Electrochemical Sensing of Hg2+ Ions Using an SWNTs/Ag@ZnBDC Composite with Ultra-Low Detection Limit
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Synthesis of ZnBDC and SWNTs/Ag@ZnBDC Composites
2.3. Fabrication of Electrochemical Sensor and Electrode
2.4. Electrochemical Measurements
2.5. Material Characterisations
3. Results and Discussion
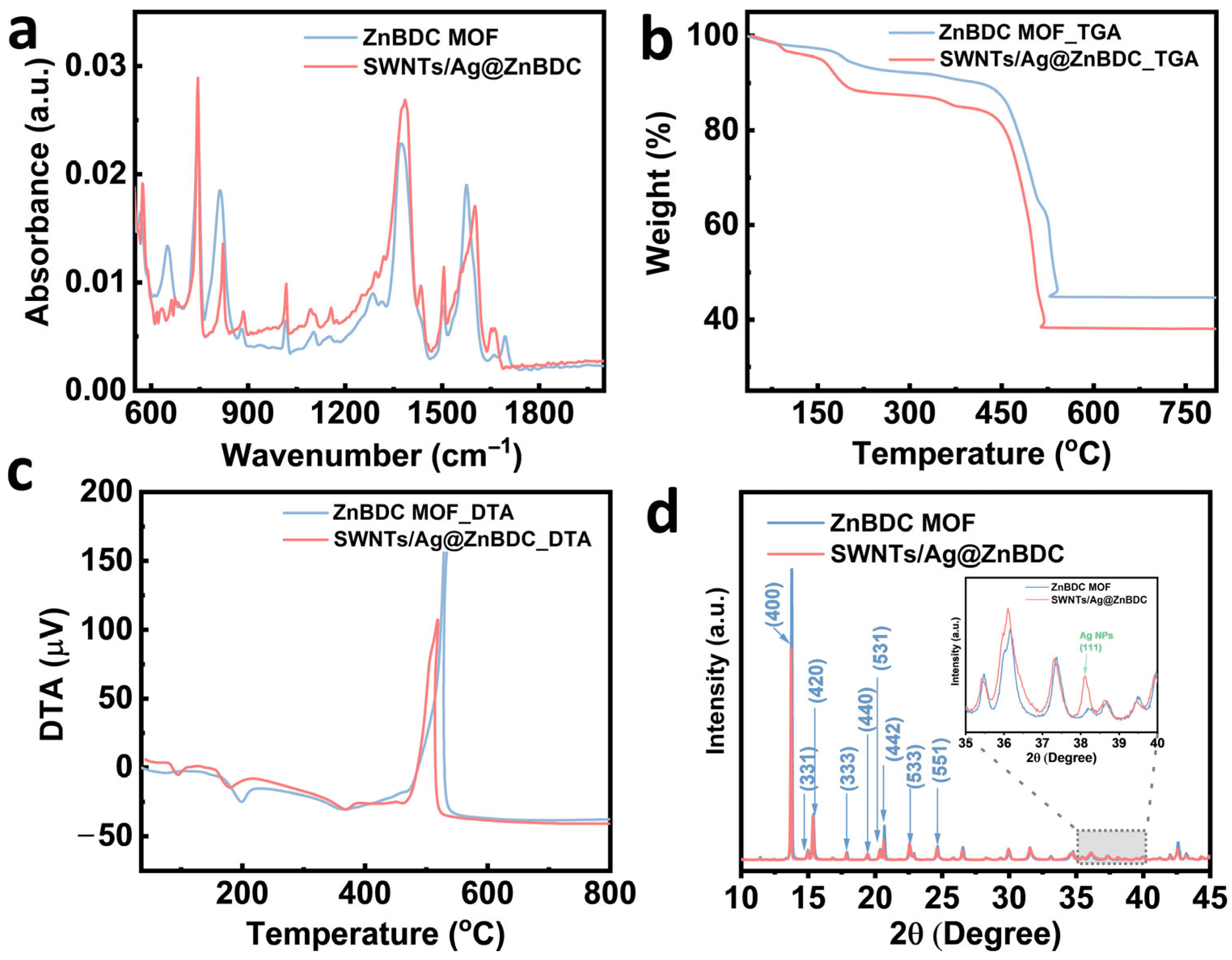
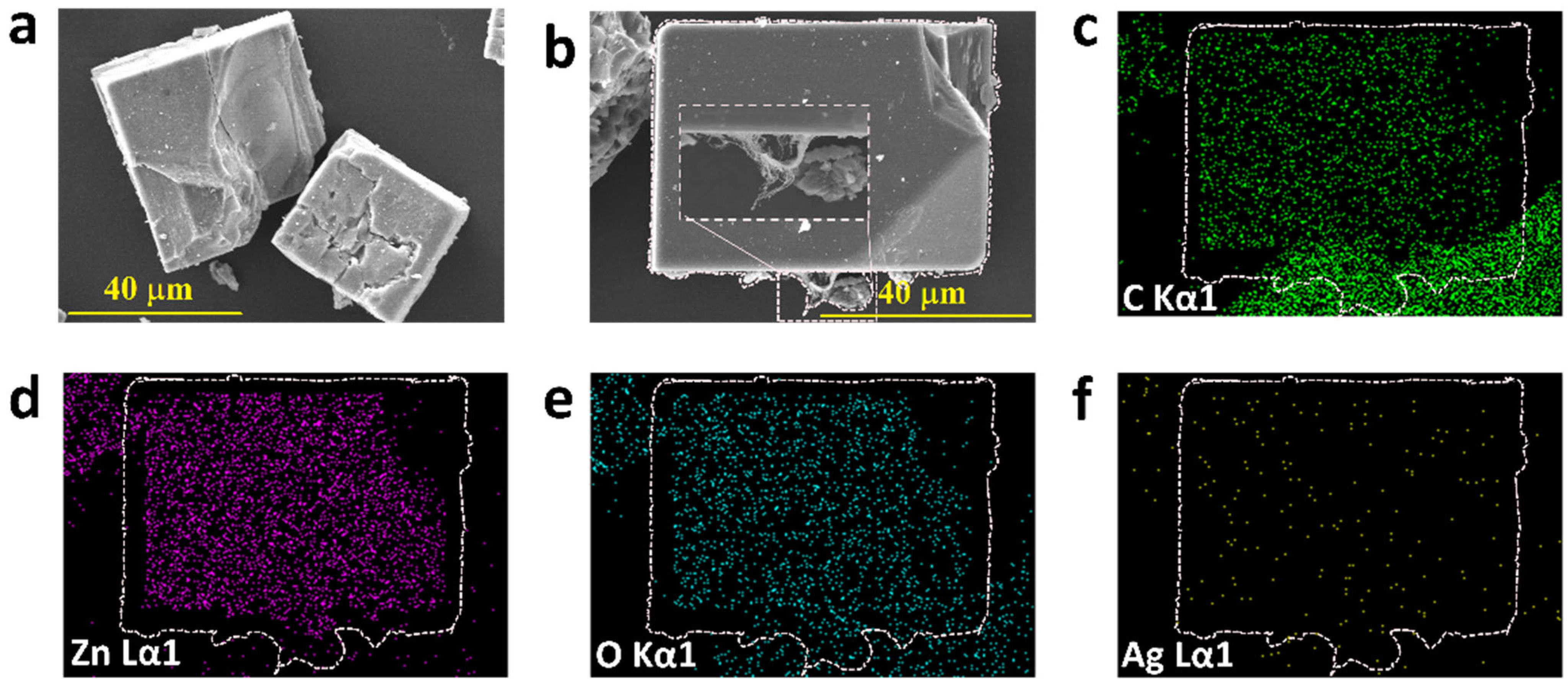
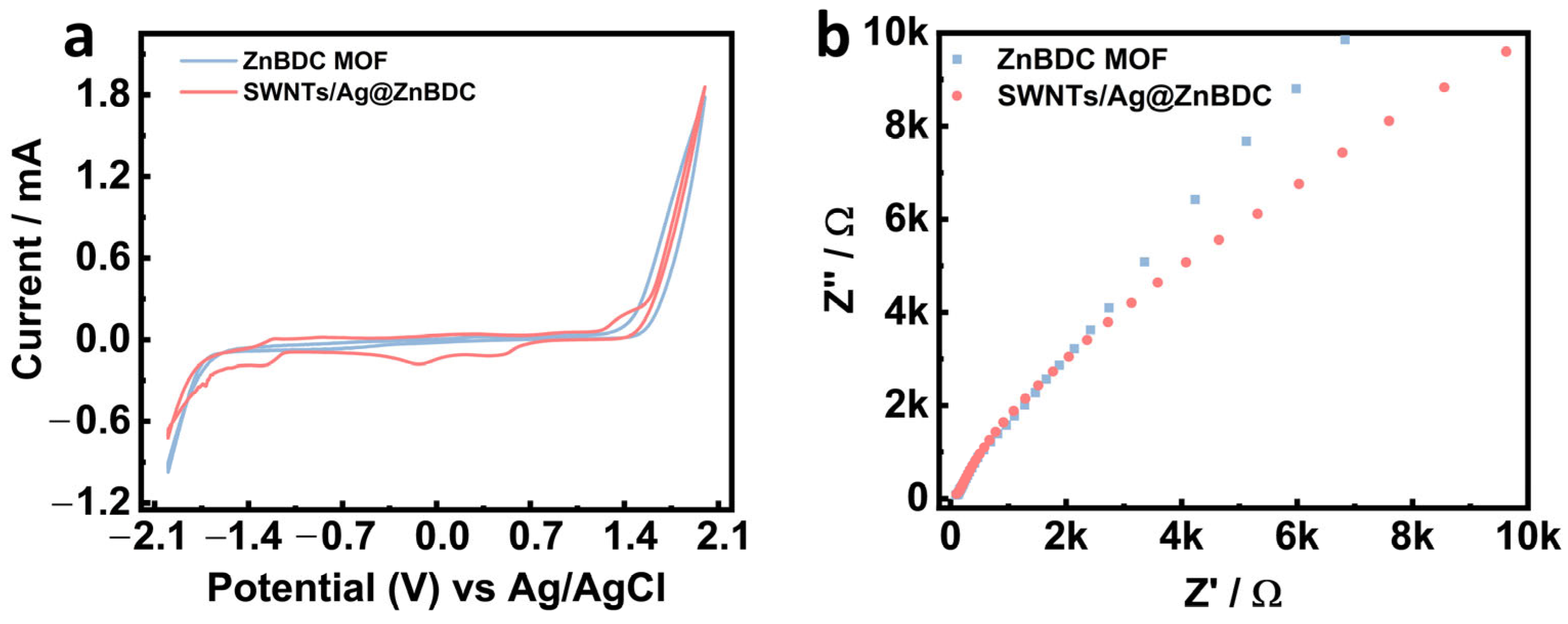
3.1. Material Analysis
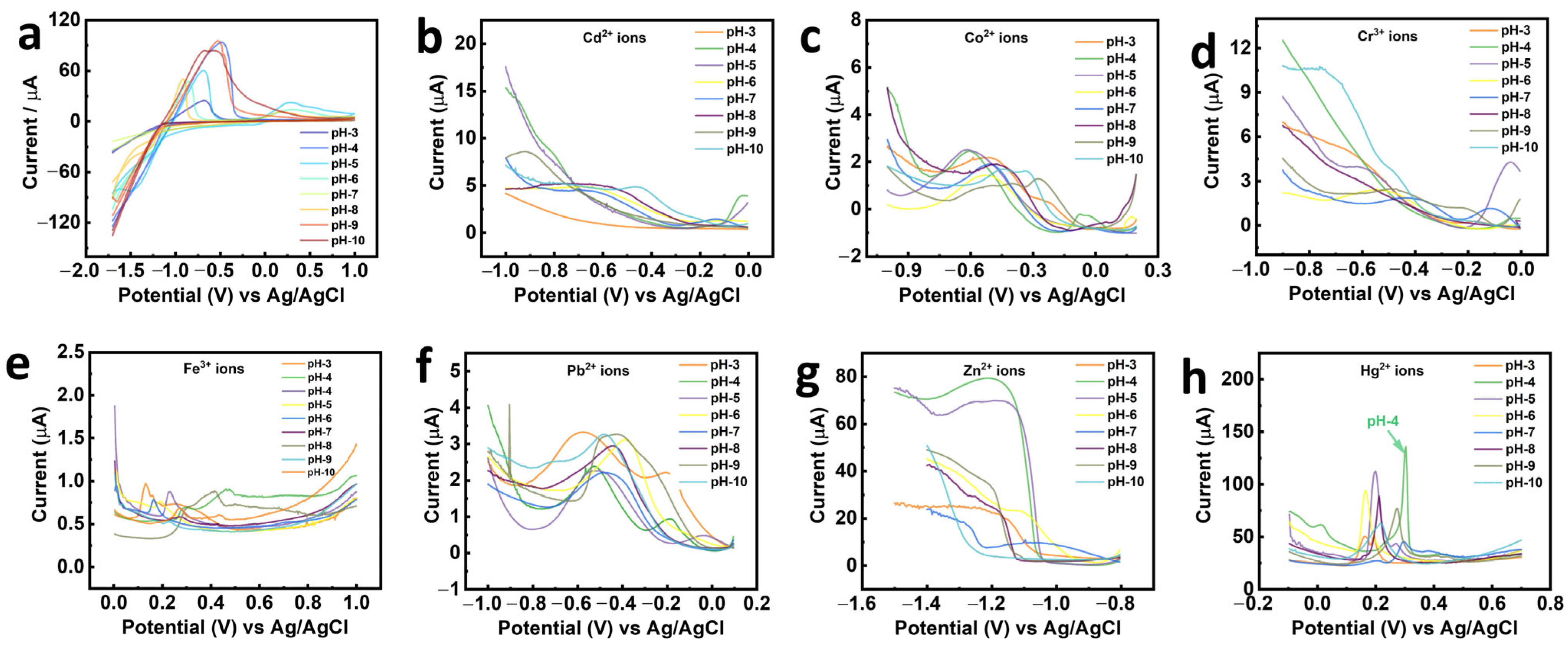
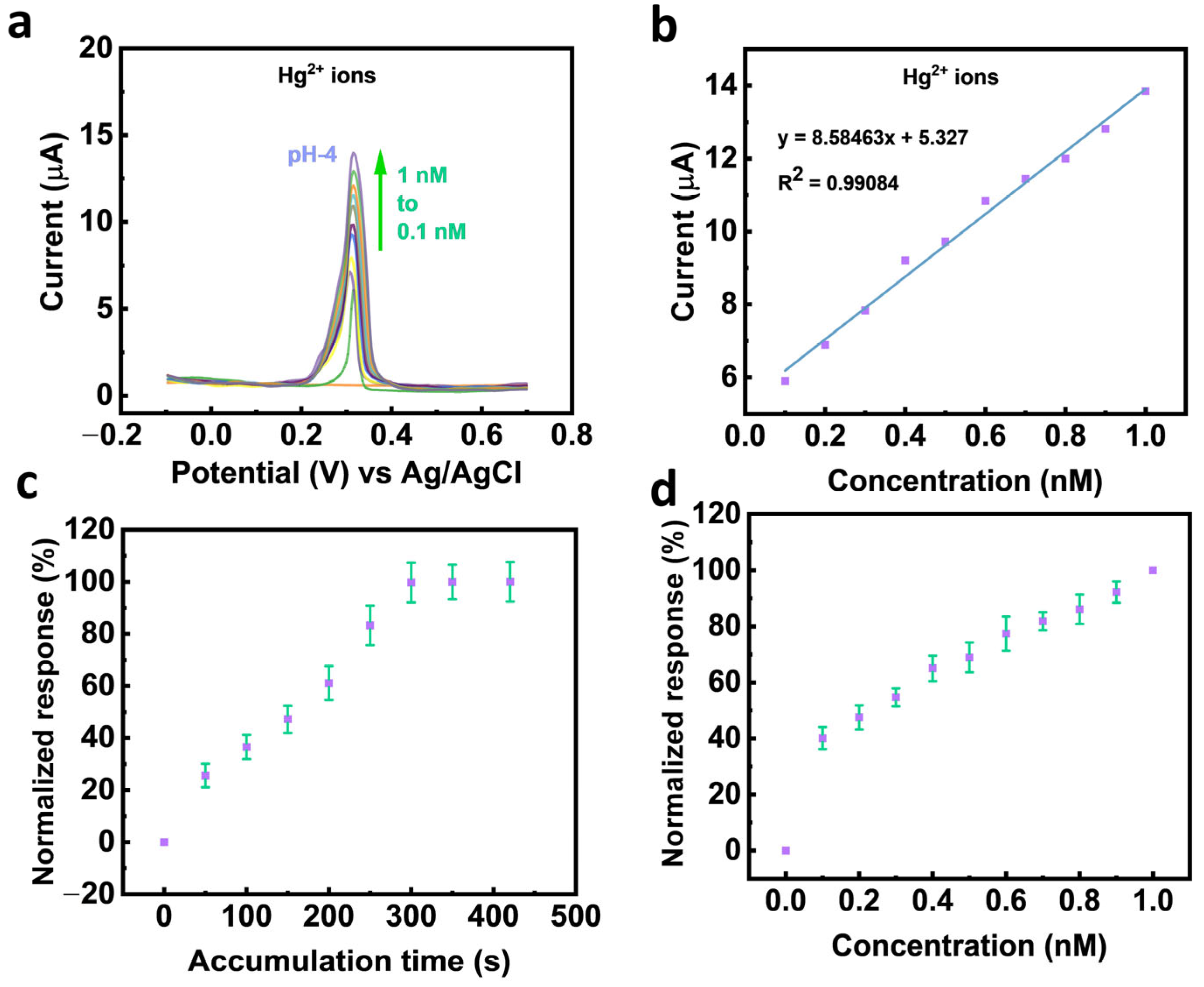
3.2. Electrochemical Sensing Performance of SWNTs/Ag@ZnBDC/GCE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, H.; Tu, Y.; Wang, H.; Wang, Z.; Li, Y.; Chai, L.; Zhang, W.; Lin, Z. Environmental behavior, human health effect and pollution control of heavy metal (loid) s towards full life cycle processes. Eco-Environ. Health 2022, 1, 229–243. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei, J.; Gu, L.; Zou, L.; Wang, T.; Chen, L.; Li, Y.; Yang, Y.; Li, P. DNAzyme-based biosensors for mercury (Ⅱ) detection: Rational construction, advances and perspectives. J. Hazard. Mater. 2022, 431, 128606. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A.; Mogaddam, M.R.A.; Kaya, S.; Katin, K.; Altunay, N. Synthesis of carbon modified with polymer of diethylenetriamine and trimesoyl chloride for the dual removal of Hg (II) and methyl mercury ([CH3Hg]+) from wastewater: Theoretical and experimental analyses. Mater. Chem. Phys. 2022, 277, 125501. [Google Scholar] [CrossRef]
