Radical TTM-DMODPA for Ascorbic Acid Non-Catalytic Visual Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipment and Characterization
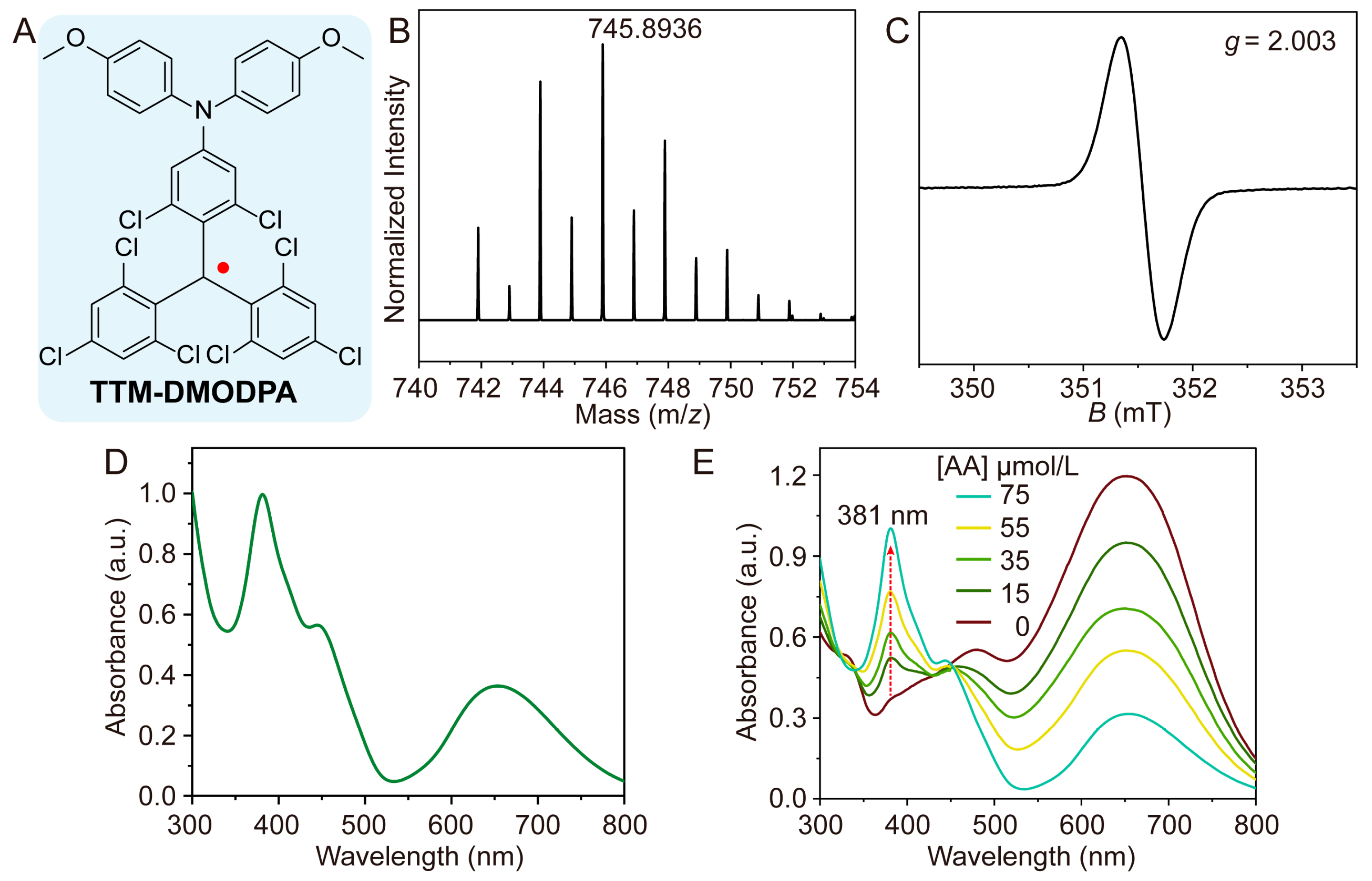
2.3. Synthesis of Compound HTTM-DMODPA
2.4. Synthesis of Radical TTM-DMODPA
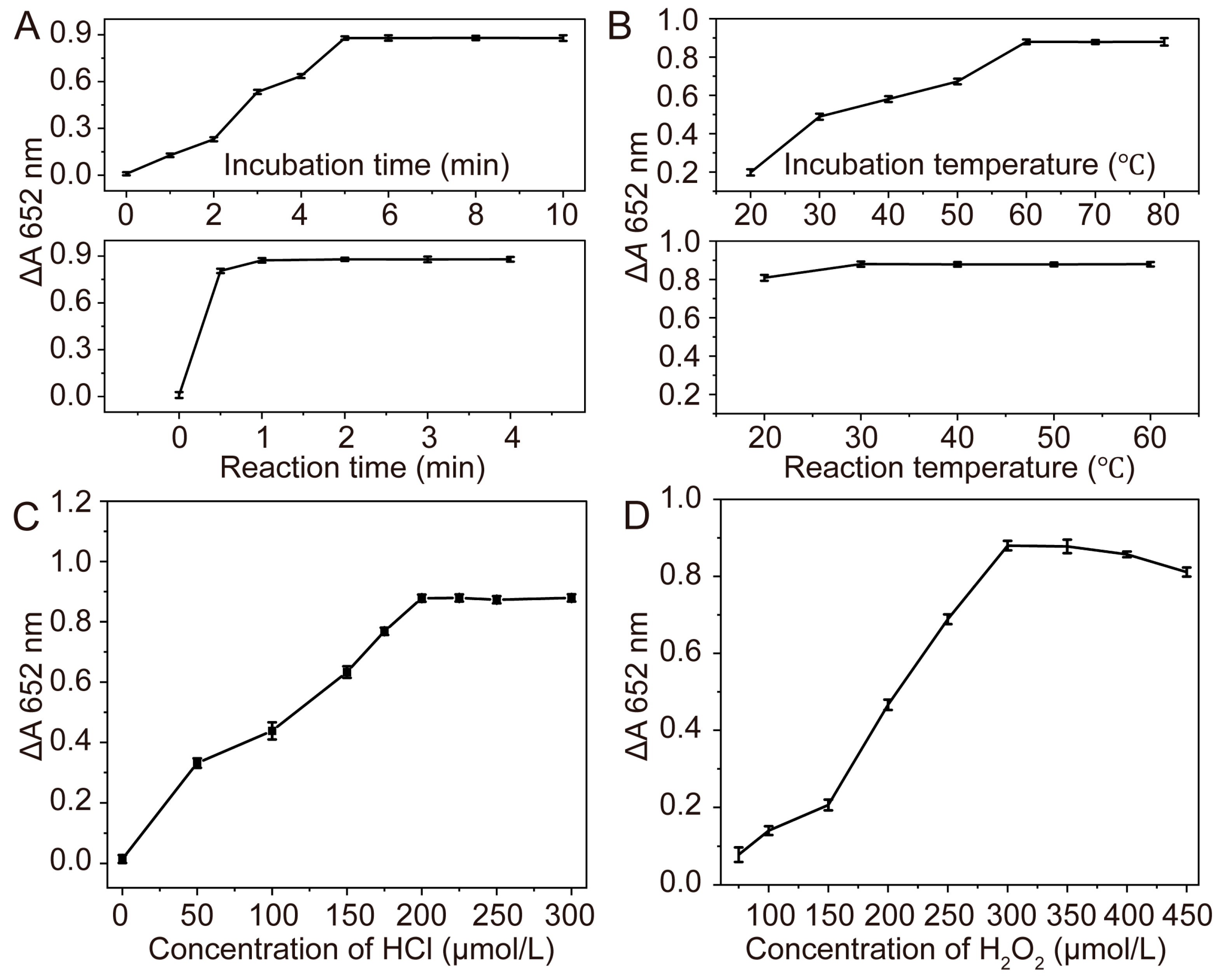
2.5. Procedures for Ascorbic Acid Assay
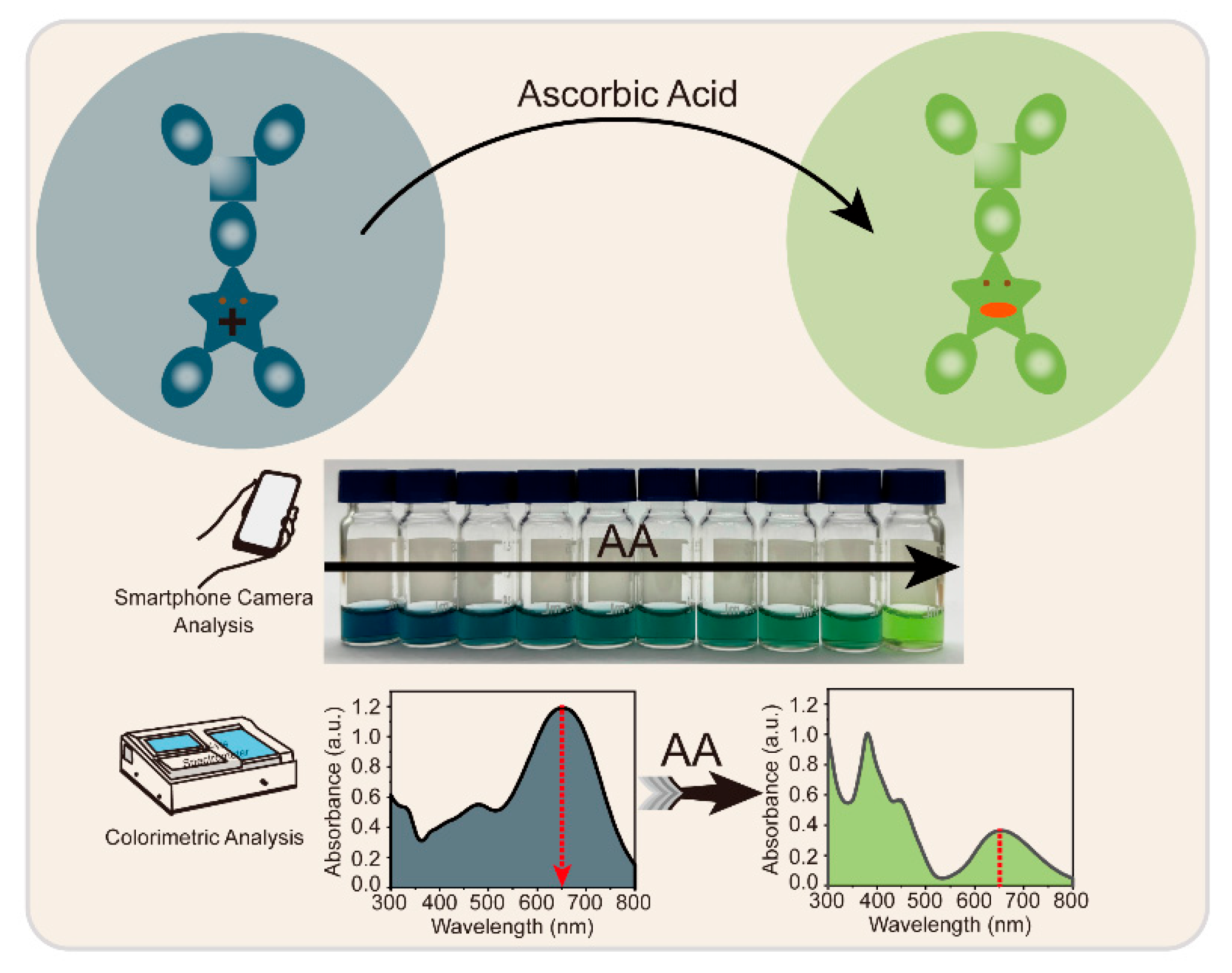
3. Results and Discussion
3.1. Synthesis and Characterization of Radical TTM-DMODPA
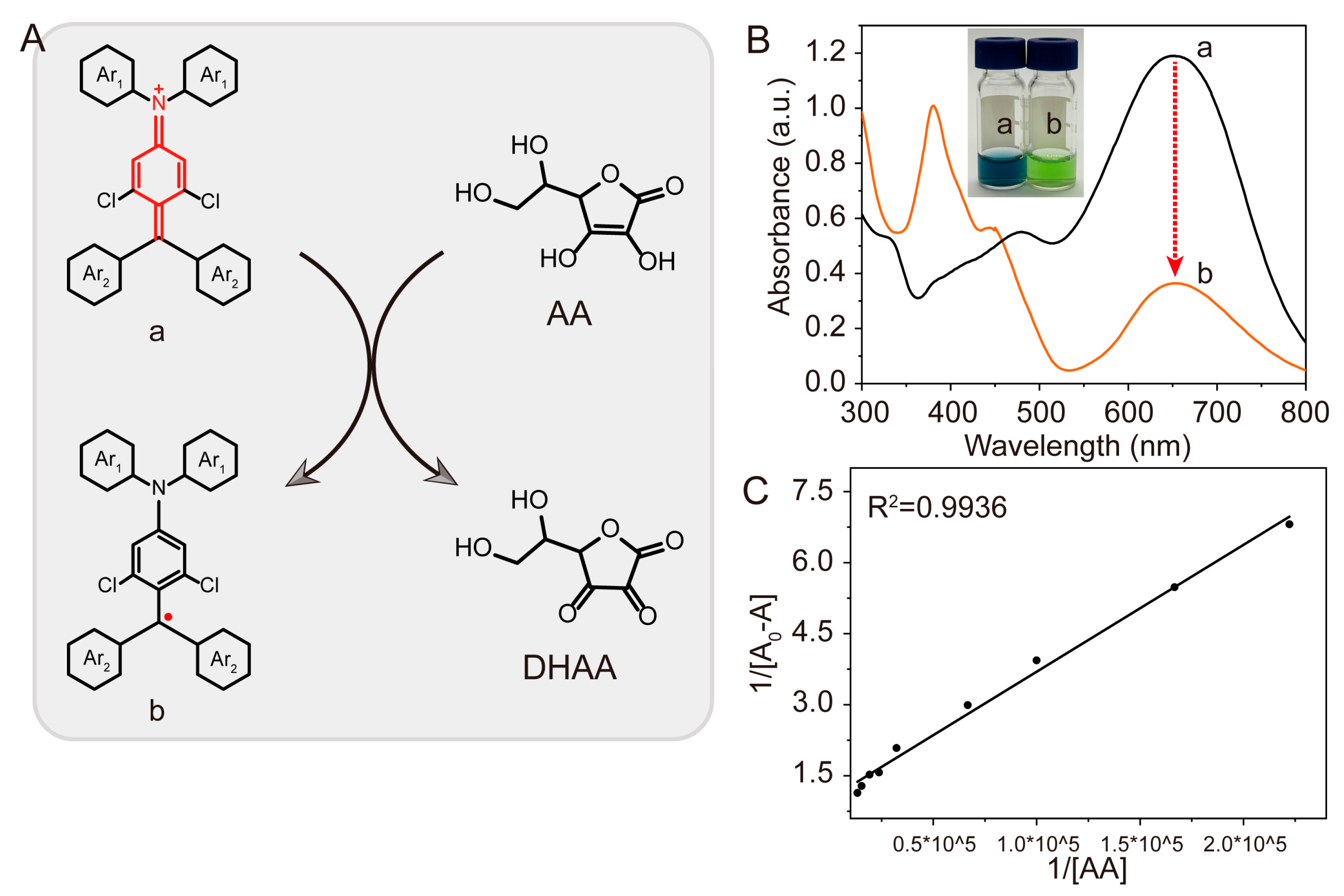
3.2. Feasibility Analysis
3.3. Colorimetric Detection of Ascorbic Acid
3.4. Camera Photography Analysis
3.5. Urine Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Z.; Liu, L.; Ouyang, S.; Chen, Y.; Lin, P.; Chen, H.; You, Y.; Zhao, P.; Huang, K.; Tao, J. In Situ Ratiometric Determination of Cerebral Ascorbic Acid after Ischemia Reperfusion. ACS Sens. 2023, 8, 4587–4596. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, J.; Zhao, S.; Zhang, Y.; Fan, J.; Wu, H.; Chen, J.; Zhang, Z.; Meng, Z.; Yang, L.; et al. A Bioinspired Flexible Sensor for Electrochemical Probing of Dynamic Redox Disequilibrium in Cancer Cells. Adv. Sci. 2023, 10, 2304079. [Google Scholar] [CrossRef]
- He, X.; Wang, Q.; Cheng, X.; Wang, W.; Li, Y.; Nan, Y.; Wu, J.; Xiu, B.; Jiang, T.; Bergholz, J.S.; et al. Lysine Vitcylation Is a Vitamin C-derived Protein Modification That Enhances STAT1-mediated Immune Response. Cell 2025, 188, 1858–1877.e1821. [Google Scholar] [CrossRef]
- Yi, Z.; Yang, X.; Liang, Y.; Tong, S. Iron Oxide Nanozymes Enhanced by Ascorbic Acid for Macrophage-based Cancer Therapy. Nanoscale 2024, 16, 14330–14338. [Google Scholar] [CrossRef]
- Qian, Y.; Gao, Y.; Wang, D.; Zhang, S.; Luo, Q.; Shan, G.; Lu, M.; Yan, D.; Tang, B.Z.; Zhang, M. A Tactfully Designed Photothermal Agent Collaborating with Ascorbic Acid for Boosting Maxillofacial Wound Healing. Natl. Sci. Rev. 2025, 12, nwae426. [Google Scholar] [CrossRef]
- Jaber, M.; Jaber, B.; Hamed, S.; AlKhatib, H.S. Preparation and Evaluation of Ascorbyl Glucoside and Ascorbic Acid Solid in Oil Nanodispersions for Corneal Epithelial WoundHealing. Int. J. Pharm. 2022, 627, 122227. [Google Scholar] [CrossRef]
