Noble Metal-Decorated In2O3 for NO2 Gas Sensor: An Experimental and DFT Study
Abstract
1. Introduction
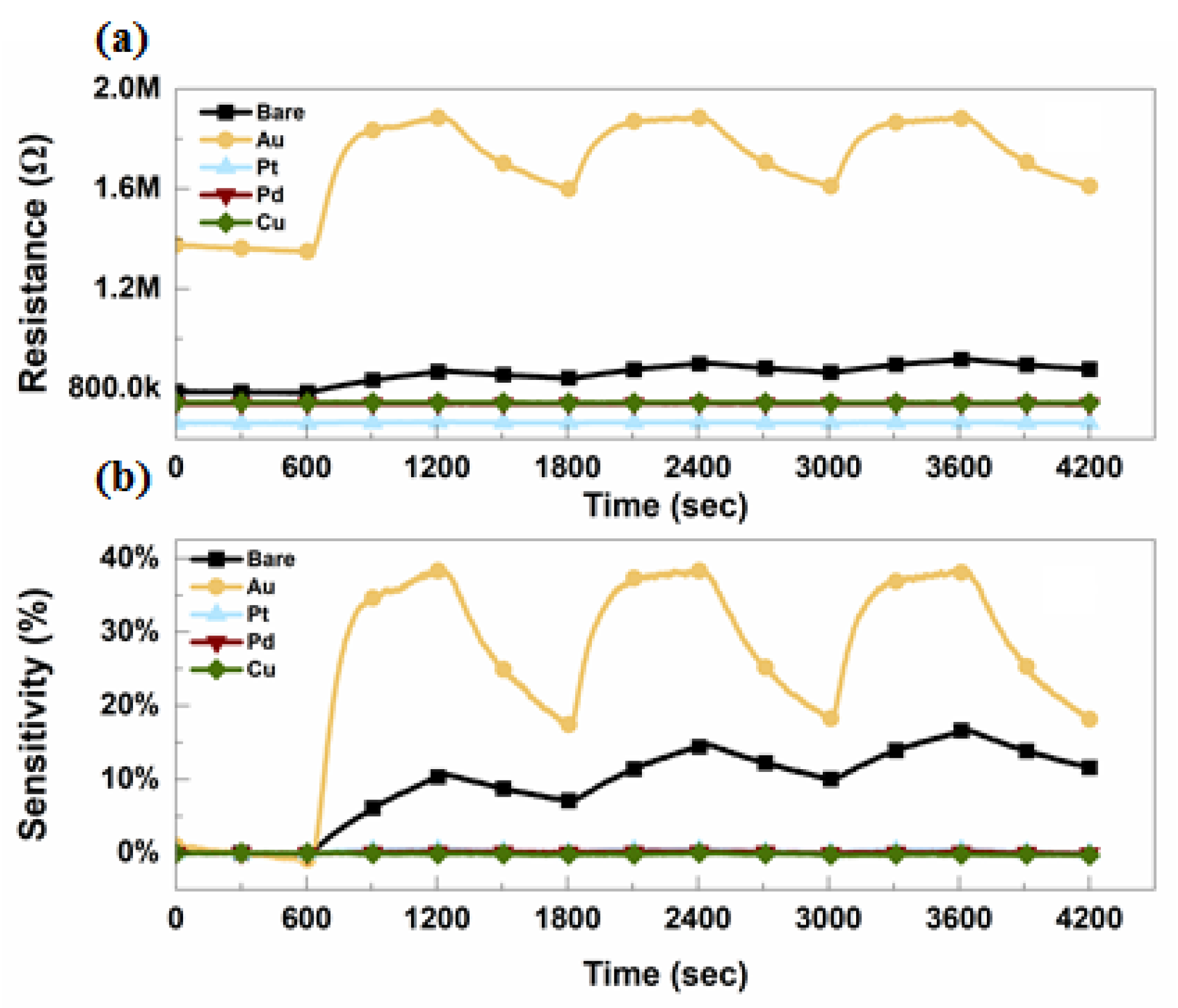
2. Device Fabrication and Characterization
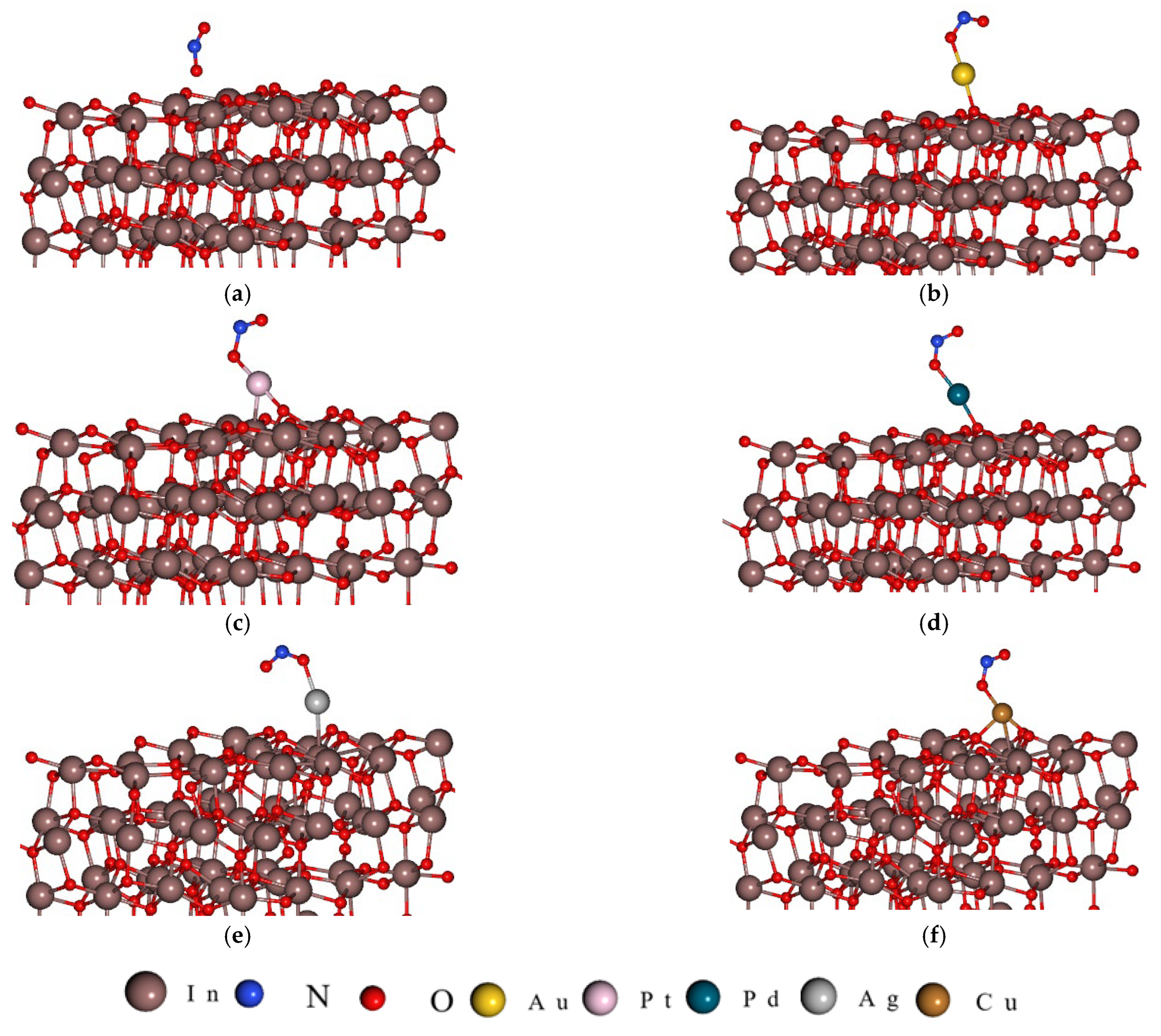
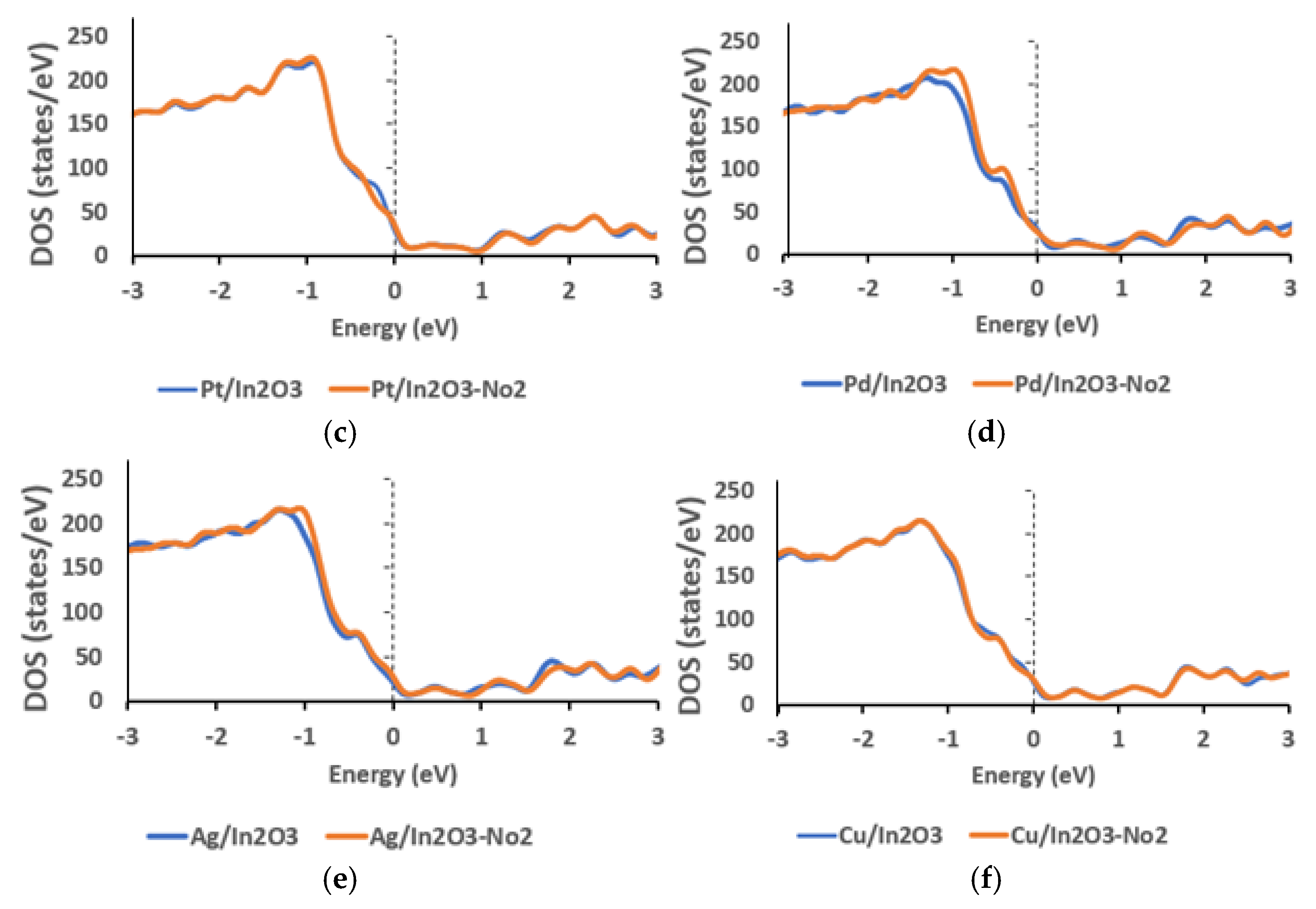
3. DFT Calculation: Results and Discussion
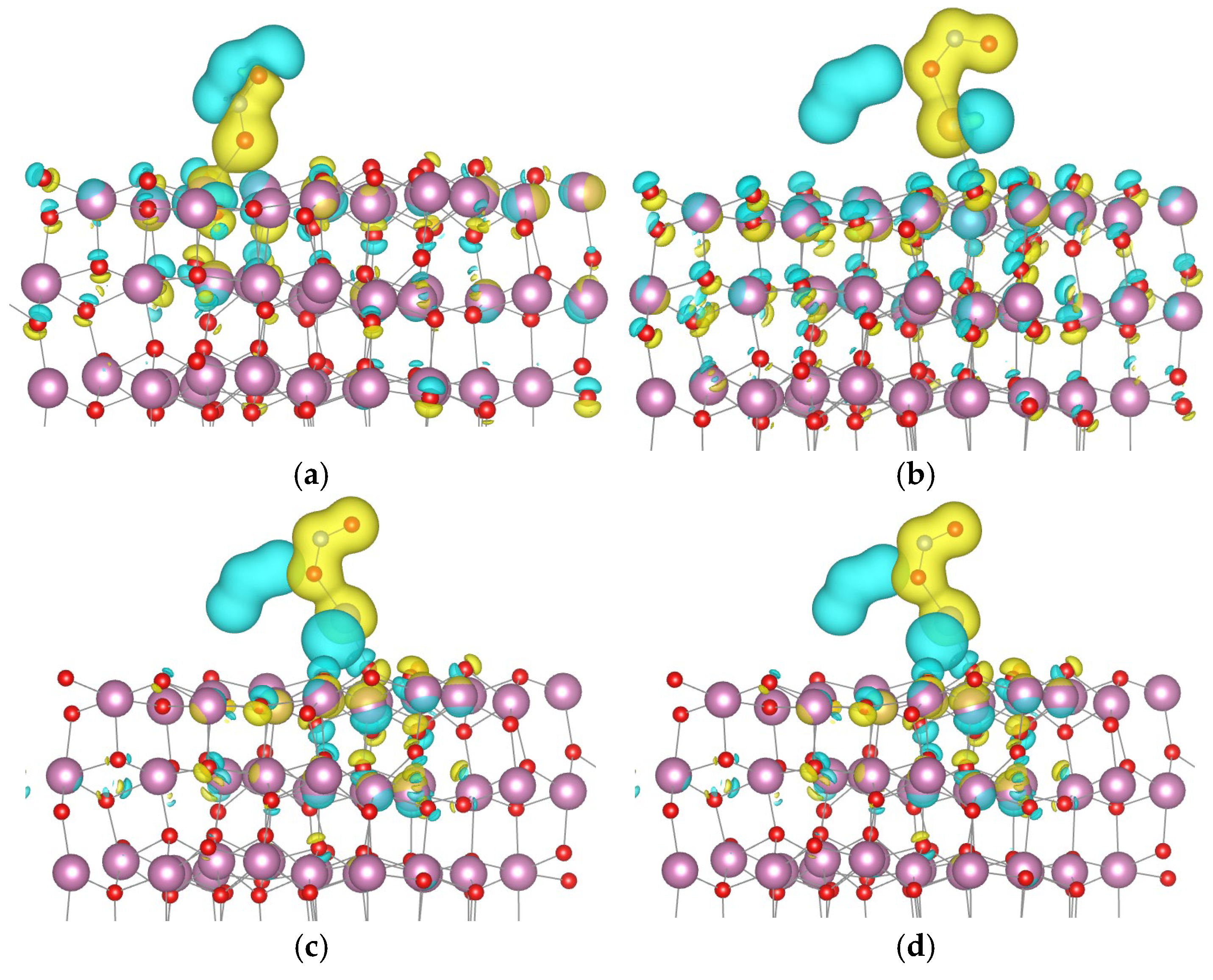
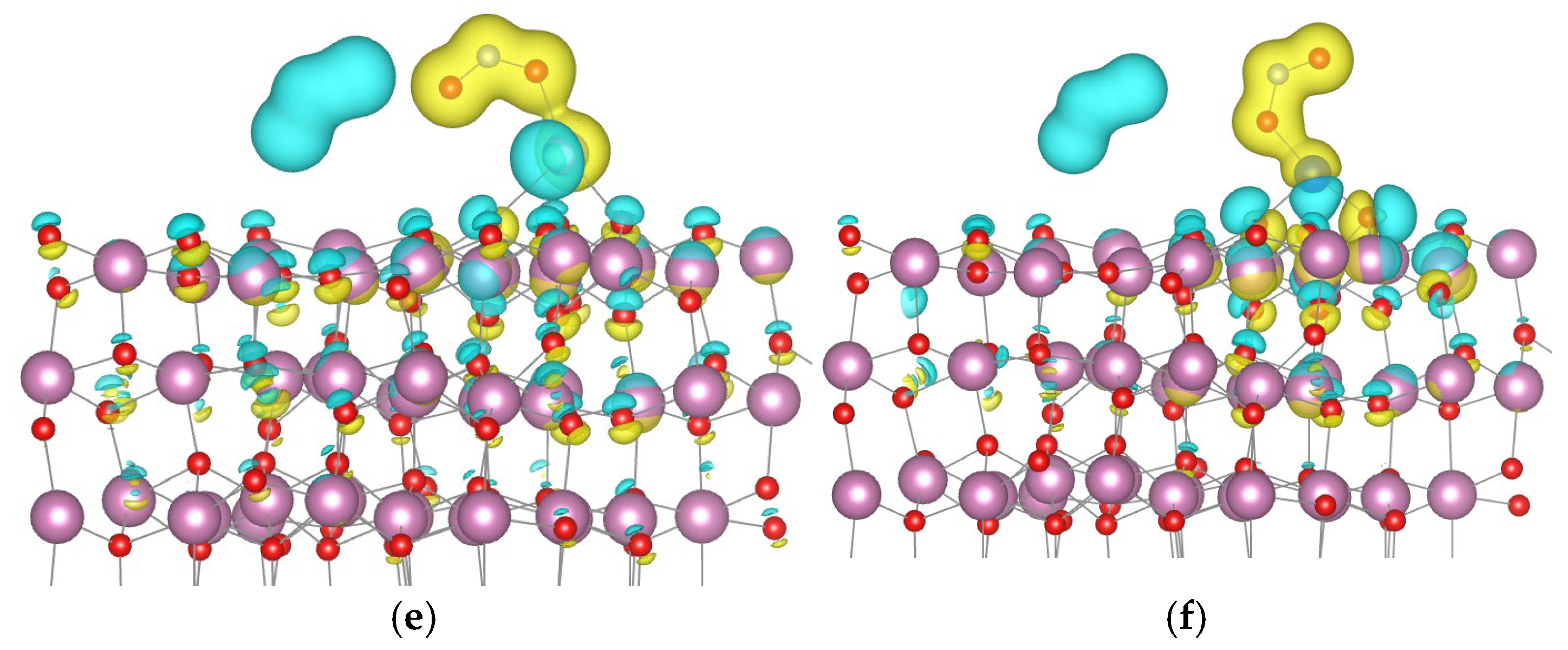
- Au and Pd each bond with one surface oxygen atom.
- Pt forms a bond with one oxygen atom and one indium atom.
- Ag binds to one indium atom.
- Cu establishes bonds with two oxygen atoms and one indium atom.
- Bare In2O3: −0.77 eV
- Au/In2O3: −1.97 eV
- Pt/In2O3: −0.99 eV
- Pd/In2O3: −0.98 eV
- Ag/In2O3: −0.94 eV
- Cu/In2O3: −0.85 eV
- Au/In2O3: 0.23 e
- Pt/In2O3: 0.09 e
- Pd/In2O3: 0.07 e
- Ag/In2O3: 0.10 e
- Cu/In2O3: 0.05 e
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raju, P.; Li, Q. Semiconductor Materials and Devices for Gas Sensors. J. Electrochem. Soc. 2022, 169, 057518. [Google Scholar] [CrossRef]
- He, Y.; Jiao, M. A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors 2024, 12, 55. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Shah, S.; Hussain, S.; Din, S.T.U.; Shahid, A.; Amu-Darko, J.N.O.; Wang, M.; Tianyan, Y.; Liu, G.; Qiao, G. A review on In2O3 nanostructures for gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112538. [Google Scholar] [CrossRef]