- Shakeel, S.; Talpur, F.N.; Sirajuddin; Anwar, N.; Iqbal, M.A.; Ibrahim, A.; Afridi, H.I.; Unar, A.; Khalid, A.; Ahmed, I.A.; et al. Xanthan gum-mediated silver nanoparticles for ultrasensitive electrochemical detection of Hg2+ ions from water. Catalysts 2023, 13, 208. [Google Scholar] [CrossRef]
- Singh, G.; Sushma; Priyanka; Diksha; Mohit; Gupta, S.; Esteban, M.A.; Espinosa-Ruíz, C.; González-Silvera, D. Designing of thiosemicarbazone-triazole linked organotriethoxysilane as UV-Visible and fluorescence sensor for the selective detection of Hg2+ ions and their cytotoxic evaluation. J. Mol. Struct. 2022, 1255, 132446. [Google Scholar] [CrossRef]
- Ariño, C.; Banks, C.E.; Bobrowski, A.; Crapnell, R.D.; Economou, A.; Królicka, A.; Pérez-Ràfols, C.; Soulis, D.; Wang, J. Electrochemical stripping analysis. Nat. Rev. Methods Primers 2022, 2, 62. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Gao, J.; Zhang, Q. Current progress in 2D metal–organic frameworks for electrocatalysis. Small Struct. 2023, 4, 2200109. [Google Scholar] [CrossRef]
- Knebel, A.; Caro, J. Metal–organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation. Nat. Nanotechnol. 2022, 17, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, M.; Liu, Y.; Wang, J.; Zhang, G.; Li, L.; Du, L.; Wang, G.; Yang, S.; Wang, X. Single atoms meet metal–organic frameworks: Collaborative efforts for efficient photocatalysis. Energy Environ. Sci. 2022, 15, 3722–3749. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Hedau, B.S.; Deshmukh, M.A.; Patil, H.K.; Shirsat, S.M.; Phase, D.M.; Pandey, K.K.; Shirsat, M.D. Detection of Pb (II): Au Nanoparticle Incorporated CuBTC MOFs. Front. Chem. 2020, 8, 803. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Hedau, B.S.; Deshmukh, M.A.; Patil, H.K.; Shirsat, S.M.; Phase, D.M.; Pandey, K.K.; Shirsat, M.D. Selective and sensitive detection of lead Pb (II) ions: Au/SWNT nanocomposite-embedded MOF-199. J. Mater. Sci. 2021, 56, 474–487. [Google Scholar] [CrossRef]
- Nivetha, R.; Gothandapani, K.; Raghavan, V.; Van Le, Q.; Pitchaimuthu, S.; Muthuramamoorty, M.; Pandiaraj, S.; Alodhayb, A.; Kwan Jeong, S.; Nirmala Grace, A. Nano-MOF-5 (Zn) Derived porous carbon as support electrocatalyst for hydrogen evolution reaction. ChemCatChem 2021, 13, 4342–4349. [Google Scholar] [CrossRef]
- Song, Y.; Gong, C.; Su, D.; Shen, Y.; Song, Y.; Wang, L. A novel ascorbic acid electrochemical sensor based on spherical MOF-5 arrayed on a three-dimensional porous carbon electrode. Anal. Methods 2016, 8, 2290–2296. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Enhanced adsorption of ammonia on metal-organic framework/graphite oxide composites: Analysis of surface interactions. Adv. Funct. Mater. 2010, 20, 111–118. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; More, M.S.; Umar, A.; Ibrahim, A.A.; Siva, S.; Deshmukh, M.A.; Ingle, N.N.; Gaikwad, D.K.; Tsai, M.-L.; Hianik, T.; et al. Enhanced detection of heavy metal ions using Ag nanoparticles and single-walled carbon nanotubes within Cu-based metal-organic frameworks. J. Environ. Chem. Eng. 2024, 12, 113024. [Google Scholar] [CrossRef]
