Research Progress on Biomarkers and Their Detection Methods for Benzene-Induced Toxicity: A Review
Abstract
1. Introduction
2. Biomarkers of Benzene Exposure
2.1. Urinary Biomarkers
2.2. Blood Biomarkers
2.3. Exhaled Air Biomarkers
3. Biomarkers of Benzene Toxicity Effects
3.1. Oxidative Stress Biomarkers
3.2. Genetic Damage Biomarkers
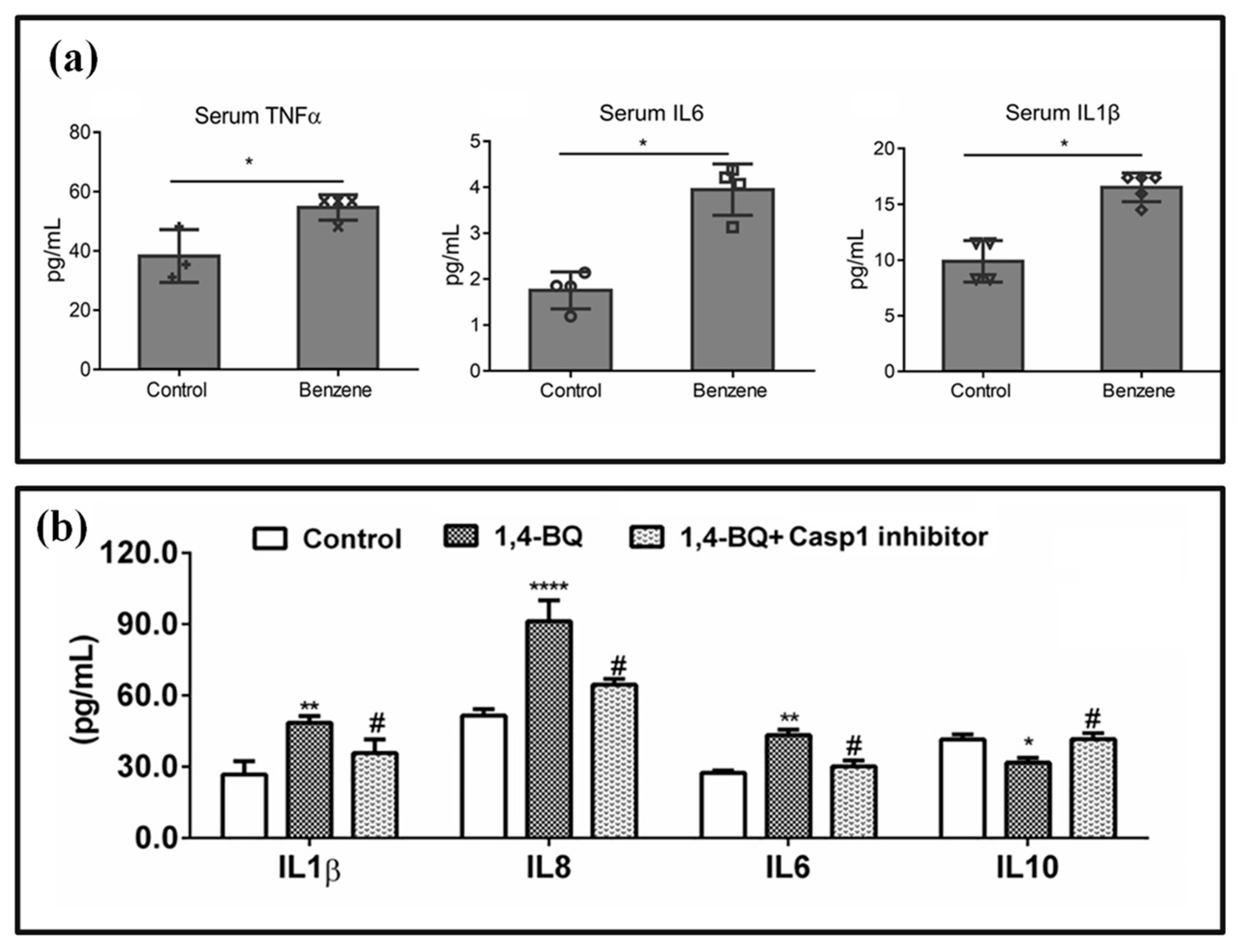
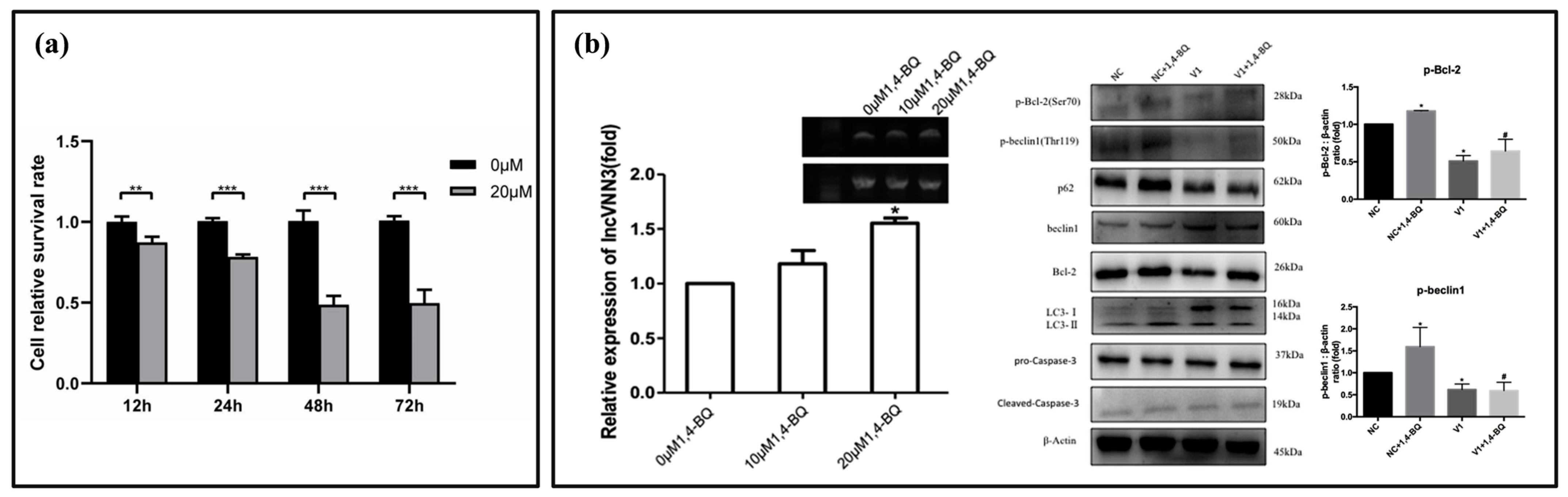
3.3. Biomarkers Related to Programmed Cell Death (PCD)
4. Detection Methods for Benzene Poisoning
4.1. Biomarker Selection
4.2. Detection Methods for Benzene in Blood, Exhaled Air, and Urine
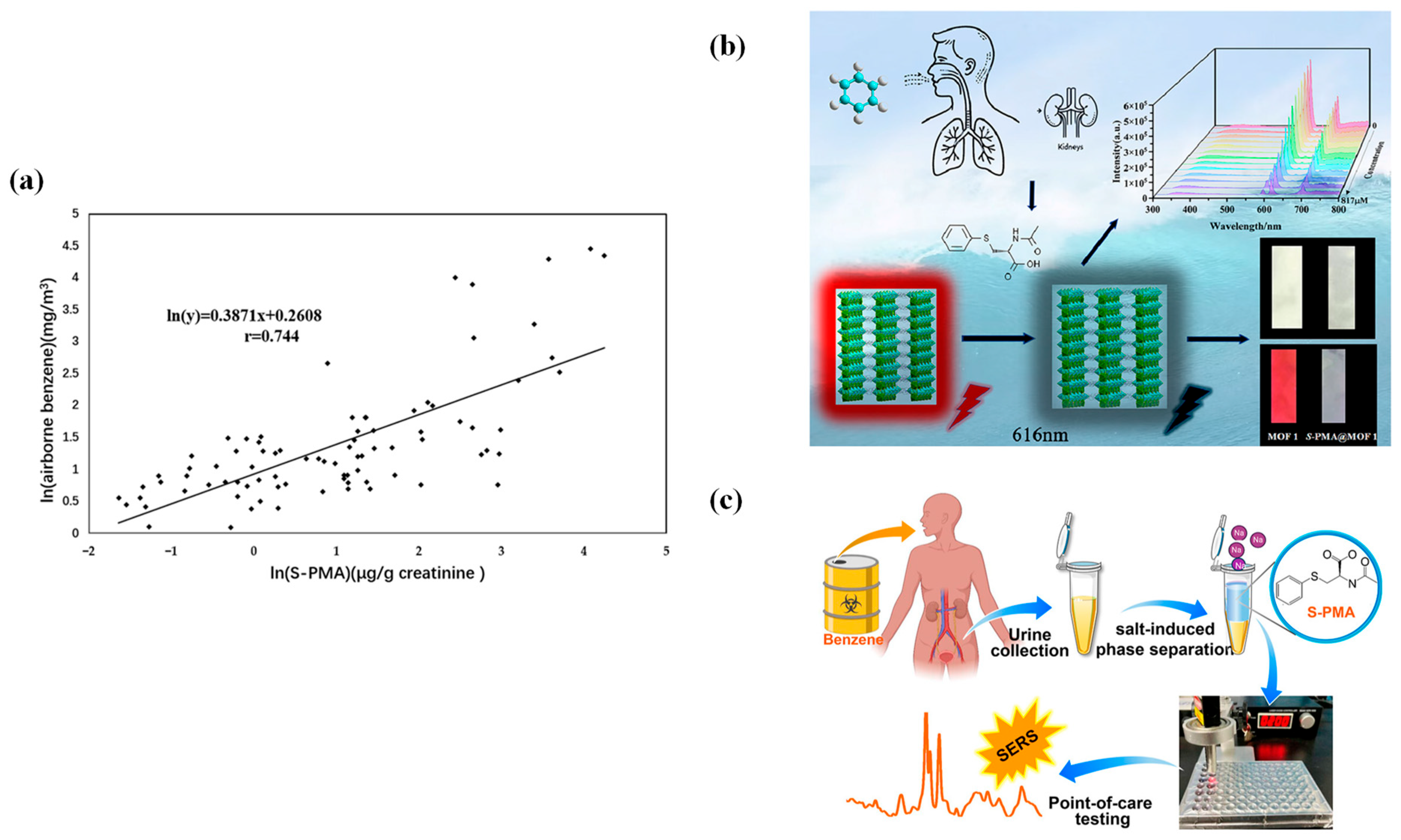
4.3. Trans, Trans Muconic Acid (t,t-MA)

4.4. S-Phenylmercapturic Acid (S-PMA)
4.5. Hippuric Acid and Methylhippuric Acid (HA and MHA)
4.6. Immunological Markers
4.7. Oxidative Stress Markers
5. Conclusions and Recommendations
Funding
Conflicts of Interest
Abbreviations
| ·O2− | superoxide radicals |
| 1,4-BQ | 1,4-Benzoquinone |
| 2-MHA | 2-methylhippuric acid |
| 3-MHA | 3-methylhippuric acid |
| 4-MHA | 4-methylhippuric acid |
| 8-OHdG | 8-hydroxydeoxyguanine nucleoside |
| AA | ascorbic acid |
| AC/DBMNs | activated carbon/diatomite-based materials |
| ACGIH | American Conference of Governmental Industrial Hygienists |
| ACSL1 | acyl coA synthetase long-chain family member 1 |
| AHR | aryl hydrocarbon receptor |
| AM | amaranth |
| BCABL | back-calculated airborne benzene levels |
| Bd | benzidine |
| BEIs | biological exposure indices |
| BSA | bovine serum albumin |
| CL | chemiluminescence |
| COF | covalent organic framework |
| COF-MEPS | covalent organic framework-microextraction by packed sorbent |
| CPT | cationic polythiophene |
| CRP | C-reactive protein |
| CYP450 | cytochrome P450 enzymes |
| DA | dopamine |
| DHGC | dynamic headspace chromatography |
| DHGC-FID | direct headspace gas chromatography with flame ionization detection |
| DI | benzene intake |
| DLLME | dispersive liquid–liquid microextraction |
| DLLME-SFOD | dispersive liquid–liquid microextraction–solidification of floating organic droplet |
| DLLME-SPE | dispersive liquid–liquid microextraction solid-phase extraction |
| DNMTs | DNA methyltransferases |
| DPV | differential pulse voltammetry |
| DTT | 1,4-Dithiothreitol |
| EA | ellagic acid |
| EIS | electrochemical impedance spectroscopy |
| ELISA | enzyme-linked immunosorbent assay |
| FID | flame ionization detector |
| GC | gas chromatography |
| GC-MS | gas chromatography-mass spectrometry |
| GC-MS/MS | gas chromatography-tandem mass spectrometry |
| GNP | gold nanoparticles |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GST | glutathione S-transferase |
| HA | hippuric acid |
| HAP-TiO2 | hydroxyapatite-titanium dioxide |
| HBROEL | health-based recommended OEL |
| hMeDIP | hydroxymethylated DNA immunoprecipitation |
| HMOF | heterometal-organic framework |
| HPLC | high-performance liquid chromatography |
| HPLC-MS/MS | high-performance liquid chromatography-tandem mass spectrometry |
| HPLC-UV | high-performance liquid chromatography with ultraviolet detector |
| HQ | hydroquinone |
| HS-SPME | headspace solid-phase microextraction |
| HS-SPME-GC-MS | headspace solid-phase microextraction-gas chromatography-mass spectrometry |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| ILs | ionic liquids |
| LC/MS | liquid chromatography-mass spectrometry |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| LOD | low limit of detection |
| MEPS | microextraction by packed sorbent |
| MHA | methylhippuric acid |
| MIP | molecular imprinting polymer |
| MISPE | molecular imprinting solid-phase extraction |
| MRI | magnetic resonance imaging |
| MS | mass spectrometry |
| MSP | methylation-specific PCR |
| MSPE | magnetic solid-phase extraction |
| MWCNT-COOH | multi-walled carbon nanotubes carboxylic acid |
| n-DEP | negative dielectrophoresis |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OEL | occupational exposure limit |
| PCD | programmed cell death |
| PAH | para-aminobenzoic acid |
| PEG | polyethylene glycol |
| POCT | point-of-care testing |
| pre-SPMA | pre-S-phenylmercapturic acid |
| PTB | photothermal biosensor |
| Pt-RTD | platinum resistance temperature detector |
| QCL | quantum cascade laser |
| qPCR | real-time quantitative PCR |
| RBCs | red blood cells |
| ROS | reactive oxygen species |
| SERS | surface-enhanced Raman spectroscopy |
| SFOD | solidification of floating organic droplet |
| SIPS | salt-induced phase separation |
| SOD | superoxide dismutase |
| S-PMA | S-Phenylmercapturic acid |
| SPME | solid-phase microextraction |
| t,t-MA | t,t-muconic acid |
| TFPA | tris (4-formyl phenyl) amine |
| UA | uric acid |
| UPLC-MS/MS | ultra-high-performance liquid chromatography-tandem mass spectrometry |
| UV-VIS | ultraviolet-visible spectrophotometry |
| WBCs | white blood cells |
| ZIF | zeolitic imidazolate framework |
References
- Cao, Y.; Wang, T.; Xi, J.; Tian, W.; Liu, W.; Sun, Y.; Liu, W.; You, X.; Li, A.; Zhang, G.; et al. Benchmark dose estimation for benzene-exposed workers in China: Based on quantitative and multi-endpoint genotoxicity assessments. Environ. Pollut. 2023, 330, 121765. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, H.-S.; Lim, D.-S.; Kim, K.-Y. Benzene exposure assessment of printing workers treating petroleum-based cleaner in South Korea. Ind. Health 2023, 61, 283–290. [Google Scholar] [CrossRef]
- Wan, W.; Peters, S.; Portengen, L.; Olsson, A.; Schüz, J.; Ahrens, W.; Schejbalova, M.; Boffetta, P.; Behrens, T.; Brüning, T. Occupational benzene exposure and lung cancer risk: A pooled analysis of 14 case-control studies. Am. J. Respir. Crit. Care Med. 2024, 209, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Talibov, M.; Sormunen, J.; Hansen, J.; Kjaerheim, K.; Martinsen, J.-I.; Sparen, P.; Tryggvadottir, L.; Weiderpass, E.; Pukkala, E. Benzene exposure at workplace and risk of colorectal cancer in four Nordic countries. Cancer Epidemiol. 2018, 55, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Lupatsch, J.E.; Huss, A.; Rischewski, J.; Schindera, C.; Spoerri, A.; Vermeulen, R.; Kuehni, C.E.; The Swiss Paediatric Oncology Group; The Swiss National Cohort Study Group. Parental occupational exposure to benzene and the risk of childhood cancer: A census-based cohort study. Environ. Int. 2017, 108, 84–91. [Google Scholar] [CrossRef]
- Werder, E.J.; Engel, L.S.; Blair, A.; Kwok, R.K.; McGrath, J.A.; Sandler, D.P. Blood BTEX levels and neurologic symptoms in Gulf states residents. Environ. Res. 2019, 175, 100–107. [Google Scholar] [CrossRef]
- Thetkathuek, A.; Polyong, C.P.; Jaidee, W. Benzene health risk assessment for neurological disorders of gas station employees in Rayong Province, Thailand. Rocz. Państwowego Zakładu Hig. 2023, 74, 231–241. [Google Scholar]
