Breathprint-Based Endotyping of COPD and Bronchiectasis COPD Overlap Using Electronic Nose Technology: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Study Population
2.3. Clinical and Functional Assessment
2.4. Breath Sample Collection and Pre-Analytical Conditions
2.5. Electronic Nose Device and Signal Acquisition
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Study Population Characteristics
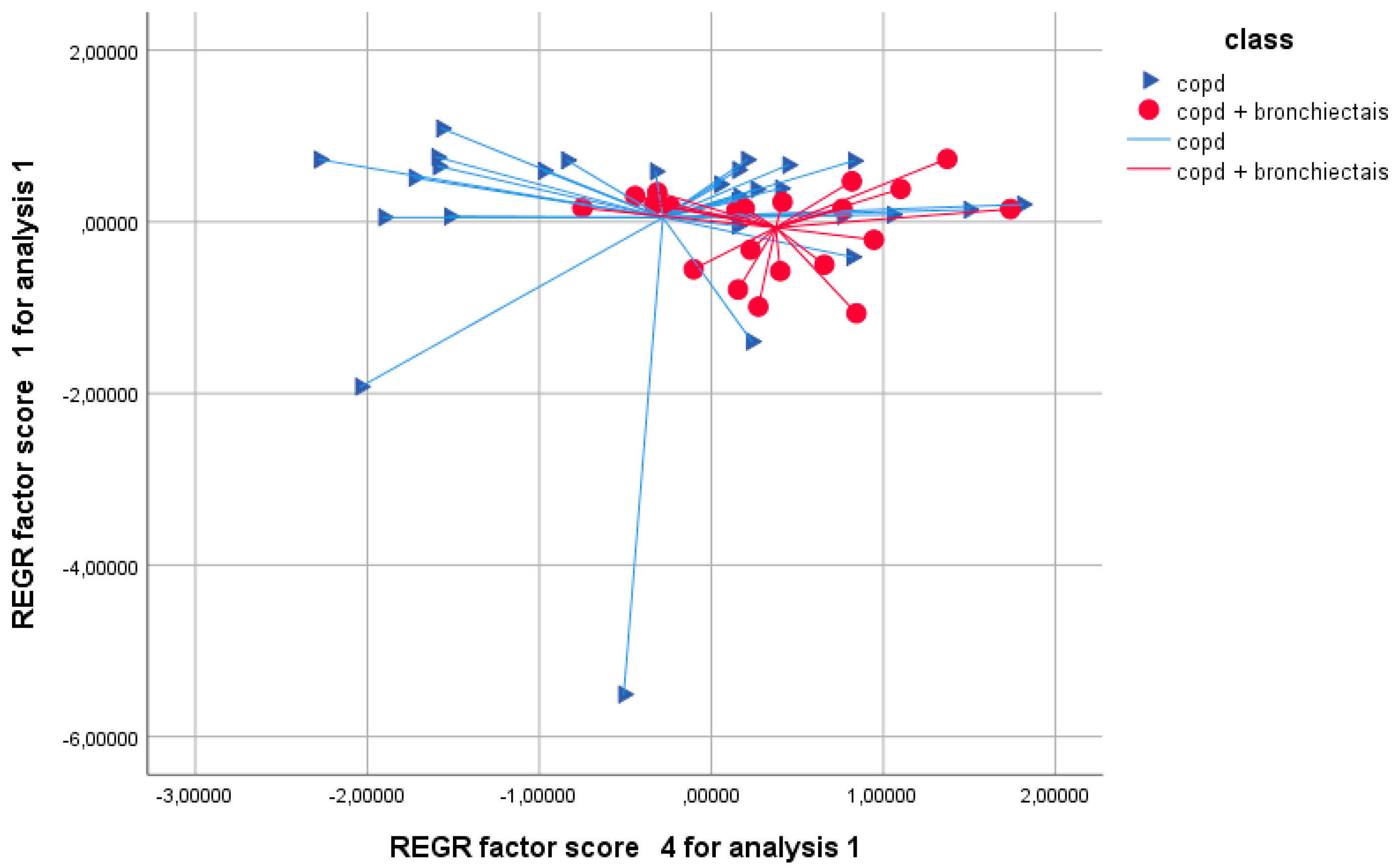
3.2. Principal Component Analysis
3.3. Discriminant Analysis and Model Performance
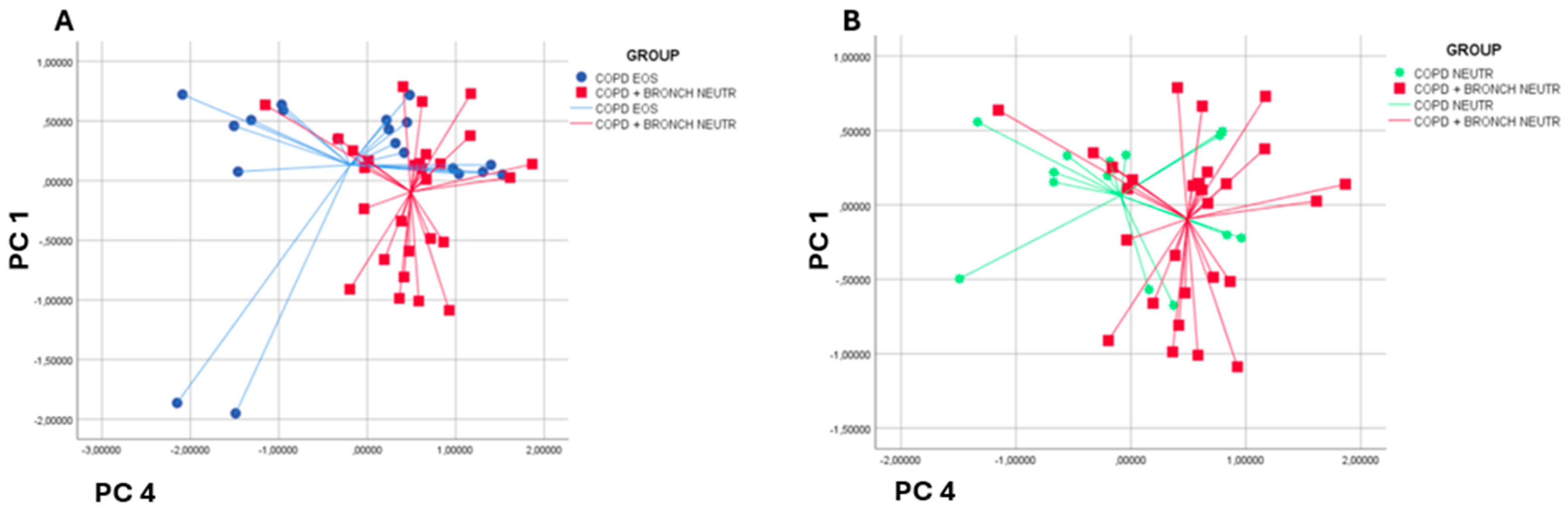
3.4. Subgroup Analysis by Inflammatory Endotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 22 October 2024).
- Wang, Z.; Lin, J.; Liang, L.; Huang, F.; Yao, X.; Peng, K.; Gao, Y.; Zheng, J. Global, regional, and national burden of chronic obstructive pulmonary disease and its attributable risk factors from 1990 to 2021: An analysis for the Global Burden of Disease Study 2021. Respir. Res. 2025, 26, 2. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open. 2023, 6, 2346598. [Google Scholar] [CrossRef]
- Huang, J.T.J.; Cant, E.; Keir, H.R.; Barton, A.K.; Kuzmanova, E.; Shuttleworth, M.; Pollock, J.; Finch, S.; Polverino, E.; Mathieu, B.; et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the “Chronic Obstructive Pulmonary Disease-Bronchiectasis Association”. Am. J. Respir. Crit. Care Med. 2022, 206, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Mangapuram, P.; Fredrick, F.C.; Singh, B.; Singla, A.; Kumar, A.; Jain, R. Bronchiectasis-COPD Overlap Syndrome: A Comprehensive Review of its Pathophysiology and Potential Cardiovascular Implications. Ther. Adv. Pulm. Crit. Care Med. 2024, 19, 29768675241300808. [Google Scholar] [CrossRef]
- Aliberti, S.; Sotgiu, G.; Lapi, F.; Gramegna, A.; Cricelli, C.; Blasi, F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm. Med. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Miravitlles, M. Bronchiectasis in COPD patients: More than a comorbidity? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1401–1411. [Google Scholar] [CrossRef]
- Polverino, E.; Dimakou, K.; Hurst, J.; Martinez-Garcia, M.A.; Miravitlles, M.; Paggiaro, P.; Shteinberg, M.; Aliberti, S.; Chalmers, J.D. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J. 2018, 52, 1800328. [Google Scholar] [CrossRef]
