Electrochemical Monitoring of Bisphenol A Degradation in Leachate by Trichoderma harzianum Using a Sensitive Sensor of Type SPE in Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
2.3. Elaboration Protocol of SPE-A-polyNiTSPc Electrochemical Sensor
2.4. Electrochemical Measurements
2.5. T. harzianum Strain Information
2.6. Bioanode and Biocathode Elaboration
2.7. Fungal Strain Preparation Protocol for SEM Observations
2.8. MFC Configuration
2.9. Composition and Characteristics of Landfill Leachate Wastewater
3. Results
3.1. Phenolic Composition of Both Influent and Effluent of Landfill Leachates
3.2. Preparation of the Electrode: Activation of SPE and Electrodeposition of poly-NiTSPc Film
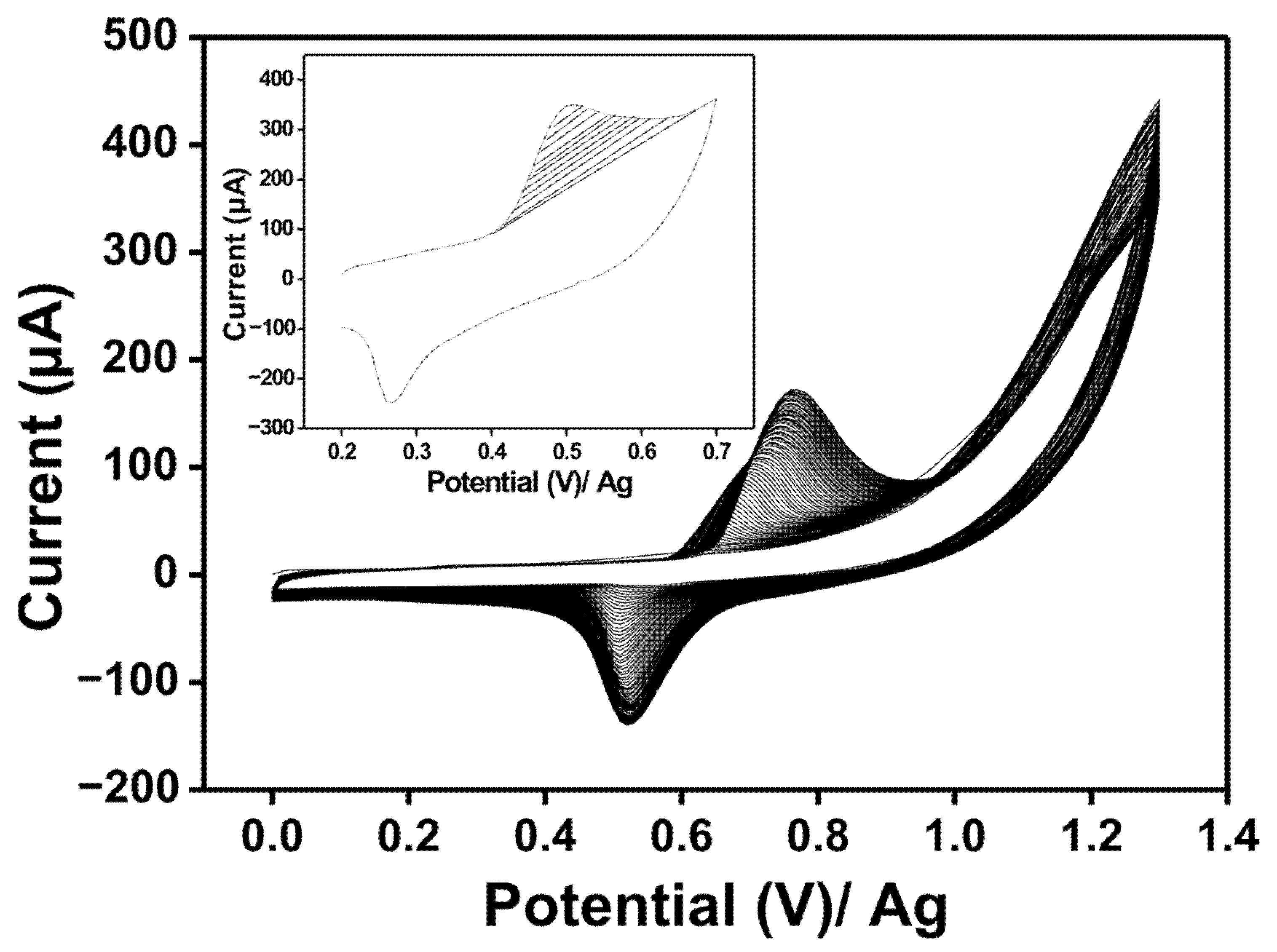
3.3. Optimization of the Number of Cycles for the Electrodeposition of poly-NiTSPc on the Activated SPE
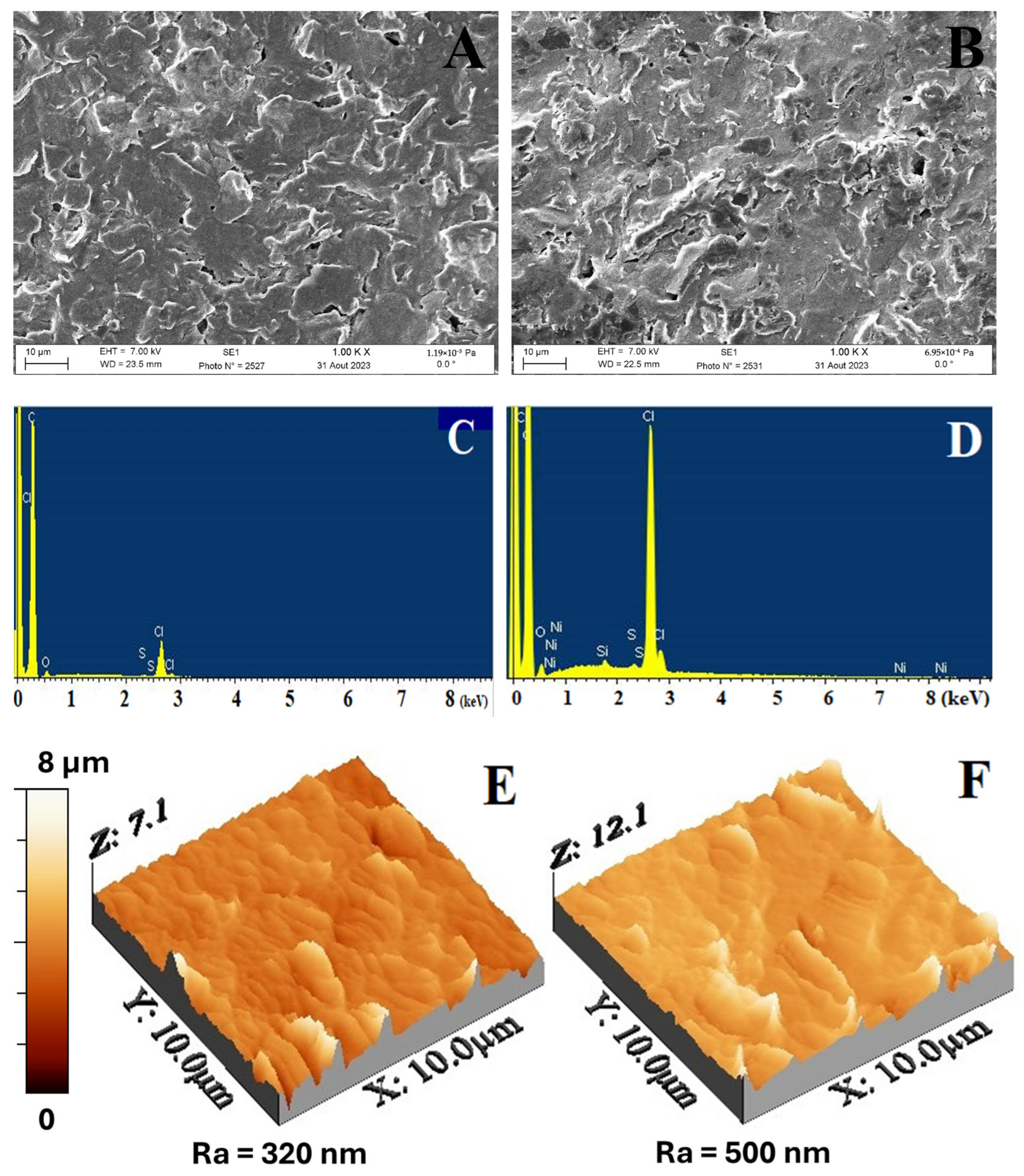
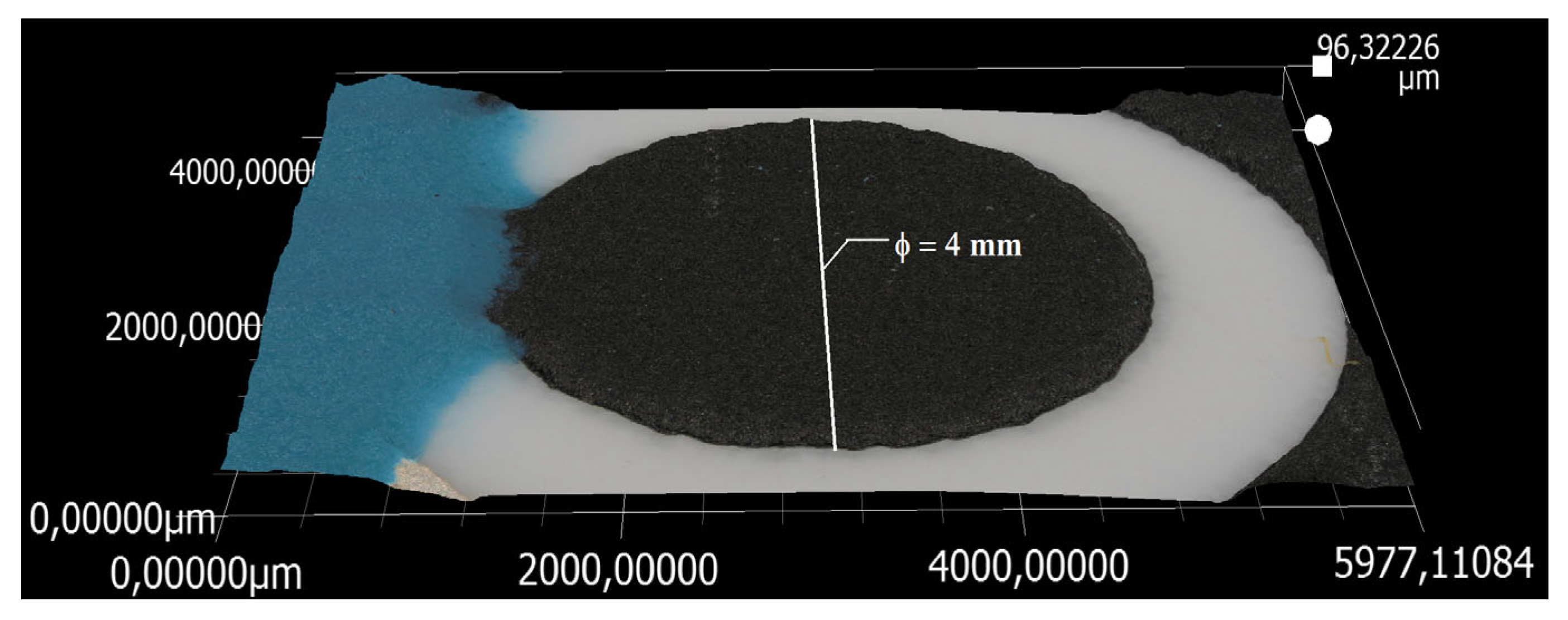
3.4. SEM, EDX, and AFM Characterizations of the SPEs
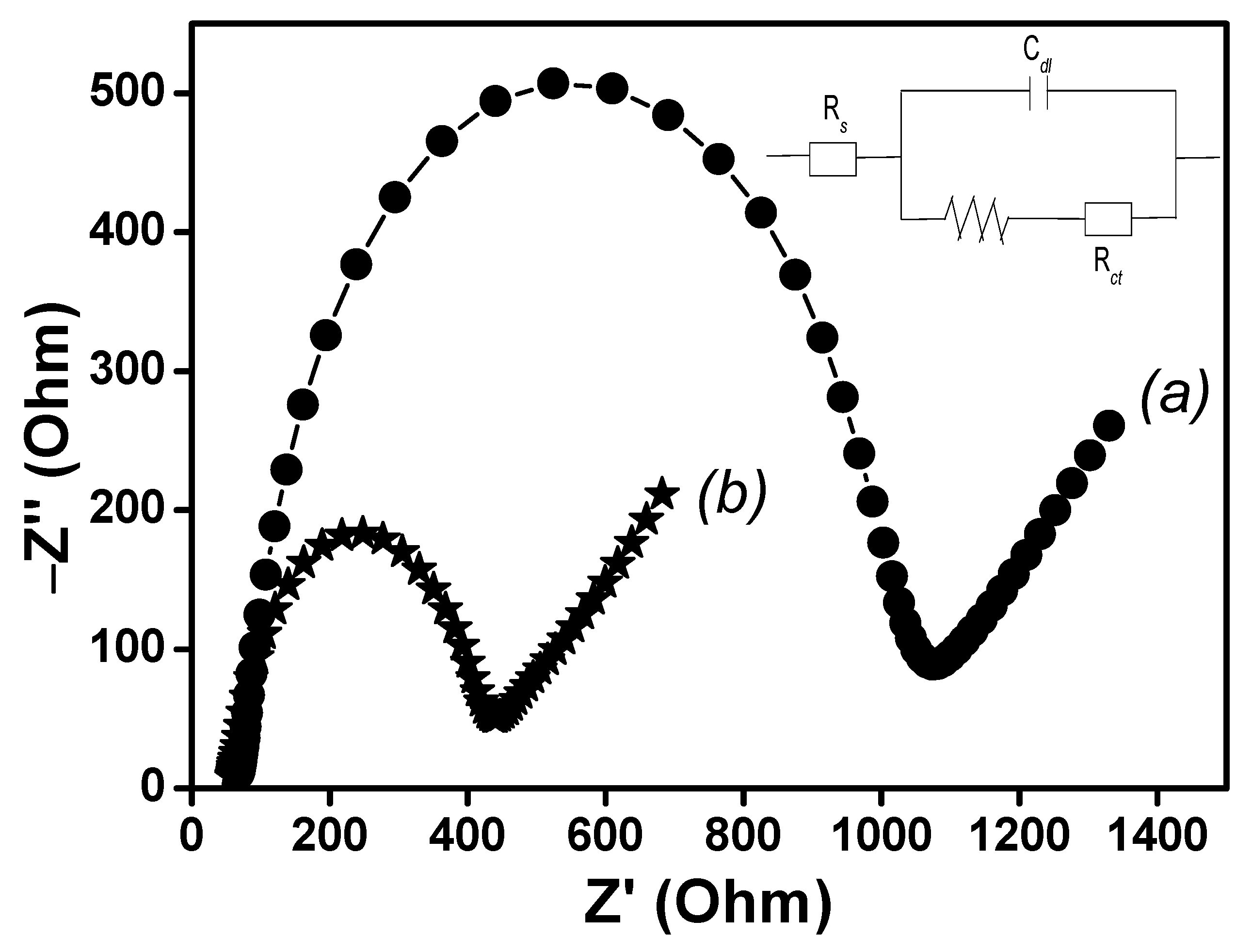
3.5. Electrochemical Impedance Spectroscopy (EIS) and Real Surface Area Determination of the Elaborated SPEs
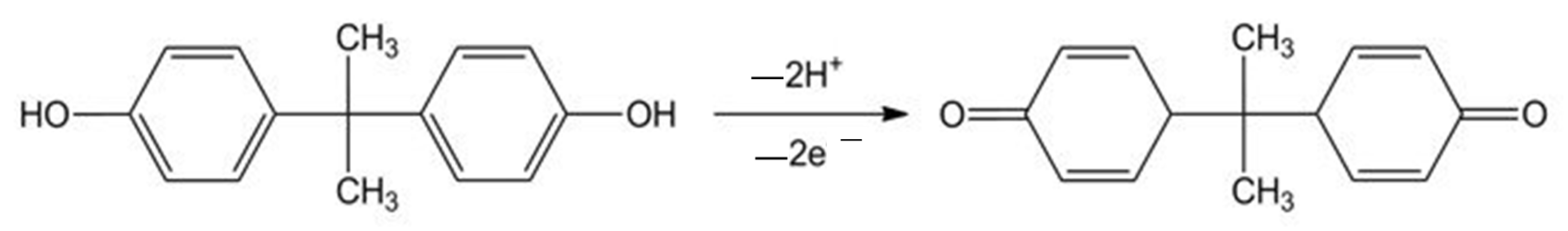
3.6. Electrochemical Behavior of BPA on Unmodified SPE and SPE-A-polyNiTSPc
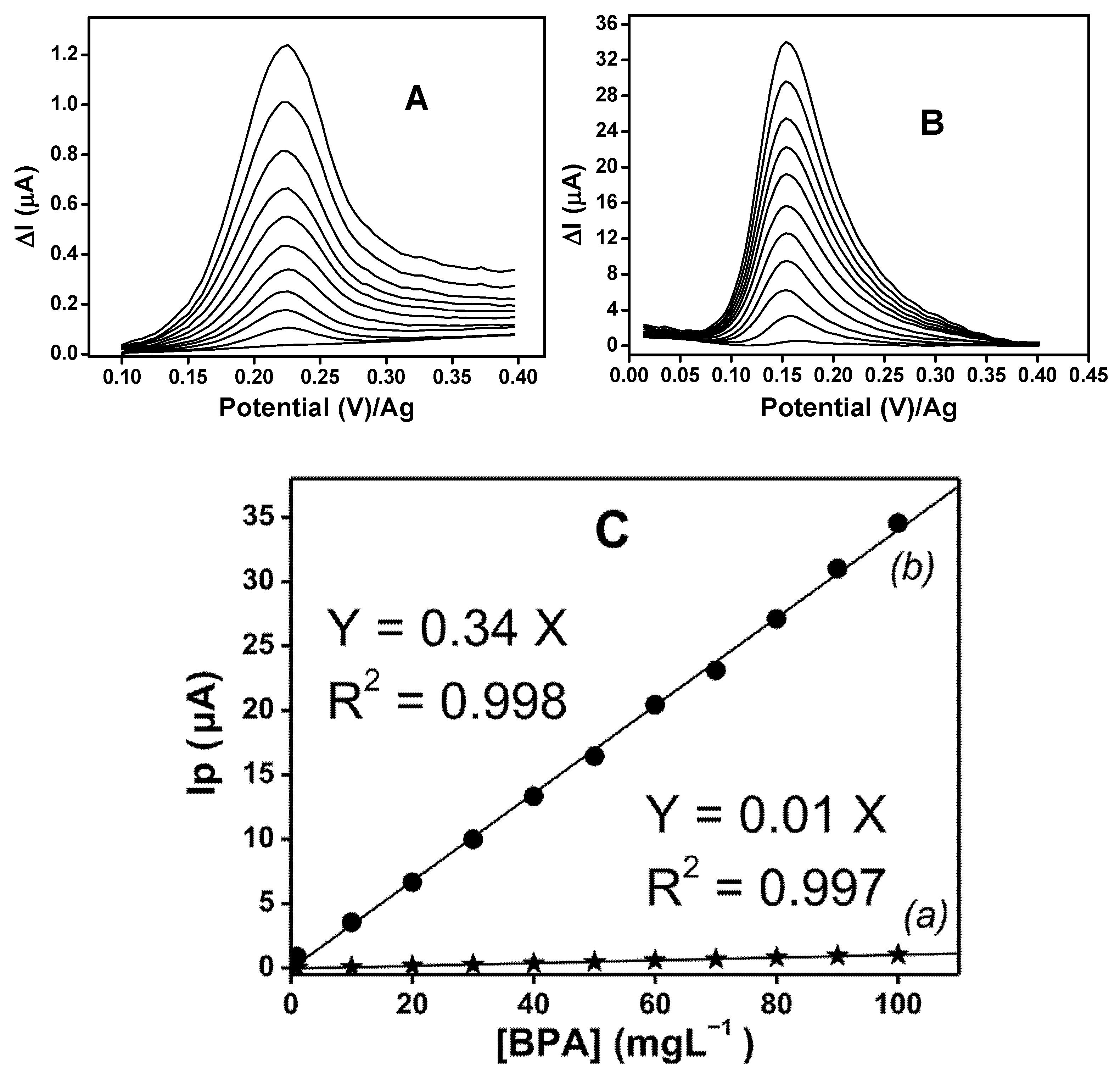
3.7. Calibration Curves and Detection Limits of BPA on the Elaborated SPEs in DPV
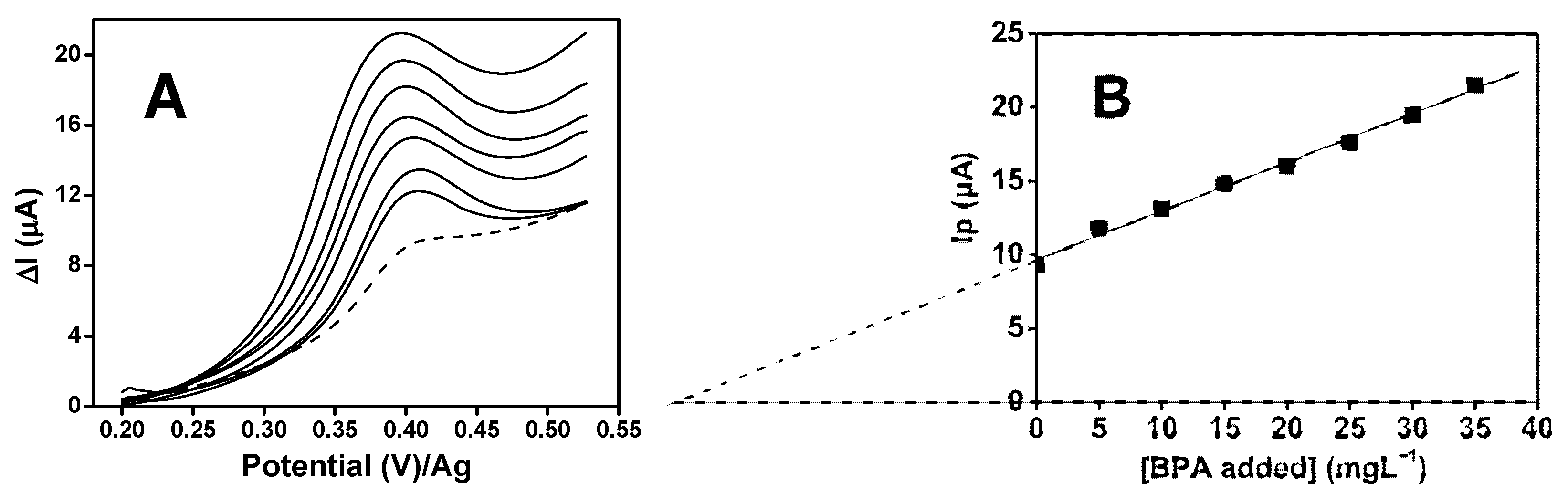
3.8. Reproducibility, Stability, Interference Study, and Application of SPE-A-polyNiTSPc for BPA Determination in Leachate Sample and RO Permeate in Crete
3.9. Application of poly-NiTSPc/activated SPE for BPA Biodegradation Study in MFCs
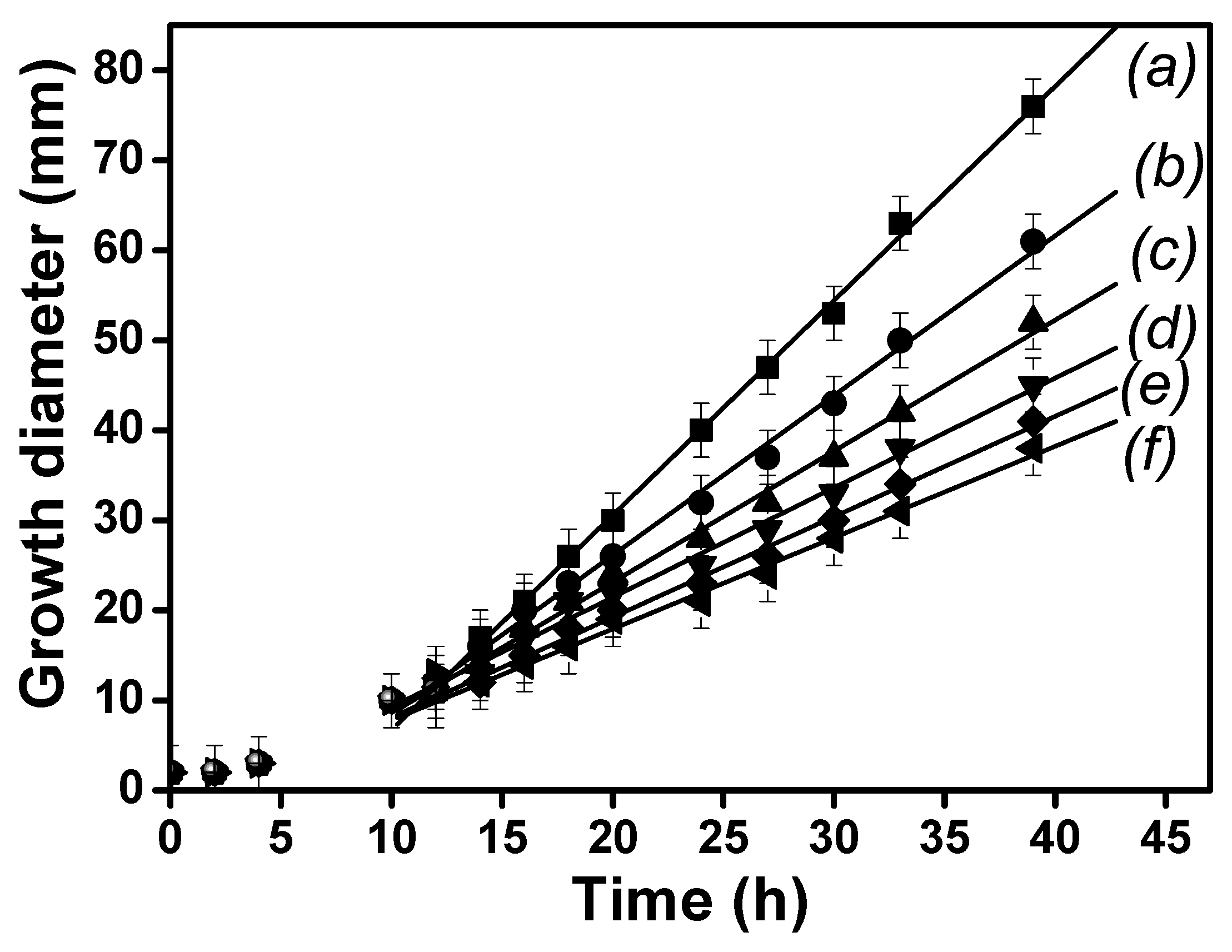
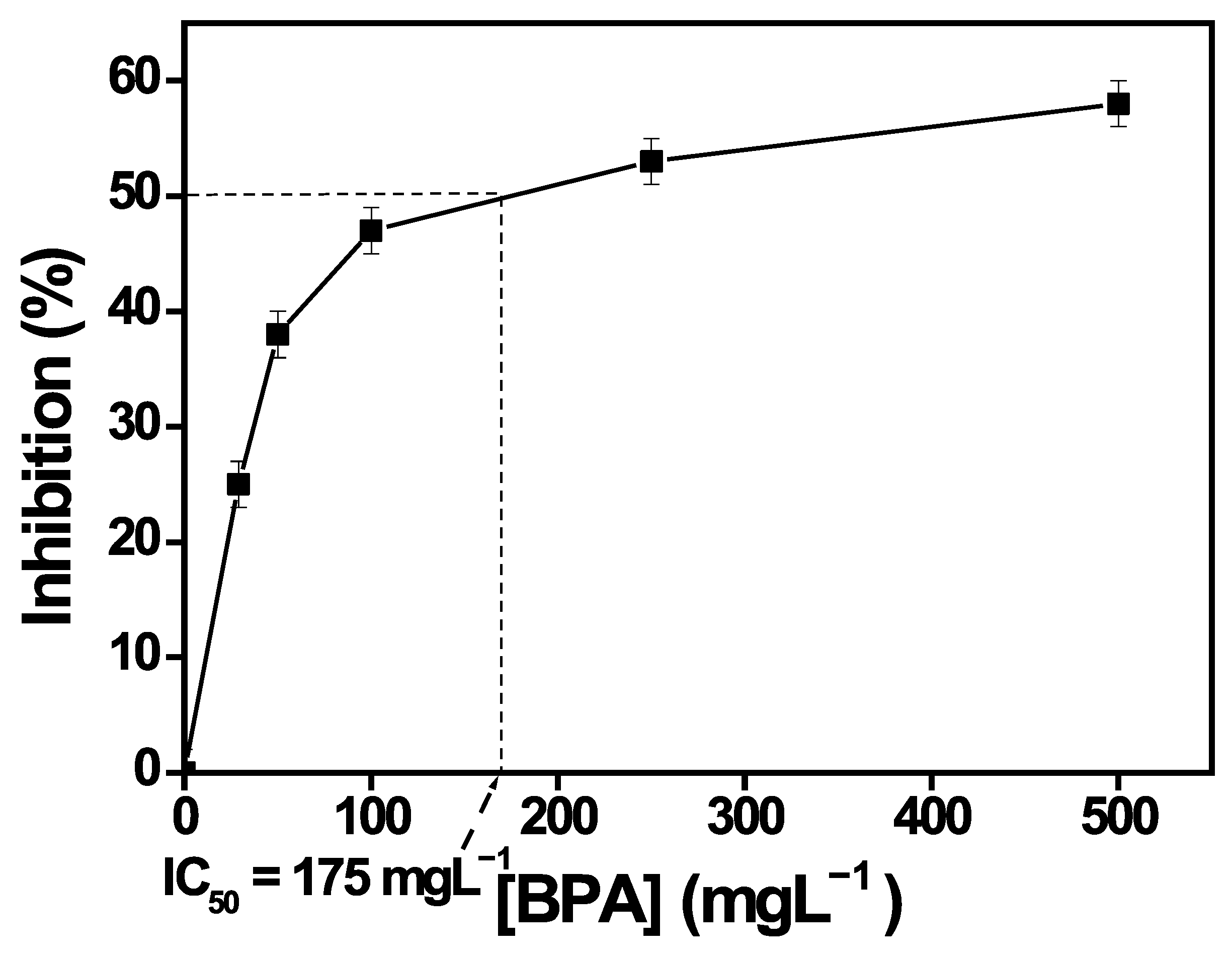
3.9.1. IC50 of T. harzianum in Presence of BPA and Inhibition Tests
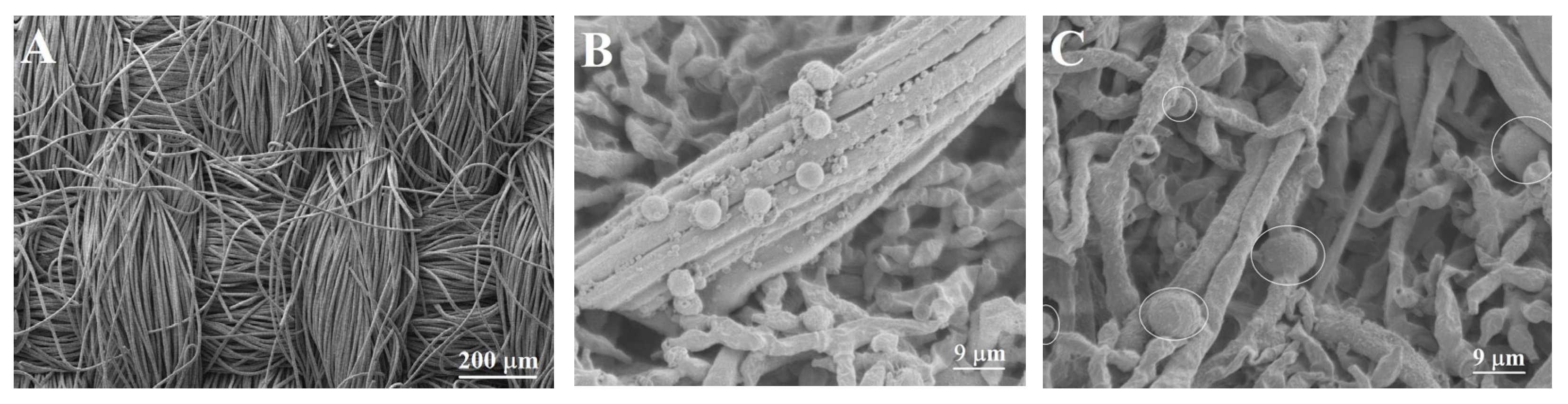
3.9.2. Morphological Characterization of the Bioanode
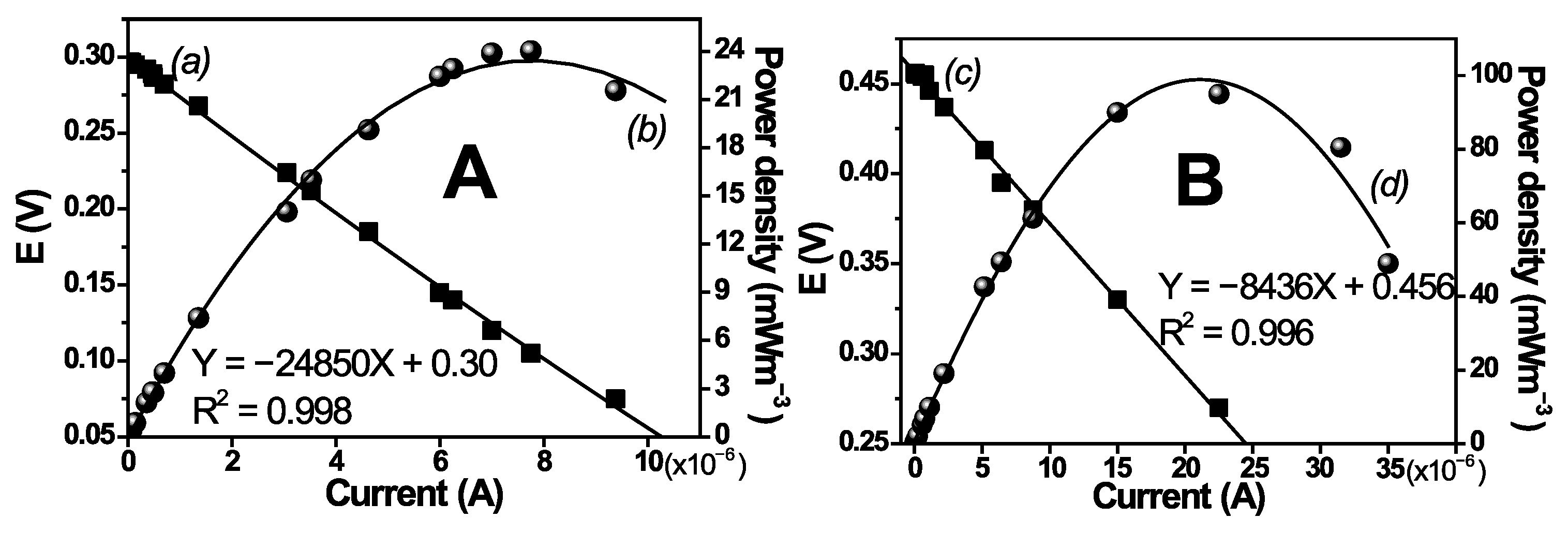
3.9.3. Polarization Curves and Setup of MFC Flowing Conditions
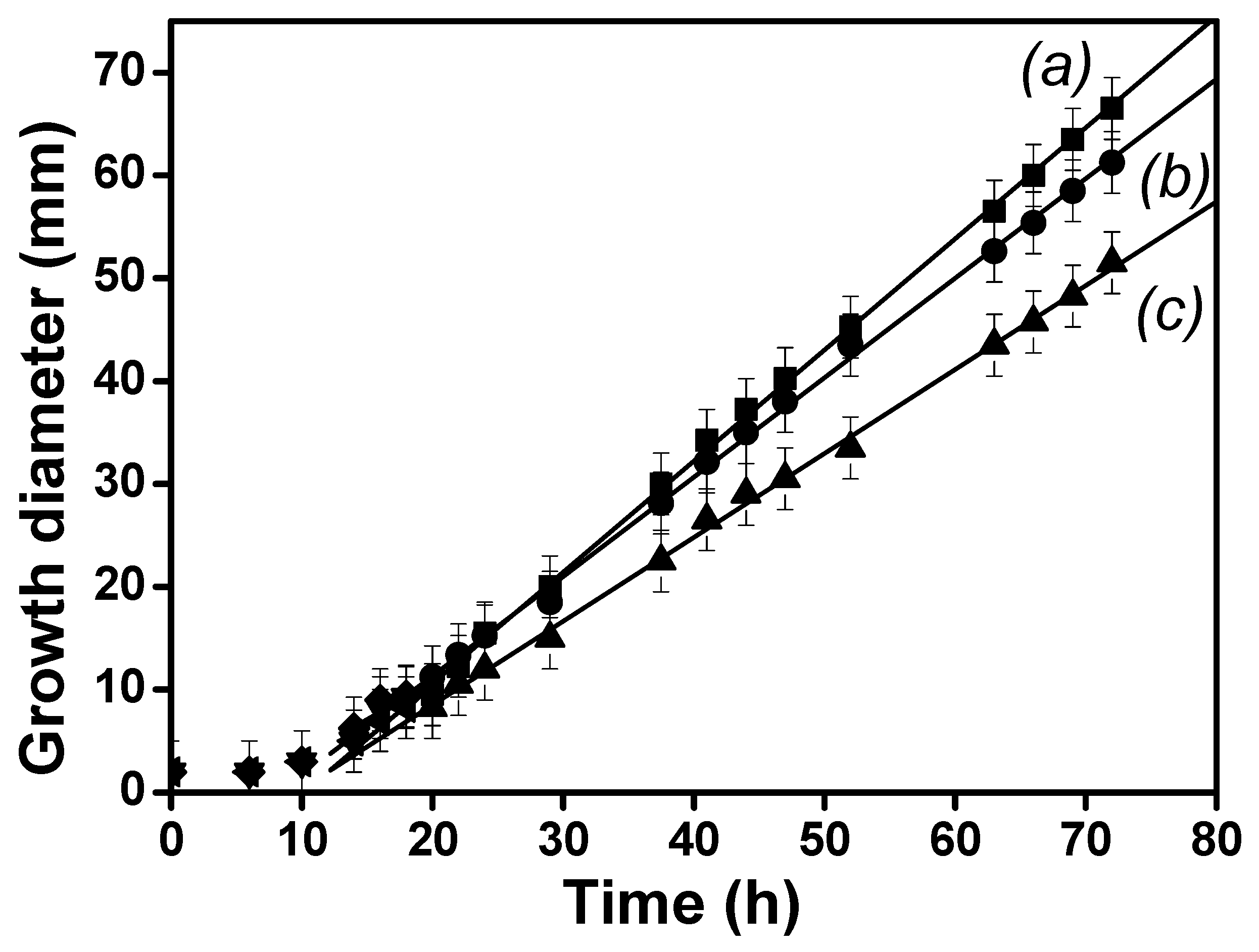
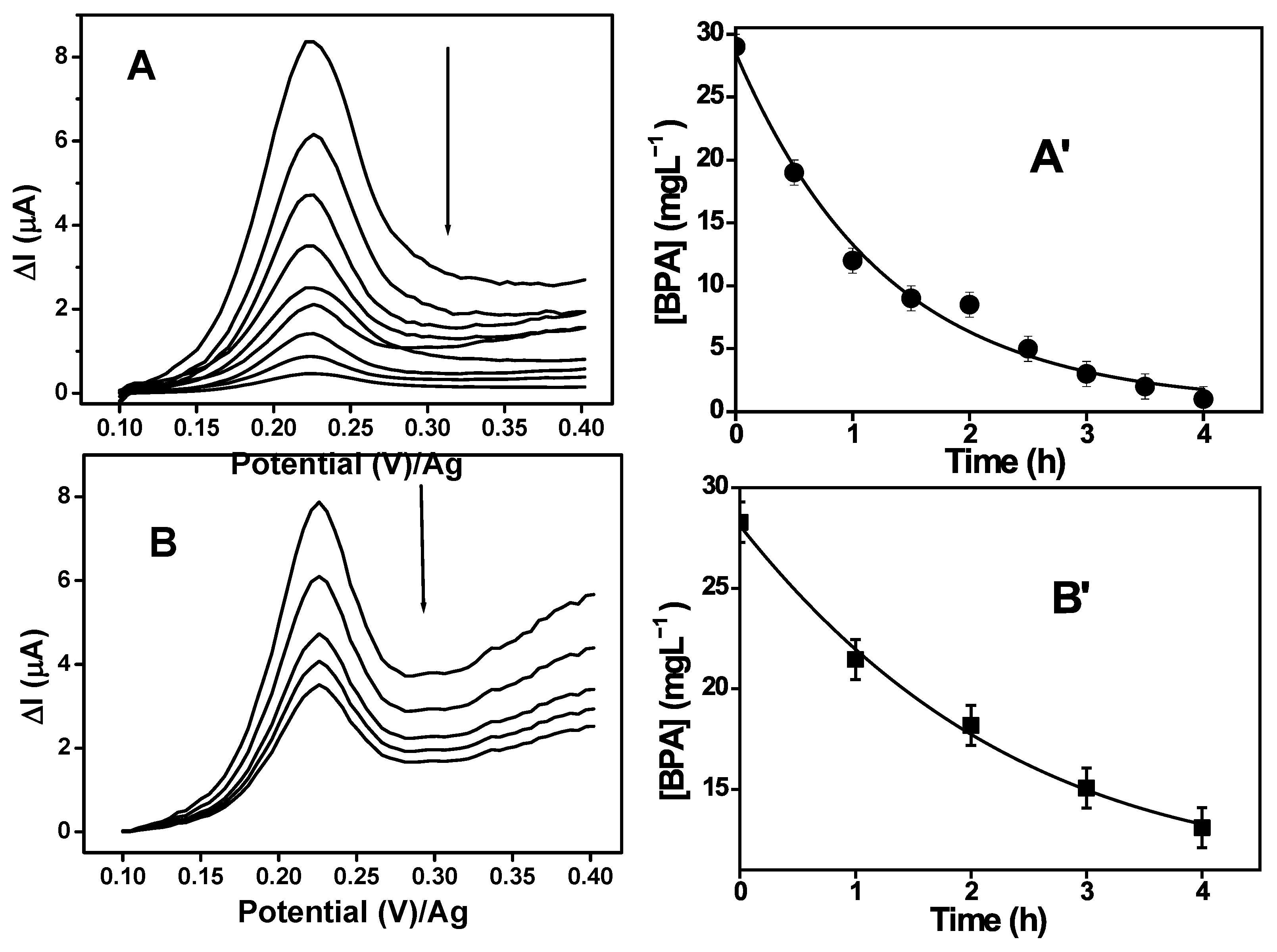
3.9.4. Biodegradation Study of BPA in the MFC1 and MFC2
- Oxidation–reduction reaction of BPA in the MFC:
- Half-reaction of the potassium ferricyanide reduction at the cathode:
- Half-reaction of the reduction of oxygen at the cathode (with the hypothesis that dioxygen also plays a role in the regeneration of the ferrocyanide into potassium ferricyanide):
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| BOD | Biochemical Oxygen Demand |
| BPA | Bisphenol A |
| CC | Carbon Cloth |
| CEM | Cation Exchange Membrane |
| COD | Chemical Oxygen Demand |