- Favarin, F.R.; Forrati, É.M.; Bassoto, V.A.; da Silva Gündel, S.; Velho, M.C.; Ledur, C.M.; Verdi, C.M.; Lemos, J.G.; Sagrillo, M.R.; Fagan, S.B.; et al. Ascorbic Acid and Ascorbyl Palmitate-loaded Liposomes: Development, Characterization, Stability Evaluation, in Vitro Security Profile, Antimicrobial and Antioxidant Activities. Food Chem. 2024, 460, 140569. [Google Scholar] [CrossRef]
- Meacham, C.E.; DeVilbiss, A.W.; Morrison, S.J. Metabolic Regulation of Somatic Stem Cells in Vivo. Nat. Rev. Mol. Cell Biol. 2022, 23, 428–443. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Abu Hejleh, T.; et al. O2- and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2017, 31, 487–500.e488. [Google Scholar] [CrossRef]
- Wang, H.; You, Q.; Kang, B.; Jing, H.; Shi, Z.; Krizkova, S.; Heger, Z.; Adam, V.; Chen, X.; Li, N. Pulling the Rug Out from Under: Biomechanical Microenvironment Remodeling for Induction of Hepatic Stellate Cell Quiescence. Adv. Mater. 2024, 36, 2406590. [Google Scholar] [CrossRef]
- Leesang, T.; Yap, Y.S.; Brabson, J.P.; Klimas, A.; Ahearn, E.L.; Barbieri, D.A.; Lam, M.Q.; Lyon, P.D.; Figueroa, M.K.E.; Beckedorff, F.; et al. Retinoic Acid and Ascorbate Remodel the Epigenome of Leukemia Cells to Improve Therapeutic Efficacy By Enhancing TET Activity. Blood 2023, 142, 5706. [Google Scholar] [CrossRef]
- Fu, D.; Liu, H.; Chen, T.; Cheng, Y.; Cao, M.; Liu, J. A Bio-analytic Nanoplatform Based on Au Post-functionalized CeFeO3 for The Simultaneous Determination of Melatonin and Ascorbic Acid through Photo-assisted Electrochemical technology. Biosens. Bioelectron. 2022, 213, 114457. [Google Scholar] [CrossRef]
- Liang, X.-H.; Yu, A.-X.; Bo, X.-J.; Du, D.-Y.; Su, Z.-M. Metal/Covalent-organic Frameworks-based Electrochemical Sensors for The Detection of Ascorbic Acid, Dopamine and Uric acid. Coord. Chem. Rev. 2023, 497, 215427. [Google Scholar] [CrossRef]
- Yang, X.; Lin, X.; Li, J.; Viitala, T.; Zhao, Y.; Zhang, H.; Shang, L. Vitamin C Encapsulated Biomimetic Nanogels with Macrophage Membrane Decoration for Chronic Wound Healing. Chem. Eng. J. 2025, 505, 159080. [Google Scholar] [CrossRef]
