SiO2-Al2O3-ZrO2-Ag Composite and Its Signal Enhancement Capacity on Raman Spectroscopy
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Ceramic Membranes
2.3. Characterization
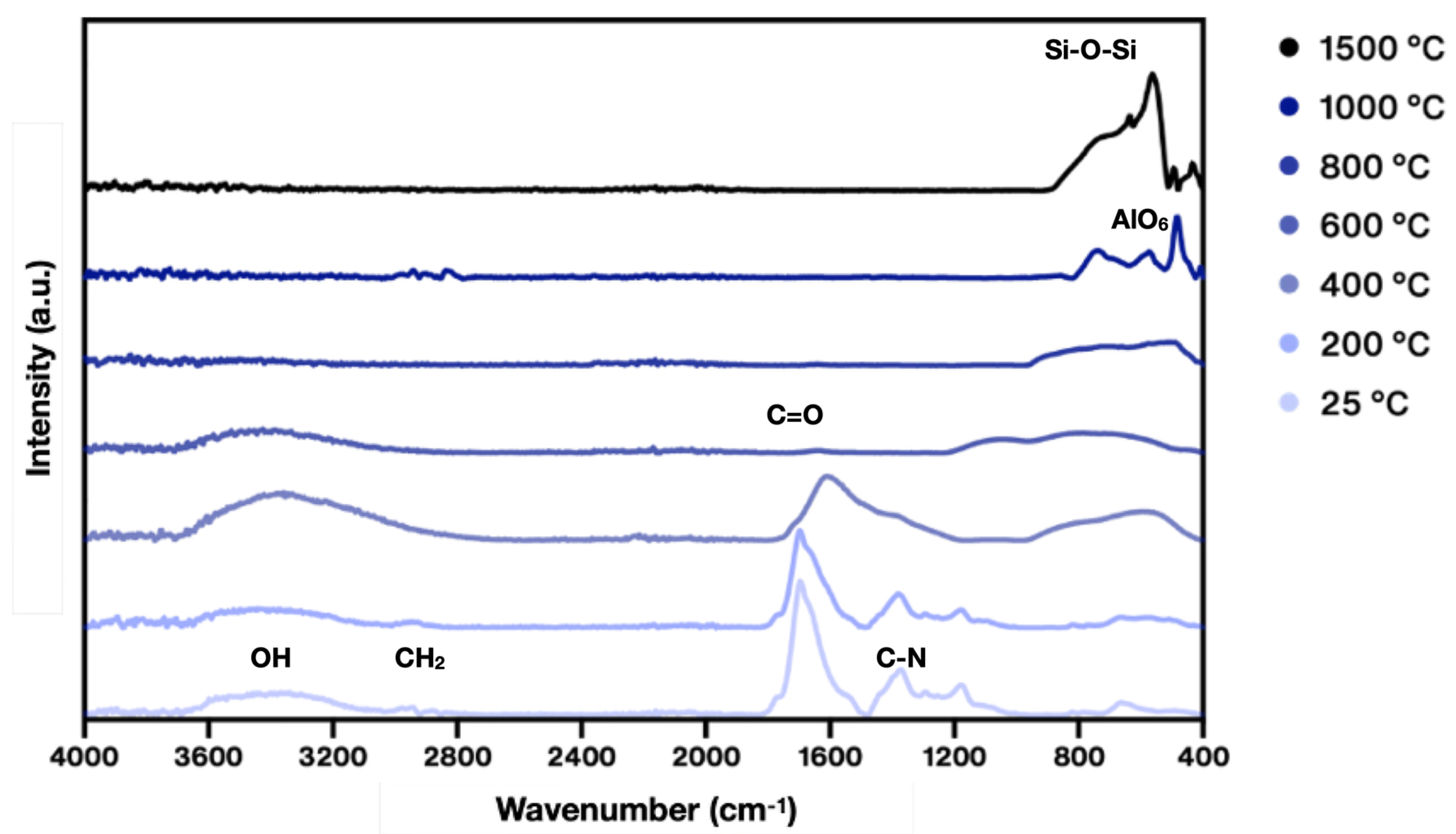
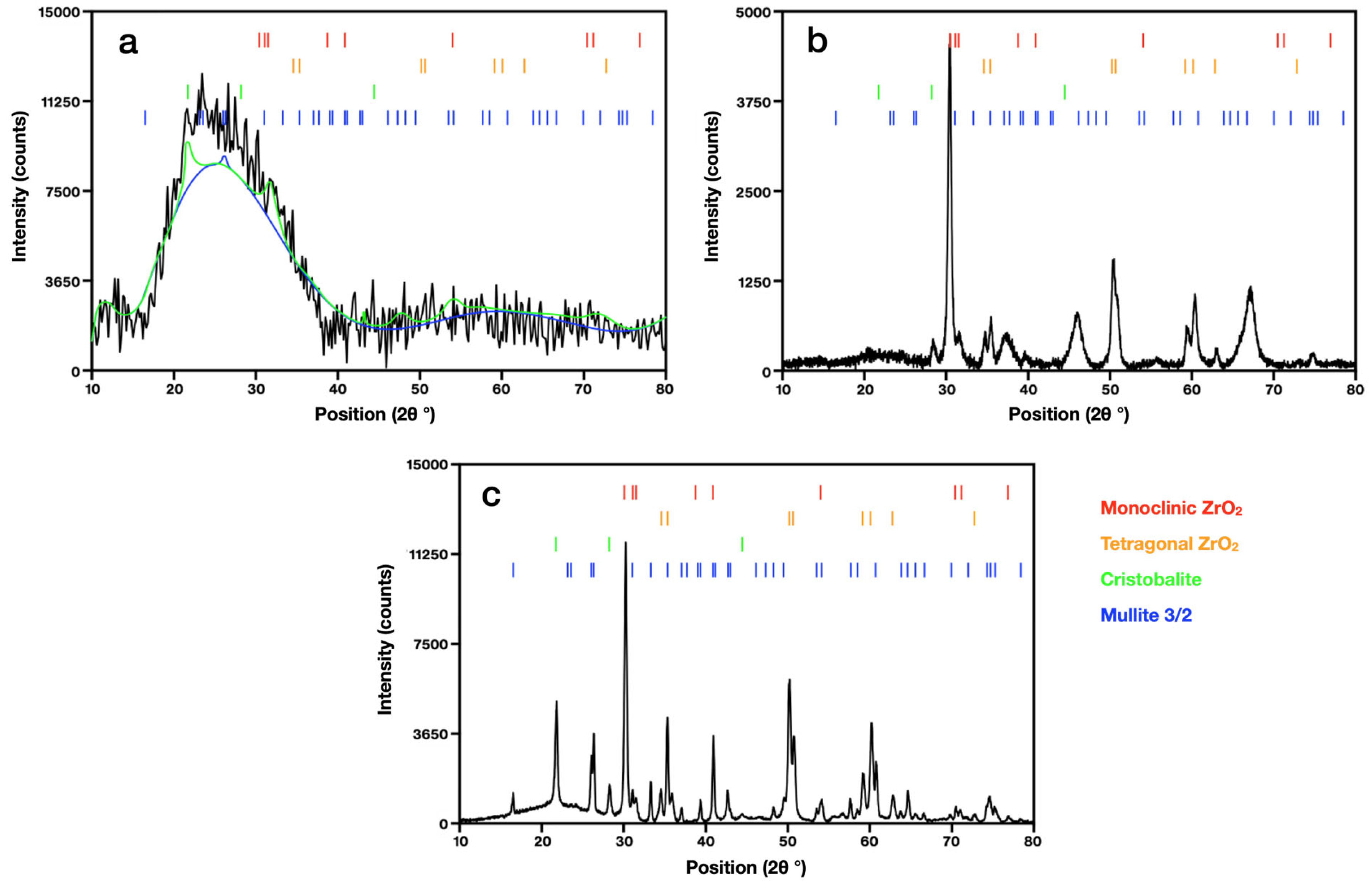
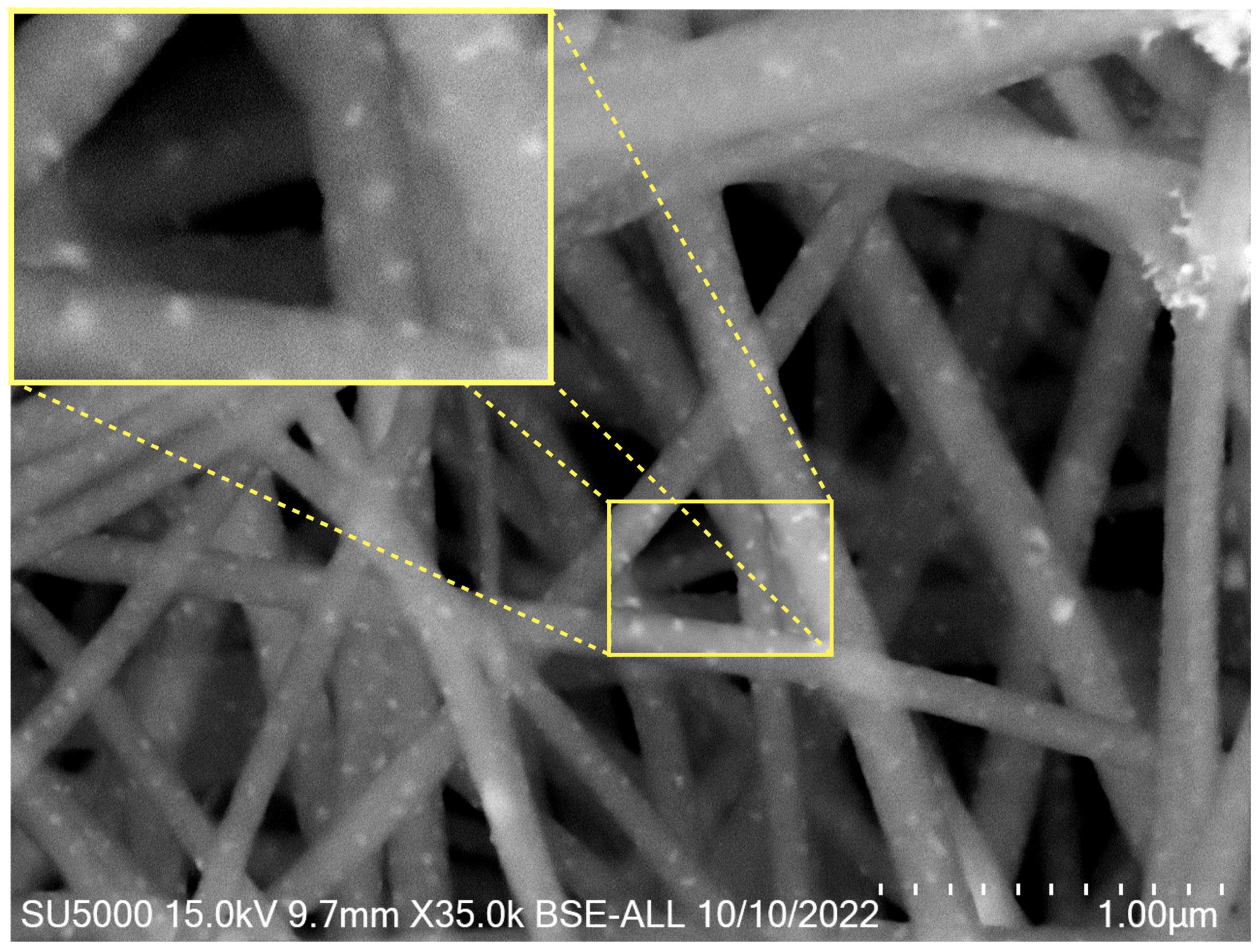
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Py | pyridine |
| MO | methyl orange |
| MB | methylene blue |
| CV | crystal violet |
| EB | Eriochrome black T |
References
- Cao, Y.; Zhang, J.; Yang, Y.; Huang, Z.; Long, N.V.; Fu, C. Engineering of SERS Substrates Based on Noble Metal Nanomaterials for Chemical and Biomedical Applications. Appl. Spectrosc. Rev. 2015, 50, 499–525. [Google Scholar] [CrossRef]
- Tong, L.; Xu, H.; Käll, M. Nanogaps for SERS applications. MRS Bull. 2014, 39, 163–168. [Google Scholar] [CrossRef]
- Kim, J.; Sim, K.; Cha, S.; Oh, J.; Nam, J. Single-Particle Analysis on Plasmonic Nanogap Systems for Quantitative SERS. J. Raman Spectrosc. 2020, 52, 375–385. [Google Scholar] [CrossRef]
- Kanzaki, S.; Tabata, H.; Kumazawa, T.; Ohta, S. Sintering and Mechanical Properties of Stoichiometric Mullite. J. Am. Ceram. Soc. 1985, 68, C‐6. [Google Scholar] [CrossRef]
- Bagchi, B.; Das, S.; Bhattacharya, A.; Basu, R.; Nandy, P. Nanocrystalline Mullite Synthesis at a Low Temperature: Effect of Copper Ions. J. Am. Ceram. Soc. 2009, 92, 748–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Gao, J.; Yang, J. Mullite fibres prepared by sol–gel method using polyvinyl butyral. J. Eur. Ceram. Soc. 2009, 29, 1101–1107. [Google Scholar] [CrossRef]
- Kong, L.B.; Zhang, T.S.; Ma, J.; Boey, F.Y.C. Mullitization behavior and microstructural development of B2O3–Al2O3–SiO2 mixtures activated by high-energy ball milling. Solid State Sci. 2009, 11, 1333–1342. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Tulyagavov, D.U.; Ferreira, J.M.; Labrincha, J.A. High temperature mullite dissolution in ceramic bodies derived from Al-rich sludge. J. Eur. Ceram. Soc. 2005, 25, 703–710. [Google Scholar] [CrossRef]