- Hu, J.; Yu, E.; Liao, Z. Changes in cognitive function and related brain regions in chronic benzene poisoning: A case report. Ann. Transl. Med. 2021, 9, 81. [Google Scholar] [CrossRef]
- Wang, D.; Nie, L.; Shao, X.; Yu, H. Exposure profile of volatile organic compounds receptor associated with paints consumption. Sci. Total Environ. 2017, 603, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Crossin, R.; Qama, A.; Andrews, Z.B.; Lawrence, A.J.; Duncan, J.R. The effect of adolescent inhalant abuse on energy balance and growth. Pharmacol. Res. Perspect. 2019, 7, e00498. [Google Scholar] [CrossRef]
- Wang, D.; Lin, D.; Yang, X.; Wu, D.; Li, P.; Zhang, Z.; Zhang, W.; Guo, Y.; Fu, S.; Zhang, N. Alterations in leukocyte telomere length and mitochondrial DNA copy number in benzene poisoning patients. Mol. Biol. Rep. 2024, 51, 309. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.C.; Wang, T.; Wu, H.; Zhang, G.H.; Sun, D.; Guo, K.; Li, H.; Zhang, F.; Wu, W.; Xia, Z.; et al. Promoter hypermethylation in CSF3R induces peripheral neutrophil reduction in benzene-exposure poisoning. Environ. Mol. Mutagen. 2020, 61, 786–796. [Google Scholar] [CrossRef]
- Jin, Y.S.; Yi, Z.C.; Zhang, Y.J.; Long, R.; Yu, C.H. Proteomics Study of Benzene Metabolite Hydroquinone Induced Hematotoxicity in K562 Cells. Biomed. Environ. Sci. 2024, 37, 341–353. [Google Scholar] [PubMed]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Benzene and childhood acute leukemia in Oklahoma. Environ. Res. 2017, 158, 167–173. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, Y.; Ma, R.; Zhang, Z.; Ji, P.; Xiao, M.; Du, R.; Liu, X.; Cui, Y.; Xing, X. Risk assessment and dose-effect of co-exposure to benzene, toluene, ethylbenzene, xylene, and styrene (BTEXS) on pulmonary function: A cross-sectional study. Environ. Pollut. 2022, 310, 119894. [Google Scholar] [CrossRef]
- Weaver, C.; Liu, S.-P.; Lu, J.-F.; Lin, B.-S. The effects of benzene exposure on apoptosis in epithelial lung cells: Localization by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) and the immunocytochemical localization of apoptosis-related gene products. Cell Biol. Toxicol. 2007, 23, 201–220. [Google Scholar] [CrossRef]
- Warden, H.; Richardson, H.; Richardson, L.; Siemiatycki, J.; Ho, V. Associations between occupational exposure to benzene, toluene and xylene and risk of lung cancer in Montréal. Occup. Environ. Med. 2018, 75, 696–702. [Google Scholar] [CrossRef]
- Meo, S.A.; Alrashed, A.H.; Almana, A.A.; Altheiban, Y.I.; Aldosari, M.S.; Almudarra, N.F.; Alwabel, S.A. Lung function and fractional exhaled nitric oxide among petroleum refinery workers. J. Occup. Med. Toxicol. 2015, 10, 37. [Google Scholar] [CrossRef]
- Wichmann, F.A.; Müller, A.; Busi, L.E.; Cianni, N.; Massolo, L.; Schlink, U.; Porta, A.; Sly, P.D. Increased asthma and respiratory symptoms in children exposed to petrochemical pollution. J. Allergy Clin. Immunol. 2009, 123, 632–638. [Google Scholar] [CrossRef]
- Sauer, E.; Gauer, B.; Nascimento, S.; Nardi, J.; Göethel, G.; Costa, B.; Correia, D.; Matte, U.; Charão, M.; Arbo, M. The role of B7 costimulation in benzene immunotoxicity and its potential association with cancer risk. Environ. Res. 2018, 166, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hanke, J.; Dutkiewicz, T.; Piotrowski, J. The absorption of benzene through human skin. Int. J. Occup. Environ. Health 2000, 6, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Frasch, H.F.; Barbero, A.M. In vitro human skin permeation of benzene in gasoline: Effects of concentration, multiple dosing and skin preparation. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, J.; Zhang, L.; Jing, J.; Zhang, W.; Liu, Z.; Gao, A. The role of N6-methyladenosine modification in benzene-induced testicular damage and the protective effect of melatonin. Chemosphere 2023, 319, 138035. [Google Scholar] [CrossRef]
- Mottola, F.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. In vitro combination of ascorbic and ellagic acids in sperm oxidative damage inhibition. Int. J. Mol. Sci. 2022, 23, 14751. [Google Scholar] [CrossRef]
- Marchetti, F.; Eskenazi, B.; Weldon, R.H.; Li, G.; Zhang, L.; Rappaport, S.M.; Schmid, T.E.; Xing, C.; Kurtovich, E.; Wyrobek, A.J. Occupational exposure to benzene and chromosomal structural aberrations in the sperm of Chinese men. Environ. Health Perspect. 2012, 120, 229–234. [Google Scholar] [CrossRef]
- Anigilaje, E.A.; Nasir, Z.A.; Walton, C. Exposure to benzene, toluene, ethylbenzene, and xylene (BTEX) at Nigeria’s petrol stations: A review of current status, challenges and future directions. Front. Public Health 2024, 12, 1295758. [Google Scholar] [CrossRef]
- Reutman, S.R.; LeMasters, G.K.; Knecht, E.A.; Shukla, R.; Lockey, J.E.; Burroughs, G.E.; Kesner, J.S. Evidence of reproductive endocrine effects in women with occupational fuel and solvent exposures. Environ. Health Perspect. 2002, 110, 805–811. [Google Scholar] [CrossRef]
- Lupo, P.J.; Symanski, E.; Waller, D.K.; Chan, W.; Langlois, P.H.; Canfield, M.A.; Mitchell, L.E. Maternal exposure to ambient levels of benzene and neural tube defects among offspring: Texas, 1999–2004. Environ. Health Perspect. 2011, 119, 397–402. [Google Scholar] [CrossRef]
- Yusoff, N.A.; Abd Hamid, Z.; Budin, S.B.; Taib, I.S. Linking benzene, in utero carcinogenicity and fetal hematopoietic stem cell niches: A mechanistic review. Int. J. Mol. Sci. 2023, 24, 6335. [Google Scholar] [CrossRef]
- Binsaleh, N.; Eltayeb, R.; Bashir, E.; Idris, H.; Althobiti, M.; Ahmed, H.; Khan, M.; Qanash, H. Insight into hematological parameters of petrol station workers. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3135–3143. [Google Scholar] [PubMed]
- Sharma, S.; Rana, S.V.S. Melatonin improves liver function in benzene-treated rats. Arh. Za Hig. Rada I Toksikol. 2013, 64, 219–226. [Google Scholar] [CrossRef]
- Pérez-Herrera, N.; de León-Martínez, L.D.; Flores-Ramírez, R.; Barbier, O.; Ortega-Romero, M.; May-Euán, F.; Saldaña-Villanueva, K.; Perera-Rios, J.; Pérez-Vázquez, F.J. Evaluation of benzene exposure and early biomarkers of kidney damage in children exposed to solvents due to precarious work in Ticul, Yucatán, México. Ann. Glob. Health 2019, 85, 94. [Google Scholar] [CrossRef]
- Abd El-Shakour, A.; El-Ebiarie, A.S.; Ibrahim, Y.H.; Moneim, A.E.A.; El-Mekawy, A.M. Effect of benzene on oxidative stress and the functions of liver and kidney in rats. J. Environ. Occup. Health 2015, 4, 34–39. [Google Scholar]
- Boogaard, P.J. Human biomonitoring of low-level benzene exposures. Crit. Rev. Toxicol. 2022, 52, 799–810. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Peng, Z.J.; Cao, J.; Bao, J.M.; Li, L.; Wang, X.Z.; Ji, Y.Y.; Chen, Z.J. Meta-analysis of the effect of low-level occupational benzene exposure on human peripheral blood leukocyte counts in China. J. Environ. Sci. 2022, 114, 204–210. [Google Scholar] [CrossRef]
