioactive aglycone, quercetin (4), increased significantly. HPLC analysis revealed that quercetin (4) content rose from 0.07 mg/g DW in the control to 0.29 mg/g DW in the Viscozyme® L-treated sample, while the levels of glycosylated compounds such as rutin (1), acteoside (2), and isoacteoside (3) declined. These findings indicate that enzymatic hydrolysis effectively converted glycosidic forms into their corresponding aglycones.
The limited bioavailability of flavonoid glycosides, such as rutin (1), is well-documented and is primarily attributed to their low intestinal permeability and partial absorption in the gastrointestinal tract [23,24,25]. In the small intestine, glycosides can undergo enzymatic hydrolysis by brush-border lactase-phloridzin hydrolase or intracellular β-glucosidases, releasing aglycones that more readily diffuse across epithelial membranes [27]. However, unabsorbed glycosides pass into the colon, where gut microbiota further metabolize them into smaller phenolic acids, which are subsequently absorbed and excreted [28,29]. Such enzymatic deglycosylation improves bioavailability and modulates biological activity, as aglycones are often more pharmacologically potent yet chemically less stable [23]. Supporting this, previous studies have shown that enzymatic treatment of rutin with α-l-rhamnosidase produces isoquercitrin, a glucoside derivative with improved antioxidant and anti-proliferative activity compared to rutin itself [30]. In this context, the present findings—that A. distichum leaves are enriched with flavonoid glycosides—suggest that targeted enzymatic hydrolysis, using multi-enzyme complexes such as Viscozyme® L, could be employed to enhance the therapeutic potential of these phytochemicals by releasing their corresponding aglycones or more bioactive glycoside forms.
The observed biotransformation can be attributed to the dual effect of Viscozyme® L, a multi-enzyme complex from Aspergillus aculeatus [13,31,32]. Its enzymatic composition facilitates a two-step process: initially, cellulase, hemicellulase, and xylanase activities degrade the plant cell wall, maximizing the release of phytochemicals. Subsequently, the complex’s notable β-glucosidase activity directly modifies these released compounds by cleaving β-1,4-glycosidic bonds [33]. This deglycosylation converts stable glycosides into their corresponding aglycones, as evidenced by the increase in quercetin (4). This process explains how the treatment leads to not just an enhanced release but a targeted modulation of the extract’s chemical makeup, favoring more bioavailable and bioactive forms [34]. Indeed, previous studies have demonstrated that flavone glycoside levels significantly decrease following enzymatic treatment, while aglycone concentrations initially increase but may subsequently decline with prolonged exposure due to secondary degradation [9,35]. Experimental findings confirm that treatment with Viscozyme® L leads to a significant reduction in flavone glycosides in plant extracts, accompanied by a transient increase in their corresponding aglycones [31,36]. These results suggest that Viscozyme® L selectively hydrolyzed glycosidic forms of flavonoids such as rutin (quercetin-3-O-rutinoside) (1), potentially releasing the corresponding aglycone quercetin (4), while concurrently degrading or transforming other glycosylated compounds such as acteoside (2) and isoacteoside (3).
Collectively, these findings underscore the potential of enzyme-assisted biotransformation—particularly using Viscozyme® L—as an effective strategy to modulate chemical composition, enhance bioactivity, and improve the pharmacological relevance of A. distichum extracts by converting glycosides into more bioavailable and bioactive forms.
5. Conclusions
This study demonstrated that enzymatic biotransformation of A. distichum extracts using the multi-enzyme complex Viscozyme® L effectively modulated their metabolite composition by converting glycosylated forms into their corresponding aglycones. LC-ESI/MS-based metabolite profiling revealed a reduction in the overall diversity of detectable compounds, alongside an enrichment of specific classes—particularly flavonoid glycosides and aglycones—as confirmed by targeted HPLC quantification. MSEA further identified a distinct sub-class distribution pattern between the untreated and enzymatically treated extracts, highlighting the structural and functional changes induced by enzymatic processing. The significant decrease in glycosylated flavonoids such as rutin (1), acteoside (2), and isoacteoside (3), accompanied by an increase in the bioactive aglycone quercetin (4), suggests that Viscozyme® L selectively hydrolyzed glycosidic bonds to yield more bioavailable and pharmacologically relevant metabolites. These findings highlight the utility of enzyme-assisted processing as a promising approach for enhancing the functional and therapeutic potential of A. distichum by improving the bioavailability and biological activity of its polyphenolic constituents. Future studies evaluating the in vivo pharmacokinetics and biological efficacy of transformed metabolites are warranted to further validate these benefits.
Author Contributions
Investigation and original draft preparation, C.-D.L.; conceptualization, resources, and funding acquisition, E.-A.K.; validation and data curation, H.S.R.; project administration, supervision, and writing—review and editing, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the 2024 R&D Support Project funded by Hwaseong City, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
This work was supported by Gyeonggido Business & Science Accelerator (GBSA), Suwon, Republic of Korea.