- Xuan, J.; Zhao, G.; Sun, M.; Jia, F.; Wang, X.; Zhou, T.; Yin, G.; Liu, B. Low-temperature operating ZnO-based NO2 sensors: A review. RSC Adv. 2020, 10, 39786–39807. [Google Scholar] [CrossRef] [PubMed]
- Khort, A.; Haiduk, Y.; Taratyn, I.; Moskovskikh, D.; Podbolotov, K.; Usenka, A.; Lapchuk, N.; Pankov, V. High-performance selective NO2 gas sensor based on In2O3–graphene–Cu nanocomposites. Sci. Rep. 2023, 13, 7834. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Liu, Q.; Zang, D.; You, R. Visible Light-Activated Room Temperature NO2 Gas Sensing Based on the In2O3@ZnO Heterostructure with a Hollow Microtube Structure. ACS Sens. 2024, 9, 3741–3753. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Luo, Z.; Luo, Z.; Sun, Y. Enhanced NO2 Gas Sensing Properties Based on Rb-Doped ZnO/In2O3 Heterojunctions at Room Temperature: A Combined DFT and Experimental Study. Sensors 2024, 24, 5311. [Google Scholar] [CrossRef]
- Luo, C.; Dong, Y.; Sun, Y.; Huang, Y.; Xue, L.; Ren, L.; Wang, D.; Qin, M.; Li, X. In2O3 and Au Nanoparticles Co-Modified WS2 Microsheets for Enhanced NO2 Gas Sensing at Room Temperature. IEEE Sens. J. 2024, 24, 25291–25298. [Google Scholar] [CrossRef]
