Abstract
Mass spectrometry (MS) and coupled gas chromatography-mass spectrometry (GC-MS) are globally recognized as the primary techniques for the analysis of gases or vapors due to their selectivity, sensitivity, accuracy, and reproducibility. When thermal stress is applied, vapors or gases are released as a result of the reactions and changes that occur. The analysis of these gases during the thermally induced reaction is scientifically referred to as evolved gas analysis (EGA), which is essential for confirming the occurrence of the induced reactions. Pyrolyzers, thermobalances, or simple heaters can increase the temperature of the analyzed samples according to a programmed and software-managed ramp, allowing for control over both the heating rate and isothermal stages. The atmosphere can also be varied to simulate pyrolysis or thermo-oxidative processes. This way, each induced reaction generates a unique evolved gas, which can be linked to a theoretically hypothesized mechanism. Mass spectrometry (MS) and coupled gas chromatography–mass spectrometry (GC-MS) are fundamental analytical methods used for on-line thermally induced evolved gas analysis (OLTI-EGA).
1. Introduction
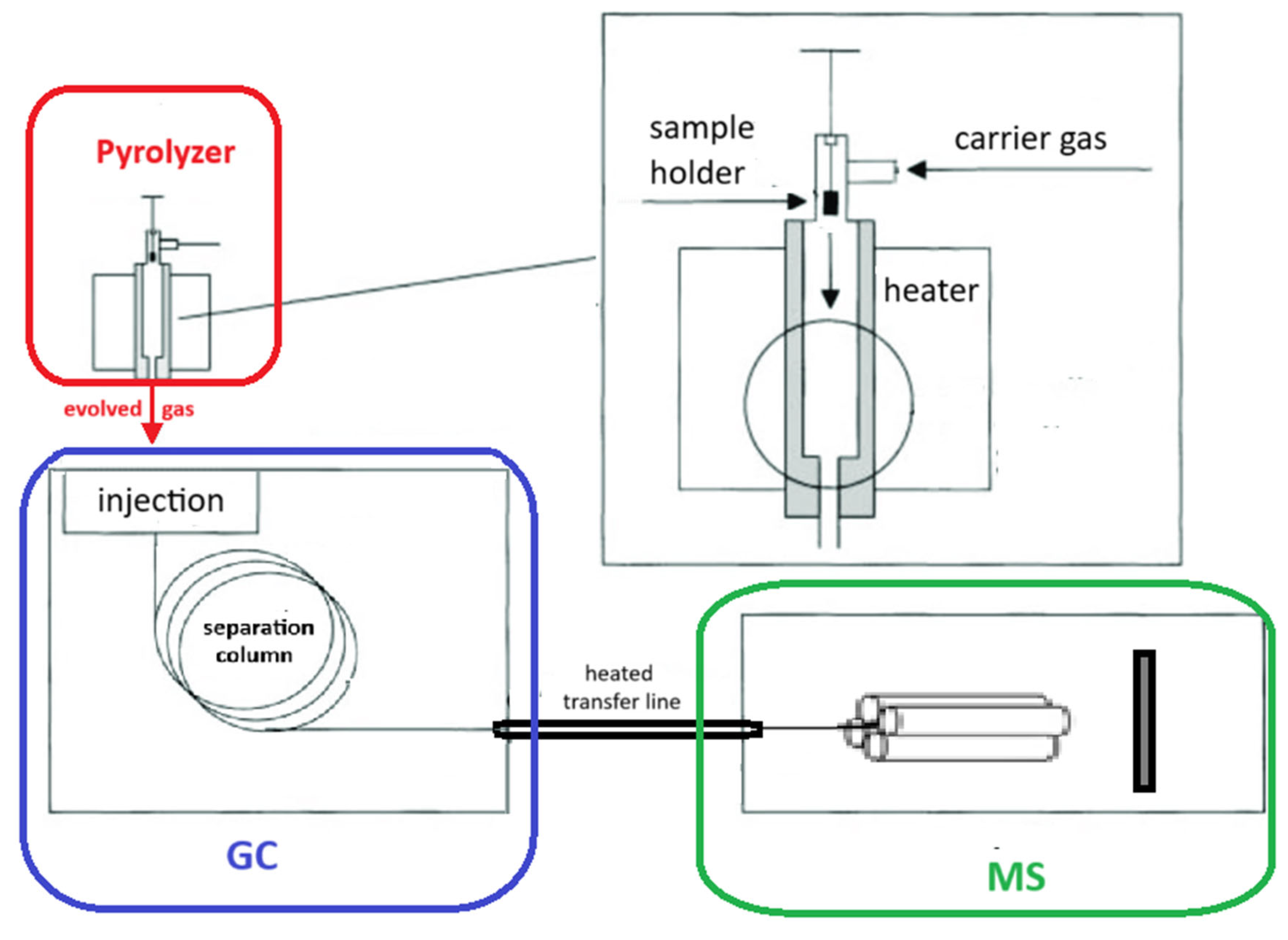
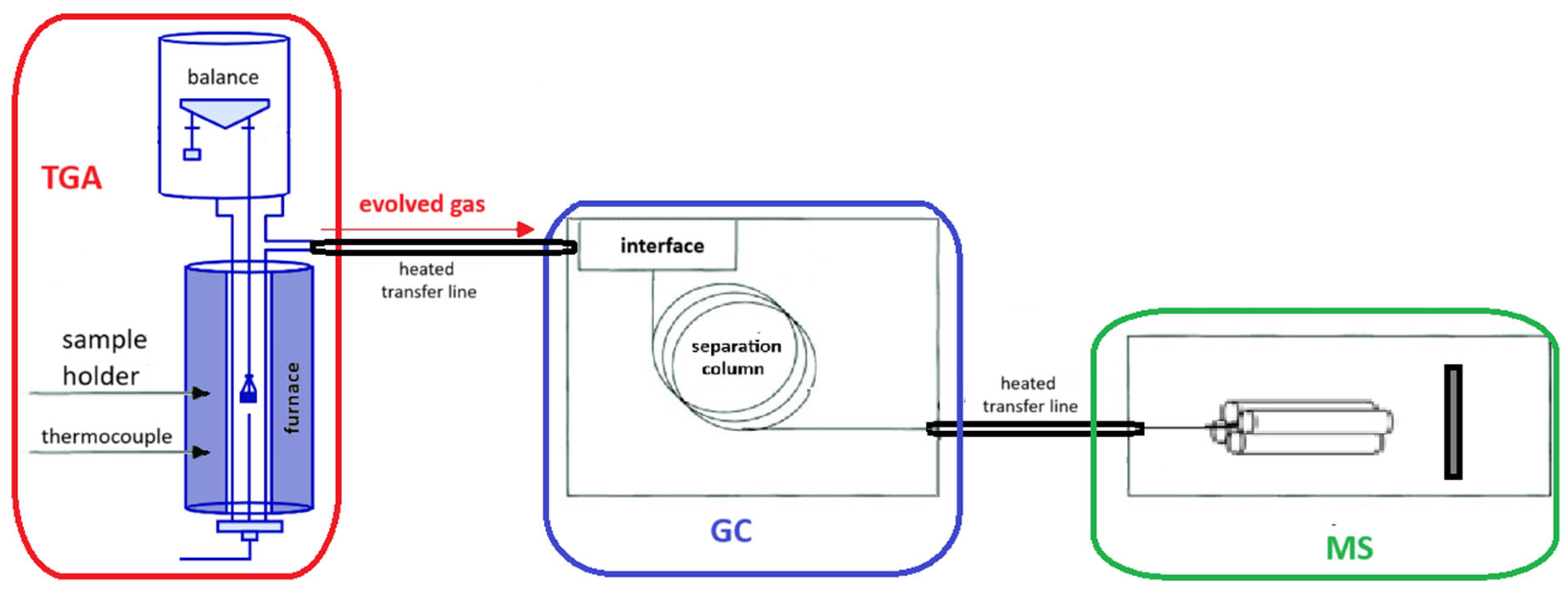
Whenever thermally induced stress occurs and critical or characteristic temperatures are reached, vapors or gases resulting from various types of reactions are released. The evaporation of solvents or chemical reactions can be monitored through evolved gas analysis (EGA) to confirm the hypothesized mechanism. EGA is carried out by coupling heating tools, such as pyrolyzers (Pys) or thermobalances (TGAs), to mass spectrometers (MSs) or gas chromatography/mass spectrometers (GC-MS). This coupling is typically achieved via a heated transfer line, which essentially consists of a deactivated tube whose sole task is to transfer the gas evolved by the heater (Py or TGA) to the GC-MS or directly to the MS, without losses or overlaps. Figure 1 and Figure 2 illustrate the two coupling modes.
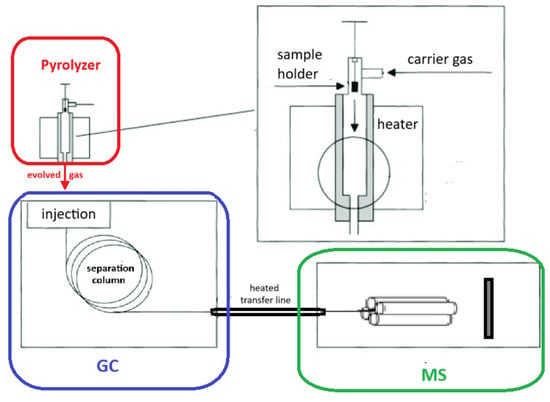
Figure 1.
Scheme of evolved gas analysis by Py-GC/MS.
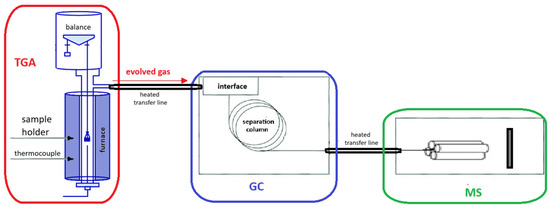
Figure 2.
Scheme of evolved gas analysis using TGA-GC/MS.
Each thermally induced reaction generates a different evolved gas, and the same reaction can be influenced physically or chemically to evaluate specific behaviors. For example, this can be carried out by changing the conditions from an inert to an oxidative atmosphere. It is therefore possible to correlate and define the connection between the reaction and the corresponding evolved gas. This correlation is scientifically used to confirm what was theoretically hypothesized and is defined as evolved gas analysis (EGA).
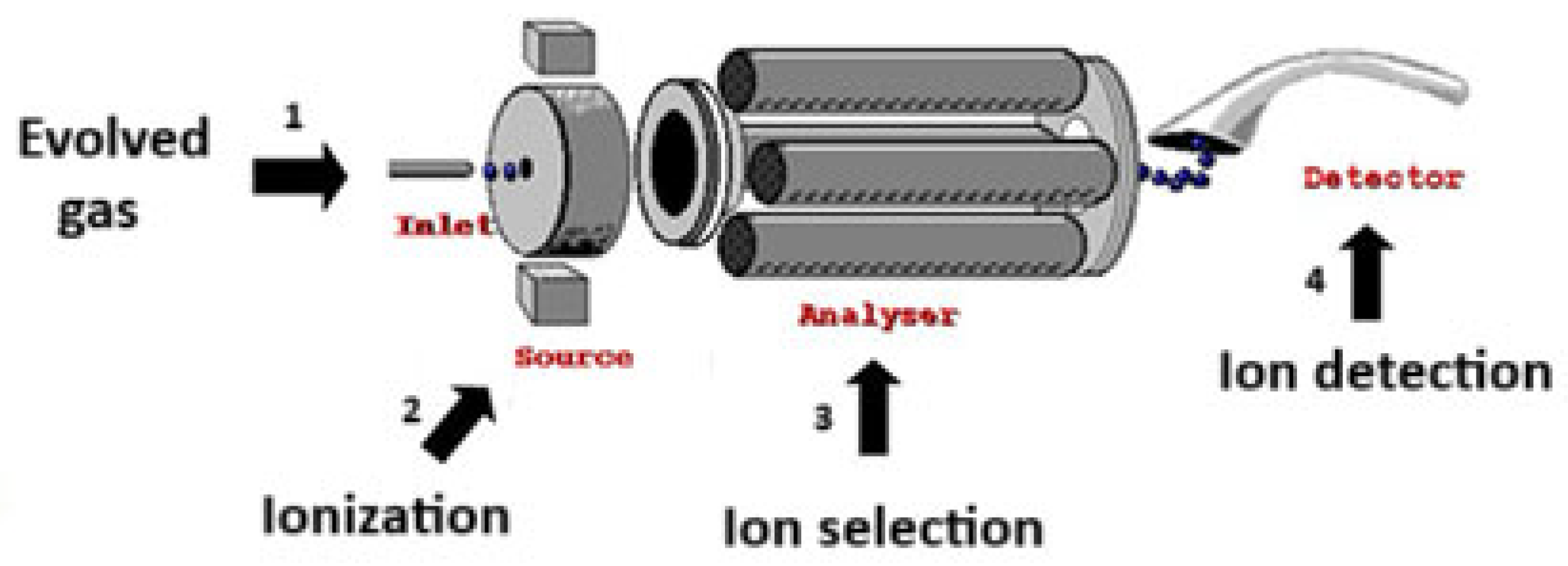
Based on the setting of the thermally induced conditions, different gaseous compounds are released, showing typical fragments derived from partial reaction mechanisms. Figure 3 shows the MS analysis of the evolved gas.
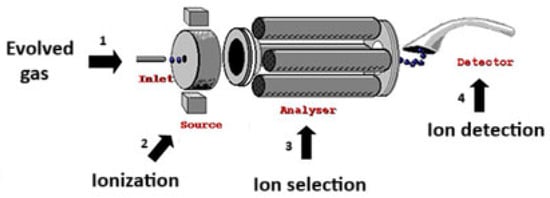
Figure 3.
Evolved Gas Analysis by MS.
A possible limitation in assigning small molecule fragments arises during decomposition steps. For example, m/z = 28 could correspond to N2, CO, or CH2=CH2. This analytical limitation of mass spectrometry can be addressed by changing the thermobalance or pyrolyzer purging flow from inert to oxidizing conditions, which enables confirmation of the thermally induced process.