- Yang, T.; Wang, E.; Wang, F.; Chou, K.; Hou, X. Fabrication of Ordered Mullite Nanowhisker Array with Surface Enhanced Raman Scattering Effect. Sci. Rep. 2015, 5, 9690. [Google Scholar] [CrossRef]
- Camerucci, M.A.; Urretavizcaya, G.; Castro, M.S.; Cavalieri, A.L. Electrical properties and thermal expansion of cordierite and cordierite-mullite materials. J. Eur. Ceram. Soc. 2001, 21, 2917–2923. [Google Scholar] [CrossRef]
- da Silva, V.J.; de Almeida, E.P.; Gonçalves, W.P.; da Nóbrega, R.B.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; de Lima Santana, L.N. Mineralogical and dielectric properties of mullite and cordierite ceramics produced using wastes. Ceram. Ceram. Int. 2019, 45, 4692–4699. [Google Scholar] [CrossRef]
- Kumar, N.; Wondergem, C.S.; Wain, A.J.; Weckhuysen, B.M. In Situ Nanoscale Investigation of Catalytic Reactions in the Liquid Phase Using Zirconia-Protected Tip-Enhanced Raman Spectroscopy Probes. J. Phys. Chem. Lett. 2019, 10, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Abe, H.; Ohara, S. Selective Growth of Monoclinic and Tetragonal Zirconia Nanocrystals. J. Am. Chem. Soc. 2010, 132, 2538–2539. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Karim, M.N.; Ghosh, K.; Murshed, M.M.; Rezwan, K.; Maas, M. Plasmonic porous ceramics based on zirconia-toughened alumina functionalized with silver nanoparticles for surface-enhanced Raman scattering. Open Ceram. 2022, 9, 100228. [Google Scholar] [CrossRef]
- Zhang, T.S.; Kong, L.B.; Du, Z.H.; Ma, J.; Li, S. In situ interlocking structure in gel-derived mullite matrix induced by mechanoactivated commercial mullite powders. Scr. Mater. 2010, 63, 1132–1135. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Du, H.; Li, Z.; Li, S.; Li, S.; Xu, R. Molten salt synthesis of mullite whiskers from various alumina precursors. J. Alloys Compd. 2010, 491, 447–451. [Google Scholar] [CrossRef]
- Fischer, R.X.; Schneider, H.; Schmucker, M. Crystal structure of Al-rich mullite. Am. Mineral. 1994, 79, 983–990. [Google Scholar]