- Koga, T.; Kawahara, N.; Aburada, M.; Ono, A.; Mae, S.; Yoshida, A.; Iwaoka, Y.; Ito, H.; Tai, A. Antiallergic Activity of 3-O-Dodecyl-l-ascorbic Acid. Molecules 2024, 29, 69. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Li, S.; Cai, Q.; Zhang, K.; She, J. Structural Basis of Vitamin C Recognition and Transport by Mammalian SVCT1 Transporter. Nat. Commun. 2023, 14, 1361. [Google Scholar] [CrossRef]
- Pouptsis, A.; Zaragoza, R.; Garcia-Trevijano, E.R.; Vina, J.R.; Ortiz-Zapater, E. Nutrition, Lifestyle, and Environmental Factors in Lung Homeostasis and Respiratory Health. Nutrients 2025, 17, 954. [Google Scholar] [CrossRef]
- Costa, M.G.; da Silva, B.C.; Alves, D.M.R.; de Lima, P.S.R.; Prado, R.d.M. Silicon, by modulating homeostasis and nutritional efficiency, increases the antioxidant action and tolerance of bell peppers to phosphorus deficiency. Sci. Hortic. 2025, 343, 114093. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, N.; Gao, Z.; Gao, J.; Wang, X.; Xie, H.; Wang, C.-Y.; Zhang, S. Reductive stress: The key pathway in metabolic disorders induced by overnutrition. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Sato, A.; Kondo, Y.; Ishigami, A. The evidence to date: Implications of l-ascorbic acid in the pathophysiology of aging. J. Physiol. Sci. 2024, 74, 29. [Google Scholar] [CrossRef]
- Edzeamey, F.J.; Ramchunder, Z.; Gomez, A.V.; Ge, H.; Marobbio, C.M.T.; Pourzand, C.; Virmouni, S.A. Therapeutic combination of L-ascorbic acid, N-acetylcysteine, and dimethyl fumarate in Friedreich’s ataxia: Insights from in vitro models. Redox Rep. 2025, 30, 2505303. [Google Scholar] [CrossRef]
- Kumar, R.; Shafique, M.S.; Chapa, S.O.M.; Madou, M.J. Recent Advances in MOF-Based Materials for Biosensing Applications. Sensors 2025, 25, 2473. [Google Scholar] [CrossRef]
- Galal, S.A.B.; Elzanfaly, E.S.; Hussien, E.M.; Amer, E.A.H.; Zaazaa, H.E. Sustainable Thin-Layer Chromatographic Method for the Determination of Ipriflavone and Vitamins in Bone Health Supplements. J. Anal. Chem. 2025, 80, 480–489. [Google Scholar] [CrossRef]