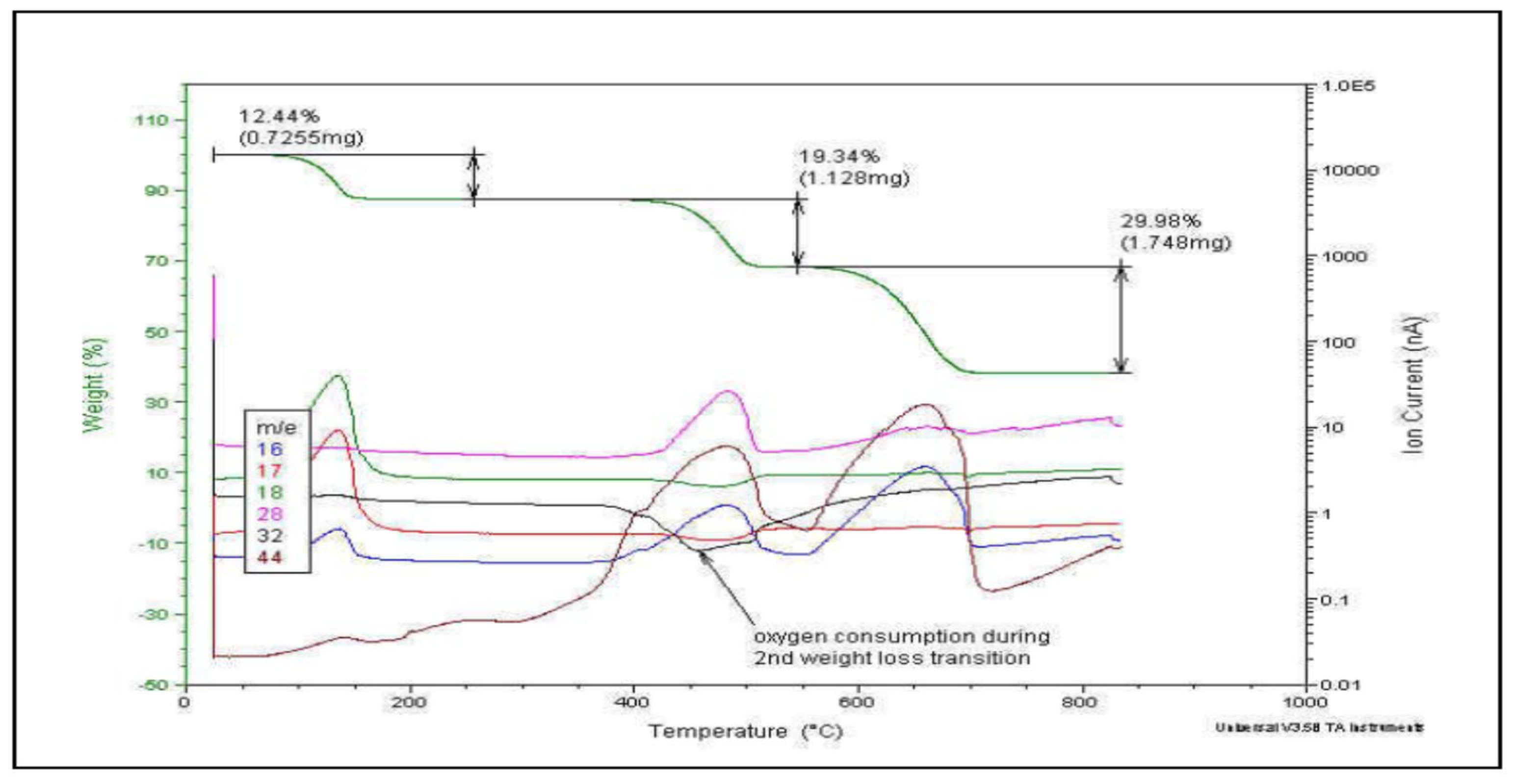
Thermogravimetry, although more expensive, is often preferred over pyrolysis due to the additional information it provides regarding the quantification of each induced process and its characteristic temperature. Figure 4 shows a typical example.
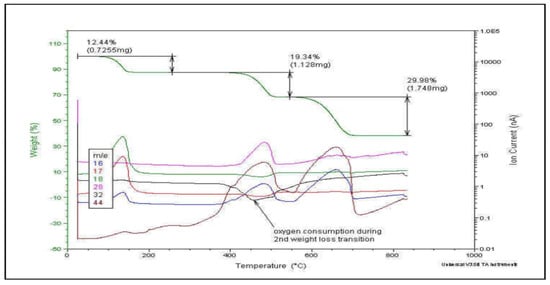
Figure 4.
Evolved Gas Analysis by TGA-MS: m/z characteristic pattern for each thermally induced step.
The analysis of gases evolved during programmed heating using MS or GC/MS is scientifically defined as “On-Line Thermally Induced Evolved Gas Analysis by Mass Spectrometry (OLTI-EGA-(GC)MS)”.
The potential of this methodology for analyzing the thermally generated gas phase lies in the synergy of the characteristics of each individual technique when coupled together. Mass spectrometry characterizes molecules based on their mass-to-charge ratio (m/z), and the combination of various generated fragments allows the nature of the incoming gas to be uniquely identified. The spectrometer can also be preceded by a gas chromatograph, which separates complex gas mixtures to enhance MS characterization and provide additional information, such as the purity of the sample under examination.
To highlight the analytical potential of OLTI-EGA-(GC)MS, some of the most recent scientific publications have been selected and are briefly discussed below. This selection does not claim to be exhaustive, given the high and ever-increasing number of articles published in the past five years. However, it aims to demonstrate the wide range of possibilities for using the results of this analytical technique, which enhances MS and GC/MS as fundamental methods of investigation.
2. Applications to Polymer Characterization
Polymers are the ideal matrix for coupling gas chromatography and mass spectrometry, as this combination provides the best analytical responses. Thermogravimetric analysis (TGA) and pyrolysis (Py) allow for the analysis of polymer behavior as a function of temperature and purging flow, sending the evolved gases to GC-MS for separation and unambiguous characterization. A growing number of articles and reviews report on the applications of evolved gas analysis using MS or GC/MS, with useful reference publications suggested [1,2,3,4,5,6].
Historically, the coupling of GC and MS has been applied to the study of combustion or pyrolysis products in polymers, related additives, and resins. The main advantages of this approach include eliminating preparation steps, limiting solvent interference, and determining the contribution of small particles. Additionally, hyphenated EGA-GC/MS enabled the characterization of the most common polymeric materials.
The innovation in recent applications, compared to the classic approach, stems from the development of “fast” pyrolysis, which has made it possible to study the performance of specific catalysts. For example, Zhang et al. used the pyrolysis of polyolefins to hypothesize a behavioral model and mechanism related to the formation of products in plastic waste. This model could serve as a reference in reactor design and parameter definition [7]. Luo et al. studied polyethylene fast pyrolysis to determine the catalytic effects. The coupling of mass spectrometry with thermogravimetry (TG/MS) and pyrolysis (Py-GC/MS) revealed the different products produced via catalyzed thermal treatment [8].
Fast and catalytic pyrolysis of polyethylene (HDPE) was reported by Yao et al., who studied product distribution and temperature dependence using Py-GC/MS [9]. Meanwhile, Peng et al. proposed a new approach to recycle PVC [10]. Ylitervo and Richards utilized the coupling of GC and MS to characterize the main reactions and correlate the gaseous emissions following the fast pyrolysis of various polymers, including LDPE, PS, and PP [11].
Coralli et al. proposed several studies on polymers, including siloxane copolymers used as cosmetic ingredients in sewage sludge and personal care products [12], poly(butylene adipate-co-terephthalate) in the field of bioplastics [13], and the potential of Py-GC-MS as a screening tool to determine poly(ethylene imine), which is used in several applications, such as CO2 adsorbents [14]. Polyimide, a high-performance polymer, has been studied for its thermal and fire behavior characteristics by Ramgobin et al., who highlighted from the results of evolved gas analysis using GC/MS that stability is linearly dependent on oxygen concentration [15].
The possibility of recycling plastic packaging waste through catalysts has been studied by Pal et al. with the aim of producing monoaromatic hydrocarbons [16]. Yang et al. reported the PVC and PE dichlorination process, followed by pyrolysis, which was monitored using the GC/MS coupled technique to optimize the parameters and obtain carbon nanotubes [17]. Polymer blends were created to simulate waste electronics and test the effects of several catalysts used in the pyrolytic process [18]. Coatings of polyamide on carbon nanotubes were characterized via thermal analysis [19].
To optimize the conversion of precursors into polyacrylonitrile-based carbon fibers, GC/MS evolved gas analysis was used to monitor the effects of cobalt and iron addition on temperature and peroxidation reactions, leading to pyrolysis products [20].
The ability to realistically predict polymer aging is of great importance. Wang et al. proposed a simulation of polylactic acid and polyethylene modifications in the natural environment to assess potential risks [21].
Polyester coatings on aluminum pose a problem in recycling processes, not only due to potential environmental contamination, but also because of their impact on the quality of aluminum recycling. The pyrolysis of waste aluminum-coated cans was studied by Yu et al. to determine the mechanism and reaction products [22].
3. Applications to Biomass Characterization
Catalytic pyrolysis experiments can be effectively used to specifically drive the production of species of interest or to monitor biomass heating processes. Fundamental information is obtained through the coupled GC/MS characterization of the gases evolved during pyrolysis, often in combination with other evolved gas analysis techniques.
Volli et al. combined data from both spectroscopy and mass spectrometry in a study of lignocellulosic biomass, characterized by TGA-FTIR and Py-GC/MS [23]. Biomass can become problematic when large amounts of invasive species are generated. Fernandez et al. studied the potential of these wastes to be converted into fuels and chemicals [24].
Fuel production by microorganisms presents a significant challenge in the context of a circular bioeconomy. Lin et al. tested Actinobacillus succinogenes with pyrolysis, combustion, and torrefaction to explore the potential conversion of this biomass into a renewable fuel [25]. In a similar vein, Liu et al. studied the combustion of litchi peels and the related emissions in the presence of different catalysts to optimize the byproducts obtained through pyrolysis [26], while banana peel was pyrolyzed to gather information on its bioenergy potential [27]. Biomass-to-biodiesel conversion was explored by Barati et al. and Xu et al. for microalgae [28,29], by Yuan et al. for clay oil [30], and by Uzoejinwa et al. for the co-pyrolysis of rice husk and seaweeds [31]. Xu et al. also reported the pyrolytic behavior of pine needles [32].
Fast pyrolysis coupled with gas chromatography–mass spectrometry (Py-GC/MS) was used to produce and confirm the formation of 2,3-dihydrobenzofuran as the major compound from Dendrocalamus asper biomass, a high-value chemical compound for the pharmaceutical industry [33]. The effect of acid pretreatment was shown to be crucial when obtaining valuable chemicals through biomass pyrolysis [34,35].
Product yields from common biomass waste materials, such as wheat straw, tobacco, or furniture, have been evaluated to chemically characterize the evolved products from the pyrolysis process. Gas chromatography coupled with mass spectrometry (GC-MS) has proven to be a useful monitoring technique for this purpose [36,37,38]. Biomass can provide a clean and efficient conversion of low-rank coal, and the volatile compounds can be easily studied using TG-FTIR and Py-GC/MS analysis to monitor the process [39]. The pyrolysis of coffee biomass has demonstrated its potential for pulp production [40] and can be efficiently converted into bio-oil and bio-gas when monitored via GC/MS analysis of the pyrolysis reactions [41].
Composting is an environmentally friendly recycling method for residues from olive oil production. Information derived from TGA-GC/MS or Py-GC/MS analysis of process residues allowed for the characterization of the organic fraction. It has been shown to be fundamental for specifying stability and maturity, as well as evaluating the potential for use as a soil amendment, as reported by Rueda et al. [42]. The pyrolysis of lobster shells was studied by Ding et al. to produce bio-oil [43].
Magdziarz et al. [44] and Sun et al. [45] investigated the co-pyrolysis of wasted cellulosic biomass to determine the optimal conditions for renewable fuel and energy production, a crucial issue when considering environmental sustainability and economic impact. Patel et al. determined the composition of biodiesel derived from eucalyptus biomass [46], while Jiang et al. reported the main bio-oil composition when tobacco underwent pyrolysis [47].
The coupling of GC and MS with thermal analysis or pyrolysis is the fundamental methodology to assess the effective action of new catalysts. Biomass-catalyzed reactions were studied by Sun et al. [48], Chen et al. [49,50], Zhou et al. [51], and Ma et al. [52,53] to produce aromatics; by Li et al. to produce Levoglucosenone [54] and aromatics [55]; and by many others to produce bio-oil [56,57], including from sugarcane molasses [58], bagasse [59,60,61], and marine biomass [62].
Mariyam reviewed publications aimed at improving the yield of volatiles generated from feedstocks and municipal waste materials [63]. Xia focused on a review of product yield and composition of biomass [64]. A State-of-the-Art review by Amenaghawon et al. gathered recent publications on biomass pyrolysis conversion to biofuels [65].
4. Applications to Coals Characterization
Coal pyrolysis is one of the primary methods for producing aromatic hydrocarbons, such as xylene, toluene, and benzene. The ability to track the conversion process and the characteristic products using mass spectrometry (MS) or gas chromatography–mass spectrometry (GC-MS) allows for process optimization.
In this context, Zhang et al. described the distribution of products resulting from the pyrolysis of aromatic model compounds in coal over calcium hydroxide [66]. In the realm of hydrogen production, the low-temperature coal tar reforming process is a key environmental and energy goal, as reported by Deng et al. in 2024 [67]. Yang et al. proposed a method for improving the quality and yield of tar through the rapid catalytic co-pyrolysis of coal [68].
Fast pyrolysis of Chinese alkaline coal was studied by Wei et al. [69], while Guo et al. studied the extraction of light components from long-flame coal to reduce moisture content [70]. He et al. pretreated coal residue via swelling, which altered the product distribution [71].
Lv et al. jointly used 13C CP/MAS NMR and GC/MS evolved gas analysis to study the carbon structures and the resulting behavior of various coals during pyrolysis treatment [72,73]. The effect of alkali metals on coal pyrolysis mechanisms was investigated in a Py-GC/MS study by Wei et al. [74], while Wu et al. reported on the influence of cobalt and magnesium on molecular sieves [75]. Wang et al. monitored the evolution of phenols from coal using GC/MS evolved gas analysis [76].
The ability to fully characterize coal is enabled by the synergistic analysis of different techniques. In this regard, Yu et al. proposed a multiparametric approach to coal analysis by combining the results from two different mass spectrometers coupled with thermogravimetry and pyrolysis [77]. Additionally, He et al. suggested a combination of fast Py-GC/MS, TG-FTIR, and rapid infrared heating to define the properties and composition of several types of coal [78].
5. Fire Simulations and Flame Retardant Characterization
Thermal decomposition mechanisms are crucial for understanding material behavior in the event of a fire. Combustion behaviors are fundamental for determining the safety of materials and testing the flame-retardant potential of additives. Mass spectrometry (MS) and gas chromatography–mass spectrometry (GC/MS) are valuable detection methods for characterizing evolved gases during these processes.
Polyether ether ketone (PEEK) is a high-performance polymer widely used in industrial applications. To assess its fire behavior, several methods can be employed, including thermally induced decomposition via Py-GC/MS. Numerous studies have shown that the initial decomposition step releases non-combustible gases and forms graphite-like structures [79].
Feng et al. investigated the effects of silsesquioxanes as flame retardants when added to aluminum diethylphosphonate (a phosphorus-based flame retardant) in polyamide 6. This combination aimed to minimize the significant smoke and heat released during combustion [80].
A high phosphorus-doped polyphosphonate (DOPO) was synthesized and tested for flame retardancy with PET materials [81]. Yang et al. [82] and Fang et al. [83] reported the evolved gas analysis characterization of a flame retardant synthesized from DOPO and phytic acid or PET. Li et al. studied the reduction in flammability due to a DOPO-based imidazolone derivative [84]. The GC/MS results confirmed the flame-retardant mechanism of the bio-based intumescent product. Wang et al. synthesized a bifunctional flame retardant and tested its effectiveness on polyurethane foam. GC/MS was used to clarify the retardant mechanism [85].
Thermogravimetric analysis (TGA) and pyrolysis were employed to study the flame-retardant behavior of a new halogen-free polystyrene composite, and the results helped to determine the associated mechanism [86].
Liu et al. proposed a greener strategy to improve flame retardancy by eliminating gases and smoke through a cross-linking reaction, with emissions monitored via GC/MS evolved gas analysis [87]. Wang et al. reported an enhancement in the temperature resistance and flammability of nanocomposites with a coating of dimethyl-methyl-phosphate [88]. A study by Liu et al. explored the creation of new materials with strength in all dimensions. For this purpose, oxidized cellulose fibers were cross-linked with metal ions and alginate polysaccharides to create a three-dimensional structure. GC/MS analysis showed that carbon dioxide emerged as the primary product, suggesting that the composite wood possesses flame-retardant properties and could be used in various applications [89].
6. Applications to Cellulose and Lignin Characterization
Packaging, coatings, and composites are increasingly based on cellulose as a sustainable alternative to fossil-based polymers. However, lignin-containing nanocellulose is characterized by complex structures that significantly influence its properties.
Non-bleached softwood or hardwood kraft pulps have been used to obtain microfibrillated cellulose. GC/MS characterization of the evolved gases during pyrolysis under thermally induced stress has been used to identify the distinct features of lignin structures [90]. Papers subjected to both natural and artificial aging were analyzed to determine cellulose degradation products using GC/MS evolved gas analysis. The results showed that the difference between natural and artificial aging can be monitored by the cleavage of β(1–4)-glycosidic bonds [91].
Yu et al. investigated the potential of cotton cellulose as a fuel and innovative chemical. Their study demonstrated that activated cotton waste results in the reduced complexity of the derived bio-oil [92].
