Molecularly Imprinted Electrochemical Sensor Electrodes Based on Poly-Pyrrole for Sensitive Detection of Morphine in Wastewater
Abstract
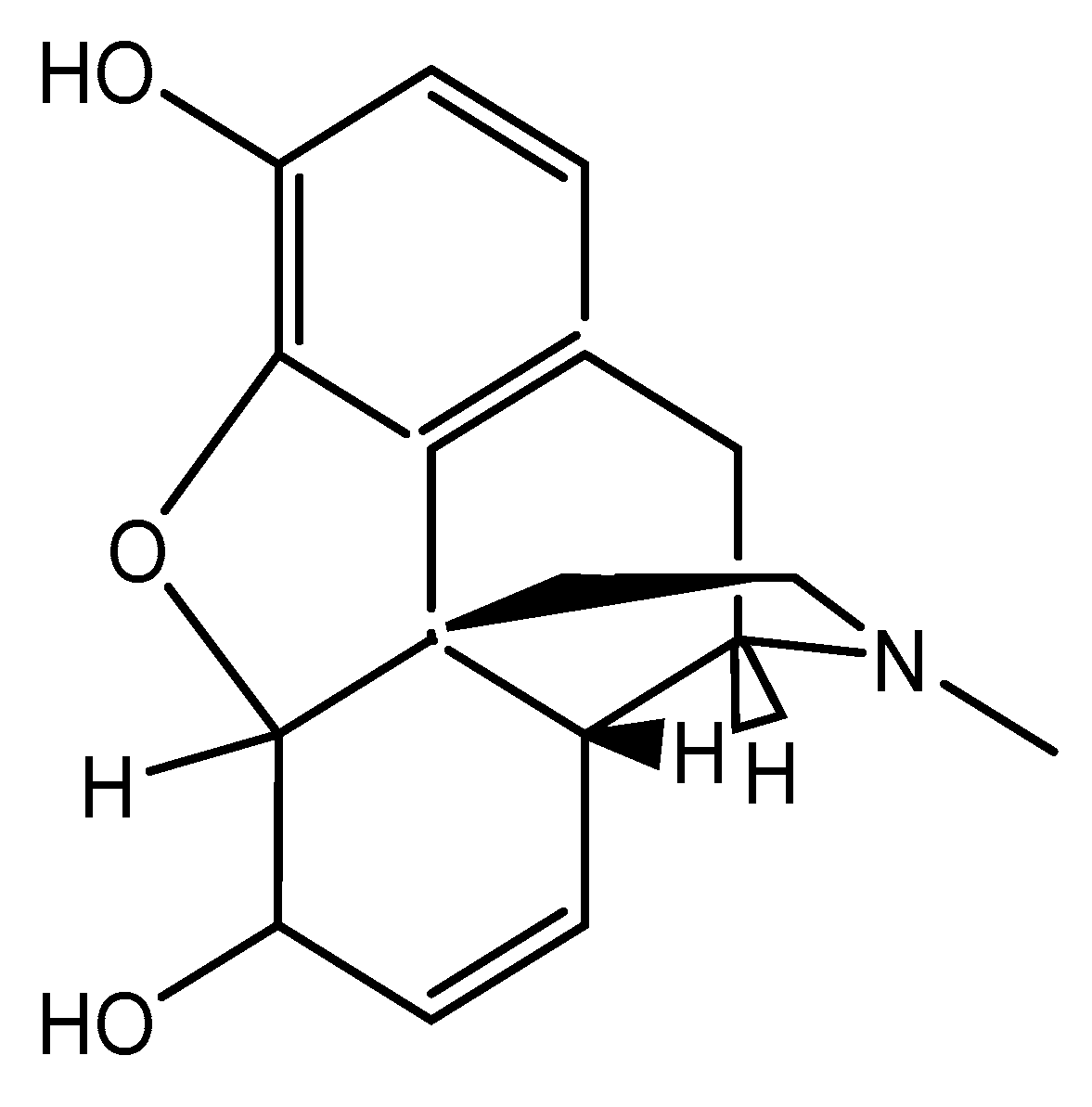
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Electrochemical Apparatus
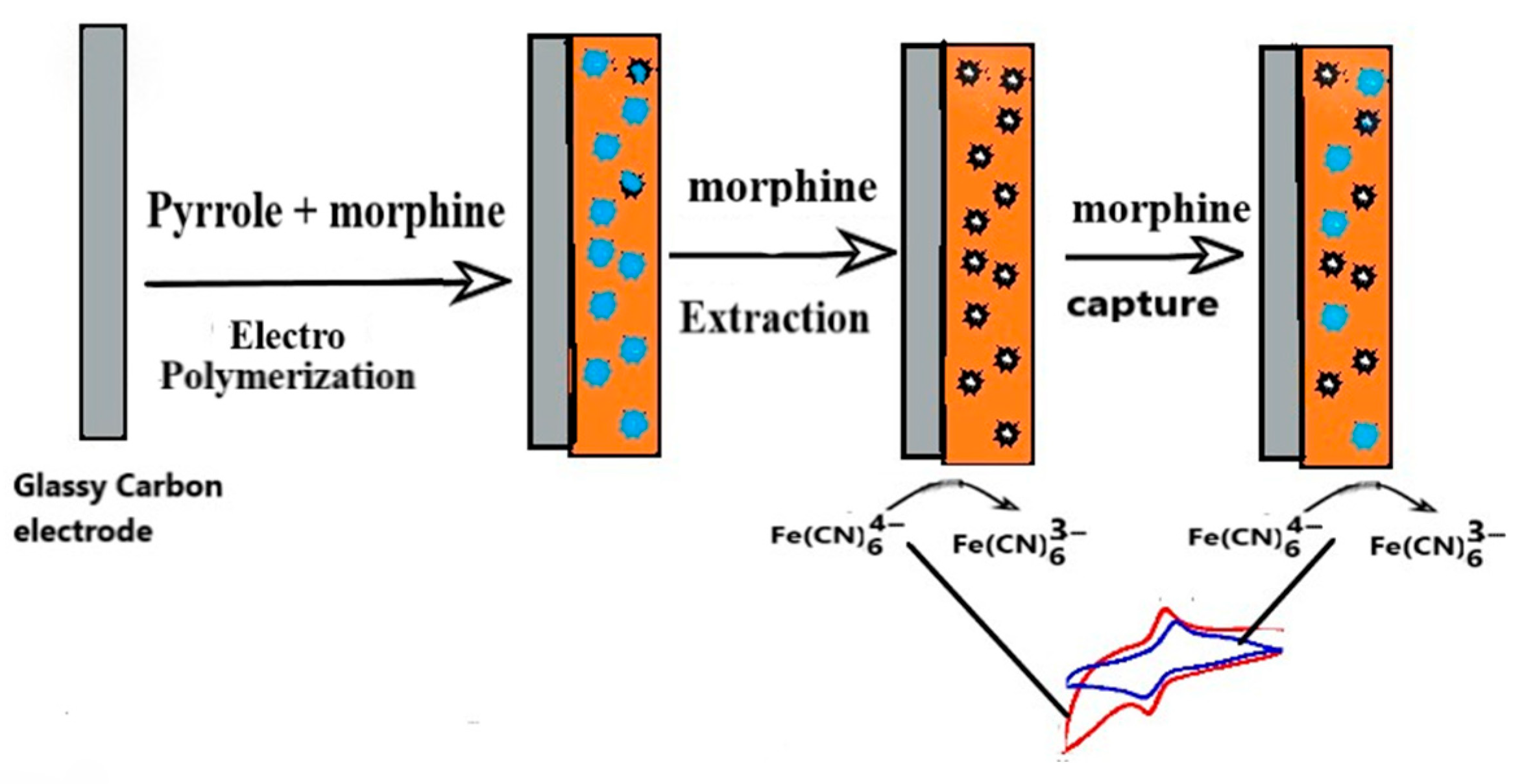
2.3. Preparation of MIP Electrode Sensor
3. Results
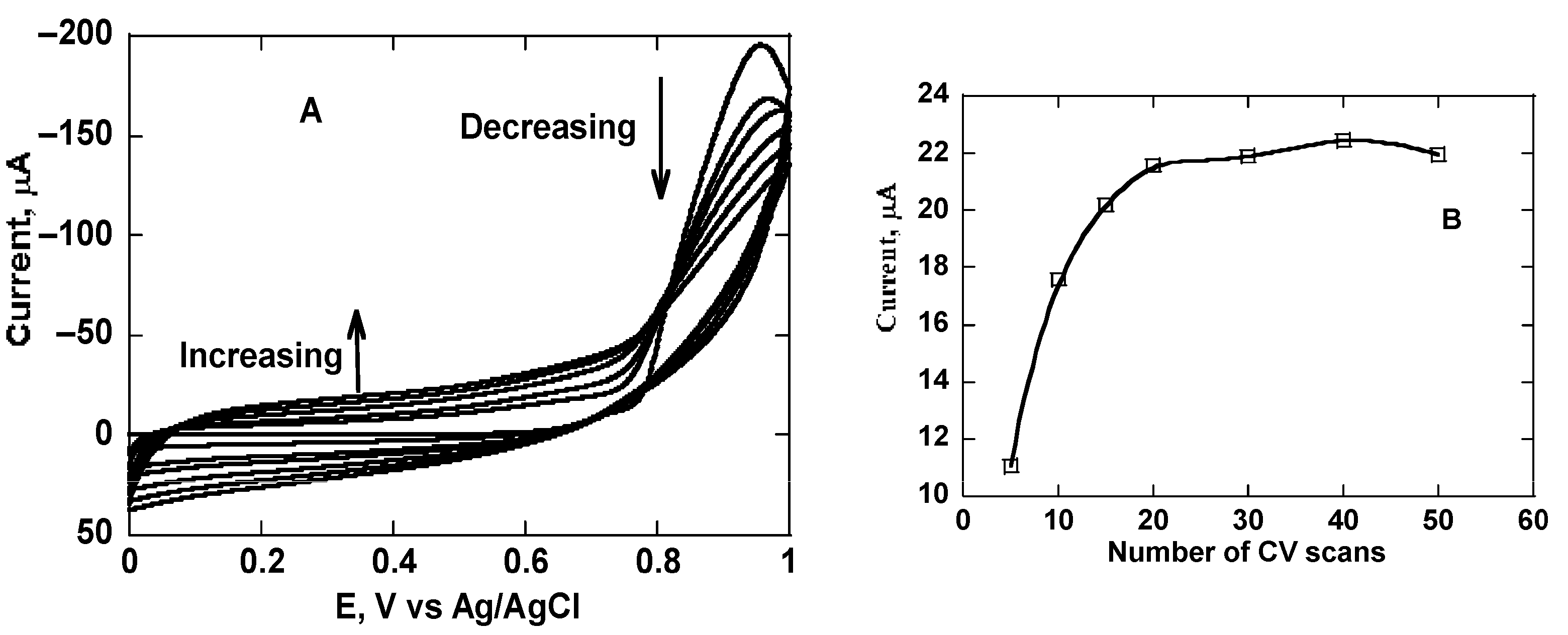
3.1. Electrochemical Characterization of the Imprinted Sensor Electrodes
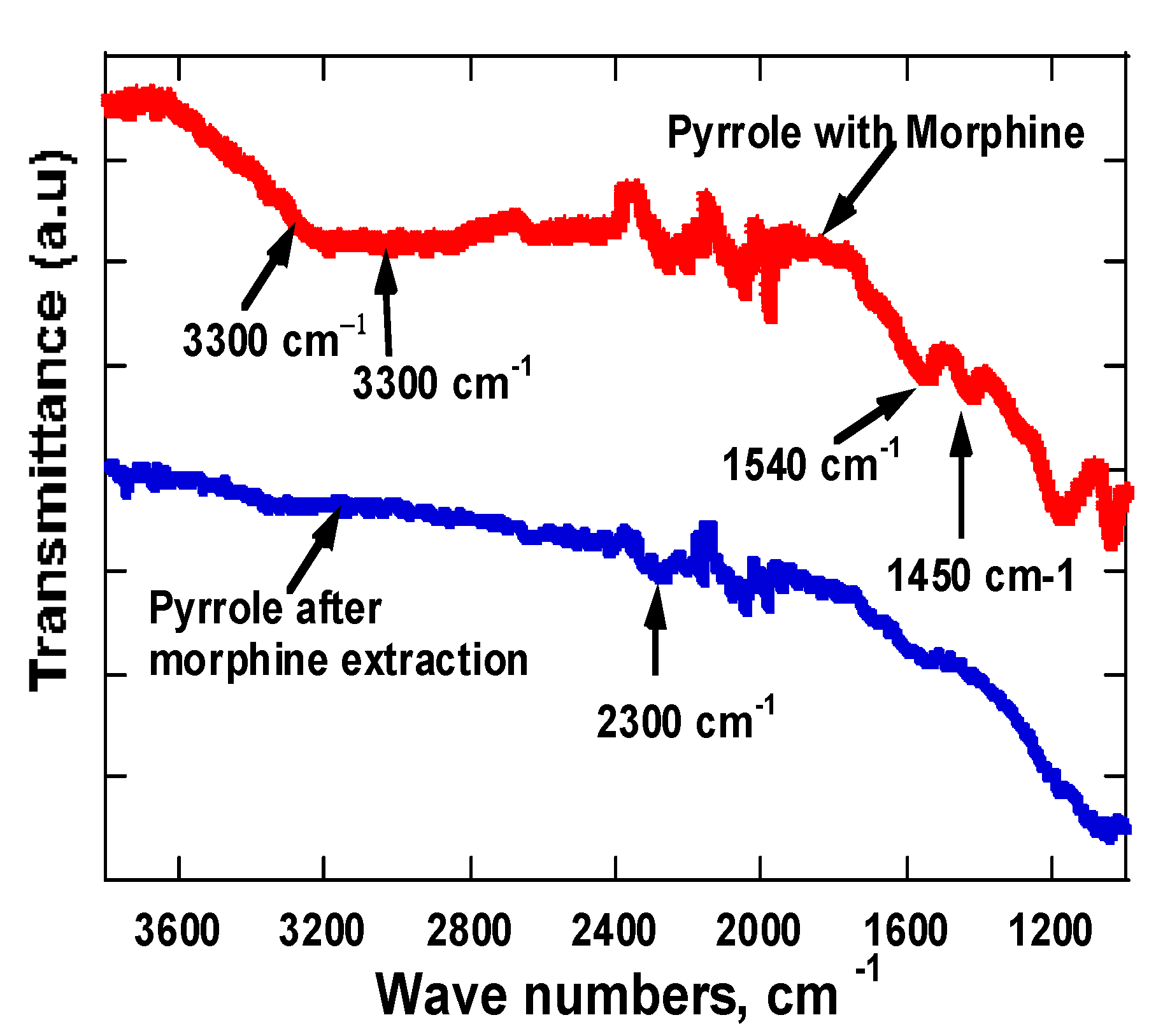
3.2. FTIR Spectroscopy
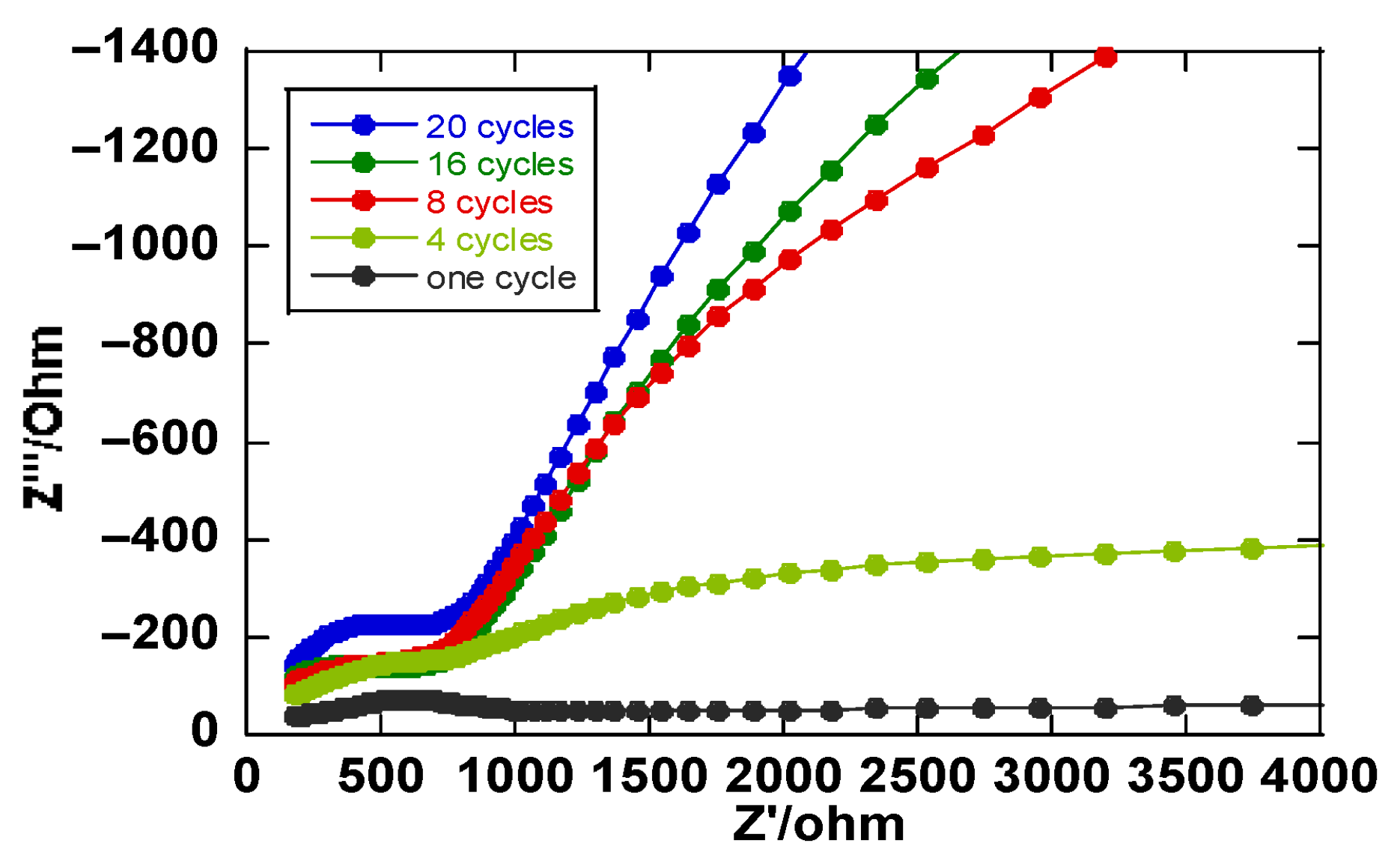
3.3. Electrochemical Impedance Spectroscopy (EIS)
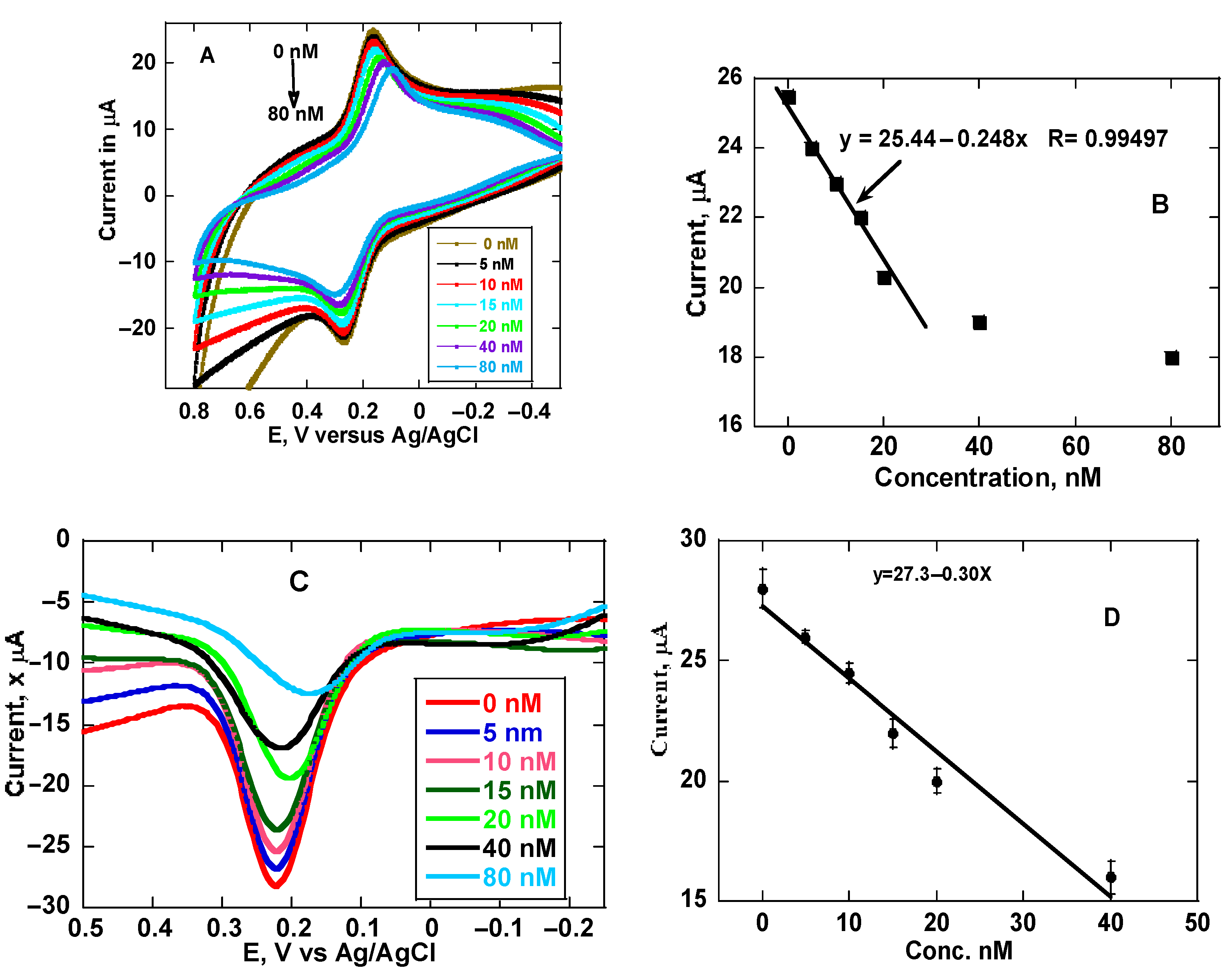
3.4. Analysis of Morphine and Calibration Methods on MIP
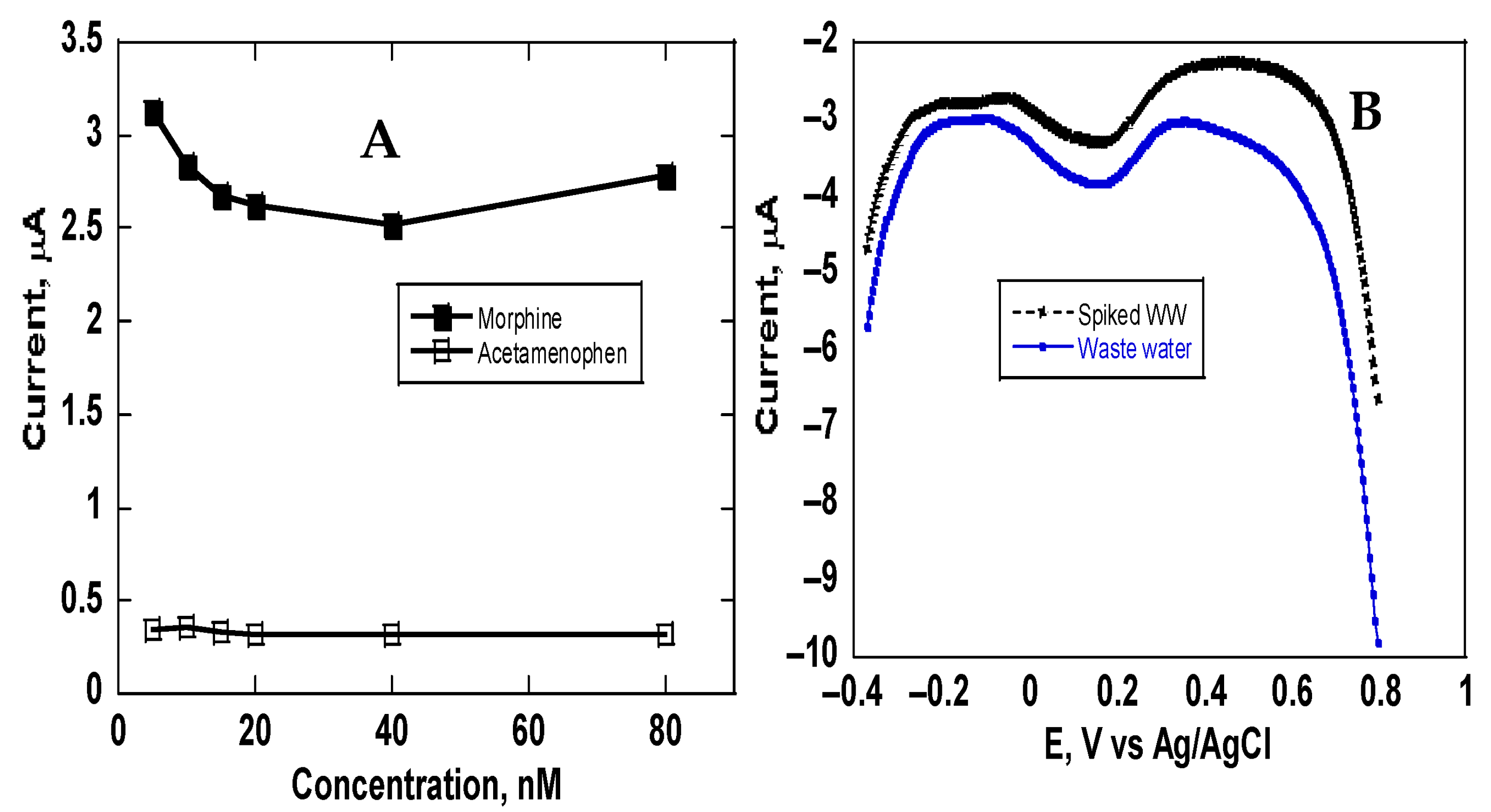
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamilton, G.R.; Baskett, T.F. In the arms of morpheus: The development of morphine for postoperative pain relief. Can. J. Anesth. 2000, 47, 367–374. [Google Scholar] [CrossRef]
- Gupta, K.; Kshirsagar, S.; Chang, L.; Schwartz, R.; Law, P.Y.; Yee, D.; Hebbel, R.P. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002, 62, 4491–4498. [Google Scholar] [PubMed]
- Osborne, R.; Thompson, P.; Joel, S.; Trew, D.; Patel, N.; Slevin, M. The analgesic activity of morphine-6-glucuronide. Br. J. Clin. Pharmacol. 1992, 34, 130–138. [Google Scholar] [CrossRef]