- Li, H.; Sun, Q.; Li, F.; Wang, B.; Zhu, B. Metabolomics of benzene exposure and development of biomarkers for exposure hazard assessment. Metabolites 2024, 14, 377. [Google Scholar] [CrossRef]
- Kivistö, H.; Pekari, K.; Peltonen, K.; Svinhufvud, J.; Veidebaum, T.; Sorsa, M.; Aitio, A. Biological monitoring of exposure to benzene in the production of benzene and in a cokery. Sci. Total Environ. 1997, 199, 49–63. [Google Scholar] [CrossRef]
- Thetkathuek, A.; Polyong, C.P.; Jaidee, W.; Sirivarasai, J. Comparison of urinary biomarkers concentrations in exposed and non-exposed petrol station workers in the Eastern Economic Corridor (EEC), Thailand. Rocz. Państwowego Zakładu Hig. 2022, 73, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Rahimpoor, R.; Jalilian, H.; Mohammadi, H.; Rahmani, A. Biological exposure indices of occupational exposure to benzene: A systematic review. Heliyon 2023, 9, e21576. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 2E1 and its roles in disease. Chem.-Biol. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Qu, X.-L.; Yan, B. Covalent-coordination tandem functionalization of a metal–organic framework (UiO-66) as a hybrid probe for luminescence detection of trans, trans-muconic acid as a biomarker of benzene and Fe3+. Analyst 2021, 146, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.; Renner, T.; Meger, M. Analysis and evaluation of trans, trans-muconic acid as a biomarker for benzene exposure. J. Chromatogr. B Biomed. Sci. Appl. 1998, 717, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Tocco, M.G.; Ibba, A.; Scano, L.; Ennas, M.G.; Flore, C.; Randaccio, F.S. trans, trans-Muconic acid excretion in relation to environmental exposure to benzene. Int. Arch. Occup. Environ. Health 2003, 76, 456–460. [Google Scholar] [CrossRef]
- Weaver, V.M.; Buckley, T.; Groopman, J.D. Lack of specificity of trans, trans-muconic acid as a benzene biomarker after ingestion of sorbic acid-preserved foods. Cancer Epidemiol. Biomark. Prev. 2000, 9, 749–755. [Google Scholar]
- Tevis, D.S.; Willmore, A.; Bhandari, D.; Bowman, B.; Biren, C.; Kenwood, B.M.; Jacob, P.; Liu, J.; Bello, K.; Hecht, S.S. Large differences in urinary benzene metabolite S-phenylmercapturic acid quantitation: A comparison of five LC–MS-MS methods. J. Anal. Toxicol. 2021, 45, 657–665. [Google Scholar] [CrossRef]
- Dougherty, D.; Garte, S.; Barchowsky, A.; Zmuda, J.; Taioli, E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure—A literature review. Toxicol. Lett. 2008, 182, 7–17. [Google Scholar] [CrossRef]
- Jalai, A.; Ramezani, Z.; Ebrahim, K. Urinary trans, trans-muconic acid is not a reliable biomarker for low-level environmental and occupational benzene exposures. Saf. Health Work 2017, 8, 220–225. [Google Scholar] [CrossRef]
- Wang, T.; Cao, Y.; Xia, Z.; Christiani, D.C.; Au, W.W. Review on novel toxicological effects and personalized health hazard in workers exposed to low doses of benzene. Arch. Toxicol. 2024, 98, 365–374. [Google Scholar] [CrossRef]
- Kebamo, T.E.; Yemane, T.; Arkew, M.; Walano, G.A.; Tantu, A.; Abose, A.; Haile, K.; Bawore, S.G.; Kiya, G.T. Hematological Parameters of Gasoline Station Workers at Hosanna Town, Southwest Ethiopia: A Comparative Cross-Sectional Study. J. Blood Med. 2024, 15, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Elkama, A.; Şentürk, K.; Karahalil, B. Assessment of genotoxicity biomarkers in gasoline station attendants due to occupational exposure. Toxicol. Ind. Health 2024, 40, 337–351. [Google Scholar] [CrossRef]
- Giardini, I.; da Poça, K.S.; da Silva, P.V.B.; Andrade Silva, V.J.C.; Cintra, D.S.; Friedrich, K.; Geraldino, B.R.; Otero, U.B.; Sarpa, M. Hematological Changes in Gas Station Workers. Int. J. Environ. Res. Public Health 2023, 20, 5896. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Moradabadi, A.; Abdollahdokht, D.; Mehrabani, M.; Nematollahi, M.H. Association of environmental exposure with hematological and oxidative stress alteration in gasoline station attendants. Environ. Sci. Pollut. Res. 2019, 26, 20411–20417. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Huang, J.; Pu, Y.; Liang, G.; Yin, L.; Zhang, J.; Sun, R.; Pu, Y. Characterization of lymphocyte subsets and intestinal short-chain fatty acids in benzene-induced immunosuppressive mice. Environ. Sci. Pollut. Res. 2023, 30, 60907–60919. [Google Scholar] [CrossRef]
- Kirkeleit, J.; Ulvestad, E.; Riise, T.; Bråtveit, M.; Moen, B.E. Acute suppression of serum IgM and IgA in tank workers exposed to benzene. Scand. J. Immunol. 2006, 64, 690–698. [Google Scholar] [CrossRef]
- Li, P.; Wu, Y.; Zhang, Z.; Lin, D.; Wang, D.; Huang, X.; Zhang, Y. Proteomics analysis identified serum biomarkers for occupational benzene exposure and chronic benzene poisoning. Medicine 2019, 98, e16117. [Google Scholar] [CrossRef] [PubMed]
- Egeghy, P.P.; Tornero-Velez, R.; Rappaport, S.M. Environmental and biological monitoring of benzene during self-service automobile refueling. Environ. Health Perspect. 2000, 108, 1195–1202. [Google Scholar] [CrossRef]
- Riedel, K.; Ruppert, T.; Conze, C.; Scherer, G.; Adlkofer, F. Determination of benzene and alkylated benzenes in ambient and exhaled air by microwave desorption coupled with gas chromatography-mass spectrometry. J. Chromatogr. A 1996, 719, 383–389. [Google Scholar] [CrossRef]
- Demirci-Cekic, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Guo, X.; Zhang, W.; Ren, J.; Gao, A. LncRNA OBFC2A modulated benzene metabolites-induced autophagy and apoptosis by interacting with LAMP2. Food Chem. Toxicol. 2023, 178, 113889. [Google Scholar] [CrossRef]
- Han, L.; Zhang, W.; Wang, J.; Jing, J.; Zhang, L.; Liu, Z.; Gao, A. Shikonin targets to m6A-modified oxidative damage pathway to alleviate benzene-induced testicular injury. Food Chem. Toxicol. 2022, 170, 113496. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.A.; Abd El-Wahab, E.W.; El-Marakby, F.A.; El-Gazzar, R.M. Assessment of oxidative stress among refueling workers in an Egyptian setting. Environ. Sci. Pollut. Res. 2020, 27, 18099–18108. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, S.; Li, C.; Li, M.; Xing, C.; He, J.; Peng, C.; Shao, H.; Jia, Q. Hydroquinone triggers pyroptosis and endoplasmic reticulum stress via AhR-regulated oxidative stress in human lymphocytes. Toxicol. Lett. 2023, 376, 39–50. [Google Scholar] [CrossRef]