- Raboso, B.; Pou, C.; Abril, R.; Erro, M.; Sánchez, C.; Manzano, C.; Zamarrón, E.; Suarez-Cuartin, G.; González, J. Bronchiectasis. Open Respir. Arch. 2024, 6, 100339. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Carr, L.; Cordell, R.; Wilde, M.J.; Salman, D.; Monks, P.S.; Thomas, P.; Brightling, C.E.; Siddiqui, S.; Greening, N.J. Breathomics for the clinician: The use of volatile organic compounds in respiratory diseases. Thorax 2021, 76, 514–521. [Google Scholar] [CrossRef]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Ibrahim, B.; Basanta, M.; Cadden, P.; Singh, D.; Douce, D.; Woodcock, A.; Fowler, S.J. Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax 2011, 66, 804–809. [Google Scholar] [CrossRef]
- Ibrahim, W.; Natarajan, S.; Wilde, M.; Cordell, R.; Monks, P.S.; Greening, N.; Brightling, C.E.; Evans, R.; Siddiqui, S. A systematic review of the diagnostic accuracy of volatile organic compounds in airway diseases and their relation to markers of type-2 inflammation. ERJ Open Res. 2021, 7, 00030-2021. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.M.; Lawal, O.; Nijsen, T.M.; Goodacre, R.; Fowler, S.J. Exhaled Volatile Organic Compounds of Infection: A Systematic Review. ACS Infect. Dis. 2017, 3, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Ager, C.; Troppmair, J. Predicting the future from the past: Volatile markers for respiratory infections. Eur. Respir. J. 2017, 49, 1700264. [Google Scholar] [CrossRef]
- Kwon, J.W.; Park, H.W.; Kim, W.J.; Kim, M.G.; Lee, S.J. Exposure to volatile organic compounds and airway inflammation. Environ. Health 2018, 17, 65. [Google Scholar] [CrossRef]
- Angrill, J.; Agustí, C.; De Celis, R.; Filella, X.; Rañó, A.; Elena, M.; De La Bellacasa, J.P.; Xaubet, A.; Torres, A. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am. J. Respir. Crit. Care Med. 2001, 164, 1628–1632. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Aliberti, S.; Filonenko, A.; Shteinberg, M.; Goeminne, P.C.; Hill, A.T.; Fardon, T.C.; Obradovic, D.; Gerlinger, C.; Giovanni, S.; et al. Characterization of the “Frequent Exacerbator Phenotype” in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2018, 197, 1410–1420. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Metersky, M.; Aliberti, S.; Morgan, L.; Fucile, S.; Lauterio, M.; McDonald, P.P. Neutrophilic inflammation in bronchiectasis. Eur. Respir. Rev. 2025, 34, 240179. [Google Scholar] [CrossRef]