- Oliveira, A.; Carvalho, F.; Pinho, P.G.; Remião, F.; Medeiros, R.; Dinis-Oliveira, R.J. Quantification of morphine and its major metabolites M3G and M6G in antemortem and postmortem samples. Biomed. Chromatogr. 2014, 28, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Opfermann, G.; Schänzer, W. Urinary concentrations of morphine and codeine after consumption of poppy seeds. J. Anal. Toxicol. 2003, 27, 53–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rashid, B.A.; Aherne, G.W.; Katmeh, M.F.; Kwasowski, P.; Stevenson, D. Determination of morphine in urine by solid-phase immunoextraction and high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 1998, 797, 245–250. [Google Scholar] [CrossRef]
- Sartori, D.; Lewis, T.; Breaud, A.; Clarke, W. The development of a high-performance liquid chromatography–tandem mass spectrometric method for simultaneous quantification of morphine, morphine-3-β-glucuronide, morphine-6-β-glucuronide, hydromorphone, and normorphine in serum. Clin. Biochem. 2015, 48, 1283–1290. [Google Scholar] [CrossRef]
- Lewis, S.W.; Francis, P.S.; Lim, K.F.; Jenkins, G.E.; Wang, X.D. Pulsed flow chemistry: A new approach to solution handling for flow analysis coupled with chemiluminescence detection. Analyst 2000, 125, 1869–1874. [Google Scholar] [CrossRef]
- Sakai, G.; Ogata, K.; Uda, T.; Miura, N.; Yamazoe, N. A surface plasmon resonance-based immunosensor for highly sensitive detection of morphine. Sens. Actuators B Chem. 1998, 49, 5–12. [Google Scholar] [CrossRef]
- Atta, N.F.; Atta, N.F.; Hassan, H.K.; Galal, A. Rapid and simple electrochemical detection of morphine on graphene–palladium-hybrid-modified glassy carbon electrode. Anal. Bioanal. Chem. 2014, 406, 6933–6942. [Google Scholar] [CrossRef]