| DPV | Differential Pulse Voltammetry |
| EDX | Energy Dispersive X-Ray |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMF | Electromotive Force |
| LC/MS | Liquid Chromatography/Mass Spectrometry |
| LSV | Linear Sweep Voltammetry Method |
| MFC | Microbial Fuel Cell |
| PBS | Phosphate Buffer Solution |
| PI | Phenol Index |
| NiTSPc | Nickel(II) Tetrasulfonated Phthalocyanine |
| RO | Reverse Osmosis |
| SEM | Scanning Electron Microscopy |
| SPE | Screen-Printed Electrode |
| TDS | Total Dissolved Solids |
| TN | Total Nitrogen |
| TOC | Total Organic Carbon |
| TP | Total Phosphorus |
| TS | Total Solids |
| TSS | Total Suspended Solids |
References
- Howdeshell, K.L.; Hotchkiss, A.K.; Thayer, K.A.; Vandenbergh, J.G.; Vomsaal, F.S. Exposure to bisphenol A advances puberty. Nature 1999, 401, 763–764. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.X.; Liu, J.T.; Tong, J.; Su, X.G. Detection of bisphenol A in food packaging based on fluorescent conjugated polymer PPESO3 and enzyme system. Food Chem. 2015, 185, 233–238. [Google Scholar] [CrossRef]
- Liguori, F.; Moreno-Marrodan, C.; Barbaro, P. Biomass-derived chemical substitutes for bisphenol A: Recent advancements in catalytic synthesis. Chem. Soc. Rev. 2020, 49, 6329–6363. [Google Scholar] [CrossRef]
- Seewoo, B.J.; Wong, E.V.S.; Mulders, Y.R.; Gozt, A.; Elagali, A.; Symeonides, C.; Dunlop, S.A. A systematic evidence map protocol for mapping global exposure to bisphenols and their alternatives and social and environmental justice implications. Environ. Inter. 2024, 194, 109091. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer-mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, A.M.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and metabolic approach of plastic additive effects: Immune cells responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar] [CrossRef]
- Soto, M.A.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370. [Google Scholar] [CrossRef]
- Walker, D.M.; Gore, A.C. Transgenerational neuroendocrine disruption of reproduction. Nat. Rev. Endocrinol. 2011, 7, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Xie, B.; Dionysiou, D.D.; Zhao, Y.P. Microplastics as Both a Sink and a Source of Bisphenol A in the Marine Environment. Environ. Sci. Technol. 2019, 53, 10188–10196. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, X.G.; Wu, D.S.; Wang, X. Study on bisphenol A induced primary cultured mesencephalic neuronal cell injury by oxidative stress. J. Hyg. Res. 2006, 35, 419–422. [Google Scholar]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Petrovic, M.; Mihajlovic, I.; Tubic, A.; Novakovic, M. Microplastics in municipal solid waste landfills Maja. Curr. Opin. Environ. Sci. Health 2023, 31, 100428. [Google Scholar] [CrossRef]