Lignocellulosic biomass presents a sustainable process due to its high-value lignin byproduct. The chemistry of lignin plays a critical role in cellulose extraction, as studied by Tang et al. [93] and Ma et al. [94]. The properties of lignin in black liquor from sugarcane leaves were examined by Nakason et al. with the assistance of GC/MS evolved gas analysis [95].
The catalytic pyrolysis of lignin to obtain aromatic hydrocarbons was studied by Zheng et al. [96] and Sun et al. [97].
Catalyzed cellulose reactions have been studied by Li et al. to produce chiral chemicals [98], and Levoglucosenone [99],by Ma et al. to produce alkylphenols [100], and by several other authors to produce bio-oil [101,102]. Liu et al. focused on the production of hydrocarbons [103,104].
When treating cellulose biomass, the characteristics of tar are closely related to the cellulose and lignin content. Jia et al. used GC/MS coupling to determine the mechanism and distinguish between primary and secondary products. Their study proposed a tar production index to evaluate the potential origins of these products [105].
Py-GC-MS and spectroscopic data have often been correlated, especially with NIR spectra. Gordobil et al. applied principal component analysis (based on evolved gas analysis (EGA) via mass spectrometry and NIR results) as a tool to enhance the understanding of different behaviors when lignin is dried at 25 °C (room temperature) or 55 °C (in an oven). Spectroscopic correlations revealed how H2O molecules influence the properties of kraft lignin and its pyrolytic results [106]. Mei et al. characterized the evolution of functional groups in lignin using two-dimensional correlation infrared spectroscopy (2D-PCIS) during low-temperature deoxidation [107]. Similar spectroscopic approaches have been proposed to jointly solve analytical challenges [108,109,110,111]. A chemometric modeling of GC/MS data after wood pyrolysis demonstrated that drought stress is closely related to lignin content (up to 4–5% higher) and can serve as a quantitative molecular marker of climate change [112].
Chen et al. reviewed publications that highlight the usefulness of coupled Py-GC/MS to characterize both non-catalytic and catalytic pyrolysis, as well as the co-pyrolysis of lignocellulosic and algal biomass [113].
7. Characterization of Microplastics, Nanoplastics, and Waste Plastics
Microplastic contamination has evolved from an emerging issue to a widespread environmental concern, with increasing evidence of its presence in both the environment and living organisms.
Poly(styrene-d5) and poly(4-fluorostyrene) have been proposed as internal standards for microplastic quantification using thermoanalytical methods [114]. Kwon et al. (2025) reported the presence of microplastics in edible salts, including sea salts (even deep-sea salts), rock salts, and lake salts. Using thermally induced on-line evolved gas analysis via GC/MS, they were able to characterize and quantify the various forms of microplastic contamination, which was further confirmed by comparing similarly shaped microplastic particles [115]. Biale et al. also determined microplastic contamination in mussels, detecting 40 different contaminants in their analysis [116].
End-of-life plastics often become waste when not recycled. The presence of specifically doped plastics may pose significant contamination risks. Kumagai et al. applied various analytical techniques to analyze sulfur-based plastics, examining their thermal-oxidative degradation behavior using thermogravimetric analysis and pyrolysis. This allowed them to determine decomposition temperatures and quantify the different stages of degradation. GC/MS coupling confirmed the evolved substances, directly correlating them with thermally induced oxidation steps [117]. Similarly, experiments on low-density and high-density polymers under UV irradiation were conducted to determine the decomposition mechanisms of microplastics [118]. Airborne microplastics in atmospheric deposits were also studied by Jarosz et al. [119]. Additionally, critical evaluations of microplastic analysis methodologies, focusing on cost optimization, were published by Pipkin et al. [120] and Primpke et al. [121]. Matsui et al. developed a standardized method for the qualitative analysis of various synthetic polymers, which was automated and implemented in a procedure for data processing from a Py-GC/MS analysis of plastic mixtures. This method allowed for the collection of both qualitative and quantitative data on microplastic mixtures by selecting a set of pyrolysis products characteristic of different polymers [122]. Li et al. examined the influence of microplastic mass, size, and source based on GC/MS evolved gas analytical data to optimize pyrolysis efficiency and product distribution [123].
Biale et al. aged microplastics in a solar box and used GC/MS evolved gas analysis to determine the degradation steps [124].
Microplastics have gained attention due to their potential impact on human health. The presence of microplastics in urban water systems was determined via Py-GC/MS, with Amsterdam serving as a case study [125,126]. Yu et al. studied blood samples from patients with extracranial artery stenosis and found higher concentrations of microplastics compared to control samples from healthy donors [127]. Microplastics were also detected in the arteries of the analyzed subjects by Liu et al. [128]. Tian et al. demonstrated the presence of microplastics in amniotic fluid, although no correlation with adverse pregnancy outcomes was found [129]. A quali-quantitative analysis of microplastics in testis, semen, and prostate samples from mice was proposed by Yang et al. [130], while Zhao studied similar contamination in humans [131]. Garcia et al. detected microplastics in placental specimens [132]. He and Zhang examined the presence of microplastics in endometrial polyps in women of a childbearing age, exploring potential correlations with gynecological pathologies [133]. Guo et al. characterized five polymer types in human bone marrow [134]. Song et al. found a significant difference in microplastic and nanoparticle content between urine samples from urban and rural residents [135].
Microwave procedures to prepare samples for microplastic contamination analysis were proposed by various authors, including Andersone [136], La Nasa [137], Hermabessiere/Rochman [138] (2021), and Liu [139] (2024). Lou et al. focused on evaluating the interferences affecting accuracy in the evolved gas analysis from pyrolytic reactions [140]. Jiang et al. determined the limit of quantification for four different microplastics [141].
Detecting plastic nanoparticles in the environment remains a challenge, primarily due to the lack of sensitive methods for determining their minimum concentrations. Hildebrandt et al. studied anti-corrosion coatings to detect the potential release of coating particles into the environment. By optimizing a Py-GC/MS approach, supported by ICP-MS/MS analysis and multivariate statistics, they were able to characterize the multi-elemental and organic additive fingerprints of paint particles [142]. Li et al. developed an efficient method to increase the nanoparticle concentration in samples [143]. Le Juge et al. identified nanoplastics from polystyrene in complex environmental matrices [144]. A year-long study on a wastewater treatment plant demonstrated plastic contamination using a combination of infrared and Py-GC/MS techniques [145].
Nanoplastics are emerging contaminants even in food products. Their size and concentration need to be monitored due to potential health risks. Li et al. proposed an analytical platform dedicated to nanoplastic monitoring via Py-GC/MS [146]. Zhang et al. published a study on plastic pollution in marine ecosystems, focusing on the quantification of contamination in saltmarsh sediments at the ocean–land interface [147].
The determination of trace nanoplastics in water, a growing problem in ecosystems, requires very sensitive techniques and accurate sample preparation. Zhang et al. used Py-GC/MS to detect nanoplastics in the 20–100 μg/L range [148], while Xu developed a method to determine microplastics in wastewater treatment plants [149,150,151]. Ye et al. reported nanoplastic contamination in edible crop parts, using GC/MS coupling to a pyrolyzer for analysis [152].
Several reviews have been published in this field. La Nasa (2020) [153], Ahmed (2021) [154], Wang (2023) [155], Liu et al. [156], Moteallemi [157], and Zheng (2024) [158] have provided comprehensive reviews on the sampling methods, analytical techniques, and exposure risks associated with microplastics, with a focus on GC/MS coupled to pyrolysis and thermogravimetric analysis. Nanoplastic analysis has also been reviewed by Cai et al. [159].
8. Applications in Cultural Heritage
Frauds and artifacts in cultural heritage are common, and analytical methods for their detection have seen significant advancements over time. Mass spectrometry (MS), particularly when coupled with gas chromatography (GC), has become a powerful tool in solving the authentication challenges of cultural objects.
Amber, a resin historically used in pergamens and ancient documents, serves as an important reference for identifying items from different eras. In particular, amber from China provides valuable insights into ancient trade routes and cultural exchanges. Li et al. successfully applied Py-GC/MS, showing that specific compounds could act as markers for confirming the authenticity of these objects [160]. Yao et al. demonstrated that GC-MS analysis of evolved gases from paper relics can distinguish between different types of ancient papers, allowing for the identification of historical treatments (such as waxing or cementing), which is crucial for conservation and restoration efforts [161,162]. Na et al. further used this approach to differentiate between ancient original waxes and modern restoration waxes [163].
In restoration projects, characterizing the original chemical composition of ancient materials is essential. Py-GC/MS has proven particularly useful for this purpose. For example, Pozzi et al. conducted a surface analysis of a famous museum collection, determining the presence of inorganic pigments to guide the cleaning of specific colored areas [164]. This technique has also been applied to the study of graffiti and urban artworks, where EGA-MS and Py-GC/MS were used to analyze binders, plasticizers, organic pigments, and other components. These methods helped to understand the modifications these materials undergo over time and how they age [165].
GC/MS evolved gas analysis via pyrolysis has been employed to study the aging effects of aqueous emulsions of acrylic copolymers and waxes. The analysis revealed that a decrease in wettability in aged paints may be attributed to phase separation between the acrylates and waxes [166]. In paper dating, GC/MS coupled with near-infrared spectroscopy has also been explored as a method to analyze and date historical papers [167]. Additionally, four historical leather bookbindings were characterized using thermogravimetry and pyrolysis coupled with MS to identify specific aging markers and better understand the effects of time on the materials used in bookbinding [168].
In the study of ancient coatings, GC/MS pyrolysis was used to identify the organic media in overpainted areas of historical plaster surfaces. A diterpenic resin was confirmed as the surface coating, containing both silicon and aluminum [169]. Pozzi et al. also studied three paintings by Vincent van Gogh, examining surface alterations using a range of techniques, including pyrolysis GC-MS. Their findings confirmed that the brushwork and pigment characteristics were consistent with other works by Van Gogh, aligning with descriptions found in the artist's letters [170].
Manfredda et al. reported on the characterization of painting materials that had undergone cleaning with hydrogels. Polyvinyl alcohol-based gels proved to be effective and safe for cleaning water-sensitive painted surfaces, such as ancient Egyptian artifacts [171]. In ancient mural paintings, egg whites were used as cementing agents. Py-GC/MS analysis showed that this technique is reliable for identifying and confirming the true composition of ancient coatings [172]. GC/MS coupling continues to be an invaluable tool for the study of paints in cultural heritage research [173,174].
For the preservation of 20th-century industrial heritage, especially items related to the Second World War, such as aircraft aluminum alloys and their protective coatings, it is crucial to identify the organic binders in paints and primers. Montanè et al. utilized Py-GC/MS to characterize the original protective coatings on these materials, providing key insights for their conservation [175].
9. Analysis of Polluttants
Py-GC/MS has been used to evaluate the impact of two different cyanotoxins on mussels, both individually and in combination. The pyrolysis of mussels resulted in over a hundred distinct peaks in the chromatograms. By applying chemometric analysis, the complex data successfully discriminated between mussels exposed to cyanotoxins and those unexposed, highlighting the effectiveness of this method in environmental monitoring [176].
Gautam et al. simulated the decomposition of mosquitoes via thermogravimetry to collect mosquito residues and described the GC/MS analysis of the vapors released from dead insects. This analysis helped to characterize the chemical composition of the decomposition process [177].
In a 2022 study, Steinmetz et al. investigated the potential soil pollution caused by plastic covers used in agriculture to enhance crop yields and quality. GC/MS analysis of evolved gases during pyrolysis revealed the presence of polyethylene, polypropylene, and polystyrene in the soil of several fields, confirming the contamination of soil by microplastics from agricultural plastic debris [178].
The potential of biochar to remove metal contamination from water was explored by De Andrade et al. They demonstrated that GC/MS evolved gas analysis after pyrolysis could effectively determine the adsorption capacity of biochar, providing a valuable tool for assessing biochar's environmental remediation capabilities [179].
Environmental pollution from tire particulates, particularly PM 2.5, poses a significant health risk. Chae et al. monitored exposure to tire-generated particulate matter at bus stops [180] and in indoor parking garages [181], aiming to assess the associated health risks. GC/MS evolved gas analysis was employed to determine the particulate composition, confirming the tire-derived contribution to the pollution.
In foundries, evolved gas analysis using GC/MS has been a fundamental tool to assess the health risks associated with the release of toxic gases during metal casting. However, proposed solutions to mitigate environmental and worker exposure are often not proportionate to the cost and quality of the casting. Zymankowska-Kumon et al. investigated the relationship between the casting process conditions, atmosphere, and the price/quality ratio. Their study used GC/MS evolved gas analysis to track resin decomposition processes under different atmospheric conditions, providing insights into optimizing the casting environment for both safety and cost-effectiveness [182].
10. Applications for Food Characterization
On-line coupled mass spectrometry for evolved gas analysis (EGA-MS) and Py-GC/MS were applied to determine the specific composition of both volatile and non-volatile thermally induced products from six spices. This approach was proposed as an alternative to the traditional solid-phase microextraction methods commonly used in similar analyses [183].
Soy sauce residues can be effectively converted into aromatic hydrocarbons using a catalytic process, as demonstrated by Li et al. [184].
Cylindrospermopsin, a toxin found in raw fish, poses a significant risk to both the environment and human health. Prieto et al. proposed a method to determine the presence of this toxin via GC-MS after a simple pyrolysis ramp, offering a straightforward and useful analytical technique for its detection [185].
Milk quality was evaluated using a multi-component analytical platform, integrating GC/MS and near-infrared spectroscopy, providing a comprehensive assessment of its composition and quality [186].
The co-combustion of household waste and sewage sludge has emerged as a promising method for sustainable waste management. Ahmad et al. studied the combustion mechanisms and distribution of products, highlighting the positive effects of co-combustion on reducing pollutants and improving waste-to-energy conversion [187]. In a similar context, kitchen waste, viewed as a valuable raw material, can be easily converted into bioenergy and chemicals of biological origin, as shown in Zhao et al.’s study [188].
GC/MS studies on the pyrolysis of peanut husks by Silveira Jr. et al. [189] and date pit waste biomass by Hamid et al. [190] revealed that food waste can be efficiently utilized to produce levoglucosan.
Tahir et al. explored the potential of mango peel as a source of phenols and bio-based chemicals, in addition to its use for bioenergy production [191].
11. Forensic Analysis
Biological matrix analysis plays a crucial role in forensic investigations, and new approaches are continually being proposed by scientific research.
Condoms are often implicated in sexual assault cases, with vaginal swabs being the primary method of analysis. While the characterization of silicone-based condom lubricants has been widely studied, Hermelin et al. proposed an innovative approach to analyze condom residues within the vaginal matrix. Their study utilized GC/MS characterization after an initial pyrolysis process, which provided additional insights into the composition of the vaginal matrix [192]. Similarly, Burnier et al. [193,194,195] and Chen et al. [196] employed Py-GC/MS to characterize various condoms and lubricants available on the international market, with the aim of identifying key characteristics useful for forensic investigations.
In cases of ship collisions, proving the incident can be challenging, especially when visible damage is minimal or inconsistent. However, the responsible collision can often be identified through a comparative characterization of residual paint. Py-GC/MS serves as an effective analytical tool for distinguishing similar paints, identifying key markers such as styrene and phthalic anhydride, which can serve as evidence of the collision [197]. Additionally, a chemometric and thermal analysis coupled approach was proposed to estimate the time since death by characterizing the vitreous humor [198].
12. Thermal Behavior of Waste
One of the major global challenges is waste production, as a significant portion is not recycled but instead incinerated, leading to pollution and harmful residual byproducts. Mass spectrometry (MS) and gas chromatography (GC) are essential techniques for tracking the fate of waste, as they can help predict the impact of improper disposal and the associated environmental risks. Thermogravimetry and pyrolysis, when coupled with GC and MS, are invaluable tools in these studies.
