Abstract
Bisphenol S (BPS), a key ingredient in polycarbonate plastics and epoxy resins, is a known endocrine-disrupting compound that poses significant risks to human health and the environment. As such, the development of rapid and reliable analytical techniques for its detection is essential. In this work, we present a newly engineered electrochemical sensor designed for the sensitive and selective detection of BPS using a straightforward and effective fabrication approach. The sensor was constructed by grafting molecularly imprinted polymers (MIPs) onto vinyl-functionalized multiwalled carbon nanotubes (f-MWCNTs). Ethylene glycol dimethacrylate and acrylamide were used as the cross-linker and functional monomer, respectively, in the synthesis of the MIP layer. The resulting MIP@f-MWCNT nanocomposite was characterized using Fourier-transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). The MIP@f-MWCNT material was then combined with chitosan, a biocompatible binder, to fabricate the final MIP@f-MWCNT/chitosan-modified glassy carbon electrode (GCE). Electrochemical evaluation showed a broad linear detection range from 1 to 60 µM (R2 = 0.992), with a sensitivity of 0.108 µA/µM and a detection limit of 2.00 µM. The sensor retained 96.0% of its response after four weeks and exhibited high selectivity against structural analogues. In spiked plastic extract samples, recoveries ranged from 95.6% to 105.0%. This robust, cost-effective, and scalable sensing platform holds strong potential for environmental monitoring, food safety applications, and real-time electrochemical detection of endocrine-disrupting compounds like BPS.
1. Introduction
Bisphenol A (BPA), a hazardous phenolic compound, is a xenoestrogen known for being an endocrine disruptor [1]. It is extensively used in plastic production and remains the most studied bisphenol, having been linked to numerous health complications [2,3,4]. In response to these concerns, several countries have restricted its use. For example, in 2008, Canada restricted BPA in baby bottles. The European Union followed suit in 2011, and, in 2023, the European Food Safety Authority recommended a significant reduction in the acceptable daily intake. The United States and China have also implemented similar restrictions [5,6]. As a result of these regulations, Bisphenol S (BPS) has emerged as a potential substitute for BPA because of its superior photo-stability and thermal stability, along with its reduced toxicological and genotoxic effects [7]. However, BPS remains less studied, and current detection methods are limited. Many existing techniques involve complex sample preparation, making them unsuitable for real-time or on-site analysis. Established methods such as chromatography and mass spectrometry, while reliable, require costly equipment, advanced instrumentation, and specialized expertise [8]. This study addresses the growing demand for simpler, more affordable, rapid, and selective methods for BPS detection.
Electrochemical sensors have gained popularity in environmental monitoring, food safety analysis, and pharmaceutical applications due to their excellent sensitivity, low cost, miniaturization potential, and rapid response [9]. BPS, with its two electroactive phenolic hydroxyl groups, is particularly suitable for electrochemical detection. Studies show that chemically modified electrodes can significantly improve conductivity, selectivity, and signal strength, resulting in more accurate detection. Among modification strategies, molecularly imprinted polymers (MIPs) stand out as highly innovative and versatile materials. As biomimetic materials, MIPs offer superior selectivity, robustness, effective binding ability, cost-effectiveness, chemical stability, and recyclability—qualities that make them perfect for sensor applications [1,10]. However, despite these advantages, MIP synthesis often involves reagents that pose environmental and safety risks. These reagents, including some used in this study, are derived from petrochemical sources, exhibit toxicity, and produce waste that requires special disposal procedures [11]. Regardless, in this work, we aimed to reduce environmental impact by optimizing reagent amounts and adopting a simplified fabrication approach. Notably, most MIPs are inherently non-conductive, which hampers electron transfer and limits sensor sensitivity. To address this, researchers commonly integrate MIPs with conductive nanomaterials or nanoparticles (NMs/NPs), such as multiwalled carbon nanotubes (MWCNTs) [12], metal NPs (e.g., Au-NPs, Ni-NPs) [13], and graphene quantum dots (GQDs) [14]. These combinations enhance electron transfer and increase surface area, effectively merging the selectivity of MIPs with the high sensitivity of NMs to improve overall sensor performance.
Ertürk and Mattiasson [15] identified bulk, surface (e.g., grafting), and epitope imprinting as the most common techniques used in sensor development. Bulk polymerization, though widely used in MIP synthesis, often suffers from low binding efficiency, poor site accessibility, complex production processes, slow kinetics, and challenges in template removal. Surface imprinting, particularly via grafting onto materials like MWCNTs, effectively overcomes these limitations [12]. By placing recognition cavities on the surface of nanomaterials, surface imprinting improves site accessibility and binding kinetics [16]. Recent studies show that surface-imprinted MIPs on MWCNTs enhance electrochemical detection by forming high-affinity binding sites directly on the substrate. This configuration reduces diffusion limitations and accelerates interactions between the template and polymer [11]. While MIP-based sensors often exhibit slower response times due to diffusion constraints, surface imprinting enables faster and more efficient detection by improving analyte accessibility. Epitope imprinting, which targets specific fragments of larger molecules, is ideal for recognizing macromolecules such as proteins or antibodies. Despite its advantages, surface imprinting is underutilized for small molecules, with bulk imprinting remaining more common. It is more frequently applied to biomolecules like proteins [16], revealing a significant research gap in small-molecule detection, including targets like BPS. This study explores the potential of surface imprinting for small molecules and establishes a basis for future work in this area.
MWCNTs are widely appreciated for their exceptional mechanical strength, high electrical conductivity, and excellent thermal properties. However, in sensor applications, their main limitation lies in their poor dispersibility and strong tendency to aggregate [17]. Covalent functionalization addresses these issues by introducing functional groups, such as carboxylic acids, through oxidation, which also creates reactive sites for further modification. Vinyl-functionalized MWCNTs (f-MWCNTs) can serve as a platform for MIP synthesis, forming MIP@f-MWCNT nanocomposites. These materials combine the high conductivity and surface area of MWCNTs with the selective recognition properties of MIPs, making them highly suitable for fabricating sensitive and selective sensors [18]. Compared to other functionalized variants (e.g., carboxylated or amine-functionalized MWCNTs), vinyl-functionalized MWCNTs offer better stability, conductivity, and compatibility with MIP synthesis [19].
Functionalizing MWCNTs with vinyl groups typically begins with carboxylation, often achieved through oxidation using reagents such as nitric acid (HNO3), aqua regia (HCl and HNO3), piranha solution (H2SO4 and H2O2), or sulfonitric acid (H2SO4 and HNO3 in a 3:1 ratio). Among these, sulfonitric acid is considered the most effective for oxidation and dispersion [17]. This study highlights a novel room-temperature carboxylation method that offers a simpler and faster alternative to conventional techniques, which typically require reflux heating at 60–85 °C and extended reaction times [20]. Traditional carboxylation can degrade the MWCNT structure and disrupt its π-electron system, negatively affecting electronic properties and sensor performance [18]. While acid reflux oxidation for up to 24 h can cause material losses of 60–90%, morphological studies indicate minimal structural damage within 6–9 h [21]. A recent study [22] demonstrated efficient carboxylation at 30 °C using sulfonic acid and ultrasonication for 3 h, and Hoel et al. [23] successfully carried out room-temperature carboxylation of MWCNTs for pharmaceutical applications. However, the further modification of carboxylated MWCNTs under mild conditions for sensor development remains underexplored.
Sharipov et al. (2024) [24] developed a paper-based surface-enhanced Raman scattering (SERS) substrate for BPS detection. However, its high cost and poor stability under variable environmental conditions limit its usability in resource-constrained regions [25,26]. Hyder et al. developed a sensor based on NiO nanostructures functionalized with supramolecular systems, which showed good selectivity for BPS. Still, their system faced potential interference from organic and inorganic substances in complex samples [27]. Additionally, their study lacked a detailed evaluation of selectivity against structural analogues like hydroquinone and provided limited data on long-term stability, leaving questions about the sensor’s robustness. Hanbing et al. [28] fabricated a BPS MIP sensor using graphene quantum dots (GQDs) coated on nickel nanospheres, improving surface area and conductivity. However, GQDs are known to suffer from stability and aggregation issues [29], which limit their reliability. In contrast, vinyl-functionalized MWCNTs offer superior mechanical stability, long-term durability, and potential for scalable fabrication of robust composites [30,31].
Herein, we developed a robust electrochemical sensor by integrating MIPs and vinyl-functionalized MWCNTs. The resulting MIP@f-MWCNT composite was immobilized on a glassy carbon electrode (GCE) using chitosan as a biocompatible and cost-effective binder, creating an efficient sensing interface. This approach takes advantage of the synergistic properties of surface imprinting and f-MWCNTs to improve sensor performance for BPS detection. By combining only MIPs with f-MWCNTs, the sensor effectively addresses common challenges in electrochemical sensing, such as limited selectivity, poor stability, and complex fabrication processes. To the best of our knowledge, this is the first report of this specific electrode configuration for BPS detection. Comprehensive characterization and validation demonstrated that the sensor exhibits high sensitivity and selectivity for BPS in spiked plastic bottle extracts using differential pulse voltammetry (DPV), offering a promising and scalable platform for environmental and food safety monitoring.