- Urase, T.; Miyashita, K. Factors affecting the concentration of bisphenol A in leachates from solid waste disposal sites and its fate in treatment processes. J. Mater. Cycles Waste Manag. 2003, 5, 77–82. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yasuhara, A.; Shiraishi, H.; Nakasugi, O. Bisphenol A in hazardous waste landfill leachates. Chemosphere 2001, 42, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Narevski, A.C.; Novaković, M.I.; Petrović, M.Z.; Mihajlović, I.J.; Maoduš, B.N.; Vujić, G.V. Occurrence of bisphenol A and microplastics in landfill leachate: Lessons from Southeast Europe. Environ. Sci. Pollut. Res. Int. 2021, 28, 42196–42203. [Google Scholar] [CrossRef] [PubMed]
- No 10/2011 of 14; European Commission, Commission Regulation (EU) on Plastic Materials and Articles Intended to Come into Contact with Food. Office for Official Publications of the European Union: Luxembourg, Luxembourg, 2011.
- Ren, L.; Fang, J.Z.; Liu, G.H.; Zhang, J.Q.; Zhu, Z.; Liu, H.G.; Lin, K.; Zhang, H.M.; Lu, S.Y. Simultaneous determination of urinary parabens, bisphenol A, triclosan, and 8-hydroxy-2′-deoxyguanosine by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, L.N.; Liu, R.T. Deciphering the toxicity of bisphenol a to Candida rugosa lipase through spectrophotometric methods. J. Photochem. Photobiol. B 2016, 163, 40–46. [Google Scholar] [CrossRef]
- Hou, C.J.; Zhao, L.X.; Geng, F.L.; Wang, D.; Guo, L.H. Donor/acceptor nanoparticle pair-based singlet oxygen channeling homogenous chemiluminescence immunoassay for quantitative determination of bisphenol A. Anal. Bioanal. Chem. 2016, 408, 1–10. [Google Scholar] [CrossRef]
- Stanic, B.; Nenadov, D.S.; Fa, S.; Pogrmic-Majkic, K.; Andric, N. Integration of data from the cell-based ERK1/2 ELISA and the Comparative Toxicogenomics Database deciphers the potential mode of action of bisphenol A and benzo [a] pyrene in lung neoplasm. Chemosphere 2021, 285, 285131527. [Google Scholar] [CrossRef] [PubMed]
- Vaghela, C.; Kulkarni, M.; Karve, M.; Zinjarde, S. Selective electrochemical sensing of bisphenol derivatives using novel bioelectrode of agarose-guar gum-graphene oxide immobilized with tyrosinase. J. Environ. Chem. Eng. 2022, 10, 107360. [Google Scholar] [CrossRef]