- Costa-Amaral, I.C.; Carvalho, L.V.; Santos, M.V.C.; Valente, D.; Pereira, A.C.; Figueiredo, V.O.; Souza, J.M.d.; Castro, V.S.; Trancoso, M.d.F.; Fonseca, A.S.A.; et al. Environmental assessment and evaluation of oxidative stress and genotoxicity biomarkers related to chronic occupational exposure to benzene. Int. J. Environ. Res. Public Health 2019, 16, 2240. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Khodaparast, F.; Bohlooli, S.; Hashemidanesh, N.; Baghal, E.; Rezagholizadeh, L. Linalool reverses benzene-induced cytotoxicity, oxidative stress and lysosomal/mitochondrial damages in human lymphocytes. Drug Chem. Toxicol. 2022, 45, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Kubo, N.; Morita, M.; Nakashima, Y.; Kitao, H.; Egashira, A.; Saeki, H.; Oki, E.; Kakeji, Y.; Oda, Y.; Maehara, Y. Oxidative DNA damage in human esophageal cancer: Clinicopathological analysis of 8-hydroxydeoxyguanosine and its repair enzyme. Dis. Esophagus 2014, 27, 285–293. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Teodoro, M.; Rapisarda, V.; Golokhvast, K.; Docea, A.O.; Tsatsakis, A.M.; Costa, C. 8-Hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to low-dose benzene. Toxicol. Rep. 2017, 4, 291–295. [Google Scholar] [CrossRef]
- Mancini, M.; Mandruzzato, M.; Garzia, A.C.; Sahnane, N.; Magnani, E.; Macchi, F.; Oulad-Abdelghani, M.; Oudet, P.; Bollati, V.; Fustinoni, S.; et al. In vitro hydroquinone–induced instauration of histone bivalent mark on human retroelements (LINE-1) in HL60 cells. Toxicol. Vitr. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Seow, W.J.; Pesatori, A.C.; Dimont, E.; Farmer, P.B.; Albetti, B.; Ettinger, A.S.; Bollati, V.; Bolognesi, C.; Roggieri, P.; Panev, T.I. Urinary benzene biomarkers and DNA methylation in Bulgarian petrochemical workers: Study findings and comparison of linear and beta regression models. PLoS ONE 2012, 7, e50471. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Zhang, W.; Wang, J.; Zhang, L.; Jing, J.; Han, L.; Gao, A. Oxidative stress-affected ACSL1 hydroxymethylation triggered benzene hematopoietic toxicity by inflammation and senescence. Food Chem. Toxicol. 2023, 180, 114030. [Google Scholar] [CrossRef]
- Scholten, B.; Vlaanderen, J.; Stierum, R.; Portengen, L.; Rothman, N.; Lan, Q.; Pronk, A.; Vermeulen, R. A quantitative meta-analysis of the relation between occupational benzene exposure and biomarkers of cytogenetic damage. Environ. Health Perspect. 2020, 128, 087004. [Google Scholar] [CrossRef]
- Maciel, L.A.; Feitosa, S.B.; Trolly, T.S.; Sousa, A.L. Genotoxic effects of occupational exposure among gas station attendants in Santarem, Para, Brazil. Rev. Bras. Med. Trab. 2019, 17, 247. [Google Scholar] [CrossRef]
- Singaraju, M.; Singaraju, S.; Parwani, R.N.; Wanjari, S.P. Cytogenetic biomonitoring in petrol station attendants: A micronucleus study. J. Cytol. 2012, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lovreglio, P.; Doria, D.; Fracasso, M.E.; Barbieri, A.; Sabatini, L.; Drago, I.; Violante, F.S.; Soleo, L. DNA damage and repair capacity in workers exposed to low concentrations of benzene. Environ. Mol. Mutagen. 2016, 57, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Rusyn, I. Environmental toxicants, epigenetics, and cancer. Adv. Exp. Med. Biol. 2012, 745, 215–232. [Google Scholar]
- Spatari, G.; Allegra, A.; Carrieri, M.; Pioggia, G.; Gangemi, S. Epigenetic effects of benzene in hematologic neoplasms: The altered gene expression. Cancers 2021, 13, 2392. [Google Scholar] [CrossRef]
- Lubbert, M.; Oster, W.; Ludwig, W.; Ganser, A.; Mertelsmann, R.; Herrmann, F. A switch toward demethylation is associated with the expression of myeloperoxidase in acute myeloblastic and promyelocytic leukemias. Blood 1992, 80, 2066–2073. [Google Scholar] [CrossRef]
- Li, W.; Zou, C. NXNL2 Promotes Colon Cancer Proliferation and Metastasis by Regulating AKT Pathway. Appl. Biochem. Biotechnol. 2023, 195, 7685–7696. [Google Scholar] [CrossRef]
- Rivandi, M.; Khorrami, M.S.; Fiuji, H.; Shahidsales, S.; Hasanzadeh, M.; Jazayeri, M.H.; Hassanian, S.M.; Ferns, G.A.; Saghafi, N.; Avan, A. The 9p21 locus: A potential therapeutic target and prognostic marker in breast cancer. J. Cell. Physiol. 2018, 233, 5170–5179. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, Y.-K.; Park, S.H.; Lim, S.B.; Choi, Y.W.; Shin, J.S.; Kim, Y.H.; Kim, J.-H.; Park, T.J. p15INK4B is an alternative marker of senescent tumor cells in colorectal cancer. Heliyon 2023, 9, e13170. [Google Scholar] [CrossRef]
- Jamebozorgi, I.; Majidizadeh, T.; Pouryaghoub, G.; Mahjoubi, F. Aberrant DNA methylation of two tumor suppressor genes, p14ARF and p15INK4b, after chronic occupational exposure to low level of benzene. Int. J. Occup. Environ. Med. 2018, 9, 145. [Google Scholar] [CrossRef]
- Xing, C.; Wang, Q.-f.; Li, B.; Tian, H.; Ni, Y.; Yin, S.; Li, G. Methylation and expression analysis of tumor suppressor genes p15 and p16 in benzene poisoning. Chem. Interact. 2010, 184, 306–309. [Google Scholar] [CrossRef]
- Fustinoni, S.; Rossella, F.; Polledri, E.; Bollati, V.; Campo, L.; Byun, H.-M.; Agnello, L.; Consonni, D.; Pesatori, A.C.; Baccarelli, A.P. Global DNA methylation and low-level exposure to benzene. Med. Lav. 2012, 103, 84–95. [Google Scholar]
- Nishikawa, T.; Izumo, K.; Miyahara, E.; Horiuchi, M.; Okamoto, Y.; Kawano, Y.; Takeuchi, T. Benzene induces cytotoxicity without metabolic activation. J. Occup. Health 2011, 53, 84–92. [Google Scholar] [CrossRef]
- Hu, J.; Ma, H.; Zhang, W.; Yu, Z.; Sheng, G.; Fu, J. Effects of benzene and its metabolites on global DNA methylation in human normal hepatic L02 cells. Environ. Toxicol. 2014, 29, 108–116. [Google Scholar] [CrossRef]
- Xuan, M.; Wu, Y.; Wang, H.; Ye, Z.; Wu, H.; Chen, Y.; Yang, H.; Tang, H. Effect of mir-92a-3p on hydroquinone induced changes in human lymphoblastoid cell cycle and apoptosis. Environ. Toxicol. 2023, 38, 1420–1430. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Zeng, M.; Wu, H.; Zou, X.; Fang, T.; Zhai, L.; Liang, H.; Luo, H.; Tian, G.; et al. Long non-coding RNA LINC01480 is activated by Foxo3a and promotes hydroquinone-induced TK6 cell apoptosis by inhibiting the PI3K/AKT pathway. Ecotoxicol. Environ. Saf. 2023, 255, 114786. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Yang, E.J.; Kim, I.S. Hydroquinone-induced apoptosis of human lymphocytes through caspase 9/3 pathway. Mol. Biol. Rep. 2012, 39, 6737–6743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, P.; Bai, W.; Gao, A. MiR-133a regarded as a potential biomarker for benzene toxicity through targeting Caspase-9 to inhibit apoptosis induced by benzene metabolite (1, 4-Benzoquinone). Sci. Total Environ. 2016, 571, 883–891. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Guo, X.; Ren, J.; Gao, A. lncRNAVNN3 mediated benzene-induced hematotoxicity through promoting autophagy and apoptosis. Ecotoxicol. Environ. Saf. 2019, 185, 109672. [Google Scholar] [CrossRef]