- Carrupt, P.A.; Testa, B.; Bechalany, A.; El Tayar, N.; Descas, P.; Perrissoud, D. Morphine 6-glucuronide and morphine 3-glucuronide as molecular chameleons with unexpected lipophilicity. J. Med. Chem. 1991, 34, 1272–1275. [Google Scholar] [CrossRef]
- Zhao, L.; Blackburn, J.; Brosseau, C.L. Quantitative detection of uric acid by electrochemical-surface enhanced Raman spectroscopy using a multilayered Au/Ag substrate. Anal. Chem. 2015, 87, 441–447. [Google Scholar] [CrossRef]
- Chen, W.; Yang, L.; Yan, C.; Yao, B.; Lu, J.; Xu, J.; Liu, G. Surface-Confined Building of Au@Pt-Centered and Multi-G-Quadruplex/Hemin Wire-Surrounded Electroactive Super-nanostructures for Ultrasensitive Monitoring of Morphine. ACS Sens. 2020, 5, 2644–2651. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Weng, C.-H.; Yeh, W.-M.; Ho, K.-C.; Lee, G.-B. A microfluidic system utilizing molecularly imprinted polymer films for amperometric detection of morphine. Sens. Actuators B Chem. 2007, 121, 576–582. [Google Scholar] [CrossRef]
- Rezaei, B.; Foroughi-Dehnavi, S.; Ensafi, A.A. Fabrication of electrochemical sensor based on molecularly imprinted polymer and nanoparticles for determination trace amounts of morphine. Ionics 2015, 21, 2969–2980. [Google Scholar] [CrossRef]
- Lanza, F.; Sellergren, B. The application of molecular imprinting technology to solid phase extraction. Chromatographia 2001, 53, 599–611. [Google Scholar] [CrossRef]
