The Development, Characteristics, and Challenges of Biosensors: The Example of Blood Glucose Meters
Abstract
1. Introduction
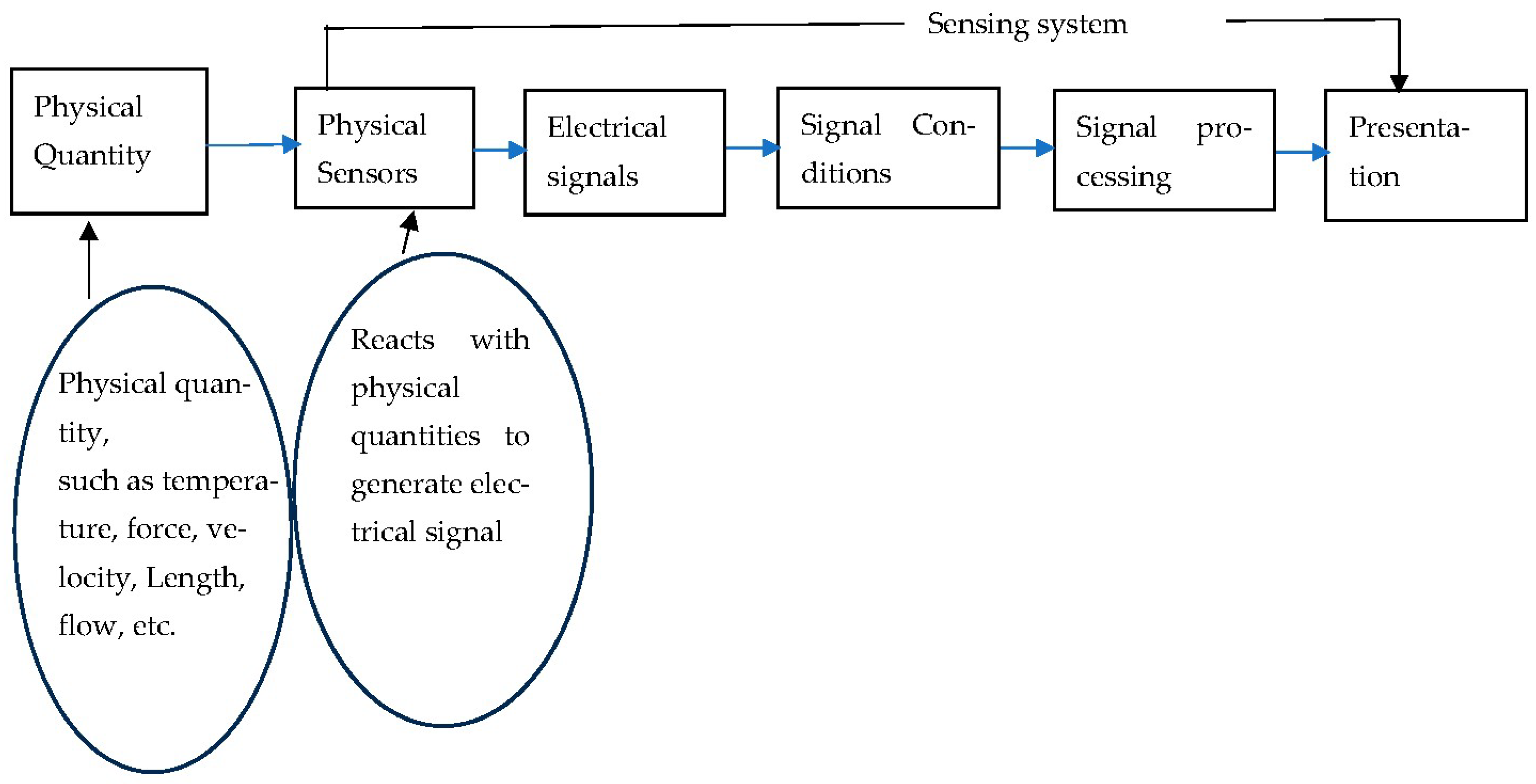
1.1. Physical Sensors
1.2. Subsystems of the Physical Sensors
- Sensing element
- 2.
- Signal conditioning element
- 3.
- Signal processing element
- 4.
- Data presentation element
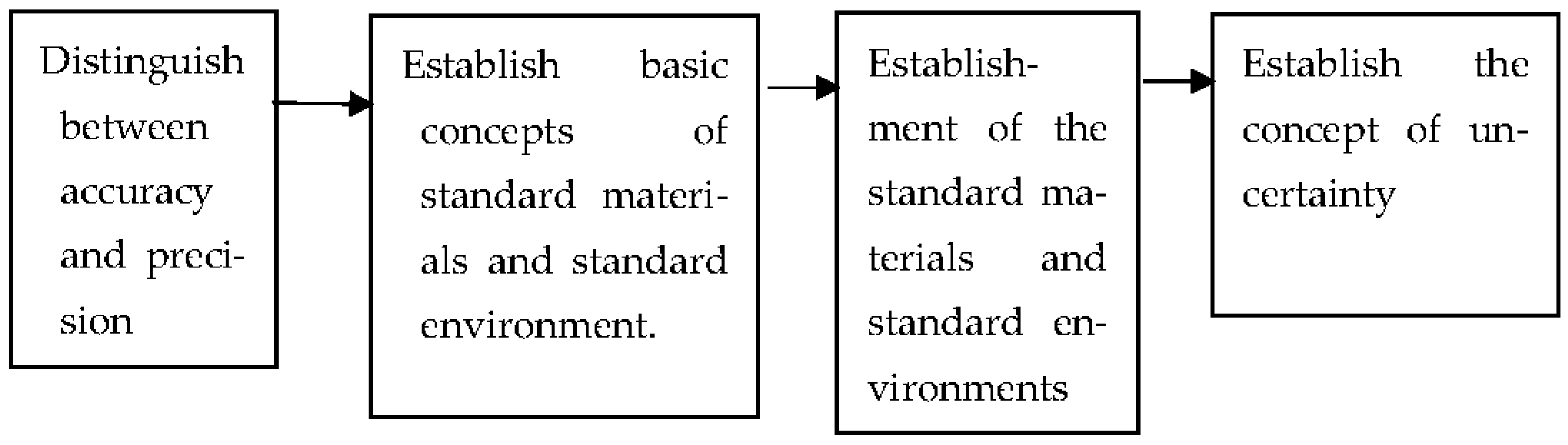
1.3. The Characteristics of the Physical Sensor
- Versatility and standardization
- 2.
- 3.
- Each sensing element has its specific calibration equation.
- 4.
- Establishment of the calibration equation
- 5.
- Impact of the working environment
Measurement Theories for Physical Sensing
- Standard materials and standard environments are traceable, and seven benchmark quantities, including length, mass, time, temperature, electrical quantity, mole, and illumination, are established. The accuracy of standard quantities at different levels is then established based on these benchmark quantities [83,84].
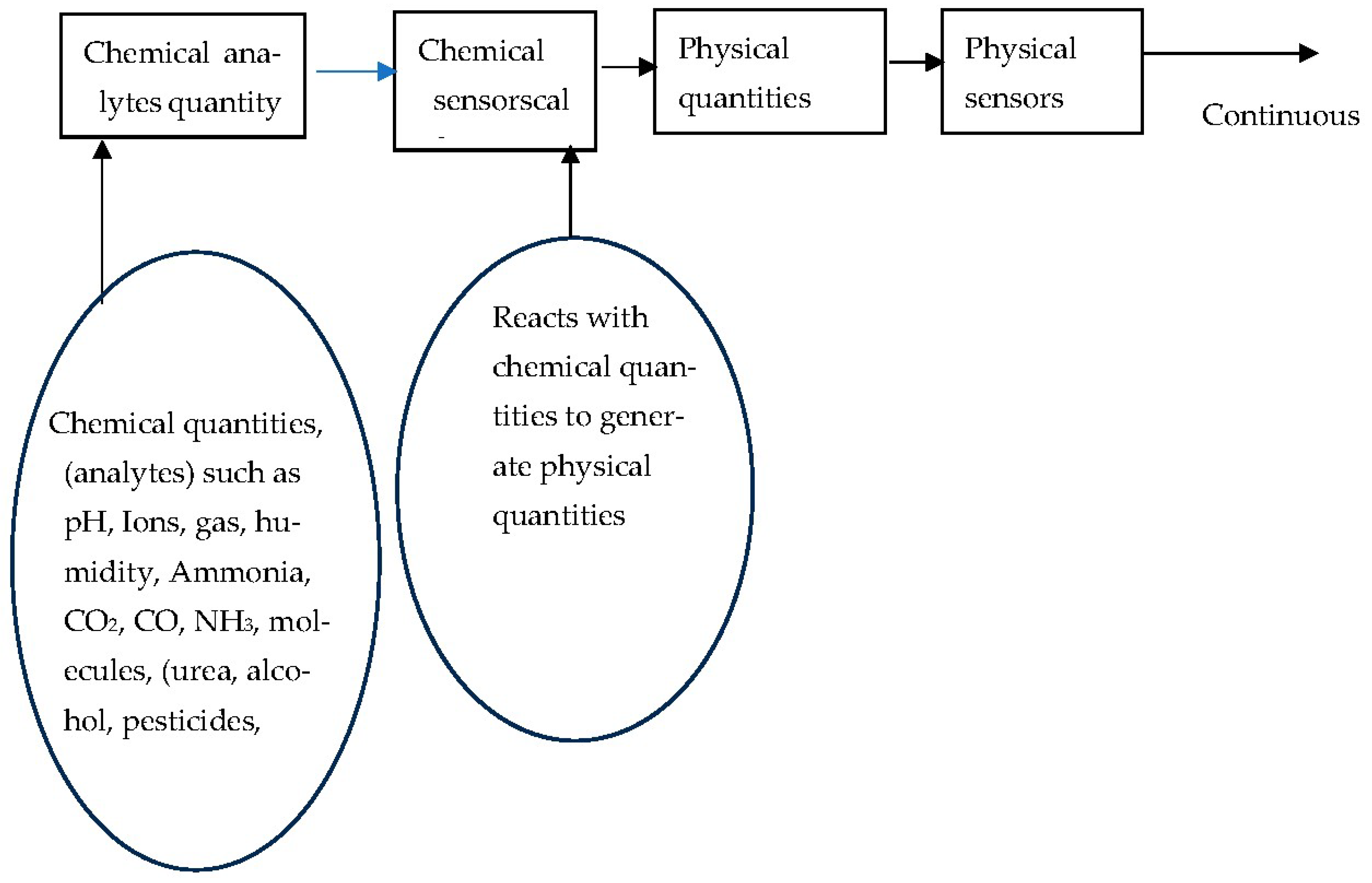
1.4. Chemical Sensors
- Frequent calibration
- 2.