Conflicts of Interest
Author Eun-A Kim was employed by R&D Center, Cleanhill Co., Ltd. The remaining authors declare no conflict of interest.
References
- Lee, K.; Jang, Y.J.; Lee, H.; Kim, E.; Kim, Y.; Yoo, T.K.; Hyun, T.K.; Park, J.I.; Yi, S.J.; Kim, K. Transcriptome Analysis Reveals That Abeliophyllum distichum Nakai Extract Inhibits RANKL-Mediated Osteoclastogenensis Mainly through Suppressing Nfatc1 Expression. Biology 2020, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.G.; Jung, E.K.; Kim, Y.I.; Lee, J.H.; Kim, B.Y.; Kang, D.H.; Shin, J.S.; Kim, Y.D. Population Connectivity and Size Reductions in the Anthropocene: The Consequence of Landscapes and Historical Bottlenecks in White Forsythia Fragmented Habitats. BMC Ecol. Evol. 2024, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.D.; Kim, J.H.; Pang, Q.Q.; Jung, P.M.; Cho, E.J.; Lee, S. Antioxidant Activity and Acteoside Analysis of Abeliophyllum distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kang, H.; Kim, M.; Kim, J.; Shin, H.; Kim, K. Analysis on the Components and Safety Evaluation of Abeliophyllum distichum Nakai Leaves and Stems. Korean J. Environ. Health Sci. 2014, 40, 234–244. [Google Scholar] [CrossRef]
- Moon, H.J.; Cha, Y.S.; Kim, K.A. Anti-Inflammatory Effects of Ethanolic Extract from Abeliophyllum distichum (Miseon Tree) Leaves in Mice with Dextran Sulfate Sodium-Induced Ulcerative Colitis. Food Nutr. Res. 2025, 69, 11052. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Kim, M.; Lee, Y.; Kang, D.; Kwon, D.; Sohn, Y.; Jung, H.-S. Ethyl Acetate Fraction of Abeliophyllum distichum Nakai Alleviates Atopic Dermatitis-like Symptoms in Vivo and in Vitro Model. J. Tradit. Complement. Med. 2025. [Google Scholar] [CrossRef]
- Yoo, T.K.; Kim, J.S.; Hyun, T.K. Polyphenolic Composition and Anti-Melanoma Activity of White Forsythia (Abeliophyllum distichum Nakai) Organ Extracts. Plants 2020, 9, 757. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, H.; Hyun, T.K. Transcriptome Analysis of Abeliophyllum distichum Nakai Reveals Potential Molecular Markers and Candidate Genes Involved in Anthocyanin Biosynthesis Pathway. S. Afr. J. Bot. 2018, 116, 34–41. [Google Scholar] [CrossRef]
- Jang, T.W.; Park, J.H. Anti-Inflammatory Effects of Abeliophyllum distichum Nakai (Cultivar Okhwang 1) Callus through Inhibition of PI3K/Akt/NF-κB, and MAPK Signaling Pathways in Lipopolysaccharide-Induced Macrophages. Processes 2021, 9, 1071. [Google Scholar] [CrossRef]
- Jang, T.W.; Choi, J.S.; Han, S.Y.; Park, H.J.; Lee, D.Y.; Min, Y.S.; Park, J.H. Comparison of the Bioactive Compounds and Anti-Inflammatory Effects Found in Different Flower Colors from Abeliophyllum distichum Nakai. J. Appl. Biol. Chem. 2022, 65, 203–213. [Google Scholar] [CrossRef]
- Liu, Y.; Angelov, A.; Übelacker, M.; Baudrexl, M.; Ludwig, C.; Rühmann, B.; Sieber, V.; Liebl, W. Proteomic Analysis of Viscozyme L and Its Major Enzyme Components for Pectic Substrate Degradation. Int. J. Biol. Macromol. 2024, 266, 131309. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ma, J.Y. Insight into the Hydrolytic Selectivity of β-Glucosidase to Enhance the Contents of Desired Active Phytochemicals in Medicinal Plants. Biomed Res. Int. 2018, 2018, 4360252. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Jurčević Šangut, I.; Karalija, E.; Šarkanj, B.; Zelić, B.; Šalić, A. 3′-8″-Biflavones: A Review of Their Structural Diversity, Natural Occurrence, Role in Plants, Extraction and Identification. Molecules 2024, 29, 4634. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.D.; Cho, H.; Lee, C.D.; Tran, G.H.; Kim, H.; Moon, S.K.; Lee, S. Antioxidative Phenolic Compounds from the Aerial Parts of Cyperus exaltatus var. iwasakii and Their HPLC Analysis. Appl. Biol. Chem. 2023, 66, 61. [Google Scholar] [CrossRef]
- Thomas, S.S.; Eom, J.; Sung, N.Y.; Kim, D.S.; Cha, Y.S.; Kim, K.A. Inhibitory Effect of Ethanolic Extract of Abeliophyllum distichum Leaf on 3T3–L1 Adipocyte Differentiation. Nutr. Res. Pract. 2021, 15, 555–567. [Google Scholar] [CrossRef]