- Chen, W.; Ran, S.; Li, C.; Li, Z.; Wei, N.; Li, J.; Li, N. Elevated Eosinophil Counts in Acute Exacerbations of Bronchiectasis: Unveiling a Distinct Clinical Phenotype. Lung 2024, 202, 53–61. [Google Scholar] [CrossRef]
- Oscullo, G.; Gómez-Olivas, J.D.; Ingles, M.; Mompean, S.; Martinez-Perez, R.; Suarez-Cuartin, G.; Rosa-Carrillo, D.; Martinez-Garcia, M.A. Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment. J. Clin. Med. 2023, 12, 6417. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.A.; Olveira, C.; Girón, R.; García-Clemente, M.; Máiz, L.; Sibila, O.; Golpe, R.; Rodríguez-Hermosa, J.L.; Barreiro, E.; Raúl, M.; et al. Reliability of blood eosinophil count in steady-state bronchiectasis. Pulmonology 2025, 31, 2416836. [Google Scholar] [CrossRef]
- Shoemark, A.; Shteinberg, M.; De Soyza, A.; Haworth, C.S.; Richardson, H.; Gao, Y.; Perea, L.; Dicker, A.J.; Goeminne, P.C.; Cant, E.; et al. Characterization of Eosinophilic Bronchiectasis: A European Multicohort Study. Am. J. Respir. Crit. Care Med. 2022, 205, 894–902. [Google Scholar] [CrossRef]
- Alahmadi, F.H.; Wilkinson, M.; Keevil, B.; Niven, R.; Fowler, S.J. Short- and medium-term effect of inhaled corticosteroids on exhaled breath biomarkers in severe asthma. J. Breath. Res. 2022, 16, 047101. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Elborn, S.; Greene, C.M. Basic, translational and clinical aspects of bronchiectasis in adults. Eur. Respir. Rev. 2023, 32, 230015. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Katerina, D.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Shteinberg, M.; Mall, M.A.; O’Donnell, A.E.; Watz, H.; Gupta, A.; Frahm, E.; Eleftheraki, A.; Rauch, J.; Sanjay, H.C.; et al. Cathepsin C (dipeptidyl peptidase 1) inhibition in adults with bronchiectasis: AIRLEAF, a phase II randomised, double-blind, placebo-controlled, dose-finding study. Eur. Respir. J. 2025, 65, 2401551. [Google Scholar] [CrossRef]