- Wang, B.; Xu, S.; Wang, T.; Xu, K.; Yin, L.; Li, X.; Sun, R.; Pu, Y.; Zhang, J. LincRNA-p21 promotes p21-mediated cell cycle arrest in benzene-induced hematotoxicity by sponging miRNA-17-5p. Environ. Pollut. 2022, 296, 118706. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, S.; Sun, Q.; Li, X.; Wang, T.; Xu, K.; Yin, L.; Sun, R.; Pu, Y.; Zhang, J.; et al. Let-7e-5p, a promising novel biomarker for benzene toxicity, is involved in benzene-induced hematopoietic toxicity through targeting caspase-3 and p21. Ecotoxicol. Environ. Saf. 2022, 246, 114142. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Chen, Y.; Yun, L.; Chen, S.; Zhou, K.; Lai, B.; Song, L.; Yang, H.; Liang, H.; et al. Inhibition of autophagy enhances Hydroquinone-induced TK6 cell death. Toxicol. Vitr. 2017, 41, 123–132. [Google Scholar] [CrossRef]
- Qian, S.; Han, Y.; Shi, Y.; Xu, W.; Zhu, Y.; Jiang, S.; Chen, Y.; Yu, Z.; Zhang, S.; Yang, Y.; et al. Benzene induces haematotoxicity by promoting deacetylation and autophagy. J. Cell. Mol. Med. 2019, 23, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Bizargity, P.; Schröppel, B. Autophagy: Basic principles and relevance to transplant immunity. Am. J. Transplant. 2014, 14, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Harrath, A.H.; Alrezaki, A.; Jalouli, M.; Al-Dawood, N.; Dahmash, W.; Mansour, L.; Sirotkin, A.; Alwasel, S. Benzene exposure causes structural and functional damage in rat ovaries: Occurrence of apoptosis and autophagy. Environ. Sci. Pollut. Res. 2022, 29, 76275–76285. [Google Scholar] [CrossRef]
- Ren, J.; Wang, J.; Guo, X.; Zhang, W.; Chen, Y.; Gao, A. Lnc-TC/miR-142-5p/CUL4B signaling axis promoted cell ferroptosis to participate in benzene hematotoxicity. Life Sci. 2022, 310, 121111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, H.; Zhang, W.; Wang, J.; Liu, Z.; Jing, J.; Han, L.; Gao, A. Probiotics ameliorate benzene-induced systemic inflammation and hematopoietic toxicity by inhibiting Bacteroidaceae-mediated ferroptosis. Sci. Total Environ. 2023, 899, 165678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Liu, Z.; Zhang, L.; Jing, J.; Han, L.; Gao, A. Iron-dependent ferroptosis participated in benzene-induced anemia of inflammation through IRP1-DHODH-ALOX12 axis. Free Radic. Biol. Med. 2022, 193, 122–133. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Xu, K.; Pu, Y.; Huang, J.; Liu, J.; Zhang, J.; Yin, L.; Pu, Y. Ferroptosis is involved in the benzene-induced hematotoxicity in mice via iron metabolism, oxidative stress and NRF2 signaling pathway. Chem. Interact. 2022, 362, 110004. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Chen, Y.; Zhang, W.; Ren, J.; Gao, A. Association between benzene exposure, serum levels of cytokines and hematological measures in Chinese workers: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 207, 111562. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhong, W.; Chen, Y.; Zhang, W.; Ren, J.; Gao, A. Benzene metabolites trigger pyroptosis and contribute to haematotoxicity via TET2 directly regulating the Aim2/Casp1 pathway. EBioMedicine 2019, 47, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Loprieno, N. International Agency for Research on Cancer (IARC) monographs on the evaluation of carcinogenic risk of chemicals to man: “Relevance of data on mutagenicity”. Mutat. Res. Mutagen. Relat. Subj. 1975, 31, 201. [Google Scholar] [CrossRef]
- World Health Organization (WHO). International Agency for Research on Cancer; World Health Organization (WHO): Geneva, Switzerland, 2019. [Google Scholar]
- Nichols, L.; Sorahan, T. Cancer incidence and cancer mortality in a cohort of UK semiconductor workers, 1970–2002. Occup. Med. 2005, 55, 625–630. [Google Scholar] [CrossRef]
- Nethery, R.C.; Vega, S.; Frazier, A.L.; Laden, F. Mobile Source Benzene Regulations and Risk of Childhood and Young Adult Hematologic Cancers in Alaska: A Quasi-experimental Study. Epidemiology 2023, 34, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.A.; Schnatter, A.R.; Boogaard, P.J.; Banton, M.; Ketelslegers, H.B. Non-parametric estimation of low-concentration benzene metabolism. Chem. Interact. 2017, 278, 242–255. [Google Scholar] [CrossRef]
- Dugheri, S.; Mucci, N.; Cappelli, G.; Bonari, A.; Campagna, M.; Arcangeli, G.; Bartolucci, G. New fully automated gas chromatographic analysis of urinary S-phenylmercapturic acid in isotopic dilution using negative chemical ionization with isobutane as reagent gas. J. Mass Spectrom. 2019, 55, e4481. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Benzene—Health-Based Recommended Occupational Exposure Limit; Health Council of the Netherlands: The Hague, The Netherlands, 2014. [Google Scholar]
- Apelblat, A.; Manzurola, E.; Balal, N.A. The solubilities of benzene polycarboxylic acids in water. J. Chem. Thermodyn. 2006, 38, 565–571. [Google Scholar] [CrossRef]
- Bleasdale, C.; Kennedy, G.; MacGregor, J.O.; Nieschalk, J.; Pearce, K.; Watson, W.P.; Golding, B.T. Chemistry of muconaldehydes of possible relevance to the toxicology of benzene. Environ. Health Perspect. 1996, 104, 1201. [Google Scholar] [PubMed]
- Lovreglio, P.; Stufano, A.; Andreoli, R.; Tomasi, C.; Cagnazzi, P.; Barbieri, A.; Soleo, L.; De Palma, G. Urinary biomarkers of nucleic acid oxidation and methylation in workers exposed to low concentrations of benzene. Toxicol. Lett. 2020, 331, 235–241. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, X.; Chen, L.; Chen, S.; Li, H.; Du, R.; You, W.; Peng, J.; Guo, P.; Zhang, R.; et al. Moderate body lipid accumulation in mice attenuated benzene-induced hematotoxicity via acceleration of benzene metabolism and clearance. Environ. Int. 2023, 178, 108113. [Google Scholar] [CrossRef]
- Soleimani, E. Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring. Rev. Anal. Chem. 2020, 39, 168–187. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Zhou, X.L.; Wang, X.H.; Nie, H.X.; Li, X.; Zhang, H.M. Association of Urinary Phenol Concentration and Blood Biochemical Indices in Coke Oven Workers. Chin. J. Ind. Hyg. Occup. Dis. 2020, 38, 440–443. [Google Scholar]
- Sisto, R.; Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Paci, E.; Pigini, D.; Gherardi, M.; Gordaini, A.; et al. Direct and oxidative DNA damage in a group of painters exposed to VOCs: Dose—Response relationship. Front. Public Health 2020, 8, 445. [Google Scholar] [CrossRef]