Xu et al. investigated the co-hydrothermal carbonization of cotton waste and PVC waste to produce solid fuel [199]. Similarly, Liang et al. studied the behavior of PLA and tobacco biomass [200], while Wu et al. focused on the pyrolysis of PLA and plastic blends [201]. GC and MS techniques are indispensable for studying co-pyrolysis behavior, especially when waste materials are involved. Several studies have been published involving different types of waste materials. For example, Guo et al. co-pyrolyzed agricultural and plastic wastes [202], while others examined the co-pyrolysis of wheat or corn straw with plastics [203,204]. Nandakumar et al. tested modified nanoparticles on waste plastics and biomass, demonstrating catalytic potential for producing α-olefins and aromatics from the co-pyrolysis of wheat straw and end-of-life HDPE waste [205].
Wang et al. explored the co-pyrolysis of waste tires and maize stalks [206,207]. Other research demonstrated the possibility of generating high-value chemicals from used car seat leather and PVC [208], as well as quality oils from the pyrolysis of plastic waste and vineyard residues [209]. Chen et al. investigated the pyrolysis of waste wind turbine blades and found that an appropriate pyrolysis process can prevent the formation of secondary products [210].
Waste tires are a growing environmental and industrial problem, with significant hazards that remain largely unaddressed. One often-overlooked issue is the dispersion of tire particles into aquatic environments through road drainage. GC/MS analysis has been used to quantify the impact of tire particle release, with benzothiazole selected by Parker-Jurd et al. as a molecular marker [211]. Studies also investigated the formation of BTX compounds when waste tires are pyrolyzed [212]. Multifunctional liquids can be produced from tire pyrolysis, with their characterization mainly conducted using GC/MS. Chavez-Delgado et al. proposed micro-pyrolysis (micro-Py-GC-MS) to identify thermal degradation products under specific conditions [213]. Additionally, waste tires have been converted into value-added chemicals via pyrolysis processes, monitored using GC/MS to determine the optimal catalytic systems [214,215]. Tang et al. studied bicycle waste tires, primarily made of polyurethane, and proposed a pyrolysis process for cleaner energy production [216].
Multi-waste biomass is gaining attention as a promising renewable and sustainable energy source. Studies have focused on bio-oil and biofuel yields from thermal degradation, with volatile molecules quantified using GC/MS analysis [217].
The issue of waste printed circuit boards (PCBs) has persisted since the last century, and research continues to seek effective solutions. Krishna et al. proposed a kinetic study using GC/MS evolved gas analysis to determine optimal pyrolysis temperatures [218], while Li et al. modeled the influence of copper on shortening pyrolysis reaction times [219].
Medical waste presents another significant challenge due to its need for proper recycling. Liu et al. studied the catalyzed conversion of medical waste and the related evolved gases after thermal treatment using GC/MS [220], while Qu et al. analyzed the pyrolysis of medical gloves [221]. Studies have also focused on the pyrolysis of medical infusion tubes and masks to assess the products generated when used for energy production [222].
13. Other Applications
Ranalli et al. developed a bio-removal protocol for tattoos, addressing the increasing demand for esthetic or personal reasons. Their work profiles both the main chemical constituents and other ambiguous compounds involved in tattoo removal [223].
Wu et al. conducted research on a submerged fermentation process that produced a novel, water-insoluble melanin isolated from a Gram-negative bacterium. This melanin was characterized using several techniques, including pyrolysis-GC/MS (Py-GC/MS) [224].
The use of lignocellulosic materials, such as walnut and hazelnut shells, in cosmetics has risen due to their natural active ingredients that benefit skin health. Gordobil et al. studied the structural characteristics and functional properties of these materials through various analytical techniques, including GC/MS, to characterize the thermally induced products [225].
The growing interest in deep eutectic solvents (DESs) has spurred a deeper understanding of their characteristics and functions. Zhou et al. investigated the condensed products generated during DES pretreatment, demonstrating the potential to significantly reduce the formation of undesirable compounds [226]. Additionally, Ji et al. explored the effect of a DES green solvent pretreatment in the extraction of lignin from lignocellulose, achieving notable yields and high purity [227].
Ionic liquids are of great scientific and industrial interest, especially azole-based heterocyclic systems, which are potentially energetic due to their high nitrogen content and positive heat of formation. These systems can be effectively studied using Py-GC-MS [228]. Ma et al. applied a degumming method using a deep eutectic solvent on hemp fibers, followed by Py-GC/MS analysis to evaluate the purification effect [229]. Tan et al. and Zhu et al. investigated the influence of “natural-DES” on volatile molecules released from heated tobacco [230,231].
In the field of flame retardants, Ali et al. used GC/MS coupled with thermal analysis and pyrolysis to demonstrate that the characteristic flame-retardant activity in cotton fabrics is attributable to catalyst-generated carbon acting as a physical barrier [232]. Kim et al. proposed an alternative to the use of antimony trioxide, a suspected carcinogen, by suggesting zinc hydroxystannate as a safer, non-toxic flame retardant. Their study utilized TGA and Py-GC/MS to evaluate the fiber composition and flame resistance of several military uniforms [233,234].
Micheluz et al. addressed the issue of urethane degradation in the skin masks of humanoid robots. They used EGA-MS and TD/Py-GC/MS to characterize the degradation process [235].
Gasparini et al. introduced an innovative method for quantifying volatile molecules to validate eco-friendly formulations designed for the microencapsulation of perfumes [236].
14. Discussion, Conclusions, and Future Directions
GC, MS, and coupled GC/MS are analytical techniques universally recognized for their ability to provide both qualitative and quantitative characterizations of analytes. Known for their high sensitivity and reproducibility, these methods are the most powerful tools for analyzing complex real-world matrices.
The examples discussed in this review illustrate the essential role of GC/MS in characterizing evolved gases, particularly when thermal stresses are applied through techniques like thermal analysis, pyrolysis, or simple heating. On-line thermally induced evolved gas analysis (OLTI-EGA), coupled with GC/MS detection, helps to solve analytical challenges, confirm reaction mechanisms, and compare various pretreatment methods, catalysts, additives, and processes. This approach is particularly valuable when addressing real-world matrices and issues with significant social and environmental impacts.
As mentioned in the introduction, the selected examples highlight some of the most representative and innovative applications of GC/MS, ranging from advanced research studies to industrial applications and public interest issues. The final section of the review presents more specific applications, where the data obtained from GC/MS analyses either complement or replace information from other techniques, showcasing the versatility of GC/MS in addressing complex scientific inquiries and analytical challenges.
The goal of this review is to emphasize the potential of GC/MS methods in on-line evolved gas analysis and demonstrate how these techniques are often critical in solving emerging and current problems. The range of potential applications for on-line EGA is vast, and as analytical challenges evolve, these methods will continue to play an integral role in solving a growing array of issues, even in unconventional contexts.
Author Contributions
Conceptualization, R.R. and S.M.; methodology, R.R., S.M. and G.G.; software, R.R., S.M. and G.G.; validation, G.P., G.R., M.R., C.A. and E.P.; resources, G.P., G.R., M.R., C.A. and E.P.; data curation, R.R., S.M. and G.G.; writing—review and editing, R.R., S.M. and G.G.; supervision, R.R. and S.M.; project administration, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akoueson, F.; Chbib, C.; Monchy, S.; Paul-Pont, I.; Doyen, P.; Dehaut, A.; Duflos, G. Identification and Quantification of Plastic Additives Using Pyrolysis-GC/MS: A Review. Sci. Total Environ. 2021, 773, 145073. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; Barceló, D. Pyrolysis Gas Chromatography-Mass Spectrometry in Environmental Analysis: Focus on Organic Matter and Microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Seeley, M.E.; Wang, Q.; Bacosa, H.; Rosenheim, B.E.; Liu, Z. Environmental Petroleum Pollution Analysis Using Ramped Pyrolysis-Gas Chromatography–Mass Spectrometry. Org. Geochem. 2018, 124, 180–189. [Google Scholar] [CrossRef]
- Seeley, M.E.; Lynch, J.M. Previous Successes and Untapped Potential of Pyrolysis–GC/MS for the Analysis of Plastic Pollution. Anal. Bioanal. Chem. 2023, 415, 2873–2890. [Google Scholar] [CrossRef] [PubMed]
- Gnoffo, C.; Frache, A. Identification of Plastics in Mixtures and Blends through Pyrolysis-Gas Chromatography/Mass Spectrometry. Polymers 2024, 16, 71. [Google Scholar] [CrossRef]
- Harata, K.; Kitagawa, S.; Iiguni, Y.; Ohtani, H. Identification of Polymer Species in a Complex Mixture by Pyrolysis-Gas Chromatography-Atmospheric Pressure Chemical Ionization-High Resolution Time-of-Flight Mass Spectrometry as a Basis for Environmental Microplastic Analysis. J. Anal. Appl. Pyrolysis 2020, 148, 104828. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Wang, W.; Ji, G.; Zhao, M.; Li, A. Kinetics, Product Evolution, and Mechanism for the Pyrolysis of Typical Plastic Waste. ACS Sustain. Chem. Eng. 2022, 10, 91–103. [Google Scholar] [CrossRef]
- Luo, B.; Liu, H.; Shan, R.; Zhang, J.; Yuan, H.; Chen, Y. Enhanced Gas/Oil Production from Catalytic Fast Pyrolysis of Polyethylene over Cu/Ce Co-Modified ZSM-5. Waste Biomass Valorization 2024, 16, 1645–1657. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, J.; Li, S.; Ma, Y.; Yue, C. Analysis of Liquid Products and Mechanism of Thermal/Catalytic Pyrolysis of HDPE. J. Therm. Anal. Calorim. 2022, 147, 14257–14266. [Google Scholar] [CrossRef]
- Peng, Y.; Dai, L.; Dai, A.; Wu, Q.; Zou, R.; Liu, Y.; Ruan, R.; Wang, Y. Catalytic Process toward Green Recycling of Polyvinyl Chloride: A Study on Thermodynamic, Kinetic and Pyrolysis Characteristics. J. Anal. Appl. Pyrolysis 2022, 168, 105719. [Google Scholar] [CrossRef]
- Ylitervo, P.; Richards, T. Gaseous Products from Primary Reactions of Fast Plastic Pyrolysis. J. Anal. Appl. Pyrolysis 2021, 158, 105248. [Google Scholar] [CrossRef]
- Coralli, I.; Rombolà, A.G.; Torri, C.; Fabbri, D. Analytical Pyrolysis of Poly(Dimethylsiloxane) and Poly(Oxyethylene) Siloxane Copolymers. Application to the Analysis of Sewage Sludges. J. Anal. Appl. Pyrolysis 2021, 158, 105236. [Google Scholar] [CrossRef]
- Coralli, I.; Rombolà, A.G.; Fabbri, D. Analytical Pyrolysis of the Bioplastic PBAT Poly(Butylene Adipate-Co-Terephthalate). J. Anal. Appl. Pyrolysis 2024, 181, 106577. [Google Scholar] [CrossRef]
- Coralli, I.; Fabbri, D.; Facchin, A.; Torri, C.; Stevens, L.A.; Snape, C.E. Analytical Pyrolysis of Polyethyleneimines. J. Anal. Appl. Pyrolysis 2023, 169, 105838. [Google Scholar] [CrossRef]
- Ramgobin, A.; Fontaine, G.; Bourbigot, S. Investigation of the Thermal Stability and Fire Behavior of High Performance Polymer: A Case Study of Polyimide. Fire Saf. J. 2021, 120, 103060. [Google Scholar] [CrossRef]
- Pal, S.K.; Prabhudesai, V.S.; Vinu, R. Catalytic Upcycling of Post-Consumer Multilayered Plastic Packaging Wastes for the Selective Production of Monoaromatic Hydrocarbons. J. Environ. Manag. 2024, 351, 119630. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Lei, S.; Xiao, H.; Yang, H.; Chen, H. Coupling Dechlorination and Catalytic Pyrolysis to Produce Carbon Nanotubes from Mixed Polyvinyl Chloride and Polyethylene. Waste Manag. 2024, 178, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Vouvoudi, E.C.; Rousi, A.T.; Achilias, D.S. Effect of the Catalyst Type on Pyrolysis Products Distribution of Polymer Blends Simulating Plastics Contained in Waste Electric and Electronic Equipment. Sustain. Chem. Pharm. 2023, 34, 101145. [Google Scholar] [CrossRef]
- Celluzzi, A.; Paolini, A.; D’Oria, V.; Risoluti, R.; Materazzi, S.; Pezzullo, M.; Casciardi, S.; Sennato, S.; Bordi, F.; Masotti, A. Biophysical and Biological Contributions of Polyamine-Coated Carbon Nanotubes and Bidimensional Buckypapers in the Delivery of Mirnas to Human Cells. Int. J. Nanomed. 2017, 13, 1–18. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, X.; Xuan, D.; Lu, Z.; Luo, F.; Li, S.; Ye, Y.; Wang, D.; Miao, C.; Liu, Z.; et al. Insights into Pyrolysis Behavior of Polyacrylonitrile Precursors Using Py-GC/MS. Chem. Pap. 2021, 75, 5297–5311. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Jia, W.; Huang, K.; Ma, Y. Comparing the Aging Processes of PLA and PE: The Impact of UV Irradiation and Water. Processes 2024, 12, 635. [Google Scholar] [CrossRef]
