Ligand Differentiation Ability of Insect Odorant Receptors in Heterologously Expressed Cells as Potential Biosensor Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Odorant Preparation
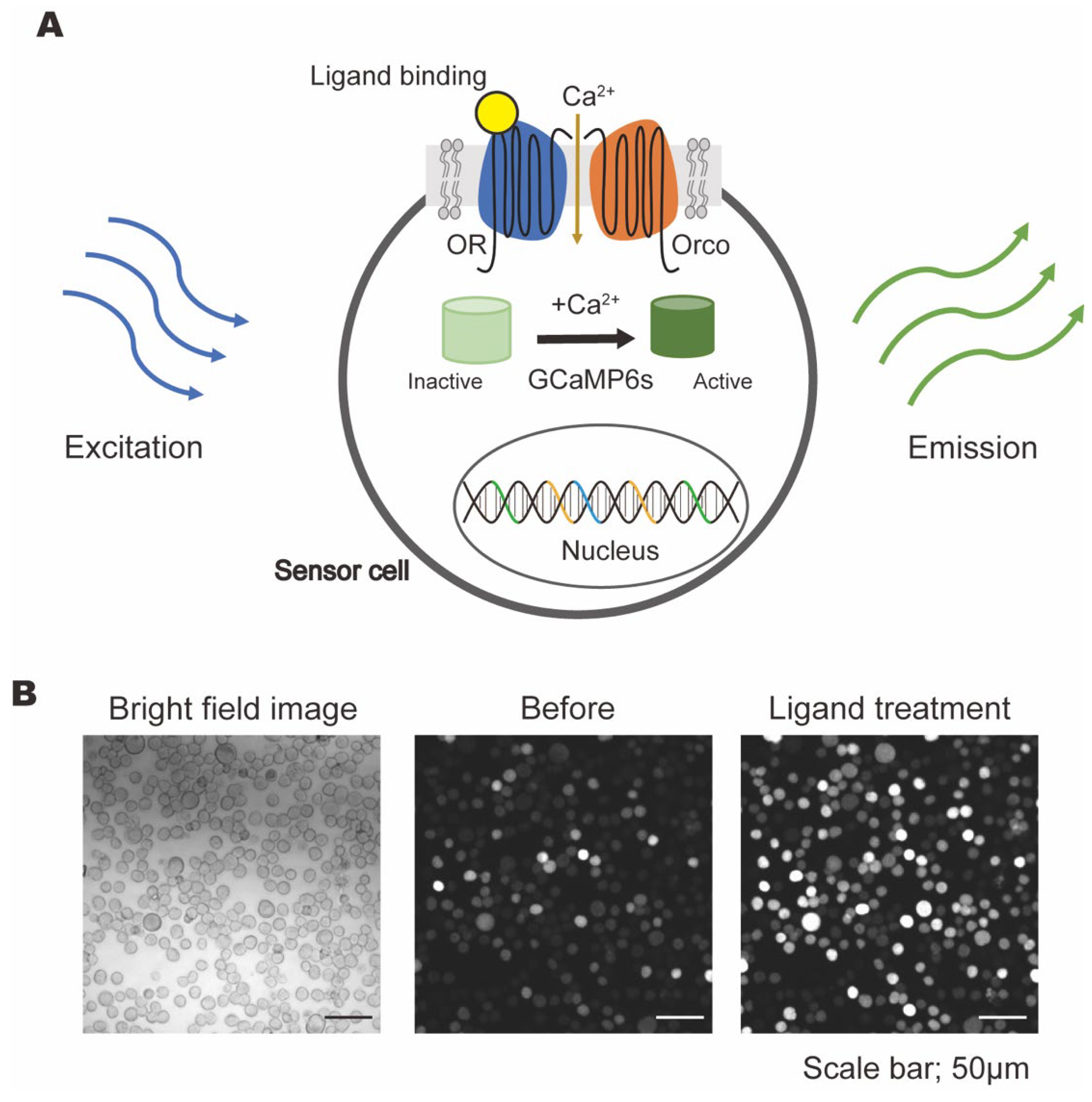
2.2. Construction of Odorant-Sensing Cells
2.3. Microplate Reader Calcium Imaging
2.4. Data Analysis
3. Results and Discussion
3.1. Response Quantification and Ligand Selectivity
3.2. Ligand Selectivity with Structurally Similar Alcohols
3.3. Ligand Selectivity with Certain Chemical Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaissling, K.E.; Priesner, E. Die riechschwelle des seidenspinners. Naturwissenschaften 1970, 57, 23–28. [Google Scholar] [CrossRef]
- Dweck, H.K.; Ebrahim, S.A.; Kromann, S.; Bown, D.; Hillbur, Y.; Sachse, S.; Hansson, B.S.; Stensmyr, M.C. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 2013, 23, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Stensmyr, M.C.; Dweck, H.K.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Farnum, A.; Parnas, M.; Hoque Apu, E.H.; Cox, E.; Lefevre, N.; Contag, C.H.; Saha, D. Harnessing insect olfactory neural circuits for detecting and discriminating human cancers. Biosens. Bioelectron. 2023, 219, 114814. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.; Warr, C.G.; de Bruyne, M. Detection of volatile indicators of illicit substances by the olfactory receptors of Drosophila melanogaster. Chem. Senses 2010, 35, 613–625. [Google Scholar] [CrossRef]
- Kato, A.; Touhara, K. Mammalian olfactory receptors: Pharmacology, G protein coupling and desensitization. Cell. Mol. Life Sci. 2009, 66, 3743–3753. [Google Scholar] [CrossRef]
- Benton, R.; Sachse, S.; Michnick, S.W.; Vosshall, L.B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006, 4, e20. [Google Scholar] [CrossRef]
- Larsson, M.C.; Domingos, A.I.; Jones, W.D.; Chiappe, M.E.; Amrein, H.; Vosshall, L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 2004, 43, 703–714. [Google Scholar] [CrossRef]
- Nakagawa, T.; Sakurai, T.; Nishioka, T.; Touhara, K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 2005, 307, 1638–1642. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Montagné, N.; De Fouchier, A.; Newcomb, R.D.; Jacquin-Joly, E. Advances in the identification and characterization of olfactory receptors in insects. Prog. Mol. Biol. Transl. Sci. 2015, 130, 55–80. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Amrein, H.; Morozov, P.S.; Rzhetsky, A.; Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 1999, 96, 725–736. [Google Scholar] [CrossRef]
- Hallem, E.A.; Ho, M.G.; Carlson, J.R. The molecular basis of odor coding in the Drosophila antenna. Cell 2004, 117, 965–979. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Martin, F.; Garcia-Fernandez, J.M.; Alcorta, E. The two main olfactory receptor families in Drosophila, ORs and IRs: A comparative approach. Front. Cell. Neurosci. 2018, 12, 253. [Google Scholar] [CrossRef]
