Advanced Chemometric Techniques for Environmental Pollution Monitoring and Assessment: A Review
Abstract
1. Introduction
2. Environmental Monitoring and Assessment
2.1. In Situ Monitoring
2.2. Remote Monitoring
3. Types of Chemometric Techniques
3.1. Unsupervised Learning Methods
3.1.1. Cluster Analysis
3.1.2. Artificial Neural Networks
3.2. Supervised Learning Methods
Discriminant Analysis
3.3. Factorial Methods
Principal Component Analysis
4. Environmental Application of Chemometric Techniques
4.1. Air Quality
4.2. Water Quality
4.3. Soil Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aerosol Optical Depth | AOD |
| Active Pharmaceutical Ingredients | API |
| Affinity Tensor-Based Matching | ATBM |
| Agglomerative Hierarchical Cluster | AHC |
| Air Pollution Index | API |
| Aluminum | Al |
| Ammonia | NH4 |
| Arsenic | As |
| Artificial Intelligence | AI |
| Artificial Neural Network | ANN |
| Asymmetric Least Squares Splines Regression | AsLSSR |
| Atomic Absorption Spectroscopy | AAS |
| Attenuated Total Reflection | ATR |
| Average air Temperature between current and previous day | TMean |
| Average relative Humidity between current and previous day | RHMean |
| Average wind Speed between current and previous day | WSMean |
| Bicarbonate | HCO3 |
| Biochemical Oxygen Demand | BOD |
| Boron | B |
| Cadmium | Cd |
| Calcium | Ca |
| Calcium Carbonate | CaCO3 |
| Carbon Monoxide | CO |
| Chemical Oxygen Demand | COD |
| Chloride | Cl |
| Chromium | Cr |
| Cluster Analysis | CA |
| Collected total precipitation past 48 h | PR48H |
| Collected total precipitation past 72 h | PR72H |
| Colored dissolved organic matters | CDOM |
| Continuous Wavelet Transform | CWT |
| Copper | Cu |
| Correlation Optimized Warping | COW |
| Current-day air temperature | TT |
| Current-day relative humidity | RHT |
| Current-day total precipitation | PRT |
| Current-day wind speed | WST |
| Discrete Wavelet Transform | DWT |
| Discriminant Analysis | DA |
| Dissolved Organic Carbon | DOC |
| Dissolved Oxygen | DO |
| Discriminant Analysis of Multi-Aspect Cytometry | DAMACY |
| Electrical Conductivity | EC |
| Electrothermal Atomic Absorption Spectroscopy | ETAAS |
| Energy Dispersive-X-ray Fluorescence | EDXRF |
| Environmental Carrying Capacity | ECC |
| Extreme Gradient Boosting | XGBoost |
| eXtensible Computational Mass Spectrometry | XCMS |
| Factor Analysis | FA |
| Flame Ionization Detector | FID |
| Fast Fourier Transform | FFT |
| Functional Analysis of Variance | FANOVA |
| Fourier Transform Infrared | FTIR |
| GAS Chromatography–Mass Spectrophotometry | GCMS |
| Geographically Weighted Regression | GWR |
| Gigahertz | GHz |
| Hazard Quotient | HQ |
| Hierarchical Agglomerative Cluster Analysis | HACA |
| Hierarchical Cluster Analysis | HCA |
| High-Performance Liquid Chromatography | HPLC |
| Hydrochloride | HCl |
| Hyperspectral Vegetation Indices | HVIs |
| Inductively Coupled Plasma Optical Emission Spectrometry | ICP-OES |
| Internet of Things | IoT |
| Interval Correlation Optimized Shifting | icoshift |
| Ion Chromatography | IC |
| Iron | Fe |
| Land Use and Land Cover | LULC |
| Life Cycle Assessment | LCA |
| Linear Discriminant Analysis | LDA |
| Liquid Chromatography–Mass Spectrophotometry | LCMS |
| Low Pollution Source | LPS |
| Manganese | Mg |
| Mean Absolute Percentage Error | MAPE |
| Mercury | Hg |
| Metabolomic Analysis and Visualization ENgine | MAVEN |
| Methane | CH4 |
| Moderate Pollution Source | MPS |
| Moderate Resolution Imaging Spectroradiometer | MODIS |
| Molecular descriptors | MDs |
| Multi-Angle Imaging Spectroradiometer | MISR |
| Multiple Linear Regression | MLR |
| Multiplicative Scatter Correction | MSC |
| National Institute of Standard Technology | NIST |
| Near-Infrared | NIR |
| Near-Infrared Reflectance Spectroscopy | NIRS |
| Nickel | Ni |
| Nitrate | NO2 |
| Nitrogen Dioxide | NO2 |
| Non-methane Hydrocarbons | NmHC |
| Normalized Difference Vegetation Index | NDVI |
| Not Available | NA |
| Norris-Williams derivation | NW |
| Orthogonal Partial Least Square | OPLS |
| Ozone | O3 |
| Partial Least Square | PLS |
| Partial Least Square Regression | PLSR |
| Particulate Matter with a Diameter of 2.5 or Less | PM2.5 |
| Particulate Matter with a Diameter of 10 or Less | PM10 |
| Pb | Lead |
| Phosphorus | P |
| Polycyclic Aromatic Hydrocarbons | PAHs |
| Positive Matrix Factorization | PMF |
| Potassium permanganate | KMnO4 |
| Previous-day air Temperature | TT-1 |
| Previous-day relative Humidity | RHT-1 |
| Previous-day wind Speed | WST-1 |
| Principal Component Analysis | PCA |
| Quadratic Discriminant Analysis | QDA |
| Quantitative Structure–Activity Relationship | QSAR |
| Random Forest | RF |
| Root Mean Square Error of Cross-Validation | RMSECV |
| Root Mean Squared Error of Prediction | RMSEP |
| Savitzky–Golay polynomial filters | SG |
| Sea surface salinity | SSS |
| Secchi disk depth | SDD |
| Selenium | Se |
| Sentinel-5 Precursor | S5P |
| Short-Wavelength Infrared | SWIR |
| Slightly High Pollution Source | SHPS |
| Sodium | Na |
| Sodium Absorption Ratio | SAR |
| Soil Organic Matter | SOM |
| Solid-Phase Microextraction | SPME |
| Standard Normal Variate | STV |
| Sulfur Dioxide | SO2 |
| Support Vector Machines | SVM |
| Suspended Solid | SS |
| Tin Dioxide | SnO2 |
| Titanium | Ti |
| Total Dissolved Solid | TDS |
| Total Hardness | TH |
| Total Hydrocarbons | THC |
| Total Kjeldahl Nitrogen method | TKN |
| Total Organic Carbon | TOC |
| Total Phosphorus | TP |
| Total Suspended Solids | TSS |
| Trihalomethanes | THMs |
| Ultraviolet-Visible | UV–Vis |
| United Kingdom | UK |
| Unmanned Aerial Vehicles | UAVs |
| Vanadium | V |
| Visible Infrared Imaging Radiometer Suite | VIIRS |
| Volatile Organic Compounds | VOCs |
| Water Quality Index | WQI |
| Wireless Sensor Networks | WSNs |
| Zinc | Zn |
References
- Sara, T.; Cinti, S. How Can Chemometrics Support the Development of Point of Need Devices? Anal. Chem. 2021, 93, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; Sayago, A.; Fernández-Recamales, A. An Overview on the Application of Chemometrics Tools in Food Authenticity and Traceability. Foods 2022, 11, 3940. [Google Scholar] [CrossRef] [PubMed]
- El-Rawy, M.; Fathi, H.; Abdalla, F.; Alshehri, F.; Eldeeb, H. An Integrated Principal Component and Hierarchical Cluster Analysis Approach for Groundwater Quality Assessment in Jazan, Saudi Arabia. Water 2023, 15, 1466. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Sun, C.; Li, H.; Gao, Y. Multivariate statistical approaches to identify the major factors governing groundwater quality. Appl. Water Sci. 2018, 8, 215. [Google Scholar] [CrossRef]
- Raphaëlle, V.; Judith, G.-A.; Florent, M.; Nicole, L.M.; Jordi, D.B.; Gemma, M.; Christophe, P.; Isabelle, R.; Francine, K.; Jean, M. Assessment of dietary patterns in nutritional epidemiology: Principal component analysis compared with confirmatory factor analysis. Am. J. Clin. Nutr. 2012, 96, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.; Patrick, J.F.G.; Trevor, H.; Alfonso, I.D.; Angelos, M.; Elena, T. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar]