- Ma, J.P.; Yuan, J.H.; Xu, Y.Y.; Jiang, Y.; Bai, W.S.; Zheng, J.B. Ultrasensitive electrochemical determination of bisphenol A in food samples based on a strategy for activity enhancement of enzyme: Layer-by-layer self-assembly of tyrosinase between two-dimensional porphyrin metal-organic framework nanofilms. Chem. Eng. J. 2022, 446, 137001. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol A on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.T.; Dong, G.T.; Zhu, S.S.; Yan, F.; Liu, J.Y. Direct and Sensitive Electrochemical Detection of Bisphenol A in Complex Environmental Samples Using a Simple and Convenient Nanochannel Modified Electrode. Front. Chem. 2022, 10, 900282. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsuda, R.; Haishima, Y.; Yagami, T.; Nakamura, A. Validation of HPLC and GC-MS systems for bisphenol-A leached from hemodialyzers on the basis of FUMI theory. J. Pharm. Biomed. Anal. 2002, 28, 421–429. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalytical Overview: Screen-Printed Electrochemical Sensing Platforms. ChemElectroChem 2024, 11, e202400370. [Google Scholar] [CrossRef]
- Alam, A.U.; Deen, M.J. Bisphenol A electrochemical sensor using graphene oxide and β-cyclodextrin-functionalized multi-walled carbon nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef]
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Luis-Sunga, M.; Carinelli, S.; García, G.; González-Mora, J.L.; Salazar-Carballo, P.A. Electrochemical Detection of Bisphenol A Based on Gold Nanoparticles/Multi-Walled Carbon Nanotubes: Applications on Glassy Carbon and Screen-Printed Electrodes. Sensors 2024, 24, 2570. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z.; Demeestere, K.; Hulle, S.V. Ozonation in view of micropollutant removal from biologically treated landfill leachate: Removal efficiency, OH exposure, and surrogate-based monitoring. Chem. Eng. J. 2021, 410, 128413. [Google Scholar] [CrossRef]
- Gorito, A.M.; Lado Ribeiro, A.R.; Pereira, M.F.R.; Almeida, C.R.M.; Silva, M.A.T. Advanced oxidation technologies and constructed wetlands in aquaculture farms: What do we know so far about micropollutant removal. Environ. Res. 2022, 204, 111955. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, H.; Belhadj, A.E.; Moula, N.; Bouranene, S.; Amrane, A. Optimisation and Prediction of the Coagulant Dose for the Elimination of Organic Micropollutants Based on Turbidity. Kem. Ind. 2021, 70, 675–691. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W. Numerical modeling of an electrically enhanced membrane bioreactor (MBER) treating medium-strength wastewater. J. Environ. Manag. 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Chakraborty, P.; Kaur, K.; Duhan, L.; Sarojini, S.; Thazeem, B.; Pasrija, R.; Vangnai, A.S.; et al. Micropollutants characteristics, fate, and sustainable removal technologies for landfill leachate: A technical perspective. J. Water Proc. Eng. 2023, 53, 103649. [Google Scholar] [CrossRef]
