Synergistic Tuning of Active Sites and π-Conjugation in 2D Conductive MOFs Boosts Uric Acid Electrosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Preparation and Testing of Electrodes
2.3. Calculation of Langmuir Adsorption Isotherms
3. Results and Discussion
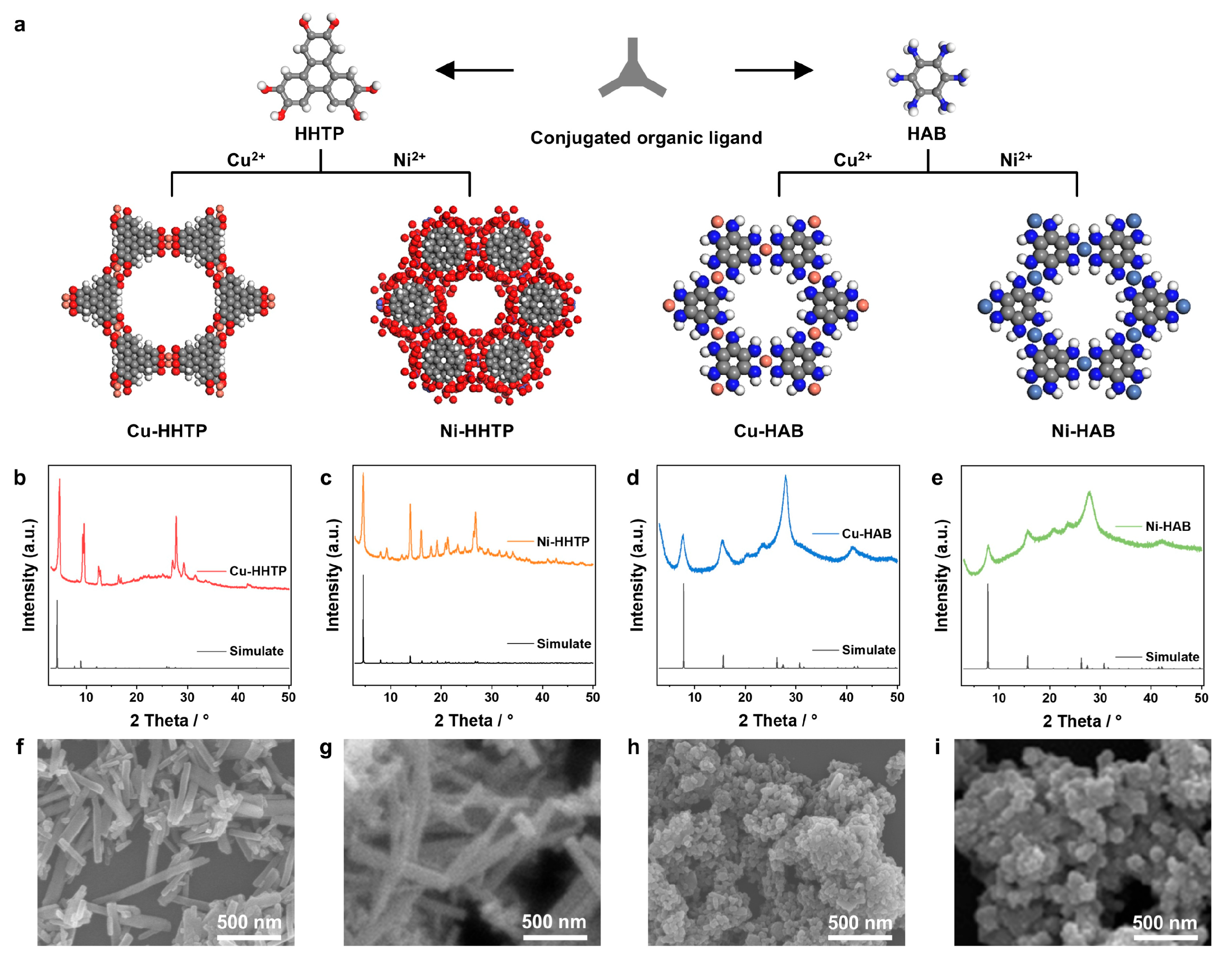
3.1. Synthesis and Characteristics of 2D c-MOF Electrodes with Different [MX4] Active Sites
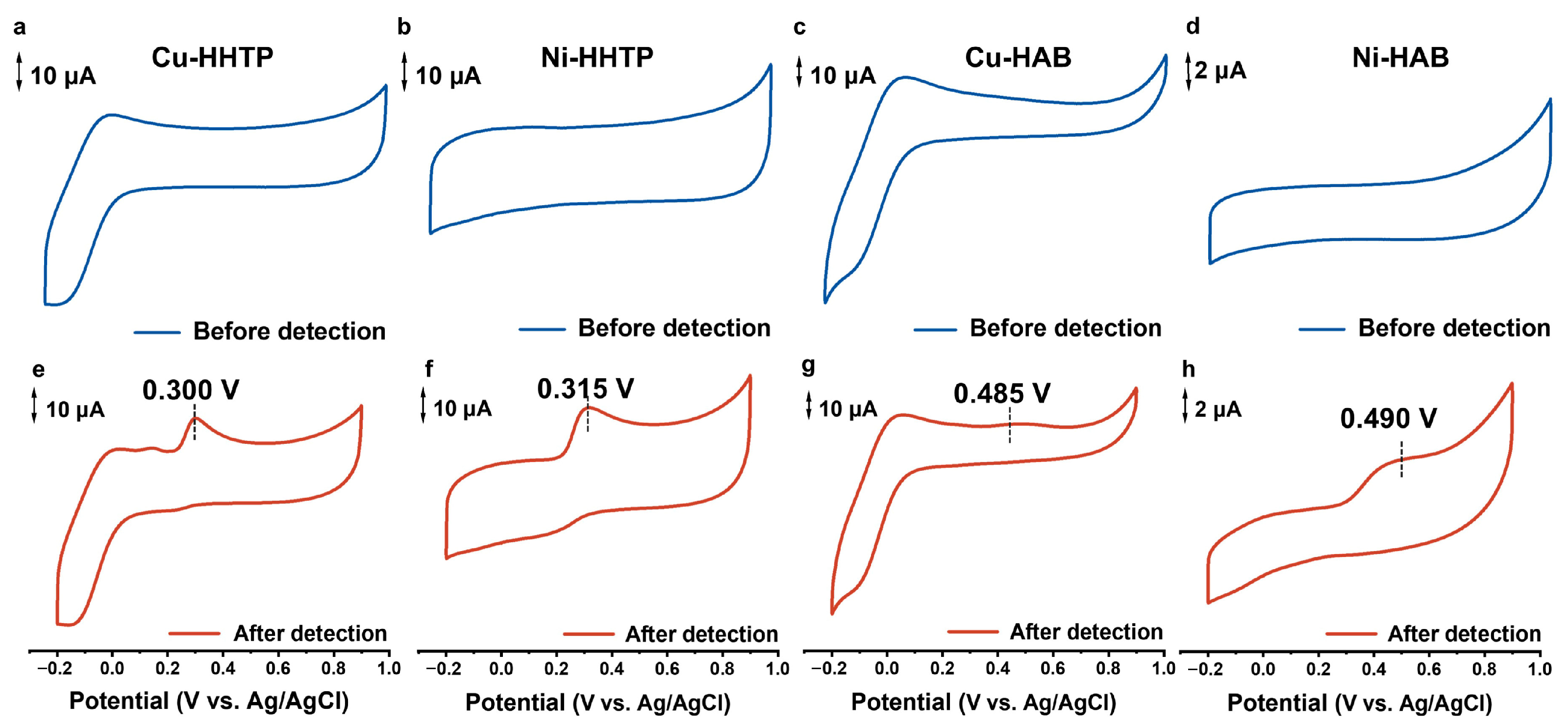
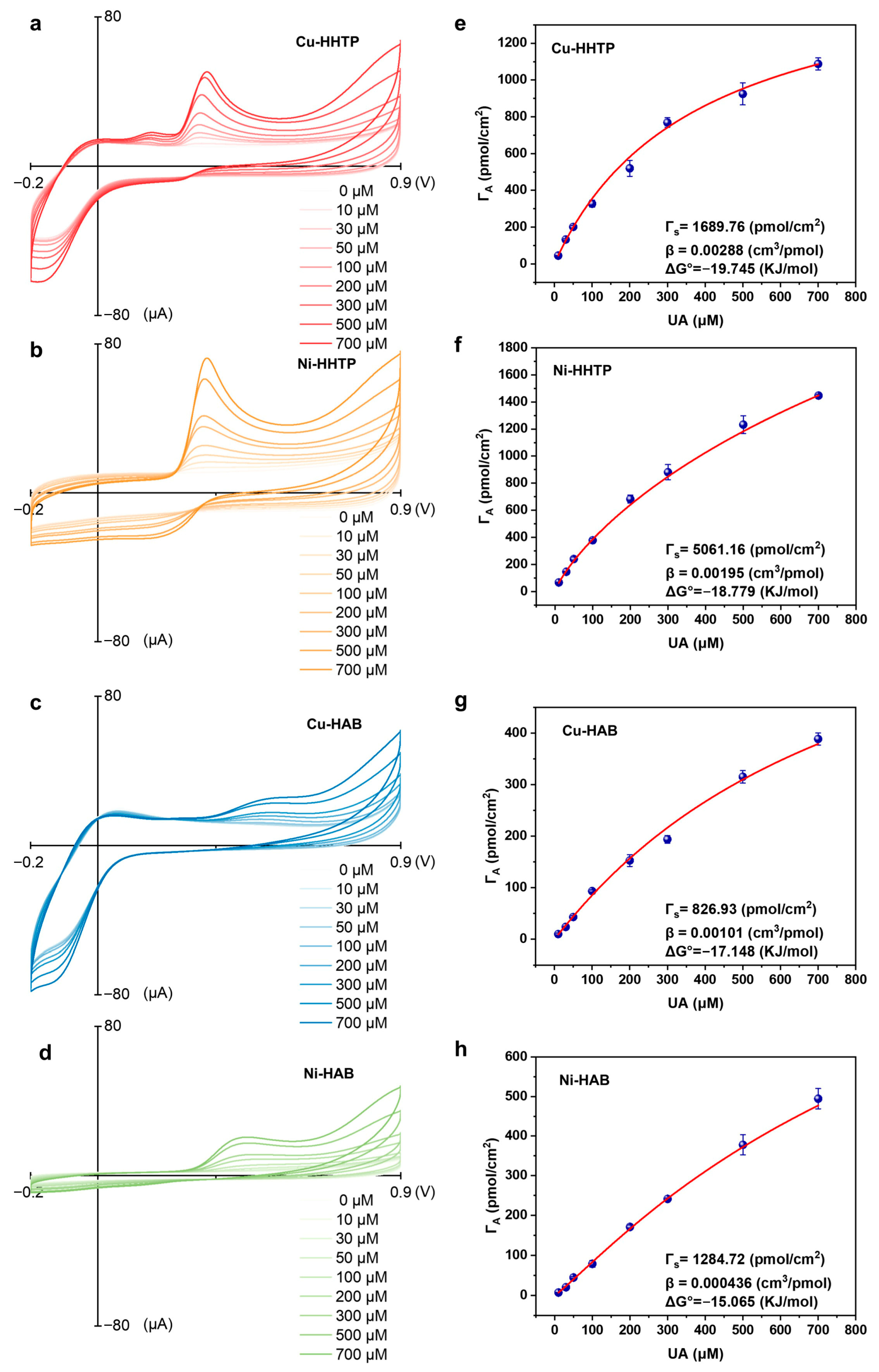
3.2. Assessing the Electrochemical Response of 2D c-MOF Electrodes with Different [MX4] Active Sites Toward UA
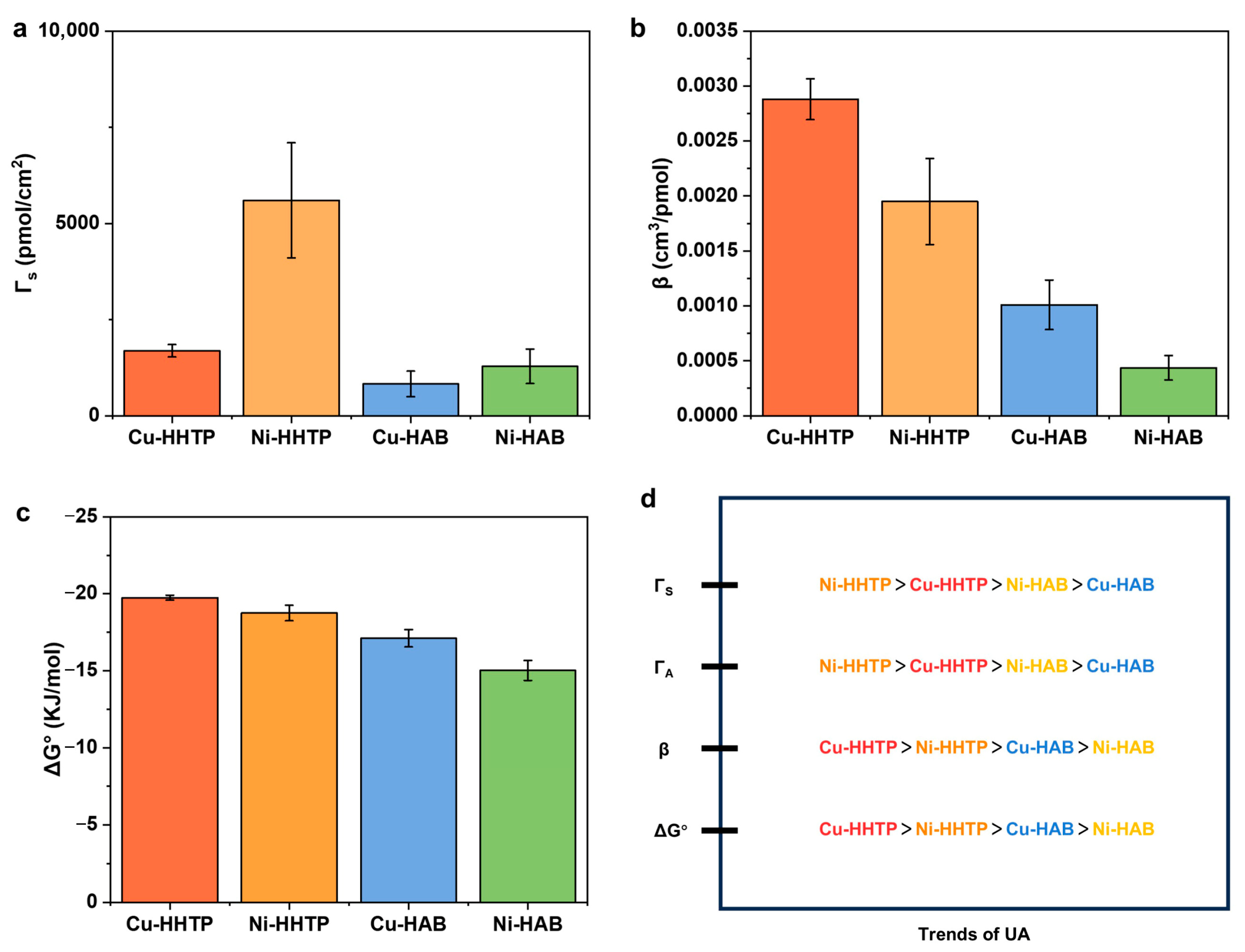
3.3. Probing the Response Mechanisms of Different [MX4] Active Sites in 2D c-MOF Electrodes to UA Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| UA | Uric acid |
| 2D c-MOF | 2D conjugated metal–organic frameworks |
| PBS | Phosphate-buffered saline |
| CNT | Carbon nanotube |
| HHTP | 2,3,6,7,10,11-hexahydroxytriphenylene |
| HAB | Hexaaminobenzene |
| DMF | N,N-Dimethylformamide |
| ITO | Indium tin oxide |
| PXRD | Powder X-ray diffraction |
| SEM | Scanning electron microscopy |
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| ΓA | Surface coverage |
| ΓS | Saturated surface coverage |
| β | Thermodynamic equilibrium constant |
| ΔG° | Gibbs adsorption free energy |
| Glu | Glucose |
| DA | Dopamine |
| AA | Ascorbic acid |
References