- Ryu, J.; Liu, K.B.; McCloskey, T.A. The use of multivariate PCA dataset in identifying the underlying drivers of critical stressors, looking at global problems through a local lens. Data Brief 2022, 41, 107946. [Google Scholar] [CrossRef] [PubMed]
- Gregory, O.N.; Jean, V.; Isabelle, C.; Olivier, T. Using a multivariate regression tree to analyze trade-offs between ecosystem services: Application to the main cropping area in France. Sci. Total Environ. 2021, 764, 142815. [Google Scholar]
- Chu, K.; Liu, W.; She, Y.; Hua, Z.; Tan, M.; Liu, X.; Gu, L.; Jia, Y. Modified Principal Component Analysis for Identifying Key Environmental Indicators and Application to a Large-Scale Tidal Flat Reclamation. Water 2018, 10, 69. [Google Scholar] [CrossRef]
- Benjamin, P.; Per, A. Principal component analyses for integrated ecosystem assessments may primarily reflect methodological artefacts. ICES J. Mar. Sci. 2018, 75, 1021–1028. [Google Scholar]
- Abel, I.; Vanya, N.; Tsado, J.M.; Stanley, O.; Lucky, E.; Alexander, I.A.; Esther, B.; Jonathan, I.; Mariam, A.M.; Singh, K.R.B. Chemometric approach in environmental pollution analysis: A critical review. J. Environ. Manag. 2022, 309, 114653. [Google Scholar] [CrossRef] [PubMed]
- Jelena, V.P.; Sladana, C.A.; Snezana, M.M.; Snezana, B.T.; Mile, M.B. Chemometric characterization of heavy metals in soils and shoots of the two pioneer species sampled near the polluted water bodies in the close vicinity of the copper mining and metallurgical complex in Bor (Serbia): Phytoextraction and biomonitoring contexts. Chemosphere 2021, 262, 127808. [Google Scholar] [CrossRef] [PubMed]
- Nur, Z.S.; Ahmad, S.M.S.; Jyh, C.P.; Izuddin, F.A.; Norzahir, S.; Mohd, K.A.K.; Hammad, F.M.S. Application of chemometrics techniques to solve environmental issues in Malaysia. Heliyon 2019, 5, e02534. [Google Scholar] [CrossRef] [PubMed]
- Abhijeet, D. An optimized approach for predicting water quality features and a performance evaluation for mapping surface water potential zones based on Discriminant Analysis (DA), Geographical Information System (GIS) and Machine Learning (ML) models in Baitarani River Basin, Odisha. Desalina. Water Treat. 2025, 321, 101039. [Google Scholar]
- Jafar, R.; Awad, A.; Hatem, I.; Jafar, K.; Awad, E.; Shahrour, I. Multiple Linear Regression and Machine Learning for Predicting the Drinking Water Quality Index in Al-Seine Lake. Smart Cities 2023, 6, 2807–2827. [Google Scholar] [CrossRef]
- Dargahi, P.; Nasseri, S.; Hadi, M.; Nodehi, R.N.; Mahvi, A.H. Prediction models for groundwater quality parameters using a multiple linear regression (MLR): A case study of Kermanshah, Iran. J. Environ. Health Sci. Eng. 2022, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Nur, F.S.Z.; Mohd, S.M.N.; Fatimah, A.R.; Munira, I.; Mohd, A.A. Hybridization of hierarchical clustering with persistent homology in assessing haze episodes between air quality monitoring stations. J. Environ. Manag. 2022, 306, 114434. [Google Scholar] [CrossRef] [PubMed]
- Zulkepli, N.F.S.; Noorani, M.S.M.; Razak, F.A.; Ismail, M.; Alias, M.A. Cluster Analysis of Haze Episodes Based on Topological Features. Sustainability 2020, 12, 3985. [Google Scholar] [CrossRef]
- Srinivasan, K.; Thirumalini, P.K. Assessment of Chennai’s Ambient Air Quality Data using Multivariate Analysis from 2005 to 2015. Asian J. Appl. Sci. 2017, 5, 320–329. [Google Scholar] [CrossRef]
- Azman, A.; Hafizan, J.; Ezureen, E.; Mohd, E.T.; Azizah, E.; Mohd, N.A.R.; Kamaruzzaman, Y.; Mohd, K.A.K.; Che, N.C.H.; Ahmad, S.M.S.; et al. Identification Source of Variation on Regional Impact of Air Quality Pattern Using Chemometric. Aerosol Air Qual. Res. 2015, 15, 1545–1558. [Google Scholar] [CrossRef]
- Thara, S.; Kamonrat, K.; Chatchawal, W. A comprehensive review on advancements in sensors for air pollution applications. Sci. Total Environ. 2024, 951, 175696. [Google Scholar]
- Masthurah, A.; Juahir, H.; Mohd, Z.N.B. Case study Malaysia: Spatial water quality assessment of Juru, Kuantan and Johor River Basins using environmetric techniques. J. Surv. Fish. Sci. 2021, 7, 19–40. [Google Scholar] [CrossRef]
- Veerasingan, A.S.; Hafizan, J.; Ananthy, R.; Ali, M.M. Chemometric Interpretation on the Occurrence of Endocrine Disruptors in Source Water from Malaysia. Clean Soil Air Water 2015, 43, 804–810. [Google Scholar]
- Arshad, K.; Hussain, N.; Ashraf, M.H.; Saleem, M.Z. Air pollution and climate change as grand challenges to sustainability. Sci. Total Environ. 2024, 928, 172370. [Google Scholar]
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2017, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Xinghui, L.; Kuppusamy, S.; Huichao, Z.; Kuldeep, K.S.; Fuchun, Z.; Saraschandra, N.; Anbarasu, K.; Ramya, R.; Aruliah, R.; Xiang, G. Frontiers in environmental cleanup: Recent advances in remediation of emerging pollutants from soil and water. J. Hazard. Mater. Adv. 2024, 16, 100461. [Google Scholar] [CrossRef]
- Miller, T.; Durlik, I.; Kostecka, E.; Kozlovska, P.; Łobodzińska, A.; Sokołowska, S.; Nowy, A. Integrating Artificial Intelligence Agents with the Internet of Things for Enhanced Environmental Monitoring: Applications in Water Quality and Climate Data. Electronics 2025, 14, 696. [Google Scholar] [CrossRef]
- Sing, W.A.; Vrontos, S.; Taylor, M.L. An assessment of people living by coral reefs over space and time. Glob. Change Biol. 2022, 28, 7139–7153. [Google Scholar] [CrossRef] [PubMed]
- Abrego, D.; Howells, E.J.; Smith, S.D.A.; Madin, J.S.; Sommer, B.; Schmidt-Roach, S.; Cumbo, V.R.; Thomson, D.P.; Rosser, N.L.; Baird, A.H. Factors Limiting the Range Extension of Corals into High-Latitude Reef Regions. Diversity 2021, 13, 632. [Google Scholar] [CrossRef]
- Madeleine, F.D.; Aaron, E.; Daniel, C.; James, C.; Vi, K.T.; Russell, J.C.; Kay, L. Chemometrics for environmental monitoring: A review. Anal. Methods 2020, 12, 4597–4620. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Li, Y.; Chu, K.; Shi, B.; De La Paz, L.; Bakre, P.; Foti, C.; Rucker, V.; Lai, C. Developing In Situ Chemometric Models with Raman Spectroscopy for Monitoring an API Disproportionation with a Complex Polymorphic Landscape. Pharmaceuticals 2023, 16, 327. [Google Scholar] [CrossRef] [PubMed]
- Frau, I.; Wylie, S.; Byrne, P.; Onnis, P.; Cullen, J.; Mason, A.; Korostynska, O. Microwave Sensors for In Situ Monitoring of Trace Metals in Polluted Water. Sensors 2021, 21, 3147. [Google Scholar] [CrossRef] [PubMed]
- Inobeme, A.; Natarajan, A.; Pradhan, S.; Adetunji, C.O.; Ajai, A.I.; Inobeme, J.; Tsado, M.J.; Jacob, J.O.; Pandey, S.S.; Singh, K.R.; et al. Chemical Sensor Technologies for Sustainable Development: Recent Advances, Classification, and Environmental Monitoring. Adv. Sens. Res. 2024, 3, 2400066. [Google Scholar] [CrossRef]
- Felemban, S.; Vazquez, P.; Moore, E. Future Trends for In Situ Monitoring of Polycyclic Aromatic Hydrocarbons in Water Sources: The Role of Immunosensing Techniques. Biosensors 2019, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Bojko, B.; Onat, B.; Boyaci, E.; Psillakis, E.; Dailianis, T.; Pawliszyn, J. Application of in situ Solid-Phase Microextraction on Mediterranean Sponges for Untargeted Exometabolome Screening and Environmental Monitoring. Front. Mar. Sci. 2019, 6, 632. [Google Scholar] [CrossRef]