- Pardieu, E.; Cheap, H.; Vedrine, C.; Lazerges, M.; Lattach, Y.; Garnier, F.; Remita, S.; Pernelle, C. Molecularly imprinted conducting polymer based electrochemical sensor for detection of atrazine. Anal. Chim. Acta 2009, 649, 236–245. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Electrochemical Detection of Atrazine by Platinum Nanoparticles/Carbon Nitride Nanotubes with Molecularly Imprinted Polymer. Ind. Eng. Chem. Res. 2017, 56, 7631–7639. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Huang, J.; Wei, Z.; Chen, J. Molecular imprinted polypyrrole nanowires for chiral amino acid recognition. Sens. Actuators B Chem. 2008, 134, 573–578. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Luo, X.; Yang, L.; Tu, X. Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 183–189. [Google Scholar] [CrossRef]
- Jin, J.-H.; Alocilja, E.C.; Grooms, D.L. Fabrication and electroanalytical characterization of label-free DNA sensor based on direct electropolymerization of pyrrole on p-type porous silicon substrates. J. Porous Mater. 2010, 17, 169–176. [Google Scholar] [CrossRef]
- Corman, M.E.; Çorman, M.E.; Cetinkaya, A.; Armutcu, C.; Bellur Atici, E.; Uzun, L.; Ozkan, S.A. A sensitive and selective electrochemical sensor based on molecularly imprinted polymer for the assay of teriflunomide. Talanta 2022, 249, 123689. [Google Scholar] [CrossRef] [PubMed]
- Ferdosi, F.; Amiri, M.; Alizadeh, N. Sulfonated reduced graphene oxide-doped polypyrrole film prepared by in situ electropolymerization and coating in fabrication of ammonia gas sensor for exhaled breath analysis of patients with kidney failure. Mikrochim. Acta 2025, 192, 222. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Tang, S.; Liu, M.; Yi, Y.; Xie, B.; Hou, H.; Luo, A. A molecularly imprinted electrochemical sensor with tunable electrosynthesized Cu-MOFs modification for ultrasensitive detection of human IgG. Bioelectrochemistry 2022, 146, 108154. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, K.; Ma, H.; Duan, R.; Sun, M.; Zou, P.; Yin, J.; Wang, X.; Wang, Y.; Wu, C.; et al. Bimetallic MOF synergy molecularly imprinted ratiometric electrochemical sensor based on MXene decorated with polythionine for ultra-sensitive sensing of catechol. Anal. Chim. Acta 2023, 1251, 340983. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Q.; Yu, S.; Sun, Y.; Kang, Q.; Shen, D. Spectrum-resolved photoelectrochemical molecularly imprinted sensors for simultaneous determination of ascorbic acid and uric acid in a differential mode. Sens. Actuators B Chem. 2024, 403, 135154. [Google Scholar] [CrossRef]
- Aliabadi, A.; Rounaghi, G.H. A novel electrochemical sensor for determination of morphine in a sub-microliter of human urine sample. J. Electroanal. Chem. 2019, 832, 204–208. [Google Scholar] [CrossRef]
- Erdem, A.; Muti, M.; Karadeniz, H.; Congur, G.; Canavar, E. Electrochemical monitoring of indicator-free DNA hybridization by carbon nanotubes–chitosan modified disposable graphite sensors. Colloids Surf. B Biointerfaces 2012, 95, 222–228. [Google Scholar] [CrossRef]
- Pires, C.K.; Reis, B.F.; Galhardo, C.X.; Martelli, P.B. A Multicommuted Flow Procedure for the Determination of Cholesterol in Animal Blood Serum by Chemiluminescence. Anal. Lett. 2003, 36, 3011–3024. [Google Scholar] [CrossRef]