- Chauhan, N.; Soni, S.; Agrawal, P.; Balhara, Y.P.S.; Jain, U. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process Biochem. 2020, 91, 241–259. [Google Scholar] [CrossRef]
- Oh, Y.; Heien, M.L.; Park, C.; Kang, Y.M.; Kim, J.; Boschen, S.L.; Shin, H.; Cho, H.U.; Blaha, C.D.; Bennet, K.E.; et al. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens. Bioelectron. 2018, 121, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Lario, B.; Macarrón-Vicente, J. Uric acid and evolution. Rheumatology 2010, 49, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Jee, J.H.; Bae, J.C.; Jin, S.-M.; Baek, J.-H.; Lee, M.-K.; Kim, J.H. Serum uric acid: A strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism 2016, 65, 432–440. [Google Scholar] [CrossRef]
- Yao, B.; Scalco de Vasconcelos, L.; Cui, Q.; Cardenas, A.; Yan, Y.; Du, Y.; Wu, D.; Wu, S.; Hsiai, T.K.; Lu, N.; et al. High-stability conducting polymer-based conformal electrodes for bio-/iono-electronics. Mater. Today 2022, 53, 84–97. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. TrAC Trends Anal. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: An overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Liu, J.; Xing, G.; Chen, L. 2D Conjugated Metal-Organic Frameworks: Defined Synthesis and Tailor-Made Functions. Acc. Chem. Res. 2024, 57, 1032–1045. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Zhang, T.; Chen, L. 2D conductive metal-organic frameworks for electronics and spintronics. Sci. China Chem. 2020, 63, 1391–1401. [Google Scholar] [CrossRef]