- Yu, Z.-Q.; Hong, G.-D.; Zhao, W.; Liang, D.; Huang, Z.; Zhao, C.; Shan, R.; Yuan, H.-R.; Chen, Y. Investigation in the Pyrolysis of Polyester Coated on Aluminum-Based Beverage: Thermodynamic Properties, Product and Mechanism. J. Anal. Appl. Pyrolysis 2025, 185, 106878. [Google Scholar] [CrossRef]
- Volli, V.; Gollakota, A.R.K.; Shu, C.-M. Comparative Studies on Thermochemical Behavior and Kinetics of Lignocellulosic Biomass Residues Using TG-FTIR and Py-GC/MS. Sci. Total Environ. 2021, 792, 148392. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Amutio, M.; Artetxe, M.; Lopez, G.; Santamaria, L.; Lopez, J.E.; Olazar, M.; Saldarriaga, J.F. Exploring the Potential of Fast Pyrolysis of Invasive Biomass Species for the Production of Chemicals. J. Anal. Appl. Pyrolysis 2024, 183, 106817. [Google Scholar] [CrossRef]
- Lin, B.-J.; Chen, W.-H.; Lin, Y.-Y.; Chang, J.-S.; Farooq, A.; Singh, Y.; Ong, H.C.; Show, P.L. An Evaluation of Thermal Characteristics of Bacterium Actinobacillus Succinogenes for Energy Use and Circular Bioeconomy. Bioresour. Technol. 2020, 301, 122774. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Evrendilek, F.; Xie, W.; Kuo, J.; Buyukada, M. Bioenergy and Emission Characterizations of Catalytic Combustion and Pyrolysis of Litchi Peels via TG-FTIR-MS and Py-GC/MS. Renew. Energy 2020, 148, 1074–1093. [Google Scholar] [CrossRef]
- Chen, L.; Tu, Z.; Chen, Y.; Hu, J.; Wang, H. Bioenergy and Value-Added Chemicals of Banana Peel Waste (BPW): Deeper Insights from Thermal Kinetics, Thermodynamics, in-Situ Volatile Products Analysis, and Bio-Chars Application for Cd(II) Highly-Efficient Removal. Biomass Bioenergy 2024, 185, 107238. [Google Scholar] [CrossRef]
- Barati, B.; Zafar, F.F.; Qian, L.; Wang, S.; El-Fatah Abomohra, A. Bioenergy Characteristics of Microalgae under Elevated Carbon Dioxide. Fuel 2022, 321, 123958. [Google Scholar] [CrossRef]
- Xu, D.; Lin, J.; Ma, R.; Hou, J.; Sun, S.; Ma, N. Fast Pyrolysis of Algae Model Compounds for Bio-Oil: In-Depth Insights into the Volatile Interaction Mechanisms Based on DFT Calculations. Fuel 2023, 333, 126449. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Q.; Li, P.; Barati, B.; Viswanathan, K.; Zhao, S.; Wang, S.; Cao, B.; Hu, Y. Biofuel Characteristic of Waste Clay Oil Pyrolysis. J. Anal. Appl. Pyrolysis 2021, 156, 105117. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; Cao, B.; Wang, S.; Hu, X.; Hu, Y.; Pan, C.; Li, B.; Anyadike, C.C.; Asoiro, F.U.; Oji, N.A.; et al. Catalytic Co-Pyrolysis of Macroalgal Components with Lignocellulosic Biomass for Enhanced Biofuels and High-Valued Chemicals. Int. J. Energy Res. 2022, 46, 2674–2697. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Wang, Z.; Guo, S.; Hao, Y.; Gao, Y.; Xin, M.; Ran, Y.; Li, S.; Ji, R.; et al. Kinetic Analysis and Pyrolysis Behavior of Pine Needles by TG-FTIR and Py-GC/MS. BioResources 2023, 18, 6412–6429. [Google Scholar] [CrossRef]
- da Costa, M.M.; Guimarães, T.; França, K.D.; Silva, L.S.; de Almeida, R.F.; Henrique, T.C.; Fernandes, S.A.; Tuler, G.V.; Bittencourt, R.C.; de Paula Barbosa, V.O.; et al. 2,3-Dihydrobenzofuran Production Eco-Friendly by Fast Pyrolysis from Dendrocalamus Asper Biomass. Biomass Convers. Biorefin. 2023, 15, 575–584. [Google Scholar] [CrossRef]
- Usino, D.O.; Sar, T.; Ylitervo, P.; Richards, T. Effect of Acid Pretreatment on the Primary Products of Biomass Fast Pyrolysis. Energies 2023, 16, 2377. [Google Scholar] [CrossRef]
- Güdücü, I.; Alper, K.; Evcil, T.; Tekin, K.; Ohtani, H.; Karagöz, S. Effects of Hydrothermal Carbonization on Products from Fast Pyrolysis of Cellulose. J. Energy Inst. 2021, 99, 299–306. [Google Scholar] [CrossRef]
- Muzyka, R.; Sobek, S.; Dudziak, M.; Ouadi, M.; Sajdak, M. A Comparative Analysis of Waste Biomass Pyrolysis in Py-GC-MS and Fixed-Bed Reactors. Energies 2023, 16, 3528. [Google Scholar] [CrossRef]
- Calabuig, E.; Marcilla, A. Effect of a Mesoporous Catalyst on the Flash Pyrolysis of Tobacco. Thermochim. Acta 2021, 705, 179032. [Google Scholar] [CrossRef]
- Gu, W.; Yu, Z.; Fang, S.; Dai, M.; Chen, L.; Ma, X. Effects of Hydrothermal Carbonization on Catalytic Fast Pyrolysis of Tobacco Stems. Biomass Convers. Biorefin. 2020, 10, 1221–1236. [Google Scholar] [CrossRef]
- Yin, N.; Song, Y.; Wu, L.; Dong, P.; Wang, C.; Zhou, J.; Zhang, X. Analysis of Tar and Pyrolysis Gas from Low-Rank Coal Pyrolysis Assisted by Apple Branch. J. Renew. Sustain. Energy 2023, 15, 043102. [Google Scholar] [CrossRef]
- Coura, M.R.; Demuner, A.J.; Demuner, I.F.; Firmino, M.J.M.; Ribeiro, R.A.; Gomes, F.J.B.; Carvalho, A.M.M.L.; Costa, M.M.; Martins, C.A.; Blank, D.E.; et al. Coffee Biomass Residue as a Raw Material for Cellulose Production and Py-GC/MS Analysis. Waste Biomass Valorization 2024, 15, 349–364. [Google Scholar] [CrossRef]
- Gan, X.; Chen, Z.; Ma, W.; Luo, P.; Xie, R. Comprehensive Evaluation of the Physicochemical Properties and Pyrolysis Mechanism of Products from the Slow Pyrolysis of Waste Coffee Shells. Renew. Energy 2024, 237, 121680. [Google Scholar] [CrossRef]
- Rueda, M.P.; Comino, F.; Aranda, V.; Domínguez-Vidal, A.; Ayora-Cañada, M.J. Analytical Pyrolysis (Py-GC-MS) for the Assessment of Olive Mill Pomace Composting Efficiency and the Effects of Compost Thermal Treatment. J. Anal. Appl. Pyrolysis 2022, 168, 105711. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Qiu, W.; Cheng, Q.; Fan, G.; Song, G.; Zhang, S. Kinetics and Behavior Analysis of Lobster Shell Pyrolysis by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2022, 165, 105580. [Google Scholar] [CrossRef]
- Magdziarz, A.; Jerzak, W.; Wądrzyk, M.; Sieradzka, M. Benefits from Co-Pyrolysis of Biomass and Refuse Derived Fuel for Biofuels Production: Experimental Investigations. Renew. Energy 2024, 230, 120808. [Google Scholar] [CrossRef]
- Sun, T.; Chen, Z.; Wang, R.; Yang, Y.; Zhang, L.; Li, Y.; Liu, P.; Lei, T. Influences of the Reaction Temperature and Catalysts on the Pyrolysis Product Distribution of Lignocellulosic Biomass (Aspen Wood and Rice Husk). Polymers 2023, 15, 3104. [Google Scholar] [CrossRef]
- Patel, A.; Agrawal, B.; Rawal, B.R. Elemental Composition of Biodiesel Produced by Fast Pyrolysis of Eucalyptus Leaves. J. Eng. Res. Kuwait 2021, 2021. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, A.; Wang, J.; Zhou, G.; Li, Y.; Wang, K.; Wang, S. Experimental Study of Tobacco Waste Pyrolysis in a Fluidized Bed Reactor. Chem. Ind. For. Prod. 2022, 42, 47–54. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Chen, L.; Yang, S.; Xie, X.; Gao, M.; Zhao, B.; Si, H.; Li, J.; Hua, D. Catalytic Fast Pyrolysis of Biomass into Aromatic Hydrocarbons over Mo-Modified ZSM-5 Catalysts. Catalysts 2020, 10, 1051. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Li, S.; Xia, S.; Cai, N.; Chen, W.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Catalytic Pyrolysis of Biomass to Produce Aromatic Hydrocarbons over Calcined Dolomite and ZSM-5. Energy Fuels 2021, 35, 16629–16636. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Chen, W.; Yang, H.; Chen, H. Catalytic Pyrolysis of Cotton Stalk to Produce Aromatic Hydrocarbons over Fe Modified CaO Catalysts and ZSM-5. J. Anal. Appl. Pyrolysis 2022, 166, 105635. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, H.; Xie, S.; Li, G.; Du, Z.; Wang, X.; Luo, Y. Highly Selective Production of Valuable Aromatic Hydrocarbons/Phenols from Forestry and Agricultural Residues Using Ni/ZSM-5 Catalyst. Processes 2022, 10, 1970. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, F.; Wu, G.; Fu, J.; Qiao, K. Catalytic Fast Pyrolysis of Biomass to Aromatics over Hierarchical HZSM-5. Huagong XuebaoCIESC J. 2020, 71, 5200–5207. [Google Scholar] [CrossRef]
- Ma, C.; Kumagai, S.; Sato, M.; Nakai, Y.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshioka, T. Investigating the Degradation and Products of Thermo-Oxidation of Polyimide-Based Engineering Plastics. J. Anal. Appl. Pyrolysis 2024, 181, 106575. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Bolatibieke, D.; Nan, D.-H.; Zhang, Z.-X.; Cui, M.-S.; Lu, Q. Catalytic Fast Pyrolysis of Biomass with Ni-P-MCM-41 to Selectively Produce Levoglucosenone. J. Anal. Appl. Pyrolysis 2020, 148, 104824. [Google Scholar] [CrossRef]
- Li, C.; Yellezuome, D.; Li, Y.; Liu, R. Catalytic Pyrolysis of Rice Straw for High Yield of Aromatics over Modified ZSM-5 Catalysts and Its Kinetics. Renew. Energy 2023, 209, 569–580. [Google Scholar] [CrossRef]
- Grams, J. Chromatographic Analysis of Bio-Oil Formed in Fast Pyrolysis of Lignocellulosic Biomass. Rev. Anal. Chem. 2020, 39, 65–77. [Google Scholar] [CrossRef]
- Prabhakara, H.M.; Bramer, E.A.; Brem, G. Hydrotalcite as a Deoxygenation Catalyst in Fast Pyrolysis of Biomass for the Production of High Quality Bio-Oil. J. Anal. Appl. Pyrolysis 2022, 161, 105431. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, Z. Exploiting Sugarcane Waste Molasses and Dephenolized Cottonseed Protein as the Promising Component for Eco-Friendly Wood-Based Panel Adhesive Formulation. Wood Mater. Sci. Eng. 2024, 19, 858–867. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosero, M.; García, A.N.; Marcilla, A. Effect of Alkaline Catalysts on the Valorization of Sugarcane Bagasse via Pyrolysis. Ind. Crops Prod. 2024, 211, 118225. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Fu, H.; Zhang, Z.-X.; Guo, Z.-T.; Zhou, G.-Z.; Zhu, L.-J.; Liu, J.; Lu, Q. Fast Pyrolysis of Bagasse Catalyzed by Mixed Alkaline-Earth Metal Oxides for the Selective Production of 4-Vinylphenol. J. Anal. Appl. Pyrolysis 2022, 164, 105531. [Google Scholar] [CrossRef]
- Imman, S.; Khongchamnan, P.; Wanmolee, W.; Laosiripojana, N.; Kreetachat, T.; Sakulthaew, C.; Chokejaroenrat, C.; Suriyachai, N. Fractionation and Characterization of Lignin from Sugarcane Bagasse Using a Sulfuric Acid Catalyzed Solvothermal Process. RSC Adv. 2021, 11, 26773–26784. [Google Scholar] [CrossRef]
- Chaerusani, V.; Zahra, A.C.A.; Anniwaer, A.; Zhang, P.; Chaihad, N.; Rizkiana, J.; Kusakabe, K.; Kasai, Y.; Abudula, A.; Guan, G. Catalytic Upgrading of Bio-Oils Derived from Terrestrial and Marine Biomass over Various Types of Zeolites. J. Anal. Appl. Pyrolysis 2022, 168, 105735. [Google Scholar] [CrossRef]
- Mariyam, S.; Zuhara, S.; Parthasarathy, P.; McKay, G. A Review on Catalytic Fast Co-Pyrolysis Using Analytical Py-GC/MS. Molecules 2023, 28, 2313. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Cai, L.; Zhang, H.; Zuo, L.; Shi, S.Q.; Lam, S.S. A Review on the Modeling and Validation of Biomass Pyrolysis with a Focus on Product Yield and Composition. Biofuel Res. J. 2021, 8, 1296–1315. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass Pyrolysis Technologies for Value-Added Products: A State-of-the-Art Review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Lu, W.; Ren, D.; Yuan, S. Exploring the Catalytic Conversion of Aromatic Model Compounds of Coal Pyrolysis over Ca(OH)2. J. Energy Inst. 2024, 117, 101850. [Google Scholar] [CrossRef]
- Deng, J.; Feng, Y.; Li, C.; Yuan, Z.; Shang, R.; Yuan, S. Highly Efficiency H2 Production for Real Coal Tar Steam Reforming over Ni-ca/H-Al Catalyst: Effects of Oxygen Vacancy, CaO Doping and Synthesis Methods. Appl. Energy 2024, 367, 123354. [Google Scholar] [CrossRef]
- Yang, Q.; Yao, Q.; Ma, D.; Liu, Y.; He, L.; Zhou, R.; Sun, M.; Ma, X. Investigation of the (Catalytic) Co-Pyrolysis of Shendong Coal and Coal Tar Based on Rapid Pyrolysis and ANN Modelling. J. Anal. Appl. Pyrolysis 2022, 163, 105486. [Google Scholar] [CrossRef]
- Wei, L.; Fan, Y.; Fang, F.; Guo, L.; Chen, Y.; Yang, T. Effect of Sodium and Mineral Types on Distribution of Tar and BTEXN under High Alkali Coal Fast Pyrolysis. Huagong XuebaoCIESC J. 2021, 72, 1702–1711. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, M.; Mo, W.; Wang, Y.; Yuan, J.; Wu, R.; Niu, J.; Ma, K.; Guo, W.; Wei, X.; et al. Effect of Solvent Treatment on the Composition and Structure of Santanghu Long Flame Coal and Its Rapid Pyrolysis Products. Molecules 2023, 28, 7074. [Google Scholar] [CrossRef]
- He, X.-Q.; Mo, W.-L.; Wang, Q.; Ma, Y.-Y.; Ma, F.-Y.; Fan, X.; Wei, X.-Y. Effect of Swelling Treatment by Organic Solvent on the Structure and Pyrolysis Performance of the Direct Coal Liquefaction Residue. Energy Fuels 2020, 34, 8685–8696. [Google Scholar] [CrossRef]
- Lv, T.; Xia, Z.; Fang, M.; Cen, J.; Yan, J.; Zeng, X.; Wang, Q. Insight into Carbon Structures and Pyrolysis Behaviors of Coal from the 13C CP/MAS NMR Spectra. J. Anal. Appl. Pyrolysis 2024, 182, 106693. [Google Scholar] [CrossRef]
- Lv, P.; Bai, Y.; Wang, J.; Song, X.; Su, W.; Yu, G.; Ma, Y. Investigation into the Interaction of Biomass Waste with Industrial Solid Waste during Co-Pyrolysis and the Synergetic Effect of Its Char Gasification. Biomass Bioenergy 2022, 159, 106414. [Google Scholar] [CrossRef]
- Wei, L.; Cui, B.; Guo, L.; Sun, Y. Effect of Sodium on Three-Phase Nitrogen Transformation during Coal Pyrolysis: A Qualitative and Semi-Quantitative Investigation. Fuel Process. Technol. 2021, 213, 106638. [Google Scholar] [CrossRef]
- Wu, C.-H.; Du, M.-L.; Cheng, X.; Ai, Q.-T.; Zhang, Y.; Lin, P.-C. Effects of Co and Mg Modified USY on Tar Product Distribution of Bark Coal Pyrolysis. Xiandai HuagongModern Chem. Ind. 2021, 41, 108–112. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Cao, R.; He, L.; Sun, M.; Ma, X. Mechanism of Phenols Evolution during Pyrolysis of Shendong Coal Macerals Swelled with Oxygen-Containing Organic Solvents: Experimental and DFT Study. Chem. Eng. J. 2024, 493, 152648. [Google Scholar] [CrossRef]