- Nie, H.; Liu, Z.; Marks, B.C.; Taylor, L.S.; Byrn, S.R.; Marsac, P.J. Analytical approaches to investigate salt disproportionation in tablet matrices by Raman spectroscopy and Raman mapping. J. Pharm. Biomed. Anal. 2016, 118, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Wray, P.S.; Sinclair, W.E.; Jones, J.W.; Clarke, G.S.; Both, D. The use of in situ near infrared imaging and Raman mapping to study the disproportionation of a drug HCl salt during dissolution. Int. J. Pharm. 2015, 493, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ewing, A.V.; Wray, P.S.; Clarke, G.S.; Kazarian, S.G. Evaluating drug delivery with salt formation: Drug disproportionation studied in situ by ATR-FTIR imaging and Raman mapping. J. Pharm. Biomed. Anal. 2015, 111, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Klinzing, G.; Xu, W. A comparative study of applying backscattering and transmission Raman spectroscopy to quantify solid-state form conversion in pharmaceutical tablets. Int. J. Pharm. 2022, 617, 121608. [Google Scholar] [CrossRef] [PubMed]
- Sabins, F.F. Remote Sensing: Principles and Interpretation, 3rd ed.; Waveland Press Inc.: Long Grove, IL, USA, 2007. [Google Scholar]
- Hadi; Krasovskii, A.; Maus, V.; Yowargana, P.; Pietsch, S.; Rautiainen, M. Monitoring Deforestation in Rainforests Using Satellite Data: A Pilot Study from Kalimantan, Indonesia. Forests 2018, 9, 389. [Google Scholar] [CrossRef]
- Gu, Z.; Zeng, M. The Use of Artificial Intelligence and Satellite Remote Sensing in Land Cover Change Detection: Review and Perspectives. Sustainability 2024, 16, 274. [Google Scholar] [CrossRef]
- Aleksey, V.; Denis, K.; Andrey, O.; Dmitry, K.; Irina, I. Scientific Bases Development for Oil Spill Accidents Automated Detection Using Drones. Transport. Res. Procedia 2023, 68, 585–590. [Google Scholar]
- Muksimova, S.; Umirzakova, S.; Mardieva, S.; Abdullaev, M.; Cho, Y.I. Revolutionizing Wildfire Detection Through UAV-Driven Fire Monitoring with a Transformer-Based Approach. Fire 2024, 7, 443. [Google Scholar] [CrossRef]
- Singh, Y.; Walingo, T. Smart Water Quality Monitoring with IoT Wireless Sensor Networks. Sensors 2024, 24, 2871. [Google Scholar] [CrossRef] [PubMed]
- Udaya, D.; Lumini, B.; Ridma, W.; Kishanga, K.; Bathiya, J. Forest fire detection system using wireless sensor networks and machine learning. Sci. Rep. 2022, 46, 12. [Google Scholar]
- Majumder, A.; Losito, M.; Paramasivam, S.; Kumar, A.; Gatto, G. Buoys for marine weather data monitoring and LoRaWAN communication. Ocean Eng. 2024, 313, 119521. [Google Scholar] [CrossRef]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L. A Comprehensive Review on Water Quality Parameters Estimation Using Remote Sensing Techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, X.; Yuan, W.; Niu, J.; Xu, F.; Zhang, Y. Matching Multi-Sensor Remote Sensing Images via an Affinity Tensor. Remote Sens. 2018, 10, 1104. [Google Scholar] [CrossRef]
- Donkelaar, A.V.; Martin, R.V.; Brauer, M.; Hsu, N.C.; Kahn, R.A.; Levy, R.C.; Apte, J.S. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol. 2015, 50, 3762–3772. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Chen, F.; Fan, L.; Sun, D.; He, H.; Dai, Y.; Li, L.; Chen, Y. Conversion of surface CH4 concentrations from GOSAT satellite observations using XGBoost algorithm. Atmos. Environ. 2023, 301, 119694. [Google Scholar] [CrossRef]
- Tan, Y.-C.; Duarte, L.; Teodoro, A.C. Comparative Study of Random Forest and Support Vector Machine for Land Cover Classification and Post-Wildfire Change Detection. Land 2024, 13, 1878. [Google Scholar] [CrossRef]
- Kumar, M.; Khamis, K.; Stevens, R.; Hannah, D.M.; Bradley, C. In-situ optical water quality monitoring sensors—Applications, challenges, and future opportunities. Front. Water 2024, 6, 1380133. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, H.; Liu, J.; Zhang, Z.; Lu, Y.; Yuan, X.; Wu, D. A novel electrochemical method to evaluate the cytotoxicity of heavy metals. J. Hazard. Mater. 2014, 271, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Gao, G.; Shen, J.; Yu, Y.; Zhi, J. A reagentless electrochemical biosensor based on thionine wrapped E. coli and chitosan-entrapped carbon nanodots film modified glassy carbon electrode for wastewater toxicity assessment. Electrochim. Acta 2016, 222, 303–311. [Google Scholar] [CrossRef]
- Shivani, D.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar]
- James, C.; Vi, K.T.; Aaron, E.; Sheeana, G.; Samuel, C.; Piumie, R.; Kay, L.; Russell, J.C.; Daniel, C. Combining Chemometrics and Sensors: Toward New Applications in Monitoring and Environmental Analysis. Chem. Rev. 2020, 120, 6048–6069. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Gonçalves, J.V.F.; de Oliveira, K.M.; de Oliveira, C.A.; Reis, A.S.; Crusiol, L.G.T.; Furlanetto, R.H.; Antunes, W.C.; Cezar, E.; de Oliveira, R.B.; et al. Chemometric Analysis for the Prediction of Biochemical Compounds in Leaves Using UV-VIS-NIR-SWIR Hyperspectroscopy. Plants 2023, 12, 3424. [Google Scholar] [CrossRef] [PubMed]
- Krzebietke, S.; Daszykowski, M.; Czarnik-Matusewicz, H.; Stanimirova, I.; Pieszczek, L.; Sienkiewicz, S.; Wierzbowska, J. Monitoring the concentrations of Cd, Cu, Pb, Ni, Cr, Zn, Mn and Fe in cultivated Haplic Luvisol soils using near-infrared reflectance spectroscopy and chemometrics. Talanta 2023, 251, 123749. [Google Scholar] [CrossRef] [PubMed]
- Gerjen, H.T.; Olga, L.; Dillen, A.; Mathijs, L.; Rinze, W.G.; Machteld, R.; Harrie, K.; George, D.; Arnold, V.; Lutgarde, M.C.B.; et al. Water quality monitoring based on chemometric analysis of high-resolution phytoplankton data measured with flow cytometry. Environ. Int. 2022, 170, 107587. [Google Scholar] [CrossRef] [PubMed]
- Malavi, D.; Nikkhah, A.; Raes, K.; Van, H.S. Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS. Foods 2023, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.J.; Kuriachan, L. Chemometric appraisal of groundwater quality for domestic, irrigation and industrial purposes in Lower Bhavani River basin, Tamil Nadu, India. Int. J. Environ. Anal. Chem. 2020, 102, 3437–3460. [Google Scholar] [CrossRef]
- Gao, H.; Lv, C.; Song, Y.; Zhang, Y.; Zheng, L.; Wen, Y.; Peng, J.; Yu, H. Chemometrics data of water quality and environmental heterogeneity analysis in Pu River, China. Environ. Earth Sci. 2015, 73, 5119–5129. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Zakaria, M.P.; Zali, M.A.; Juahir, H.; Yaziz, M.I.; Surif, S. Application of chemometrics in understanding the spatial distribution of human pharmaceuticals in surface water. Environ. Monit. Assess. 2012, 184, 6735–6748. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.M.; Ballabio, D.; Amigo, J.M.; Viguri, J.R.; Bro, R. A chemometric approach to the environmental problem of predicting toxicity in contaminated sediments. J. Chemom. 2010, 24, 379–386. [Google Scholar] [CrossRef]