- Yildiz, G.; Yildiz, G. Insights into Ultrasound-Assisted Postharvest Preservation: Metabolic, Structural, and Quality Changes in Strawberries During Storage. Food Bioprocess Technol. 2025. [Google Scholar] [CrossRef]
- Ma, J.; Zou, M.; Peijnenburg, W.; Chen, F. Priming agents combat copper stress in wheat (Triticum aestivum L.) under hydroponic conditions: Insights in impacts on morpho–physio–biochemical traits and health risk assessment. Ecotoxicol. Environ. Saf. 2025, 291, 117899. [Google Scholar] [CrossRef]
- Lv, J.; Chen, S.; Xu, W.; Zhang, X.; Wang, M.; Xu, J.; Zhu, Z.; Hu, Q.; Niu, L. Lectin-Mediated Labeling of Alkaline Phosphatase for Enzymatic Silver Deposition-Based Electrochemical Detection of Glycoprotein Tumor Markers. Anal. Chem. 2025, 97, 2479–2485. [Google Scholar] [CrossRef]
- Agar, M.; Laabei, M.; Leese, H.S.; Estrela, P. Multi-Template Molecularly Imprinted Polymeric Electrochemical Biosensors. Chemosensors 2025, 13, 11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Wan, Q.; Sun, T.; Gao, G. A smartphone sensing colorimetric detection of ascorbic acid in fruit samples based on Cu2Fe(CN)6@PVP nanozyme. Talanta 2025, 293, 128125. [Google Scholar] [CrossRef]
- Rocha, F.R.P.; Zagatto, E.A.G. Chemical Derivatization in Flow Analysis. Molecules 2022, 27, 1563. [Google Scholar] [CrossRef]
- Wu, N.; Liu, W.; Yang, S.; Wang, G.; Zhang, Z.; Guo, S. Construction of ratio fluorescence sensor based on fluorescence scattering characteristics and its analytical application. Microchem. J. 2025, 213, 113829. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Mu, X.; Liu, B.; Xia, M.; Wang, F.; Tong, Z. Carbon quantum dots in spectrofluorimetric analysis: A comprehensive review of synthesis, mechanisms and multifunctional applications. Talanta 2025, 293, 128066. [Google Scholar] [CrossRef]
- Zhu, Q.; Dong, D.; Zheng, X.; Song, H.; Zhao, X.; Chen, H.; Chen, X. Chemiluminescence determination of ascorbic acid using graphene oxide@copper-based metal–organic frameworks as a catalyst. RSC Adv. 2016, 6, 25047–25055. [Google Scholar] [CrossRef]