- Yu, G.; Bai, X.; Fan, X.; He, X.-Y.; Zou, H.-X.; Dilixiati, Y.; Wei, X.-Y.; Pidamaimaiti, G.; Pan, Y. In-Situ Evaluation of Volatile Products Released during Pyrolysis of Coals with Different Ranks. J. Energy Inst. 2024, 115, 101660. [Google Scholar] [CrossRef]
- He, L.; Yao, Q.; Cao, R.; Wang, L.; Wang, W.; Ma, D.; Sun, M.; Ma, X. Indentification of Coal-Origin Structural Units by Multi-Step Pyrolysis through Py-GC/MS and by DFT Calculation. Chem. Eng. J. 2024, 492, 152410. [Google Scholar] [CrossRef]
- Ramgobin, A.; Fontaine, G.; Bourbigot, S. A Case Study of Polyether Ether Ketone (I): Investigating the Thermal and Fire Behavior of a High-Performance Material. Polymers 2020, 12, 1789. [Google Scholar] [CrossRef]
- Feng, H.; Li, D.; Cheng, B.; Song, T.; Yang, R. A Cross-Linked Charring Strategy for Mitigating the Hazards of Smoke and Heat of Aluminum Diethylphosphonate/Polyamide 6 by Caged Octaphenyl Polyhedral Oligomeric Silsesquioxanes. J. Hazard. Mater. 2022, 424, 127420. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, S.; Zhao, S.; Wang, X. A High-Phosphorus-Content Polyphosphonate with Combined Phosphorus Structures for Flame Retardant PET. Polymers 2023, 15, 1713. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Wu, G.; Chen, W.; Huang, G. A Novel Biobased Intumescent Flame Retardant through Combining Simultaneously Char-Promoter and Radical-Scavenger for the Application in Epoxy Resin. Polym. Degrad. Stab. 2022, 196, 109841. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, X.; Wu, Y. High Efficient Flame Retardant Finishing of PET Fabric Using Eco-Friendly DOPO. J. Text. Inst. 2022, 113, 1248–1255. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Wang, H.; Zhu, Z.; Yin, X.; Mao, J. Low Flammability and Smoke Epoxy Resins with a Novel DOPO-Based Imidazolone Derivative. Polym. Adv. Technol. 2021, 32, 294–303. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Wang, X.; Liu, Y. A Phosphorous-Based Bi-Functional Flame Retardant for Rigid Polyurethane Foam. Polym. Degrad. Stab. 2021, 186, 109516. [Google Scholar] [CrossRef]
- Wang, X.; Tu, H.; Xiao, H.; Lu, J.; Xu, J.; Gu, G. A Novel Halogen-Free Flame-Retardant Fabrication for the Study of Smoke Suppression and Flame Retardancy of Polystyrene. Polymer 2023, 283, 126240. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Chen, L.; Chen, L.; Wang, X.-L.; Wang, Y.-Z. Eco-Friendly Synergistic Cross-Linking Flame-Retardant Strategy with Smoke and Melt-Dripping Suppression for Condensation Polymers. Compos. Part B Eng. 2021, 211, 108664. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Zhao, J. Enhancing the High Temperature Resistance of Nanocomposite Materials through Dimethyl Methyl Phosphate Impregnation-Coating Treatment. J. Polym. Sci. 2025, 63, 270–290. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Sun, N.; Duan, J.; Nan, S.; Dong, Z.; Zhang, Y.; Long, X.; Wang, B.; Xia, Y. Flame-Retardant Wood Composite Based on Carboxylated Cellulose Fibers and Algal Polysaccharides. ACS Appl. Polym. Mater. 2024, 6, 9353–9363. [Google Scholar] [CrossRef]
- Li, H.; Chen, B.; Kulachenko, A.; Jurkjane, V.; Mathew, A.P.; Sevastyanova, O. A Comparative Study of Lignin-Containing Microfibrillated Cellulose Fibers Produced from Softwood and Hardwood Pulps. Cellulose 2024, 31, 907–926. [Google Scholar] [CrossRef]
- Kaszonyi, A.; Izsák, L.; Králik, M.; Jablonsky, M. Accelerated and Natural Aging of Cellulose-Based Paper: Py-GC/MS Method. Molecules 2022, 27, 2855. [Google Scholar] [CrossRef]
- Yu, Z.; Ahmad, M.S.; Shen, B.; Li, Y.; Ibrahim, M.; Bokhari, A.; Klemeš, J.J. Activated Waste Cotton Cellulose as Renewable Fuel and Value-Added Chemicals: Thermokinetic Analysis, Coupled Pyrolysis with Gas Chromatography and Mass Spectrometry. Energy 2023, 283, 128341. [Google Scholar] [CrossRef]
- Tang, K.; Hao, X.-W.; Wei, Q.-F.; Zhou, X.-F. Effects of Lignin Chemistry on Cellulose Extractioperformance towards Crop Straw/Stalk. Chiang Mai J. Sci. 2020, 47, 1204–1215. [Google Scholar]
- Ma, Z.; Wang, J.; Huang, M.; Cai, W.; Xu, J.; Yang, Y. Effects of Lignin Species and Catalyst Addition on Pyrolysis Products. Nongye Gongcheng XuebaoTransactions Chin. Soc. Agric. Eng. 2020, 36, 274–282. [Google Scholar] [CrossRef]
- Nakason, K.; Chukaew, P.; Utrarachkij, F.; Kuboon, S.; Kraithong, W.; Pichaiyut, S.; Wanmolee, W.; Panyapinyopol, B. Antimicrobial and Antioxidant Activities of Lignin By-Product from Sugarcane Leaf Conversion to Levulinic Acid and Hydrochar. Sustain. Mater. Technol. 2024, 40, e00973. [Google Scholar] [CrossRef]
- Zheng, X.; Zhong, Z.; Zhang, B.; Du, H.; Wang, W.; Li, Q.; Yang, Y.; Qi, R.; Li, Z. Catalytic Pyrolysis of Enzymatic Hydrolysis Lignin by Transition-Metal Modified HZSM-5/MCM-41 Core–Shell Catalyst for the Enhancement of Monocyclic Aromatic Hydrocarbons. J. Anal. Appl. Pyrolysis 2023, 169, 105849. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, L.; Yang, Y.; Li, Y.; Ren, S.; Dong, L.; Lei, T. Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution. Int. J. Environ. Res. Public Health 2022, 19, 16837. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Hu, B.; Zhang, Z.-X.; Zhang, G.; Feng, S.-Y.; Wang, T.-P.; Lu, Q. Catalytic Fast Pyrolysis of Cellulose for Selective Production of 1-Hydroxy-3,6-Dioxabicyclo [3.2.1]Octan-2-One Using Nickel-Tin Layered Double Oxides. Ind. Crops Prod. 2021, 162, 113269. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Fu, H.; Wu, Y.-L.; Zhang, Z.-X.; Liu, J.; Zhang, B.; Lu, Q. Catalytic Fast Pyrolysis of Cellulose for the Selective Production of Levoglucosenone Using Phosphorus Molybdenum Tin Mixed Metal Oxides. Energy Fuels 2022, 36, 10251–10260. [Google Scholar] [CrossRef]
- Ma, S.; Li, H.; Zhang, G.; Iqbal, T.; Li, K.; Lu, Q. Catalytic Fast Pyrolysis of Walnut Shell for Alkylphenols Production with Nitrogen-Doped Activated Carbon Catalyst. Front. Environ. Sci. Eng. 2021, 15, 25. [Google Scholar] [CrossRef]
- Ma, S.-W.; Zhang, G.; Li, H.; Zhang, Z.-X.; Li, K.; Lu, Q. Catalytic Fast Pyrolysis of Walnut Shell with K/AC Catalyst for the Production of Phenolic-Rich Bio-Oil. Biomass Convers. Biorefin. 2022, 12, 2451–2462. [Google Scholar] [CrossRef]
- Zhong, W.-R.; Liu, H.-L.; Liu, H.; Hu, J.-H. Characterization of the Composition and Molecular Size Distribution of Lignin Pyrolysis Bio-Oil. J. Mol. Catal. 2023, 37, 151–163. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Li, C.; Chai, M.; Sarker, M.; Wang, Y.; Cai, J. Catalytic Pyrolysis of Microcrystalline Cellulose Extracted from Rice Straw for High Yield of Hydrocarbon over Alkali Modified ZSM-5. Fuel 2021, 285, 119038. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Zhang, H.; Xiao, R. Fast Pyrolysis of Holocellulose for the Preparation of Long-Chain Ether Fuel Precursors: Effect of Holocellulose Types. Bioresour. Technol. 2021, 338, 125519. [Google Scholar] [CrossRef]
- Jia, X.; Che, Y.; Li, J.; Yan, B.; Cheng, Z.; Chen, G.; Zhao, J. From Cellulose to Tar: Analysis of Tar Formation Pathway with Distinguishing the Primary and Secondary Reactions. Bioresour. Technol. 2023, 390, 129846. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Poohphajai, F.; Sandak, J.; Sandak, A. Impact of Drying Process on Kraft Lignin: Lignin-Water Interaction Mechanism Study by 2D NIR Correlation Spectroscopy. J. Mater. Res. Technol. 2021, 12, 159–169. [Google Scholar] [CrossRef]
- Mei, Y.; Zhang, S.; Wang, H.; Jing, S.; Hou, T.; Pang, S. Low-Temperature Deoxidization of Lignin and Its Impact on Liquid Products from Pyrolysis. Energy Fuels 2020, 34, 3422–3428. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. “lab-on-Click” Detection of Illicit Drugs in Oral Fluids by MicroNIR-Chemometrics. Anal. Chem. 2019, 91, 6435–6439. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Carcassi, E.; Masotti, A.; Materazzi, S. TGA/Chemometrics Addressing Innovative Preparation Strategies for Functionalized Carbon Nanotubes. J. Pharm. Anal. 2020, 10, 351–355. [Google Scholar] [CrossRef]
- Aiello, D.; Siciliano, C.; Mazzotti, F.; Di Donna, L.; Risoluti, R.; Napoli, A. Protein Extraction, Enrichment and MALDI MS and MS/MS Analysis from Bitter Orange Leaves (Citrus Aurantium). Molecules 2020, 25, 1485. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. MicroNIR/Chemometrics: A New Analytical Platform for Fast and Accurate Detection of Δ9-Tetrahydrocannabinol (THC) in Oral Fluids. Drug Alcohol Depend. 2019, 205, 107578. [Google Scholar] [CrossRef]
- Barker-Rothschild, D.; Stoyanov, S.R.; Gieleciak, R.; Cruickshank, M.; Filipescu, C.N.; Dunn, D.; Choi, P. Assessing the Impact of Drought-Induced Abiotic Stress on the Content and Composition of Douglas-Fir Lignin. ACS Sustain. Chem. Eng. 2023, 11, 13519–13526. [Google Scholar] [CrossRef]
- Chen, W.-H.; Ho, K.-Y.; Aniza, R.; Sharma, A.K.; Saravanakumar, A.; Hoang, A.T. A Review of Noncatalytic and Catalytic Pyrolysis and Co-Pyrolysis Products from Lignocellulosic and Algal Biomass Using Py-GC/MS. J. Ind. Eng. Chem. 2024, 134, 51–64. [Google Scholar] [CrossRef]
- Lauschke, T.; Dierkes, G.; Schweyen, P.; Ternes, T.A. Evaluation of Poly(Styrene-D5) and Poly(4-Fluorostyrene) as Internal Standards for Microplastics Quantification by Thermoanalytical Methods. J. Anal. Appl. Pyrolysis 2021, 159, 105310. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, H.; Siddiqui, M.Z.; Kang, H.-S.; Choi, J.-H.; Kumagai, S.; Watanabe, A.; Teramae, N.; Kwon, E.E.; Kim, Y.-M. A Comprehensive Pyrolysis-Gas Chromatography/Mass Spectrometry Analysis for the Assessment of Microplastics in Various Salts. Food Chem. 2025, 467, 142193. [Google Scholar] [CrossRef]
- Biale, G.; La Nasa, J.; Fiorentini, L.; Ceccarini, A.; Carnaroglio, D.; Mattonai, M.; Modugno, F. Characterization and Quantification of Microplastics and Organic Pollutants in Mussels by Microwave-Assisted Sample Preparation and Analytical Pyrolysis. Environ. Sci. Adv. 2023, 3, 76–84. [Google Scholar] [CrossRef]
- Kumagai, S.; Sato, M.; Ma, C.; Nakai, Y.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshioka, T. A Comprehensive Study into the Thermo-Oxidative Degradation of Sulfur-Based Engineering Plastics. J. Anal. Appl. Pyrolysis 2022, 168, 105754. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging Effects on Low- and High-Density Polyethylene, Polypropylene and Polystyrene under UV Irradiation: An Insight into Decomposition Mechanism by Py-GC/MS for Microplastic Analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Jarosz, K.; Janus, R.; Wądrzyk, M.; Wilczyńska-Michalik, W.; Natkański, P.; Michalik, M. Airborne Microplastic in the Atmospheric Deposition and How to Identify and Quantify the Threat: Semi-Quantitative Approach Based on Kraków Case Study. Int. J. Environ. Res. Public Health 2022, 19, 12252. [Google Scholar] [CrossRef]
- Pipkin, W.; Belganeh, R.; Robberson, W.; Allen, H.L.; Cook, A.-M.; Watanabe, A. Identification of Microplastics in Environmental Monitoring Using Pyrolysis–GC–MS Analysis. LC-GC N. Am. 2021, 39, 179–186. [Google Scholar]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef]
- Matsui, K.; Ishimura, T.; Mattonai, M.; Iwai, I.; Watanabe, A.; Teramae, N.; Ohtani, H.; Watanabe, C. Identification Algorithm for Polymer Mixtures Based on Py-GC/MS and Its Application for Microplastic Analysis in Environmental Samples. J. Anal. Appl. Pyrolysis 2020, 149, 104834. [Google Scholar] [CrossRef]
- Li, Q.; Bai, Q.; Sheng, X.; Li, P.; Zheng, R.; Yu, S.; Liu, J. Influence of Particle Characteristics, Heating Temperature and Time on the Pyrolysis Product Distributions of Polystyrene Micro- and Nano-Plastics. J. Chromatogr. A 2022, 1682, 463503. [Google Scholar] [CrossRef] [PubMed]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Vinciguerra, V.; Castelvetro, V.; Modugno, F. A Systematic Study on the Degradation Products Generated from Artificially Aged Microplastics. Polymers 2021, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of Microplastics in Wastewater after Cascade Filtration Using Pyrolysis-GC–MS. MethodsX 2020, 7, 100778. [Google Scholar] [CrossRef]
- Sefiloglu, F.Ö.; Stratmann, C.N.; Brits, M.; van Velzen, M.J.M.; Groenewoud, Q.; Vethaak, A.D.; Dris, R.; Gasperi, J.; Lamoree, M.H. Comparative Microplastic Analysis in Urban Waters Using μ-FTIR and Py-GC-MS: A Case Study in Amsterdam. Environ. Pollut. 2024, 351, 124088. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Cui, C.; Han, Y.; Xiao, Y.; Zhang, B.; Li, G. Association between Blood Microplastic Levels and Severity of Extracranial Artery Stenosis. J. Hazard. Mater. 2024, 480, 136211. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in Three Types of Human Arteries Detected by Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef]
- Tian, J.; Liang, L.; Li, Q.; Li, N.; Zhu, X.; Zhang, L. Association between Microplastics in Human Amniotic Fluid and Pregnancy Outcomes: Detection and Characterization Using Raman Spectroscopy and Pyrolysis GC/MS. J. Hazard. Mater. 2025, 482, 136637. [Google Scholar] [CrossRef]
