Electrostatic Self-Assembly of Heterostructured In2O3/Ti3C2Tx Nanocomposite for High-Selectivity NO2 Gas Sensing at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
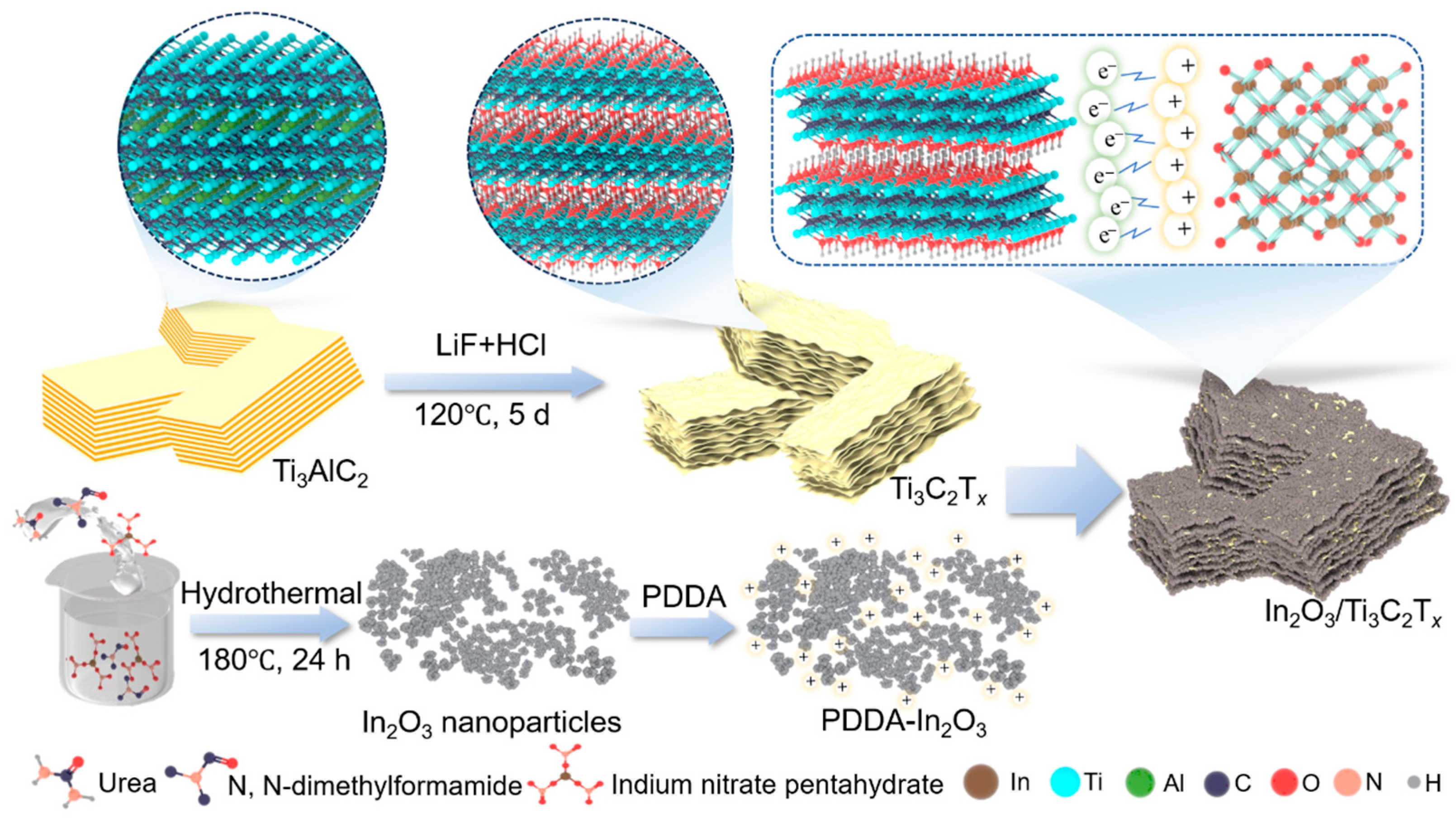
2.1. Sample Preparation
- (1)
- Synthesis of zero-dimensional In2O3 nanoparticles
- (2)
- Synthesis of 2D Ti3C2Tx
- (3)
- Synthesis of In2O3/Ti3C2Tx Composite
2.2. Characterization Method
2.3. Fabrication of Gas Sensors and Tests
3. Results and Discussion
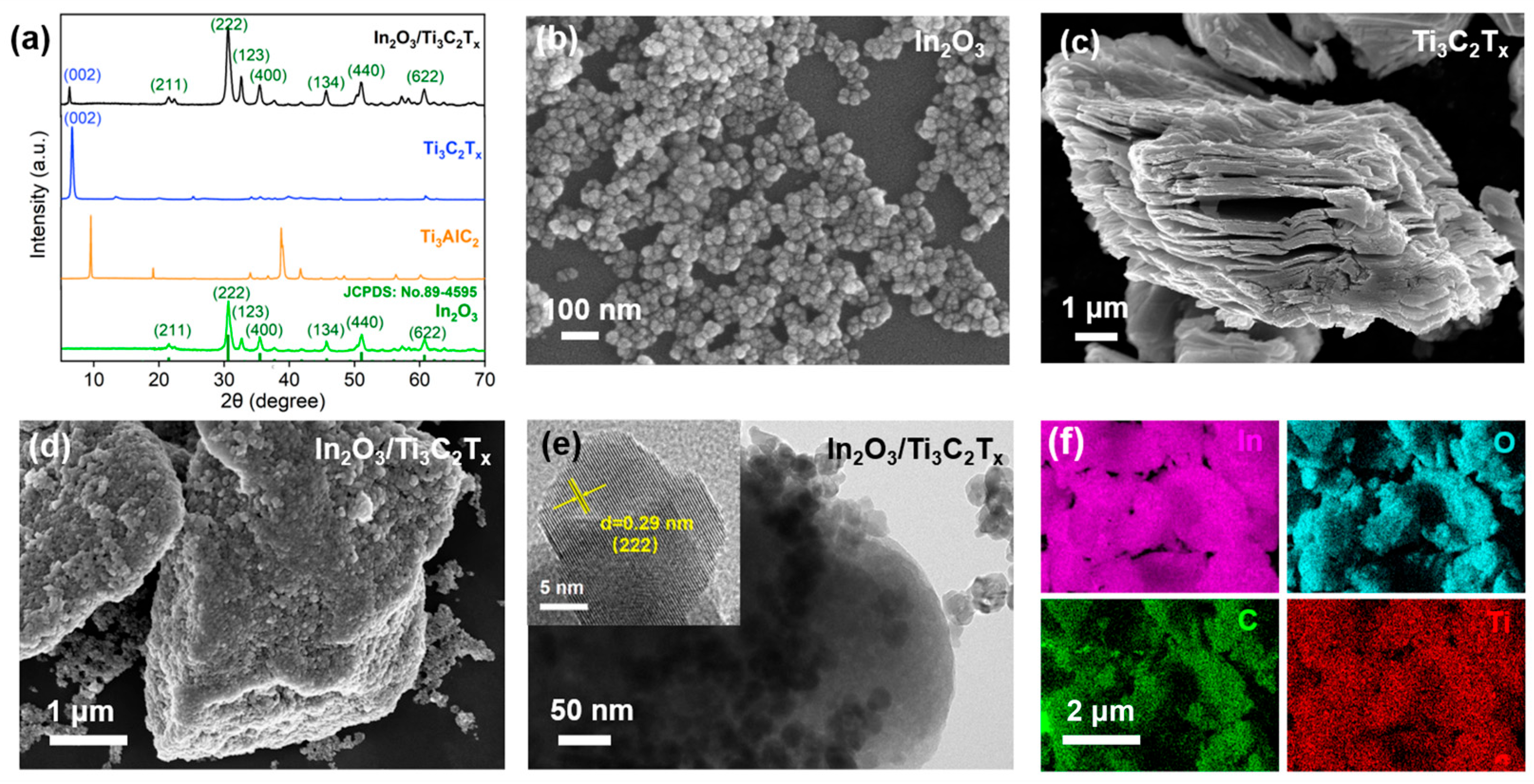
3.1. Characterization
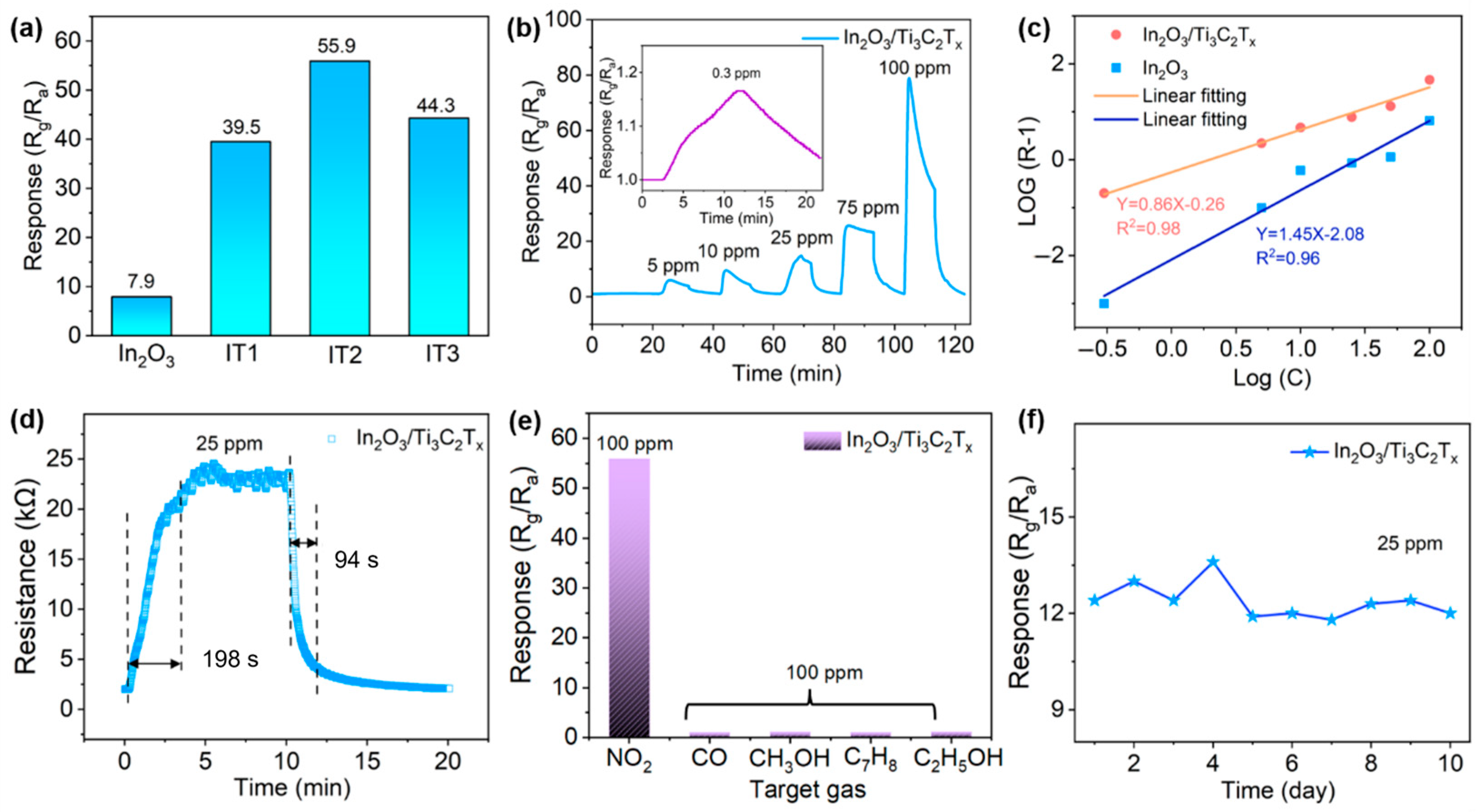
3.2. Gas-Sensing Performances
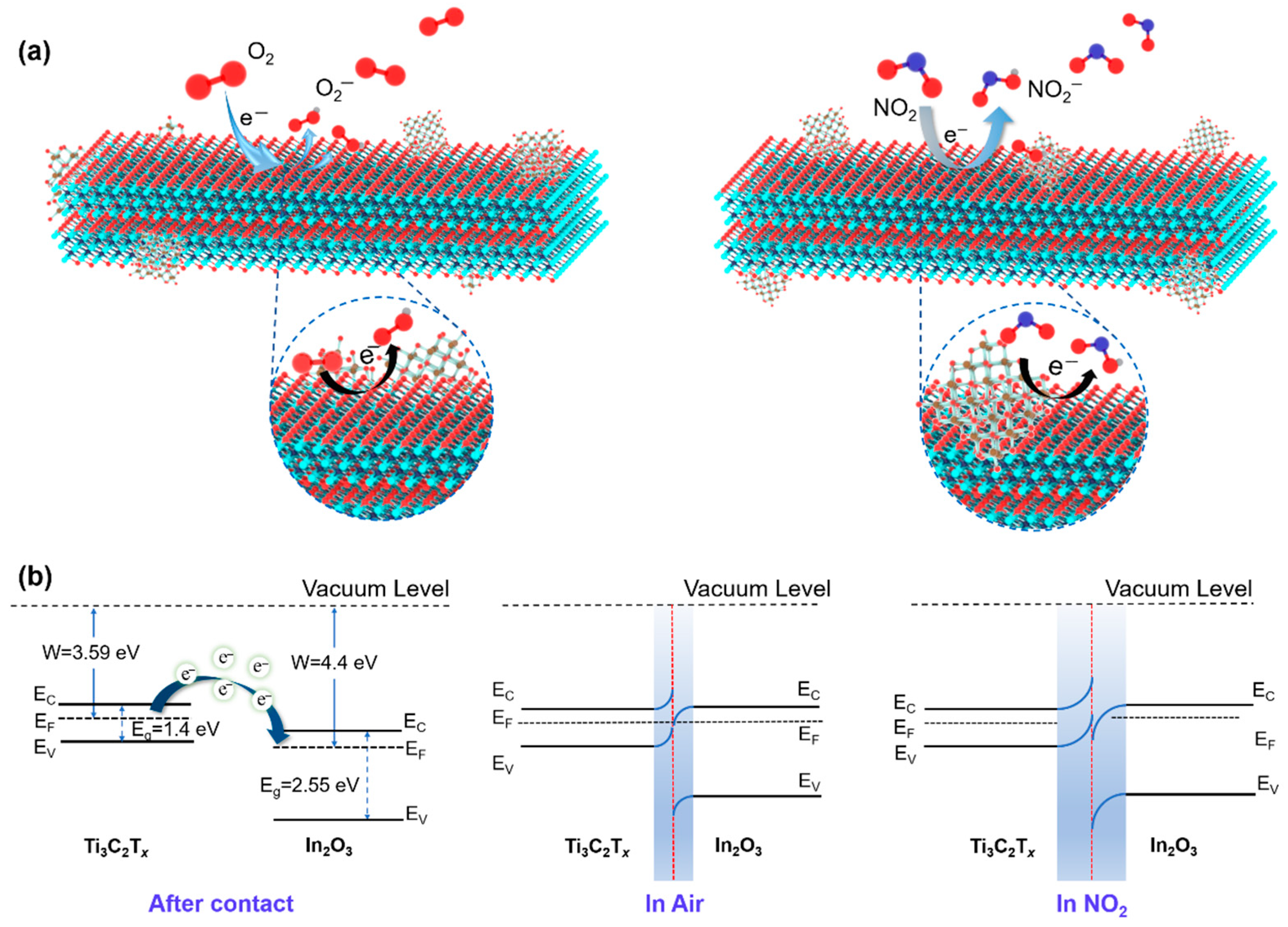
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhowmick, T.; Ghosh, A.; Nag, S.; Majumder, S. Sensitive and selective CO2 gas sensor based on CuO/ZnO bilayer thin-film architecture. J. Alloys Compd. 2022, 903, 163871. [Google Scholar] [CrossRef]
- Keerthana, S.; Rathnakannan, K. Hierarchical ZnO/CuO nanostructures for room temperature detection of carbon dioxide. J. Alloys Compd. 2022, 897, 162988. [Google Scholar] [CrossRef]
- Ji, H.; Guo, S.; Gao, L.; Yang, L.; Yan, H.; Zeng, H. Revolutionizing titanium production: A comprehensive review of thermochemical and molten salt electrolysis processes. Int. J. Min. Met. Mater. 2025. [Google Scholar] [CrossRef]
- Qian, Y.; Li, H.; Rosenberg, A.; Li, Q.; Sarnat, J.; Papatheodorou, S.; Schwartz, J.; Liang, D.; Liu, Y.; Liu, P. Long-term exposure to low-level NO2 and mortality among the elderly population in the southeastern United States. Environ. Health Perspect. 2021, 129, 127009. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Wu, P.; Qiu, X.; Wu, Y.; Duan, Z.; Ma, Y.; Yu, H.; Yuan, Z.; Jiang, Y.; Tai, H. Linear Model for Concentration Measurement of Mixed Gases. ACS Sens. 2025, 10, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhao, Q.; Duan, Z.; Xie, C.; Duan, X.; Li, S.; Ye, Z.; Jiang, Y.; Tai, H. Ag2Te nanowires for humidity-resistant trace-level NO2 detection at room temperature. Sens. Actuators B Chem. 2022, 363, 131790. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Ge, C.; Lei, S.; Hussain, S.; Wang, M.; Qiao, G.; Liu, G. Enhanced room-temperature NO2 sensing performance of SnO2/Ti3C2 composite with double heterojunctions by controlling co-exposed {221} and {110} facets of SnO2. Sens. Actuators B Chem. 2022, 365, 131919. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Huang, L.; Zhang, Y.; Hu, Z.; Sun, C.; Yang, X.; Pan, G.; Cheng, Y. AuPd bimetallic functionalized monodisperse In2O3 porous spheres for ultrasensitive trimethylamine detection. Sens. Actuators B Chem. 2023, 381, 133355. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, Z.; Jiang, C.; Zhang, X.; Qiao, Z.; Zhang, J.; Liang, J.; Wang, S.; Duan, Z.; Wu, Y. Ultrafast Hydrogen Detection System Using Vertical Thermal Conduction Structure and Neural Network Prediction Algorithm Based on Sensor Response Process. ACS Sens. 2025, 10, 2181–2190. [Google Scholar] [CrossRef]
- Bonardo, D.; Septiani, N.L.W.; Amri, F.; Humaidi, S.; Yuliarto, B. Recent development of WO3 for toxic gas sensors applications. J. Electrochem. Soc. 2021, 168, 107502. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.; Zhang, L.; Wang, Z.; Lin, S. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping. Sens. Actuators B Chem. 2023, 379, 133294. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, Y.; Liu, B.; Duan, Z.; Zhang, Y.; Yuan, Z.; Tai, H. Enhancing the carbon dioxide sensing performance of LaFeO3 by Co doping. Sens. Actuators B Chem 2024, 402, 135136. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, Y.C.; Liu, J.L.; Qu, Z.H.; Tian, S.Y.; Liu, H. Anti-humidity and high sensitivity sensor for detecting acetone with Ce-ZnO nanosheets. Ceram. Int. 2024, 50, 29787–29798. [Google Scholar] [CrossRef]
- Meghana, N.; Zimba, V.; Nayak, J. Enhancement of room temperature sensitivity and reduction of baseline drift in WO3/g-C3N4 nanocomposite based volatile organic compound gas sensors. Ceram. Int. 2025, 51, 6233–6243. [Google Scholar]
- Liu, X.C.; Wang, J.H.; Zhang, Y.T.; Zhang, D.Z. Hydrogen sulfide gas sensing characteristics based on copper oxide/molybdenum diselenide heterojunction. J. Alloys Compd. 2023, 963, 171197. [Google Scholar] [CrossRef]
- Pinna, N.; Neri, G.; Antonietti, M.; Niederberger, M. Nonaqueous synthesis of nanocrystalline semiconducting metal oxides for gas sensing. Angew. Chem. Int. Ed. 2004, 43, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. Ordered mesoporous In2O3: Synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar] [CrossRef]
- Zhang, B.; Bao, N.; Wang, T.; Xu, Y.; Dong, Y.; Ni, Y.; Yu, P.; Wei, Q.; Wang, J.; Guo, L. High-performance room temperature NO2 gas sensor based on visible light irradiated In2O3 nanowires. J. Alloys Compd. 2021, 867, 159076. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhang, W.; Zhang, J.; Wei, D.; Lu, R.; Zhu, L.; Li, H.; Shen, Y. In-situ growth of ZnO nanowire arrays on the sensing electrode via a facile hydrothermal route for high-performance NO2 sensor. Appl. Surf. Sci. 2018, 435, 1096–1104. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, J.; Hao, J.; Sun, Q.; Wan, P.; Li, Y.; Zhou, X.; Yuan, Y.; Zhang, X.; Wang, Y. Engineering SnO2 nanorods/ethylenediamine-modified graphene heterojunctions with selective adsorption and electronic structure modulation for ultrasensitive room-temperature NO2 detection. Nanotechnology 2021, 32, 155505. [Google Scholar] [CrossRef]
- Zhao, Q.-N.; Zhang, Y.-J.; Duan, Z.-H.; Wang, S.; Liu, C.; Jiang, Y.-D.; Tai, H.-L. A review on Ti3C2Tx-based nanomaterials: Synthesis and applications in gas and humidity sensors. Rare Met. 2021, 40, 1459–1476. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The rise of MXenes. In MXenes; Jenny Stanford Publishing: New York, NY, USA, 2023; pp. 3–11. [Google Scholar]
- Xu, B.; Gogotsi, Y. MXenes-The fastest growing materials family in the two-dimensional world. Chin. Chem. Lett. 2020, 31, 919–921. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-M.; Byun, J.-H.; Kim, S.S. Influence of grain size on gas-sensing properties of chemiresistive p-type NiO nanofibers. Sens. Actuators B Chem. 2016, 227, 149–156. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Sreedhar, A.; Tri, N.N.; Noh, J.-S. In situ growth of TiO2 on Ti3C2Tx MXene for improved gas-sensing performances. Ceram. Int. 2024, 50, 27227–27236. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Liu, G.; Wan, N.; Ge, C.; Hussain, S.; Meng, H.; Wang, M.; Qiao, G. Enhanced NO2 gas-sensing performance of 2D Ti3C2/TiO2 nanocomposites by in-situ formation of Schottky barrier. Appl. Surf. Sci. 2021, 567, 150747. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Q.; Yang, L.; Gao, J.; Yi, J.; Hou, M.; Guo, S.; Zeng, H. High-Throughput Experimental Technology: Rapid Identification of the Precious Metal Modified In 2 O 3 for NO 2 Low-Temperature Sensing. IEEE Sens. J. 2023, 23, 8101–8108. [Google Scholar] [CrossRef]
- Maleski, K.; Shuck, C.E.; Fafarman, A.T.; Gogotsi, Y. The broad chromatic range of two-dimensional transition metal carbides. Adv. Opt. Mater 2021, 9, 2001563. [Google Scholar] [CrossRef]
- Peng, M.; Wang, L.; Li, L.; Tang, X.; Huang, B.; Hu, T.; Yuan, K.; Chen, Y. Manipulating the interlayer spacing of 3D MXenes with improved stability and zinc-ion storage capability. Adv. Funct. Mater. 2022, 32, 2109524. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, W.; Zhang, M.; Wang, Y.; Duan, Z.; Tan, C.; Liu, B.; Ouyang, F.; Yuan, Z.; Tai, H. Edge-enriched Mo2TiC2Tx/MoS2 heterostructure with coupling interface for selective NO2 monitoring. Adv. Funct. Mater. 2022, 32, 2203528. [Google Scholar] [CrossRef]
- Yang, E.; Park, K.H.; Oh, T.; Kim, S.J. Ultra-dense SnO2 QDs-decorated MXene nanosheets with high water dispersibility for rapid NH3 sensing at room temperature. Sens. Actuators B Chem. 2024, 409, 135542. [Google Scholar] [CrossRef]
- Qin, W.; Lu, B.; Xu, X.; Shen, Y.; Meng, F. Metal organic framework-derived porous Ni-doped In2O3 for highly sensitive and selective detection to hydrogen at low temperature. Sens. Actuators B Chem. 2024, 417, 136123. [Google Scholar] [CrossRef]