- Lazic, B.; Krueger, H.; Kaindl, R.; Perfler, L.; Kremenovic, A.; Cvetkovic, V.; Withers, R.L. ChemInform Abstract: Superstructure of Mullite-Type KAl9O14. ChemInform 2013, 44, 496–502. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. Spinel structured MgAl2O4 nanoparticles as a low-cost and stable SERS substrate for rapid simultaneous detection of neurological drugs in biofluids. Ceram. Int. 2022, 48, 18667–18675. [Google Scholar] [CrossRef]
- Shi, T.; Tang, Z.; Liang, P.; Zhang, X.; Zhang, D.; Shao, Q.; Huang, J.; Yu, Z.; Xu, Y.; Chen, H. ZrO2@Ag@SiO2 Sandwich Structure with High SERS Enhancement Effect and Stability. J. Phys. Chem. C 2020, 124, 25967–25974. [Google Scholar] [CrossRef]
- Sitjar, J.; Liao, J.D.; Lee, H.; Liu, B.H.; Fu, W.E. SERS-active substrate with collective amplification design for trace analysis of pesticides. Nanomaterials 2019, 9, 664. [Google Scholar] [CrossRef]
- Garibay-Alvarado, J.A.; Ruiz-Esparza-Rodríguez, M.A.; Zaragoza-Contreras, E.A.; Reyes-López, S.Y. Ag Nanoparticle-Decorated SiO2–Al2O3–ZrO2 Composites as a Low-Cost Substrate for Enhanced Signal Infrared Spectroscopy. ACS Appl. Nano Mater. 2024, 7, 4658–4666. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Dai, X.; Song, R.; Wang, B.; Wang, Z. A strong charge-transfer effect in surface-enhanced Raman scattering induced by valence electrons of actinide elements. RSC Adv. 2015, 5, 32198–32204. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; Xu, H.; Wang, Z.; Li, Z.; Zhang, R.Q. The mechanism of N–Ag bonding determined tunability of surface-enhanced Raman scattering of pyridine on MAg (M = Cu, Ag, Au) diatomic clusters. Phys. Chem. Phys. 2014, 16, 20665–20671. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Chen, C.; Peng, J.; Lee, S. Enhancement in SERS intensities of azo dyes adsorbed on ZnO by plasma treatment. J. Raman Spectrosc. 2014, 45, 332–337. [Google Scholar] [CrossRef]
- Yu, T.; Ho, C.; Wu, C.; Chien, C.; Lin, C.; Lee, S. Metal–organic frameworks: A novel SERS substrate. J. Raman Spectrosc. 2013, 44, 1506–1511. [Google Scholar] [CrossRef]