- Geraldino, B.R.; Nunes, R.F.N.; Gomes, J.B.; da Poça, K.S.; Giardini, I.; Silva, P.V.B.; Souza, H.P.; Otero, U.B.; Sarpa, M.; Silva, D.A.S. Evaluation of exposure to toluene and xylene in gasoline station workers. Adv. Prev. Med. 2021, 2021, 5553633. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Yuan, L.; Wei, C.; Zhao, Y.; Qian, Y.; Ma, P.; Ding, S.; Yang, X.; Wang, X. Effects of combined exposure to formaldehyde and benzene on immune cells in the blood and spleen in Balb/c mice. Environ. Toxicol. Pharmacol. 2016, 45, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Li, W.; Zhao, D.; Li, W.; Liu, X.; Liu, L.; Yin, J.; Muddassir, M.; Wen, R.; Fan, L. A bifunctional luminescence sensor for biomarkers detection in serum and urine based on chemorobust Nickel(II) metal-organic framework. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 306, 123585. [Google Scholar] [CrossRef]
- Weisel, C.P. Benzene exposure: An overview of monitoring methods and their findings. Chem. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef]
- Kim, S.; Vermeulen, R.; Waidyanatha, S.; Johnson, B.A.; Lan, Q.; Smith, M.T.; Zhang, L.; Li, G.; Shen, M.; Yin, S.; et al. Modeling human metabolism of benzene following occupational and environmental exposures. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2246–2252. [Google Scholar] [CrossRef]
- Angerer, J.; Scherer, G.; Schaller, K.H.; Müller, J. The determination of benzene in human blood as an indicator of environmental exposure to volatile aromatic compounds. Fresenius’ J. Anal. Chem. 1991, 339, 740–742. [Google Scholar] [CrossRef]
- Mirzaei, N.; Naddafi, K.; Raminnabizadeh; Yaghmaeian, K.; Assanvand, M.S.; Maroufizadeh, S.; Hoseini, M.; Adabi, S.; Yunesian, M. Urinary benzene as a biomarker of environmental exposure to benzene in males in the general population. Acta Medica Mediterr. 2016, 32, 1471–1475. [Google Scholar]
- Geng, Y.; Zhang, S.; Lin, J.; Zhu, X.; Gao, W.; Li, J.; Wang, C.; Wu, Y.; Han, R.; Tang, K.; et al. Determination of Ethanol and Aromatics in Blood by Headspace Portable Gas Chromatography-Mass Spectrometry (HS-PGC-MS). Anal. Lett. 2024, 58, 724–735. [Google Scholar] [CrossRef]
- Petrick, M.E.; Royster, L.H.; Royster, J.D.; Reist, P. Comparison of daily noise exposures in one workplace based on noise criteria recommended by ACGIH and OSHA. Am. Ind. Hyg. Assoc. J. 1996, 57, 924–928. [Google Scholar] [CrossRef]
- Amorim, L.C.; Carneiro, J.P.; Cardeal, Z.L. An optimized method for determination of benzene in exhaled air by gas chromatography–mass spectrometry using solid phase microextraction as a sampling technique. J. Chromatogr. B 2008, 865, 141–146. [Google Scholar] [CrossRef]
- Plebani, C.; Tranfo, G.; Salerno, A.; Panebianco, A.; Marcelloni, A.M. An optimized sampling and GC–MS analysis method for benzene in exhaled breath, as a biomarker for occupational exposure. Talanta 1999, 50, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Menezes, H.C.; Amorim, L.C.A.; Cardeal, Z.L. Sampling of benzene in environmental and exhaled air by solid-phase microextraction and analysis by gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2009, 395, 2583–2589. [Google Scholar] [CrossRef]
- Ayache, D.; Trzpil, W.; Rousseau, R.; Kinjalk, K.; Teissier, R.; Baranov, A.N.; Bahriz, M.; Vicet, A. Benzene sensing by quartz enhanced photoacoustic spectroscopy at 14.85 µm. Opt. Express 2022, 30, 5531–5539. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.d.P.; Sanson, A.L.; Lobo, F.A.; Afonso, R.J.d.C.F.; Coutrim, M.X. Method for the determination of benzene metabolite t, t-muconic acid in urine by HPLC-UV with an Ion exclusion column. Separations 2016, 3, 14. [Google Scholar] [CrossRef]
- Kim, S.; Vermeulen, R.; Waidyanatha, S.; Johnson, B.A.; Lan, Q.; Rothman, N.; Smith, M.T.; Zhang, L.; Li, G.; Shen, M.; et al. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinog. 2005, 27, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Pang, B.; Yan, H.; Wu, B.; Li, M.; Xing, C.; Li, J. Using urinary biomarkers to estimate the benzene exposure levels in individuals exposed to benzene. Toxics 2022, 10, 636. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Yousefinejad, S.; Jafari, S.; Soleimani, E. In-syringe ionic liquid-dispersive liquid–liquid microextraction coupled with HPLC for the determination of trans, trans-muconic acid in human urine sample. J. Sep. Sci. 2021, 44, 3126–3136. [Google Scholar] [CrossRef]
- Pacenti, M.; Dugheri, S.; Villanelli, F.; Bartolucci, G.; Calamai, L.; Boccalon, P.; Arcangeli, G.; Vecchione, F.; Alessi, P.; Kikic, I.; et al. Determination of organic acids in urine by solid-phase microextraction and gas chromatography–ion trap tandem mass spectrometry previous ‘in sample’ derivatization with trimethyloxonium tetrafluoroborate. Biomed. Chromatogr. 2008, 22, 1155–1163. [Google Scholar] [CrossRef]
- Omidi, F.; Khadem, M.; Dehghani, F.; Seyedsomeah, M.; Shahtaheri, S.J. Ultrasound-assisted dispersive micro-solid-phase extraction based on N-doped mesoporous carbon and high-performance liquid chromatographic determination of 1-hydroxypyrene in urine samples. J. Sep. Sci. 2020, 43, 2602–2609. [Google Scholar] [CrossRef]
- Mansour, F.R.; Danielson, N.D. Solidification of floating organic droplet in dispersive liquid-liquid microextraction as a green analytical tool. Talanta 2017, 170, 22–35. [Google Scholar] [CrossRef]
- Vieira, A.C.; Zampieri, R.A.; de Siqueira, M.E.P.B.; Martins, I.; Figueiredo, E.C. Molecularly imprinted solid-phase extraction and high-performance liquid chromatography with ultraviolet detection for the determination of urinary trans, trans-muconic acid: A comparison with ionic exchange extraction. Analyst 2012, 137, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Omidi, F.; Heravizadeh, O.; Yousefinejad, S. Solidified floating organic droplet microextraction coupled with HPLC for rapid determination of trans, trans muconic acid in benzene biomonitoring. Sci. Rep. 2021, 11, 15751. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.; Blandez, J.F.; Lozano-Torres, B.; de la Torre, C.; Licchelli, M.; Mangano, C.; Amendola, V.; Sancenón, F.; Martínez-Máñez, R. A Nanoprobe Based on Gated Mesoporous Silica Nanoparticles for The Selective and Sensitive Detection of Benzene Metabolite t,t-Muconic Acid in Urine. Chem. Eur. J. 2021, 27, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Waidyanatha, S.; Yeowell-O’Connell, K.; Rothman, N.; Smith, M.T.; Zhang, L.; Qu, Q.; Shore, R.; Li, G.; Yin, S. Protein adducts as biomarkers of human benzene metabolism. Chem.-Biol. Interact. 2005, 153, 103–109. [Google Scholar] [CrossRef]
- Gomes, A.P.; Barbosa, E.; Santos, A.L.A.d.; Lizot, L.F.; Sauer, E.; Garcia, S.C.; Linden, R.; Antunes, M.V.; Charao, M.F. A simple and sensitive LC-MS/MS method for the determination of S-phenylmercapturic acid in human urine. Química Nova 2021, 44, 334–340. [Google Scholar] [CrossRef]
- Mendes, M.P.R.; Silveira, J.N.; Andre, L.C. An efficient analytical method for determination of S-phenylmercapturic acid in urine by HPLC fluorimetric detector to assessing benzene exposure. J. Chromatogr. B 2017, 1063, 136–140. [Google Scholar] [CrossRef]