- Dalapati, R.; Biswas, S. Aqueous Phase Sensing of Fe3+ and Ascorbic Acid by a Metal–Organic Framework and Its Implication in the Construction of Multiple Logic Gates. Chem. Asian J. 2019, 14, 2822–2830. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Liu, X.; Song, K.; Wang, H.; Wang, J.; Li, R.; Liu, S.; Peng, Z. A Novel Electrochemical Sensor Based on Pd Confined Mesoporous Carbon Hollow Nanospheres for the Sensitive Detection of Ascorbic Acid, Dopamine, and Uric Acid. Molecules 2024, 29, 2427. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, H.; Tong, Y.; Zhuang, Q.-F.; Wang, Y. Colorimetric Detection of Ascorbic Acid Based on Oxidase-like Activity of Fe2O3/Nitrogen-doped Carbon Nanomaterials. Chin. J. Anal. Chem. 2025, 53, 346–355. [Google Scholar] [CrossRef]
- Sang, Y.; Wu, P.; Ge, J.; Cao, Y.; Xiao, Y.; Yang, Y.; Wang, C.; Li, Z.; Cai, R. Bimetallic CuAg nanoflowers with high nanozyme activity for detection of acid phosphatase. Microchem. J. 2025, 213, 113808. [Google Scholar] [CrossRef]
- Qi, J.; Tian, L.; Pang, Y.; Wu, F. Manganese Phthalocyanine-Based Magnetic Core-Shell Composites with Peroxidase Mimetic Activity for Colorimetric Detection of Ascorbic Acid and Glutathione. Molecules 2025, 30, 1484. [Google Scholar] [CrossRef]
- Kukreja, E.A.; Tripathi, R.M.; Chung, S.J. Colorimetric and smartphone-based dual modality sensing platform for detection of ascorbic acid. Sens. Actuators A 2025, 389, 116573. [Google Scholar] [CrossRef]
- Pu, Z.; Xu, Z.; Zhang, X.; Guo, Y.; Sun, Z. Unlocking the Multistage Redox Property of Graphenic Radicals by π-Extension. Angew. Chem. Int. Ed. 2024, 63, e202406078. [Google Scholar] [CrossRef]
- Kodama, T.; Aoba, M.; Hirao, Y.; Rivero, S.M.; Casado, J.; Kubo, T. Molecular and Spin Structures of a Through-Space Conjugated Triradical System. Angew. Chem. Int. Ed. 2022, 61, e202200688. [Google Scholar] [CrossRef]
- Chen, L.; Arnold, M.; Kittel, Y.; Blinder, R.; Jelezko, F.; Kuehne, A.J.C. 2,7-Substituted N-Carbazole Donors on Tris(2,4,6-trichlorophenyl)methyl Radicals with High Quantum Yield. Adv. Optical Mater. 2022, 10, 2102101. [Google Scholar] [CrossRef]
- Matsuda, K.; Xiaotian, R.; Nakamura, K.; Furukori, M.; Hosokai, T.; Anraku, K.; Nakao, K.; Albrecht, K. Photostability of Luminescent Tris(2,4,6-trichlorophenyl)methyl Radical Enhanced by Terminal Modification of Carbazole Donor. Chem. Commun. 2022, 58, 13443–13446. [Google Scholar] [CrossRef]