- Tan, N.H.; Le, D.T.T.; Hoang, T.T.; Duy, N.M.; Tonezzer, M.; Xuan, C.T.; Van Duy, N.; Hoa, N.D. Metal-decorated indium oxide nanofibers used as nanosensor for triethylamine sensing towards seafood quality monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135268. [Google Scholar] [CrossRef]
- Wu, M.-J.; Wu, Y.-Z.; Tung, Y.-F.; Chang, T.-C.; Lee, C.-T.; Lee, H.-Y. Surface Modification and Decoration of Nano-Honeycombed ZnO Structure with Gold-Black Nanoparticles for High Responsivity Detection of NO2 Gas Sensors. IEEE Sens. J. 2024, 24, 38699–38707. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, W.; Li, Y.; Ma, J.; Li, J.; Liu, Y.; Zou, Y.; Deng, Y. Noble Metal Nanoparticles Decorated Metal Oxide Semiconducting Nanowire Arrays Interwoven into 3D Mesoporous Superstructures for Low-Temperature Gas Sensing. ACS Cent. Sci. 2021, 7, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Xiao, S.; Du, K. In2O3 microtubes decorated with Ag nanoparticles for NO2 gas detection at room temperature. Vacuum 2022, 202, 111197. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Gao, H.; Zhang, R.; Meng, F. Ppb-Level N-Propanol Gas Sensor Based on Ce-Doped Flower-like In2O3 Compounded on rGO Nanosheets. IEEE Trans. Instrum. Meas. 2024, 73, 9517109. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, H.; Li, J.; Meng, F.; Yang, Z.; Zhang, H. Ag Nanoparticle-Modified ZnO–In2O3 Nanocomposites for Low-Temperature Rapid Detection Hydrogen Gas Sensors. IEEE Trans. Instrum. Meas. 2023, 72, 9503712. [Google Scholar] [CrossRef]