- Hafizan, J.; Sharifuddin, M.Z.; Ahmad, Z.A.; Mohd, K.Y.; Mazlin, B.M. Spatial assessment of Langat river water quality using chemometrics. J. Environ. Monit. 2010, 12, 287–295. [Google Scholar]
- Manuel, D.P.D.; Artur, K. A guide to good practice in chemometric methods for vibrational spectroscopy, electrochemistry, and hyphenated mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116157. [Google Scholar] [CrossRef]
- Adejuwon, E.O.; Ogwueleka, T.C.; Ogungbemi, E.O.; Prabhu, R.; Nava, A.R.; Yates, K. Assessment of Surface Water Quality Using Chemometric Tools: A Case Study of Jabi Lake, Abuja, Nigeria. Iran. J. Sci. Technol. Trans. Civ. Eng. 2025, 49, 829–852. [Google Scholar] [CrossRef]
- Curcic, L.; Loncar, B.; Pezo, L.; Stojic, N.; Prokic, D.; Filipovic, V.; Pucarevic, M. Chemometric Approach to Pesticide Residue Analysis in Surface Water. Water 2022, 14, 4089. [Google Scholar] [CrossRef]
- Rocha, W.F.D.C.; Prado, C.B.D.; Blonder, N. Comparison of Chemometric Problems in Food Analysis using Non-Linear Methods. Molecules 2020, 25, 3025. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, M.; You, Y.; Park, J.; Lee, B.J.; Kang, H.G.; Lee, S. Artificial neural network implementation for dissolved organic carbon quantification using fluorescence intensity as a predictor in wastewater treatment plants. Chemosphere 2023, 335, 139032. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhao, W.; Kinouchi, T.; Nagano, T.; Tanaka, S. Development of statistical regression and artificial neural network models for estimating nitrogen, phosphorus, COD, and suspended solid concentrations in eutrophic rivers using UV–Vis spectroscopy. Environ. Monit. Assess. 2023, 195, 1114. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.A.; Tahmasebi, B.Y.; Baboli, Z.; Heydar Maleki, H.; Angali, K.A. Using water quality parameters to prediction of the ion-based trihalomethane by an artificial neural network model. Environ. Monit. Assess. 2023, 195, 917. [Google Scholar] [CrossRef] [PubMed]
- Zarra, T.; Galang, M.G.; Ballesteros, F.; Belgiorno, V.; Naddeo, V. Environmental odour management by artificial neural network—A review. Environ. Int. 2019, 133, 105189. [Google Scholar] [CrossRef] [PubMed]
- de Lima, B.D.; de Cassia, M.A.R.; de Oliveira, G.G.; Paim, B.L. The performance of artificial neural networks for modeling daily concentrations of particulate matter from meteorological data. Environ. Monit. Assess. 2023, 195, 1305. [Google Scholar] [CrossRef] [PubMed]
- Amaya, J.A.G.; Colmenares, A.N.N.; Rodríguez, A.F.C.; Pulido, J.G. Artificial neural network-based QSAR model for predicting degradation techniques of pharmaceutical contaminants in water bodies with experimental verification. Environ. Sci. Water Res. Technol. 2024, 10, 1492–1498. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Chen, R.; Li, Y.C.; Peng, Y.; Liu, C. Multielemental Analysis Associated with Chemometric Techniques for Geographical Origin Discrimination of Tea Leaves (Camelia sinensis) in Guizhou Province, SW China. Molecules 2018, 23, 3013. [Google Scholar] [CrossRef] [PubMed]
- Athamena, A.; Gaagai, A.; Aouissi, H.A.; Burlakovs, J.; Bencedira, S.; Zekker, I.; Krauklis, A.E. Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis. Sustainability 2023, 15, 20. [Google Scholar] [CrossRef]
- Reta, C.; Asmellash, T.; Atlabachew, M.; Mehari, B. Multielement analysis coupled with chemometrics modelling for geographical origin classification of teff [Eragrostis tef (Zuccagni) Trotter] grains from Amhara Region, Ethiopia. BMC Chem. 2023, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Benkov, I.; Varbanov, M.; Venelinov, T.; Tsakovski, S. Principal Component Analysis and the Water Quality Index—A Powerful Tool for Surface Water Quality Assessment: A Case Study on Struma River Catchment, Bulgaria. Water 2023, 15, 1961. [Google Scholar] [CrossRef]
- Kamal, M.A.; Almohana, A.I. Assessment of Physicochemical Water Quality using Principal Component Analysis: A Case Study Wadi Hanifa, Riyadh. Civ. Eng. Res. J. 2022, 12, 555850. [Google Scholar]
- Su, Q.; Yu, H.; Xu, X.; Chen, B.; Yang, L.; Fu, T.; Liu, W.; Chen, G. Using Principal Component Analysis (PCA) Combined with Multivariate Change-Point Analysis to Identify Brine Layers Based on the Geochemistry of the Core Sediment. Water 2023, 15, 1926. [Google Scholar] [CrossRef]
- Martini, E.; Wollschläger, U.; Musolff, A.; Werban, U.; Zacharias, S. Principal Component Analysis of the Spatiotemporal Pattern of Soil Moisture and Apparent Electrical Conductivity. Vadose Zone J. 2017, 16, 1–12. [Google Scholar] [CrossRef]
- Acal, C.; Aguilera, A.M.; Sarra, A.; Evangelista, A.; Di Battista, T.; Palermi, S. Functional ANOVA Approaches for Detecting Changes in Air Pollution During the COVID-19 Pandemic. Stoch. Environ. Res. Risk Assess. 2022, 36, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Karamizadeh, S.; Abdullah, S.; Manaf, A.; Zamani, M.; Hooman, A. An Overview of Principal Component Analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-samha, M.; Halwani, J.; Murshid, N. Application of Unsupervised Machine Learning for the Evaluation of Aerogels’ Efficiency towards Ion Removal—A Principal Component Analysis (PCA) Approach. Gels 2023, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Wang, S.; Wang, Y.; Zhao, A. Assessment of Environmental Carrying Capacity Using Principal Component Analysis. J. Geosci. Environ. Protect. 2018, 6, 54–65. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Kang, H.; Tao, W.; Li, H.; He, D.; Ma, L.; Tang, H.; Wu, S.; Yang, K.; Li, X. A spatial distribution—Principal component analysis (SD-PCA) model to assess pollution of heavy metals in soil. Sci. Total Environ. 2023, 859, 160112. [Google Scholar] [CrossRef] [PubMed]
- Mamouei, M.; Zhu, Y.; Nazarzadeh, M.; Hassaine, A.; Salimi-Khorshidi, G.; Cai, Y.; Rahimi, K. Investigating the association of environmental exposures and all-cause mortality in the UK Biobank using sparse principal component analysis. Sci. Rep. 2022, 12, 9239. [Google Scholar] [CrossRef] [PubMed]
- Akbar, T.A.; Javed, A.; Ullah, S.; Ullah, W.; Pervez, A.; Akbar, R.A.; Javed, M.F.; Mohamed, A.; Mohamed, A.M. Principal Component Analysis (PCA)–Geographic Information System (GIS) Modeling for Groundwater and Associated Health Risks in Abbottabad, Pakistan. Sustainability 2022, 14, 14572. [Google Scholar] [CrossRef]
- Chen, S.; Yu, L.; Zhang, C.; Wu, Y.; Li, T. Environmental impact assessment of multi-source solid waste based on a life cycle assessment, principal component analysis, and random forest algorithm. J. Environ. Manag. 2023, 339, 117942. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Keithley, R.B.; Wightman, R.M.; Heien, M.L. Multivariate concentration determination using principal component regression with residual analysis. TrAC Trends Anal. Chem. 2009, 28, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Mejia, A.F.; Nebel, M.B.; Eloyan, A.; Caffo, B.; Lindquist, M.A. PCA leverage: Outlier detection for high-dimensional functional magnetic resonance imaging data. Biostatistics 2017, 18, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Biancolillo, A.; Marini, F.; Ruckebusch, C.; Vitale, R. Chemometric Strategies for Spectroscopy-Based Food Authentication. Appl. Sci. 2020, 10, 6544. [Google Scholar] [CrossRef]