- Yan, C.; Fang, J.; Zhu, J.; Chen, W.; Wang, M.; Chi, K.; Lu, X.; Zhou, G.; Liu, Y. Donor Engineering of Diphenylamine-substituted Tris(2,4,6-trichlorophenyl)methyl Radicals for Controlling the Intramolecular Charge Transfer and Near-infrared Photothermal Conversion. J. Mater. Chem. C 2023, 11, 2729–2736. [Google Scholar] [CrossRef]
- Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic Light-Emitting Diodes Using a Neutral π Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7095. [Google Scholar] [CrossRef]
- Ai, X.; Chen, Y.; Feng, Y.; Li, F. A Stable Room-Temperature Luminescent Biphenylmethyl Radical. Angew. Chem. Int. Ed. 2018, 57, 2869–2873. [Google Scholar] [CrossRef]
- Murto, P.; Li, B.; Fu, Y.; Walker, L.E.; Brown, L.; Bond, A.D.; Zeng, W.; Chowdhury, R.; Cho, H.-H.; Yu, C.P.; et al. Steric Control of Luminescence in Phenyl-Substituted Trityl Radicals. J. Am. Chem. Soc. 2024, 146, 13133–13141. [Google Scholar] [CrossRef]
- Yan, C.; An, D.; Chen, W.; Zhang, N.; Qiao, Y.; Fang, J.; Lu, X.; Zhou, G.; Liu, Y. Stable Diarylamine-Substituted Tris(2,4,6-trichlorophenyl)methyl Radicals: One-Step Synthesis, Near-Infrared Emission, and Redox Chemistry. CCS Chem. 2022, 4, 3190–3203. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Mercante, L.A.; Gogotsi, Y.; Correa, D.S. ZnO–Co3O4 Nanofibers/MXene Composite with Peroxidase-Like Activity for Ascorbic Acid Detection. ACS Appl. Nano Mater. 2025, 8, 4291–4299. [Google Scholar] [CrossRef]
- Hebbar, S.D.; Trivedi, D.R. Discriminative ion detection of Hg2+ and Cu2+ and selective recognition of PO43− ions: Real time monitoring in food and water samples and molecular keypad lock integration. Spectrochim. Acta Part A 2025, 331, 125706. [Google Scholar] [CrossRef]
- Mane, P.V.; Patil, P.; Mahishi, A.A.; Kigga, M.; Bhat, M.P.; Lee, K.-H.; Kurkuri, M. Rhodamine 6G derivative for the selective copper detection and remediation using nanoporous diatomaceous earth-engineered functional receptor. Heliyon 2023, 9, e16600. [Google Scholar] [CrossRef]