- Guo, L.; Zeng, H.; Feng, Y.; Wang, Y.; Zhao, W.; Liu, X. Ar Plasma-Treated In2O3 Microtubes with Rich Oxygen Vacancies, High Surface Area, and Large Pores for Enhanced Formaldehyde Detection. IEEE Sens. J. 2023, 23, 22207–22216. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, J.; Zhu, H.; Zhang, H.; Meng, F. Ag-Modified Co3O4-In2O3 Nanohollow Microspheres for H2 Detection. IEEE Sens. J. 2024, 24, 25299–25307. [Google Scholar] [CrossRef]
- Cai, Z.; Park, J.; Jun, D.; Park, S. High-performance UV-activated room temperature NO2 sensors based on TiO2/In2O3 composite. CrystEngComm 2023, 25, 2546–2556. [Google Scholar] [CrossRef]
- Ilin, A.; Martyshov, M.; Forsh, E.; Forsh, P.; Rumyantseva, M.; Abakumov, A.; Gaskov, A.; Kashkarov, P. UV effect on NO2 sensing properties of nanocrystalline In2O3. Sens. Actuators B Chem. 2016, 231, 491–496. [Google Scholar] [CrossRef]
- Rambeloson, J.; Ioannou, D.E.; Raju, P.; Wang, X.; Motayed, A.; Yun, H.J.; Li, Q. Photoactivated In2O3-GaN Gas Sensors for Monitoring NO2 with High Sensitivity and Ultralow Operating Power at Room Temperature. Chemosensors 2022, 10, 405. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Hou, W.; Zhou, Q.; Zeng, W. Pristine and Ag decorated In2O3 (110): A gas-sensitive material to selective detect NO2 based on DFT study. J. Mater. Res. Technol. 2022, 18, 4236–4247. [Google Scholar] [CrossRef]
- Khalili, S.; Majidi, M.; Bahrami, M.; Roshanaei, M.; Madrakian, T.; Afkhami, A. A portable gas sensor based on In2O3@CuO P–N heterojunction connected via Wi-Fi to a smartphone for real-time carbon monoxide determination. Sci. Rep. 2024, 14, 13594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, S.; Wang, M.; Liu, S.; Liu, G.; Meng, X.; Xu, Z.; Wang, M.; Qiao, G. Electrospun Cu-doped In2O3 hollow nanofibers with enhanced H2S gas sensing performance. J. Adv. Ceram. 2022, 11, 427–442. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Kim, K.; Kim, H. Performance Optimization of Nitrogen Dioxide Gas Sensor Based on Pd-AlGaN/GaN HEMTs by Gate Bias Modulation. Micromachines 2021, 12, 400. [Google Scholar] [CrossRef]
- Almaev, A.V.; Kopyev, V.V.; Novikov, V.A.; Chikiryaka, A.V.; Yakovlev, N.N.; Usseinov, A.B.; Karipbayev, Z.T.; Akilbekov, A.T.; Koishybayeva, Z.K.; Popov, A.I. ITO Thin Films for Low-Resistance Gas Sensors. Materials 2023, 16, 342. [Google Scholar] [CrossRef]