- Sterz, K.; Köhler, D.; Schettgen, T.; Scherer, G. Enrichment and properties of urinary pre-S-phenylmercapturic acid (pre-SPMA). J. Chromatogr. B 2010, 878, 2502–2505. [Google Scholar] [CrossRef]
- Shan, X.; Tan, S.; Shi, Y.; Shao, J.; Su, K.; Zhang, L.; Feng, H.; Ye, H. Activated carbon/diatomite-based magnetic nanocomposites for magnetic solid-phase extraction of S-phenylmercapturic acid from human urine. Biomed. Chromatogr. 2020, 34, e4834. [Google Scholar] [CrossRef]
- Bowman, B.A.; Lewis, E.V.; Goldy, D.W.; Kim, J.Y.; Elio, D.M.; Blount, B.C.; Bhandari, D. Assessment of urinary 6-hydroxy-2, 4-cyclohexadienyl mercapturic acid as a novel biomarker of benzene exposure. J. Anal. Toxicol. 2023, 47, 597–605. [Google Scholar] [CrossRef]
- Li, W.T.; Li, D.C.; Yang, Y.F.; Su, S.; Qie, S.W.; Jia, Y.J.; Hu, M. Identification of S-phenylmercapturic acid using heterometallic Zn-Eu MOF as a fluorescence sensor. J. Mol. Struct. 2025, 1321, 139974. [Google Scholar] [CrossRef]
- Wei, H.Y.; Zou, W.; Feng, R.; Liu, L.Y.; Zhang, M.P.; Meng, X.; Chen, W.W.; Jia, Q.; Wang, C.J. Point-of-Care Testing of Benzene Metabolite S-Phenylmercapturic Acid Using Salt-Induced Phase Separation Combined with Nanoparticle-Based Surface-Enhanced Raman Spectroscopy. ACS Appl. Nano Mater. 2024, 7, 16237–16244. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, X.D.; Meng, X.J.; Li, J.; Niu, D.S.; Ding, X.W.; Nie, J. Determination of seven urinary metabolites of benzene, toluene and xylene by ultra-high performance liquid chromatography-triple quadrupole mass spectrometry. Chin. J. Ind. Hyg. Occup. Dis. 2019, 37, 303–307. [Google Scholar]
- Tzanetou, E.N.; Manea-Karga, E.; Baira, E.; Boutsikou, T.; Iliodromiti, Z.; Iacovidou, N.; Machera, K.; Kasiotis, K.M. Gas and Liquid Chromatography Mass Spectrometry as a Tool for Elucidating Volatile Organic Compounds (VOCs) and Metabolites in Maternal Milk: A Perspective on Infants’ Health Risk Assessment. Chemosensors 2024, 12, 30. [Google Scholar] [CrossRef]
- Kurd, N.; Bahrami, A.; Afkhami, A.; Shahna, F.G.; Assari, M.J.; Farhadian, M. Application of Fe3O4@ TbBd nanobeads in microextraction by packed sorbent (MEPS) for determination of BTEXs biomarkers by HPLC–UV in urine samples. J. Chromatogr. B 2022, 1197, 123197. [Google Scholar] [CrossRef] [PubMed]
- Kurd, N.; Bahrami, A.; Afkhami, A.; Shahna, F.G.; Assari, M.J.; Farhadian, M. A Novel Magnetized Imine-linked Covalent Organic Framework Sorbent (Fe3O4@ TFPA-Bd) for Microextraction of BTEX Biomarkers in Urinary Samples. J. Health Saf. Work 2023, 13, 474–479. [Google Scholar]
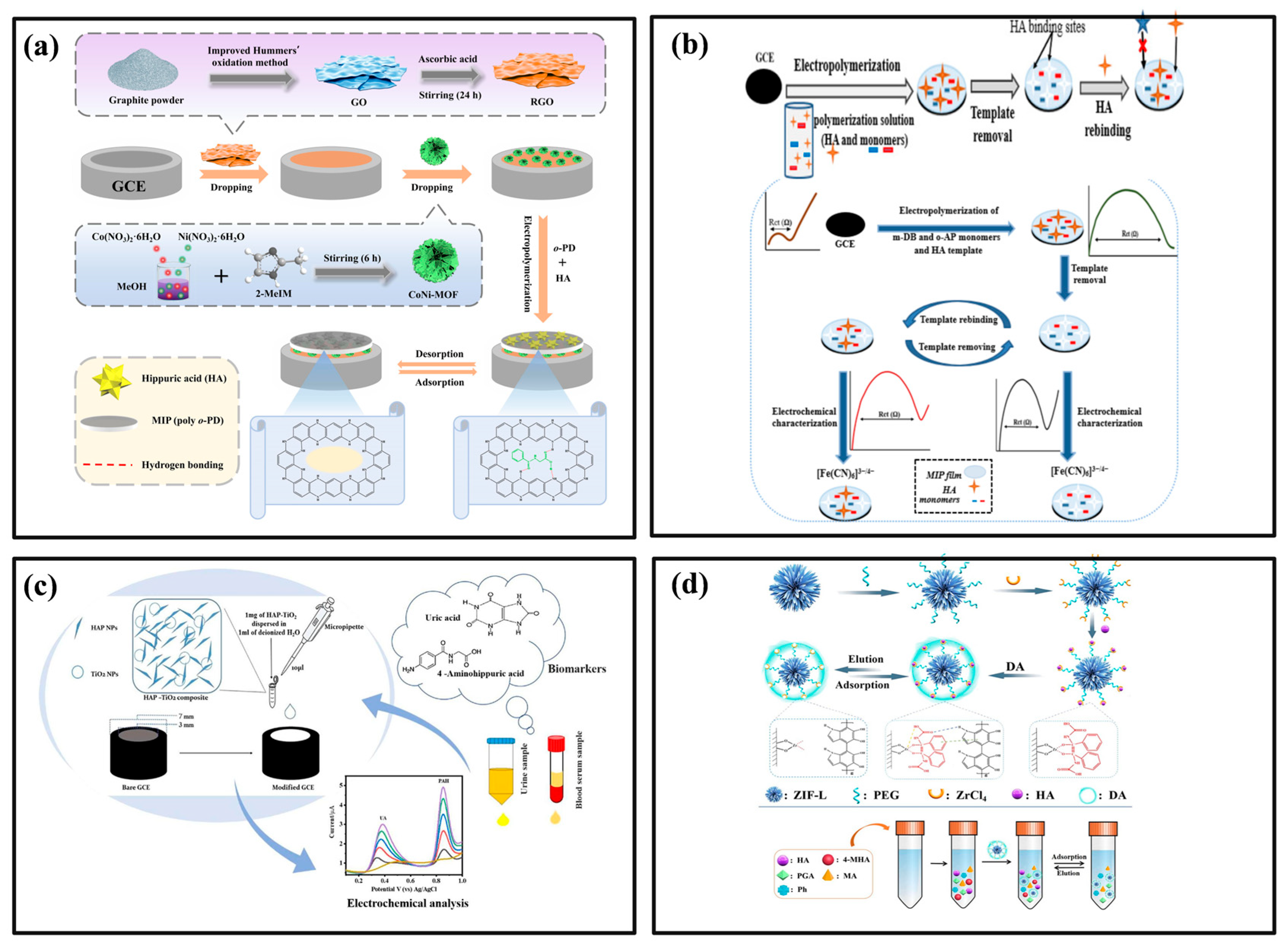
- Han, J.; Wang, H.; Li, Z.; Wang, Z. Preparation of chitosan-modified magnetic Schiff base network composite nanospheres for effective enrichment and detection of hippuric acid and 4-methyl hippuric acid. J. Chromatogr. A 2021, 1652, 462373. [Google Scholar] [CrossRef]
- Gao, H.; Chai, J.; Jin, C.; Tian, M. Molecularly imprinted electrochemical sensor based on CoNi-MOF/RGO nanocomposites for sensitive detection of the hippuric acid. Anal. Chim. Acta 2024, 1296, 342307. [Google Scholar] [CrossRef]
- Karazan, Z.M.; Roushani, M. A new method for electrochemical determination of Hippuric acid based on molecularly imprinted copolymer. Talanta 2022, 246, 123491. [Google Scholar] [CrossRef]
- Anitta, S.; Sekar, C. HAP-TiO2 nanocomposites based electrochemical sensor for selective and simultaneous detection of para-aminohippuric acid and uric acid. Microchem. J. 2022, 181, 107704. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, H.; Jin, C.; Tian, M. Selective sorption of hippuric acid and 4-methylhippuric acid by polyethylene glycol modified ZIF based molecular imprinted polymer. Microchem. J. 2024, 199, 110026. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Amer, N.M.; El-dossuky, E.A.; Emara, A.M.; Abd El-Fattah, A.E.-S.M.; Shahy, E.M. Hepatic Dysfunction and Immune Suppression among Egyptian Workers Occupationally Exposed to Benzene. Int. Public Health Forum 2014, 1, 1. [Google Scholar]
- Vermeulen, R.; Portengen, L.; Li, G.; Gilbert, E.S.; Dores, G.M.; Ji, B.-T.; Hayes, R.; Yin, S.; Rothman, N.; Linet, M.S. Benzene exposure and risk of benzene poisoning in Chinese workers. Occup. Environ. Med. 2022, 79, 610–617. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Zeng, W.; Lin, Q.; Liu, Y. A study on the effects of exposure to benzene on the activity of immunoglobulin E. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi = Zhonghua Laodong Weisheng Zhiyebing Zazhi = Chin. J. Ind. Hyg. Occup. Dis. 2017, 35, 380–382. [Google Scholar]
- Sajid Jabbar, A.; Ali, E.T. Impact of Petroleum Exposure on Some Hematological Indices, Interleukin-6, and Inflammatory Markers of Workers at Petroleum Stations in Basra City. J. Environ. Public Health 2020, 2020, 7693891. [Google Scholar] [CrossRef]
- Hashemi, F.; Hamidinejad, F.S.; Hoepner, L.; Rafiee, A.; Abbasi, A.; Hoseini, M. BTEX exposure of pregnant women and associations with pro-inflammatory cytokines (IL-6 and TNF-α). Air Qual. Atmos. Health 2021, 15, 707–719. [Google Scholar] [CrossRef]
- Werder, E.J.; Beier, J.I.; Sandler, D.P.; Falkner, K.C.; Gripshover, T.; Wahlang, B.; Engel, L.S.; Cave, M.C. Blood BTEXS and heavy metal levels are associated with liver injury and systemic inflammation in Gulf states residents. Food Chem. Toxicol. 2020, 139, 111242. [Google Scholar] [CrossRef] [PubMed]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef]
- Sangaramoorthy, M.; Yang, J.; Tseng, C.; Wu, J.; Ritz, B.; Larson, T.V.; Fruin, S.; Stram, D.O.; Park, S.-S.L.; Franke, A.A. Particulate matter, traffic-related air pollutants, and circulating C-reactive protein levels: The Multiethnic Cohort Study. Environ. Pollut. 2023, 332, 121962. [Google Scholar] [CrossRef]
- Li, Q.; Ke, N.; Sundaram, R.; Wong-Staal, F. NR4A1, 2, 3 an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol. Histopathol. 2006, 21, 533–540. [Google Scholar] [PubMed]
- Songjaroen, T.; Feeny, R.M.; Mensack, M.M.; Laiwattanapaisal, W.; Henry, C.S. Label-free detection of C-reactive protein using an electrochemical DNA immunoassay. Sens. Bio-Sens. Res. 2016, 8, 14–19. [Google Scholar] [CrossRef]
- Sheen, H.-J.; Panigrahi, B.; Kuo, T.-R.; Hsu, W.-C.; Chung, P.-S.; Xie, Q.-Z.; Lin, C.-Y.; Chang, Y.-S.; Lin, C.-T.; Fan, Y.-J. Electrochemical biosensor with electrokinetics-assisted molecular trapping for enhancing C-reactive protein detection. Biosens. Bioelectron. 2022, 210, 114338. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, Y.; He, B.; Tan, C.S. A Simple Label-Free Aptamer-Based Electrochemical Biosensor for the Sensitive Detection of C-Reactive Proteins. Biosensors 2022, 12, 1180. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, S.; Kwon, K.; Bae, N.-H.; Kwak, B.S.; Cho, W.C.; Lee, S.J.; Jung, H.-I. A photothermal biosensor for detection of C-reactive protein in human saliva. Sens. Actuators B Chem. 2017, 246, 471–476. [Google Scholar] [CrossRef]