- The recovery time
- 3.
- Dynamic time of response
- 4.
- Cross-sensitivity
- 5.
- The trends of chemical sensors
1.5. Reference Materials
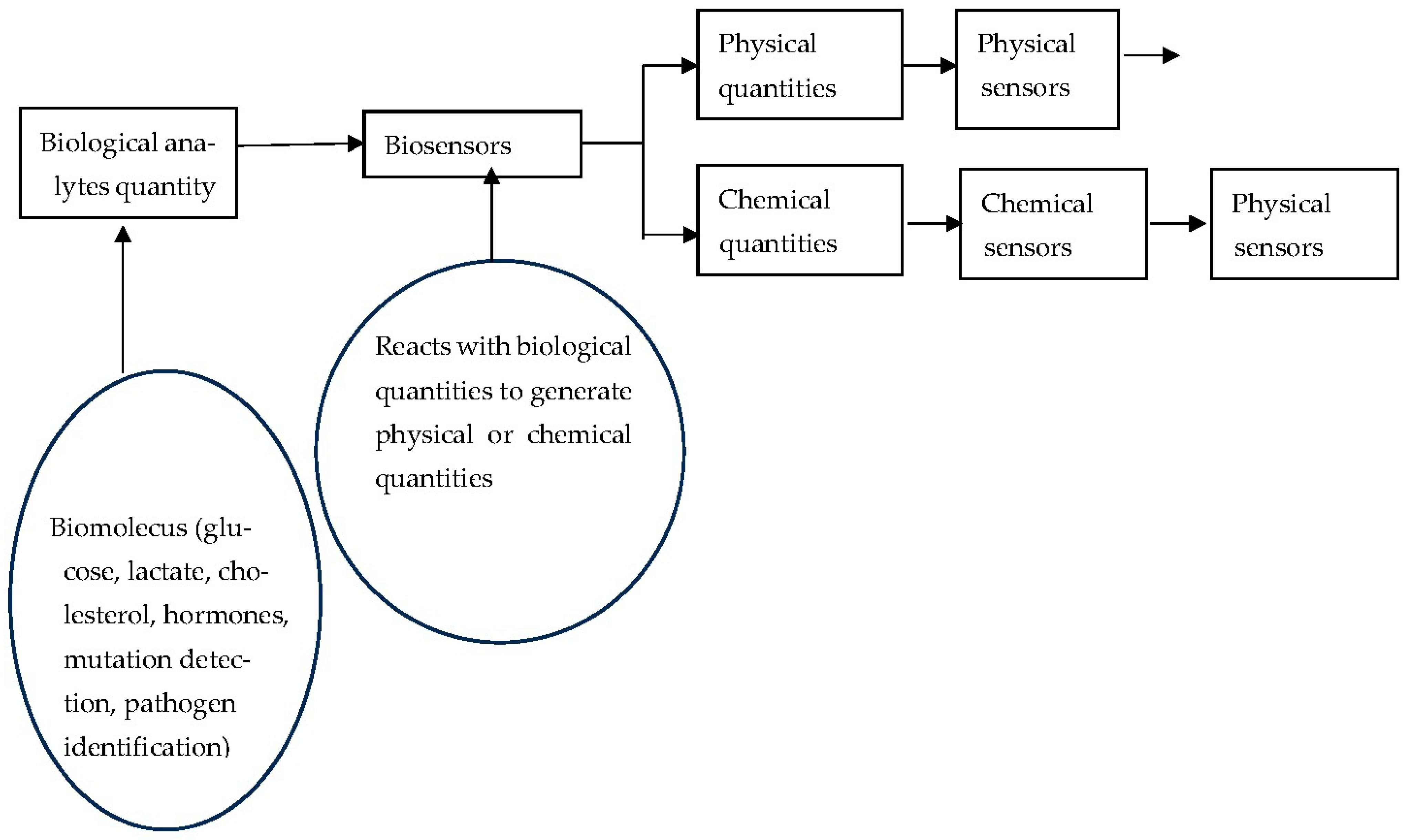
1.6. Biosensors
2. The Characteristics of the Glucose Meter
2.1. The History of the Glucose Biosensors
2.2. Characteristics of Glucosidases for Glucose Biosensors
- Catalytic activity
- 2.
- Specificity
- 3.
- Stability
- 4.
- Enzyme immobilization capability
- 5.
- Enzyme kinetics
- 6.
- Coupling with transduction mechanisms
- 7.
- Signal amplification
- 8.
- Biocompatibility and safety
2.3. The Control Solutions of Glucose Biosensors
2.4. Success Factors of Blood Glucose Meters
2.4.1. Technical Aspects
- Mature technology
- 2.
- Accessibility of sensing elements
- 3.
- Availability of the standard reference materials
- 4.
- Easy to use
- 5.
- Continuous innovative development.
- 6.
- Development of continuous glucose monitors (CGM).
2.4.2. Larger Market
- Huge demand
- 2.
- The characteristics of diabetes
- 3.
- Strict blood sugar control
- 4.
- Controlled insulin dosage
2.4.3. Institutional Aspects
- Regulatory approval and standardization
- 2.
- Reimbursement of purchase funds
2.4.4. Financial Aspects
3. Highly Developed and Competitive Biosensors
3.1. Continuous Glucose Monitoring (CGM)
3.2. Paper-Based Biosensors
4. Challenges in the Development of Biosensors
4.1. Issues in the Commercialization of Biosensors
4.1.1. Completeness of Scientific Development
- Reproducibility: many biosensors developed in laboratories operate under controlled conditions, but reproducing consistent performance in real-world settings is significantly more challenging [230]. Ensuring that every sensor behaves the same way under the same conditions is difficult, particularly for biosensors that rely on biological components [231].
4.1.2. Oversimplifying the Practical Application
4.1.3. Challenges of Mass Production
- Scalability: laboratory biosensors might work well in research, but scaling them for consistent mass production can be expensive and technically demanding [236].
- Cost of materials: high-quality biological recognition elements (e.g., monoclonal antibodies) can be expensive or difficult to produce in large quantities [237].
- Batch-to-batch variability: maintaining quality across mass production is a hurdle [239].
4.1.4. Market Demand and Development Costs [236]
4.2. The Methods of Immobilization
4.3. Market Competitors
5. Future Trends for Glucose Detection
5.1. Non-Invasive and Minimally Invasive Methods [181,260,261]
5.2. Integration with Wearable Devices [191,192,262,263]
5.3. Enhancement of Continuous Glucose Monitoring (CGM) [179,181,182,264]
5.4. Alternative Biofluids for Detection [187,188,265]
5.5. Remote Monitoring and Telehealth Integration [193,194,266]
5.6. AI and Predictive Analytics [195,267]
5.7. Sustainability and Accessibility [268]
6. Conclusions
- Short lifespan and stability
- 2.
- Complex fabrication and calibration
- 3.
- Invasiveness and user discomfort
- 4.
- Biofouling and surface contamination
- 5.
- Data interpretation and user interface
- 6.
- High cost of materials and devices
- 7.
- Environmental and storage constraints
- 8.
- Regulatory and Standardization Barriers
- Enhance sensitivity and selectivity
- 2.
- Advanced wearable and implantable biosensors
- 3.
- Development of the environment-friendly paper-based biosensors
- 4.
- Focus on global health and resource-limited regions
- 5.
- Integrate with AI and data analytics
- 6.
- Promote interdisciplinarity and innovation
- Can the characteristics of the biosensing element be comparable to glucosidases?
- Can all technical limitations, selectivity, sensitivity, and reproducibility be solved?
- What are the service life and storage methods of the biological components?
- How are biological components and transducers connected?
- How should the biological reference materials be prepared?
- How can mass production and quality be controlled?
- How do we define usage time and storage time?
- What is the cost and sale price after commercialization?
- Does any competitor exist in the market?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
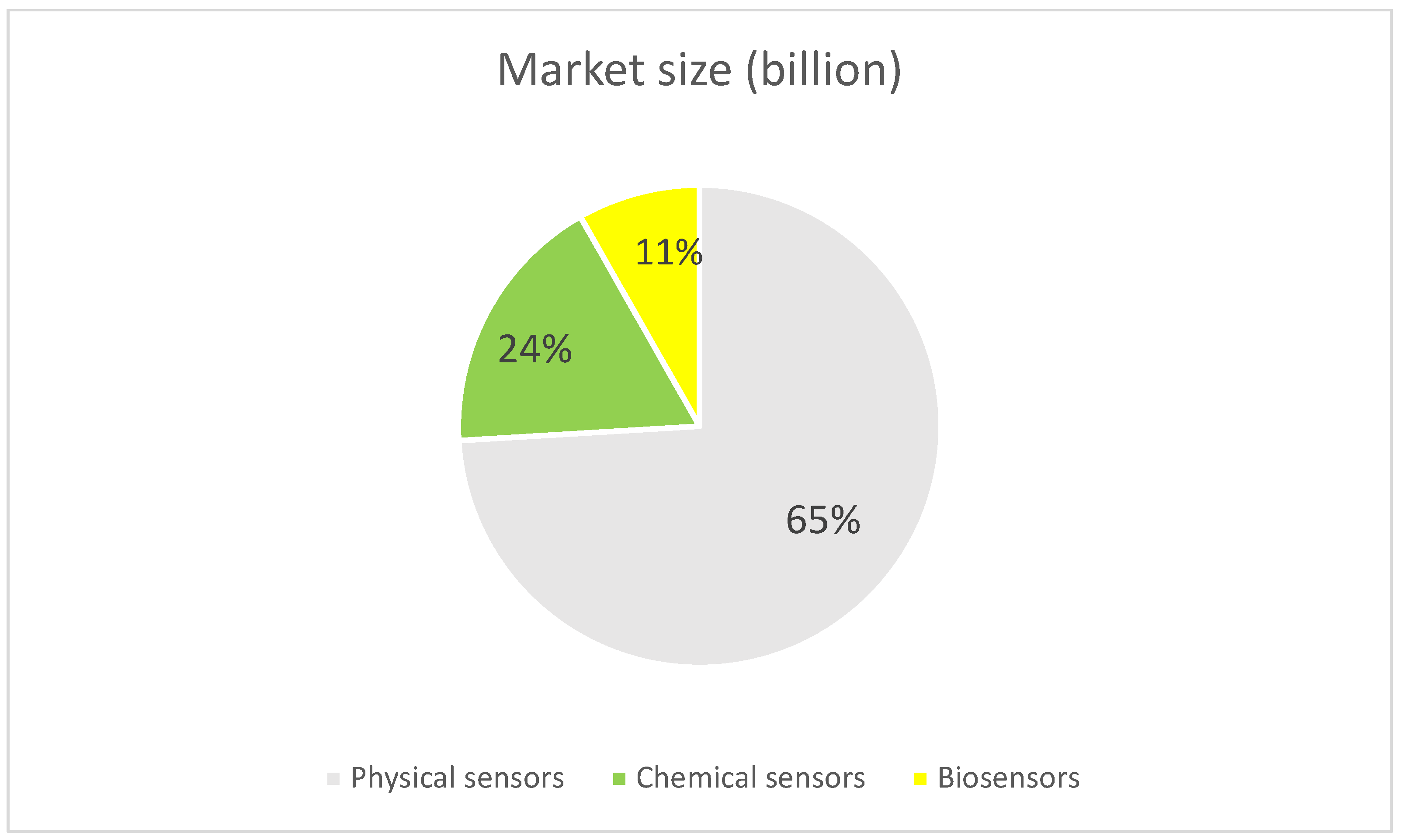
- Mordor Intelligence. Sensor Market Size—Industry Report on Share, Growth Trends & Forecasts Analysis (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/global-smart-sensors-market-industry (accessed on 20 June 2025).