- Xia, Y.; Le, T.; Peng, J.; Ravindra, A.; Xu, L. Pt quantum dots decorated nest-like 3D porous ZnO nanostructures for enhanced visible-light degradation of RhB. J. Porous Mater. 2020, 27, 1339–1348. [Google Scholar] [CrossRef]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.; Wang, J.; Sun, B.; Zhang, Y.; Wang, R.; Shi, K. Thin-layered MoS2 nanoflakes vertically grown on SnO2 nanotubes as highly effective room-temperature NO2 gas sensor. J. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Gu, X.; Li, D.; Zhang, D.; Zhang, D.; Huang, H.; Mao, B.; Kang, Z.; Shi, W. Carbon dots mediated charge sinking effect for boosting hydrogen evolution in Cu-In-Zn-S QDs/MoS2 photocatalysts. Appl. Catal. B-Environ. 2022, 301, 120755. [Google Scholar] [CrossRef]
- Xiang, J.C.; Zhang, Z.X.; Li, X.; Yang, L.; Guo, S.H.; Xia, Y. In2O3/MoS2 composite prepared by electrostatic self-assembly for NO2 sensing at room-temperature. J. Porous Mater. 2025, 5, 1–10. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, J.; Xu, X.; Zheng, C.; Wu, L. Highly sensitive NO2 gas sensor based on VS2/Ti3C2Tx/TiO2 nanocomposites. Microchem. J. 2025, 212, 113563. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.; Han, Y.; Xie, L.; Zhao, X.; Shahrokhian, S.; Barsan, N.; Zhu, Z. Conductometric Cr2O3/TiO2/Ti3C2Tx gas sensor for detecting triethylamine at room temperature. Sens. Actuators B 2023, 381, 133412. [Google Scholar] [CrossRef]
- Qiu, L.; Huo, Y.; Pan, Z.; Wang, T.; Yu, H.; Liu, X.; Tong, X.; Yang, Y. Resister-type sensors based on Ti3C2Tx MXene decorated In2O3 p-n heterojunction for ppb-level NO2 detection at room temperature. J. Environ. Chem. Eng 2025, 13, 115249. [Google Scholar] [CrossRef]