- Tian, L.; Tadepalli, S.; Farrell, M.E.; Liu, K.K.; Gandra, N.; Pellegrino, P.M.; Singamaneni, S. Multiplexed charge-selective surface enhanced Raman scattering based on plasmonic calligraphy. J. Mater. Chem. C 2014, 2, 5438. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Vu, D.C.; Pham, V.H.; Pham, T.B.; Pham, V.H.; Bui, H. Improvement of SERS for detection of ultra-low concentration of methyl orange by nanostructured silicon decorated with Ag nanoparticles. Optik 2021, 231, 166431. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y. Influence of adsorption orientation of methyl orange on silver colloids by Raman and fluorescence spectroscopy: pH effect. Chem. Phys. 2006, 331, 55–60. [Google Scholar] [CrossRef]
- Santos Ede, B.; Lima, E.C.N.L.; Oliveira CSde Sigoli, F.A.; Mazali, I.O. Fast detection of paracetamol on a gold nanoparticle–chitosan substrate by SERS. Anal. Methods 2014, 6, 3564–3568. [Google Scholar] [CrossRef]
- Pham, T.T.; Vu, X.H.; Dien, N.D.; Trang, T.T.; Chi, T.T.; Phuong, P.H.; Nghia, N.T. Ag nanoparticles on ZnO nanoplates as a hybrid SERS-active substrate for trace detection of methylene blue. RSC Adv. 2022, 12, 7850–7863. [Google Scholar] [CrossRef]
- Meng, W.; Hu, F.; Jiang, X.; Lu, L. Preparation of silver colloids with improved uniformity and stable surface-enhanced Raman scattering. Nanoscale Res. Lett. 2015, 10, 34. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Yang, S.; Li, L.; Guo, L. Facile synthesis of spinous-like Au nanostructures for unique localized surface plasmon resonance and surface-enhanced Raman scattering. New J. Chem. 2015, 39, 2551–2556. [Google Scholar] [CrossRef]
- Meng, W.; Hu, F.; Zhang, L.Y.; Jiang, X.H.; Lu, L.D.; Wang, X. SERS and DFT study of crystal violet. J. Mol. Struct. 2013, 1035, 326–331. [Google Scholar] [CrossRef]
- Szabó, L.; Herman, K.; Leopold, N.; Buzumurgă, C.; Chiş, V. Surface-enhanced Raman scattering and DFT investigation of Eriochrome Black T metal chelating compound. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 226–231. [Google Scholar] [CrossRef]