- Charkravarthula, P.; Mugweru, A. Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water. Electrochem 2023, 4, 435–446. [Google Scholar] [CrossRef]
- Afkhami, A.; Gomar, F.; Madrakian, T. CoFe2O4 nanoparticles modified carbon paste electrode for simultaneous detection of oxycodone and codeine in human plasma and urine. Sens. Actuators B Chem. 2016, 233, 263–271. [Google Scholar] [CrossRef]
- Wang, C.; Luo, J.; Dou, H.; Raise, A.; Ali, M.S.; Fan, W.; Li, Q. Optimization and analytical behavior of a morphine electrochemical sensor in environmental and biological samples based on graphite rod electrode using graphene/Co3O4 nanocomposite. Chemosphere 2023, 326, 138451. [Google Scholar] [CrossRef]
- Verrinder, E.; Wester, N.; Leppänen, E.; Lilius, T.; Kalso, E.; Mikladal, B.; Varjos, I.; Koskinen, J.; Laurila, T. Electrochemical Detection of Morphine in Untreated Human Capillary Whole Blood. ACS Omega 2021, 6, 11563–11569. [Google Scholar] [CrossRef]
- Atta, N.F. Sensitive electrochemical determination of morphine using gold nanoparticles-ferrocene modified carbon paste electrode. Int. J. Electrochem. Sci. 2012, 7, 10501–10518. [Google Scholar] [CrossRef]
- Krumbiegel, F.; Hastedt, M.; Westendorf, L.; Niebel, A.; Methling, M.; Parr, M.K.; Tsokos, M. The use of nails as an alternative matrix for the long-term detection of previous drug intake: Validation of sensitive UHPLC-MS/MS methods for the quantification of 76 substances and comparison of analytical results for drugs in nail and hair samples. Forensic Sci. Med. Pathol. 2016, 12, 416–434. [Google Scholar] [CrossRef]