- Alessandra, B.; Federico, M. Chemometric Methods for Spectroscopy-Based Pharmaceutical Analysis. Front. Chem. 2018, 6, 576. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C. Chemometrics and Statistics, Experimental Design. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miro, M., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 420–424. [Google Scholar]
- Veron, J.E.N.; Hoegh-Guldberg, O.; Lenton, T.M.; Lough, J.M.; Obura, D.O.; Pearce-Kelly, P.; Sheppard, C.R.C.; Spalding, M.; Stafford-Smith, M.G.; Rogers, A.D. The coral reef crisis: The critical importance of <350 ppm CO2. Mar. Pollut. Bull. 2009, 58, 1428–1436. [Google Scholar]
- Mutalib, S.N.S.A.; Juahir, H.; Azid, A.; Sharif, S.M.; Latif, M.T.; Aris, A.Z.; Zain, S.M.; Dominick, D. Spatial and temporal air quality pattern recognition using environmetric techniques: A case study in Malaysia. Environ. Sci. Process. Impacts 2013, 15, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Saudi, A.S.M.; Shafii, N.Z.; Kamarudin, M.K.A.; Sukki, F.M. Temporal analysis and predictive modeling of ambient air quality in Hulu Langat district, Selangor, Malaysia: A chemometric approach. J. Malays. Inst. Plan. 2024, 22, 394–408. [Google Scholar] [CrossRef]
- Pavel, M.R.S.; Zaman, S.U.; Jeba, F.; Islam, M.S.; Salam, A. Long-term (2003–2019) air quality, climate variables, and human Health Consequences in Dhaka, Bangladesh. Front. Sustain. Cities 2021, 3, 681759. [Google Scholar] [CrossRef]
- Muzyka, R.; Chrubasik, M.; Pogoda, M.; Sajdak, M. Chemometric analysis of air pollutants in raw and thermally treated coals–low-emission fuel for domestic applications, with a reduced negative impact on air quality. J. Environ. Manag. 2021, 281, 111787. [Google Scholar] [CrossRef] [PubMed]
- Hua, A.K. Applied chemometric approach in identification sources of air quality pattern in Selangor, Malaysia. Sains Malays. 2018, 47, 471–479. [Google Scholar] [CrossRef]
- Azid, A.; Amran, M.A.; Samsudin, M.S.; Rani, N.L.A.; Khalit, S.I.; Gasim, M.B.; Yunus, K.; Saudi, A.S.M.; Amin, S.N.S.M.; Yusof, K.M.K.K. Assessing indoor air quality using chemometric models. Pol. J. Environ. Stud. 2018, 27, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Hanapiah, S.M.; Saudi, A.S.M.; Rizman, Z.I. Assessment on pattern of urban air quality by using chemometric technique: A case study in Kota Kinabalu, Sabah. J. Fundam. Appl. Sci. 2017, 9, 861–870. [Google Scholar] [CrossRef]
- Azid, A.; Juahir, H.; Toriman, M.E.; Endut, A.; Kamarudin, M.K.A.; Rahman, M.N.A.; Hasnam, C.N.C.; Saudi, A.S.M.; Yunus, K. Source apportionment of air pollution: A case study in Malaysia. J. Tek. 2015, 72, 83–88. [Google Scholar] [CrossRef]
- Thomas, E.O. Evaluation of groundwater quality using multivariate, parametric and non-parametric statistics, and GWQI in Ibadan, Nigeria. Water Sci. 2023, 37, 117–130. [Google Scholar] [CrossRef]
- Kaur, H.; Rajor, A.; Kaleka, A.S. Application of chemometric modeling for identification of pollution sources from drains of Ghaggar River, Punjab, India. Sadhana 2022, 47, 251. [Google Scholar] [CrossRef]
- Elkorashey, R.M. Utilizing chemometric techniques to evaluate water quality spatial and temporal variation. A case study: Bahr El-Baqar drain–Egypt. Environ. Technol. Innov. 2022, 26, 102332. [Google Scholar] [CrossRef]
- Kustomo; Rasidah; Oktaviano, D. Chemometrics analysis for the groundwater quality assessment in UIN Walisongo Semarang. Adv. Eng. Res. 2021, 211, 53–60. [Google Scholar]
- Pathak, H. Chemometric analysis of drinking water quality parameters of Sagar city, Madhya Pradesh, India. Ovidius Univ. Ann. Chem. 2020, 31, 99–105. [Google Scholar] [CrossRef]
- Benamar, A.; Mahjoubi, F.Z.; Ali, G.A.M.; Kzaiber, F.; Oussama, A. A chemometric method for contamination sources identification along the Oum Er Rbia river (Morocco). Bulg. Chem. Commun. 2020, 52, 159–171. [Google Scholar]
- Ioele, G.; De Luca, M.; Grande, F.; Durante, G.; Trozzo, R.; Crupi, C.; Ragno, G. Assessment of surface water quality using multivariate analysis: Case study of the Crati River, Italy. Water 2020, 12, 2214. [Google Scholar] [CrossRef]
- Miller, T.I. Assessment of water quality using chemometric methods—A case study of Rusałka Lake, NW-Poland. Ecol. Montenegrina 2020, 27, 80–89. [Google Scholar] [CrossRef]
- Bam, E.K.P.; Akumah, A.M.; Bansah, S. Geochemical and chemometric analysis of soils from a data scarce river catchment in West Africa. Environ. Res. Commun. 2020, 2, 035001. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Simeonov, V. Chemomertic risk assessment of soil pollution. Open Chem. 2019, 17, 711–721. [Google Scholar] [CrossRef]
- Dimitrov, D.S.; Nedyalkova, M.A.; Donkova, B.V.; Simeonov, V.D. Chemometric assessment of soil pollution and pollution source apportionment for an industrially impacted region around a non-ferrous metal smelter in Bulgaria. Molecules 2019, 24, 883. [Google Scholar] [CrossRef] [PubMed]
- Bellino, A.; Colombo, C.; Iovieno, P.; Alfani, A.; Palumbo, G.; Baldantoni, D. Chemometric technique performances in predicting forest soil chemical and biological properties from UV-Vis-NIR reflectance spectra with small, high dimensional datasets. IForest 2015, 9, 101–108. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.I.; Choi, J.H.; Lim, K.J.; Mo, C. Development of a Soil Organic Matter Content Prediction Model Based on Supervised Learning Using Vis-NIR/SWIR Spectroscopy. Sensors 2022, 22, 5129. [Google Scholar] [CrossRef] [PubMed]
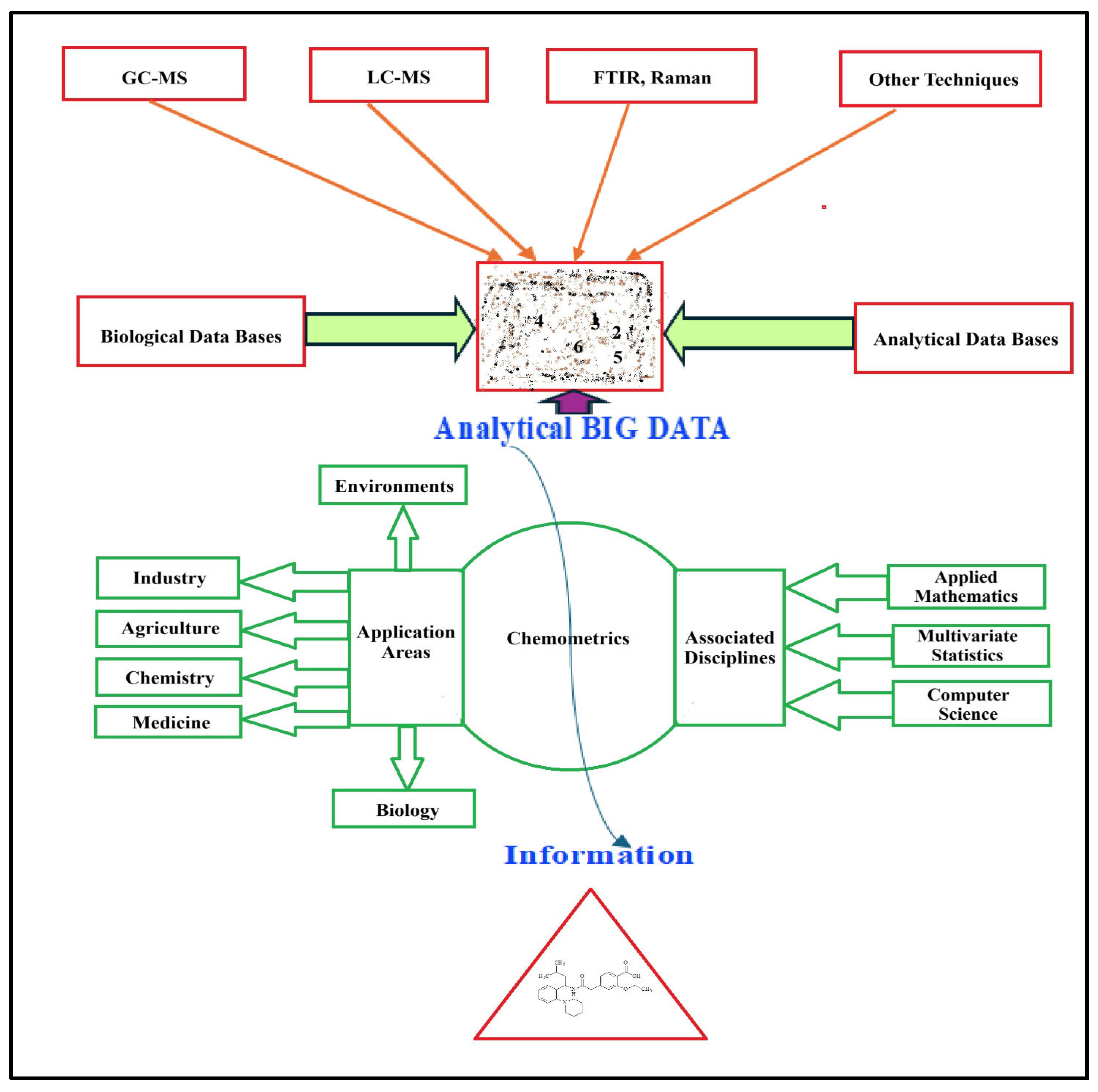
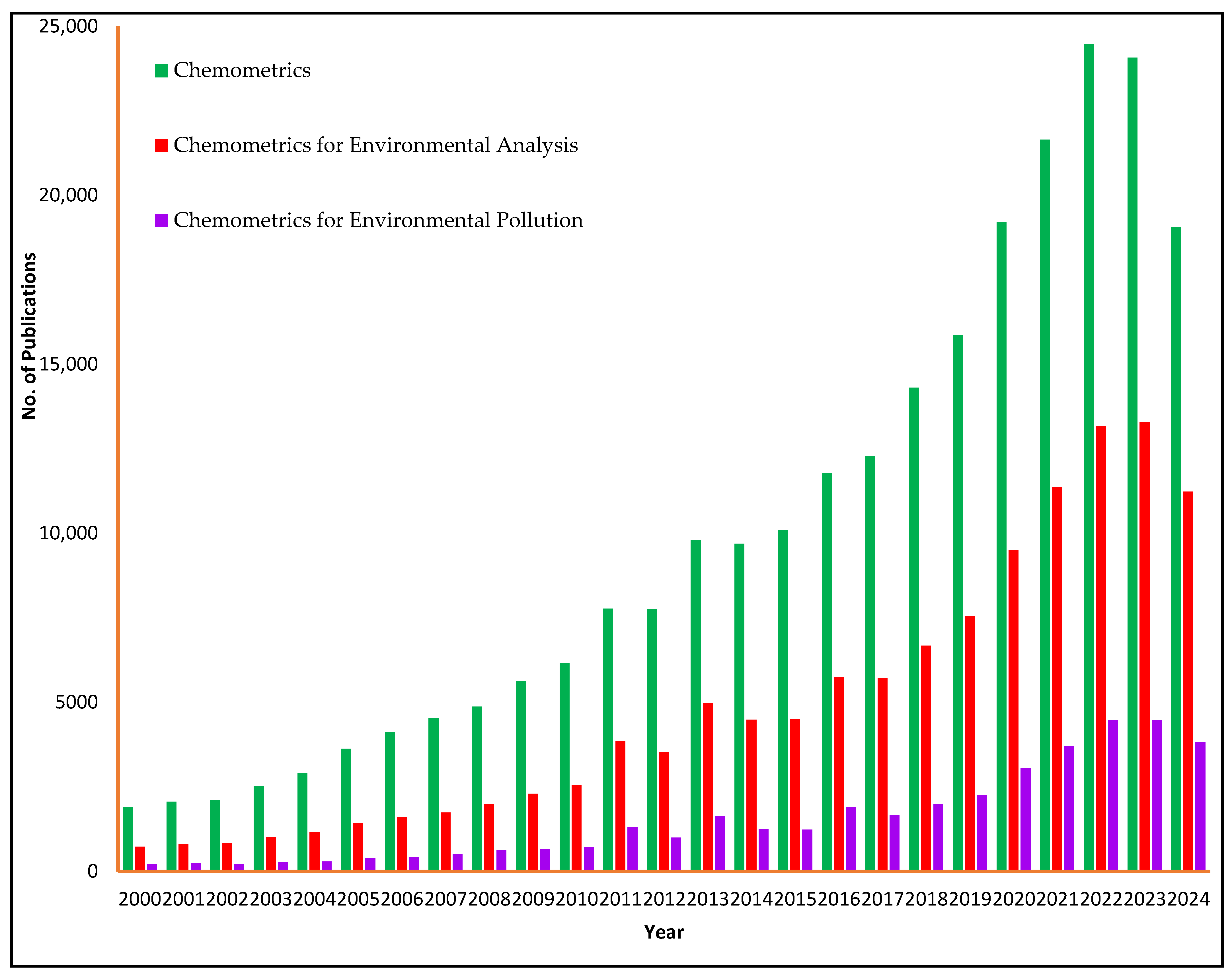
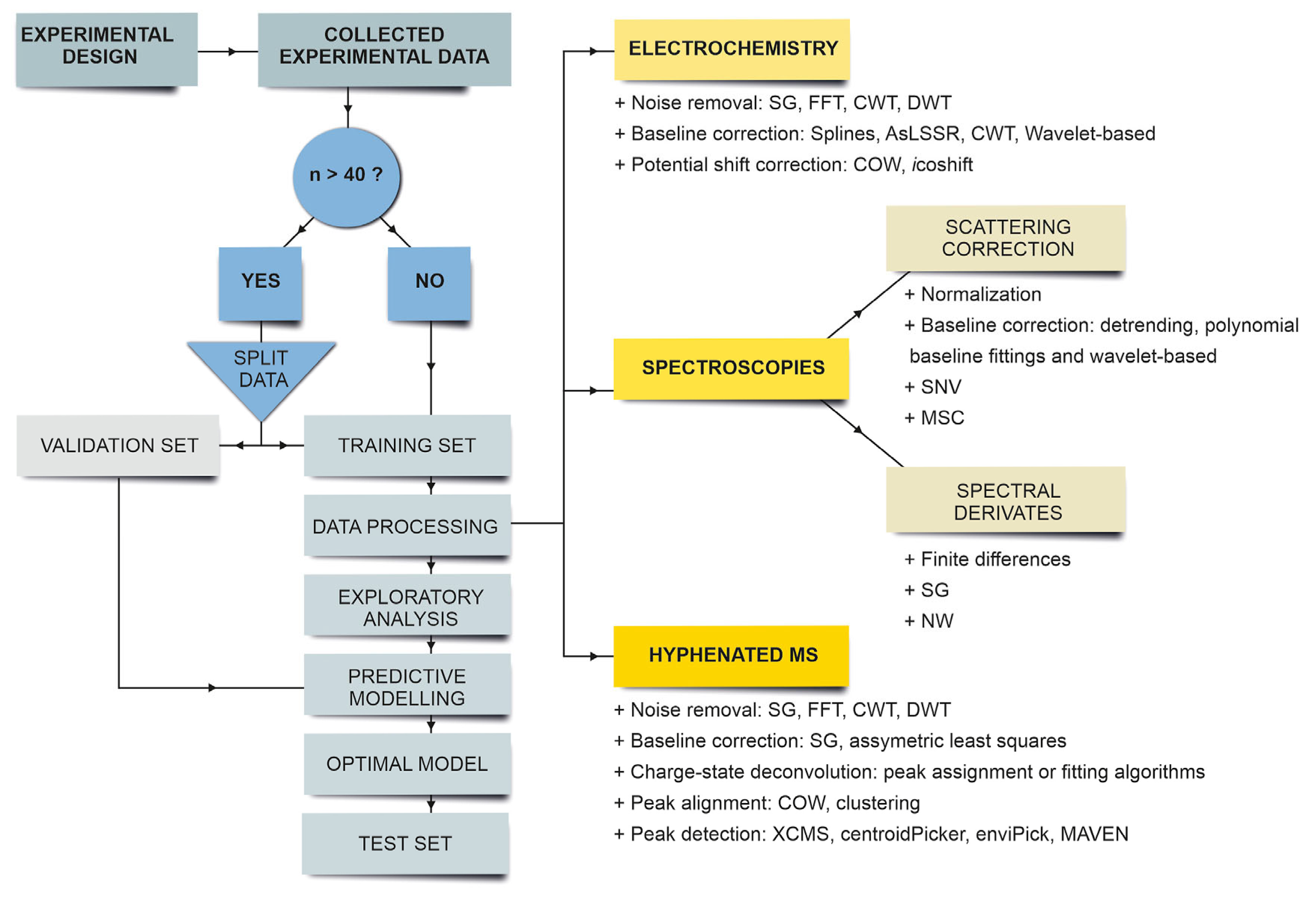
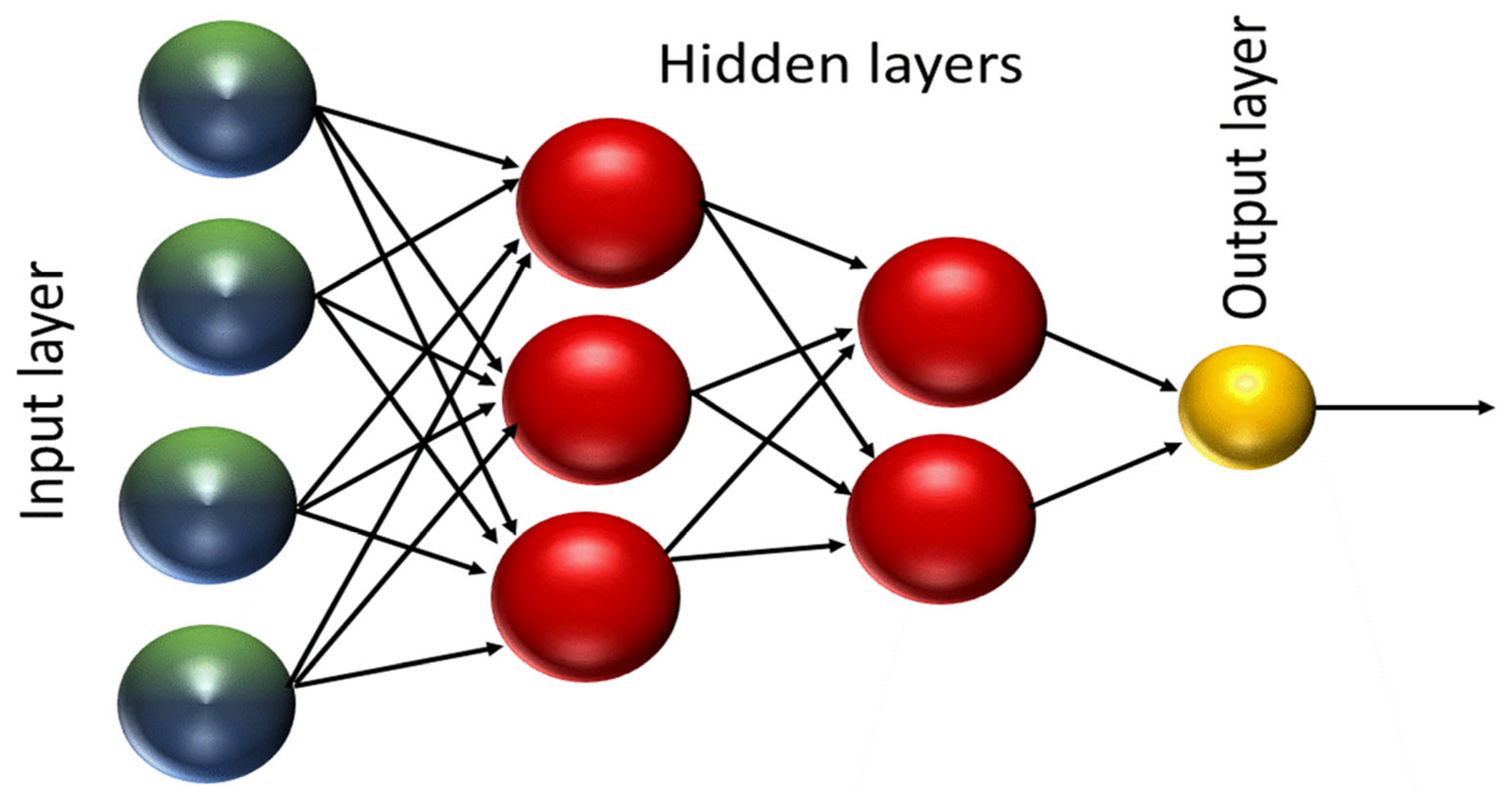


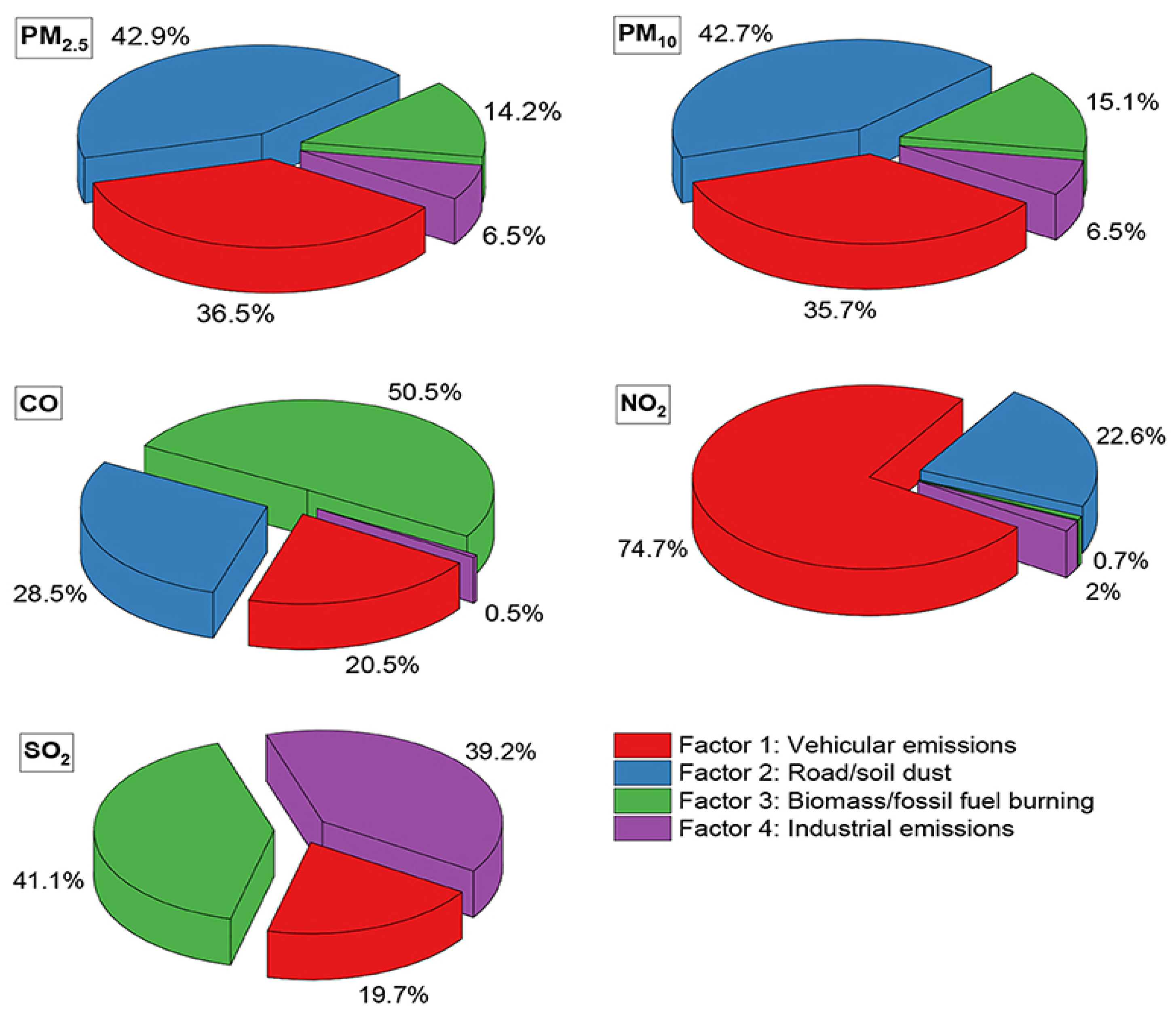
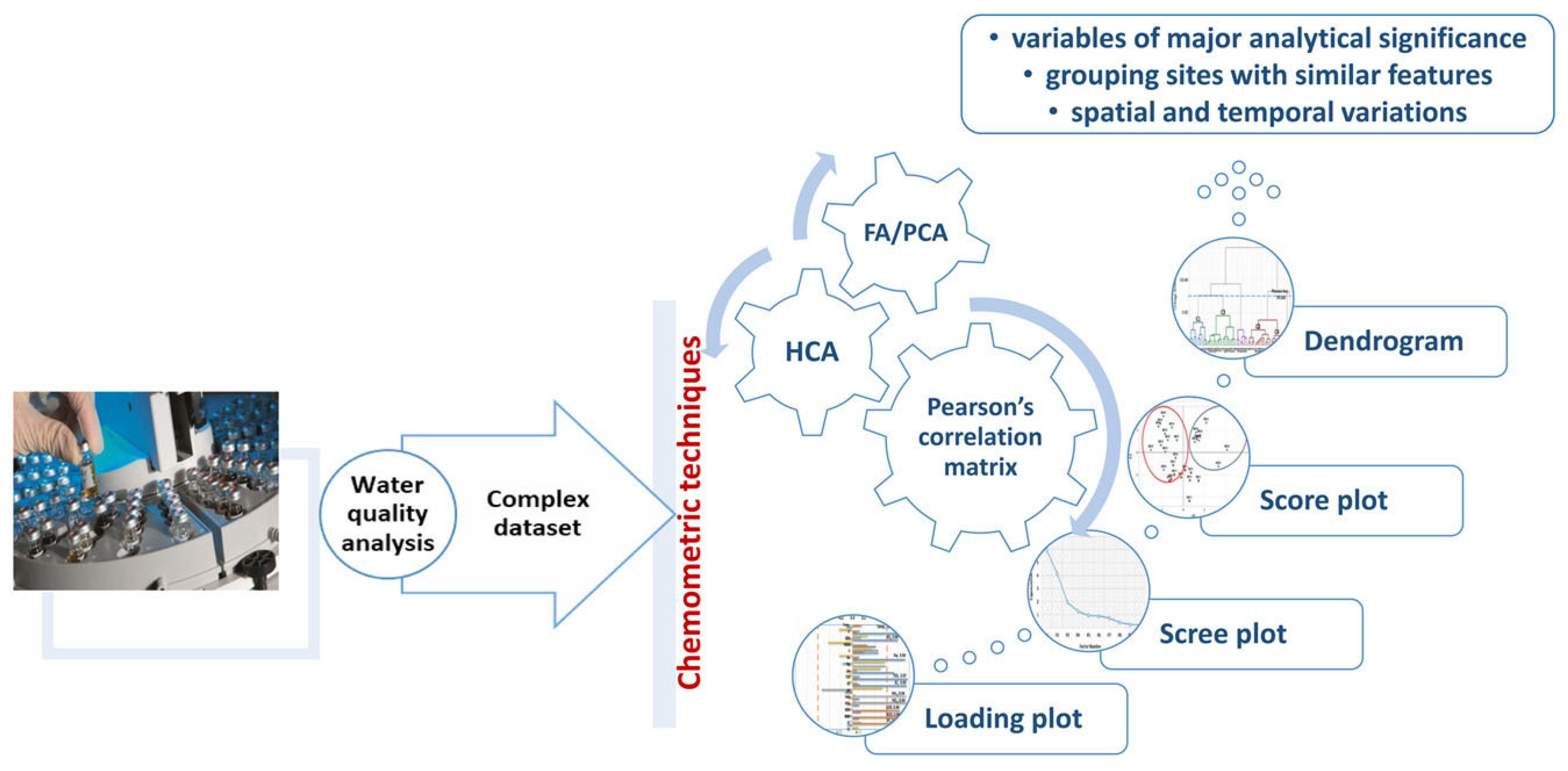
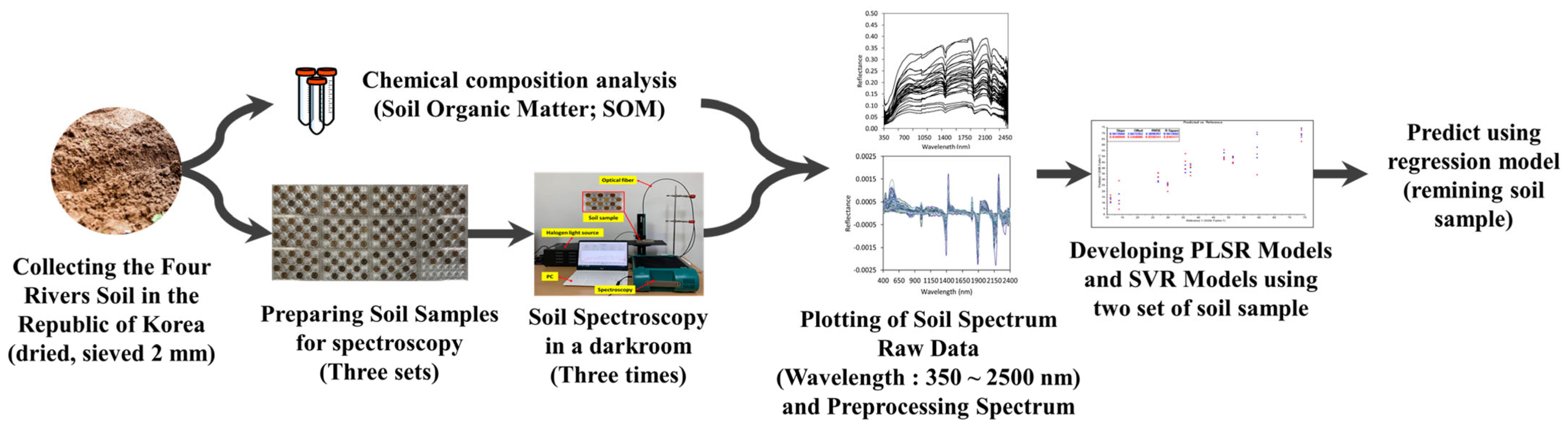
| Focus | Chemometric Approach | Analytical Method | Analytes | Accuracy/Precision & R2 | Application | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Monitoring of polymorphic transitions of pharmaceutical compounds | PCA, PLS | In situ Raman spectroscopy and X-ray diffraction | API salts (HCl and maleate) | For HCl salt, RMSECV = 0.056, 0.034, and 0.022 For maleate salt, RMSECV = 0.016 and 0.023 | Real-time monitoring for quality control and stability assessment | Detects multiple polymorphs to enhance calibration for complex systems | Requirements of complex calibration | [31] |
| Real-time monitoring of trace metal contamination | PLS | Microwave spectroscopy and planar sensors | Trace metals (e.g., Pb, Cd, As, and Hg) | R2 > 0.96 | Continuous environmental monitoring of water quality | Immediate response, in situ monitoring, and cost-effective | Non-specific metal detection | [32] |
| In situ SPME for untargeted exometabolome screening | PCA, PLS, DA | GC-MS/LC-MS | Primary (amino acids and fatty acids), secondary (alkaloids and terpenes), and pollutants | 80.92–100% p < 0.05 | Marine health monitoring, chemical ecology, and natural product discovery | Non-invasive and eco-friendly with minimal contamination | Specific for polar compounds | [35] |