2. Materials and Methods
2.1. Reagents
MWCNTs (95% purity, diameter 10–20 nm, and length 10–30 µm), BPS (99%), ethylene glycol dimethacrylate (EGDMA), KCl, and azobisisobutyronitrile (AIBN) were purchased from Aladdin Industrial Corporation (Shanghai, China). Acetonitrile (ACN), toluene, acrylamide (AAm), and acetic acid were obtained from Sigma–Aldrich (Shanghai, China). Dimethylformamide (DMF), tetrahydrofuran (THF), and thionyl chloride (SOCl2) were sourced from Macklin (Shanghai, China). Potassium ferricyanide [K3Fe(CN)6] and potassium ferrocyanide [K4Fe(CN)6] were acquired from Shanghai Lingfeng Chemicals (Shanghai, China). Sodium chloride (NaCl), disodium phosphate (Na2HPO4), sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O), nitric acid (HNO3, 65% w/w), and sulfuric acid (H2SO4, 98% w/w) were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Chitosan was obtained from Meryer (Shanghai, China). Phosphate-buffered saline (PBS, 0.1 M, pH 7.4) was freshly prepared and used as the supporting electrolyte. Double-distilled water was purified using a Milli-Q deionized (DI) water system. All chemicals were of analytical grade and used without further purification.
2.2. Instruments
The surface morphology of the engineered materials was investigated using a ZEISS Gemini Crossbeam 350 Scanning Electron Microscope (SEM) machine (Shanghai, China). Functional group elucidation was performed via Fourier-transform infrared (FT-IR) spectroscopy using a Nicolet iS50 machine (Waltham, MA, USA), within a wavenumber range of 400–4000 cm−1. Electrochemical characterization and analyte detection were performed using a CHI660E workstation (Shanghai, China) with a three-electrode setup: a modified GCE (3 mm diameter) as the working electrode, a bare GCE as control, a saturated calomel electrode (SCE) as a reference, and a platinum wire as the auxiliary electrode. The pH of the solutions was measured using a calibrated PHS-3C pH meter from Shanghai INESA Scientific Instrument Co. (Shanghai, China). All measurements were conducted at room temperature.
2.3. Vinyl Functionalization of MWCNTs
Carboxylation of pristine MWCNTs was performed using a modified version of a previously reported method [32]. Briefly, 0.6 g of crude MWCNTs was oxidized in 60 mL of a concentrated sulfuric–nitric acid solution (3:1 v/v) at room temperature for 4, 6, and 8 h to evaluate the effect of oxidation time. After the reaction, the products were centrifuged and thoroughly washed with deionized water and ethanol until a neutral pH was achieved, then dried in a vacuum oven at 50 °C.
The preparation of f-MWCNTs was conducted as follows: Functionalization was conducted according to a previously established method [33]. First, 0.4 g of MWCNTs-COOH was reacted with 10 mL of SOCl2 in 30 mL of chloroform. The mixture was sonicated and then refluxed at 60 °C for 24 h to convert the carboxylic groups into acyl chlorides (MWCNTs-COCl). The solid product was washed three times with THF, chosen for its polar aprotic nature, low boiling point, and chemical stability, to ensure the complete removal of residual SOCl2 [20,32]. The resulting MWCNTs-COCl were then dispersed in DMF and reacted with 0.8 g of AAm under reflux at 60 °C for 24 h to yield vinyl-functionalized MWCNTs (f-MWCNTs). The product was washed with THF and dried under vacuum.
2.4. Synthesis of MIP@f-MWCNTs Nanocomposites
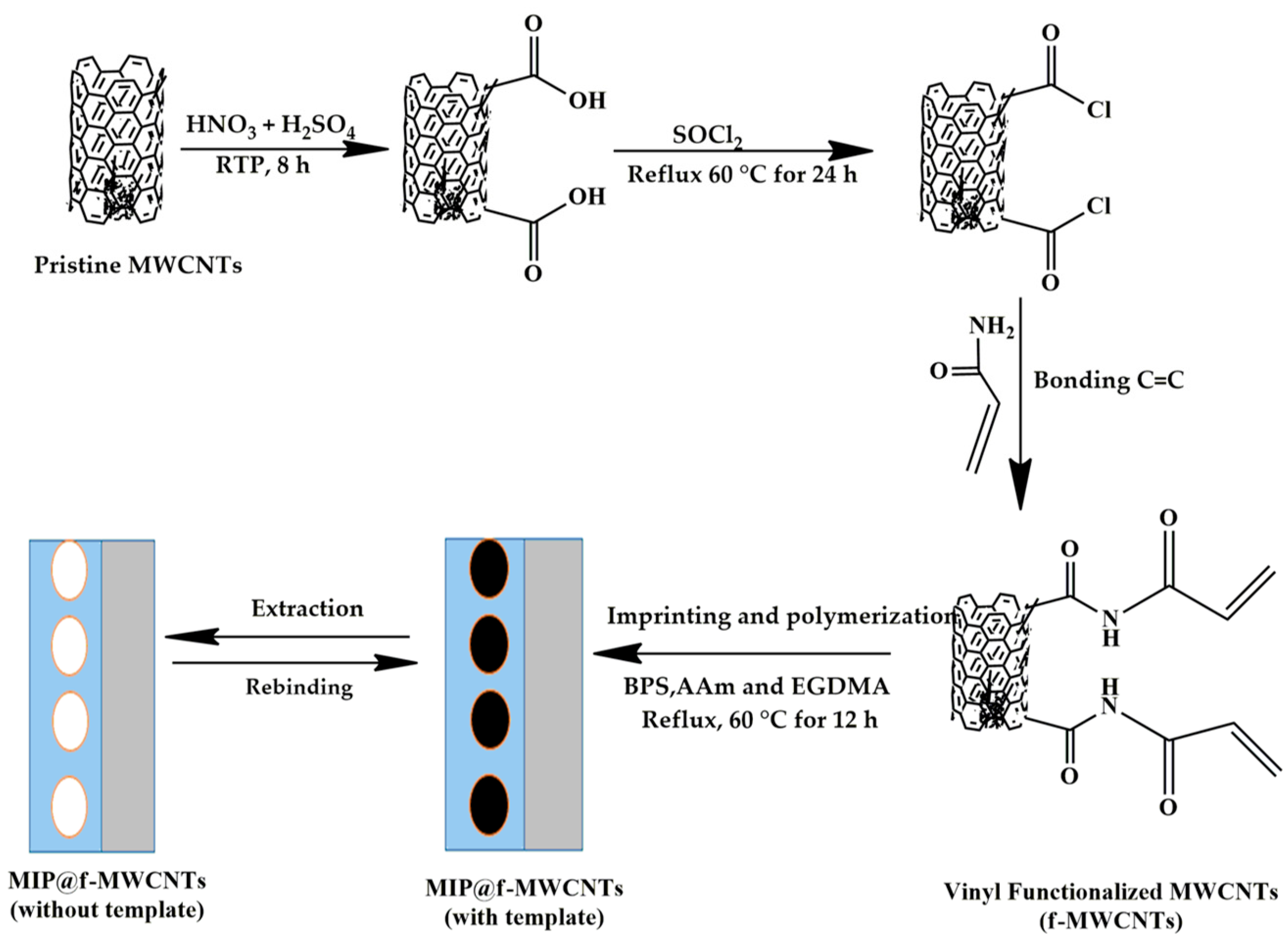
The MIP@f-MWCNTs composite (referred to simply as MIP) was synthesized as illustrated in Scheme 1. First, 0.02 g of f-MWCNTs was dispersed in a mixture of 15 mL acetonitrile and 2 mL toluene. Then, 3 mL of a DMF solution containing 0.05 mmol of BPS (the template) and 0.25 mmol of the biodegradable monomer AAm was added. The mixture was stirred under a nitrogen atmosphere to promote the formation of the template–monomer complex. Subsequently, 1.25 mmol of EGDMA (the cross-linker) and 10 mg of AIBN (the initiator) were introduced. The molar ratio of template, functional monomer, and cross-linker was maintained at 1:5:25. Polymerization was carried out at 60 °C for 12 h. After polymerization, the resulting MIP was washed with ethanol, followed by solvent extraction using a methanol–acetic acid solution (9:1 v/v) to remove the BPS template. Successful template removal was confirmed by UV–Vis spectroscopy, with the corresponding spectra shown in Figure S1 of the Supporting Information. The final product was dried in a vacuum oven at 50 °C. Non-imprinted polymers (NIP@f-MWCNTs) were synthesized under identical conditions, excluding the template molecule, for comparison.
