Emergency Wound Infection Monitoring and Treatment Based on Wearable Electrochemical Detection and Drug Release with Conductive Hydrogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Fabrication of the Wound Infection-Detecting Electrode
2.3. Preparation and Characterization of the Sensor
2.4. Preparation of the Gel
2.5. Preparation and Characterization of the Treatment Module
2.6. Quantitative Drug Release in the Treatment Module
2.7. Antibacterial Activity and Biocompatibility Testing
2.8. In Vivo Wound Healing
3. Results
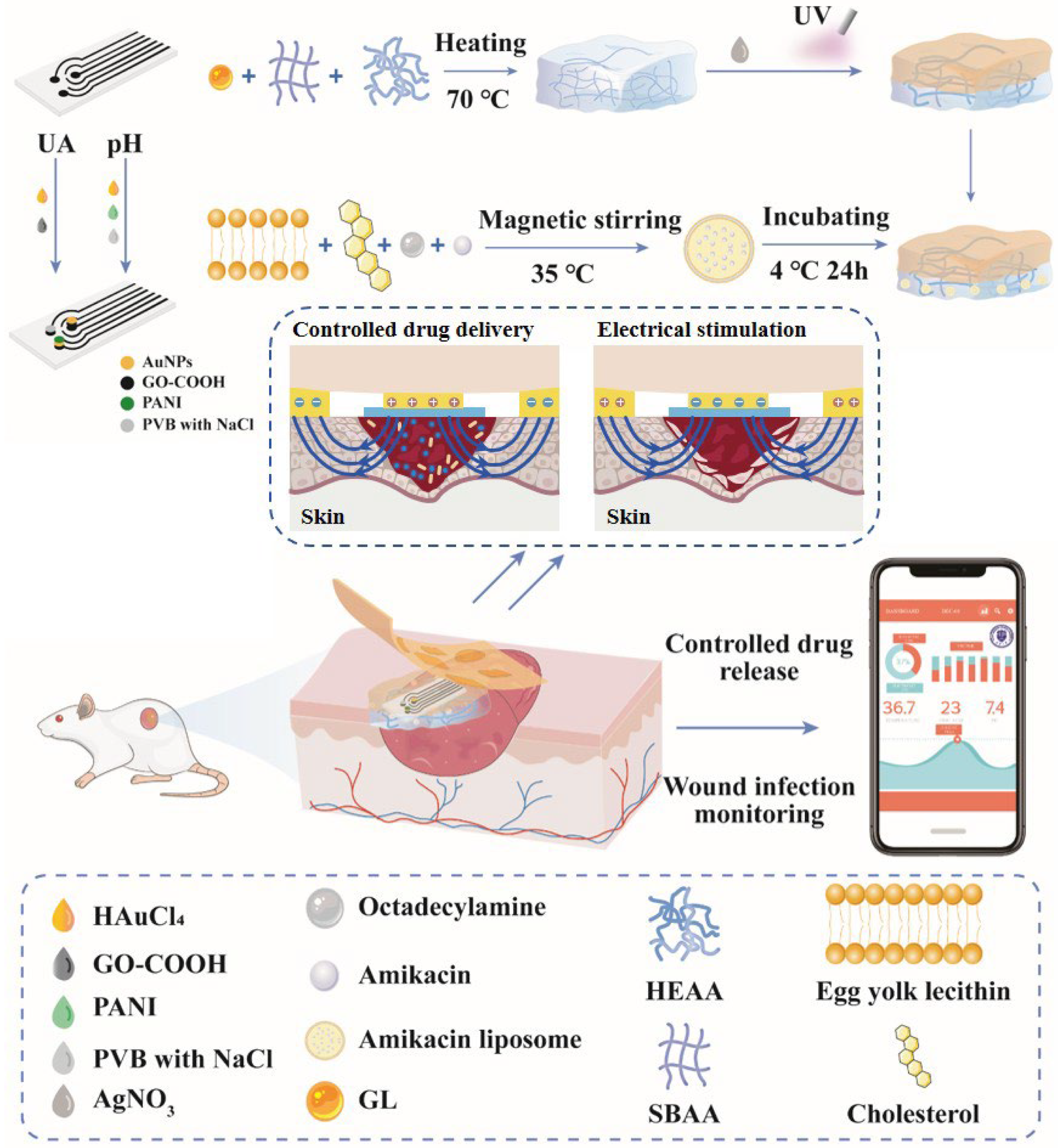
3.1. Wound Infection Detection and Treatment System Overview
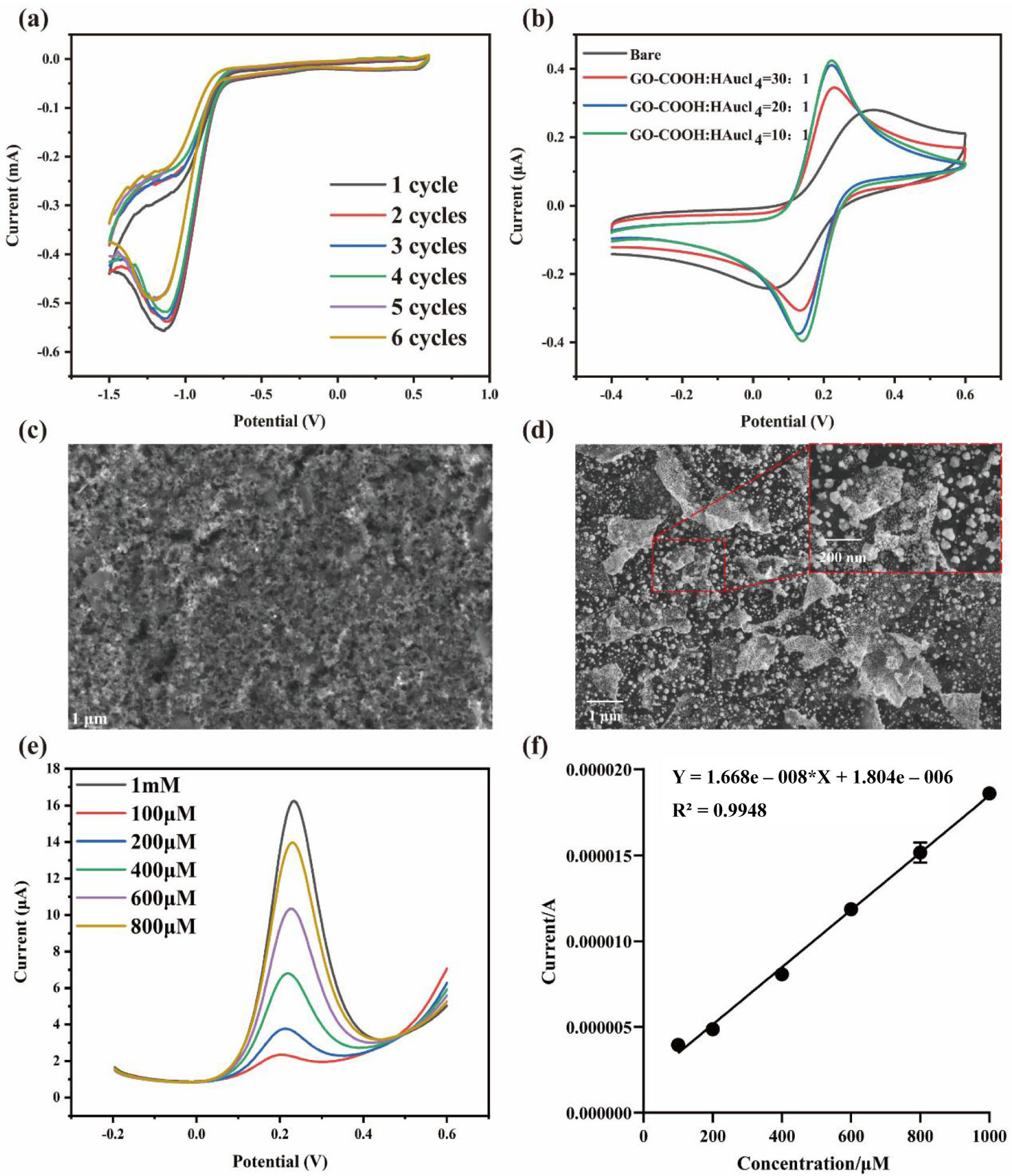
3.2. Modification of the Integrated Screen-Printed Electrodes
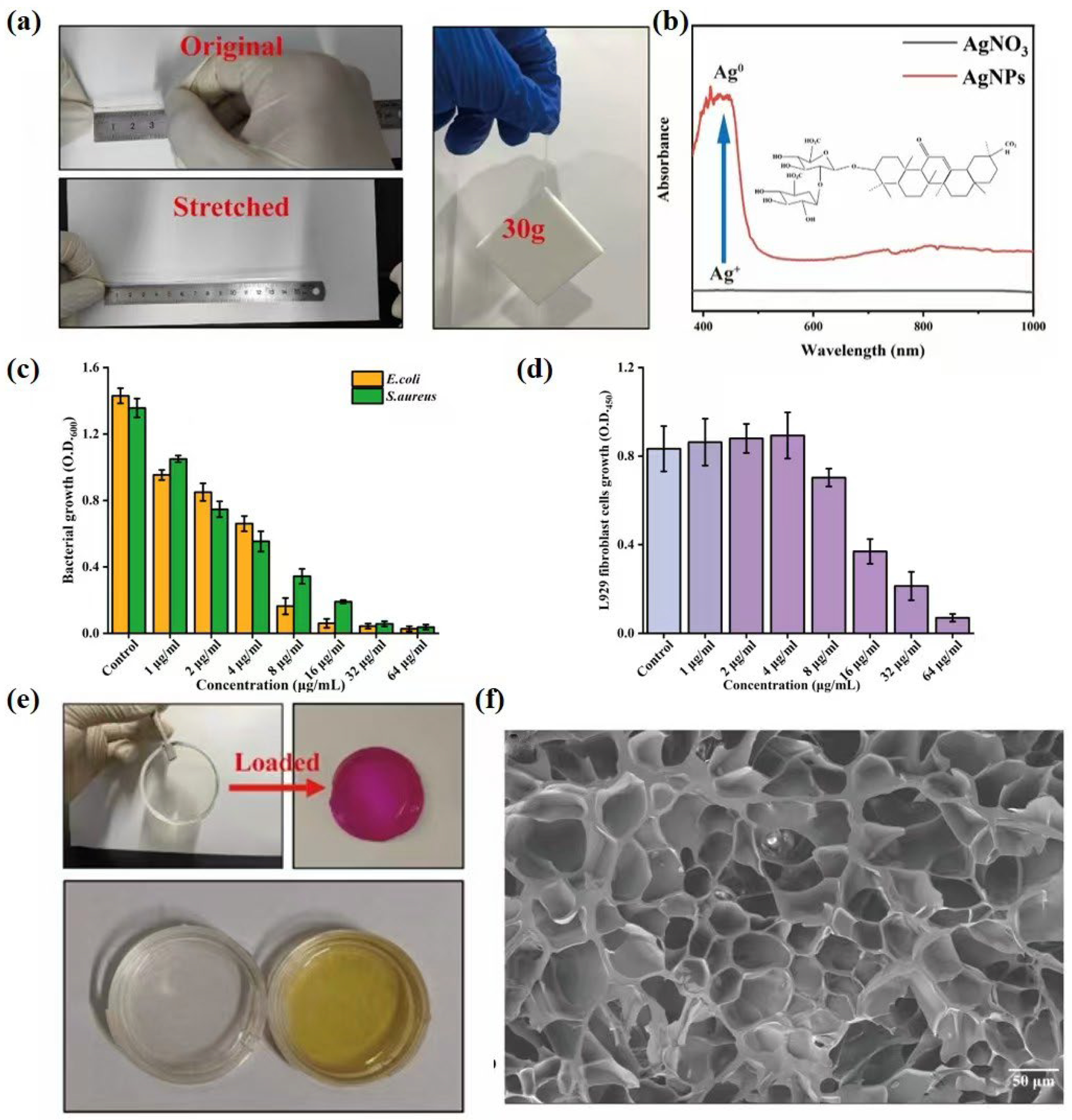
3.3. Characterization of Gel
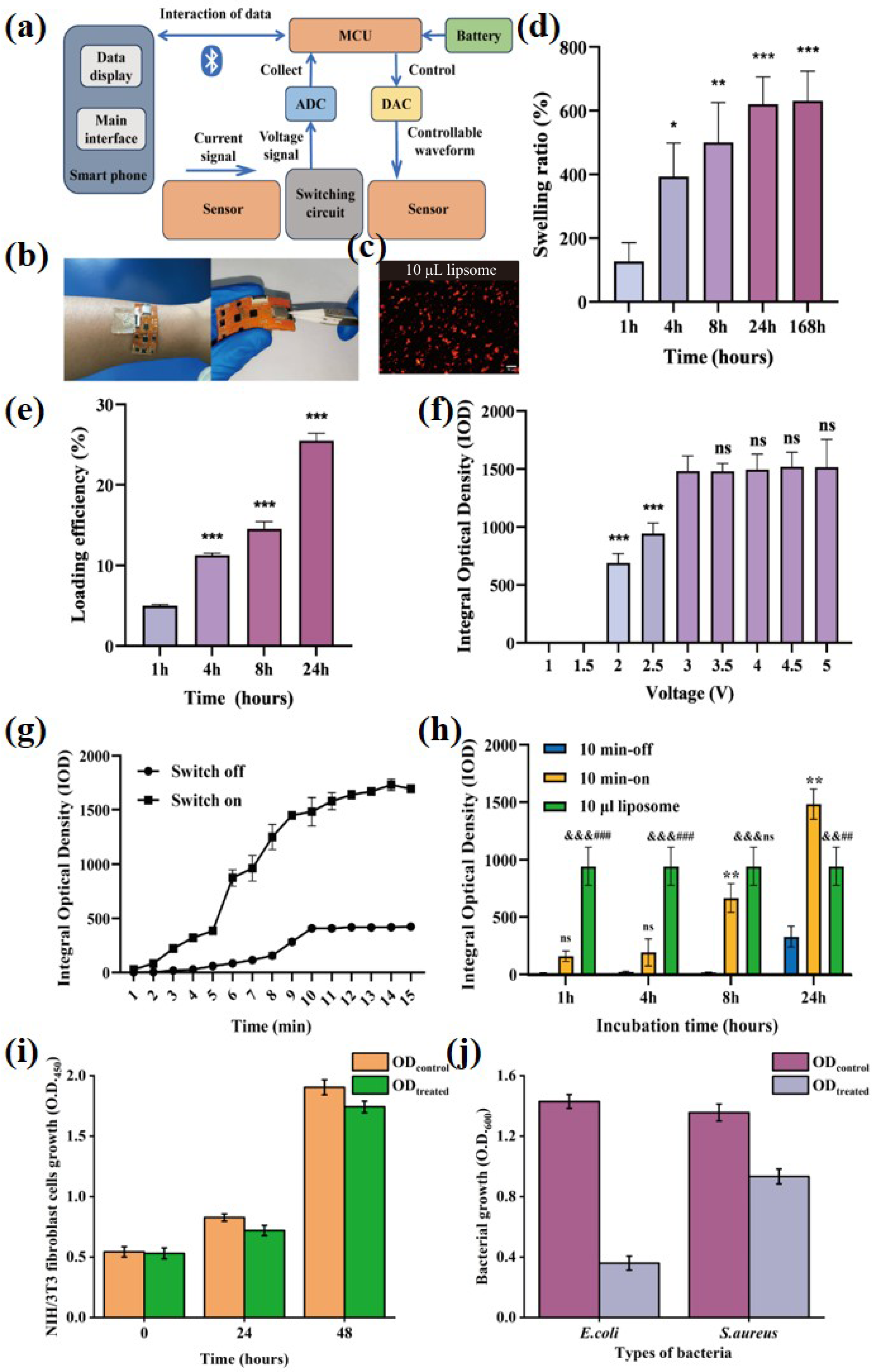
3.4. Optimization and Characterization of Lipo@Ami
3.5. Electronic Control Gel-Lipo@Ami Drug Release Test
3.6. Integrated System Promotes In Vivo Evaluation of Infected Wound Healing
3.7. Coordinated Wound Repair Systems
3.7.1. Infection–Inflammation Control System
3.7.2. Extracellular Matrix Restoration System
3.7.3. Angiogenic Revascularization System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UA | Uric acid |
| AgNPs | Silver nanoparticles |
| SBMA | [2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide |
| HEAA | N-(2-Hydroxyethyl)-2-acrylamide |
| GL | Glycyrrhizic acid |
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| IOD | Integrated optical density |
| SEM | Scanning electron microscopy |
| Ami | Amikacin |
| Lipo | Cationic liposomes |
| Gel | Zwitterionic conductive hydrogel |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus |
| FPCB | Flexible printed circuit board |
| CCK-8 | Cell counting kit-8 |
| CE | Counter electrode |
| RE | Reference electrode |
| WE | Working electrode |
| PVB | Polyvinyl Butyral |
| OCPT | Open circuit potential-time |
| OD | Optical density |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| PA | Pseudomonas aeruginosa |