- Shabani, M.; Pontié, M.; Younesi, H.; Nacef, M.; Rahimpour, A.; Rahimnejad, M.; Medjda, R.; Khelladi, R.M.B. Biodegradation of acetaminophen and its main by-product 4-aminophenol by Trichoderma harzianum versus mixed bioflm of Trichoderma harzianum/Pseudomonas fuorescens in a fungal microbial fuel cell. J. Appl. Electrochem. 2021, 51, 581–596. [Google Scholar] [CrossRef]
- Kyrila, G.; Katsoulas, A.; Schoretsaniti, V.; Rigopoulos, A.; Rizou, E.; Doulgeridou, S.; Sarli, V.; Samanidou, V.; Touraki, M. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties. J. Hazard. Mater. 2021, 413, 125363. [Google Scholar] [CrossRef]
- Gorin, M.; Shabani, M.; Votat, S.; Lebrun, L.; Mbokou, S.F.; Pontie, M. Application of fungal-based microbial fuel cells for biodegradation of pharmaceuticals: Comparative study of individual vs. mixed Contaminant Solutions. Chemosphere 2024, 363, 142849. [Google Scholar] [CrossRef]
- Segundo, R.F.; Rocío, P.C.; Luis, C.C.; Angelats Silva, L.M. Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells. Processes 2024, 12, 2904. [Google Scholar] [CrossRef]
- Bint-e-Zahira, S.; Khalid, A.N.; Yousaf, N.; Iqbal, M.; Anwar, T.; Qureshi, H.; Salmen, S.H.; Ansari, M.J. Exploring Trichoderma Species in Industrial Wastewater: Morphological and Molecular Insights from Isolates. Life 2024, 14, 750. [Google Scholar] [CrossRef]
- Silga, J.P.T.; Foukmeniok, S.M.; Bako, Y.F.R.; Ramdane, A.C.; Nazerifar, M.; Younesi, H.; Tapsoba, I.; Maxime, P. Ultrasensitive screen-printed-electrodes for p-aminophenol analysis and applications in bioremediation and photodegradation processes. J. Electrochem. Soc. 2025, 172, 027501. [Google Scholar] [CrossRef]
- Votat, S.; Pontié, M.; Jaspard, E.; Lebrun, L. Crystal Violet (CV) Biodegradation Study in a Dual Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies 2024, 17, 247. [Google Scholar] [CrossRef]
- Abiriga, D.; Jenkins, A.; Vestgarden, L.S.; Klempe, H. A nature-based solution to a landfill-leachate contamination of a confined aquifer. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill leachate: Sources, nature, organic composition, and treatment: An environmental overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, N.O., Jr. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J. Electroanal. Chem. 2019, 848, 113319. [Google Scholar] [CrossRef]
- Mbokou Foukmeniok, S.; Bako, F.R.Y.; Ilboudo, O.; Karanga, Y.; Njanja, E.; Pontie, M.; Tapsoba, I.; Kenfack, I.T.; Djouaka, R. Sensitive Carbon Fiber Microelectrode for the Quantification of Diuron in Quality Control of a Commercialized Formulation. Int. J. Anal. Chem. 2022, 2022, 9994639. [Google Scholar] [CrossRef]