- Yadav, D.K.; Ganesan, V.; Marken, F.; Gupta, R.; Sonkar, P.K. Metal@ MOF materials in electroanalysis: Silver-enhanced oxidation reactivity towards nitrophenols adsorbed into a zinc metal organic framework—Ag@ MOF-5 (Zn). Electrochim. Acta 2016, 219, 482–491. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Pütter, H.; Müller, U. Hydrogen adsorption in metal–organic frameworks: Cu-MOFs and Zn-MOFs compared. Adv. Funct. Mater. 2006, 16, 520–524. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Siva, S.; Gaikwad, D.K.; Tsai, M.-L.; Hianik, T.; Kim, M.; Shirsat, M.D. Ag nanoparticles incorporated metal organic framework (Ag@ ZnBDC): Highly sensitive and selective detection of Hg2+ ions. J. Phys. Chem. Solids 2024, 193, 112142. [Google Scholar] [CrossRef]
- Munawar, T.; Bashir, A.; Mukhtar, F.; Nadeem, M.S.; Manzoor, S.; Ashiq, M.N.; Khan, S.A.; Koc, M.; Iqbal, F. Scalable synthesis of MOFs-derived ZnO/C nanohybrid: Efficient electrocatalyst for oxygen evolution reaction in alkaline medium. J. Korean Ceram. Soc. 2023, 60, 918–934. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Khandagale, D.D.; More, M.S.; Deshmukh, M.A.; Ingle, N.N.; Sayyad, P.W.; Mahadik, M.M.; Shirsat, S.M.; Al-Buriahi, M.S.; Tsai, M.L.; et al. Ag@MOF-199 Metal Organic Framework for Selective Detection of Nickel Ions in Aqueous Media. Ceram. Int. 2023, 49, 6772–6779. [Google Scholar] [CrossRef]