| PANI | Polyaniline |
References
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, H.; Zhang, Z.; Chen, K.; Zhang, Q.; Zhang, C.; Gao, W.; Zheng, Y. Antibacterial hydrogels for wound dressing applications: Current status, progress, challenges, and trends. Gels 2024, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Salleh, N.A.B.; Tanaka, Y.; Sutarlie, L.; Su, X. Detecting bacterial infections in wounds: A review of biosensors and wearable sensors in comparison with conventional laboratory methods. Analyst 2022, 147, 1756–1776. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel wound dressings accelerating healing process of wounds in movable parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef]
- Valdez, M.K.; Sexton, J.D.; Lutz, E.A.; Reynolds, K.A. Spread of infectious microbes during emergency medical response. Am. J. Infect. Control 2015, 43, 606–611. [Google Scholar] [CrossRef]
- Lu, S.-H.; Samandari, M.; Li, C.; Li, H.; Song, D.; Zhang, Y.; Tamayol, A.; Wang, X. Multimodal sensing and therapeutic systems for wound healing and management: A review. Sens. Actuators Rep. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Guo, J. Uric Acid Monitoring with a Smartphone as the Electrochemical Analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered electrochemical sensors on gauze for rapid quantification of wound biomarkers. Biosens. Bioelectron. 2017, 98, 189–194. [Google Scholar] [CrossRef]
- Yapor, J.P.; Alharby, A.; Gentry-Weeks, C.; Reynolds, M.M.; Alam, A.; Li, Y.V. Polydiacetylene Nanofiber Composites as a Colorimetric Sensor Responding To Escherichia coli and pH. ACS Omega 2017, 2, 7334–7342. [Google Scholar] [CrossRef]
- Chanmugam, A.; Langemo, D.; Thomason, K.; Haan, J.; Altenburger, E.A.; Tippett, A.; Henderson, L.; Zortman, T.A. Relative Temperature Maximum in Wound Infection and Inflammation as Compared with a Control Subject Using Long-Wave Infrared Thermography. Adv. Skin Wound Care 2017, 30, 406–414. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, R.; Han, F.; Zheng, Z.; Liu, Y.; Zhou, X.; Li, Q.; Zhai, X.; Wu, J.; Pan, X.; et al. A soft intelligent dressing with pH and temperature sensors for early detection of wound infection. RSC Adv. 2022, 12, 3243–3252. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Edwards, H.; Finlayson, K.; Shooter, G.K. Elevated uric acid correlates with wound severity. Int. Wound J. 2012, 9, 139–149. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Z.; Gan, L.; Zhang, L.; Yang, L.; Wu, P. Application of Biomedical Microspheres in Wound Healing. Int. J. Mol. Sci. 2023, 24, 7319. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, M.; Cheng, W.; Niu, W.; Chen, M.; Luo, M.; Xie, C.; Leng, T.; Zhang, L.; Lei, B. Bioactive antiinflammatory antibacterial hemostatic citrate-based dressing with macrophage polarization regulation for accelerating wound healing and hair follicle neogenesis. Bioact. Mater. 2021, 6, 721–728. [Google Scholar] [CrossRef]
- Song, J.W.; Ryu, H.; Bai, W.; Xie, Z.; Vázquez-Guardado, A.; Nandoliya, K.; Avila, R.; Lee, G.; Song, Z.; Kim, J.; et al. Bioresorbable, wireless, and battery-free system for electrotherapy and impedance sensing at wound sites. Sci. Adv. 2023, 9, eade4687. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Qi, R.; Yuan, H. Recent Advances of Natural-Polymer-Based Hydrogels for Wound Antibacterial Therapeutics. Polymers 2023, 15, 3305. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Zhang, Q. Injectable redox and light responsive MnO(2) hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, e1703509. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Ou, X.; Wang, Z.; Li, X.; Feng, Y.; Yang, X.; Qu, W.; Yang, B.; Lin, Q. Electrical stimulation-based conductive hydrogel for immunoregulation, neuroregeneration and rapid angiogenesis in diabetic wound repair. Sci. China Mater. 2023, 66, 1237–1248. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Ma, C.; Tang, H.; Hao, H.; Li, M.; Luo, X.; Yang, M.; Gao, L.; Li, J. Advances in Hydrogels of Drug Delivery Systems for the Local Treatment of Brain Tumors. Gels 2024, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent Progress in Biomedical Sensors Based on Conducting Polymer Hydrogels. ACS Appl. Bio Mater. 2023, 6, 1720–1741. [Google Scholar] [CrossRef]