- Fortune Business Insight. Sensor Market Size, Share & Industry Analysis, by Technology (MEMS, CMOS, and Others), by Types. Available online: https://www.fortunebusinessinsights.com/sensor-market-109899 (accessed on 20 June 2025).
- Sezgintürk, M.K. Commercial Biosensors and Their Applications: Clinical, Food, and Beyond; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Eggins, B.R. Chemical Sensors and Biosensors; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Spichiger-Keller, U.E. Chemical Sensors and Biosensors for Medical and Biological Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Janata, J. Principles of Chemical Sensors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Lalauze, R. Chemical Sensors and Biosensors; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- De Marcellis, A.; Ferri, G. Physical and Chemical Sensors. In Analog Circuits and Systems for Voltage-Mode and Current-Mode Sensor Interfacing Applications, Analog Circuits and Signal Processing; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Banica, F.G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Mathew, R.; Ajayan, J. Biosensors: Developments, Challenges and Perspectives; Springer Nature: Singapore, 2024. [Google Scholar]
- Serna-Cock, L.; Perenguez-Verdugo, J.G. Biosensors applications in agri-food industry. In Environmental Biosensors; IntechOpen: London, UK, 2011; pp. 43–64. [Google Scholar]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral. Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Yurish, S. Chemical Sensors and Biosensors; Advances in Sensors: Reviews (6); International Frequency Sensor Association (IFSA) Publishing: Barcelona, Spain, 2018. [Google Scholar]
- Otero, F.; Magner, E. Biosensors-recent advances and future challenges in electrode materials. Sensors 2020, 20, 3561. [Google Scholar] [CrossRef]
- Khan, R.; Mohammad, A.; Asiri, A.M. Advanced Biosensors for Health Care Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef]
- Laha, S.; Rajput, A.; Laha, S.S.; Jadhav, R. A concise and systematic review on non-invasive glucose monitoring for potential diabetes management. Biosensors 2022, 12, 965. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Liu, L.; Qiao, H. A review of biosensor technology and algorithms for glucose monitoring. J. Diabetes Complicat. 2021, 35, 107929. [Google Scholar] [CrossRef]
- Kumar, A.; Mahato, K.; Purohit, B.; Chandra, P. Commercial aspects and market pull of biosensors in diagnostic industries. In Miniaturized Biosensing Devices: Fabrication and Applications; Springer Nature: Singapore, 2022; pp. 351–368. [Google Scholar]
- Chakraborty, T.; Senthamizh, R.; Hazra, S.; Patra, S. Current challenges and future prospects for biosensor application in healthcare. Appl. Biosens. Healthc. 2025, 3, 731–749. [Google Scholar]
- Mak, G.C.; Lau, S.S.; Wong, K.K.; Chow, N.L.; Lau, C.S.; Lam, E.T.; Chan, C.W.; Tsang, D.N. Evaluation of rapid antigen detec tion kit from the WHO Emergency Use List for detecting SARS-CoV-2. J. Clin. Virol. 2021, 134, 104712. [Google Scholar] [CrossRef]
- Khalid, M.F.; Selvam, K.; Jeffry, A.J.N.; Salmi, M.F.; Najib, M.A.; Norhayati, M.N.; Aziah, I. Performance of rapid antigen tests for COVID-19 diagnosis: A systematic review and meta-analysis. Diagnostics 2022, 12, 110. [Google Scholar] [CrossRef]
- Larcher, R.; Lottelier, M.; Badiou, S.; Dupuy, A.M.; Bargnoux, A.S.; Cristol, J.P. Analytical performances of the novel i-STAT alinity point-of-care analyzer. Diagnostics 2023, 13, 297. [Google Scholar] [CrossRef]
- Beattie, C.; Thibodeau, L. A-349 Evaluation of i-STAT® point of care blood gas cartridges and competitor blood gas devices against reference standard for PCO2 and PO2. Clin. Chem. 2024, 70 (Suppl. S1), hvae106.343. [Google Scholar] [CrossRef]
- Ferasin, L.; Ferasin, H.; Farminer, J.; Hudson, E.; Lamb, K. Diagnostic value of a point-of-care cardiac troponin-I assay (i-STAT®) for clinical application in canine and feline cardiology. J. Vet. Cardiol. 2024, 56, 35–43. [Google Scholar] [CrossRef] [PubMed]
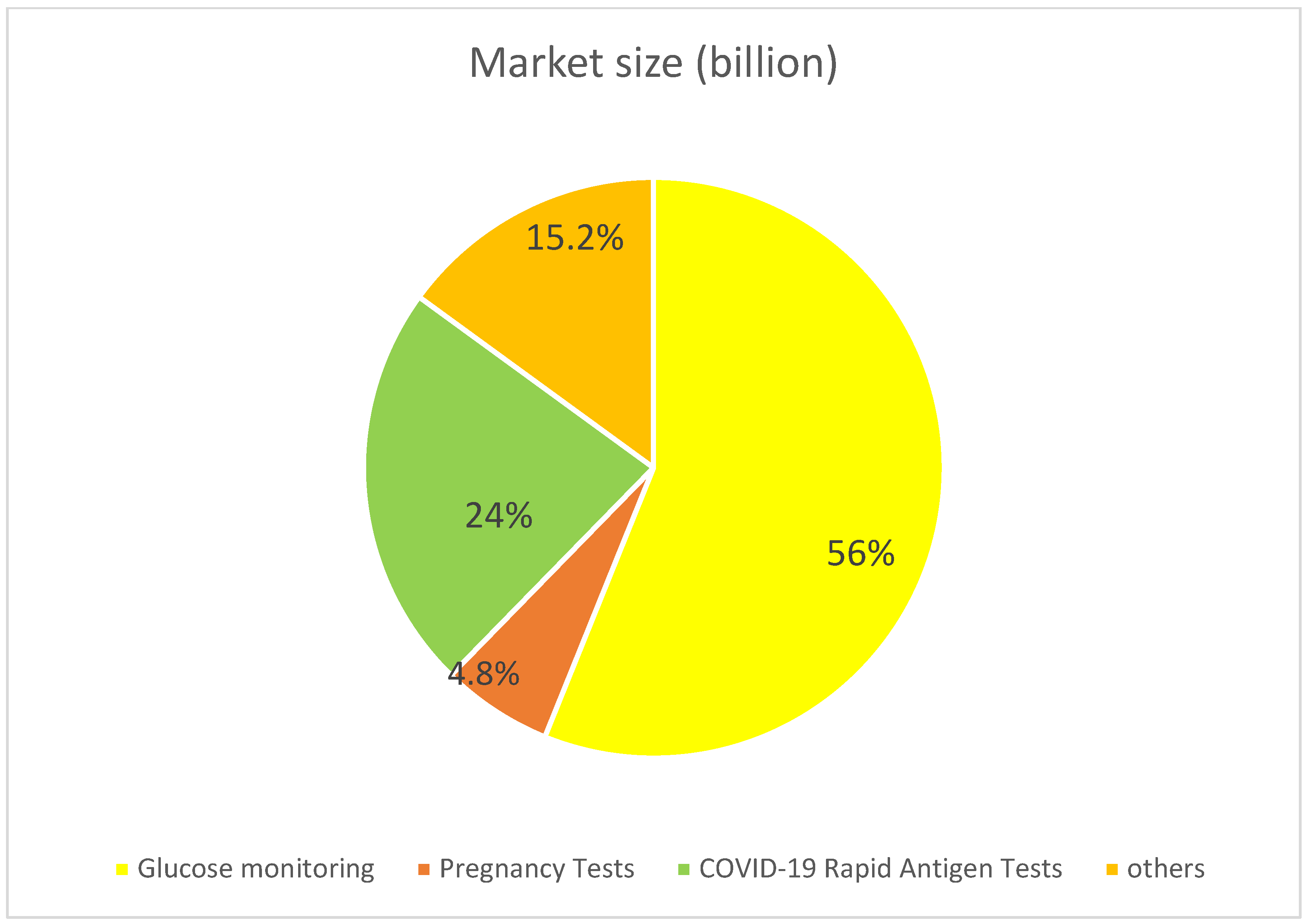
- Global Market Insights. Pregnancy Detection Kits Market Size. Available online: https://www.gminsights.com/industry-analysis/pregnancy-detection-kits-market (accessed on 20 June 2025).
- Grand View Research. COVID-19 Antigen Test Market Size & Trends. Available online: https://www.grandviewresearch.com/industry-analysis/covid-19-antigen-tests-market (accessed on 21 June 2025).
- Precedence Research. Biosensors Market Size and Forecast 2025 to 2034. Available online: https://www.precedenceresearch.com/biosensors-market (accessed on 21 June 2025).