| Detection of salt disproportionation | PCA, PLS | Raman spectroscopy and X-ray diffraction | Pioglitazone HCl salt | RMSECV = 0.45 R2 = 0.996 | Quality control and stability assessment | Detects minor species, provides spatial distribution, and enhances sensitivity | Spectral interference requires advanced techniques | [36] |
| Disproportionation of the drug HCl salt | NA | NIR and Raman spectroscopy | Avicel and API salt | NA | Formulation stability, drug release, and efficacy | Non-destructive, spatially resolved monitoring, and better process understanding | Requires advanced data analysis | [37] |
| Disproportionation of drugs | NA | ATR-FTIR and Raman spectroscopy | Avicel and API salt | NA | Particularly for salt-based drug delivery systems, drug solubility, and bioavailability | High spatial and chemical specificity | Requires complex data interpretation and spectral deconvolution | [38] |
| Solid-state form conversion within intact pharmaceutical tablets | PCA, PLS | Raman spectroscopy | Pioglitazone hydrochloride salt | RMSECV = 0.506, 0.837 R2 = 0.928, 0.981 | Process monitoring, quality control, and regulatory | Reducing surface bias, non-destructive, and rapid | Requires careful calibration and uniform sample density | [39] |
| Technology | Description | Example | Ref. |
|---|---|---|---|
| Satellites | Use optical, thermal, or radar sensors to observe Earth from space | Monitoring deforestation, glacial retreat, and land use changes | [41,42] |
| Drones (UAVs) | Provide high-resolution aerial images and multispectral data | Mapping crop health, detecting oil spills, and wildfire monitoring | [43,44] |
| IoT Sensors | Ground-based devices connected via wireless networks | Air/water quality sensors in urban areas or rivers | [45] |
| WSNs | Networks of sensors transmitting environmental data | Forest fire detection and weather stations | [46] |
| Buoys and Floating Platforms | For marine environments | Ocean temperature, salinity, pH, and wave height monitoring | [47] |
| Satellite/Sensor | Environmental Parameters Retrieved | Common Chemometric Methods | Applications | Ref. |
|---|---|---|---|---|
| MODIS | CDOM, SDD, TSS, TP, SSS, DO, BOD, COD | PCA, ANN | Evaluating and quantifying the water quality | [48] |
| ATBM, UAV | Geometric and radiometric information | ANN | Tensor power iteration and detection process | [49] |
| MODIS, MISR | AOD | GWR | PM2.5 estimation | [50] |
| GOSAT | CH4 | XGBoost | Atmospheric profiling and greenhouse gas monitoring | [51] |
| Sentinel 2A | LULC, NDVI | SVM, RF | Water quality assessment, agricultural monitoring, and land cover change detection | [52] |
| Focus | Chemometric Approach | Analytical Method | Analytes | Accuracy/Precision & R2 | Application | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Development of non-destructive, rapid, and accurate method | HVIs, PCA, PLSR | UV–VIS-NIR-SWIR | Chlorophylls, carotenoids, flavonoids, and lignin | R2 > 0.75 p < 0.01 | Real-time plant health monitoring and breeding ecophysiological studies | Non-invasive, Rapid, and high throughput | High hyperspectral equipment cost, and complex data analysis | [58] |
| Estimate the concentrations of heavy metals in soil | PLS, PCA | NIRS | Heavy metals (Cd, Cu, Pb, Ni, Cr, Zn, Mn, and Fe) | RMSEP = 9.63, 11.5% R2 = 0.86, 0.58 | Assessing soil contamination | Non-destructive, rapid, cost-effective, and high throughput | Calibration dependency, matrix effects, and limited analyte scope | [59] |
| Detection of highly polar pesticide residues | OPLS, PCA | LC-MS/MS | 50 medium to highly polar pesticides | R2 = 0.49–0.73 | Monitor sediment contamination | High sensitivity and selectivity | Requires advanced equipment and technical skill | [60] |
| The study evaluates the use of plant-based ingredients | PLS, PCA, DA | FTIR, UV–Vis, Raman, GC-MS | Plant-derived ingredients and microbial counts | 100, 99.8, 99.6, 96.6 and 93.7% RMSEP-1.1% R2 = 0.97 | Useful in the industry for producing reduced fat and natural preservation | Reduces fat content, and enhances safety and shelf life | Limited details on the specific plant ingredients | [61] |
| Improving water quality assessment | OPLS, PCA | Flow cytometry | 50 medium to highly polar pesticides | R2 = 0.49–0.73 | Early detection of environmental changes, pollution events, and ecosystem health monitoring | Rapid, real-time tracking, and high-sensitivity | Requires specialized equipment and expertise, high cost, and complex data interpretation | [60] |
| Assessing groundwater quality | PCA | WQI, SAR, EC, UV–Vis, IC | Ca, Mg, and Cl | WQI = 17–47% EC = 17–64% | Water quality assessment | Identification of patterns in water quality data | Depending on the quality of the data available | [62] |
| Heavy metal contamination in the groundwater | HCA, PCA | AAS | Fe, Mn, Pb, Cd, Cr, and As | 34.21–82.97% p < 0.05 | Assessing health risks and guiding safe water | Identifies health-threatening pollutants | It does not cover all possible contaminants. | [63] |
| Assessing human pharmaceuticals in water | CA, PCA, DA | LC-MS/MS | Nineteen pharmaceuticals | 100% R2 > 0.75 p < 0.05 | River water quality monitoring | Identifies pharmaceuticals | Focus limited to selected pharmaceuticals | [64] |
| Evaluating sediment quality | PCA, DA, PLS, ANN | NA | Heavy metals and polycyclic aromatic hydrocarbons | 92.3–97.2% | Broad use in environmental analysis | Enhances accuracy, and reduces experimental trials | Requires high-quality, representative data | [65] |
| Quantifying inorganic arsenic species | DA, ANN | ETAAS | As(III) and As(V) | 92–98% 88–91% | Assessment of arsenic contamination | High selectivity and sensitivity | It requires careful pH control and multiple extraction steps. | [66] |
| Environmental Target | Input Data | ANN Role | Performance Highlights | Ref. |
|---|---|---|---|---|
| DOC in Wastewater | Fluorescence intensity and UV absorbance | Quantification of DOC | R2 = 0.9079; RMSE = 0.2989 mg/L | [71] |
| Nutrient and COD levels in Rivers | UV–Vis spectral data | Estimation of N, P, COD, and SS | ANN outperformed regression models | [72] |
| THMs in Water | Physicochemical water parameters | Prediction of THM levels | High accuracy vs. traditional models | [73] |
| PM10 in the Air | Meteorological variables | Forecasting air pollution | R2 = 0.81; RMSE = 7.40 µg/m3 | [75] |
| Pharmaceutical Degradation in Water | Molecular descriptors | Predicting optimal degradation techniques | Experimentally validated predictions | [76] |
| Environmental Matrix | Chemometric Approach | Type of DA | Key Discriminating Variables | Accuracy/Precision & R2 | Main Application | Ref. |
|---|---|---|---|---|---|---|
| Tea leaf samples | Multielemental analysis + chemometrics | LDA | As, K, La, and Pb | 98.9% | Discriminating tea origins based on geochemical fingerprint | [77] |
| Surface water (lake) | Physicochemical analysis + DA | Not specified | pH, EC, BOD, and TDS | 98.5–100% | Assessment and classification of water quality | [68] |
| Groundwater | Hydrochemical analysis + multivariate statistics | Not explicitly DA-only | Major ion such as Ca2+, Mg2+, Cl−, NO3−, etc. | R2 = 0.62–0.96 | Characterizing groundwater facies and pollution sources | [78] |