- Bako, Y.F.R.; Mbokou Foukmeniok, S.; Kaboré, B.; Tapsoba, I.; Pontié, M. Indirect Electroanalysis of 3-Methyl-4-Nitrophenol in Water Using Carbon Fiber Microelectrode Modified with Nickel Tetrasulfonated Phthalocyanine Complex. Mater. Sci. Appl. 2024, 15, 25–35. [Google Scholar] [CrossRef]
- Pontié, M.; Sikpo, L.; Thouand, G.; Lahan, R.; Tapsoba, I.; Mallet, R.; Feng, T. Direct Electroanalysis of p-Nitrophenol (PNP) in Estuarine and Surface Waters by a High Sensitive Type C/p-NiTSPc CoatingCarbon Fiber Microelectrode (CFME). Electroanalysis 2011, 23, 433–441. [Google Scholar] [CrossRef]
- Bako, Y.F.R.; Silga, J.P.T.; Mbokou Foukmeniok, S.; Pontié, M.; Tapsoba, I. Molecular imprinted polymer modified carbon ultramicroelectrode for a selective detection of 3-methyl-4-nitrophenol and its bioremediation in a fungal microbial fuel cell. J. Appl. Electrochem. 2024, 54, 1253–1265. [Google Scholar] [CrossRef]
- Deffo, G.; Temgoua, R.T.C.; Mbokou Foukmeniok, S.; Njanja, E.; Tonlé, I.K.; Ngameni, E. A sensitive voltammetric analysis and detection of Alizarin Red S onto a glassy carbon electrode modified by an organosmectite. Sens. Int. 2021, 2, 100126. [Google Scholar] [CrossRef]
- Yi, G.R.; Wu, X.; Tang, K.H.D.; Li, R.H. Microbial degradation of bisphenol A-A mini-review. Curr. Opin. Environ. Sci. Health 2025, 43, 100595. [Google Scholar] [CrossRef]
- Liu, J.; Sun, K.; Zhu, R.; Wang, X.; Waigi, M.G.; Li, S.Y. Biotransformation of bisphenol A in vivo and in vitro by laccase-producing Trametes hirsuta La-7: Kinetics, products, and mechanisms. Environ Pollut. 2023, 321, 121155. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.; Kaur, N.; Rahman, P.K.S.M.; Sharma, S. Solubilization and enhanced degradation of benzene phenolic derivatives-bisphenol A/Triclosan using a biosurfactant producing white rot fungus Hypocrea lixii S5 with plant growth promoting traits. Front. Microbiol. 2024, 15, 1433745. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, A.; Sobon, A.; Rózalska, S.; Srednicka, P. Bisphenol A removal by the fungus Myrothecium roridumIM 6482—Analysis of the cellular and subcellular level. Int. J. Mol. Sci. 2021, 22, 10676. [Google Scholar] [CrossRef]
- Ademakinwa, A.N. A heat-resistant intracellular laccase immobilized via cross-linked enzyme aggregate preparation: Characterization, application in bisphenol A removal and phytotoxicity evaluation. J. Hazard. Mater. 2021, 419, 126480. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Liu, Q.Z.; Liu, J.; Sun, K.; Yang, W.; Si, Y.B.; Li, Y.C.; Gao, Y.Z. Inhibition mechanisms of Fe2+/Fe3+ and Mn2+ on fungal laccase enabled bisphenol a polyreaction. Chemosphere 2022, 307, 135685. [Google Scholar] [CrossRef]