- Yan, B.; Yang, Y.; Xie, Y.; Li, J.; Li, K. Fe Doping Enhances the Peroxidase-Like Activity of CuO for Ascorbic Acid Sensing. Chemistry 2023, 5, 1302–1316. [Google Scholar] [CrossRef]
- Gu, Y.; Cao, Z.; Zhao, M.; Xu, Y.; Lu, N. Single-Atom Fe Nanozyme with Enhanced Oxidase-like Activity for the Colorimetric Detection of Ascorbic Acid and Glutathione. Biosensors 2023, 13, 487. [Google Scholar] [CrossRef]
- Meng, R.; Li, S.; Li, W.; Wang, Q.; Wang, Y.; Hao, S.; Zhang, D.; Zhou, X. Highly dispersed Co9S8 loaded on Fe3O4 modified by graphene quantum dots for ascorbic acid detection. Microchem. J. 2025, 212, 113411. [Google Scholar] [CrossRef]
- Lian, Q.; Zheng, X.; Peng, G.; Liu, Z.; Chen, L.; Wu, S. Oxidase mimicking of CuMnO2 nanoflowers and the application in colorimetric detection of ascorbic acid. Colloids Surf. A 2022, 652, 129887. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Cui, X.; Zhang, Y.; Zhu, R.; Zhao, H.; Li, Z. Sensitive and facile colorimetric sensing strategy for ascorbic acid determination based on CoOOH nanoflakes-ABTs oxidative system. Colloids Surf. A 2019, 575, 66–74. [Google Scholar] [CrossRef]
- Ma, F.; Luo, J.; Li, X.; Liu, S.; Yang, M.; Chen, X. A “switch-on” fluorescence assay based on silicon quantum dots for determination of ascorbic acid. Spectrochim. Acta, Part A 2021, 249, 119343. [Google Scholar] [CrossRef]
- Huang, L.; Qin, S.; Yang, K.; Xu, Y.; Wu, X.; Lin, Z.; Wang, Y. Dual signal AA detection based on fluorescence and local surface plasmon resonance absorption technology. Spectrochim. Acta Part A 2024, 306, 123570. [Google Scholar] [CrossRef]
- Hu, F.; Mao, Y.; Huang, J.; Wan, L.; Liu, S.; Wen, Z.; Ai, F. “On–off–on” fluorescent sensor based on Ti3C2 quantum dots and CoOOH nanosheets for the detection of ascorbic acid. New J. Chem. 2024, 48, 5258–5266. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Z.-J.; Li, J.-J.; Zhao, J.-W. Fluorescent detection of ascorbic acid based on the emission wavelength shift of CdTe quantum dots. J. Lumin. 2017, 192, 47–55. [Google Scholar] [CrossRef]