| Phytoplankton (flow cytometry) | DAMACY algorithm (DA-based) | LDA-based (with anomaly detection) | Cell size, fluorescence, and scattering | R2 = 0.49–0.73 | Real-time monitoring and early pollution detection | [60] |
| Teff grain | Multielemental ICP-OES + chemometrics | LDA | Fe, Mn, Zn, Ca, etc. | 96% | Origin authentication of grains from different zones | [79] |
| Environmental Matrix | Main Objective | Factorial Method | Key Findings | Chemometric Contribution | Ref. |
|---|---|---|---|---|---|
| Surface water (river) | Assess pollution sources and seasonal changes in water quality | PCA | Identified major pollution sources; separated seasonal trends | PCA revealed key influencing parameters (DO, BOD, NO3−, etc.) and anthropogenic vs. natural impact | [80] |
| Surface water (wadi) | Evaluate spatial variation in water quality in Wadi Hanifa | PCA | Explained 85% of total variance with 3 PCs; salinity and nutrients were the main drivers | PCA helped classify water quality zones and contamination levels | [81] |
| Groundwater (plain) | Characterize hydrochemical processes and pollution sources | PCA | Identified geochemical processes: silicate weathering, evaporation, and salinization | PCA simplified hydrochemical data into manageable PCs for interpretation | [78] |
| Sediment core samples | Identify brine layers and geochemical change points | PCA + Change-Point Analysis | Revealed stratification and historical geochemical transitions | PCA reduced complexity; change-point detection linked transitions to salinity | [82] |
| Soil sample | Determine the pattern of soil moisture and apparent electrical conductivity | PCA | Provided insights into controlling factors and the major soil water changing aspects responsible for the soil moisture spatial pattern | PCA accounts for 86% of the total dataset’s variance, and all are significant in illustrating the spatial association between the topsoil and its sequential variations in soil moisture. | [83] |
| Air quality data sample (several monitoring stations) | Air quality changes in terms of air pollution | Variance for functional data (FANOVA). | Significant reduction of NO2 but increased PM10 and P2.5 in the lockdown period | FANOVA analysis was feasible, allowing for the comparison and rejection of the null hypothesis of impartiality for mean functions of all contaminants | [84] |
| Chemometric Techniques | Analytical Method | Pollutants and Levels | Accuracy/Precision & R2 | Ref. |
|---|---|---|---|---|
| DA, HACA, PCA, ANNs | API | Station 1 SO2: Maximum—0.1 ppm, Average—0.015 ppm; NO2: Maximum—0.22 ppm, Average—0.053 ppm; O3: Maximum—0.15 ppm, Average—0.034 ppm; CO: Maximum—10.41 ppm, Average—2.138 ppm; PM10: Maximum—806 µg/m3, Average—88.24 µg/m3; API: Maximum—392, Average—57.651 Station 2 SO2: Maximum—0.06 ppm, Average—0.006 ppm; NO2: Maximum—0.05 ppm, Average—0.022 ppm; O3: Maximum—0.14 ppm, Average—0.045 ppm; CO: Maximum—5.72 ppm, Average—1.393 ppm; PM10: Maximum—640 µg/m3, Average—83.27 µg/m3; API: Maximum—153, Average—51.068 Station 3 SO2: Maximum—0.02 ppm, Average—0.003 ppm; NO2: Maximum—0.12 ppm, Average—0.012 ppm; O3: Maximum—0.08 ppm, Average—0.024 ppm; CO: Maximum—4.13 ppm, Average—0.978 ppm; PM10: Maximum—411 µg/m3, Average—70.616 µg/m3; API: Maximum—188, Average—41.762 | 87.2% R2 > 0.75 p < 0.05 | [99] |
| PCA, SPC, ANNs | API | SO2: Maximum—0.084 ppm, Average—0.003 ppm; NO2: Maximum—1.325 ppm, Average—0.013 ppm; O3: Maximum—0.149 ppm, Average—0.022 ppm; CO: Maximum—5.658 ppm, Average—0.702 ppm; PM10: Maximum—438.61 µg/m3, Average—51.866 µg/m3; API: Maximum—323, Average—56.431 | R2 = 0.9 | [100] |
| PCA, PMF | HQ | PM2.5: 65 ppm; PM10: 150 ppm; CO: 35 ppm; O3: 0.12 ppm; NO3: 0.053 ppm; SO2: 0.014 ppm | R2 = 0.37 p < 0.05 | [101] |
| CA, PCA | GC-FID | Anthracene: Maximum—6420 ppm; Phenanthrene: Maximum—13,880 ppm; Fluorene: 5200 ppm; Acenaphthene: 5791 ppm | 99% | [102] |
| HCA, DA, PCA, MLR | API | O3: Average—0.1 ppm; CO: Average—30 ppm; NO2: Average—0.18 ppm, SO2: Average—0.15 ppm; PM10: Average—120 µg/m3 | 95.38% p < 0.05 | [103] |
| PCA, PLS-DA, LDA, AHC | Turnkey dust mate detector, and gas meter | O3: 0.467 ppm; CO: 0.781 ppm; CO2: 0.892 ppm; PM1: 0.798 µg/m3; PM2.5: 0.752 µg/m3; PM10: 0.751 µg/m3 | 89.05% R2 > 0.75 | [104] |
| PCA, SPC | API | CO: CL—0.631 ppm, Upper control limit (UCL)—0.915 ppm, Lower control limit (LCL)—0.347 ppm, Maximum—37 ppm; PM10: CL—47.304 µg/m3, UCL—68.463 µg/m3, LCL—26.146 µg/m3 | R2 = 0.49–1 | [105] |
| PCA | UV-fluoresenece, and Teledyne API-FID | Station 1 CO: Maximum—4.85 ppm, Average—1.24 ppm; O3: Maximum—0.12 ppm, Average—0.03 ppm; PM10: Maximum—780 µg/m3, Average—81.24 µg/m3; SO2: Maximum—0.13 ppm, Average—0.01 ppm; NO2: Maximum—0.06 ppm, Average—0.02 ppm; CH4: Maximum—9.75 ppm, Average—2.49 ppm; NmHC: Maximum—5.15 ppm, Average—0.055 ppm; THC: Maximum—10.5 ppm, Average—2.96 ppm; API: Maximum—125.88, Average—57.84 Station 4 CO: Maximum—2.84 ppm, Average—0.86 ppm; O3: Maximum—0.16 ppm, Average—0.04 ppm; PM10: Maximum—202 µg/m3, Average—58.70 µg/m3; SO2: Maximum—0.1 ppm, Average—0.01 ppm; NO2: Maximum—0.06 ppm, Average—0.02 ppm; CH4: Maximum—9.33 ppm, Average—2.91 ppm; NmHC: Maximum—4.81 ppm, Average—0.41 ppm; THC: Maximum—9.6 ppm, Average—3.24 ppm; API: Maximum—158, Average–50.14 Station 7 CO: Maximum—3.82 ppm, Average—0.99 ppm; O3: Maximum—0.12 ppm, Average—0.02 ppm; PM10: Maximum—760 µg/m3, Average—94.66 µg/m3; SO2: Maximum—0.06 ppm, Average—0.01 ppm; NO2: Maximum—0.06 ppm, Average—0.01 ppm; CH4: Maximum—6.4 ppm, Average—2.24 ppm; NmHC: Maximum—6.17 ppm, Average—0.58 ppm; THC: Maximum—8.2 ppm, Average—2.75 ppm; API: Maximum—151, Average—57.32 Station 10 CO: Maximum—3.32 ppm, Average—0.57 ppm; O3: Maximum—0.06 ppm, Average—0.02 ppm; PM10: Maximum—357 µg/m3, Average—55.56 µg/m3; SO2: Maximum—0.04 ppm, Average—0.00 ppm; NO2: Maximum—0.04 ppm, Average—0.01 ppm; CH4: Maximum—6.64 ppm, Average—2.2 ppm; NmHC: Maximum—4.54 ppm, Average—0.4 ppm; THC: Maximum—7.6 ppm, Average—2.54 ppm; API: Maximum—97, Average—38.41 | 1% R2 > 0.75 p < 0.05 | [106] |
| HACA, DA, PCA, FA, MLR | API | LPS region CO: 0.896 ppm; NO2: 0.939 ppm; SO2: 0.697 ppm; PM10: 0.646 µg/m3; O3: 0.343 ppm; CH4: 0.263 ppm; NO:0.873 ppm; Non-methane hydrocarbon: 0.887 ppm MPS region CO: 0.933 ppm; NO2: 0.733 ppm; SO2: 0.906 ppm; O3: 0.213 ppm; CH4: 0.913 ppm; NO: 0.857 ppm SHPS region CO: 0.801 ppm; NO2: 0.747 ppm; SO2: 0.108 ppm; O3: 0.024 ppm; CH4: 0.263 ppm; NO:0.918 ppm; Non-methane hydrocarbon: 0.218 ppm | 91.67–97.22% | [20] |
| Chemometric Techniques | Analytical Method | Parameters and Concentration | Accuracy/Precision & R2 | Ref. |
|---|---|---|---|---|
| HCA, PCA | pH meter, conductivity meter, spectrophotometry, and flame photometer | pH: Minimum—4.4, Maximum—7.10, Mean—6.49; EC: Minimum—270, Maximum—1870, Mean—893.56; TDS: Minimum—142, Maximum—1720, Mean—536.88; K+: Minimum—9.8, Maximum—99.6, Mean—56.97; Na+: Minimum—5.1, Maximum—59.52, Mean—31.96; Mg2+: Minimum—3.73, Maximum—9.5, Mean—6.02; Ca2+: Minimum—1.3, Maximum—7.37, Mean—4.71; Cl−: Minimum—57.6, Maximum—1476, Mean—445.01; SO4−: Minimum—0, Maximum—6.1, Mean—2.26; HCO3−: Minimum—100.5, Maximum—609, Mean—253; NO3−: Minimum—0, Maximum—6.09, Mean—1.36 | 69.9% R2 = 0.849, 0.968 p < 0.05 | [107] |