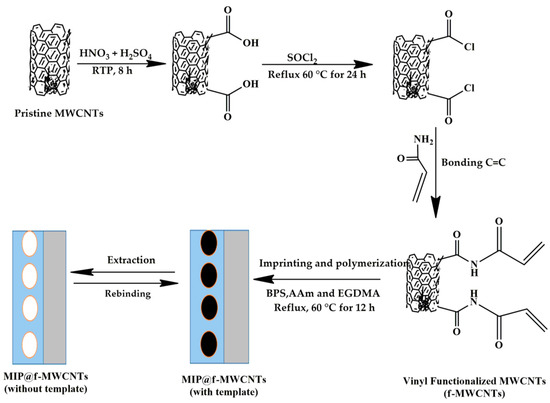
Scheme 1.
Schematic illustration of the synthesis of the MIPs@f-MWCNTs composite.
2.5. Electrode Modification and Electrochemical Measurements
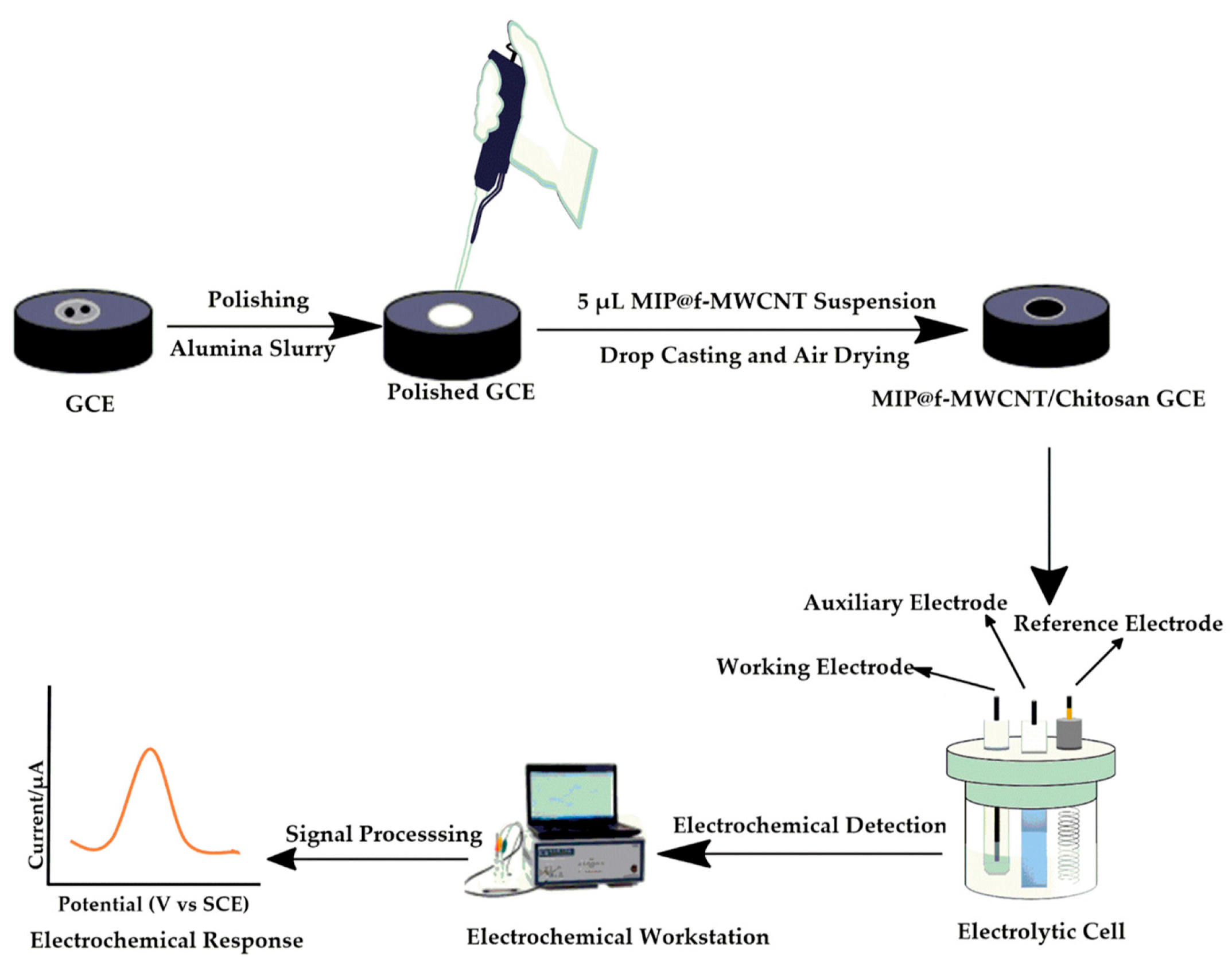
Scheme 2 illustrates the sensor fabrication and electrochemical detection process. Prior to modification, the GCEs were cleaned using standard polishing and ultrasonic procedures to ensure a clean and active surface. The synthesized MIP composite (0.02 g) was dispersed in a 500 µL 0.5% chitosan acetate solution and ultrasonicated for 15 min. Then, a 5.0 µL aliquot of the resulting suspension was drop-cast onto the cleaned GCE surface to fabricate the MIP@f-MWCNTs/Chitosan/GCE (referred to as MIP-GCE). A corresponding non-imprinted sensor (NIP-GCE) was prepared using the NIP composite under identical conditions for comparison. The electrochemical properties of the bare GCE, MIP-GCE, and NIP-GCE were evaluated using CV in a 1.0 mM [Fe(CN)6]3−/4− solution containing 0.1 M KCl as the redox probe. DPV was used to investigate the electrode’s response to BPS in 0.1 M phosphate-buffered saline (PBS, pH 7.4). To ensure reliability and enhance sensor performance, key parameters such as electrode incubation time, solution pH, and scan rate were systematically optimized. DPV was also used to determine the sensor’s linear detection range, limit of detection (LoD), sensitivity, selectivity, reproducibility, and stability. Finally, the practical applicability of the sensor was validated by detecting BPS in real samples, with measurements conducted within a potential window of +0.2 to +1.2 V.
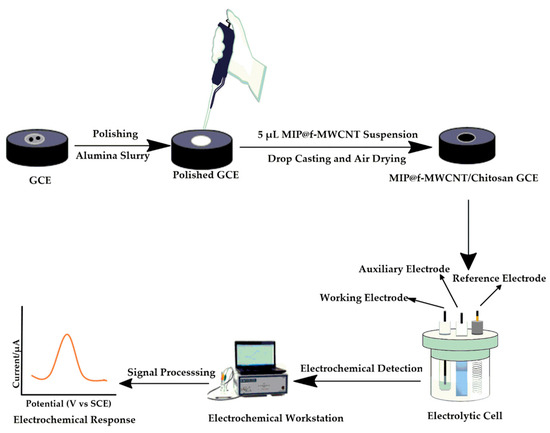
Scheme 2.
Electrode modification and electrochemical detection processes.
2.6. Quantification of BPS in Real Samples
To detect BPS in real-world samples, a modified method based on Zahedi et al. [11] was adapted with modifications. Commercially available plastic water bottles were obtained, thoroughly cleaned, dried, and cut into small pieces. A 10 g portion of these fragments was immersed in 50.0 mL of absolute ethanol and stirred at 55 °C for 24 h, followed by an additional 3 h of stirring at room temperature. The resulting mixture was then centrifuged at 10,000 rpm for 10 min and filtered using a 0.45 μm filter. The filtrate was concentrated under reduced pressure using a rotary evaporator until the volume was reduced to 5.0 mL. This concentrate was then diluted with PBS (pH 6.0) to a final volume of 50.0 mL, making it suitable for electrochemical analysis. Quantitative analysis of BPS was then performed using the standard addition method.
3. Results and Discussion
3.1. Characterization of Fabricated Materials
3.1.1. FT-IR of Synthesized Materials
FT-IR of MWCNTs-COOH: FT-IR analysis of room-temperature carboxylation reveals the time-dependent incorporation of carboxyl groups on MWCNTs and the oxidation efficiency under mild conditions (Figure S2). No significant carboxyl-related peaks were detected at 4 (Figure S2A) and 6 (Figure S2B) hours, indicating minimal or undetectable functionalization. At 8 h (Figure S2C), a sharp increase in peak intensities confirmed successful grafting. These results align with studies linking oxidation time to functionalization extent, though excessive treatment can degrade MWCNT [34]. Our study underscores the importance of optimizing reaction time to balance effective functionalization with the preservation of MWCNT structural integrity. A more comprehensive analysis and interpretation of the FT-IR spectral data relevant to this subject matter are presented in the subsequent section.