- Wang, M.; Dong, R.; Feng, X. Two-dimensional conjugated metal–organic frameworks (2D c-MOFs): Chemistry and function for MOFtronics. Chem. Soc. Rev. 2021, 50, 2764–2793. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, W.; Dong, L.; Deng, S.; Wilkinson, D.P.; Wang, X.; Zhang, L.; Zhang, J. Catalytically active sites of MOF-derived electrocatalysts: Synthesis, characterization, theoretical calculations, and functional mechanisms. J. Mater. Chem. A 2021, 9, 20320–20344. [Google Scholar] [CrossRef]
- Park, C.; Baek, J.W.; Shin, E.; Kim, I.-D. Two-Dimensional Electrically Conductive Metal–Organic Frameworks as Chemiresistive Sensors. ACS Nanosci. Au 2023, 3, 353–374. [Google Scholar] [CrossRef]
- Liang, C.; Liu, J.; Zhang, H.; Zhang, Z.; Liu, Y.; Zhang, K.; Wang, T. Mitigating lipid biofouling in wearable sweat sensors: A study on conductive MOF-based electrodes with tuned hydrophilicity. Chem. Eng. J. 2025, 518, 164477. [Google Scholar] [CrossRef]
- Liang, X.-H.; Yu, A.-X.; Bo, X.-J.; Du, D.-Y.; Su, Z.-M. Metal/covalent-organic frameworks-based electrochemical sensors for the detection of ascorbic acid, dopamine and uric acid. Coord. Chem. Rev. 2023, 497, 215427. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Zhang, X.; Ling, G.; Zhang, P. DNAzyme@MOF breaking pH limitation for the detection of dopamine in the interstitial fluid. Biosens. Bioelectron. 