- Münch, D.; Galizia, C.G. DoOR 2.0-comprehensive mapping of Drosophila melanogaster odorant responses. Sci. Rep. 2016, 6, 21841. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.H.; Behrendt, H.J.; Gisselmann, G.; Störtkuhl, K.F.; Hovemann, B.; Hatt, H. Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc. Natl. Acad. Sci. USA 2001, 98, 9377–9380. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, E.M.; Gisselmann, G.; Zhang, W.; Dooley, R.; Störtkuhl, K.; Hatt, H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 2005, 8, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.; Kiely, A.; Beale, M.; Vargas, E.; Carraher, C.; Kralicek, A.V.; Christie, D.L.; Chen, C.; Newcomb, R.D.; Warr, C.G. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem. Mol. Biol. 2008, 38, 770–780. [Google Scholar] [CrossRef]
- Tsitoura, P.; Andronopoulou, E.; Tsikou, D.; Agalou, A.; Papakonstantinou, M.P.; Kotzia, G.A.; Labropoulou, V.; Swevers, L.; Georgoussi, Z.; Iatrou, K. Expression and membrane topology of Anopheles gambiae odorant receptors in lepidopteran insect cells. PLoS ONE 2010, 5, e15428. [Google Scholar] [CrossRef]
- Kiely, A.; Authier, A.; Kralicek, A.V.; Warr, C.G.; Newcomb, R.D. Functional analysis of a Drosophila melanogaster olfactory receptor expressed in Sf9 cells. J. Neurosci. Methods. 2007, 159, 189–194. [Google Scholar] [CrossRef]
- Mitsuno, H.; Sakurai, T.; Namiki, S.; Mitsuhashi, H.; Kanzaki, R. Novel cell-based odorant sensor elements based on insect odorant receptors. Biosens. Bioelectron. 2015, 65, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Termtanasombat, M.; Mitsuno, H.; Misawa, N.; Yamahira, S.; Sakurai, T.; Yamaguchi, S.; Nagamune, T.; Kanzaki, R. Cell-based odorant sensor array for odor discrimination based on insect odorant receptors. J. Chem. Ecol. 2016, 42, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Mitsuno, H.; Araki, S.; Sukekawa, Y.; Terutsuki, D.; Niki, S.; Kuroda, E.; Fujibayashi, S.; Sakurai, T.; Oguma, K.; Yamaguchi, S.; et al. Portable geosmin detection system based on sensor cells expressing insect odorant receptors. Res. Sq. 2025; preprint. [Google Scholar] [CrossRef]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kako, H.; Yokogoshi, H. Contribution of intracellular Ca2+ concentration and protein dephosphorylation to the induction of dopamine release from PC12 cells by the green odor compound hexanal. Cell. Mol. Neurobiol. 2010, 30, 173–184. [Google Scholar] [CrossRef]
- Aratani, Y.; Uemura, T.; Hagihara, T.; Matsui, K.; Toyota, M. Green leaf volatile sensory calcium transduction in Arabidopsis. Nat. Commun. 2023, 14, 6236. [Google Scholar] [CrossRef]
- Sukekawa, Y.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Odor discrimination using cell-based odor biosensor system with fluorescent image processing. IEEE Sens. J. 2019, 19, 7192–7200. [Google Scholar] [CrossRef]