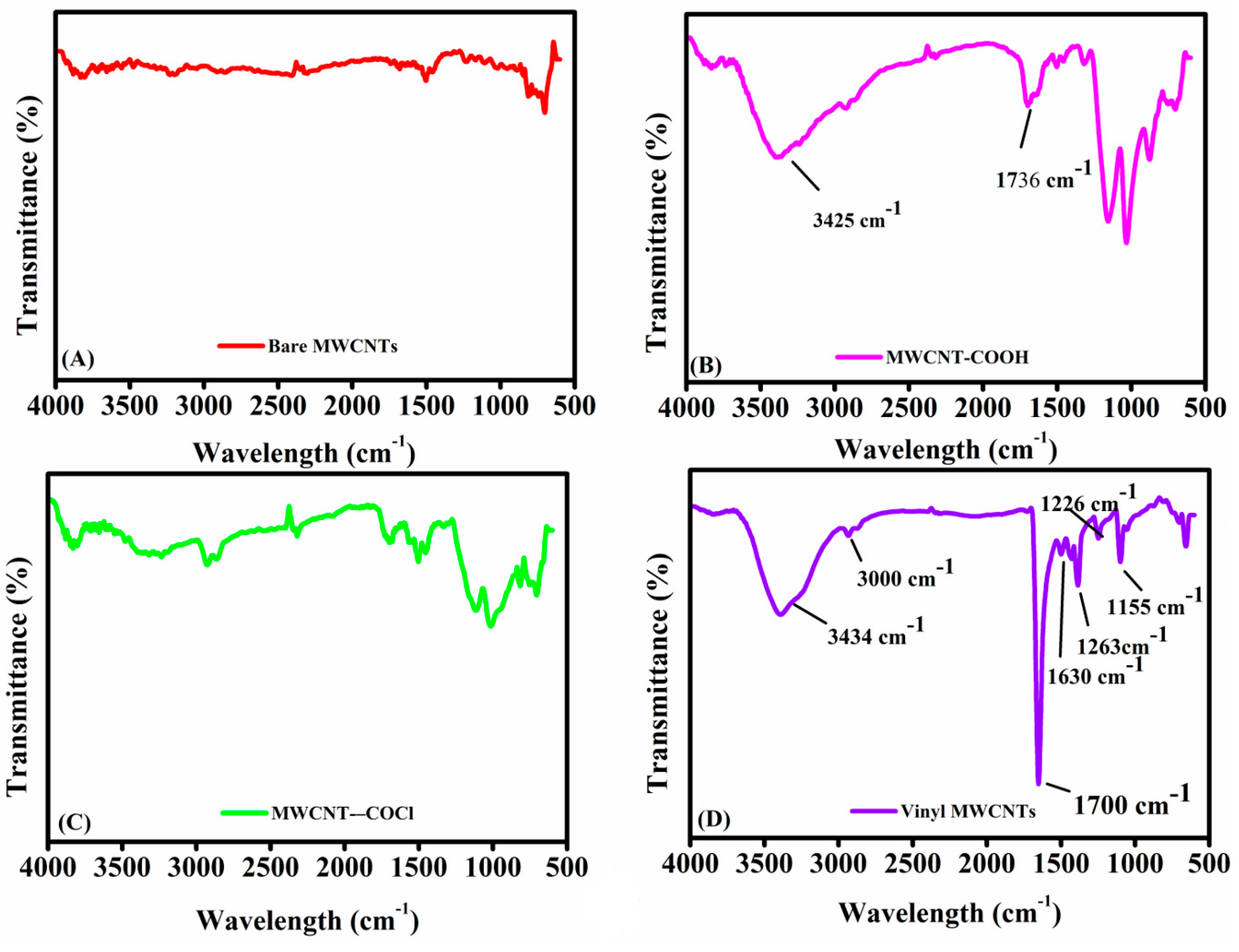
FT-IR of the vinyl-functionalization of MWCNTs: Figure 1 presents the spectra of (A) bare MWCNTs, (B) carboxylated MWCNTs, (C) MWCNTs-COCl, and (D) vinyl-functionalized MWCNTs. Bare MWCNTs (Figure 1B) show minimal absorption bands, consistent with their pristine nature and lack of surface functional groups [35,36]. In contrast, carboxylated MWCNTs (Figure 1B) exhibit peaks at 1736 cm−1 (C=O stretching) and 3425 cm−1 (O–H stretching), indicating the presence of carboxyl and hydroxyl groups introduced via oxidation [37]. The broad O–H peak suggests hydrogen bonding [38], while the peak at 1560 cm−1 corresponds to carboxylate anion stretching, confirming successful carboxylation. These findings indicate the successful introduction of -COOH groups onto the MWCNT surface through oxidation.
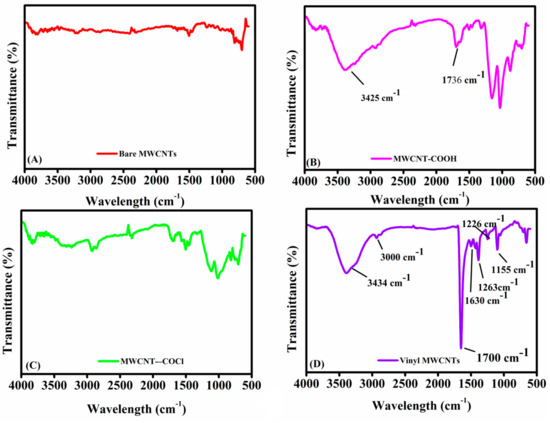
Figure 1.
FT-IR spectra for monitoring the synthesis of vinyl-functionalized MWCNTs: (A) represents bare MWCNTs, (B) carboxylated MWCNTs, (C) acyl chloride–MWCNTs, and (D) f-MWCNTs.
The FT-IR spectra of acyl chloride-functionalized MWCNTs (Figure 1C) show distinct changes in vibrational modes compared to unmodified and carboxylated MWCNTs. Specifically, when comparing MWCNT-COCl to MWCNT-COOH, the conversion of carboxylic acid groups to acyl chlorides is evident. The broad –OH stretching band typically observed between 2800 and 3400 cm−1 in carboxylated MWCNTs is significantly reduced or absent in the MWCNT-COCl spectrum, confirming the successful substitution of –COOH with –COCl. In addition, the carbonyl (C=O) stretching frequency is shifted, and the vibrational modes associated with hydroxyl and ether (C–O) groups are diminished, further supporting the modification. In the FT-IR spectrum of vinyl-functionalized MWCNTs (Figure 1D), new peaks appear at 1630 cm−1 (C=C stretching), 1720 cm−1 (C=O stretching), and around 3000 cm−1 (C–H stretching), indicating successful vinyl grafting. Reaction of MWCNT-COCl with acrylamide (AAm) leads to the formation of amide bonds, confirmed by the appearance of a broad N–H stretching band at 3300–3500 cm−1 and sharp C–N stretching peaks at 1263 and 1448 cm−1. Notably, the C=O peak at 1720 cm−1 becomes more intense in Figure 1D following AAm modification, reflecting the presence of additional carbonyl groups introduced during functionalization. These spectral features collectively confirm the successful introduction of acyl chloride, vinyl, carbonyl, amide, and C=C functionalities onto the MWCNT surface, validating the proposed multi-step synthetic strategy [39,40].