2025, 279, 117367. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Shang, X.; Liu, J.; Tang, T. A skin-mountable flexible biosensor based on Cu-MOF/PEDOT composites for sweat ascorbic acid monitoring. Biosens. Bioelectron. 2025, 267, 116852. [Google Scholar] [CrossRef]
- Shabanur Matada, M.S.; Nutalapati, V.; Velappa Jayaraman, S.; Sivalingam, Y. Tuning Mn-MOF by Incorporating a Phthalocyanine Derivative as an Enzyme Mimic for Efficient EGFET-based Ascorbic Acid Detection. ACS Appl. Mater. Interfaces 2025, 17, 20806–20819. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Y.; Zhou, Y.; Zhang, A.; Meng, J.; Li, L.; Zhang, W. Multifunctional wearable sensor using hetero-nanoforest structural Cu-HHTP/CuCoNi-LDH composite toward applications of human motion, sound, gas and light monitoring. J. Mater. Sci. Technol. 2024, 195, 197–207. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing Conductive Metal–Organic Frameworks for Voltammetric Detection of Neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717–11733. [Google Scholar] [CrossRef]
- Ortega Vega, M.R.; Luo, Y.; Werheid, M.; Weidinger, I.; Senkovska, I.; Grothe, J.; Kaskel, S. Ni-based metal-organic framework sensor material for urea detection: Mechanistic insights and performance. Electrochim. Acta 2024, 477, 143748. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Makaremi, M.; Cuniberti, G.; Rabczuk, T. First-principles investigation of Ag-, Co-, Cr-, Cu-, Fe-, Mn-, Ni-, Pd- and Rh-hexaaminobenzene 2D metal-organic frameworks. Mater. Today Energy 2018, 10, 336–342. [Google Scholar] [CrossRef]
- Stolz, R.M.; Kolln, A.F.; Rocha, B.C.; Brinks, A.; Eagleton, A.M.; Mendecki, L.; Vashisth, H.; Mirica, K.A. Epitaxial Self-Assembly of Interfaces of 2D Metal–Organic Frameworks for Electroanalytical Detection of Neurotransmitters. ACS Nano 2022, 16, 13869–13883. [Google Scholar] [CrossRef]
- Yao, M.-S.; Lv, X.-J.; Fu, Z.-H.; Li, W.-H.; Deng, W.-H.; Wu, G.-D.; Xu, G. Layer-by-Layer Assembled Conductive Metal–Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem. Int. Ed. 2017, 56, 16510–16514. [Google Scholar] [CrossRef]
- Hmadeh, M.; Lu, Z.; Liu, Z.; Gándara, F.; Furukawa, H.; Wan, S.; Augustyn, V.; Chang, R.; Liao, L.; Zhou, F.; et al. New Porous Crystals of Extended Metal-Catecholates. Chem. Mater. 2012, 24, 3511–3513. [Google Scholar] [CrossRef]
- Feng, D.; Lei, T.; Lukatskaya, M.R.; Park, J.; Huang, Z.; Lee, M.; Shaw, L.; Chen, S.; Yakovenko, A.A.; Kulkarni, A.; et al. Robust and conductive two-dimensional metal–organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Peng, Z.; Tang, X.; Xu, P.; Qiu, P. Calcium Fluoride/Manganese Dioxide Nanocomposite with Dual Enzyme-like Activities for Uric Acid Sensing: A Comparative Study of Enzyme and Nonenzyme Methods. ACS Appl. Mater. Interfaces 2024, 16, 54–65. [Google Scholar] [CrossRef]