- Hwang, J.; Jung, H.; Shin, H.-S.; Kim, D.-S.; Kim, D.S.; Ju, B.-K.; Chun, M. The Effect of Noble Metals on CO Gas Sensing Properties of In2O3 Nanoparticles. Appl. Sci. 2021, 11, 4903. [Google Scholar] [CrossRef]
- Kumar, R.; Mamta; Kumari, R.; Singh, V.N. SnO2-based NO2 gas sensor with outstanding sensing performance at room temperature. Micromachines 2023, 14, 728. [Google Scholar] [CrossRef]
- Soydan, G.; Ergenc, A.F.; Alpas, A.T.; Solak, N. Development of an NO2 Gas Sensor Based on Laser-Induced Graphene Operating at Room Temperature. Sensors 2024, 24, 3217. [Google Scholar] [CrossRef]
- Jo, S.H.; Rhyu, H.; Park, C.; Kang, M.H.; Yoon, D.H.; Song, W.; Lee, S.S.; Lim, J.; Myung, S. Light-enhanced ultra-sensitive NO2 gas detection using In2O3/MXene-based flexible sensors. Appl. Surf. Sci. 2025, 704, 163448. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Thomson, B.; Yu, J.; Debnath, R.; Motayed, A.; Rao, M.V. Scalable Metal Oxide Functionalized GaN Nanowire for Precise SO2 Detection. Sens. Actuators B Chem. 2020, 318, 128223. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Enhanced NO2-Sensing Properties of Au-Loaded Porous In2O3 Gas Sensors at Low Operating Temperatures. Chemosensors 2020, 8, 72. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Xie, G.; Yao, M.; Pan, H.; Du, H.; Tai, H.; Du, X.; Su, Y. Enhancing Visible Light-Activated NO2 Sensing Properties of Au NPs Decorated ZnO Nanorods by Localized Surface Plasmon Resonance and Oxygen Vacancies. Mater. Res. Express 2020, 7, 015924. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.F. A scaled conjugate gradient algorithm for fast supervised learning. Neural Netw. 1993, 6, 525–533. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, P.P. SF6 decomposed gas sensing performance of van der Waals layered cobalt oxyhydroxide: Insights from a computational study. J. Mol. Model. 2021, 27, 158. [Google Scholar] [CrossRef]
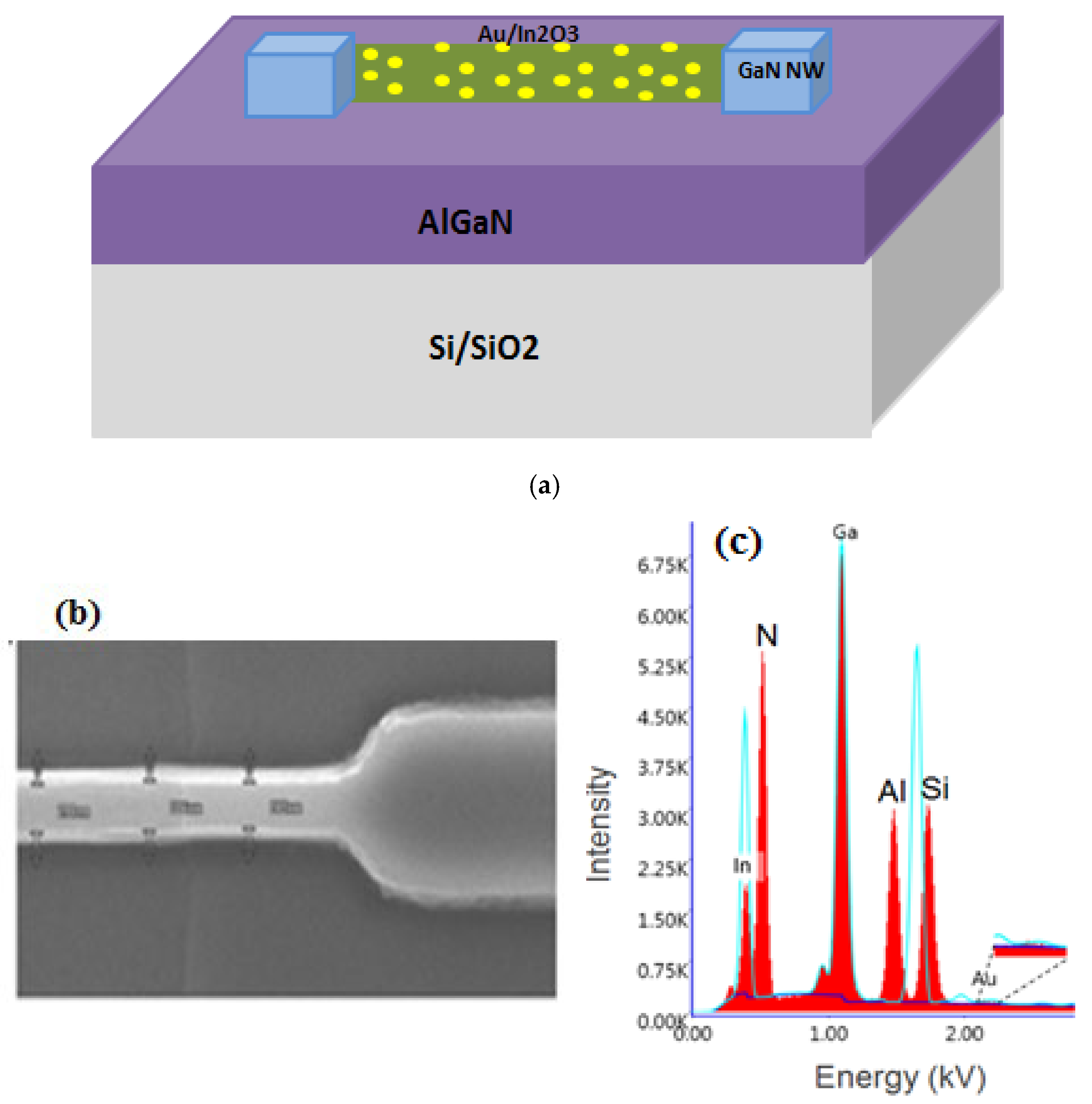
| In2O3 | Au/In2O3 | |||
|---|---|---|---|---|
| Elements | Weight % | Atomic % | Weight % | Atomic % |
| N | 31.84 | 69.93 | 33.73 | 71.73 |
| Ga | 68.16 | 30.07 | 66.15 | 28.26 |
| Au | 0 | 0 | 0.11 | 0.02 |
| Model | Bond Length (O-N) Å | Bond Angle (O-N-O) Deg | Adsorption Energy (ev) | Charge Transfer (e) | Distance (Å) |
|---|---|---|---|---|---|
| In2O3–NO2 | 1.21, 1.28 | 121.46 | −0.77 | 0.03 | 2.86 |
| Au/In2O3–NO2 | 1.22, 1.3 | 119.72 | −1.97 | 0.23 | 2.06 |
| Ag/In2O3–NO2 | 1.22, 1.26 | 124.34 | −0.94 | 0.1 | 2.17 |
| Pt/In2O3–NO2 | 1.19, 1.42 | 115.02 | −0.99 | 0.09 | 1.95 |
| Pd/In2O3–NO2 | 1.22, 1.28 | 124.07 | −0.98 | 0.07 | 1.99 |
| Cu/In2O3–NO2 | 1.21, 1.29 | 122.17 | −0.85 | 0.05 | 1.89 |
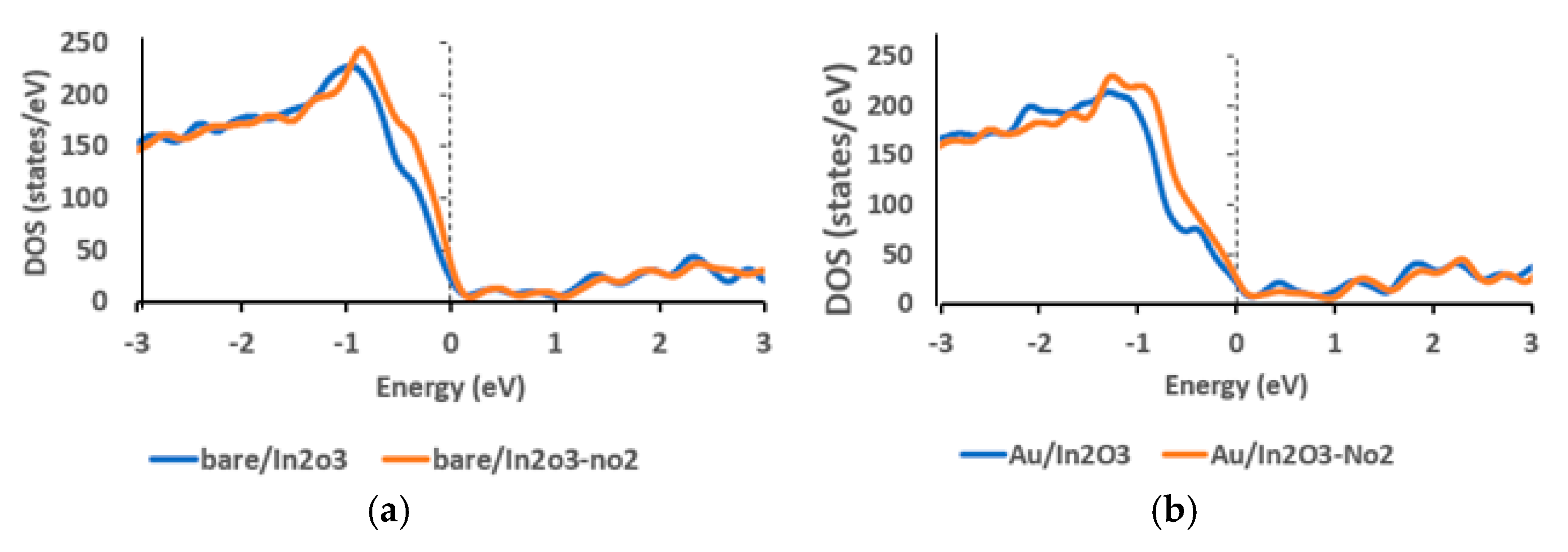
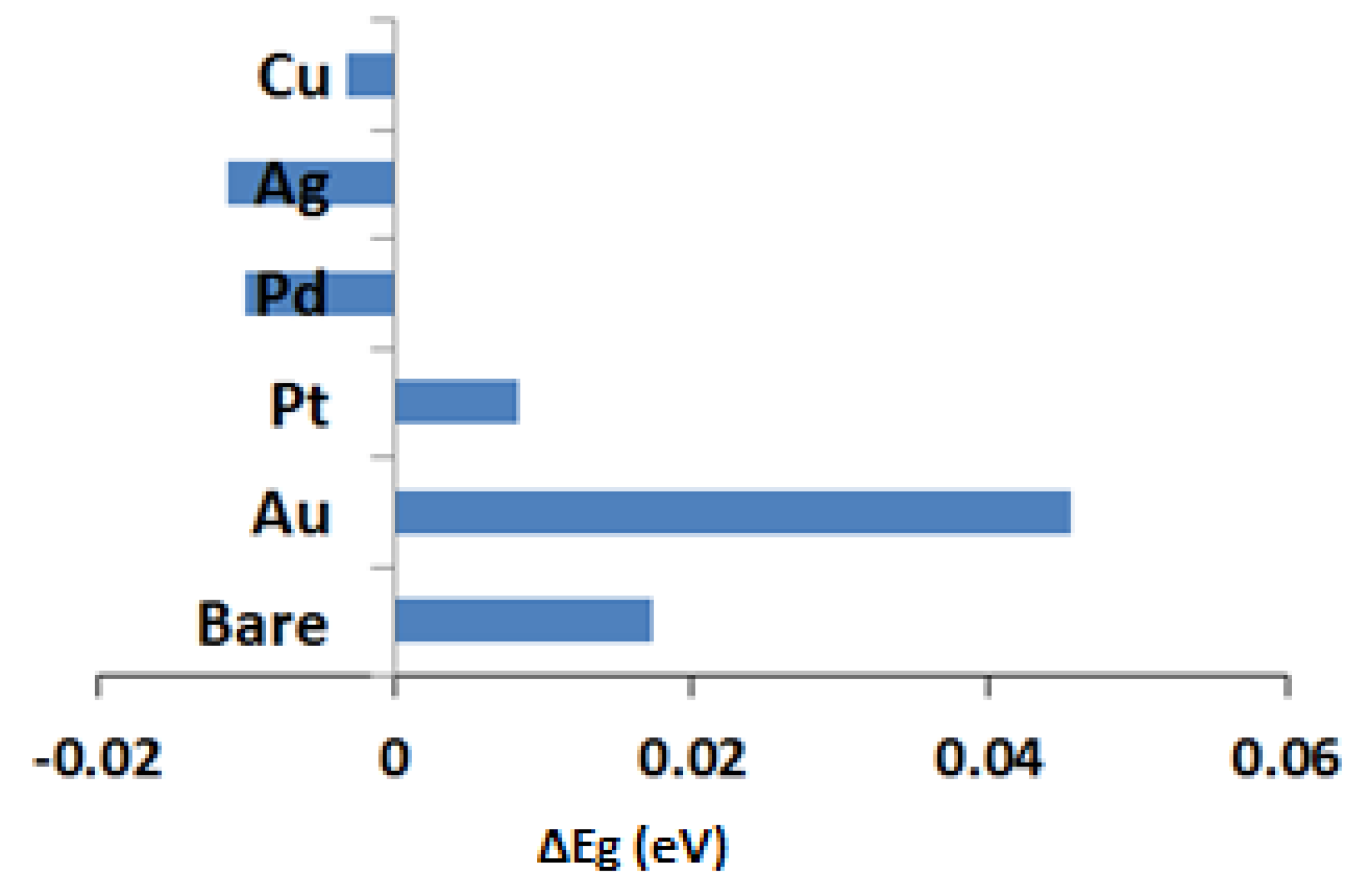