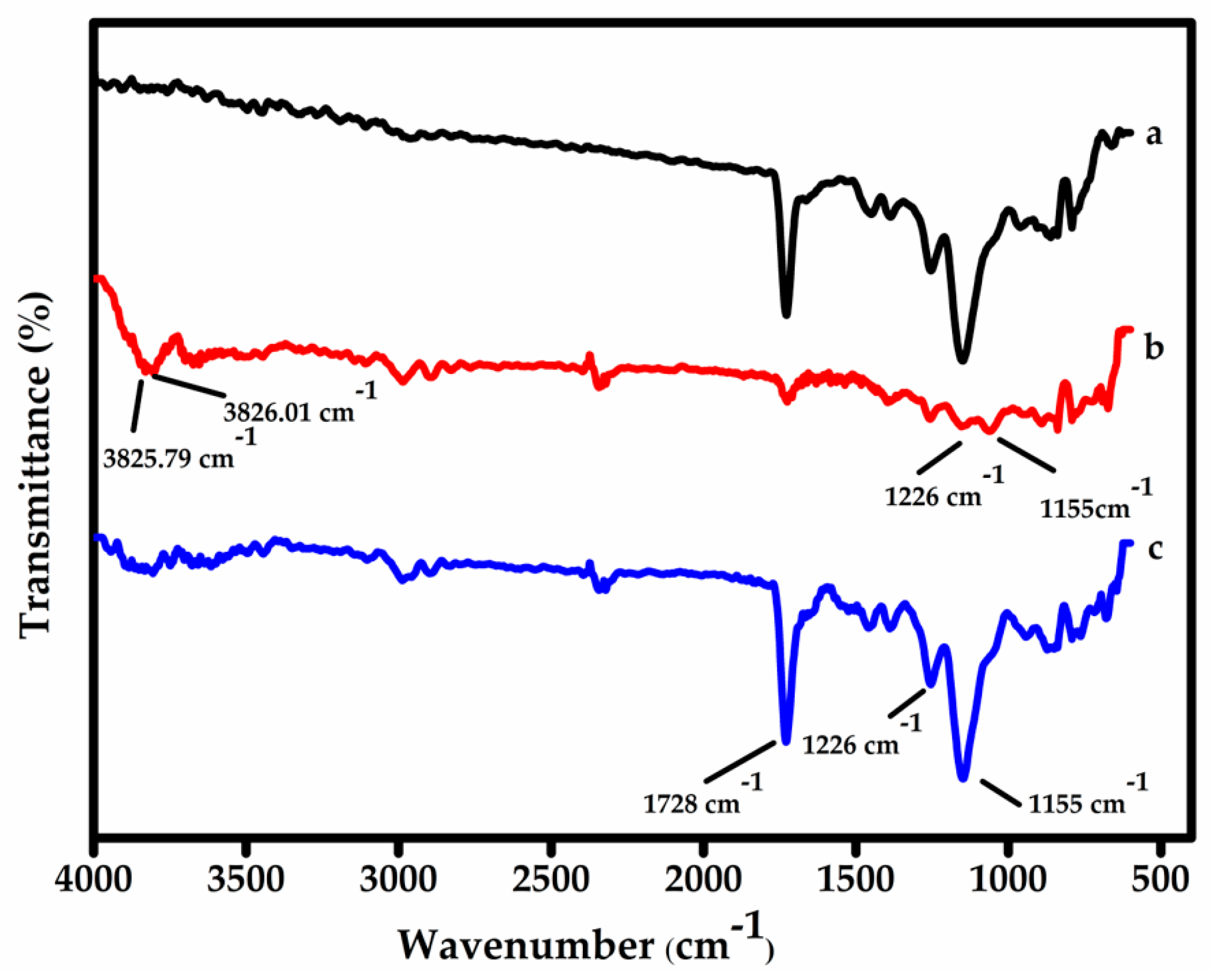
Surface imprinting on functionalized MWCNTs—FT-IR characterization: Figure 2 presents the FT-IR spectra of (a) the non-imprinted polymer (NIP) as a control, (b) the surface-imprinted MIP before template removal, and (c) the MIP after the template (BPS) has been extracted. The spectra were further interpreted using the B3LYP (Becke, 3-parameter, Lee–Yang–Parr) method, a well-established density functional theory (DFT) approach known for its accuracy in predicting vibrational frequencies and molecular structures [41]. The B3LYP simulation predicted two distinct O–H stretching vibrations at 3825.79 and 3826.06 cm−1, which correspond to the presence of BPS in spectrum (b). These appear as a single broad peak in the experimental FT-IR due to their close proximity. The experimental data also show these vibrational features in the expected regions, corresponding to the 5O–27H and 4O–26H bond stretches [42,43]. The disappearance of these peaks in spectrum (c) confirms successful removal of the BPS template from the polymer matrix. Additionally, characteristic peaks observed at 1728, 1226, and 1155 cm−1 are assigned to C=O stretching from AAm and C–O stretching from EGDMA, confirming the formation of the MIP@f-MWCNT composite [44]. The overall similarity between the MIP and NIP spectra is expected, as both share the same monomers and synthesis conditions. The main difference lies in the presence of BPS in the MIP during synthesis, which contributes to the template-specific vibrational signals before extraction.
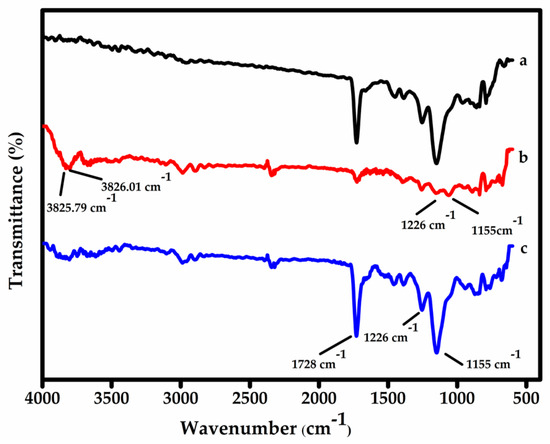
Figure 2.
FT-IR spectra monitoring the synthesis of MIPs and NIPs. Curves (a), (b), and (c) represent the NIP, the MIP before template extraction, and the MIP after template extraction, respectively.
3.1.2. Morphological Characterization
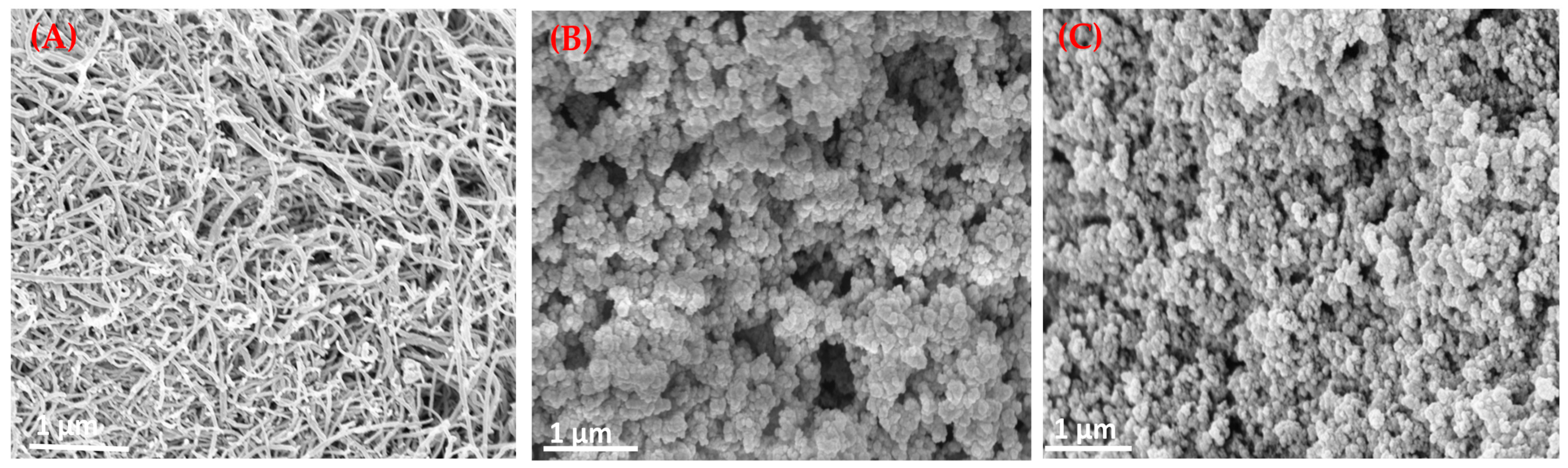
To investigate the surface morphologies of the engineered materials, SEM was employed. SEM was primarily used to characterize the morphological and surface changes of the MWCNTs before and after functionalization with MIPs, with NIPs used as controls. As expected, the surface morphologies of the pristine MWCNTs, MIP-grafted MWCNTs, and NIP-grafted MWCNTs exhibit notable differences. Figure 3A shows entangled tubular structures, characteristic of pristine MWCNTs, confirming their fibrous and one-dimensional morphology. The average diameter of the MWCNTs is estimated to be in the range of 8–25 nm. In contrast, Figure 3B reveals a significant increase in the apparent diameter of the MWCNTs following MIP grafting, likely due to surface coverage by the cross-linked poly(acrylamide) (AAm) network formed by utilizing EGDMA as a cross-linker. This morphological change provides probable evidence of successful incorporation of the imprinted polymer layer on the MWCNT surface. The fibrous structure appears masked by the polymeric matrix, leading to a rougher and more irregular surface. This modified morphology suggests that the MWCNTs are embedded within a polymer film, confirming successful surface modification. The fibrous structure of the MWCNTs has disappeared to become a polymeric matrix due to surface modification. Figure 3C shows the NIP-modified MWCNTs, which have a similar morphology to the MIP composites but with a more uniform and smoother surface, lacking the fine structural features possibly associated with binding cavities. On comparing the image of MIP and NIP [Figure 3B,C], the image of the latter seems to appear less uniform in nature, which can be ascribed to the possible presence of imprinting sites.
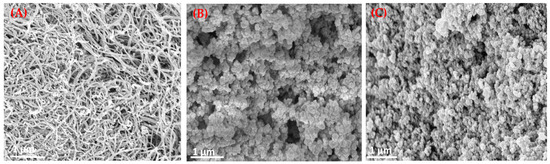
Figure 3.
SEM images of (A) pristine MWCNTs, (B) MIP@f-MWCNT, and (C) NIP@f-MWCNT.