- Sun, Q.; Miao, S.; Yu, W.; Jiang, E.-Y.; Gong, M.; Liu, G.; Luo, X.; Zhang, M.-Z. Visual detection of uric acid in serum through catalytic oxidation by a novel cellulose membrane biosensor with schiff base immobilized uricase. Biosens. Bioelectron. 2025, 268, 116912. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Chai, Y.; Xu, Y.; Yu, Q.; Huang, X.; Zhang, L.; Jiang, Z. Quantification of Uric Acid of Rat Serum by Liquid Chromatography-ultraviolet Detection and Its Comparison Study. Lab. Anim. Comp. Med. 2023, 43, 314–322. [Google Scholar] [CrossRef]
- Gaya, E.; Menendez, N.; Mazario, E.; Herrasti, P. Fe3O4-Nanoparticle-Modified Sensor for the Detection of Dopamine, Uric Acid and Ascorbic Acid. Chemosensors 2023, 11, 79. [Google Scholar] [CrossRef]
- Huang, W.; Xu, Y.; Yang, Y.; Sun, J.; Hu, M.; Hao, F.; Xiao, F. Wearable Sensor for Continuous Monitoring Multiple Biofluids: Improved Performances by Conductive Metal-Organic Framework with Dual-Redox Sites on Flexible Graphene Fiber Microelectrode. Adv. Funct. Mater. 2025, 35, 2424018. [Google Scholar] [CrossRef]