- Andersson, M.N.; Schlyter, F.; Hill, S.R.; Dekker, T. What reaches the antenna? How to calibrate odor flux and ligand–receptor affinities. Chem. Senses 2012, 37, 403–420. [Google Scholar] [CrossRef]
- Zboray, K.; Toth, A.V.; Miskolczi, T.D.; Pesti, K.; Casanova, E.; Kreidl, E.; Mike, A.; Szenes, Á.; Sági, L.; Lukacs, P. High-throughput ligand profile characterization in novel cell lines expressing seven heterologous insect olfactory receptors for the detection of volatile plant biomarkers. Sci. Rep. 2023, 13, 21757. [Google Scholar] [CrossRef]
- Olsen, S.R.; Wilson, R.I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 2008, 452, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 83, 241–272. [Google Scholar] [CrossRef]
- Guo, Y.R.; Yin, N.N.; Wu, C.; Yang, Z.X.; Wang, Z.Q.; Liu, N.Y. Expression profile and functional characterization of odorant binding proteins in a forest pest, Dioryctria abietella (Lepidoptera: Pyralidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023, 266, 110835. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef]
- Wang, G.; Carey, A.F.; Carlson, J.R.; Zwiebel, L.J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2010, 107, 4418–4423. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; de March, C.A.; van der Velden, W.J.; Ma, N.; Tewari, J.; Del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural basis of odorant recognition by a human odorant receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef]
- Bohbot, J.D.; Dickens, J.C. Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS ONE 2009, 4, e7032. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Sukekawa, Y.; Niki, S.; Kuroda, E.; Kanzaki, R.; Namiki, S.; Mitsuno, H. Ligand Differentiation Ability of Insect Odorant Receptors in Heterologously Expressed Cells as Potential Biosensor Elements. Chemosensors 2025, 13, 273. https://doi.org/10.3390/chemosensors13080273
Zhou R, Sukekawa Y, Niki S, Kuroda E, Kanzaki R, Namiki S, Mitsuno H. Ligand Differentiation Ability of Insect Odorant Receptors in Heterologously Expressed Cells as Potential Biosensor Elements. Chemosensors. 2025; 13(8):273. https://doi.org/10.3390/chemosensors13080273
Chicago/Turabian StyleZhou, Rui, Yuji Sukekawa, Sawako Niki, Eri Kuroda, Ryohei Kanzaki, Shigehiro Namiki, and Hidefumi Mitsuno. 2025. "Ligand Differentiation Ability of Insect Odorant Receptors in Heterologously Expressed Cells as Potential Biosensor Elements" Chemosensors 13, no. 8: 273. https://doi.org/10.3390/chemosensors13080273
APA StyleZhou, R., Sukekawa, Y., Niki, S., Kuroda, E., Kanzaki, R., Namiki, S., & Mitsuno, H. (2025). Ligand Differentiation Ability of Insect Odorant Receptors in Heterologously Expressed Cells as Potential Biosensor Elements. Chemosensors, 13(8), 273. https://doi.org/10.3390/chemosensors13080273