3.2. Electrochemical Characteristics of Differently Modified GCEs and Response to BPS
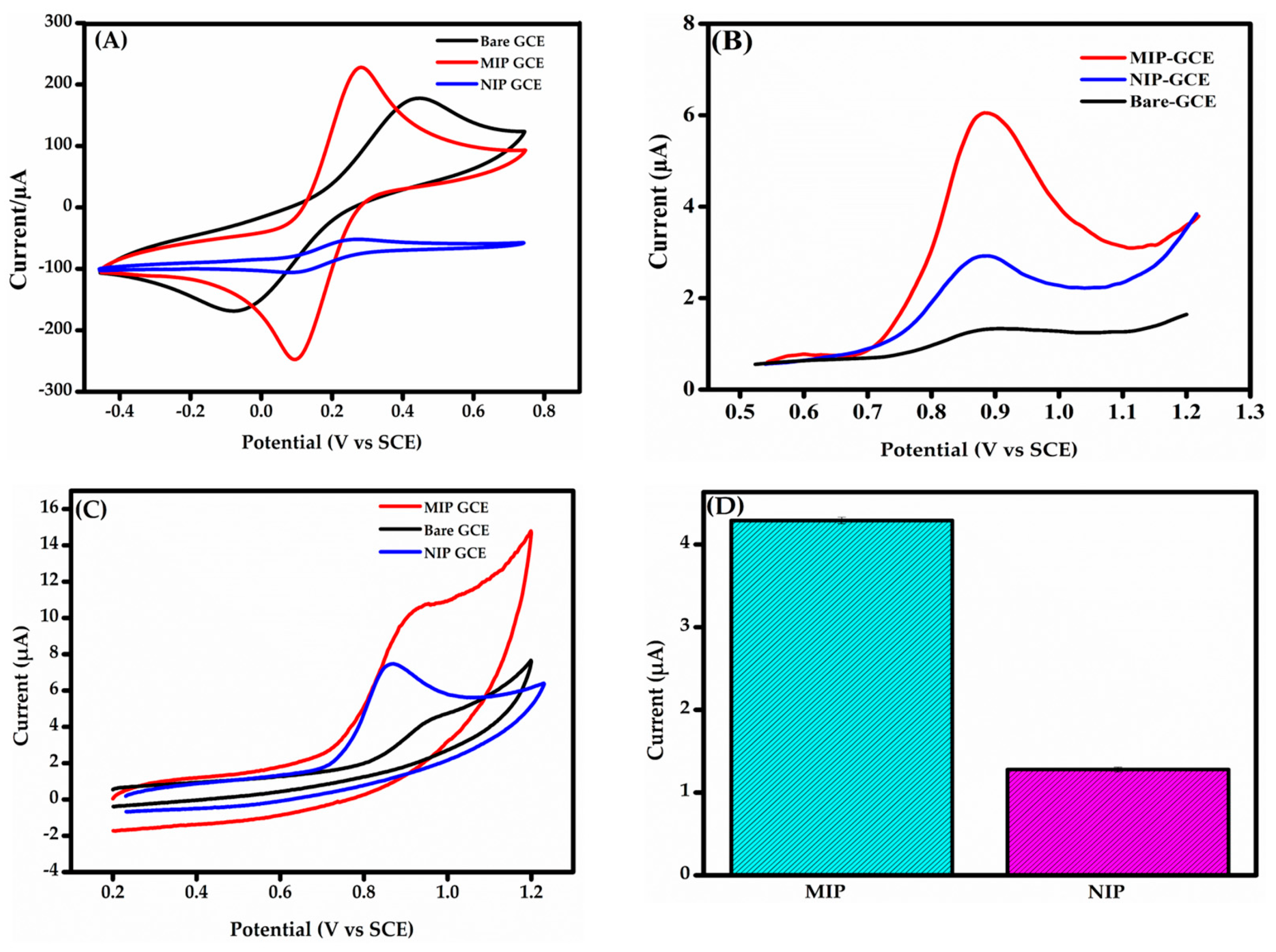
Figure 4A shows the CV responses of the bare GCE, MIP-GCE, and NIP-GCE. All electrodes display distinct anodic and cathodic peaks, reflecting the expected electrochemical behavior of the redox probe. The bare GCE exhibits broad peaks with a large ΔEp, indicating poor catalytic activity and weak interaction with the redox probe, likely due to its low surface area. In contrast, the MIP-GCE presents a smaller ΔEp and significantly higher peak currents, which are attributed to the improved conductivity and larger surface area provided by the MIP@f-MWCNTs composite. The NIP-GCE, which lacks imprinted binding sites, shows reduced peak currents compared to the MIP-GCE, underscoring the critical role of electroactive cavities in the MIP structure. The potential shifts observed for both MIP-GCE and NIP-GCE, relative to the bare GCE, are due to the conductive nature of the MWCNTs embedded in the polymer matrix. According to previous studies [45], such shifts indicate a more reversible electrochemical process, suggesting enhanced electron transfer. These findings confirm the superior electrochemical performance of the MIP-GCE, resulting from the synergistic effects of the MIP structure and the conductive MWCNTs.
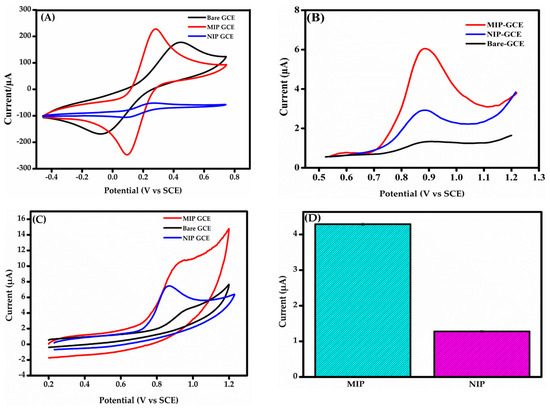
Figure 4.
(A) CV response of bare GCE, MIP-GCE, and NIP-GCE in an electrolytic solution con-taining 1.0 mM [Fe(CN)6]3−/4− and 0.1 M KCl. (B) DPV responses of bare GCE, MIP-GCE, and NIP-GCE in 0.1 M PBS (pH 7.4) containing BPS. (C) CV responses of MIP-GCE, NIP-GCE, and bare GCE in 0.1 M PBS (pH 7.4) containing BPS. (D) Bar charts illustrating the DPV response profiles of MIP-GCE ad NIP-GCE sensors (n = 3).
Initial investigations on the detection of BPS using DPV and CV: DPV in 0.1 M PBS (pH 7.4) revealed a minor anodic peak for bare GCE due to poor electron transfer kinetics during BPS oxidation (Figure 4B). The MIP@f-MWCNTs-modified GCE displayed a significantly higher peak current, highlighting enhanced BPS recognition via two key mechanisms: increased electroactive surface area and conductivity from MWCNTs, promoting efficient electron transfer and selective binding cavities in the MIP, enabling specific interactions with BPS via hydrogen bonding and π-π stacking.
The NIP-modified GCE, though it contained MWCNTs, showed a limited response due to the absence of selective binding sites. CV measurements (Figure 4C) further validated these findings: the MIP-GCE demonstrated superior oxidation currents, while the bare and NIP-modified GCEs exhibited poor responses. The irreversible nature of BPS electrooxidation was confirmed by the absence of a reduction peak, a finding consistent with those of previous studies [46]. Upon the sensing material’s interaction with the BPS solution, the characteristic oxidation peak increases, corresponding to the electrochemical reaction occurring at the specific imprinted sites. This highlights the critical role of the MIP in facilitating efficient and selective BPS recognition. In addition, the imprinting factor (I.F = 3.36) was determined by comparing the BPS signal response obtained from the MIP-GCE sensor with that of the NIP-GCE sensor (Figure 4D). This calculation was performed to assess the imprinting efficiency of the MIP-GCE sensor in selectively recognizing BPS. All in all, these results establish the MIP-GCE as a highly efficient sensor for BPS recognition and detection.