- Kaur, Y.; Jasrotia, T.; Kumar, R.; Chaudhary, G.R.; Chaudhary, S. Adsorptive removal of eriochrome black T (EBT) dye by using surface active low cost zinc oxide nanoparticles: A comparative overview. Chemosphere 2021, 278, 130366. [Google Scholar] [CrossRef] [PubMed]
- Nahali, L.; Miyah, Y.; Mejbar, F.; Benjelloun, M.; Assila, O.; Fahoul, Y.; Nenov, V.; Zerrouq, F. Assessment of Brilliant Green and Eriochrome Black T dyes adsorption onto fava bean peels: Kinetics, isotherms and regeneration study. Desalination Water Treat. 2022, 245, 255–269. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Sanchez-Cortes, S.; Castillejo, M.; Oujja, M. Comparative SERS effectiveness of silver nanoparticles prepared by different methods: A study of the enhancement factor and the interfacial properties. J. Colloid Interface Sci. 2008, 326, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ciou, S.H.; Cao, Y.W.; Huang, H.C.; Su, D.Y.; Huang, C.L. SERS Enhancement Factors Studies of Silver Nanoprism and Spherical Nanoparticle Colloids in The Presence of Bromide Ions. J. Phys. Chem. C 2009, 113, 9520–9625. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Au, L.; Rycenga, M.; Li, W.; Xia, Y. Measuring the SERS enhancement factors of dimers with different structures constructed from silver nanocubes. Chem. Phys. Lett. 2010, 484, 304–308. [Google Scholar] [CrossRef]
- Lin, W.C.; Huang, S.H.; Chen, C.L.; Chen, C.C.; Tsai, D.P.; Chiang, H.P. Controlling SERS intensity by tuning the size and height of a silver nanoparticle array. Appl. Phys. A 2010, 101, 185–189. [Google Scholar] [CrossRef]
- Babich, E.; Raskhodchikov, D.; Redkov, A.; Hmima, A.; Nashchekin, A.; Lipovskii, A. Dendritic structures by glass electrolysis: Studies and SERS capability. Curr. Appl. Phys. 2021, 24, 54–59. [Google Scholar] [CrossRef]
- Jana, D.; Mandal, A.; De, G. High Raman Enhancing Shape-Tunable Ag Nanoplates in Alumina: A Reliable and Efficient SERS Technique. ACS Appl. Mater. Interfaces 2012, 4, 3330–3334. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Gartia, M.R.; Jiang, J.; Liu, G.L. Surface plasmon enhanced broadband spectrophotometry on black silver substrates. Appl. Phys. Lett. 2011, 98, 241904. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, C.; Yang, G.; You, H.; Fang, J. Hierarchical silver mesoparticles with tunable surface topographies for highly sensitive surface-enhanced Raman spectroscopy. J. Mater. Chem. A 2014, 2, 4534–4542. [Google Scholar] [CrossRef]