- Tanabe, N.; Matsumoto, H.; Kogo, M.; Morimoto, C.; Nomura, N.; Hayashi, Y.; Sakamoto, R.; Oguma, T.; Nagasaki, T.; Sunadome, H.; et al. Exploring the roles of airway dipeptidyl peptidase 1 in obstructive airway disease. ERJ Open Res. 2025, 11, 00841–02024. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Burgel, P.R.; Daley, C.L.; De Soyza, A.; Haworth, C.S.; Mauger, D.; Mange, K.; Teper, A.; Fernandez, C.; Conroy, D.; et al. Brensocatib in non-cystic fibrosis bronchiectasis: ASPEN protocol and baseline characteristics. ERJ Open Res. 2024, 10, 00151–0202. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Keir, H.R.; Chalmers, J.D. Endotypes in bronchiectasis: Moving towards precision medicine. A narrative review. Pulmonology 2023, 29, 505–517. [Google Scholar] [CrossRef]
- Nigro, E.; Mosella, M.; Daniele, A.; Mallardo, M.; Accardo, M.; Bianco, A.; Perrotta, F.; Scialò, F. Adiponectin Increase in Patients Affected by Chronic Obstructive Pulmonary Disease with Overlap of Bronchiectasis. Life 2023, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Lu, H.W.; Bai, J.W.; Yang, J.W.; Mao, B.; Yu, L.; Xu, J.F. The role of volatile organic compounds for assessing characteristics and severity of non-cystic fibrosis bronchiectasis: An observational study. Front. Med. 2024, 11, 1345165. [Google Scholar] [CrossRef]
- Kuo, P.H.; Jhong, Y.C.; Kuo, T.C.; Hsu, Y.T.; Kuo, C.H.; Tseng, Y.J. A Clinical Breathomics Dataset. Sci. Data 2024, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath Analysis: Comparison among Methodological Approaches for Breath Sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Xie, G.; Huo, X.; Li, J.; Deng, S.; Su, Y. Implantable and Biodegradable Smart Textiles for Continuous Limb and Gastrointestinal Motility Monitoring. Small 2025, 21, 2407773. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | COPD (n = 56) | BCO (n = 42) | p-Value |
|---|---|---|---|
| Number of patients | 56 | 42 | – |
| Sex (M/F) | 38\18 | 30\12 | 0.92 |
| Age (years) | 69.2 ± 8.4 | 70.5 ± 7.9 | 0.44 |
| FEV1 (% predicted) | 67.3 ± 22.3 | 52.3 ± 16.5 | <0.01 |
| FVC (% predicted) | 83.2 ± 25.8 | 66.7 ± 17.8 | <0.01 |
| BMI (kg/m2) | 26.8 ± 4.1 | 25.9 ± 4.3 | 0.28 |