| PCA, FA, CA, DA | Conductivity meter, titration, UV-spectrophotometry, and TKN | pH: Minimum—6.8, Maximum—8.3, Mean—7.8; COD: Minimum—40, Maximum—120, Mean—81.6; BOD: Minimum—12, Maximum—48, Mean—22.2; Alkalinity: Minimum—222, Maximum—514, Mean—360; TDS: Minimum—104, Maximum—360, Mean—264; TSS: Minimum—7, Maximum—153, Mean—74.2; SO4-S: Minimum—49.4, Maximum—185.3, Mean—89.9; NO3-N: Minimum—0.1, Maximum—4.1, Mean—1.6; NO2-N: Minimum—0.7, Maximum—45.7, Mean—15.8 | 100% R2 > 0.7 | [108] |
| PCA, HCA | pH meter, conductivity meter, spectrophotometry, TKN, IC, and ICP-MS | pH: 7.33; EC: 1.03; TDS: 728; BOD: 18; COD: 22; K+: 0.56; Na+: 4.67; Mg2+: 1.72; Ca2+: 3.44; Cl−: 3.68; SO4−: 2.08; HCO3−: 4.38; NO3−: 12.55; NH4: 3.31 | 76–81.1% R2 = 0.3–1 p < 0.05 | [109] |
| PCA, CA | pH meter, conductivity meter, UV–Vis spectrophotometer, and AAS | pH: Minimum—6.83, Maximum—7.63, Mean—7.29; TDS: Minimum—148.5, Maximum—662, Mean—328.3; Fe: Minimum—0, Maximum—0.03, Mean—0.0177; SO4: Minimum—0, Maximum—2, Mean—1; NO3: Minimum—0.6, Maximum—1, Mean—0.833; CaCO3: Minimum—184, Maximum—678.8, Mean—354; Cr: Minimum—0, Maximum—0.05, Mean—0.0267; Zn: Minimum—0.09, Maximum—0.43, Mean—0.2567; CN: Minimum—0, Maximum—0.04, Mean—0.0133; KMnO4: Minimum—2.43, Maximum—3.63, Mean—3.22 | 18.43–81.57% p < 0.05 | [110] |
| FA, CA | pH meter, conductivity meter, incubation and titration, argentometric titration, and complexometric titration | pH: Minimum—7.16, Maximum—8.34, Mean—7.86; DO: Minimum—6.7, Maximum—8.8, Mean—7.786; TDS: Minimum—304.3, Maximum—452.8, Mean—348.2; BOD: Minimum—3.2, Maximum—5.8, Mean—4.72; Cl−: Minimum—24.61, Maximum—55.85, Mean—36.31; Mg2+: Minimum—18.74, Maximum—119.14, Mean—45.84; Ca2+: Minimum—115.9, Maximum—168.26, Mean—131.05 | 75.4–83.05% p < 0.05 | [111] |
| PCA | pH meter, conductivity meter, turbidimetry, nephelometric method, titrimetry, and ICP-OES | pH: Minimum—7.42, Maximum—8.59, Mean—8.21; DO: Minimum—4.62, Maximum—8.8, Mean—7.24; CE: Minimum—856, Maximum—2420, Mean—1827.58; Nitrites: Minimum—0.003, Maximum—2.09, Mean—0.447; Cl−: Minimum—134.9, Maximum—724.9, Mean—458.19; NO3−: Minimum—4.22, Maximum—13.64, Mean—9.68; Cu: Minimum—0.036, Maximum—0.539, Mean—0.135; Cd: Minimum—0.088, Maximum—0.378, Mean—0.137; Pb: Minimum—0.069, Maximum—0.307, Mean—0.109; Cr: Minimum—0.0143, Maximum—0.278, Mean—0.073 | 84–96% R2 = 0.256–0.989 | [112] |
| PCA | pH meter, conductivity meter, and GC-MS | Samples–Crati 13 pH: 8; NH4+: 0.19; N-NO2: 0.06; Al3+: 0.09; As: 0.09; Cr: 0.4; Fe: 36; Hg: 0.3; Ni: 0.5; Pb: 3; B: 5; Se: 0.04 | 29%, 49% | [113] |
| CA, FA, DA | pH meter, conductivity meter, incubation and titration, and complexometric titration | pH: 7.30–8.96; BOD: 0.6–9; DO: 4.3–16.4; NO3: 0.02–1.5; NO2: 0.006–0.953; NH4: 0.08–2.8; COD: 22; Mg2+: 4–66; Ca2+: 43–253; Cl−: 25–91; SO4−: 19–185; Pb: 1–13.6; Cd: 1–7 | 76–100% | [114] |
| Chemometric Techniques | Analytical Method | Parameters and Concentration | Accuracy/Precision & R2 | Ref. |
|---|---|---|---|---|
| HCA, PCA | pH meter and EDXRF | NIST SRM-1646a (Estuarine Sediment) K: 0.67 cg/kg; Ca: 0.456 cg/kg; Fe: 1.743 cg/kg; Ti: 0.556; V: 47.62 mg/kg; Cr: 63.9 mg/kg; Ni: 21.8 mg/kg; Cu: 7.9 mg/kg; Zn: 45.57 mg/kg IAEA SOIL-7 (Austria) K: 1.34 cg/kg; Ca: 22.75 cg/kg; Fe: 3.28 cg/kg; Ti: 4583.29; V: 68.84 mg/kg; Cr: 123.89 mg/kg; Ni: <22.9 mg/kg; Cu: 14.3 mg/kg; Zn: 104.21 mg/kg | 36.94–85.99% | [115] |
| HCA, PCA | ICP MS and UV–VIS spectroscopy | pH: minimum—5.45, maximum—8.25, mean—6.91; N total (mg/kg): minimum—794.66, maximum—2856, mean—1564.65; P total (mg/kg): minimum—268.16, maximum—1920.83, mean—744.87; TC (%): minimum—0.88, maximum—4.42, mean—2.28; TOC (%): minimum—0.93, maximum—3.73, mean—1.98; As (mg/kg): minimum—3.59, maximum—16.63, mean—7.55; Cu (mg/kg): minimum—11.76, maximum—97.42, mean—44.86; Cr (mg/kg): minimum—18.1, maximum—230.42, mean—71.87; Ni (mg/kg): minimum—9.44, maximum—85.19, mean—37.63; Cd (mg/kg): minimum—0.22, maximum—0.63, mean—0.4; Zn (mg/kg): minimum—30.58, maximum—115.03, mean—64.03; Pb (mg/kg): minimum—11.41, maximum—37.42, mean—20.78 | 90–110% R2 = 0.79–0.91 | [116] |
| CA, PCA | pH meter, ICP-OES, and ETAAS | Location: K Zn (mg/kg): 103.74; Cd (mg/kg): 0.17; Pb (mg/kg): 17.66; Cu (mg/kg): 33.55; Hg (mg/kg): 0.033 Location: KRM Zn (mg/kg): 66.33; Cd (mg/kg): 0.38; Pb (mg/kg): 14.33; Cu (mg/kg): 12.07; Hg (mg/kg): 0.036 Location: Kagri Zn (mg/kg): 384.6; Cd (mg/kg): 2.22; Pb (mg/kg): 19.69; Cu (mg/kg): 8; Hg (mg/kg): 0.106 Location: AS Zn (mg/kg): 409.7; Cd (mg/kg): 0.97; Pb (mg/kg): 31.68; Cu (mg/kg): 25.22; Hg (mg/kg): 0.072 Location: PB Zn (mg/kg): 330.4; Cd (mg/kg): 0.81; Pb (mg/kg): 14.73; Cu (mg/kg): 30.76; Hg (mg/kg): 0.043 | 80% R2 = 0.81–0.93 | [117] |
| PCA, CA | Potentiometry, UV–Vis-NIR, ICP-OES, and GC | Location: F. Sylvatica pH: 6.07 ± 0.24; TOC (mg/g): 171.30 ± 26.80; Total N (mg/g): 10.32 ± 1.85; Total Ca (mg/g): 46.81 ± 11.68; Total K (mg/g): 11.18 ± 4.08; Total Mg (mg/g): 7.10 ± 2.82; Total Mn (mg/g): 1.35 ± 0.56; Total Na (mg/g): 2.76 ± 1.17; Total Fe (mg/g): 24.49 ± 6.70; Total Al (mg/g): 42.46 ± 15.72; Py-Fe (mg/g): 6.18 ± 1.75; Py-Al (mg/g): 18.02 ± 3.97; Ox-Fe (mg/g): 12.65 ± 2.88; Ox-Al (mg/g): 23.93 ± 7.79 Location: Q. Cerris pH: 6.57 ± 0.23; TOC (mg/g): 80.10 ± 9.70; Total N (mg/g): 6.12 ± 0.71; Total Ca (mg/g): 20.89 ± 12.93; Total K (mg/g): 3.84 ± 1.70; Total Mg (mg/g): 9.33 ± 4.62; Total Mn (mg/g): 1.98 ± 1.34; Total Na (mg/g): 0.14 ± 0.06; Total Fe (mg/g): 22.59 ± 4.72; Total Al (mg/g): 22.23 ± 12.20; Py-Fe (mg/g): 1.95 ± 0.42; Py-Al (mg/g): 2.23 ± 0.69; Ox-Fe (mg/g): 8.33 ± 2.31; Ox-Al (mg/g): 6.45 ± 2.37 Location: Q. Ilex pH: 6.87 ± 0.29; TOC (mg/g): 328.80 ± 50.30; Total N (mg/g): 17.65 ± 3.47; Total Ca (mg/g): 140.06 ± 49.95; Total K (mg/g): 6.25 ± 3.04; Total Mg (mg/g): 15.12 ± 7.51; Total Mn (mg/g): 0.96 ± 0.37; Total Na (mg/g): 1.12 ± 0.69; Total Fe (mg/g): 11.14 ± 2.47; Total Al (mg/g): 25.13 ± 19.75; Py-Fe (mg/g): 1.64 ± 0.56; Py-Al (mg/g): 4.86 ± 2.24; Ox-Fe (mg/g): 5.22 ± 3.24; Ox-Al (mg/g): 13.62 ± 10.35 | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, S.M.; Umar, Y.; Kabir, A. Advanced Chemometric Techniques for Environmental Pollution Monitoring and Assessment: A Review. Chemosensors 2025, 13, 268. https://doi.org/10.3390/chemosensors13070268
Haque SM, Umar Y, Kabir A. Advanced Chemometric Techniques for Environmental Pollution Monitoring and Assessment: A Review. Chemosensors. 2025; 13(7):268. https://doi.org/10.3390/chemosensors13070268
Chicago/Turabian StyleHaque, Shaikh Manirul, Yunusa Umar, and Abuzar Kabir. 2025. "Advanced Chemometric Techniques for Environmental Pollution Monitoring and Assessment: A Review" Chemosensors 13, no. 7: 268. https://doi.org/10.3390/chemosensors13070268
APA StyleHaque, S. M., Umar, Y., & Kabir, A. (2025). Advanced Chemometric Techniques for Environmental Pollution Monitoring and Assessment: A Review. Chemosensors, 13(7), 268. https://doi.org/10.3390/chemosensors13070268