- Stevens, D.L.; Aldape, M.J.; Bryant, A.E. Life-threatening clostridial infections. Anaerobe 2012, 18, 254–259. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.; Lin, Z.; Zhang, X.; Fu, W. Microbiologic characteristics of pathogenic bacteria from hospitalized trauma patients who survived Wenchuan earthquake. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2529–2535. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, e2303326. [Google Scholar] [CrossRef]
- Lazaridou, M.; Nanaki, S.; Zamboulis, A.; Papoulia, C.; Chrissafis, K.; Klonos, P.A.; Kyritsis, A.; Vergkizi-Nikolakaki, S.; Kostoglou, M.; Bikiaris, D.N. Super absorbent chitosan-based hydrogel sponges as carriers for caspofungin antifungal drug. Int. J. Pharm. 2021, 606, 120925. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G.; et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023, 9, eadf7388. [Google Scholar] [CrossRef]
- Liu, W.S.; Liu, Y.; Gao, J.; Zheng, H.; Lu, Z.M.; Li, M. Biomembrane-Based Nanostructure- and Microstructure-Loaded Hydrogels for Promoting Chronic Wound Healing. Int. J. Nanomed. 2023, 18, 385–411. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, N.; Yu, X.; Guo, Z.; Liu, Z.; Sun, X.; Li, M.-H.; Hu, J. Natural glycyrrhizic acid-tailored hydrogel with in-situ gradient reduction of AgNPs layer as high-performance, multi-functional, sustainable flexible sensors. Chem. Eng. J. 2022, 430, 132779. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.; Gao, Y.; Kong, D.; Jiang, Y.; Cui, X.; Guo, M.; Chen, J.; Chang, F.; Zhang, M.; et al. Functional chitosan gel coating enhances antimicrobial properties and osteogenesis of titanium alloy under persistent chronic inflammation. Front. Bioeng. Biotechnol. 2023, 11, 1118487. [Google Scholar] [CrossRef]