- Rahmani, M.; Ghafoorifard, H.; Afrang, S.; Ahmadi, M.T.; Rahmani, K.; Ismail, R. Effect of solution pH and adsorbent concentration on the sensing parameters of TGN-based electrochemical sensor. IET Nanobiotechnol. 2019, 13, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Deshmukh, S.; Roy, S.S.; Dhar, B.B. Selenium-doped Graphite for Electrochemical Sensing and Adsorption of Hg (II) and Cd (II) Ions. ChemElectroChem 2023, 10, e202201044. [Google Scholar] [CrossRef]
- Liu, T.; Xue, Q.; Jia, J.; Liu, F.; Zou, S.; Tang, R.; Chen, T.; Li, J.; Qian, Y. New insights into the effect of pH on the mechanism of ofloxacin electrochemical detection in aqueous solution. Phys. Chem. Chem. Phys. 2019, 21, 16282–16287. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Siva, S.; Cong, C.; Castro, I.J.L.; Kim, S.H.; Kim, M. Electrohydrodynamically configured aflatoxin B1 probing field effect transistor biosensor. Microchem. J. 2025, 215, 114385. [Google Scholar] [CrossRef]
- Siva, S.; Bodkhe, G.A.; Cong, C.; Kim, S.H.; Kim, M. Electrohydrodynamic-printed ultrathin Ti3C2Tx-MXene field-effect transistor for probing aflatoxin B1. Chem. Eng. J. 2024, 479, 147492. [Google Scholar] [CrossRef]
- Zorn, M.E.; Gibbons, R.D.; Sonzogni, W.C. Evaluation of approximate methods for calculating the limit of detection and limit of quantification. Environ. Sci. Technol. 1999, 33, 2291–2295. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Zhan, Y.; Singh, V.; Van Hung, T.; Nam, N.D. A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework. J. Hazard. Mater. 2020, 399, 123042. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Xu, W.; Peng, J.; Liu, Y.; Du, H. A ratiometric electrochemical sensor for simultaneous detection of multiple heavy metal ions in water and herbal medicines based on methylene blue-functionalised metal–organic framework. Microchem. J. 2024, 201, 110542. [Google Scholar] [CrossRef]