- Yang, W.; Wu, L.; Li, G.; Shi, L.; Zhang, J.; Liu, L.; Chen, Y.; Yu, H.; Wang, K.; Xin, L.; et al. Atlas and Source of the Microplastics of Male Reproductive System in Human and Mice. Environ. Sci. Pollut. Res. 2024, 31, 25046–25058. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of Microplastics in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Garcia, M.A.; Liu, R.; Nihart, A.; Hayek, E.E.; Castillo, E.; Barrozo, E.R.; Suter, M.A.; Bleske, B.; Scott, J.; Forsythe, K.; et al. Quantitation and Identification of Microplastics Accumulation in Human Placental Specimens Using Pyrolysis Gas Chromatography Mass Spectrometry. Toxicol. Sci. 2024, 199, 81–88. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y. Detection and Quantification of Microplastics in Endometrial Polyps and Their Role in Polyp Formation. Reprod. Toxicol. 2025, 132, 108757. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wang, X.; Li, D.; Wang, H.; Xu, H.; Liu, Y.; Kang, R.; Chen, Q.; Zheng, L.; et al. Discovery and Analysis of Microplastics in Human Bone Marrow. J. Hazard. Mater. 2024, 477, 135266. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, T.; Chen, Z.; Du, L.; Qiu, X.; Zhang, Y.; Li, Y.; Zhu, Y.; Tan, Z.; Mo, Y.; et al. Micro(Nano)Plastics in Human Urine: A Surprising Contrast between Chongqing’s Urban and Rural Regions. Sci. Total Environ. 2024, 917, 170455. [Google Scholar] [CrossRef] [PubMed]
- Andersone, A.; Arshanitsa, A.; Akishin, Y.; Semenischev, A.; Telysheva, G. Microwave Assisted Torrefaction of Plant Biomass of Different Origin with a Focus on Solid Products Valorisation for Energy and Beyond. Chem. Eng. Trans. 2021, 86, 109–114. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Mattonai, M.; Modugno, F. Microwave-Assisted Solvent Extraction and Double-Shot Analytical Pyrolysis for the Quali-Quantitation of Plasticizers and Microplastics in Beach Sand Samples. J. Hazard. Mater. 2021, 401, 123287. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Rochman, C.M. Microwave-Assisted Extraction for Quantification of Microplastics Using Pyrolysis–Gas Chromatography/Mass Spectrometry. Environ. Toxicol. Chem. 2021, 40, 2733–2741. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, W.; Fu, J.; Siyal, A.A.; An, Q.; Zhou, C.; Liu, C.; Zhang, Y.; Chen, Z.; Yun, H.; et al. Microwave-Assisted Pyrolysis of Industrial Biomass Waste: Insights into Kinetic, Characteristics and Intrinsic Mechanisms. Energy 2024, 306, 132423. [Google Scholar] [CrossRef]
- Hildebrandt, L.; Fischer, M.; Klein, O.; Zimmermann, T.; Fensky, F.; Siems, A.; Zonderman, A.; Hengstmann, E.; Kirchgeorg, T.; Pröfrock, D. An Analytical Strategy for Challenging Members of the Microplastic Family: Particles from Anti-Corrosion Coatings. J. Hazard. Mater. 2024, 470, 134173. [Google Scholar] [CrossRef]
- Li, Q.; Lai, Y.; Li, P.; Liu, X.; Yao, Z.; Liu, J.; Yu, S. Evaluating the Occurrence of Polystyrene Nanoparticles in Environmental Waters by Agglomeration with Alkylated Ferroferric Oxide Followed by Micropore Membrane Filtration Collection and Py-GC/MS Analysis. Environ. Sci. Technol. 2022, 56, 8255–8265. [Google Scholar] [CrossRef]
- Lou, F.; Wang, J.; Sun, C.; Song, J.; Wang, W.; Pan, Y.; Huang, Q.; Yan, J. Influence of Interaction on Accuracy of Quantification of Mixed Microplastics Using Py-GC/MS. J. Environ. Chem. Eng. 2022, 10, 108012. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, X.; Jin, X. Determination of Microplastics in Soil by Pyrolizer-GC-MS. Environ. Chem. 2022, 41, 1824–1826. [Google Scholar]
- Le Juge, C.; Grassl, B.; Allan, I.J.; Gigault, J. Identification of Polystyrene Nanoplastics from Natural Organic Matter in Complex Environmental Matrices by Pyrolysis–Gas Chromatography–Mass Spectrometry. Anal. Bioanal. Chem. 2023, 415, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Roscher, L.; Halbach, M.; Nguyen, M.T.; Hebeler, M.; Luschtinetz, F.; Scholz-Böttcher, B.M.; Primpke, S.; Gerdts, G. Microplastics in Two German Wastewater Treatment Plants: Year-Long Effluent Analysis with FTIR and Py-GC/MS. Sci. Total Environ. 2022, 817, 152619. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lee, L.M.; Yu, D.; Chan, S.H.; Li, A. An Optimized Multi-Technique Based Analytical Platform for Identification, Characterization and Quantification of Nanoplastics in Water. Talanta 2024, 272, 125800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhu, P.; Jing, S.; Li, J.; Wanger, T.C.; Liu, W.; Liu, K.; Chen, X.; Li, L. Mass Concentrations, Compositions and Burial Fluxes of Nano- and Micro-Plastics in a Multi-Species Saltmarsh. Environ. Pollut. 2024, 363, 125181. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, J.-C.; Feng, Z.-Y.; Jin, B.; Liu, Y.; Meng, L.-Y. ACFs-NH2 Developed for Dispersive Solid Phase Extraction Combined with Py-GC/MS for Nanoplastic Analysis in Ambient Water Samples. J. Chromatogr. A 2024, 1736, 465382. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Wang, X.; Hou, F.; Li, P.; van der Hoek, J.P.; Liu, G. Assessing the Mass Concentration of Microplastics and Nanoplastics in Wastewater Treatment Plants by Pyrolysis Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2023, 57, 3114–3123. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Jiao, M.; Liu, G.; Van Der Hoek, J.P. Identification and Quantification of Nanoplastics in Surface Water and Groundwater by Pyrolysis Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2022, 56, 4988–4997. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Wang, X.; van der Hoek, J.P.; Liu, G. Mass Concentration and Removal Characteristics of Microplastics and Nanoplastics in a Drinking Water Treatment Plant. ACS ES T Water 2024, 4, 3348–3358. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Y.; Liu, W.; Ma, X.; He, D.; Wang, Y.; Li, J.; Wu, W. Identification and Quantification of Nanoplastics in Different Crops Using Pyrolysis Gas Chromatography-Mass Spectrometry. Chemosphere 2024, 354, 141689. [Google Scholar] [CrossRef] [PubMed]
- La Nasa, J.; Biale, G.; Fabbri, D.; Modugno, F. A Review on Challenges and Developments of Analytical Pyrolysis and Other Thermoanalytical Techniques for the Quali-Quantitative Determination of Microplastics. J. Anal. Appl. Pyrolysis 2020, 149, 104841. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.S.; Johir, M.A.H.; Mondal, M.I.H.; Lee, D.-Y.; Park, J.; Zhou, J.L.; Yoon, M.-H. Microplastic Particles in the Aquatic Environment: A Systematic Review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Huang, Q.; Yang, C.; Wang, L.; Lou, Z.; Zhou, Q.; Wang, T.; Ning, C. Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes. Water 2023, 15, 252. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Sun, M.; Li, S.; Yu, X.; Dai, J. Microplastics in Soils and Plants: Current Research Status and Progress on Detection Methods. Earth Sci. Front. 2024, 31, 183–195. [Google Scholar] [CrossRef]
- Moteallemi, A.; Dehghani, M.H.; Momeniha, F.; Azizi, S. Nanoplastics as Emerging Contaminants: A Systematic Review of Analytical Processes, Removal Strategies from Water Environments, Challenges and Perspective. Microchem. J. 2024, 207, 111884. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, P.; Lou, X.; Zhou, Z.; Zhou, L.; Hu, Y.; Luan, Y.; Quan, C.; Fang, J.; Zou, H.; et al. A Review of Airborne Micro- and Nano-Plastics: Sampling Methods, Analytical Techniques, and Exposure Risks. Environ. Pollut. 2024, 363, 125074. [Google Scholar] [CrossRef]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of Environmental Nanoplastics: Progress and Challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Liu, Q.; Li, Q. Ageing Characterization and Origin Traceability of Archaeological Amber Artefacts via FTIR Spectroscopy and Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Cult. Herit. 2024, 69, 37–46. [Google Scholar] [CrossRef]
- Yao, N.; Wang, S.; Guo, H.; Hu, H.; Liu, Y.; Wei, S. Characterization and Identification of Paper Relics Using Py-GC/MS. Sci. Conserv. Archaeol. 2024, 36, 44–53. [Google Scholar] [CrossRef]
- Yao, N.; Wei, S. Characterization and Identification of Traditional Chinese Handmade Paper via Pyrolysis-Gas Chromatography-Mass Spectrometry. BioResources 2021, 16, 3942–3951. [Google Scholar] [CrossRef]
- Na, W.; An, G.; Kai, L.; Jingyuan, L.; Yong, L. Identification of Waxes Using Pyrolysis—Gas Chromatography/Mass Spectrometry. Sci. Conserv. Archaeol. 2020, 32, 71–77. [Google Scholar] [CrossRef]
- Pozzi, F.; Basso, E.; Alderson, S.; Levinson, J.; Neiman, M.; Alcalá, S. Aiding the Cleaning of Four 19th-Century Tsimshian House Posts: Investigation of Museum-Applied Surface Coatings and Original Polychromy. Herit. Sci. 2021, 9, 42. [Google Scholar] [CrossRef]
- Pellis, G.; Bertasa, M.; Ricci, C.; Scarcella, A.; Croveri, P.; Poli, T.; Scalarone, D. A Multi-Analytical Approach for Precise Identification of Alkyd Spray Paints and for a Better Understanding of Their Ageing Behaviour in Graffiti and Urban Artworks. J. Anal. Appl. Pyrolysis 2022, 165, 105576. [Google Scholar] [CrossRef]
- Izzo, F.C.; Balliana, E.; Perra, E.; Zendri, E. Accelerated Ageing Procedures to Assess the Stability of an Unconventional Acrylic-Wax Polymeric Emulsion for Contemporary Art. Polymers 2020, 12, 1925. [Google Scholar] [CrossRef]
- Risoluti, R.; Materazzi, S.; Tau, F.; Russo, A.; Romolo, F.S. Towards Innovation in Paper Dating: A MicroNIR Analytical Platform and Chemometrics. Analyst 2018, 143, 4394–4399. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Badea, E.; Carsote, C.; Czégény, Z.; Szabó, T.; Babinszki, B.; Bozi, J.; Jakab, E. Characterization of Historical Leather Bookbindings by Various Thermal Methods (TG/MS, Py-GC/MS, and Micro-DSC) and FTIR-ATR Spectroscopy. J. Anal. Appl. Pyrolysis 2022, 162, 105428. [Google Scholar] [CrossRef]
- Risdonne, V.; Hubbard, C.; Puisto, J.; Theodorakopoulos, C. A Multi-Analytical Study of Historical Coated Plaster Surfaces: The Examination of a Nineteenth-Century V&A Cast of a Tombstone. Herit. Sci. 2021, 9, 70. [Google Scholar] [CrossRef]
- Pozzi, F.; Basso, E.; Centeno, S.A.; Smieska, L.M.; Shibayama, N.; Berns, R.; Fontanella, M.; Stringari, L. Altered Identity: Fleeting Colors and Obscured Surfaces in Van Gogh’s Landscapes in Paris, Arles, and Saint-Rémy. Herit. Sci. 2021, 9, 15. [Google Scholar] [CrossRef]
- Manfredda, N.; Buscaglia, P.; Gallo, P.; Borla, M.; Aicardi, S.; Poggi, G.; Baglioni, P.; Nervo, M.; Scalarone, D.; Borghi, A.; et al. An Ancient Egyptian Multilayered Polychrome Wooden Sculpture Belonging to the Museo Egizio of Torino: Characterization of Painting Materials and Design of Cleaning Processes by Means of Highly Retentive Hydrogels. Coatings 2021, 11, 1335. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Yu, Z.-R.; Su, B.-M. Analysis of Egg Whites from Burial Murals by Pyrolysis-Gas Chromatography/Mass Spectrometry. Chin. J. Appl. Chem. 2023, 40, 562–570. [Google Scholar] [CrossRef]
- Zhao, F.; Xing, H.; Wang, J.; Jia, Z.; Chao, X.; Wang, J.; Liu, J.; Li, Y. Analytical Investigation of Jiatang Scroll Paintings in the Seventh Year of the Guangxu Era. Coatings 2022, 12, 410. [Google Scholar] [CrossRef]
- Rizzo, D.; Casciaro, R. Analytical Investigation of the Original Painted Canvas of Santa Irene, by Giuseppe Verrio (Church of Sant’Irene, Lecce, Italy)*. Archaeometry 2022, 64, 245–255. [Google Scholar] [CrossRef]
- Montané, C.; Velino, C.; André, E.; Aufray, M.; Gayet, F.; Robbiola, L.; Brouca-Cabarrecq, C.; Sciau, P.; Brunet, M. Historical Primers and Paints Used for Aeronautical Protection and Colouring during WWII: A Multi-Techniques Approach on Archaeological Parts. J. Cult. Herit. 2023, 62, 54–64. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; de Oliveira, F.L.; Jos, Á.; Cameán, A.M.; Aparicio-Ruiz, R.; Vasconcelos, V.; Campos, A.; González-Vila, F.J.; González-Pérez, J.A. Alterations in Mediterranean Mussel (Mytilus Galloprovincialis) Composition Exposed to Cyanotoxins as Revealed by Analytical Pyrolysis. J. Anal. Appl. Pyrolysis 2020, 152, 104970. [Google Scholar] [CrossRef]
- Gautam, R.; Vinu, R.; Vaithyanathan, P. Analytical Fast Pyrolysis of Nitrogen-Rich Mosquito Species via Pyrolysis-FTIR and Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2020, 146, 104766. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Löffler, P.; Eichhöfer, S.; David, J.; Muñoz, K.; Schaumann, G.E. Are Agricultural Plastic Covers a Source of Plastic Debris in Soil? A First Screening Study. SOIL 2022, 8, 31–47. [Google Scholar] [CrossRef]
- De Andrade, M.A.M.; Monteiro, A.S.C.; Gontijo, E.S.J.; Bueno, C.C.; Macedo, J.C.A.; Rangel, E.C.; Melo, D.S.; Montero, J.I.Z.; Rosa, A.H. Combined Analytical Py-GC-MS, SEM, FTIR and 13C NMR for Investigating the Removal of Trace Metals from Aqueous Solutions by Biochar. J. Braz. Chem. Soc. 2020, 31, 1518–1530. [Google Scholar] [CrossRef]
- Chae, E.; Choi, S.-S. Comparison of Polymeric Components and Tire Wear Particle Contents in Particulate Matter Collected at Bus Stop and College Campus. Heliyon 2023, 9, e16558. [Google Scholar] [CrossRef]
- Chae, E.; Choi, S.-S. Concentrations of Particulate Matter (PM2.5) and Contributions of Tire Wear Particle to PM2.5 in an Indoor Parking Garage: Comparison with the Outside and the Differences According to the Sampling Sites. Heliyon 2024, 10, e23513. [Google Scholar] [CrossRef] [PubMed]
- Zymankowska-Kumon, S.; Kaczmarska, K.; Grabowska, B.; Bobrowski, A.; Cukrowicz, S. Influence of the Atmosphere on the Type of Evolved Gases from Phenolic Binders. Arch. Foundry Eng. 2020, 20, 31–36. [Google Scholar] [CrossRef]
- Mattonai, M.; Watanabe, A.; Ribechini, E. Characterization of Volatile and Non-Volatile Fractions of Spices Using Evolved Gas Analysis and Multi-Shot Analytical Pyrolysis. Microchem. J. 2020, 159, 105321. [Google Scholar] [CrossRef]
- Li, K.; Bolatibieke, D.; Yang, S.-G.; Wang, B.; Nan, D.-H.; Lu, Q. Ex Situ Catalytic Fast Pyrolysis of Soy Sauce Residue with HZSM-5 for Co-Production of Aromatic Hydrocarbons and Supercapacitor Materials. RSC Adv. 2020, 10, 23331–23340. [Google Scholar] [CrossRef]