- Yi, Y.T.; Sun, J.Y.; Lu, Y.W.; Liao, Y.C. Programmable and on-demand drug release using electrical stimulation. Biomicrofluidics 2015, 9, 022401. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Barańska-Rybak, W. Nanomaterials as a Successor of Antibiotics in Antibiotic-Resistant, Biofilm Infected Wounds? Antibiotics 2021, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Talló, K.; Vílchez, S.; Pons, R.; López, O. Gels formed from the interaction of lipid vesicles: Influence of charge in their structural and rheological properties. J. Mol. Liq. 2021, 322, 114957. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lodha, R.; Kabra, S.K. Current therapies for the treatment of multidrug-resistant tuberculosis in children in India. Expert Opin. Pharmacother. 2017, 18, 1595–1606. [Google Scholar] [CrossRef]
- Rukavina, Z.; Vanić, Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, 18. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Y.; Lei, K.; Liu, J.; Hu, M.; Guo, L.; Guo, Y.; Ye, Q. A “Trojan Horse” Strategy: The Preparation of Bile Acid-Modifying Irinotecan Hydrochloride Nanoliposomes for Liver-Targeted Anticancer Drug Delivery System Study. Molecules 2023, 28, 1577. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Shooter, G.K. Uric acid and xanthine oxidoreductase in wound healing. Curr. Rheumatol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef]
- Mirzaei, Y.; Hagemeister, K.; Hüffel, M.; Schwandt, T.; Tolba, R.H.; Steitz, J. A Novel In Vitro Method to Assess the Microbial Barrier Function of Tissue Adhesives Using Bioluminescence Imaging Technique. Biomed. Res. Int. 2022, 2022, 3483238. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges-A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, A.B.; Bhalla-Regev, S.K.; Tierno, P.M., Jr.; Stevens-Riley, M.; Wiygul, R.C. Proteolytic activity by multiple bacterial species isolated from chronic venous leg ulcers degrades matrix substrates. Biol. Res. Nurs. 2013, 15, 407–415. [Google Scholar] [CrossRef]