3.3. Optimization of the Electrochemical Protocol
3.3.1. Effect of pH and Incubation Time on the Response of the Electrode
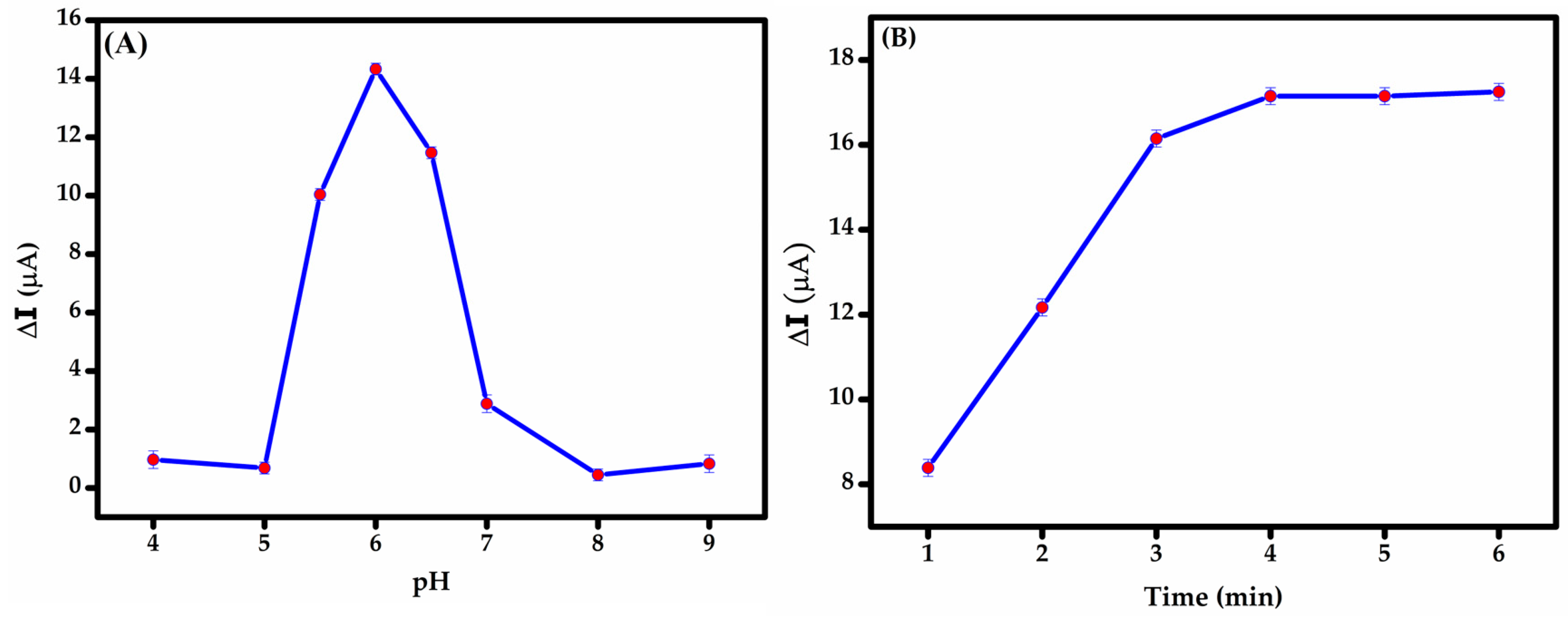
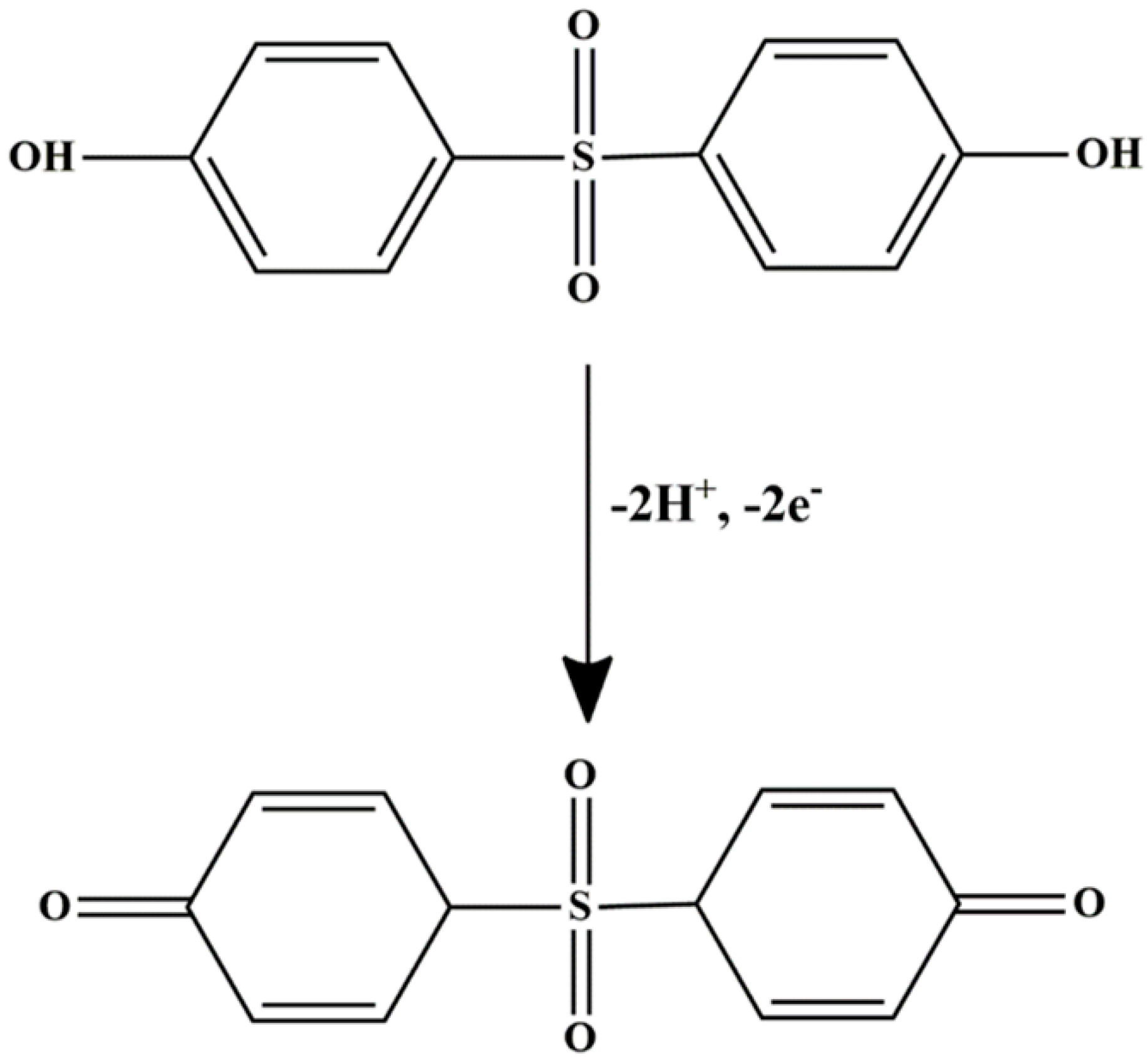
pH optimization is essential for maximizing the current response, as the redox behavior of BPS is highly pH-dependent; this is likely due to the protonation or deprotonation of its phenolic groups, as illustrated in Figure 5A. During the measurement, the peak current followed a clear trend. From pH 4.0 to 6.0, the oxidation peak current increased steadily. At pH 4–5, a slight suppression in current was observed, possibly due to partial protonation that hinders efficient electron transfer. A sharp increase occurred up to pH 6.0, where the maximum current was recorded, indicating this as the optimal pH for detection. Beyond pH 6.0, the current dropped sharply and continued to decline through pH 9.0. This can be reduction is attributed to the deprotonation of BPS, which disrupts the redox process. Below pH 8.2, BPS primarily exists in its protonated form (BPS-H), with the phenolic –OH group intact. As the pH rises past this value, a larger fraction of BPS shifts to the phenoxide ion form [47], which is generally less electrochemically active or may follow altered oxidation mechanisms. Based on these observations, pH 6.0 was identified as the optimal condition for BPS detection, providing the right balance between proton availability and redox efficiency. Scheme 3 illustrates the proposed oxidation mechanisms of BPS at the electrode surface, highlighting key interactions involved in the electrochemical process.
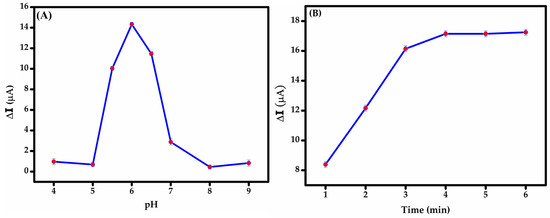
Figure 5.
Influence of (A) pH (4-9) and (B) incubation time (1-6 min) on the DPV response of the modified GCE towards 50 μM BPS solution.
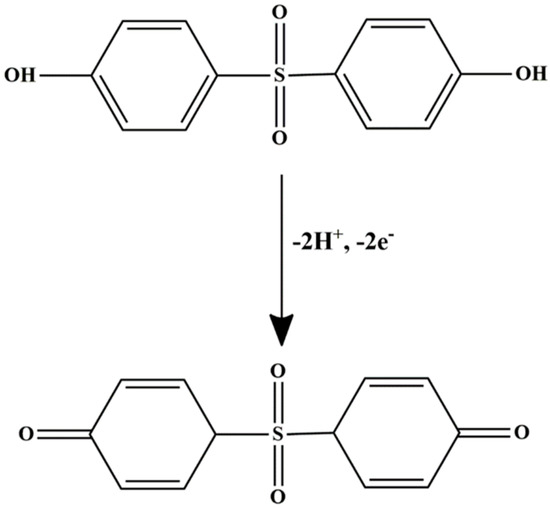
Scheme 3.