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Clarke, S.F.; Foster, J.R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [CrossRef]
- Sin, M.L.; Mach, K.E.; Wong, P.K.; Liao, J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert. Rev. Mol. Diagn. 2014, 14, 225–244. [Google Scholar] [CrossRef]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Gibson, T.D. Biosensors: The stabilité problem. Analysis 1999, 27, 630–638. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Pezzotti, G.; Pezzotti, I.; Cano, J.; Buonasera, K.; Giannini, D.; Giardi, M.T. Biosensors for effective environment tal and agrifood protection and commercialization: From research to market. Microchim. Acta 2010, 170, 215–225. [Google Scholar] [CrossRef]
- Bertok, T.; Lorencova, L.; Chocholova, E.; Jane, E.; Vikartovska, A.; Kasak, P.; Tkac, J. Electrochemical impedance spectroscopy based biosensors: Mechanistic principles, analytical examples and challenges towards commercialization for assays of protein cancer biomarkers. ChemElectroChem 2019, 6, 989–1003. [Google Scholar] [CrossRef]
- Neethirajan, S.; Ragavan, V.; Weng, X.; Chand, R. Biosensors for sustainable food engineering: Challenges and perspectives. Biosensors 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Ensafi, A.A. Perspective—Paper-based biosensors: Trending topic in clinical diagnostics developments and com mercialization. J. Electrochem. Soc. 2020, 167, 037509. [Google Scholar] [CrossRef]
- dos Santos, C.C.; Lucena, G.N.; Pinto, G.C.; Júnior, M.J.; Marques, R.F. Advances and current challenges in non-invasive wearable sensors and wearable biosensors- A mini-review. Med. Devices Sens. 2021, 4, e10130. [Google Scholar] [CrossRef]
- Zucolotto, V. Specialty grand challenges in biosensors. Front. Sens. 2020, 1, 3. [Google Scholar] [CrossRef]
- Akhlaghi, A.A.; Kaur, H.; Adhikari, B.R.; Soleymani, L. Editors’ choice—Challenges and opportunities for developing electro chemical biosensors with commercialization potential in the point-of-care diagnostics market. ECS Sens. Plus 2024, 3, 3011601. [Google Scholar] [CrossRef]
- Romanholo, P.V.; Paiva, J.V.F.; Sgobbi, L.F. Reliability issues and challenges. In Biosensor Development. Biosensors: Developments, Challenges and Perspectives; Springer Tracts in Electrical and Electronics Engineering; Springer: Berlin/Heidelberg, Germany, 2024; pp. 321–344. [Google Scholar]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose oxidase, an enzyme “ferrari”: Its structure, function, produc tion and properties in the light of various industrial and biotechnological applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef]
- Geleta, G.S. A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: An alternative approach to conventional methods. Anal. Bioanal. Chem. 2022, 414, 7103–7122. [Google Scholar] [CrossRef]
- Bakker, E. So, you have an excellent new sensor. How will you validate it? ACS Sens. 2018, 3, 1431. [Google Scholar] [CrossRef]
- Bentley, J.P. Principles of Measurement Systems, 4th ed.; Pearson Education: London, UK, 2005. [Google Scholar]
- Pallas-Areny, R.; Webster, J.G. Sensors and Signal Conditioning, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kalantar-Zadeh, K. Sensors: An Introductory Course; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Fraden, J. Physical principles of sensing. In Handbook of Modern Sensors: Physics, Designs, and Applications; Springer: Berlin/Heidelberg, Germany, 2004; pp. 37–121. [Google Scholar]
- Kalantar-zadeh, K.; Fry, B. Sensor characteristics and physical effects. In Nanotechnology-Enabled Sensors; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–62. [Google Scholar]
- Mukhopadhyay, S.C.; Mukhopadhyay, S.C. Interfacing of Sensors and Signal Conditioning. In Intelligent Sensing, Instrumentation and Measurements; Springer: Berlin/Heidelberg, Germany, 2013; pp. 29–53. [Google Scholar]
- Schmalzel, J.L.; Rauth, D.A. Sensors and signal conditioning. IEEE Instrum. Meas. Mag. 2006, 8, 48–53. [Google Scholar] [CrossRef]
- Montalvão, D. Introduction to Sensors and Signal Processing. In Mechatronics: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2015; pp. 125–220. [Google Scholar]
- Kirianaki, N.V.; Yurish, S.Y.; Shpak, N.O.; Deynega, V.P. Data Acquisition and Signal Processing for Smart Sensors; Wiley: Chichester, UK, 2002; pp. 51–60. [Google Scholar]
- Tzagkarakis, G.; Tsagkatakis, G.; Alonso, D.; Celada, E.; Asensio, C.; Panousopoulou, P.; Tsakalides, P.; Beferull-Lozano, B. Signal and data processing techniques for industrial cyber-physical systems. In Cyber Physical Systems: From Theory to Practice; CRC Press: Boca Raton, FL, USA, 2015; pp. 181–226. [Google Scholar]
- Berger, C.; Hees, A.; Braunreuther, S.; Reinhart, G. Characterization of cyber-physical sensor systems. Procedia CIRP 2016, 41, 638–643. [Google Scholar] [CrossRef]
- Hall, D.L.; Llinas, J. An introduction to multisensor data fusion. Proc. IEEE 1997, 85, 6–23. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoring and personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Liu, E.; Cai, Z.; Ye, Y.; Zhou, M.; Liao, H.; Yi, Y. An overview of flexible sensors: Development, application, and challenges. SenSors 2023, 23, 817. [Google Scholar] [CrossRef]
- Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043. [Google Scholar]
- Zhu, Z.; Wang, J.; Wu, C.; Chen, X.; Liu, X.; Li, M. A wide range and high repeatability MEMS pressure sensor based on gra phene. IEEE Sens. J. 2022, 22, 17737–17745. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Billey, W.; Begay, M.; Thomas, B.; Woody, C.; Soundappan, T. Sensor reproducibility analysis: Challenges and poten tial solutions. ECS Sens. Plus 2024, 3, 046401. [Google Scholar] [CrossRef]
- Edler, F. Reliable and traceable temperature measurements using thermocouples: Key to ensuring process efficiency and product consistency. John. Matthey Technol. Rev. 2023, 67, 65–76. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, C. Comparison of classical and inverse calibration equations in chemical analysis. Sensors 2024, 24, 7038. [Google Scholar] [CrossRef]
- Müller, R. Calibration and verification of remote sensing instruments and observations. Remote Sens. 2014, 6, 5692–5695. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, R.; Yang, Y.; Zhang, Y.; Xu, Y.; Gong, Y.; Yan, C. A calibration method for multi-sensors in efficient chiller plant systems based on thermo-physical constraints. Appl. Therm. Eng. 2025, 270, 126229. [Google Scholar] [CrossRef]
- Bordé, C.J. Base units of the SI, fundamental constants and modern quantum physics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2005, 363, 2177–2201. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.; Burnett, K. Introduction: The fundamental constants of physics, precision measurements and the base units of the SI. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2005, 363, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Fellmuth, B.; Gaiser, C. High-accuracy realization of temperature fixed and reference points. Rev. Sci. Instrum. 2023, 94, 011102. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chen, C. Determination of optimal measurement points for calibration equations—Examples by RH sensors. Sensors 2019, 19, 1213. [Google Scholar] [CrossRef]
- Foyer, G.; Haller, J.; Müller-Schöll, C. Data structures in calibrations of weights and mass standards. Meas. Sens. 2025, 38, 101468. [Google Scholar] [CrossRef]
- Wiederhold, P.R. Water Vapor Measurement: Methods and Instrumentation; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Howard, J.; Murashov, V.; Cauda, E.; Snawder, J. Advanced sensor technologies and the future of work. Am. J. Ind. Med. 2022, 65, 3–11. [Google Scholar] [CrossRef]
- Chang, H.; Tzenog, P.K. Analysis of the dynamic characteristics of pressure sensors using ARX system identification. Sens. Actuators A Phys. 2008, 141, 367–375. [Google Scholar] [CrossRef]
- Sabitov, A.F.; Tyurina, M.M.; Safina, I.A. Identification of dynamic characteristics of temperature sensors. J. Eng. Thermophys. 2020, 29, 618–631. [Google Scholar] [CrossRef]
- Lai, S.; Barbaro, M.; Bonfiglio, A. Tailoring the sensing performances of an OFET-based biosensor. Sens. Actuators B Chem. 2016, 233, 314–319. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Huo, Y.X. Application evaluation and performance-directed improvement of the native and engineered biosensors. ACS Sens. 2024, 9, 5002–5024. [Google Scholar] [CrossRef]
- Chakrabortty, T.; Varma, M.M. Accuracy and precision limits of concentration sensing using nanopore biosensors. Adv. Theory Simul. 2025, e00305. [Google Scholar] [CrossRef]
- Zhang, J.; Srivatsa, P.; Ahmadzai, F.H.; Liu, Y.; Song, X.; Karpatne, A.; Kong, Z.; Johnson, B.N. Improving biosensor accuracy and speed using dynamic signal change and theory-guided deep learning. Biosens. Bioelectron. 2024, 246, 115829. [Google Scholar] [CrossRef]
- McGrath, M.J.; Scanaill, C.N. Sensing and sensor fundamentals. In Sensor Technologies: Healthcare, Wellness, and Environmental appli Cations; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–50. [Google Scholar]
- Selvolini, G.; Marrazza, G. Sensor principles and basic designs. In Fundamentals of Sensor Technology; Woodhead Publishing: Sawston, UK, 2023; pp. 17–43. [Google Scholar]
- Jameel, R.H.; Wu, Y.C.; Pratt, K.W.; Shreiner, R.H. Primary Standards and Standard Reference Materials for Electrolytic Conductivity; US Department of Commerce, Technology Administration, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Zakaria, O.; Rezali, M.F. Reference materials as a crucial tools for validation and verification of the analytical process. Procedia Soc. Behav. Sci. 2014, 121, 204–213. [Google Scholar] [CrossRef]
- Kirkup, L.; Frenkel, R.B. An Introduction to Uncertainty in Measurement: Using the GUM (Guide to the Expression of Uncertainty in Measurement); Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- NASA-HDBK-8793; Measurement Uncertainty Analysis Principles and Methods, NASA Measurement Quality Assurance Handbook—ANNEX 3. NASA: Washington, DC, USA, 2010.