- Arcangeli, D.; Gualandi, I.; Mariani, F.; Tessarolo, M.; Ceccardi, F.; Decataldo, F.; Melandri, F.; Tonelli, D.; Fraboni, B.; Scavetta, E. Smart Bandaid Integrated with Fully Textile OECT for Uric Acid Real-Time Monitoring in Wound Exudate. ACS Sens. 2023, 8, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wei, Y.; Ju, Y.; Li, P.; Zhang, Y.; Li, L.; Gao, L.; Liu, S.; Liu, D.; Hu, Y.; et al. Anaerobic purinolytic enzymes enable dietary purine clearance by engineered gut bacteria. Cell Chem. Biol. 2023, 30, 1104–1114.e1107. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Z.; Liu, L.; Low, S.S.; Lu, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef]
- Cullen, B.; Gefen, A. The biological and physiological impact of the performance of wound dressings. Int. Wound J. 2023, 20, 1292–1303. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Ma, C.; Yuan, Q.; Wang, X.; Wan, H.; Wang, P. A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing. Biosensors 2021, 12, 10. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time pH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Lin, G.; Zhao, C.; Liao, W.; Yang, J.; Zheng, Y. Eco-Friendly Green Synthesis of Rubropunctatin Functionalized Silver Nanoparticles and Evaluation of Antibacterial Activity. Nanomaterials 2022, 12, 4052. [Google Scholar] [CrossRef]
- Widatalla, H.A.; Yassin, L.F.; Alrasheid, A.A.; Rahman Ahmed, S.A.; Widdatallah, M.O.; Eltilib, S.H.; Mohamed, A.A. Green synthesis of silver nanoparticles using green tea leaf extract, characterization and evaluation of antimicrobial activity. Nanoscale Adv. 2022, 4, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, D.; Xu, X.; Shen, Y.; Huang, Y.; Zeng, Z.; Xia, M.; Zhao, C. NIR-triggered thermo-responsive biodegradable hydrogel with combination of photothermal and thermodynamic therapy for hypoxic tumor. Asian J. Pharm. Sci. 2020, 15, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, T. Shaping and Structuring of Polymer Gels. Gels 2024, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Song, S.; Zhao, H.; Yuan, Y. Platinum-based drugs and hydrogel: A promising anti-tumor combination. Drug. Deliv. 2023, 30, 2287966. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.Y.; Li, L.; Li, Z.; Fu, S.; Xu, Y.; Zheng, B.Y.; Ke, M.; Li, X.; Huang, J.D. A Sulfur-Bridging Sulfonate-Modified Zinc(II) Phthalocyanine Nanoliposome Possessing Hybrid Type I and Type II Photoreactions with Efficient Photodynamic Anticancer Effects. Molecules 2023, 28, 2250. [Google Scholar] [CrossRef]
- Salem, H.F.; Ahmed, S.M.; Hassaballah, A.E.; Omar, M.M. Targeting brain cells with glutathione-modulated nanoliposomes: In vitro and in vivo study. Drug. Des. Devel. Ther. 2015, 9, 3705–3727. [Google Scholar] [CrossRef]
- Qi, N.; Tang, B.; Liu, G.; Liang, X. Poly(γ-glutamic acid)-coated lipoplexes loaded with Doxorubicin for enhancing the antitumor activity against liver tumors. Nanoscale Res. Lett. 2017, 12, 361. [Google Scholar] [CrossRef]
- Tsichlis, I.; Manou, A.P.; Manolopoulou, V.; Matskou, K.; Chountoulesi, M.; Pletsa, V.; Xenakis, A.; Demetzos, C. Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation. Materials 2023, 16, 5509. [Google Scholar] [CrossRef]
- Feng, L.; Ye, W.; Zhang, K.; Qu, D.; Liu, W.; Wu, M.; Han, J. In vitro Digestion Characteristics of Hydrolyzed Infant Formula and Its Effects on the Growth and Development in Mice. Front. Nutr. 2022, 9, 912207. [Google Scholar] [CrossRef]
- Gu, X.; Wang, D.; Wang, X.; Liu, Y.; Di, X. Fast Screening of Biomembrane-Permeable Compounds in Herbal Medicines Using Bubble-Generating Magnetic Liposomes Coupled with LC-MS. Molecules 2021, 26, 1742. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Pei, D.; Su, K.; Wang, L. Evaluation of Aminated Nano-Silica as a Novel Shale Stabilizer to Improve Wellbore Stability. Materials 2024, 17, 1776. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef]
- Scheeder, A.; Brockhoff, M.; Ward, E.N.; Kaminski Schierle, G.S.; Mela, I.; Kaminski, C.F. Molecular Mechanisms of Cationic Fusogenic Liposome Interactions with Bacterial Envelopes. J. Am. Chem. Soc. 2023, 145, 28240–28250. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug. Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Udeni Gunathilake, T.M.S.