- Shao, Z.; Di, K.; Ding, L.; You, F.; Fan, C.; Wang, K. Amino-enriched Zn-MOFs with self-reduction for energy-free simultaneous removal and electrochemical detection of heavy metal ions in the aquatic environment. Anal. Chim. Acta 2025, 1333, 343408. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Moghaddam, B.S.; Nodehi, M. Cu-Based MOF for simultaneous determination of trace Tl (I) and Hg (II) by stripping voltammetry. J. Electrochem. Soc. 2020, 167, 167522. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Ke, X.-X.; Weerasooriya, R.; Li, H.; Wang, L.-C.; Wu, Y.-C. A green method to synthesise AuNPs/mpg-C3N4 nanocomposites for constructing anti-interference electrochemical sensing interface toward methylmercury. J. Alloys Compd. 2021, 853, 157365. [Google Scholar] [CrossRef]
- Kim, M.-H.; Hedau, B.S.; Sayyad, P.W.; Kim, J.; Ha, T.-J. Electrochemical environmental sensors based on La-doped zeolitic imidazolate framework-67 functionalised with L-cysteine for simultaneous detection of Cd2+ and Hg2+. Appl. Surf. Sci. 2025, 689, 162549. [Google Scholar] [CrossRef]
- Shejwal, N.N.; Patil, S.S.; Lanke, H.R.; Kamble, R.B.; Gole, P.V.; Shirsat, M.D. Ag-modified La-succinate composite as a novel electrochemical sensor for Hg2+ ion detection. J. Solid State Electrochem. 2025, 1–12. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; More, M.S.; Hashem, M.; Fouad, H.; Siva, S.; Deshmukh, M.A.; Ingle, N.N.; Kim, M.; Shirsat, M.D. Hg2+ ions sensor: Single walled carbon nanotubes incorporated Zn-metal organic framework. J. Nanoelectron. Optoelectron. 2023, 18, 993–1001. [Google Scholar] [CrossRef]
- Pei, L.; Yang, H.; Chen, S.; Wang, L. UiO-66-NHC (S) NHMe/three-dimensional macroporous carbon for removal and electrochemical detection of Cd2+, Pb2+, Cu2+, and Hg2+. Ind. Eng. Chem. Res. 2022, 61, 1588–1595. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene aerogel–metal–organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2018, 91, 888–895. [Google Scholar] [CrossRef]
- Economou, A.; Fielden, P.R. Mercury film electrodes: Developments, trends and potentialities for electroanalysis. Analyst 2003, 128, 205–213. [Google Scholar] [CrossRef]
- Clavijo Morales, J.A.; Zea Ramírez, H.R. Simultaneous quantification of Hg (II) and Pb (II) by square wave anodic stripping voltammetry using Bi/graphite electrode. Heliyon 2024, 10, e34656. [Google Scholar] [CrossRef]
- Tonle, I.K.; Ngameni, E.; Walcarius, A. Preconcentration and voltammetric analysis of mercury (II) at a carbon paste electrode modified with natural smectite-type clays grafted with organic chelating groups. Sens. Actuators B Chem. 2005, 110, 195–203. [Google Scholar] [CrossRef]
| Sensing Material | Technique | LOD (nM) | Reference |
|---|---|---|---|
| GCE/Cu-MOF nanocubes | DPV | 0.0633 | [30] |
| MB-UiO-66-NH2/MWCNTs/GC | DPASV | 6.42 | [31] |
| Amino-enriched Zn-MOFs | SWASV | 2.5 | [32] |