- Liu, M.; Ji, J.; Song, P.; Wang, J.; Wang, Q. Sensing performance of α-Fe2O3/Ti3C2Tx MXene nanocomposites to NH3 at room temperature. J. Alloys Compd. 2022, 898, 162812. [Google Scholar] [CrossRef]
- Xiao, B.X.; Wang, D.X.; Song, S.L.; Zhai, C.B.; Wang, F.; Zhang, M.Z. Fabrication of mesoporous In2O3 nanospheres and their ultrasensitive NO2 sensing properties. Sens. Actuators B Chem. 2017, 248, 519–526. [Google Scholar] [CrossRef]
- Choi, S.B.; Lee, J.K.; Lee, W.S.; Ko, T.G.; Lee, C. Optimization of the Pt Nanoparticle Size and Calcination Temperature for Enhanced Sensing Performance of Pt-Decorated In2O3 Nanorods. J. Korean Phys. Soc. 2018, 73, 1444–1451. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Gang, M.G.; Patil, V.L.; Dongale, T.D.; Patil, P.S.; Kim, J.H. Enhanced Gas-Sensing Response of Zinc Oxide Nanorods Synthesized via Hydrothermal Route for Nitrogen Dioxide Gas. J. Electron. Mater. 2019, 48, 589–595. [Google Scholar] [CrossRef]
- Mulik, R.; Chougule, M.; Khuspe, G.; Patil, V. In Hydrothermal Synthesis of Tungsten Oxide for the Detection of NO2 Gas. Tec. Soc. Appl. 2020, 1, 957–961. [Google Scholar]
- Liu, M.; Song, P.; Yang, Z.; Wang, Q. MXene/In2O3 nanocomposites for formaldehyde detection at low temperature. Inorg. Chem. Commun. 2023, 148, 110302. [Google Scholar] [CrossRef]

| Materials | Working Temperature (°C) | Response (Resistance Ratio) | Detection Range (ppm) | Selectivity Ratio | Ref. |
|---|---|---|---|---|---|
| In2O3 micro flower | 125 | 19.6 (5ppm) | - | ~21.7 | [44] |