| Structure | Band Gap (eV) |
|---|---|
| Bare/In2O3 | 0.8725 |
| Bare/In2O3–NO2 | 0.8899 |
| Au/In2O3 | 0.77 |
| Au/In2O3–NO2 | 0.8155 |
| Ag/In2O3 | 0.8361 |
| Ag/In2O3–NO2 | 0.8248 |
| Pt/In2O3 | 0.8112 |
| Pt/In2O3–NO2 | 0.8196 |
| Pd/In2O3 | 0.8294 |
| Pd/In2O3–NO2 | 0.8194 |
| Cu/In2O3 | 0.8534 |
| Cu/In2O3–NO2 | 0.8503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, P.; Rambeloson, J.; Ioannou, D.E.; Motayed, A.; Li, Q. Noble Metal-Decorated In2O3 for NO2 Gas Sensor: An Experimental and DFT Study. Chemosensors 2025, 13, 350. https://doi.org/10.3390/chemosensors13090350
Raju P, Rambeloson J, Ioannou DE, Motayed A, Li Q. Noble Metal-Decorated In2O3 for NO2 Gas Sensor: An Experimental and DFT Study. Chemosensors. 2025; 13(9):350. https://doi.org/10.3390/chemosensors13090350
Chicago/Turabian StyleRaju, Parameswari, Jafetra Rambeloson, Dimitris E. Ioannou, Abhishek Motayed, and Qiliang Li. 2025. "Noble Metal-Decorated In2O3 for NO2 Gas Sensor: An Experimental and DFT Study" Chemosensors 13, no. 9: 350. https://doi.org/10.3390/chemosensors13090350
APA StyleRaju, P., Rambeloson, J., Ioannou, D. E., Motayed, A., & Li, Q. (2025). Noble Metal-Decorated In2O3 for NO2 Gas Sensor: An Experimental and DFT Study. Chemosensors, 13(9), 350. https://doi.org/10.3390/chemosensors13090350