| Cu-MOF | SWASV | 1.7 | [33] |
| Ag/SWNTs@CuBTC MOF | DPV | 3.03 | [16] |
| AuNPs/mpg-C3N4/GCE | DPSV | 10.3 | [34] |
| La-doped ZIF+L-cystine | DPV | 52 | [35] |
| Ag-La-succinate/GCE | EIS | 0.1 | [36] |
| SWNTs@ZnBDC/GCE | CV | 6.74 | [37] |
| UiO-66-NHC(S)NHMe/3D-KSC | SWASV | 9.4 | [38] |
| GA-UiO-66-NH2 | DPV | 2.0 | [39] |
| Hg-film electrode | SWASV | 0.84 | [40] |
| Bi-film electrode | DPASV | 5.0 | [41] |
| Bare GCE | SWASV | 68–87 | [42] |
| SWNTs/Ag@ZnBDC/GCE | DPV | 0.102 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodkhe, G.A.; Hedau, B.; More, M.S.; Kim, M.; Shirsat, M.D. Electrochemical Sensing of Hg2+ Ions Using an SWNTs/Ag@ZnBDC Composite with Ultra-Low Detection Limit. Chemosensors 2025, 13, 259. https://doi.org/10.3390/chemosensors13070259
Bodkhe GA, Hedau B, More MS, Kim M, Shirsat MD. Electrochemical Sensing of Hg2+ Ions Using an SWNTs/Ag@ZnBDC Composite with Ultra-Low Detection Limit. Chemosensors. 2025; 13(7):259. https://doi.org/10.3390/chemosensors13070259
Chicago/Turabian StyleBodkhe, Gajanan A., Bhavna Hedau, Mayuri S. More, Myunghee Kim, and Mahendra D. Shirsat. 2025. "Electrochemical Sensing of Hg2+ Ions Using an SWNTs/Ag@ZnBDC Composite with Ultra-Low Detection Limit" Chemosensors 13, no. 7: 259. https://doi.org/10.3390/chemosensors13070259
APA StyleBodkhe, G. A., Hedau, B., More, M. S., Kim, M., & Shirsat, M. D. (2025). Electrochemical Sensing of Hg2+ Ions Using an SWNTs/Ag@ZnBDC Composite with Ultra-Low Detection Limit. Chemosensors, 13(7), 259. https://doi.org/10.3390/chemosensors13070259