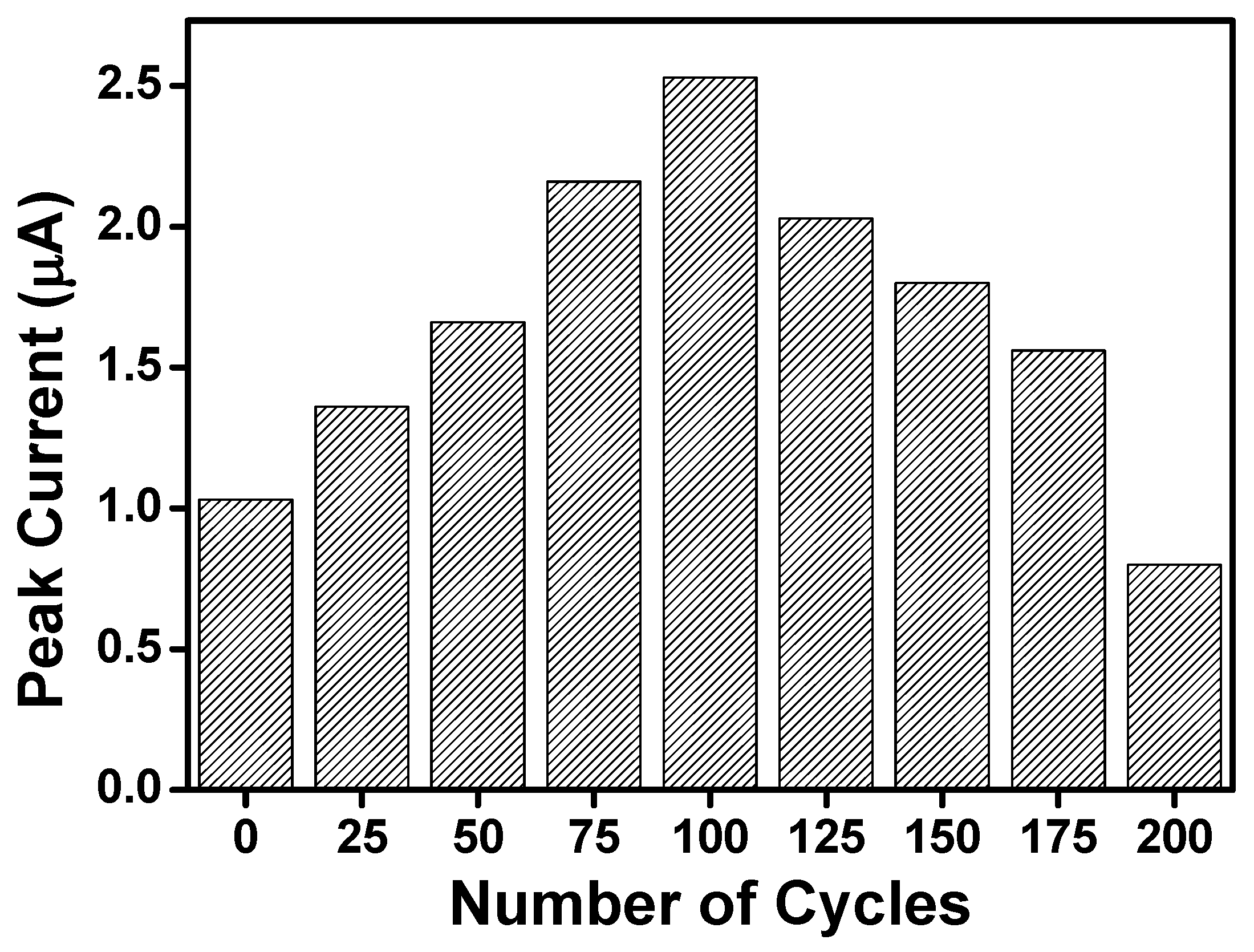


| Parameters | Units | Raw Leachate | Effluent After RO |
|---|---|---|---|
| BOD | mgL−1 | 2900 | <4 |
| COD | mgL−1 | 14,140 | 7 |
| TS | mgL−1 | 23,450 | 24 |
| TSS | mgL−1 | 24 | <2 |
| TDS | mgL−1 | 23,400 | 20 |
| TN | mgL−1 | 4400 | 34 |
| Organic N | mgL−1 | 550 | <0.5 |
| NH4-N | mgL−1 | 3840 | 27.5 |
| TOC | mgL−1 | 5550 | 1.7 |
| TP | mgL−1 | 39 | <0.05 |
| P-PO4 | mgL−1 | 35 | <0.05 |
| pH | 8 | 5.8 | |
| Conductivity | µScm−1 | 34,100 | 263 |
| Temperature | °C | 19.8 | 19.9 |
| Odor | TON | >100 | 50 |
| Turbidity | NTU | 210 | 1.9 |
| Cl | mgL−1 | 4750 | <5 |
| SO4 | mgL−1 | 480 | <5 |
| Phenol Index (PI) | mgL−1 | 71 | <0.10 |
| As | mgL−1 | 0.49 | <0.003 |
| Cd | mgL−1 | 0.0014 | <0.0005 |
| Cu | mgL−1 | 0.24 | <0.005 |
| Ni | mgL−1 | 0.92 | <0.002 |
| Zn | mgL−1 | 0.68 | <0.01 |
| Analytical Techniques | Molecules | Concentration in Landfill Leachate Solution (µgL−1) | % in Landfill Leachate Solution | Concentration in the Effluent (After RO) (µgL−1) |
|---|---|---|---|---|
| LC-MS (Accredited Lab /INOVALYS) | Bisphenol A | 29,579.00 | 97.75 | 6.00 |
| 4-tert-butylphenol | 582.00 | 1.92 | 0.27 | |
| 4-n-nonylphenols | <0.10 | - | <0.10 | |
| 4-nonylphenols | 42.00 | <0.01 | <0.10 | |
| Nonylphenols | 42.00 | <0.01 | <0.10 | |
| 4-nonylphenol monoethoxylate | 6.00 | <0.01 | <0.10 | |
| 4-nonylphenol diethoxylate | 0.88 | <0.01 | <0.10 | |
| Octylphenols | <0.10 | - | <0.10 | |
| 4-(para)-tert-octylphenol | 4.40 | <0.01 | <0.05 | |
| 4-n-ctylphenols | <0.25 | - | <0.05 | |
| 4-(para)-tert-octylphenol monoethoxylate | 1.70 | <0.01 | <0.10 |
| Electrodes | Unmodified SPE | SPE-A-polyNiTSPc |
|---|---|---|
| Parameters | ||
| Geometric surface area (cm2) | 0.126 ± 0.010 | 0.126 ± 0.010 |
| Real surface area (cm2) | 0.128 ± 0.005 | 0.186 ± 0.005 |
| Resistance of charge transfer (Ω) | 1050 ± 5 | 400 ± 5 |
| Roughness (nm) | 320 ± 0.05 | 500 ± 0.05 |
| Contact angles (°) | 111 ± 1 | 60 ± 1 |
| Sample | PI | [BPA] (µgL−1) Using LC/MS Accredited Lab | [BPA] (µgL−1) Using ECS | Regression Equation/ ECS Method | Correlation Coefficient (R2) |
|---|---|---|---|---|---|
| Raw influent | 71 | 29,579 | 29,700 | Y = 0.33X + 9.70 | 0.995 |
| Effluent after RO | <0.1 | 6.0 | 6.4 | Y = 4.40X + 0.30 | 0.995 |
| BPA Concentration (mgL−1) | 0 (Control) | 29 | 50 | 100 | 250 | 500 |
|---|---|---|---|---|---|---|
| Slope (mmh−1) | 2.321 | 1.751 | 1.429 | 1.215 | 1.081 | 0.981 |