| Pt decorated In2O3 | 300 | 11 (200 ppm) | - | ~11.2 | [45] |
| VS2/Ti3C2Tx/TiO2 nanocomposites | 180 | 4.73 (50 ppm) | 10–50 | - | [40] |
| ZnO nanorod | 150 | 6.7 (100 ppm) | - | ~12.4 | [46] |
| WO3 Nanorod | 200 | 24 (100 ppm) | - | ~15.6 | [47] |
| In2O3/Ti3C2Tx | Room temperature | 55 (100 ppm) | 0.3–100 | 45.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, Z.; Feng, H.; Dai, Q.; Zhao, Q.; Duan, Z.; Guo, S.; Yang, L.; Hou, M.; Xia, Y. Electrostatic Self-Assembly of Heterostructured In2O3/Ti3C2Tx Nanocomposite for High-Selectivity NO2 Gas Sensing at Room Temperature. Chemosensors 2025, 13, 249. https://doi.org/10.3390/chemosensors13070249
Guo Y, Zhang Z, Feng H, Dai Q, Zhao Q, Duan Z, Guo S, Yang L, Hou M, Xia Y. Electrostatic Self-Assembly of Heterostructured In2O3/Ti3C2Tx Nanocomposite for High-Selectivity NO2 Gas Sensing at Room Temperature. Chemosensors. 2025; 13(7):249. https://doi.org/10.3390/chemosensors13070249
Chicago/Turabian StyleGuo, Yongjing, Zhengxin Zhang, Hangshuo Feng, Qingfu Dai, Qiuni Zhao, Zaihua Duan, Shenghui Guo, Li Yang, Ming Hou, and Yi Xia. 2025. "Electrostatic Self-Assembly of Heterostructured In2O3/Ti3C2Tx Nanocomposite for High-Selectivity NO2 Gas Sensing at Room Temperature" Chemosensors 13, no. 7: 249. https://doi.org/10.3390/chemosensors13070249
APA StyleGuo, Y., Zhang, Z., Feng, H., Dai, Q., Zhao, Q., Duan, Z., Guo, S., Yang, L., Hou, M., & Xia, Y. (2025). Electrostatic Self-Assembly of Heterostructured In2O3/Ti3C2Tx Nanocomposite for High-Selectivity NO2 Gas Sensing at Room Temperature. Chemosensors, 13(7), 249. https://doi.org/10.3390/chemosensors13070249