- Richard, T.; Climensa, S. Chemical sensors: From fundamentals to the future—A review. Adv. Anal. Sci. 2024, 5, 2838. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Roles of shape and size of component crystals in semiconductor gas sensors: I. Response to oxygen. J. Electrochem. Soc. 2008, 155, J85. [Google Scholar] [CrossRef]
- Caliendo, C.; Verona, E.; D’Amico, A. Surface acoustic wave (SAW) gas sensors. In Gas Sensors: Principles, Operation and Developments; Springer: Dordrecht, The Netherlands, 1992; pp. 281–306. [Google Scholar]
- Mandal, D.; Banerjee, S. Surface acoustic wave (SAW) sensors: Physics, materials, and applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef]
- Länge, K. Bulk and surface acoustic wave sensor arrays for multi-analyte detection: A review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, chemical sensors, electrochemical sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef]
- Saputra, H.A. Electrochemical sensors: Basic principles, engineering, and state of the art. Monatshefte Chem. Chem. Mon. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Janshoff, A.; Galla, H.J.; Steinem, C. Piezoelectric mass-sensing devices as biosensors—An alternative to optical biosensors? Angew. Chem. Int. Ed. 2000, 39, 4004–4032. [Google Scholar] [CrossRef]
- Skládal, P. Piezoelectric biosensors. TrAC Trends Anal. Chem. 2016, 79, 127–133. [Google Scholar] [CrossRef]
- Vasuki, S.; Varsha, V.; Mithra, R.; Dharshni, R.A.; Abinaya, S.; Dharshini, R.D.; Sivarajasekar, N. Thermal biosensors and their applications. Am. Int. J. Res. Sci. Technol. Eng. Math. 2019, 1, 262–264. [Google Scholar]
- Yakovleva, M.; Bhand, S.; Danielsson, B. The enzyme thermistor—A realistic biosensor concept. A critical review. Anal. Chim. Acta 2013, 766, 1–12. [Google Scholar] [CrossRef]
- Fonollosa, J.; Fernandez, L.; Gutiérrez-Gálvez, A.; Huerta, R.; Marco, S. Calibration transfer and drift counteraction in chemical sensor arrays using Direct Standardization. Sens. Actuators B Chem. 2016, 236, 1044–1053. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Costa, A.M.S.; Delgadillo, I. Calibration update strategies for an array of potentiometric chemical sensors. Sens. Actuators B Chem. 2017, 238, 1181–1189. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, Y.; Chen, J.; Yu, Y.; Zheng, S.; Zhang, R.; Zhang, R.; Li, Q.; Chen, X.; Sun, C.; et al. Highly sensitive MoTe2 chemical sensor with fast recovery rate through gate biasing. 2D Mater. 2017, 4, 025018. [Google Scholar] [CrossRef]
- Ménil, F.; Susbielles, M.; Debéda, H.; Lucat, C.; Tardy, P. Evidence of a correlation between the non-linearity of chemical sensors and the asymmetry of their response and recovery curves. Sens. Actuators B Chem. 2005, 106, 407–423. [Google Scholar] [CrossRef]
- Nakamoto, T.; Ishida, H. Chemical sensing in spatial/temporal domains. Chem. Rev. 2008, 108, 680–704. [Google Scholar] [CrossRef] [PubMed]
- Fischerauer, G.; Dickert, F.L. An analytic model of the dynamic response of mass-sensitive chemical sensors. Sens. Actuators B Chem. 2007, 123, 993–1001. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Shynybekov, Y.; Soltabayev, B.; Yergaliuly, G.; Mentbayeva, A. The cross-sensitivity of chemiresistive gas sen sors: Nature, methods, and peculiarities: A systematic review. ACS Sens. 2024, 9, 6358–6371. [Google Scholar] [CrossRef]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-reactive chemical sensor arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J. Research on dynamic characteristics of multi-sensor system in the case of cross-sensitivity. Sci. China Technol. Sci. 2005, 48, 1–22. [Google Scholar] [CrossRef]
- Justino, C.I.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Recent trends in the design of chemical sensors based on graphene–metal oxide nanocomposites for the analysis of toxic species and biomolecules. TrAC Trends Anal. Chem. 2019, 120, 115660. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, Y.; Na, M.; Hwang, S.J.; Yoon, Y. Recent trends in chemical sensors for detecting toxic materials. Sensors 2024, 24, 431. [Google Scholar] [CrossRef]
- Uriano, G.A.; Gravatt, C.C.; Morrison, G.H. The role of reference materials and reference methods in chemical analysis. Crit. Rev. Anal. Chem. 2008, 6, 361–412. [Google Scholar]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Recent advances in tin oxide nanomaterials as electro chemical/chemiresistive sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Ellison, S.L.; Williams, A. Quantifying Uncertainty in Analytical Measurement; EURACHEM/CITAC: London, UK, 2012. [Google Scholar]
- Breja, S.K. Role of certified reference materials (CRMs) in standardization, quality control, and quality assurance. In Handbook of Metrology and Applications; Springer Nature: Singapore, 2023; pp. 613–633. [Google Scholar]
- Singh, N. Certified Reference Materials (CRMs) An Introduction. In Handbook of Metrology and Applications; Springer Nature: Singapore, 2022; pp. 1–8. [Google Scholar]
- Saito, T.; Botha, A. Guidance on the contents of accompanying documentation for reference materials (RMs). Accredit. Qual. Assur. 2016, 21, 239–241. [Google Scholar] [CrossRef]
- Kumari, M.; Vijayan, N.; Nayak, D.; Kiran; Pant, R.P. Role of Indian reference materials for the calibration of sophisticated instruments. MAPAN 2022, 37, 505–510. [Google Scholar] [CrossRef]
- Ueno, H. Standard solutions for reference and calibration: Basic facts and practical guidelines. Tech. Mag. Electron. Microsc. Anal. Instrum. 2021, 17, 1–10. [Google Scholar]
- Ricci, M.; de Boer, J. Certified reference materials for environmental monitoring of organic contaminants. In Sample Handling and Trace Analysis of Pollutants; Elsevier Science: Amsterdam, The Netherlands, 2025; pp. 379–415. [Google Scholar]
- Wise, S.A. What if using certified reference materials (CRMs) was a requirement to publish in analytical/bioanalytical chemistry journals? Anal. Bioanal. Chem. 2022, 414, 7015–7022. [Google Scholar] [CrossRef]
- Wise, S.A. What is novel about certified reference materials? Anal. Bioanal. Chem. 2018, 410, 2045–2049. [Google Scholar] [CrossRef]
- Prasad, A.D.; Thangavel, S.; Rastogi, L.; Soni, D.; Dash, K.; Kumar, S.J. Development of a certified reference material (CRM) for seven trace elements (Al, Ca, Fe, K, Mg, Na and Ti) in high purity quartz. Microchem. J. 2022, 172, 106926. [Google Scholar] [CrossRef]
- Elliott, K.; Potts, E.; Bercik, I. NIST Standard Reference Materials Catalog; Office of Reference Materials, National Institute of Stand ards and Technology: Gaithersburg, MD, USA, 2025. [Google Scholar]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent advances in optical biosensors for sensing applications: A review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Zhang, W.; Li, N.; Pu, Q.; Lin, J.M. Recent progress on microfluidic biosensors for rapid detection of pathogenic bacte ria. Chin. Chem. Lett. 2022, 33, 1743–1751. [Google Scholar] [CrossRef]
- Sarcina, L.; Macchia, E.; Tricase, A.; Scandurra, C.; Imbriano, A.; Torricelli, F.; Cioffi, N.; Trosi, L.; Bollella, P. Enzyme based field effect transistor: State-of-the-art and future perspectives. Electrochem. Sci. Adv. 2023, 3, e2100216. [Google Scholar] [CrossRef]
- Cho, S.K.; Cho, W.J. Highly sensitive and transparent urea-EnFET based point-of-care diagnostic test sensor with a triple-gate a-IGZO TFT. Sensors 2021, 21, 4748. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Singh, A.P. Market trends of biosensors. In Smart and Intelligent Nanostructured Materials for Next-Generation Biosen sors; Elsevier: Amsterdam, The Netherlands, 2025; pp. 315–335. [Google Scholar]
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 rapid antigen tests: Promises and challenges. Lancet Infect. Dis. 2021, 21, e290–e295. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chang, S.J.; Chen, C.J.; Liu, J.T. Glucose monitoring sensors: History, principle, and challenges. J. Electrochem. Soc. 2022, 169, 057514. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose meters: A review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Battelino, T.; Peters, A.L.; Chamberlain, J.J.; Aleppo, G.; Bergenstal, R.M. Role of Continuous Glucose Monitoring in Diabetes Treatment; American Diabetes Association: Arlington, VA, USA, 2018; Volume 6. [Google Scholar]
- Yadav, D.; Singh, S.P.; Dubey, P.K. Glucose monitoring techniques and their calibration. In Handbook of Metrology and Applica tions; Springer Nature: Singapore, 2023; pp. 1–23. [Google Scholar]
- Ghosh, M.; Bora, V.R. Evolution in blood glucose monitoring: A comprehensive review of invasive to non-invasive devices and sensors. Discov. Med. 2025, 2, 74. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Yin, H.; Guo, M. A new generation of sensors for non-invasive blood glucose monitoring. Am. J. Transl. Res. 2023, 15, 3825. [Google Scholar]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent advances in enzymatic and non-enzymatic electrochemical glucose sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Dalvi, N. Glucose Meter Reference Design; Application Note AN1560; Microchip Technology Inc.: Chandler, AZ, USA, 2013. [Google Scholar]
- Yanez, M.G. Glucose Meter Fundamentals and Design; Freescale Semiconductor Document Number, AN4364; Freescale Semiconductor, Inc.: Tempe, AZ, USA, 2013. [Google Scholar]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Fard, E.S.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2022, 69, 939–950. [Google Scholar] [CrossRef]
- Franceschini, F.; Taurino, I. Nickel-based catalysts for non-enzymatic electrochemical sensing of glucose: A review. Phys. Med. 2022, 14, 100054. [Google Scholar] [CrossRef]
- Xiang, Y.; Xian, S.; Ollier, R.C.; Yu, S.; Su, B.; Pramudya, I.; Webber, M.J. Diboronate crosslinking: Introducing glucose specificity in glucose-responsive dynamic-covalent networks. J. Control Release 2022, 348, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, M.; Srivastava, A.; Muthukumaran, M.K.; Tsai, P.C.; Lin, Y.C.; Raja, B.K.; Rajendran, J.; Ponnusamy, V.K.; Selvi, J.A. Current advancements and prospects of enzymatic and non-enzymatic electrochemical glucose sensors. Int. J. Biol. Macromol. 2023, 253, 126680. [Google Scholar] [CrossRef]
- Shafaat, A.; Žalnėravičius, R.; Ratautas, D.; Dagys, M.; Meškys, R.; Rutkienė, R.; Gonzalez-Martinez, J.F.; Neilands, J.; Björklund, S.; Sotres, J.; et al. Glucose-to-resistor transduction integrated into a radio-frequency antenna for chip-less and battery-less wireless sensing. ACS Sens. 2022, 7, 1222–1234. [Google Scholar] [CrossRef]