- Rekha, C.R.; Nayar, V.U.; Gopchandran, K.G. Synthesis of highly stable silver nanorods and their application as SERS substrates. J. Sci. Adv. Mater. Devices 2018, 3, 196–205. [Google Scholar] [CrossRef]
- Sun, K.; Meng, G.; Huang, Q.; Zhao, X.; Zhu, C.; Huang, Z.; Qian, Y.; Wang, X.; Hu, X. Gap-tunable Ag-nanorod arrays on alumina nanotip arrays as effective SERS substrates. J. Mater. Chem. C 2013, 1, 5015. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Zhou, Y.; Wang, Y. SERS activity of self-cleaning silver/titania nanoarray. Appl. Surf. Sci. 2014, 313, 549–557. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.; Fu, W.E.; Chen, C.C.; Chen, G.D.; Juang, Y.D. Gold Nanoparticle-Coated ZrO2-Nanofiber Surface as a SERS-Active Substrate for Trace Detection of Pesticide Residue. Nanomaterials 2018, 8, 402. [Google Scholar] [CrossRef]
- Pal, A.K.; Pagal, S.; Prashanth, K.; Chandra, G.K.; Umapathy, S.; Mohan, D.B. Ag/ZnO/Au 3D hybrid structured reusable SERS substrate as highly sensitive platform for DNA detection. Sens. Actuators B Chem. 2019, 279, 157–169. [Google Scholar] [CrossRef]
- Zhao, H.; Hasi, W.; Li, N.; Sha, X.; Han, S. Preparation of a high-performance thermally shrinkable polystyrene SERS substrate via Au@Ag nanorods self-assembled to detect pesticide residues. J. Raman Spectrosc. 2019, 50, 1679–1690. [Google Scholar] [CrossRef]
- Francis, M.K.; Sahu, B.K.; Bhargav, P.B.; Ahmed, N.; Das, A.; Dhara, S. Ag nanowires based SERS substrates with very high enhancement factor. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 137, 115080. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Silva-Holguín, P.N.; Garibay-Alvarado, J.A.; Reyes-López, S.Y. Silver nanoparticles: Multifunctional tool in environmental water remediation. Materials 2024, 17, 1939. [Google Scholar] [CrossRef]