- Mi, Z.; Xia, Y.; Dong, H.; Shen, Y.; Feng, Z.; Hong, Y.; Zhu, H.; Yin, B.; Ji, Z.; Xu, Q.; et al. Microfluidic Wearable Electrochemical Sensor Based on MOF-Derived Hexagonal Rod-Shaped Porous Carbon for Sweat Metabolite and Electrolyte Analysis. Anal. Chem. 2024, 96, 16676–16685. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, J.; Chen, L.; Lin, H.; Han, D.; Bao, Y.; Wang, W.; Niu, L. A Wearable Electrochemical Biosensor Utilizing Functionalized Ti3C2Tx MXene for the Real-Time Monitoring of Uric Acid Metabolite. Anal. Chem. 2024, 96, 3914–3924. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fu, Y.; Zhang, H.; Wang, L.; Lin, X.; Liu, J. Synergistic Tuning of Active Sites and π-Conjugation in 2D Conductive MOFs Boosts Uric Acid Electrosensing. Chemosensors 2025, 13, 318. https://doi.org/10.3390/chemosensors13090318
Liu Y, Fu Y, Zhang H, Wang L, Lin X, Liu J. Synergistic Tuning of Active Sites and π-Conjugation in 2D Conductive MOFs Boosts Uric Acid Electrosensing. Chemosensors. 2025; 13(9):318. https://doi.org/10.3390/chemosensors13090318
Chicago/Turabian StyleLiu, Yanli, Yifan Fu, Haitong Zhang, Lingyu Wang, Xuejing Lin, and Jingjuan Liu. 2025. "Synergistic Tuning of Active Sites and π-Conjugation in 2D Conductive MOFs Boosts Uric Acid Electrosensing" Chemosensors 13, no. 9: 318. https://doi.org/10.3390/chemosensors13090318
APA StyleLiu, Y., Fu, Y., Zhang, H., Wang, L., Lin, X., & Liu, J. (2025). Synergistic Tuning of Active Sites and π-Conjugation in 2D Conductive MOFs Boosts Uric Acid Electrosensing. Chemosensors, 13(9), 318. https://doi.org/10.3390/chemosensors13090318