| Inhibition (%) | 0 | 25 | 38 | 47 | 53 | 58 |
| Culture Media | [BPA] (mgL−1) | Slope (mmh−1) | Inhibition (%) |
|---|---|---|---|
| PGA alone | 0 (control) | 1.081 | 0 |
| PGA + BPA | 29 | 0.967 | 10 |
| PGA + landfill leachate | 29 | 0.815 | 25 |
| MFC Type | Open Circuit Potential (mV) | Optimal Resistance (Ω) | Optimal Power Density (mWm−3) |
|---|---|---|---|
| MFC1 | 456 | 24,850 | 24 |
| MFC2 | 300 | 8436 | 97 |
| Fungal Strains | Oxygen Condition | Temperature (°C) | pH | t1/2 (h) | References |
|---|---|---|---|---|---|
| Trametes hirsuta | Aerobic | 30 | 4.8 | 1.44 | [52] |
| Hypocrea lixii | Aerobic | 30 | 5.0 | - | [53] |
| Myrothecium roridum | Aerobic | 28 | 7.0 | - | [54] |
| Aureobasidium pullulans | Aerobic | 30 | 4.5 | 0.08 | [55] |
| Trametes versicolor | Aerobic | 25 | 3.8 | 1.14 | [56] |
| T harzianum | Aerobic | 25 | 8.0 | 3.80 | This work |
| T harzianum | Aerobic | 25 | 7.2 | 0.90 | This work |
| MFC Type | Regression Equation | R2 | k (h−1) | t1/2 (h) | t [BPA] = 0.3 mgL−1 (h) |
|---|---|---|---|---|---|
| MFC 1 | ln C/C0 = −0.795t + 6.728 | 0.974 | 0.795 | 0.9 | 5750 |
| MFC 2 | ln C/C0 = −0.183t − 0.026 | 0.992 | 0.183 | 3.8 | 25,121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbokou Foukmeniok, S.; Silga, J.-P.T.; Ait Yazza, A.; Bougna Tchoumi, H.H.; Dia, M.; Pontie, M.; Urošević, V. Electrochemical Monitoring of Bisphenol A Degradation in Leachate by Trichoderma harzianum Using a Sensitive Sensor of Type SPE in Microbial Fuel Cells. Chemosensors 2025, 13, 317. https://doi.org/10.3390/chemosensors13090317
Mbokou Foukmeniok S, Silga J-PT, Ait Yazza A, Bougna Tchoumi HH, Dia M, Pontie M, Urošević V. Electrochemical Monitoring of Bisphenol A Degradation in Leachate by Trichoderma harzianum Using a Sensitive Sensor of Type SPE in Microbial Fuel Cells. Chemosensors. 2025; 13(9):317. https://doi.org/10.3390/chemosensors13090317
Chicago/Turabian StyleMbokou Foukmeniok, Serge, Jean-Philippe Theodore Silga, Adil Ait Yazza, Honorine Hortense Bougna Tchoumi, Malak Dia, Maxime Pontie, and Vladimir Urošević. 2025. "Electrochemical Monitoring of Bisphenol A Degradation in Leachate by Trichoderma harzianum Using a Sensitive Sensor of Type SPE in Microbial Fuel Cells" Chemosensors 13, no. 9: 317. https://doi.org/10.3390/chemosensors13090317
APA StyleMbokou Foukmeniok, S., Silga, J.-P. T., Ait Yazza, A., Bougna Tchoumi, H. H., Dia, M., Pontie, M., & Urošević, V. (2025). Electrochemical Monitoring of Bisphenol A Degradation in Leachate by Trichoderma harzianum Using a Sensitive Sensor of Type SPE in Microbial Fuel Cells. Chemosensors, 13(9), 317. https://doi.org/10.3390/chemosensors13090317