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.L. Biocompatible and long-term monitoring strategies of wearable, ingestible and implantable biosensors: Reform the next generation healthcare. Sensors 2023, 23, 2991. [Google Scholar] [CrossRef]
- Xue, Y.; Thalmayer, A.S.; Zeising, S.; Fischer, G.; Lübke, M. Commercial and scientific solutions for blood glucose monitoring—A review. Sensors 2022, 22, 425. [Google Scholar] [CrossRef]
- Ahmed, I.; Jiang, N.; Shao, X.; Elsherif, M.; Alam, F.; Salih, A.; Butt, H.; Yetisen, A.K. Recent advances in optical sensors for continuous glucose monitoring. Sen. Diagn. 2022, 1, 1098–1125. [Google Scholar] [CrossRef]
- Peng, Z.; Xie, X.; Tan, Q.; Kang, H.; Cui, J.; Zhang, X.; Li, W.; Feng, G. Blood glucose sensors and recent advances: A review. J. Innov. Opt. Health Sci. 2022, 15, 2230003. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y. Review of noninvasive continuous glucose monitoring in diabetics. ACS Sens. 2023, 8, 3659–3679. [Google Scholar] [CrossRef]
- Gohumpu, J.; Lim, W.K.; Peng, Y.; Xue, M.; Hu, Y. Enhancing User Experience: Innovations in Blood Glucose Meter Design for Improved Efficiency and Convenience. In International Conference on Human-Computer Interaction; Springer Nature: Cham, Switzerland, 2024; pp. 47–69. [Google Scholar]
- Hossain, M.I.; Yusof, A.F.; Sadiq, A.S. Factors influencing adoption model of continuous glucose monitoring devices for internet of things healthcare. IoT 2021, 15, 100353. [Google Scholar] [CrossRef]
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable continuous glucose monitoring sensors: A revolution in diabetes treatment. Electronics 2017, 6, 65. [Google Scholar] [CrossRef]
- Flockhart, M.; Larsen, F.J. Continuous glucose monitoring in endurance athletes: Interpretation and relevance of measurements for improving performance and health. Sports Med. 2024, 54, 247–255. [Google Scholar] [CrossRef]
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous glucose monitoring systems-Current status and future perspectives of the flagship technologies in biosensor research. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef]
- Khosravi Ardakani, H.; Gerami, M.; Chashmpoosh, M.; Omidifar, N.; Gholami, A. Recent progress in nanobiosensors for precise detection of blood glucose level. Biochem. Res. Int. 2022, 1, 2964705. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in biosensors for continuous glucose monitoring towards wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Wyawahare, M.; Aadarsh, A.; Anuse, U. Market analysis of various types of rapid testing glucose level equipments. In Proceedings of the 2024 IEEE International Conference for Women in Innovation, Technology & Entrepreneurship (ICWITE), Bangalore, India, 16–17 February 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 311–316. [Google Scholar]
- Pan, B.L.; Pan, Y.T.; Gao, Z.H.; Tung, T.H. Blood Glucose meter buying behavior of diabetic patients: Factors influencing pur chase. Front. Public Health 2022, 10, 880088. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Mustapha, M.T.; Ozsahin, D.U.; Ozsahin, I. Comparative evaluation of point-of-care glucometer devices in the management of diabetes mellitus. In Applications of Multi-Criteria Decision-Making Theories in Healthcare and Biomedical Engineering; Academic Press: Cambridge, MA, USA, 2021; pp. 117–136. [Google Scholar]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive review on wearable sweat-glucose sensors for continuous glu cose monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Pleus, S.; Freckmann, G.; Schauer, S.; Heinemann, L.; Ziegler, R.; Ji, L.; Mohan, V.; Calliar, L.E.; Hinzmann, R. Self-monitoring of blood glucose as an integral part in the management of people with type 2 diabetes mellitus. Diabetes Ther. 2022, 13, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Tolan, N.V.; Melanson, S.E.; Kane, G.; Avery, K.R.; Fitzsimons, D.; Gregory, K.; Goonan, E.M.; Lewandrowski, K.B.; Tanasijevic, M.J. Glucose meter standardization across a large academic hospital system. Clin. Chim. Acta 2022, 531, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Dhatt, G.S.; Agarwal, M.; Bishawi, B. Evaluation of a glucose meter against analytical quality specifications for hospital use. Clin. Chim. Acta 2004, 343, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Medical 4.0 technologies for healthcare: Features, capabilities, and applications. Internet Things Cyber Phys. Syst. 2022, 2, 12–30. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, L.; Li, M.; Ye, X.; Wan, X. Questionnaire and analysis of the standardized use of home self-monitoring portable blood glucose meters. Medicine 2025, 104, e41330. [Google Scholar] [CrossRef]
- Ahmad, F.; Joshi, S.H. Self-care practices and their role in the control of diabetes: A narrative review. Cureus 2023, 15, e41409. [Google Scholar] [CrossRef]
- Vashist, S.K. Continuous glucose monitoring systems: A review. Diagnostics 2013, 3, 385–412. [Google Scholar] [CrossRef]
- Rodbard, D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther. 2016, 18, S3–S13. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- Klonoff, D.C. A review of continuous glucose monitoring technology. Diabetes Technol. Ther. 2005, 7, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, L.B.E.A.; Greven, W.L.; De Valk, H.W. Real-time continuous glucose monitoring system for treatment of diabetes: A systematic review. Diabet. Med. 2011, 28, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab. J. 2019, 43, 383. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; O’neal, D.; Furler, J.; Ekinci, E.I. Continuous glucose monitoring: A review of the evidence, opportunities for future use and ongoing challenges. Intern. Med. J. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Funtanilla, V.D.; Caliendo, T.; Hilas, O. Continuous glucose monitoring: A review of available systems. Pharm. Ther. 2019, 44, 550. [Google Scholar]
- Van Enter, B.J.; Von Hauff, E. Challenges and perspectives in continuous glucose monitoring. ChemComm 2018, 54, 5032–5045. [Google Scholar] [CrossRef]
- Rice, M.J.; Coursin, D.B.; Riou, B. Continuous measurement of glucose: Facts and challenges. Anesthesiology 2012, 116, 199–204. [Google Scholar] [CrossRef]
- Anhalt, H. Limitations of continuous glucose monitor usage. Diabetes Technol. Ther. 2016, 18, 115–117. [Google Scholar] [CrossRef]
- Moses, J.C.; Adibi, S.; Wickramasinghe, N.; Nguyen, L.; Angelova, M.; Islam, S.M.S. Non-invasive blood glucose monitoring technology in diabetes management. Mhealth 2023, 10, 9. [Google Scholar] [CrossRef]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and miniaturized sensor technologies for personalized and preventive medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Pirrera, A.; Giansanti, D. Smart Tattoo Sensors 2.0: A Ten-Year Progress Report through a Narrative Review. Bioengineering 2024, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.B.; Arunachalam, S.; Zhong, A.; Agrawal, P.; Cohen, O.; McMahon, C.M. Improved real-world glycemic control with continuous glucose monitoring system predictive alerts. J. Diabetes Sci. Technol. 2021, 15, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.; Hassan, N.; Tam, W.; Koh, S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: A systematic review of randomised controlled trials and meta-analysis. Diabetologia 2022, 65, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Chen, S.; Zheng, Y.; Peng, L.; Liu, B.; Chen, Z.; Xie, X.; Yi, C.; Jiang, L. Development of smartphone-controlled and microneedle-based wearable continuous glucose monitoring system for home-care diabetes management. ACS Sens. 2023, 8, 1241–1251. [Google Scholar] [CrossRef]
- Litchman, M.L.; Allen, N.A.; Colicchio, V.D.; Wawrzynski, S.E.; Sparling, K.M.; Hendricks, K.L.; Berg, C.A. A qualitative analysis of real-time continuous glucose monitoring data sharing with care partners: To share or not to share? Diabetes Technol. Ther. 2018, 20, 25–31. [Google Scholar] [CrossRef]
- Allen, N.A.; Grigorian, E.G.; Mansfield, K.; Berg, C.A.; Litchman, M.L. Continuous glucose monitoring with data sharing in older adults: A qualitative study. J. Clin. Nurs. 2023, 32, 7483–7494. [Google Scholar] [CrossRef]
- Vettoretti, M.; Cappon, G.; Facchinetti, A.; Sparacino, G. Advanced diabetes management using artificial intelligence and con tinuous glucose monitoring sensors. Sensors 2020, 20, 3870. [Google Scholar] [CrossRef]
- Ji, C.; Jiang, T.; Liu, L.; Zhang, J.; You, L. Continuous glucose monitoring combined with artificial intelligence: Redefining the pathway for prediabetes management. Front. Endocrinol. 2025, 16, 1571362. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Owens, D. Calibration free continuous glucose monitoring (CGM) devices: Weighing up the benefits and limitations. Diabetes Metab. 2020, 46, 79–82. [Google Scholar] [CrossRef]
- Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Calibration of minimally invasive continuous glucose monitoring sensors: State-of-the-art and current perspectives. Biosensors 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; Laffel, L.M.; Wadwa, R.P.; Garg, S.K. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol. Ther. 2018, 20, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.B.C.; Mohd-Naim, N.F.; Tamiya, E.; Ahmed, M.U. Trends in paper-based electrochemical biosensors: From design to application. Anal. Sci. 2018, 34, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Du, D.; Hua, X.; Yu, X.Y.; Lin, Y. Paper-based electrochemical biosensors: From test strips to paper-based microfluidics. Electroanalysis 2014, 26, 1214–1223. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef]
- Colozza, N.; Caratelli, V.; Moscone, D.; Arduini, F. Origami paper-based electrochemical (bio) sensors: State of the art and per spective. Biosensors 2021, 11, 328. [Google Scholar] [CrossRef]
- Wang, K.; Wang, M.; Ma, T.; Li, W.; Zhang, H. Review on the selection of aptamers and application in paper-based sensors. Biosensors 2022, 13, 39. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef]
- Benjamin, S.R.; de Lima, F.; Nascimento, V.A.D.; de Andrade, G.M.; Oriá, R.B. Advancement in paper-based electrochemical biosensing and emerging diagnostic methods. Biosensors 2023, 13, 689. [Google Scholar] [CrossRef]
- Silveira, C.M.; Monteiro, T.; Almeida, M.G. Biosensing with paper-based miniaturized printed electrodes—A modern trend. Biosensors 2016, 6, 51. [Google Scholar] [CrossRef]
- Matar, Z.; Zainon Noor, Z.; Al-Hindi, A.; Yuliarto, B. Recent advances in paper-based nano-biosensors for waterborne pathogen detection: Challenges and solutions. Chem. Biodivers. 2025, 22, e202403451. [Google Scholar] [CrossRef]