- Liederer, B.M.; Borchardt, R.T. Enzymes Involved in the Bioconversion of Ester-Based Prodrugs. J. Pharm. Sci. 2006, 95, 1177–1195. [Google Scholar] [CrossRef]
- Rooseboom, M.; Commandeur, J.N.M.; Vermeulen, N.P.E. Enzyme-Catalyzed Activation of Anticancer Prodrugs. Pharmacol. Rev. 2004, 56, 53–102. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kim, H.R.; Hou, C.T.; Kang, S.C. Bioconverted Products of Essential Fatty Acids as Potential Antimicrobial Agents. New Biotechnol. 2009, 26, 122–130. [Google Scholar] [CrossRef]
- Hong, Y.H.; Jung, E.Y.; Park, Y.; Shin, K.S.; Kim, T.Y.; Yu, K.W.; Chang, U.J.; Suh, H.J. Enzymatic Improvement in the Polyphenol Extractability and Antioxidant Activity of Green Tea Extracts. Biosci. Biotechnol. Biochem. 2013, 77, 22–29. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Lee, G.; Kim, H. Antioxidant Activities and Polyphenol Content of Morus alba Leaf Extracts Collected from Varying Regions. Planta Med. 2014, 80, 675–680. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, S.; Chang, Q.; Zhang, L.; Wang, G.; Chen, W.; Miao, X.; Zheng, Y. A Strategy for the Improvement of the Bioavailability and Antiosteoporosis Activity of BCS IV Flavonoid Glycosides through the Formulation of Their Lipophilic Aglycone into Nanocrystals. Mol. Pharm. 2013, 10, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Al-Rajhi, A.M.H.; Almashjary, M.N.; Basabrain, A.A.; Hazzazi, M.S.; Abdelghany, T.M. Inhibitory Potential of Rutin and Rutin Nano-Crystals against Helicobacter pylori, Colon Cancer, Hemolysis and Butyrylcholinesterase in Vitro and in Silico. Appl. Biol. Chem. 2023, 66, 79. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kwon, J.E.; Choi, W.S.; Baek, N.I.; Kang, S.C. Deciphering Chemical Diversity among Five Variants of Abeliophyllum distichum Flowers through Metabolomics Analysis. Plant Direct 2024, 8, e616. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary Flavonoid and Isoflavone Glycosides are Hydrolysed by the Lactase Site of Lactase Phlorizin Hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The Relative Contribution of the Small and Large Intestine to the Absorption and Metabolism of Rutin in Man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green Tea Flavan-3-Ols: Colonic Degradation and Urinary Excretion of Catabolites by Humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Sobreiro, M.A.; Della Torre, A.; de Araújo, M.E.M.B.; Canella, P.R.B.C.; de Carvalho, J.E.; Carvalho, P.d.O.; Ruiz, A.L.T.G. Enzymatic Hydrolysis of Rutin: Evaluation of Kinetic Parameters and Anti-Proliferative, Mutagenic and Anti-Mutagenic Effects. Life 2023, 13, 549. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Kwon, S.Y.; Chung, S.K. Viscozyme L Aided Flavonoid Extraction and Identification of Quercetin from Saururus chinensis (Lour.) Baill. J. Appl. Biol. Chem. 2020, 63, 197–201. [Google Scholar] [CrossRef]
- Sharma, S. Physical Characterization of Isozymes of Endo-β-1,4-Glucanase and β-1,4-Glucosidase from Aspergillus Species. FEMS Microbiol. Lett. 1991, 79, 99–104. [Google Scholar] [CrossRef]
- Yang, Y.C.; Li, J.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Wu, N.; Liu, X.L. Optimisation of Microwave-Assisted Enzymatic Extraction of Corilagin and Geraniin from Geranium sibiricum Linne and Evaluation of Antioxidant Activity. Food Chem. 2010, 122, 373–380. [Google Scholar] [CrossRef]
- Kotik, M.; Kulik, N.; Valentová, K. Flavonoids as Aglycones in Retaining Glycosidase-Catalyzed Reactions: Prospects for Green Chemistry. J. Agric. Food Chem. 2023, 71, 14890–14910. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and Ultrasound-Assisted Extractions of Polyphenols from Citrus sinensis (cv. Malta) Peel: A Comparative Study. J. Food Process. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, H.J.; Ryuk, J.A.; Jung, D.H.; Ko, B.S. Efficiency of the Enzymatic Conversion of Flavone Glycosides Isolated from Carrot Leaves and Anti-Inflammatory Effects of Enzyme-Treated Carrot Leaves. Molecules 2023, 28, 4291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).