- Soragni, C.; Rabussier, G.; Lanz, H.L.; Bircsak, K.M.; de Windt, L.J.; Trietsch, S.J.; Murdoch, C.E.; Ng, C.P. A versatile multiplexed assay to quantify intracellular ROS and cell viability in 3D on-a-chip models. Redox Biol. 2022, 57, 102488. [Google Scholar] [CrossRef]
- Ammanath, G.; Yildiz, U.H.; Palaniappan, A.; Liedberg, B. Luminescent device for the detection of oxidative stress biomarkers in artificial urine. ACS Appl. Mater. Interfaces 2018, 10, 7730–7736. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, L.; Tang, Y.; Zhang, X. A facile Pt-doped g-C3N4 photocatalytic biosensor for visual detection of superoxide dismutase in serum samples. Sens. Actuators B Chem. 2020, 318, 128238. [Google Scholar] [CrossRef]
- Gut, I.; Nedelcheva, V.; Soucek, P.; Stopka, P.; Tichavska, B. Cytochromes P450 in benzene metabolism and involvement of their metabolites and reactive oxygen species in toxicity. Environ. Health Perspect. 1996, 104, 1211–1218. [Google Scholar] [PubMed]
- Sarma, S.N.; Kim, Y.-J.; Song, M.; Ryu, J.-C. Induction of apoptosis in human leukemia cells through the production of reactive oxygen species and activation of HMOX1 and Noxa by benzene, toluene, and o-xylene. Toxicology 2011, 280, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lagorio, S.; Tagesson, C.; Forastiere, F.; Iavarone, I.; Axelson, O.; Carere, A. Exposure to benzene and urinary concentrations of 8-hydroxydeoxyguanosine, a biological marker of oxidative damage to DNA. Occup. Environ. Med. 1994, 51, 739–743. [Google Scholar] [CrossRef]
- Amin, M.M.; Rafiei, N.; Poursafa, P.; Ebrahimpour, K.; Mozafarian, N.; Shoshtari-Yeganeh, B.; Hashemi, M.; Kelishadi, R. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ. Sci. Pollut. Res. 2018, 25, 34046–34052. [Google Scholar] [CrossRef]
- Peter, A.; Jose, J.; Bhat, S.G.; Abhitha, K. A modified fluorescent probe protocol for evaluating the reactive oxygen species generation by metal and metal oxide nanoparticles in Gram-positive and Gram-negative organisms. Results Eng. 2024, 24, 102925. [Google Scholar] [CrossRef]
- Zhang, J.; Arbault, S.; Sojic, N.; Jiang, D. Electrochemiluminescence imaging for bioanalysis. Annu. Rev. Anal. Chem. 2019, 12, 275–295. [Google Scholar] [CrossRef]
- Yan, F.; Zang, Y.; Sun, J.; Sun, Z.; Zhang, H. Sensing mechanism of reactive oxygen species optical detection. TrAC Trends Anal. Chem. 2020, 131, 116009. [Google Scholar] [CrossRef]
- Lü, R. Reaction-based small-molecule fluorescent probes for dynamic detection of ROS and transient redox changes in living cells and small animals. J. Mol. Cell. Cardiol. 2017, 110, 96–108. [Google Scholar] [CrossRef]
- Rücker, H.; Amslinger, S. Identification of heme oxygenase-1 stimulators by a convenient ELISA-based bilirubin quantification assay. Free Radic. Biol. Med. 2015, 78, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.V.; Tavares, A.P.; Fortunato, E.; Sales, M.G.F. based sensing device for electrochemical detection of oxidative stress biomarker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in point-of-care. Sci. Rep. 2017, 7, 14558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Q.; Li, Z.; Ming, P.; Sun, D.; Zhai, H. An ultrasensitive aptasensor for 8-hydroxy-2′-deoxyguanosine of human urine detection based on COFTAPB-DMTP@ MWCNT-COOH nanocomposites and CeFeOx-C@ Au@ Apt nanoprobe. Microchem. J. 2024, 203, 110907. [Google Scholar] [CrossRef]
- Abdussalam, A.; Chen, Y.; Yuan, F.; Ma, X.; Lou, B.; Xu, G. Dithiothreitol–Lucigenin Chemiluminescent System for Ultrasensitive Dithiothreitol and Superoxide Dismutase Detection. Anal. Chem. 2022, 94, 11023–11029. [Google Scholar] [CrossRef] [PubMed]


| Materials | Detection Method | Linear Range | LoD | Sample Types | Reference |
|---|---|---|---|---|---|
| t,t-MA | HPLC-UV | 5−500 µg/L | 0.11 µg/L | Human urine | [132] |
| UPLC-MS/MS | 3.3−1000 μg/L | 3.3 μg/L | Human urine | [134] | |
| DLLME/HPLC-UV | 0.029−10 μg/mL | 0.011 μg/mL | Human urine | [135] | |
| MIP/HPLC-UV | 0.3−10 mg/L | 0.3 mg/L | Human urine | [139] | |
| SFOC/HPLC-UV | 0.02−5 μg/mL | 0.006 μg/mL | Human urine | [140] | |
| Nanoprobe Based on Gated Mesoporous Silica Nanoparticles | 0.025–0.225 mM | 0.017 mM | HEPES suspension/Human urine | [141] | |
| S-PMA | UPLC-MS/MS | 0.17−50 μg/L | 0.17 μg/L | Human urine | [134] |
| LC-MS/MS | 0.5−500 ng/mL | 0.5 ng/mL | Human urine | [143] | |
| SPE/HPLC | 10−100 μg/L | 0.22 μg/L | Human urine | [144] | |
| AC/DBMNs/HPLC-UV-vis | 0.03−1.0 mg/L | 0.01 mg/L | Standard sample/Human urine | [146] | |
| luminescent HMOF | 3.70−180 μM | 0.03 μM | Human urine | [148] | |
| SIPS-SERS | 0−5 ppm | 1.06 ppb | Standard sample/Human urine | [149] |
| Materials | Detection Method | Linear Range | LoD | Sample Types | Reference |
|---|---|---|---|---|---|
| HA | COFs-MEPS/HPLC-UV | 0.1–50 µg/mL | 0.05 µg/mL | Human urine | [152] |
| Fe3O4@TFPA-Bd-MEPS/HPLC | 0.16–25 µg/mL | 0.05 µg/mL | Human urine | [153] | |
| Fe3O4@SNW@Chitosan-MSPE/HPLC | 1–1000 μg/L | 0.3 μg/L | Human urine | [154] | |
| MIP/RGO/CoNi-MOF/GCE | 2–800 nM | 0.97 nM | Human urine | [155] | |
| MIP/GCE | 0.05–500 nM | 0.012 nM | Human urine and blood serum | [156] | |
| MHA | ZIF@PEG@Zr@MIPs-SPE/HPLC-UV | 0.03–100 mg/L | 0.015 mg/L | Human urine | [157] |
| COFs-MEPS/HPLC-UV | 0.1–25 µg/mL | 0.05 µg/mL | Human urine | [152] | |
| Fe3O4@TFPA-Bd-MEPS/HPLC | 0.16–25 µg/mL | 0.05 µg/mL | Human urine | [153] | |
| Fe3O4@SNW@Chitosan-MSPE/HPLC | 1–1000 μg/L | 0.2 μg/L | Human urine | [154] | |
| ZIF@PEG@Zr@MIPs-SPE/HPLC-UV | 0.02–100 mg/L | 0.011 mg/L | Human urine | [157] | |
| PAH | HAP-TiO2/GCE | 50 nM–5.76 mM | 37 nM | Human urine | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Deng, S.; Li, S. Research Progress on Biomarkers and Their Detection Methods for Benzene-Induced Toxicity: A Review. Chemosensors 2025, 13, 312. https://doi.org/10.3390/chemosensors13080312
Qin R, Deng S, Li S. Research Progress on Biomarkers and Their Detection Methods for Benzene-Induced Toxicity: A Review. Chemosensors. 2025; 13(8):312. https://doi.org/10.3390/chemosensors13080312
Chicago/Turabian StyleQin, Runan, Shouzhe Deng, and Shuang Li. 2025. "Research Progress on Biomarkers and Their Detection Methods for Benzene-Induced Toxicity: A Review" Chemosensors 13, no. 8: 312. https://doi.org/10.3390/chemosensors13080312
APA StyleQin, R., Deng, S., & Li, S. (2025). Research Progress on Biomarkers and Their Detection Methods for Benzene-Induced Toxicity: A Review. Chemosensors, 13(8), 312. https://doi.org/10.3390/chemosensors13080312