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, P.; Lu, B.; Yu, H.; Huang, W.; Hou, H.; Dai, K. Causes of infection after earthquake, China, 2008. Emerg. Infect. Dis. 2010, 16, 974–975. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q. Current research on fungi in chronic wounds. Front. Mol. Biosci. 2022, 9, 1057766. [Google Scholar] [CrossRef]
- Du, Y.; Du, W.; Lin, D.; Ai, M.; Li, S.; Zhang, L. Recent Progress on Hydrogel-Based Piezoelectric Devices for Biomedical Applications. Micromachines 2023, 14, 167. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Rational Design of Multifunctional Hydrogels for Wound Repair. J. Funct. Biomater. 2023, 14, 553. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Huang, S.; Chen, Q.; Li, Y.; Duan, L.; Yu, Z.; Li, W.; Luo, H.; Li, S.; Fan, B.; et al. Emergency Wound Infection Monitoring and Treatment Based on Wearable Electrochemical Detection and Drug Release with Conductive Hydrogel. Chemosensors 2025, 13, 267. https://doi.org/10.3390/chemosensors13070267
Wang S, Huang S, Chen Q, Li Y, Duan L, Yu Z, Li W, Luo H, Li S, Fan B, et al. Emergency Wound Infection Monitoring and Treatment Based on Wearable Electrochemical Detection and Drug Release with Conductive Hydrogel. Chemosensors. 2025; 13(7):267. https://doi.org/10.3390/chemosensors13070267
Chicago/Turabian StyleWang, Shaopeng, Songsong Huang, Qian Chen, Yanjun Li, Liyang Duan, Zhi Yu, Weixia Li, Hui Luo, Shuang Li, Bin Fan, and et al. 2025. "Emergency Wound Infection Monitoring and Treatment Based on Wearable Electrochemical Detection and Drug Release with Conductive Hydrogel" Chemosensors 13, no. 7: 267. https://doi.org/10.3390/chemosensors13070267
APA StyleWang, S., Huang, S., Chen, Q., Li, Y., Duan, L., Yu, Z., Li, W., Luo, H., Li, S., Fan, B., & Chen, Z. (2025). Emergency Wound Infection Monitoring and Treatment Based on Wearable Electrochemical Detection and Drug Release with Conductive Hydrogel. Chemosensors, 13(7), 267. https://doi.org/10.3390/chemosensors13070267