- Ghorani-Azam, A.; Balali-Mood, M.; Khatami, S.-M.; Asoodeh, A.; Es’haghi, Z.; Riahi-Zanjani, B. Plant Extract and Herbal Products as Potential Source of Sorbent for Analytical Purpose: An Experimental Study of Morphine and Codeine Determination Using HPLC and LC–MSMS. J. Chromatogr. Sci. 2021, 59, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Beck, O.; Bottcher, M. Paradoxical Results in Urine Drug Testing for 6-Acetylmorphine and Total Opiates: Implications for Best Analytical Strategy. J. Anal. Toxicol. 2006, 30, 73–79. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.-H.; Lee, M.-R.; Lee, R.-J.; Ko, W.-K.; Wu, S.-M. Hair analysis for methamphetamine, ketamine, morphine and codeine by cation-selective exhaustive injection and sweeping micellar electrokinetic chromatography. J. Chromatogr. 2007, 1145, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.M.P.J.; Delerue-Matos, C.; Borges, F.; Macedo, T.R.A.; Oliveira-Brett, A.M. Voltammetric Oxidation of Drugs of Abuse I. Morphine and Metabolites. Electroanalysis 2004, 16, 1419–1426. [Google Scholar] [CrossRef]
- Li, F.; Song, J.; Gao, D.; Zhang, Q.; Han, D.; Niu, L. Simple and rapid voltammetric determination of morphine at electrochemically pretreated glassy carbon electrodes. Talanta 2009, 79, 845–850. [Google Scholar] [CrossRef] [PubMed]
| Electrode-Based | |||
|---|---|---|---|
| Modified Electrodes | Linear Range | Detection Limit | Reference |