| CAT score | 18.5 ± 6.2 | 19.4 ± 7.1 | 0.51 |
| mMRC score | 2.1 ± 0.8 | 2.3 ± 0.9 | 0.26 |
| Eosinophils ≥ 300 cells/μL (n, %) | 20 (35.7%) | 14 (33.3%) | 0.80 |
| Sensor No. | Polymer Coating Material | General VOC Sensitivity Profile |
|---|---|---|
| S1 | Poly(4-vinylphenol) | Polar alcohols, phenols |
| S2 | Poly(ethylene-co-vinyl alcohol) | Small alcohols, ketones |
| S3 | Poly(vinylpyridine) | Amines, basic VOCs |
| S4 | Poly(styrene) | Aromatic hydrocarbons |
| S5 | Poly(vinyl acetate) | Esters, ketones |
| S6 | Poly(butadiene) | Non-polar hydrocarbons |
| S7 | Poly(vinylidene chloride-co-acrylonitrile) | Halogenated compounds |
| S8 | Poly(methyl methacrylate) | Ketones, esters |
| S9 | Poly(ethylene oxide) | Alcohols, ethers |
| S10 | Poly(isobutylene) | Non-polar VOCs |
| S11 | Poly(ethylene-co-propylene) | Alkanes, alkenes |
| S12 | Poly(caprolactone) | Esters, aldehydes |
| S13 | Poly(acrylic acid) | Polar VOCs |
| S14 | Poly(vinyl alcohol) | Alcohols, aldehydes |
| S15 | Poly(2-vinylpyridine) | Amines |
| S16 | Poly(ethylene-co-vinyl acetate) | Ketones, esters |
| S17 | Poly(tetrafluoroethylene) | Fluorinated VOCs |
| S18 | Poly(propylene glycol) | Alcohols, glycols |
| S19 | Poly(4-methyl-1-pentene) | Alkanes, non-polar VOCs |
| S20 | Poly(ethylene terephthalate) | Aromatics, esters |
| S21 | Poly(vinyl chloride) | Chlorinated hydrocarbons |
| S22 | Poly(phenylene oxide) | Aromatics, phenols |
| S23 | Poly(acrylonitrile) | Nitriles, polar VOCs |
| S24 | Poly(lactic acid) | Aldehydes, ketones |
| S25 | Poly(ethylene glycol) | Alcohols, glycols |
| S26 | Poly(butyl methacrylate) | Esters, ketones |
| S27 | Poly(oxymethylene) | Aldehydes |
| S28 | Poly(caprylic acid) | Fatty acids |
| S29 | Poly(urethane) | Ketones, aldehydes |
| S30 | Poly(dimethylsiloxane) | Non-polar VOCs |
| S31 | Poly(vinyl methyl ether) | Ethers, aldehydes |
| S32 | Poly(ethylene naphthalate) | Aromatics, hydrocarbons |
| Principal Component | COPD (Mean ± SD) | BCO (Mean ± SD) | p-Value |
|---|---|---|---|
| PC1 | 0.0501 ± 1.2590 | −0.0668 ± 0.5019 | 0.690 |
| PC2 | −0.0493 ± 1.0085 | 0.0657 ± 1.0095 | 0.695 |
| PC3 | −0.0545 ± 1.2115 | 0.0726 ± 0.6394 | 0.664 |
| PC4 | −0.2811 ± 1.1354 | 0.3748 ± 0.6337 | 0.021 |