- Hosseini, S.; Vázquez-Villegas, P.; Martínez-Chapa, S.O. Paper and fiber-based bio-diagnostic platforms: Current challenges and future needs. Appl. Sci. 2017, 7, 863. [Google Scholar] [CrossRef]
- Gutiérrez-Capitán, M.; Baldi, A.; Fernández-Sánchez, C. Electrochemical paper-based biosensor devices for rapid detection of biomarkers. Sensors 2020, 20, 967. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, C.; Feng, G.; Fang, J. Hospitals and laboratories on paper-based sensors: A mini review. Sensors 2021, 21, 5998. [Google Scholar] [CrossRef] [PubMed]
- Kummari, S.; Panicker, L.R.; Rao Bommi, J.; Karingula, S.; Sunil Kumar, V.; Mahato, K.; Goud, K.Y. Trends in paper-based sensing devices for clinical and environmental monitoring. Biosensors 2023, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Liu, X. A paper-based microfluidic biosensor integrating zinc oxide nanowires for electrochemical glucose de tection. Microsyst. Nanoeng. 2015, 1, 15014. [Google Scholar] [CrossRef]
- Yuan, M.; Alocilja, E.C.; Chakrabartty, S. Self-powered wireless affinity-based biosensor based on integration of paper-based microfluidics and self-assembled RFID antennas. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 799–806. [Google Scholar] [CrossRef]
- Ge, X.; Asiri, A.M.; Du, D.; Wen, W.; Wang, S.; Lin, Y. Nanomaterial-enhanced paper-based biosensors. TrAC Trends Anal. Chem. 2014, 58, 31–39. [Google Scholar] [CrossRef]
- Caratelli, V.; Di Meo, E.; Colozza, N.; Fabiani, L.; Fiore, L.; Moscone, D.; Arduini, F. Nanomaterials and paper-based electro chemical devices: Merging strategies for fostering sustainable detection of biomarkers. J. Mater. Chem. B 2022, 10, 9021–9039. [Google Scholar] [CrossRef]
- Younis, M.R.; Wang, C.; Younis, M.A.; Xia, X.H. Smartphone-Based Biosensors. In Nanobiosensors: From Design to Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 357–387. [Google Scholar]
- Xing, E.; Chen, H.; Xin, X.; Cui, H.; Dou, Y.; Song, S. Recent advances in smart phone-based biosensors for various applications. Chemosensors 2025, 13, 221. [Google Scholar] [CrossRef]
- Mishra, A.; Patra, S.; Srivastava, V.; Uzun, L.; Mishra, Y.K.; Syväjärvi, M.; Tiwari, A. Progress in paper-based analytical devices for climate neutral biosensing. Biosens. Bioelectron. 2022, 11, 100166. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, E.V.; Fraceto, L.F.; Palleschi, G. An eco-designed paper-based algal biosensor for nanoformulated herbicide optical detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Based electrochemical biosensors for food safety analysis. Biosensors 2022, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushal, J.B.; Lee, H.P. Sustainable sensing with paper microfluidics: Applications in health, environment, and food safety. Biosensors 2024, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, X.; Li, N. Smartphone-based mobile biosensors for the point-of-care testing of human metabolites. Mater. Today Bio. 2022, 14, 100254. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper-based diagnostics for personalized health care: Emerging technologies and com mercial aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef]
- Karim, M.E. Biosensors: Ethical, regulatory, and legal issues. In Handbook of Cell Biosensors; Springer International Publishing: Cham, Switzerland, 2021; pp. 679–705. [Google Scholar]
- Demir, E.; Kırboga, K.K.; Işık, M. An overview of stability and lifetime of electrochemical biosensors. In Novel Nanostructured Materials for Electrochemical Biosensing Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 129–158. [Google Scholar]
- Curulli, A. Electrochemical biosensors in food safety: Challenges and perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Biosensors—Recent advances and future challenges. Sensors 2020, 20, 6645. [Google Scholar] [CrossRef]
- Bucur, B.; Purcarea, C.; Andreescu, S.; Vasilescu, A. Addressing the selectivity of enzyme biosensors: Solutions and perspectives. Sensors 2021, 21, 3038. [Google Scholar] [CrossRef]
- Sekhar, P.; Soundappan, T. Sensor reproducibility: Challenges, solutions, and generic model. Electrochem. Soc. Interface 2025, 34, 45–48. [Google Scholar] [CrossRef]
- Corzo, S.P.; Bueno, P.R.; Miranda, D.A. Improving the analytical reproducibility of electrochemical capacitive sensors using the chemical hardness of the interface. IEEE Access 2021, 9, 166446–166454. [Google Scholar] [CrossRef]
- Siontorou, C.G. University-industry relationships for the development and commercialization of biosensors. In Handbook of Cell Biosensors; Springer: Berlin/Heidelberg, Germany, 2022; pp. 707–722. [Google Scholar]
- Chatterjee, N.; Manna, K.; Mukherjee, N.; Saha, K.D. Challenges and future prospects and commercial viability of biosensor-based devices for disease diagnosis. In Biosensor Based Advanced Cancer Diagnostics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 333–352. [Google Scholar]
- Prabowo, B.A.; Cabral, P.D.; Freitas, P.; Fernandes, E. The challenges of developing biosensors for clinical assessment: A re view. Chemosensors 2021, 9, 299. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential ap plications—An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Warfade, T.S.; Dhoke, A.P.; Kitukale, M.D. Biosensors in healthcare: Overcoming challenges and pioneering innovations for disease management. World J. Bio. Pharm. Health Sci. 2025, 21, 350–358. [Google Scholar] [CrossRef]
- Wilson, D.J.; Kumar, A.A.; Mace, C.R. Overreliance on cost reduction as a featured element of sensor design. ACS Sens. 2019, 4, 1120–1125. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and microfluidic biosensors: From fabrication to application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Akhlaghi, A.A.; Kaur, H.; Adhikari, B.R.; Soleymani, L. Challenges and opportunities for developing electrochemical biosensors with commercialization potential in the point-of-care diagnostics market. ECS Sens. Plus 2024, 3, 011601. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Mahalakshmi, K.; Palanivelu, V.R.; Kirubakaran, D. Global Market trends in biomedical sensors: Materials, device engineering, and healthcare applications. Biomed. Mater. Devices 2025, 1–19. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; Labjar, N.; Laasri, S.; Dalimi, M.; Labjar, H.; El Hajjaji, S. Applications and Commercialization Challenges of Voltammetry in Biosensing Applications. In Advancements in Voltammetry for Biosensing Applications; Springer Nature: Singapore, 2025; pp. 461–482. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Inter. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Tripathi, A.; Melo, J.S. Immobilization Strategies: Biomedical, Bioengineering and Environmental Applications. Springer Nature: Singapore, 2020. [Google Scholar]
- Iyer, M.; Shreshtha, I.; Baradia, H.; Chattopadhyay, S. Challenges and opportunities of using immobilized lipase as biosensor. Biotechnol. Genet. Eng. Rev. 2022, 38, 87–110. [Google Scholar] [CrossRef]
- Suni, I.I. Substrate materials for biomolecular immobilization within electrochemical biosensors. Biosensors 2021, 11, 239. [Google Scholar] [CrossRef]
- Pedersen, T.; Gurevich, L.; Magnusson, N.E. Aspects of electrochemical biosensors using affinity assays. Biosensors 2025, 15, 166. [Google Scholar] [CrossRef]
- Bhardwaj, T. A review on immobilization techniques of biosensors. Int. J. Eng. Res. 2014, 3, 294–298. [Google Scholar]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, İ. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Lin, C.T.; Wang, S.M. Biosensor commercialization strategy-a theoretical approach. Front. Biosci. 2005, 10, 99. [Google Scholar] [CrossRef]
- Luong, J.H.; Male, K.B.; Glennon, J.D. Biosensor technology: Technology push versus market pull. Biotechnol. Adv. 2008, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Siontorou, C.G.; Batzias, F.A. Innovation in biotechnology: Moving from academic research to product development—The case of biosensors. Criti. Rev. Biotechnol. 2010, 30, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Haick, H. Biodiagnostics in an era of global pandemics—From biosensing materials to data management. View 2022, 3, 20200164. [Google Scholar] [CrossRef]
- Medina, D.A.V.; Maciel, E.V.S.; Lanças, F.M. Modern automated sample preparation for the determination of organic compounds: A review on robotic and on-flow systems. TrAC Trends Anal. Chem. 2023, 166, 117171. [Google Scholar] [CrossRef]
- More, D.; Khan, N.; Tekade, R.K.; Sengupta, P. An update on current trend in sample preparation automation in bioanalysis: Strategies, challenges and future direction. Crit. Rev. Anal. Chem. 2024, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zamanabadi, M.N.; Zamanabadi, T.N.; Alizadeh, R. Measuring serum sodium levels using blood gas analyzer and auto analyzer in heart and lung disease patients: A cross-sectional study. Ann. Med. Surg. 2022, 78, 103713. [Google Scholar] [CrossRef]
- Jain, A.; Subhan, I.; Joshi, M. Comparison of the point-of-care blood gas analyzer versus the laboratory auto-analyzer for the measurement of electrolytes. Int. J. Emerg. Med. 2009, 2, 117–120. [Google Scholar] [CrossRef]
- Solak, Y. Comparison of serum sodium levels measured by blood gas analyzer and biochemistry autoanalyzer in patients with hyponatremia, eunatremia, and hypernatremia. Am. J. Emerg. Med. 2016, 34, 1473–1479. [Google Scholar] [CrossRef]
- Yılmaz, S.; Uysal, H.B.; Avcil, M.; Yılmaz, M.; Dağlı, B.; Bakış, M.; Ömürlü, I.K. Comparison of different methods for measure ment of electrolytes in patients admitted to the intensive care unit. Saudi Med. J. 2016, 37, 262. [Google Scholar] [CrossRef][Green Version]
- Min, S.; Geng, H.; He, Y.; Xu, T.; Liu, Q.; Zhang, X. Minimally and non-invasive glucose monitoring: The road toward commercialization. Sens. Diagn. 2025, 4, 370–396. [Google Scholar] [CrossRef]
- Jain, P.; Joshi, A.M.; Mohanty, S.P.; Cenkeramaddi, L.R. Non-invasive glucose measurement technologies: Recent advancements and future challenges. IEEE Access 2024, 12, 61907–61936. [Google Scholar] [CrossRef]
- Huang, X.; Yao, C.; Huang, S.; Zheng, S.; Liu, Z.; Liu, J.; Wang, J.; Chen, H.; Xie, X. Technological advances of wearable device for continuous monitoring of in vivo glucose. ACS Sens. 2024, 9, 1065–1088. [Google Scholar] [CrossRef]
- Bocu, R. Extended review concerning the integration of electrochemical biosensors into modern IoT and wearable devices. Biosensors 2024, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, D.; Tian, X.; Wang, Y.; Cui, B.; Yang, Y.; Dai, S.; Lin, W.; Zhu, J.; Wang, J.; et al. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef] [PubMed]