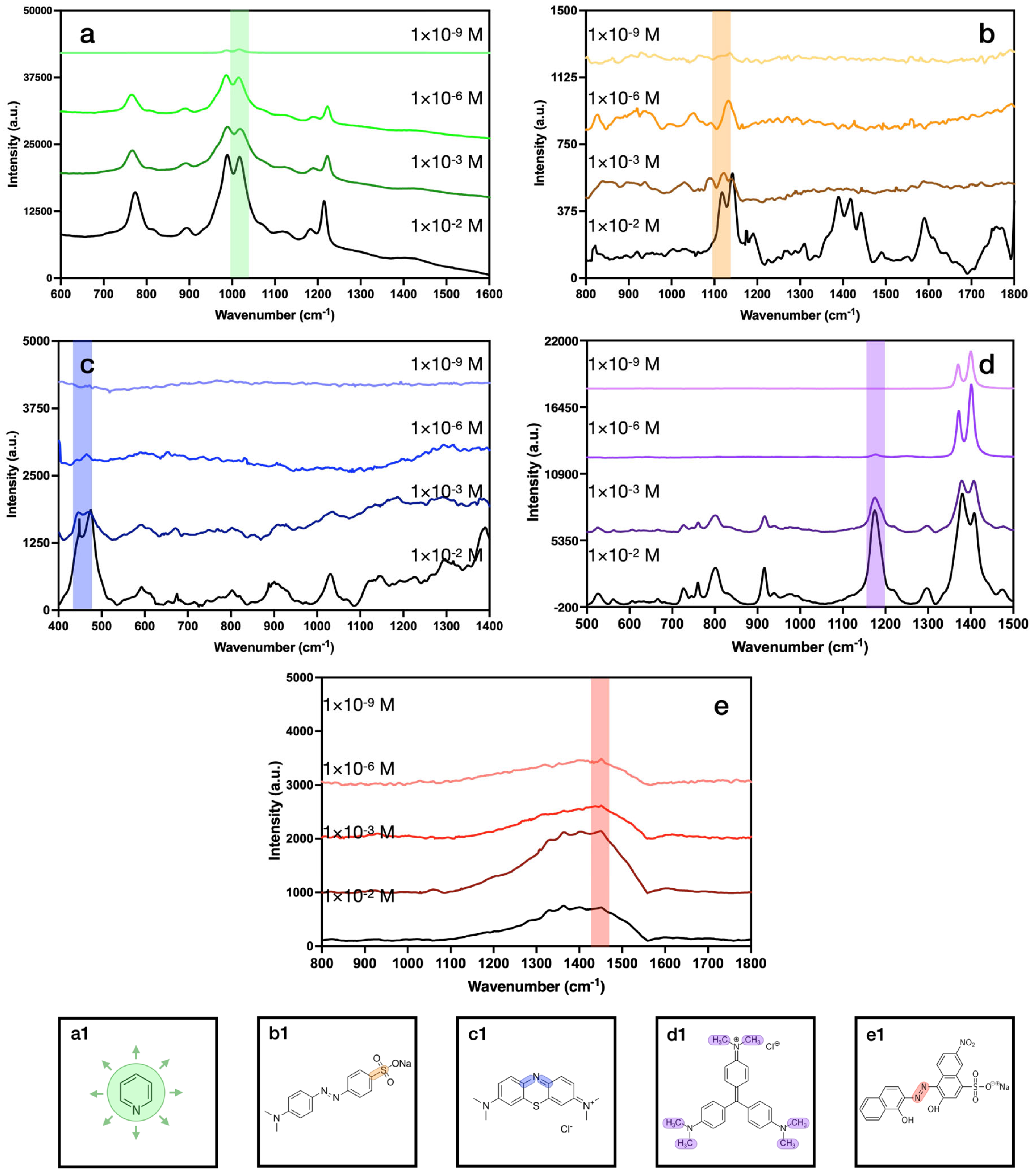
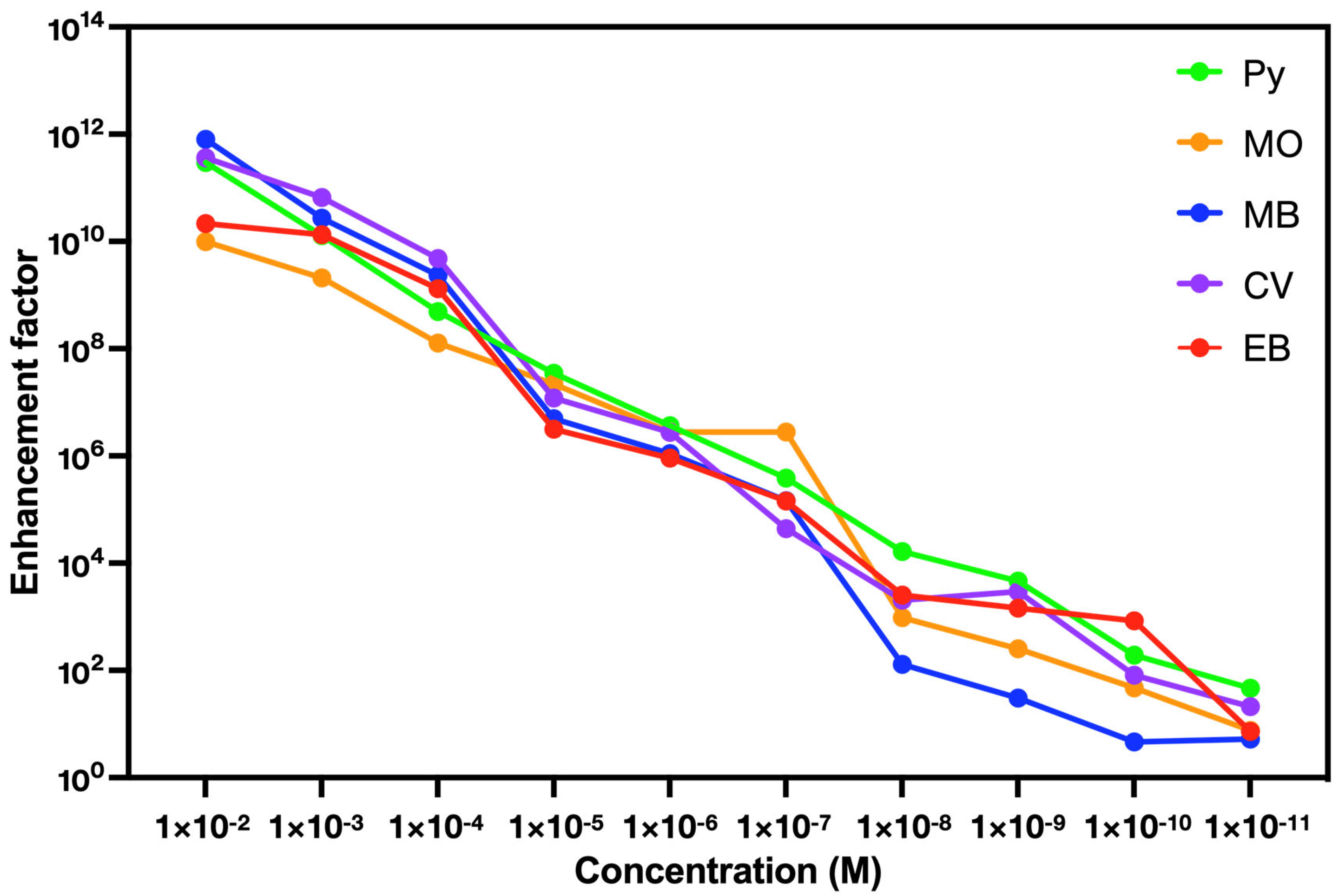
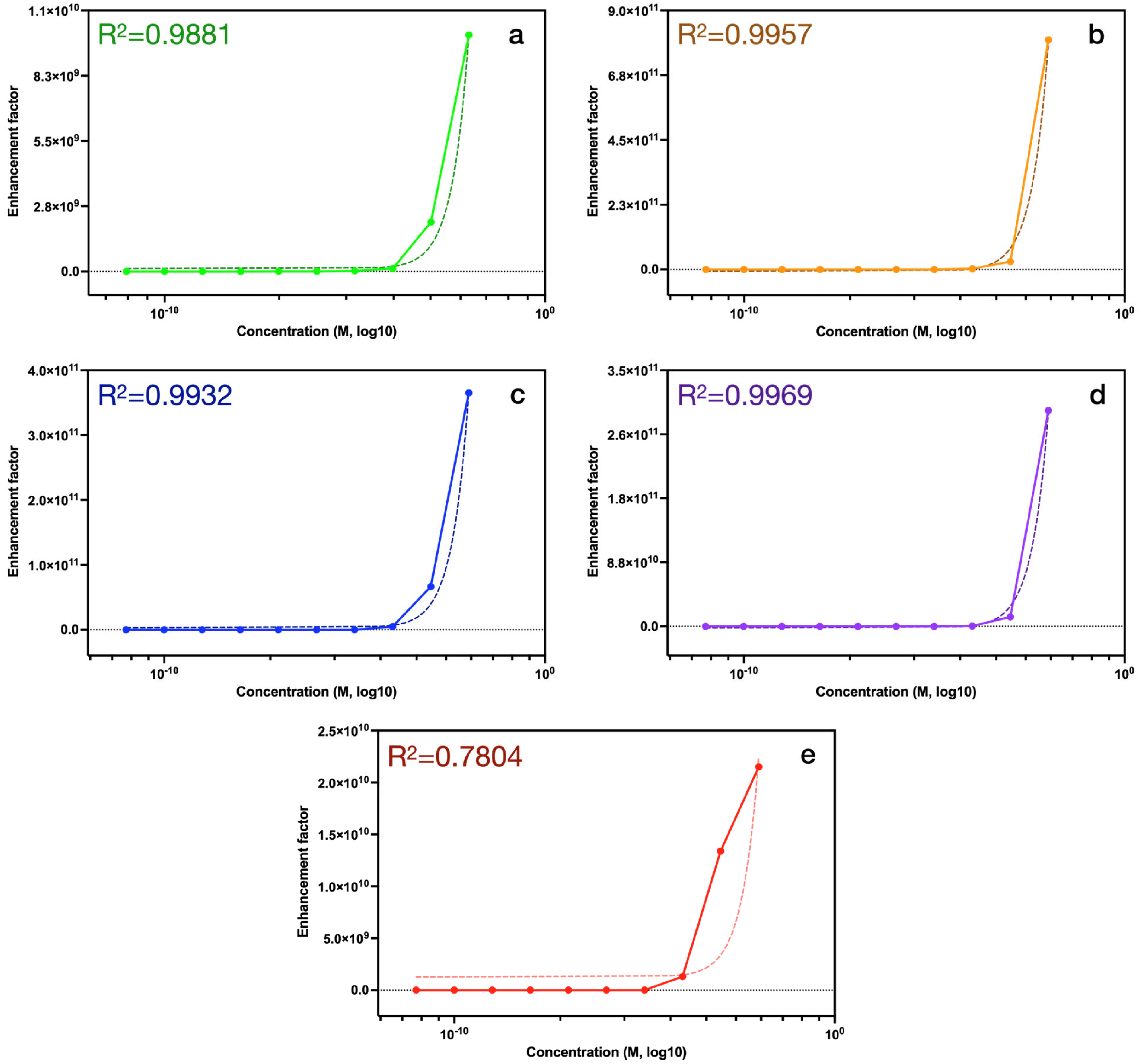
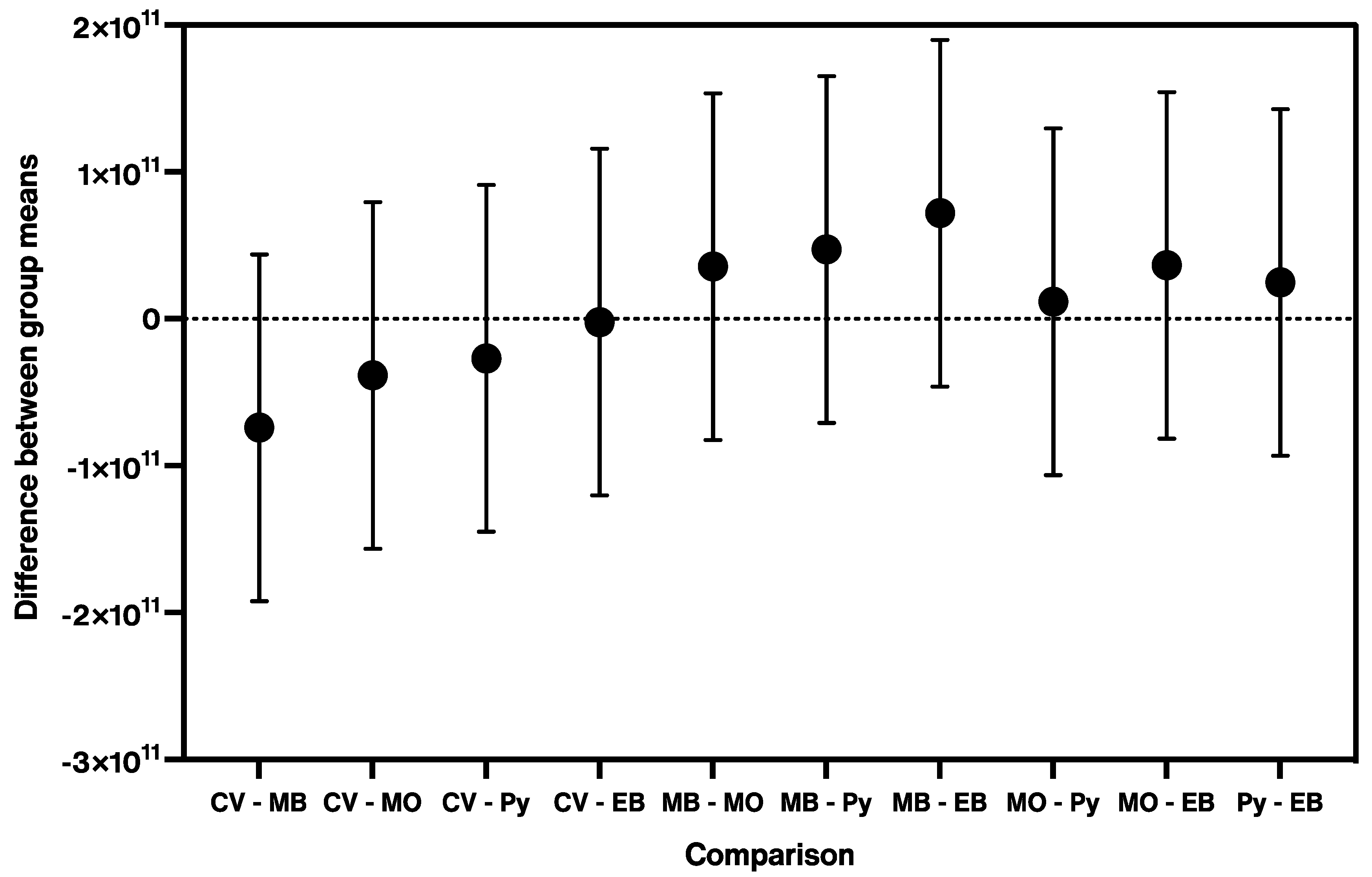
| Concentration (M) | 1 × 10−2 | 1 × 10−3 | 1 × 10−4 | 1 × 10−5 | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 | 1 × 10−9 | 1 × 10−10 | 1 × 10−11 |
|---|---|---|---|---|---|---|---|---|---|---|
| Py | 9.97 × 109 | 2.08 × 109 | 1.28 × 108 | 2.19 × 107 | 2.80 × 106 | 2.80 × 106 | 9.68 × 102 | 2.53 × 102 | 4.71 × 101 | 7.58 |
| MO | 7.98 × 1011 | 2.73 × 1010 | 2.32 × 109 | 4.94 × 106 | 1.10 × 106 | 1.46 × 105 | 1.30 × 102 | 3.06 × 101 | 4.66 | 5.26 |
| MB | 3.65 × 1011 | 6.65 × 1010 | 4.83 × 109 | 1.20 × 107 | 2.74 × 106 | 4.39 × 104 | 2.03 × 103 | 2.97 × 103 | 8.16 × 101 | 2.10 × 101 |
| CV | 2.95 × 1011 | 1.29 × 1010 | 4.91 × 108 | 3.47 × 107 | 3.67 × 106 | 3.86 × 105 | 1.64 × 104 | 4.66 × 103 | 1.92 × 102 | 4.69 × 101 |
| EB | 2.15 × 1010 | 1.34 × 1010 | 1.31 × 109 | 3.16 × 106 | 9.13 × 105 | 1.44 × 105 | 2.54 × 103 | 1.45 × 103 | 8.36 × 102 | 7.28 |
| Material | Morphology | Method | Structure Size | Maximum Enhancement Factor | Analyte | Analyte Concentration | Author |
|---|---|---|---|---|---|---|---|
| Silver | Spherical NPs | Array | ~10 nm | 2.53 × 102 | Pyridine | 1 × 10−9 | This article |
| Silver | Spherical NPs | Array | ~10 nm | 3.06 × 101 | Methyl orange | 1 × 10−9 | This article |
| Silver | Spherical NPs | Array | ~10 nm | 2.97 × 103 | Methylene blue | 1 × 10−9 | This article |
| Silver | Spherical NPs | Array | ~10 nm | 4.66 × 103 | Crystal violet | 1 × 10−9 | This article |
| Silver | Spherical NPs | Array | ~10 nm | 1.45 × 103 | Eriochrome black T | 1 × 10−9 | This article |
| Silver | Spherical NPs | Colloid | 100 nm | 1.8 × 105 | Alizarin | 10−6 | [39] |
| Silver | Nanoprisms Spherical NPs | Colloid | 70 nm 10 nm | 1.6 × 105, 3.2 × 105 | R6G | 4.5 × 10−9 | [40] |
| Silver | Nanocubes | Colloid | 100 nm | 2 × 107 | 4-MBT | 1 × 10−1 | [41] |
| Silver | Nanospheres | Array | 96 nm | 2 × 106 | R6G | 10−5 | [42] |