| PC | COPD Eos ≥ 300 Cells/μL | BCO Eos ≥ 300 Cells/μL | COPD Eos < 300 Cells/μL | BCO Eos < 300 Cells/μL | p-Value |
|---|---|---|---|---|---|
| PC1 | 0.13 ± 0.73 | −0.02 ± 1.48 | 0.06 ± 0.41 | −0.09 ± 0.55 | 0.889 |
| PC2 | −0.29 ± 0.78 | 0.11 ± 1.12 | −0.02 ± 0.99 | 0.08 ± 0.97 | 0.488 |
| PC3 | −0.14 ± 0.70 | −0.13 ± 1.31 | 0.32 ± 0.68 | 0.12 ± 0.84 | 0.430 |
| PC4 | −0.19 ± 1.19 | −0.23 ± 1.09 | −0.09 ± 0.79 | 0.49 ± 0.60 | 0.014 |
| Comparison | Cross-Validated Classification Accuracy (%) |
|---|---|
| COPD eosinophilic vs. BCO eosinophilic | 33.4 |
| COPD eosinophilic vs. COPD neutrophilic | 41.2 |
| COPD eosinophilic vs. BCO neutrophilic | 76.8 |
| BCO eosinophilic vs. COPD neutrophilic | 44.0 |
| BCO vs. BCO neutrophilic | 38.6 |
| COPD neutrophilic vs. BCO neutrophilic | 74.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaranta, V.N.; Grimaldi, M.; Dragonieri, S.; Marinelli, A.; Portacci, A.; Vulpi, M.R.; Carpagnano, G.E. Breathprint-Based Endotyping of COPD and Bronchiectasis COPD Overlap Using Electronic Nose Technology: A Prospective Observational Study. Chemosensors 2025, 13, 311. https://doi.org/10.3390/chemosensors13080311
Quaranta VN, Grimaldi M, Dragonieri S, Marinelli A, Portacci A, Vulpi MR, Carpagnano GE. Breathprint-Based Endotyping of COPD and Bronchiectasis COPD Overlap Using Electronic Nose Technology: A Prospective Observational Study. Chemosensors. 2025; 13(8):311. https://doi.org/10.3390/chemosensors13080311
Chicago/Turabian StyleQuaranta, Vitaliano Nicola, Mariafrancesca Grimaldi, Silvano Dragonieri, Alessio Marinelli, Andrea Portacci, Maria Rosaria Vulpi, and Giovanna Elisiana Carpagnano. 2025. "Breathprint-Based Endotyping of COPD and Bronchiectasis COPD Overlap Using Electronic Nose Technology: A Prospective Observational Study" Chemosensors 13, no. 8: 311. https://doi.org/10.3390/chemosensors13080311
APA StyleQuaranta, V. N., Grimaldi, M., Dragonieri, S., Marinelli, A., Portacci, A., Vulpi, M. R., & Carpagnano, G. E. (2025). Breathprint-Based Endotyping of COPD and Bronchiectasis COPD Overlap Using Electronic Nose Technology: A Prospective Observational Study. Chemosensors, 13(8), 311. https://doi.org/10.3390/chemosensors13080311