- Prieto, A.I.; Guzmán-Guillén, R.; Jos, Á.; Cameán, A.M.; de la Rosa, J.M.; González-Pérez, J.A. Detection of Cylindrospermopsin and Its Decomposition Products in Raw and Cooked Fish (Oreochromis Niloticus) by Analytical Pyrolysis (Py-GC/MS). Chemosphere 2020, 244, 125469. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Materazi, S. Assessing the Quality of Milk Using a Multicomponent Analytical Platform MicroNIR/Chemometric. Front. Chem. 2020, 8, 614718. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Embaye, T.M.; Deng, S.; Bukhsh, K.; Ruan, R.; Zhou, A.; Hu, Z.; Deng, N.; Wu, D.; Tan, H.; et al. Investigation on Co-Combustion Behavior of Domestic Waste and Sewage Sludge Using TG-FTIR-Py-GC/MS: Thermal Behavior and Gaseous Pollutants Emission. J. Energy Inst. 2025, 118, 101933. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Sun, M.; Liu, B.; Chen, W.; Dang, C.; Zhong, H.; Jiang, J.; Qin, S.; Han, Z.; et al. Evaluating the Bioenergy Potential of Kitchen Wastes Fermentation Residues through Pyrolysis Kinetics, Thermodynamics and Py-GC/MS Analysis Technique. J. Therm. Anal. Calorim. 2023, 148, 995–1010. [Google Scholar] [CrossRef]
- Silveira Junior, E.G.; da Silva, N.R.F.; Perez, V.H.; David, G.F.; Olivares, F.L.; Fernandes, S.A.; Justo, O.R.; Simionatto, E. Fast Pyrolysis of Peanut Husk Agroindustrial Waste: Intensification of Anhydro Sugar (Levoglucosan) Production. Waste Biomass Valorization 2021, 12, 5573–5585. [Google Scholar] [CrossRef]
- Hamid, A.; Alam, A.; Ali, L.; Shittu, T.; Labata, F.G.T.; Altarawneh, M. Improving the Yield of Levoglucosan Platform Chemical from the Pyrolysis of Date Pits Waste Biomass through Pre-Treatments. Sustain. Chem. Pharm. 2024, 42, 101758. [Google Scholar] [CrossRef]
- Tahir, M.H.; Irfan, R.M.; Cheng, X.; Ahmad, M.S.; Jamil, M.; Shah, T.-U.-H.; Karim, A.; Ashraf, R.; Haroon, M. Mango Peel as Source of Bioenergy, Bio-Based Chemicals via Pyrolysis, Thermodynamics and Evolved Gas Analyses. J. Anal. Appl. Pyrolysis 2021, 155, 105066. [Google Scholar] [CrossRef]
- Hermelin, A.; Fabien, L.; Fischer, J.; Saric, N.; Massonnet, G.; Burnier, C. Analysis of Condom Evidence in Forensic Science: Background Survey of the Human Vaginal Matrix Using DRIFTS and Pyrolysis-GC/MS. Forensic Sci. Int. 2021, 321, 110724. [Google Scholar] [CrossRef] [PubMed]
- Burnier, C.; Massonnet, G.; Coulson, S.; DeTata, D.; Pitts, K. Condom Evidence: Characterisation, Discrimination and Classification of Pyrolysis-GC-MS Profiles. Forensic Sci. Int. 2021, 324, 110793. [Google Scholar] [CrossRef] [PubMed]
- Burnier, C.; Kelly, M.; DeTata, D.; Pitts, K. Investigation of Condom Evidence in Cases of Sexual Assault: Case Studies. Forensic Sci. Int. Rep. 2021, 4, 100221. [Google Scholar] [CrossRef]
- Burnier, C.; Maurer, J. Microfurnace or Filament Pyrolyzer: An Example of Pyrolysis-GC/MS for Condom Lubricant Analysis. Forensic Chem. 2024, 40, 100593. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Zhao, P.-C.; Song, H.; Zhao, L. Detection of Trace Evidence of Lubricants in Condoms by Py/GCMS Method. J. Instrum. Anal. 2024, 43, 995–1002. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Seo, J.M. Analysis of Paint Traces to Determine the Ship Responsible for a Collision. Sci. Rep. 2021, 11, 134. [Google Scholar] [CrossRef]
- Materazzi, S.; Gullifa, G.; Fabiano, M.A.; Frati, P.; Santurro, A.; Scopetti, M.; Fineschi, V.; Risoluti, R. New Frontiers in Thermal Analysis: A TG/Chemometrics Approach for Postmortem Interval Estimation in Vitreous Humor. J. Therm. Anal. Calorim. 2017, 130, 549–557. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Zhang, D.; Gao, Y.; Xiong, M.; Chen, W. Co-Hydrothermal Carbonization of Cotton Textile Waste and Polyvinyl Chloride Waste for the Production of Solid Fuel: Interaction Mechanisms and Combustion Behaviors. J. Clean. Prod. 2021, 316, 128306. [Google Scholar] [CrossRef]
- Liang, M.; Pan, H.; Zhu, Y.; Zhu, H.; Su, M.; Xie, Y.; Zheng, Y.; Jiang, X.; Li, R.; Zhang, J. Co-Pyrolysis Behavior of Polylactic Acid and Biomass from Heated Tobacco Products. Biomass Convers. Biorefin. 2024, 14, 26035–26050. [Google Scholar] [CrossRef]
- Wu, X.; Bourbigot, S.; Li, K.; Zou, Y. Co-Pyrolysis Characteristics and Flammability of Polylactic Acid and Acrylonitrile-Butadiene-Styrene Plastic Blend Using TG, Temperature-Dependent FTIR, Py-GC/MS and Cone Calorimeter Analyses. Fire Saf. J. 2022, 128, 103543. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Z.; Chen, G.; Zhang, M.; Sun, T.; Wang, Q.; Du, Z.; Chen, Y.; Wu, M.; Li, Z.; et al. Co-Pyrolysis Characteristics of Forestry and Agricultural Residues and Waste Plastics: Thermal Decomposition and Products Distribution. Process Saf. Environ. Prot. 2023, 177, 380–390. [Google Scholar] [CrossRef]
- Guo, N.; Wang, Z.; Chen, G.; Zhang, M.; Zhu, H.; Wang, Q.; Guo, S.; Su, F.; You, Z.; Yang, S.; et al. Co-Pyrolysis Kinetic Characteristics of Wheat Straw and Hydrogen Rich Plastics Based on TG-FTIR and Py-GC/MS. Energy 2024, 312, 133683. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Xiao, M.; Feng, H.; Lin, R.; Wang, X. Insight into the Pyrolysis Behavior of Corn Straw through TG-FTIR, Py-GC/MS Experiments and ReaxFF MD Simulations. Fuel 2024, 372, 132036. [Google Scholar] [CrossRef]
- Nandakumar, T.; Pal, S.K.; Vinu, R.; Ramar, P.M.; Pant, K.K.; Kumar, S.; Balaraman, E. Graphene-Encapsulated Transition Metal@N/C Catalysts for Catalytic Copyrolysis of Biomass and Waste Plastics: Production of Linear α-Olefins and Aromatics. ACS Sustain. Chem. Eng. 2024, 12, 5283–5299. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, M.; Chen, G.; Zhang, M.; Sun, T.; Burra, K.G.; Guo, S.; Chen, Y.; Yang, S.; Li, Z.; et al. Co-Pyrolysis Characteristics of Waste Tire and Maize Stalk Using TGA, FTIR and Py-GC/MS Analysis. Fuel 2023, 337, 127206. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Chen, G.; Zhang, M.; Sun, T.; Wang, Q.; Zhu, H.; Yang, S.; Chen, Y.; Wu, M.; et al. Co-Pyrolysis of Waste Tire with Agricultural and Forestry Residues: Pyrolysis Behavior, Products Distribution and Synergistic Effects. J. Energy Inst. 2024, 114, 101634. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, S.; Li, C.; Shan, R.; Yuan, H.; Chen, Y. Co-Pyrolysis Mechanism of Waste Vehicle Seats Derived Artificial Leather and Foam. J. Clean. Prod. 2024, 434, 140436. [Google Scholar] [CrossRef]
- Dong, R.; Tang, Z.; Song, H.; Chen, Y.; Wang, X.; Yang, H.; Chen, H. Co-Pyrolysis of Vineyards Biomass Waste and Plastic Waste: Thermal Behavior, Pyrolytic Characteristic, Kinetics, and Thermodynamics Analysis. J. Anal. Appl. Pyrolysis 2024, 179, 106506. [Google Scholar] [CrossRef]
- Chen, W.; Ye, M.; Li, M.; Xi, B.; Hou, J.; Qi, X.; Zhang, J.; Wei, Y.; Meng, F. Characteristics, Kinetics and Product Distribution on Pyrolysis Process for Waste Wind Turbine Blades. J. Anal. Appl. Pyrolysis 2023, 169, 105859. [Google Scholar] [CrossRef]
- Parker-Jurd, F.N.F.; Abbott, G.D.; Guthery, B.; Parker-Jurd, G.M.C.; Thompson, R.C. Features of the Highway Road Network That Generate or Retain Tyre Wear Particles. Environ. Sci. Pollut. Res. 2024, 31, 26675–26685. [Google Scholar] [CrossRef]
- Zheng, D.; Cheng, J.; Wang, X.; Yu, G.; Xu, R.; Dai, C.; Liu, N.; Wang, N.; Chen, B. Influences and Mechanisms of Pyrolytic Conditions on Recycling BTX Products from Passenger Car Waste Tires. Waste Manag. 2023, 169, 196–207. [Google Scholar] [CrossRef]
- Chávez-Delgado, M.; Colina, J.R.; Segura, C.; Álvarez, C.; Osorio-Vargas, P.; Arteaga-Pérez, L.E.; Norambuena-Contreras, J. Asphalt Pyro-Rejuvenators Based on Waste Tyres: An Approach to Improve the Rheological and Self-Healing Properties of Aged Binders. J. Clean. Prod. 2024, 452, 142179. [Google Scholar] [CrossRef]
- Azócar, B.S.; Vargas, P.O.; Campos, C.; Medina, F.; Arteaga-Pérez, L.E. Dataset from Analytical Pyrolysis Assays for Converting Waste Tires into Valuable Chemicals in the Presence of Noble-Metal Catalysts. Data Brief 2022, 40, 107745. [Google Scholar] [CrossRef]
- Li, J.; Zheng, D.; Yao, Z.; Wang, S.; Xu, R.; Deng, S.; Chen, B.; Wang, J. Formation Mechanism of Monocyclic Aromatic Hydrocarbons during Pyrolysis of Styrene Butadiene Rubber in Waste Passenger Car Tires. ACS Omega 2022, 7, 42890–42900. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, Z.; Liu, J.; Chen, Z.; Xie, W.; Evrendilek, F.; Buyukada, M. Dynamic Pyrolysis Behaviors, Products, and Mechanisms of Waste Rubber and Polyurethane Bicycle Tires. J. Hazard. Mater. 2021, 402, 123516. [Google Scholar] [CrossRef]
- Mariyam, S.; Alherbawi, M.; Rashid, N.; Al-Ansari, T.; McKay, G. Bio-Oil Production from Multi-Waste Biomass Co-Pyrolysis Using Analytical Py–GC/MS. Energies 2022, 15, 7409. [Google Scholar] [CrossRef]
- Krishna, J.V.J.; Prashanth, P.F.; Vinu, R. Distributed Activation Energy Modeling and Py-GC/MS Studies on Pyrolysis of Different Printed Circuit Boards for Resource Recovery. ACS Omega 2022, 7, 31713–31725. [Google Scholar] [CrossRef]
- Li, B.; Shen, B.; Tao, R.; Hu, C.; Wu, Y.; Yuan, H.; Gu, J.; Chen, Y. Effect of Copper Content on the Pyrolysis Process of Organic Components in Waste Printed Circuit Boards: Based on Experimental and Quantum Chemical DFT Simulations. Chin. J. Chem. Eng. 2024, 73, 202–211. [Google Scholar] [CrossRef]
- Liu, J.; Jia, D.; Xu, W.; Chen, Z.; Evrendilek, F.; Cao, H.; Zhong, S.; Yang, Z.; He, Y.; Qi, J. Catalytic Pyrolysis of FeAlOx and Medical Plastic Waste: Kinetic, Slag Conversion, and Gas Emission Patterns. J. Environ. Chem. Eng. 2024, 12, 112605. [Google Scholar] [CrossRef]
- Qu, X.; Li, Y.; Zhang, X.; Li, R. Comprehensive Analysis of Pyrolysis in Medical Rubber Gloves: Pyrolysis Characteristics, Kinetics, Thermodynamics, Volatile Products, and Pathways. Waste Dispos. Sustain. Energy 2024, 6, 297–308. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J.; Ding, Z.; Fu, J.; Evrendilek, F.; Xie, W.; He, Y. Dynamic Pyrolytic Reaction Mechanisms, Pathways, and Products of Medical Masks and Infusion Tubes. Sci. Total Environ. 2022, 842, 156710. [Google Scholar] [CrossRef]
- Ranalli, G.; Andreotti, A.; Colombini, M.P.; Corti, C.; Paris, D.; Rampazzi, L.; Saviano, G.; Vecchio, R.; Caprari, C. Investigation on Tattoo Ink (Hexadecachlorinate Copper Phthalocyanine) Removal: Novel Chemical and Biological Approach. Molecules 2024, 29, 5543. [Google Scholar] [CrossRef]
- Wu, C.-C.; Li, H.; Yin, Z.-W.; Zhang, H.-T.; Gao, M.-J.; Zhu, L.; Zhan, X.-B. Isolation, Purification, and Characterization of Novel Melanin from the Submerged Fermentation of Rhizobium Radiobacter. Process Biochem. 2022, 121, 263–275. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from Agroindustrial By-Products as Natural Ingredients for Cosmetics: Chemical Structure and in Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Zhan, Y.; Bian, H.; Wu, S.; Dai, H.; Liang, F.; Meng, X.; Huang, C.; Fang, G.; et al. A Tailored Deep Eutectic Solvent for High-Yield Conversion of Poplar Residues to Bio-Based Building Blocks at Mild Conditions. Chem. Eng. J. 2024, 487, 150407. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Wu, P.; Yagoub, A.E.-G.A.; Chen, L.; Abdullateef Taiye, M.; Zhou, C. Pretreatment of Sugarcane Bagasse with Deep Eutectic Solvents Affect the Structure and Morphology of Lignin. Ind. Crops Prod. 2021, 173, 114108. [Google Scholar] [CrossRef]
- Sreelekshmi, K.R.; Thomas, D.; Nimesh, S.; Vijayalakshmi, K.P.; Prabhakaran, K. An Insight into the Thermal Decomposition Mechanism of 1-Butyl-3-Methyl-Imidazolium-5-Aminotetrazolate Guided by Py-GC–MS and DFT. J. Mol. Liq. 2023, 392, 123413. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Q.; Meng, C.; Shen, G.; Fu, J. Deep Eutectic Solvent Degumming of Hemp Fiber: Key Factors Influencing Fiber Property and Its Mechanism. Ind. Crops Prod. 2023, 203, 117125. [Google Scholar] [CrossRef]
- Tan, J.-N.; Li, N.; Wang, X.; Yan, J.; Wentao, Z.; Dou, Y. Influence of Natural Deep Eutectic Solvents on the Release of Volatile Compounds from Heated Tobacco. Ind. Crops Prod. 2021, 174, 114171. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, J.; Dai, Y.; Jiang, J.; Liao, S.; Zhou, G.; Wang, S. Mechanism Study of Tobacco Pyrolysis Based on the Analysis of Characteristic Products and In-Situ Identification of Functional Groups Evolution on Pyrolytic Char. J. Anal. Appl. Pyrolysis 2022, 167, 105681. [Google Scholar] [CrossRef]
- Ali, W.; Zilke, O.; Danielsiek, D.; Salma, A.; Assfour, B.; Shabani, V.; Caglar, S.; Phan, H.M.; Kamps, L.; Wallmeier, R.; et al. Flame-Retardant Finishing of Cotton Fabrics Using DOPO Functionalized Alkoxy- and Amido Alkoxysilane. Cellulose 2023, 30, 2627–2652. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, J.E.; Lim, D.; Ahn, H.; Jeong, W. Flame-Retardant Mechanism and Mechanical Properties of Wet-Spun Poly(Acrylonitrile-Co-Vinylidene Chloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate. Polymers 2020, 12, 2442. [Google Scholar] [CrossRef]
- McPartlin, M.W.; Italiano, B.R.; Tiano, T.M.; Pilkenton, S.J.; Lawton, T.J. An Approach to Identifying Fibers and Evolved Compounds from Flame Resistant Fabrics. J. Anal. Appl. Pyrolysis 2021, 159, 105327. [Google Scholar] [CrossRef]
- Micheluz, A.; Angelin, E.M.; Sawitzki, J.; Pamplona, M. Plastics in Robots: A Degradation Study of a Humanoid Skin Mask Made of Soft Urethane Elastomer. Herit. Sci. 2022, 10, 4. [Google Scholar] [CrossRef]
- Gasparini, G.; Semaoui, S.; Augugliaro, J.; Boschung, A.; Berthier, D.; Seyfried, M.; Begnaud, F. Quantification of Residual Perfume by Py-GC-MS in Fragrance Encapsulate Polymeric Materials Intended for Biodegradation Tests. Molecules 2020, 25, 718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).