| Graphene/Co3O4 (Gr/Co3O4) | 0.5–100.0 μM | 80 nM | [33,34] |
| Polymer/SWCNT | 0.5–10 μM | 0.48 μM | [35] |
| Carbon Paste GNP | 1.0 × 10−6–18.0 × 10−4 M | 3.507 × 10−9 M | [36] |
| Poly-pyrrole polymer imprint | 0–20 nM and 0 to 40 nM | 2.75 and 1.9 nM | This work |
| Other methods | |||
| UHPLC-MS/MS | 0.025–12.5 ng/mg | 20.27 pg/mg | [37] |
| (LC-MS/MS) with CNTs extraction | 25–2000 ng/mL | 2.0 ng/mL | [38] |
| DRI immunoassay | 0 to 1466 ug/L | 300 ug/L | [39] |
| Electrokinetic chromatography | 0.5–50 ng/mg | 200 pg/mg | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkravarthula, P.; Mugweru, A. Molecularly Imprinted Electrochemical Sensor Electrodes Based on Poly-Pyrrole for Sensitive Detection of Morphine in Wastewater. Chemosensors 2025, 13, 284. https://doi.org/10.3390/chemosensors13080284
Charkravarthula P, Mugweru A. Molecularly Imprinted Electrochemical Sensor Electrodes Based on Poly-Pyrrole for Sensitive Detection of Morphine in Wastewater. Chemosensors. 2025; 13(8):284. https://doi.org/10.3390/chemosensors13080284
Chicago/Turabian StyleCharkravarthula, Pranaya, and Amos Mugweru. 2025. "Molecularly Imprinted Electrochemical Sensor Electrodes Based on Poly-Pyrrole for Sensitive Detection of Morphine in Wastewater" Chemosensors 13, no. 8: 284. https://doi.org/10.3390/chemosensors13080284
APA StyleCharkravarthula, P., & Mugweru, A. (2025). Molecularly Imprinted Electrochemical Sensor Electrodes Based on Poly-Pyrrole for Sensitive Detection of Morphine in Wastewater. Chemosensors, 13(8), 284. https://doi.org/10.3390/chemosensors13080284