- Jarnda, K.V.; Dai, H.; Ali, A.; Bestman, P.L.; Trafialek, J.; Roberts-Jarnda, G.P.; Anaman, R.; Kamara, M.G.; Wu, P.; Ding, P. A review on optical biosensors for monitoring of uric acid and blood glucose using portable POCT devices: Status, challenges, and future horizons. Biosensors 2025, 15, 222. [Google Scholar] [CrossRef]
- Getie, A.; Amlak, B.T.; Ayenew, T.; Gedfew, M. Assessing the impact of telehealth on blood glucose management among patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. BMC Health Serv. Res. 2025, 25, 285. [Google Scholar] [CrossRef]
- Rajeswari, S.V.K.R.; Vijayakumar, P. Development of sensor system and data analytic framework for non-invasive blood glucose prediction. Sci. Rep. 2024, 14, 9206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Maiti, P. Based sustainable biosensors. Mater. Adv. 2024, 5, 3563–3586. [Google Scholar] [CrossRef]
| Category | Challenges | Sources |
|---|---|---|
| Commercialization Gap | Few biosensors (e.g., for glucose, pregnancy, and COVID-19) have been widely commercialized despite a vast number of research publications | [14,31,41] |
| Market Translation | Difficulty in testing long-term performance High production costs Need for scalable, easy-to-manufacture components | [31,36] |
| Sample Complexity | Biological samples are heterogeneous. Performance varies between real and laboratory conditions High specificity required | [33,37,40,42] |
| Device Stability | Enzyme/protein degradation over time Shelf stability (for disposable use) Operational stability (for reusable devices) | [35,43] |
| Sensitivity and Specificity | High detection limits Cross-reactivity in complex matrices Inadequate specificity in real samples | [39,40,42,43] |
| Reproducibility | Fabrication inconsistencies Signal variation in optical and electrochemical transducers Environmental influence on measurements | [40,43,45] |
| Integration and Scalability | Difficulties in component integration Lack of robust and scalable manufacturing for large-scale production | [38,41] |
| Ease of Use | Complex sample preparation Lengthy procedures Complex operation for end users | [34,42] |
| Analytical Validation | Need for real-sample testing Cross-validation with standard methods (e.g., GC-MS for gas sensors) | [37,46] |
| Wearable and Portable Sensors | Biocompatibility issues Power requirements Difficulty detecting targets in low concentrations within biological fluids | [40] |
| Transducer Limitations | Electrochemical: reproducibility, ink resistivity Optical: color intensity variation, matrix interference | [45] |
| Biomarker Challenges | Lack of specific, validated biomarkers (e.g., for cancer types) | [3,43] |
| Detection Time and Signal Quality | Long detection time Low signal-to-noise ratio | [39] |
| Environmental Sensitivity | Ambient conditions affect sensor performance Expensive to maintain sensor activity | [43] |
| Aspect | Physical Sensors | Chemical Sensors | Biosensors |
|---|---|---|---|
| Sensing Element | Physical phenomena (e.g., temperature, pressure, acceleration) | Chemical-sensitive materials (e.g., metal oxides, polymers, SAW films) | Biological elements (e.g., enzymes, antibodies, cells, DNA) |
| Detection Principle | Electrical or mechanical response to physical change | changes in electrical properties, the emission of light, the accumulation of mass on the sensing element, and heat reactions, due to chemical interactions | Biochemical reactions leading to physical or chemical signal generation |
| Signal Processing | Direct (ADC, DAC, amplifiers, display interfaces) | Direct (resistance, current, wave speed); often needs calibration | Direct (processed via transducers) or indirect (requires chemical/physical transducers) |
| Typical Applications | Temperature, pressure, displacement, humidity, acceleration | Gas sensing, pH, ion concentration, chemical composition | Medical diagnostics, food safety, environmental monitoring, personalized healthcare |
| Key Features | High detection limits Cross-reactivity in complex matrices Inadequate specificity in real samples | - Specific to certain chemicals - Requires frequent calibration - Sensitive to the environment. - Can exhibit cross-sensitivity - Integration with IoT trending | - Highly selective due to biological specificity - Can detect trace biological substances - Interface with chemical/physical sensors - Require biological immobilization |
| Interference and Cross-Sensitivity | Generally well-compensated in design (EM interference, temperature) | Significant issue; can be affected by non-target analytes | High selectivity, but can be influenced by complex sample matrices (e.g., blood, food) |
| Standardization | High, well-defined standards, protocols, materials | Increasing reference materials and protocols are emerging | Low; immobilization methods and biological stability hinder standardization |
| Trends and Advances | Enhanced miniaturization, wireless integration | Improved materials, sensitivity, selectivity, and IoT integration | Focus on stability, reproducibility, scalability, and practical usability |
| Commercialization Status | Widely used and mature in the industry | Mature in some applications (e.g., gas sensors), still advancing | Mostly in research/prototype stage; few widely commercialized examples (e.g., glucose meters, pregnancy tests) |
| Trend | Description | Future Implications |
|---|---|---|
| 1. Transition to non-invasive and minimally invasive technologies [181,186] | Development of CGMs using interstitial fluid, sweat, saliva, or optical signals rather than subcutaneous sensors. | Increased patient comfort, improved compliance, and broader use among prediabetic individuals and health-conscious populations. |
| 2. Miniaturization and skin-adherent technologies [187,188] | The inner, smaller, flexible sensors with skin-like properties are being developed. | Improved wearability, particularly for children and active users, with potential applications in consumer wellness wearables. |
| 3. Real-time data analytics and alerts [189,190] | Advanced CGMs offer continuous, real-time glucose readings and trend analysis with predictive alerts. | Early intervention for hypo- and hyperglycemia; enhanced safety during sleep, exercise, or illness; improved decision support for users and clinicians. |
| 4. Smartphone and wearable integration [191,192] | CGM devices sync with smartphones, smartwatches, and fitness trackers for data visualization and sharing. | A provides a more user-friendly experience, enhanced remote monitoring by caregivers and healthcare providers, and integration into digital health ecosystems. |
| 5. Data sharing and cloud connectivity [193,194] | CGM platforms enable cloud storage, data sharing, and AI-based analytics. | Personalized treatment strategies, improved telemedicine services, and population-level diabetes management insights |
| 6. AI and predictive modeling integration [195,196] | Use of machine learning to predict glucose trends and recommend actions. | Precision medicine for diabetes management: potential to extend predictive analytics to comorbidities and lifestyle patterns. |
| 7. Extended sensor lifespan and calibration-free devices [197,198,199] | New CGMs are now factory calibrated and can function continuously for 10–14 days or longer. | Reduced user burden and maintenance; broader acceptance among users who previously resisted CGMs due to inconvenience. |
| Trend | Description | Future Implications |
|---|---|---|
| 1. Low-cost and disposable designs [211,212] | Increasing use of inexpensive materials (e.g., cellulose paper) and simple fabrication methods (e.g., wax printing). | Widely accessible diagnostics in low-resource and rural settings; scalable production for large-scale use in pandemics and screening programs. |
| 2. Integration with microfluidics [213,214] | Development of microfluidic paper-based analytical devices (μPADs) for fluid handling, mixing, and multiplex testing. | More complex, multi-analyte detection in a single test strip; applications in environmental, food, and clinical testing. |
| 3. Use of nanomaterials and advanced functional inks [215,216] | Incorporation of nanoparticles (e.g., gold, carbon, graphene) to enhance sensitivity and specificity. | Improved performance rivals that of traditional biosensors, with potential for early disease detection and analysis of ultra-low concentrations. |
| 4. Digital and smartphone-based readout systems [217,218] | Coupling with smartphones for data acquisition, image analysis, cloud connectivity, and even result interpretation using AI. | Making healthcare diagnostics accessible and interpretable by non-experts; enables telemedicine and remote health monitoring; and provides real-time data tracking for epidemiology and personalized medicine. |
| 5. Eco-friendly and sustainable development [219,220] | Emphasis on biodegradable materials and greener manufacturing processes. | Reduced environmental impact of disposable diagnostics; aligns with global sustainability goals. |
| 6. Environmental and food safety monitoring [221,222] | Expanding application from medical diagnostics to detecting pesticides, heavy metals, or pathogens in food/water. | Preventive public health strategies and rapid screening tools for agricultural and environmental surveillance. |
| 7. Personalized and home-based testing [218,223] | Movement toward user-friendly, at-home test kits for chronic disease management and infectious disease screening. | Empowerment of individuals in health monitoring; potential to reduce the burden on healthcare systems |
| 8. Mass production and commercialization [210,224] | Scalable manufacturing techniques like screen printing, inkjet printing, and laser cutting—standardization efforts for regulatory approval and industrial adoption. | Leads to wider market availability and lower per-unit cost; Drives competition and innovation among medical device startups and established companies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Chen, C. The Development, Characteristics, and Challenges of Biosensors: The Example of Blood Glucose Meters. Chemosensors 2025, 13, 300. https://doi.org/10.3390/chemosensors13080300
Chen H-Y, Chen C. The Development, Characteristics, and Challenges of Biosensors: The Example of Blood Glucose Meters. Chemosensors. 2025; 13(8):300. https://doi.org/10.3390/chemosensors13080300
Chicago/Turabian StyleChen, Hsuan-Yu, and Chiachung Chen. 2025. "The Development, Characteristics, and Challenges of Biosensors: The Example of Blood Glucose Meters" Chemosensors 13, no. 8: 300. https://doi.org/10.3390/chemosensors13080300
APA StyleChen, H.-Y., & Chen, C. (2025). The Development, Characteristics, and Challenges of Biosensors: The Example of Blood Glucose Meters. Chemosensors, 13(8), 300. https://doi.org/10.3390/chemosensors13080300