- Dong, X.-X.; Chen, T.-L.; Kong, X.-J.; Wu, S.; Kong, F.-F.; Xiao, Q. A facile fluorescence Eu MOF sensor for ascorbic acid and ascorbate oxidase detection. Anal. Methods 2024, 16, 704–708. [Google Scholar] [CrossRef]
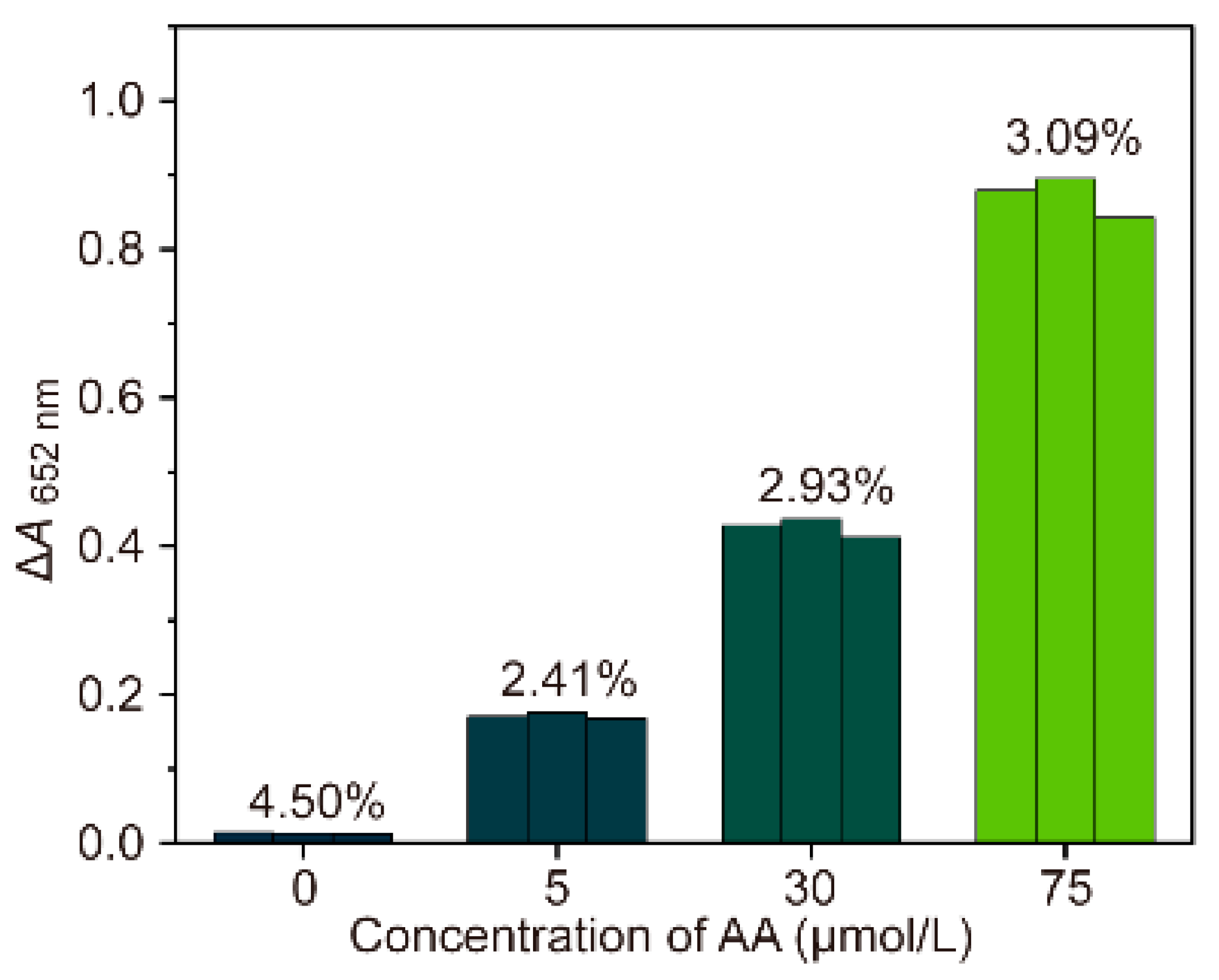
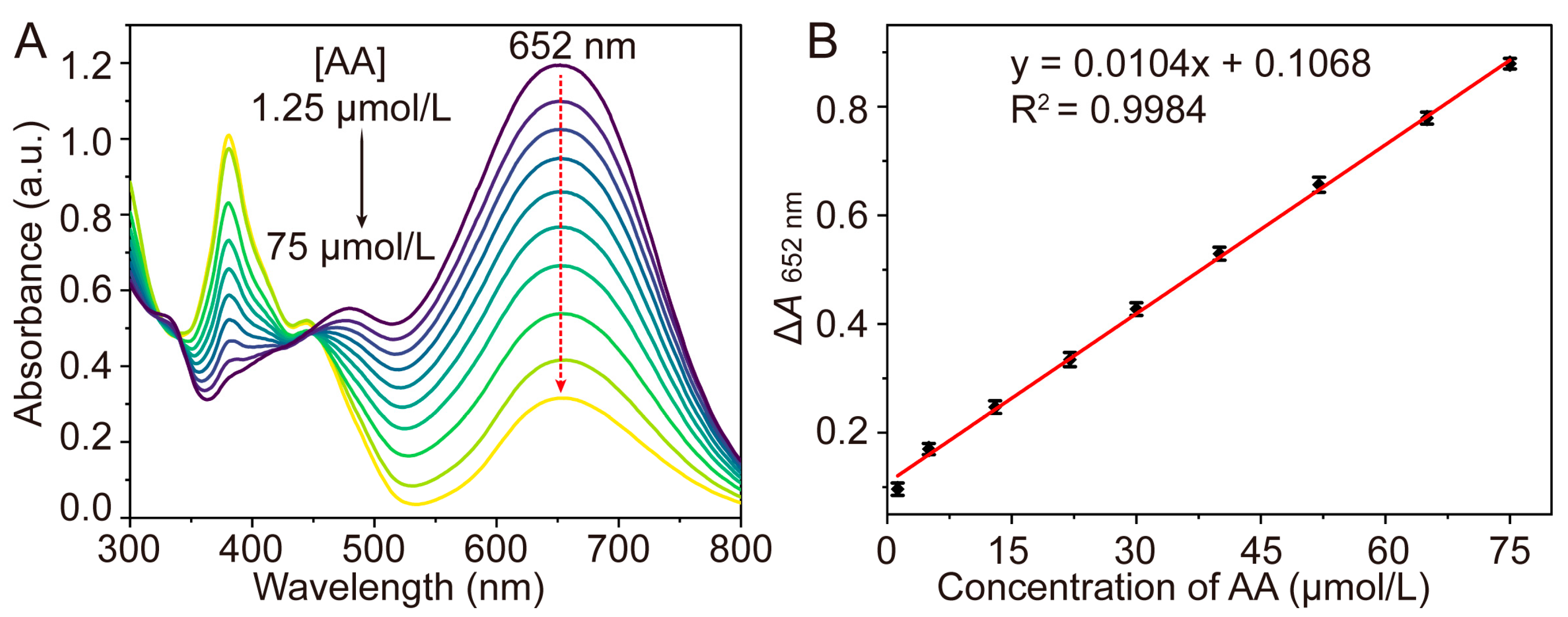
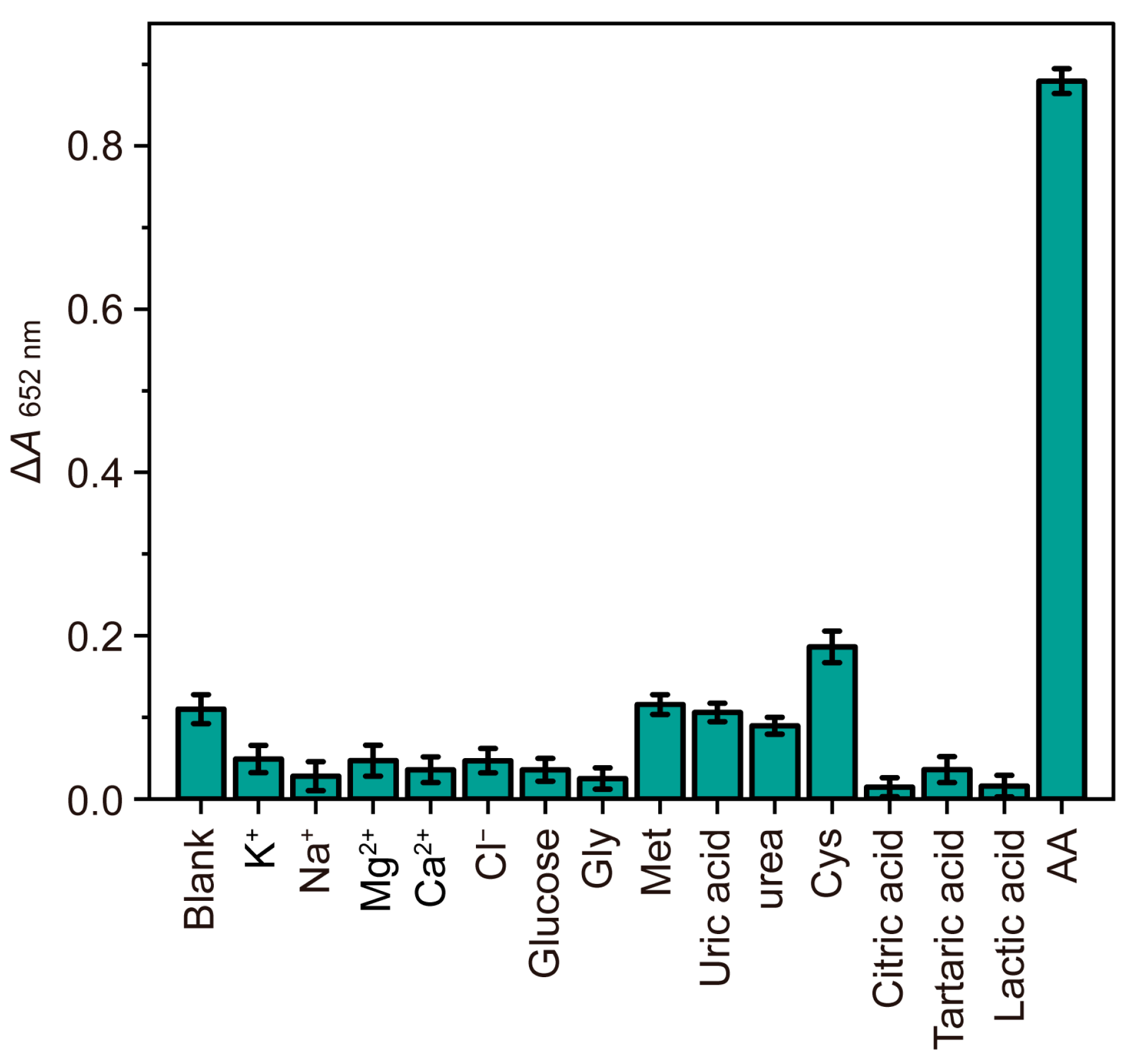
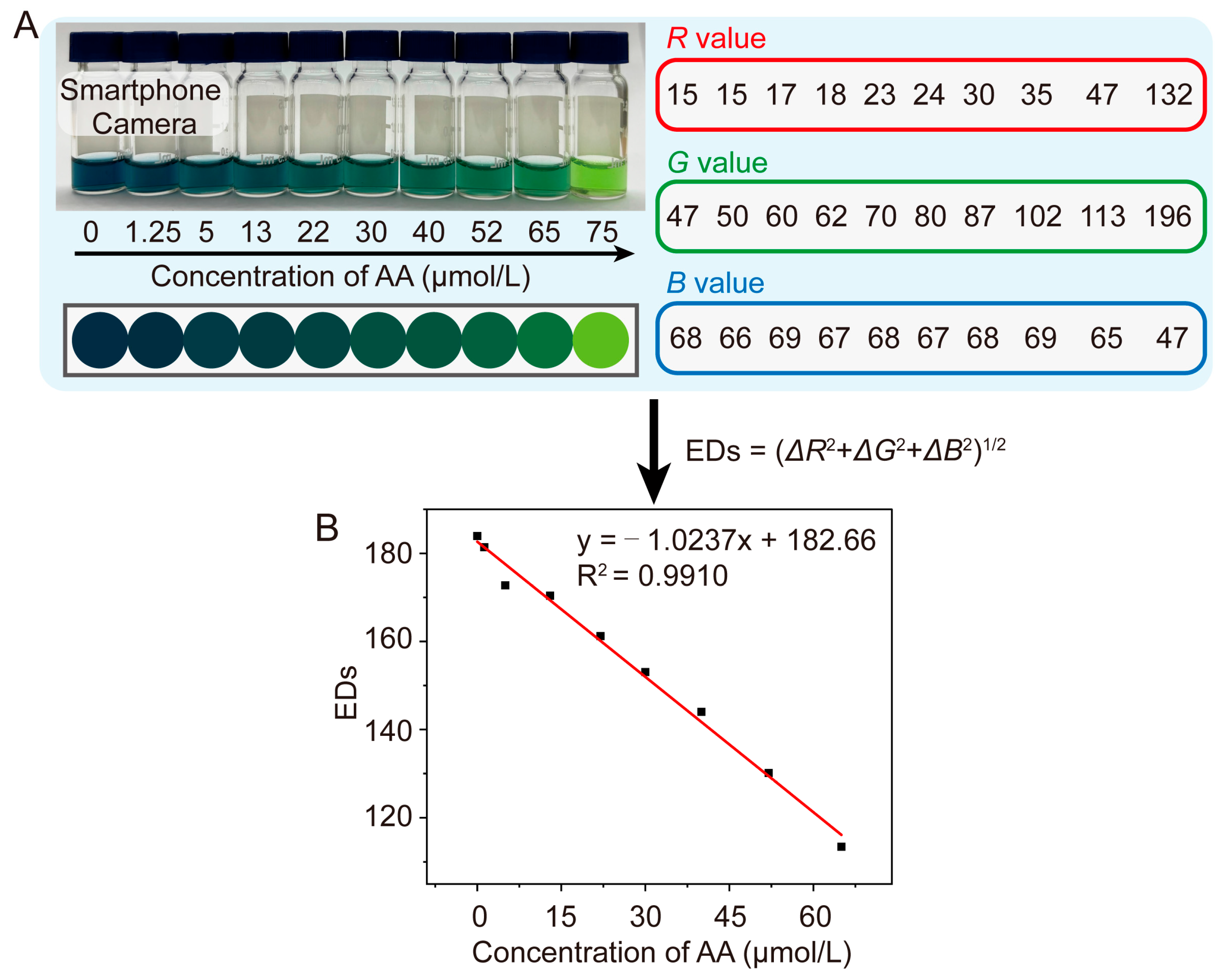
| Detection System | Analytical Method | Linear Range (μM) | LOD (μM) | References |
|---|---|---|---|---|
| Fe-CuO + TMB | colorimetric | 5–50 | 4.66 | [51] |
| Fe-N-C + TMB | colorimetric | 0.1–2 | 0.1 | [52] |
| N-GQDs/Fe3O4@Co9S8 + TMB | colorimetric | 1–70 | 0.404 | [53] |
| CuMnO2 + TMB | colorimetric | 1–105 | 0.39 | [54] |
| CoOOH-ABTS | colorimetric | 0.5–15 | 0.16 | [55] |
| SiQDs + MnO2 | fluorometric | 1–80 | 0.48 | [56] |
| Au@MnO2 | fluorometric | 0.75–17.5 | 0.47 | [57] |
| Ti3C2QDs + CoOOH | fluorometric | 0–100 | 0.19 | [58] |
| CdTe QDs | fluorometric | 10–250 | 1.3 | [59] |
| Eu MOF | fluorometric | 0–3.0 | 0.32 | [60] |
| TTM-DMODPA cation | colorimetric | 1.25–75 | 0.288 | This work |
| Samples 1 | Added (μM) | Found (μM) | Recovery (%) | RSD (n = 3, %) |
|---|---|---|---|---|
| Urine 1 | 0.0 | 12.15 | − | 2.69 |
| 5.0 | 17.27 | 102.4 | 4.03 | |
| 10.0 | 22.09 | 99.4 | 3.88 | |
| Urine 2 | 0.0 | 9.38 | − | 3.28 |
| 5.0 | 14.27 | 97.8 | 1.12 | |
| 10.0 | 19.79 | 104.1 | 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Q.; Zong, H.; Xie, X.; Rong, X.; Yan, C. Radical TTM-DMODPA for Ascorbic Acid Non-Catalytic Visual Detection. Chemosensors 2025, 13, 277. https://doi.org/10.3390/chemosensors13080277
Zhong Q, Zong H, Xie X, Rong X, Yan C. Radical TTM-DMODPA for Ascorbic Acid Non-Catalytic Visual Detection. Chemosensors. 2025; 13(8):277. https://doi.org/10.3390/chemosensors13080277
Chicago/Turabian StyleZhong, Qingmei, Huixiang Zong, Xiaohui Xie, Xiaomei Rong, and Chuan Yan. 2025. "Radical TTM-DMODPA for Ascorbic Acid Non-Catalytic Visual Detection" Chemosensors 13, no. 8: 277. https://doi.org/10.3390/chemosensors13080277
APA StyleZhong, Q., Zong, H., Xie, X., Rong, X., & Yan, C. (2025). Radical TTM-DMODPA for Ascorbic Acid Non-Catalytic Visual Detection. Chemosensors, 13(8), 277. https://doi.org/10.3390/chemosensors13080277