| Silver | Dendrites | Film | 100 nm | 106, 107 | BPE | 6 × 10−12 | [43] |
| Silver | Plates | Colloid | 180 nm | 1 × 109 | Methylene blue | 1 × 10−12 | [44] |
| Silver | Nanocones | Array | 180 nm | 6.38 × 107 | R6G | 1 × 10−4 | [45] |
| Silver | Nanoparticles | Colloid | 100 nm | 4.1 × 109 | Crystal violet | 1 × 10−12 | [46] |
| Silver | Nanorods | Array | ~600 nm | 1.44 × 108 | Nile blue chloride | 4.2 × 10−7 | [47] |
| Silver | Nanorods | Array | 60 nm | 3.2 × 107 | 4-MBN | 10−6 | [48] |
| SilverTiO2 | Nanorod NPs | Array | 75 nm | 7.8 × 105 | PMBA | 5 × 10−12 | [49] |
| Gold, ZrO2 | Nanofiber NPs | Mesh | 50 nm | 2.1 × 107 | Phosmet | 10−8 | [50] |
| Silver, Gold, ZnO | Nanorod Nanoparticles | Array | 1380 nm | 1 × 1010 | R6G | 10−16 | [51] |
| Gold, Silver | Nanorods | Colloid | 18.3 nm | 1.25 × 106 | 4-MBA | 10−5 | [52] |
| Silver | Nanowires | Colloid | 1000 nm | 6.93 × 1013 | R6G | 10−14 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garibay-Alvarado, J.A.; Pizá-Ruiz, P.; Zaragoza-Contreras, A.E.; Espinosa-Magaña, F.; Reyes-López, S.Y. SiO2-Al2O3-ZrO2-Ag Composite and Its Signal Enhancement Capacity on Raman Spectroscopy. Chemosensors 2025, 13, 266. https://doi.org/10.3390/chemosensors13070266
Garibay-Alvarado JA, Pizá-Ruiz P, Zaragoza-Contreras AE, Espinosa-Magaña F, Reyes-López SY. SiO2-Al2O3-ZrO2-Ag Composite and Its Signal Enhancement Capacity on Raman Spectroscopy. Chemosensors. 2025; 13(7):266. https://doi.org/10.3390/chemosensors13070266
Chicago/Turabian StyleGaribay-Alvarado, Jesús Alberto, Pedro Pizá-Ruiz, Armando Erasto Zaragoza-Contreras, Francisco Espinosa-Magaña, and Simón Yobanny Reyes-López. 2025. "SiO2-Al2O3-ZrO2-Ag Composite and Its Signal Enhancement Capacity on Raman Spectroscopy" Chemosensors 13, no. 7: 266. https://doi.org/10.3390/chemosensors13070266
APA StyleGaribay-Alvarado, J. A., Pizá-Ruiz, P., Zaragoza-Contreras, A. E., Espinosa-Magaña, F., & Reyes-López, S. Y. (2025). SiO2-Al2O3-ZrO2-Ag Composite and Its Signal Enhancement Capacity on Raman Spectroscopy. Chemosensors, 13(7), 266. https://doi.org/10.3390/chemosensors13070266