Proposed electrochemical oxidation mechanism of BPS occurring on the surface of MIP@f-MWCNT-modified GCE.
Optimizing incubation time balances effective BPS binding to the modified GCE while minimizing non-specific adsorption. Adequate incubation ensures analyte–surface interaction for robust electrochemical signals and stable sensing layers, while prolonged durations trigger interference (e.g., false positives) by promoting non-target binding. Optimal time hinges on analyte-sensor binding kinetics [46]. In this study, incubation time was optimized by evaluating DPV responses over a range of 1 to 6 min (Figure 6B) using BPS in PBS at the optimized pH of 6. Peak current increased sharply until 3 min, stabilizing at 4 min, indicating equilibrium adsorption of BPS onto the MIP cavities. The rapid signal stabilization reflects the fast rebinding dynamics facilitated by the porous MWCNT-MIP composite. Our findings indicate that the MIP film reached adsorption equilibrium within 4 min, optimizing analyte binding while minimizing non-specific adsorption.
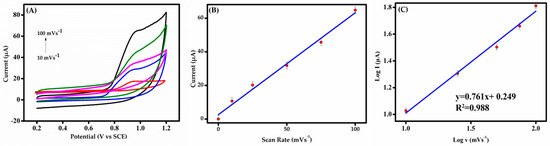
Figure 6.
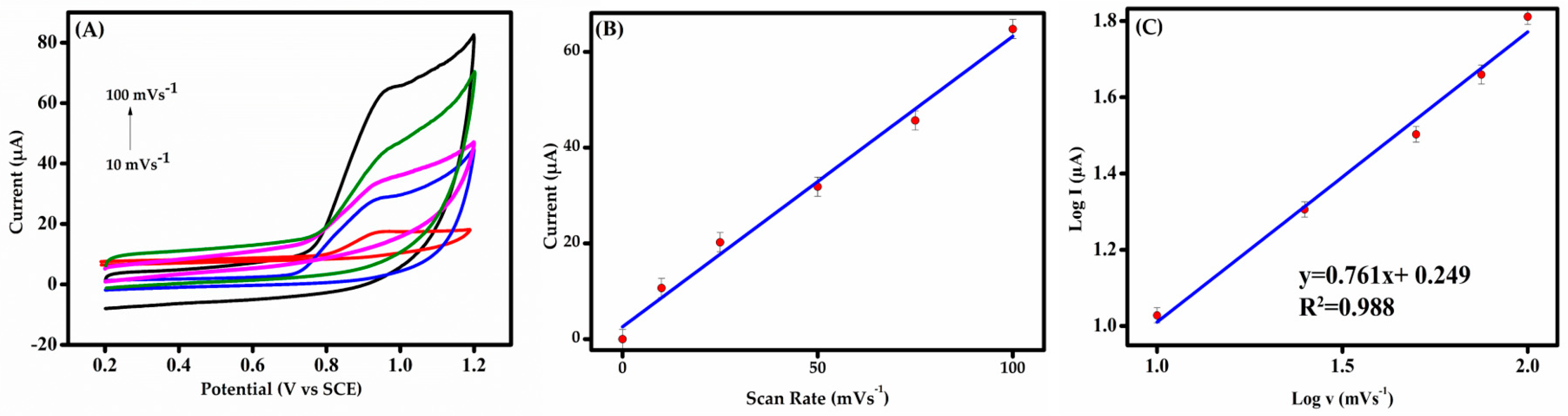
(A) CVs of 50 μM BPS at various scan rates (10–100 mV s−1) in 0.1 M PBS (pH 6.0) at MIP-GCE. (B) Graph of anodic peak current against scan rate. (C) Plot of peak current against the scan rate on the logarithmic scale.
3.3.2. Effect of Scan Rate
The electrochemical response of BPS in 0.1 M PBS (pH 6.0) was analyzed via CV to evaluate the scan rate (υ) effects. Superimposed voltammograms (Figure 6A) reveal υ-dependent behavior at the modified electrode, critical for probing redox kinetics and analyte diffusion dynamics [48]. Figure 6A reveals a single oxidation peak on the molecularly imprinted electrode, further confirming irreversible BPS electrooxidation. As the scan rate increases, it is observed that the anodic peak potential of BPS shifts toward more positive potential values. The regression equation was found as follows:
IBPS (µA) = 0.607 v (mV/s) + 2.560, (R2 = 0.988)
In Figure 6B, the oxidation current exhibits a linear increase with the scan rate (10–100 mV s−1), indicating nanocomposite stability and adsorption-controlled kinetics at the modified GCE. The linear relationship between the peak current and the scan rate suggests that the oxidation process is predominantly adsorption-driven. A log-scale plot (Figure 6C) shows peak current proportionality to scan rate (slope ≈ 0.8), further validating that the process is predominantly adsorption-driven and validating the electrode’s electrochemical efficiency. A slope near 0.5 in electrode processes is indicative of a diffusion-controlled process, where analyte transport to the electrode is a limiting factor. A slope closer to 1.0 suggests adsorption control, dominated by analyte–electrode surface interactions [49]. The adsorption of BPS could be driven by specific interactions such as π–π interactions between the aromatic rings of BPS and the adsorbent surface of the MIP composite. Note that, since DPV measurement could be obtained in less than 125 s under the optimized parameters, the whole analysis period for BPS detection was about 6 min.
3.4. Analytical Performance: The Analytical Curve and Limit of Detection
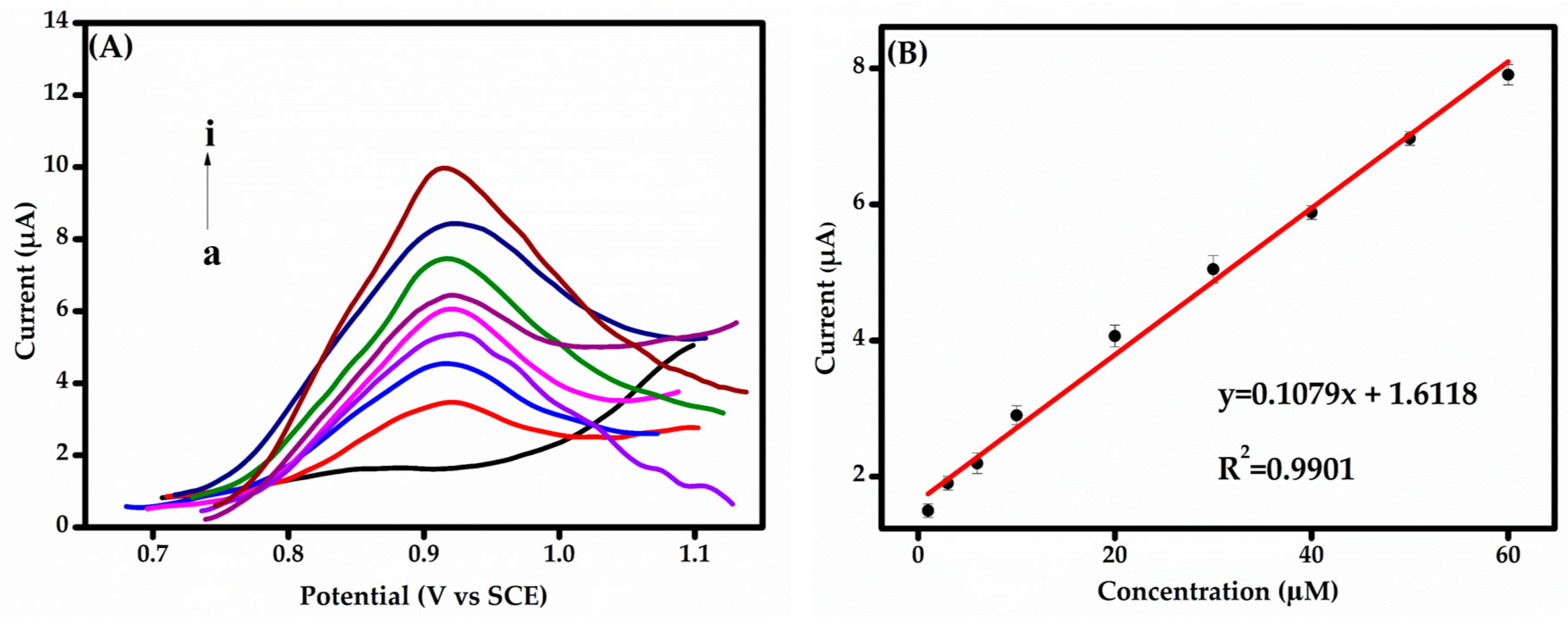
For each concentration, the fabricated sensors were used to detect BPS using the DPV technique under optimized conditions. Current responses were recorded in 0.1 M PBS (pH 6.0) over a BPS concentration range of 1.0–60.0 μM, as shown in Figure 7A. A clear linear relationship between BPS concentration and the DPV current response is observed in Figure 7B, demonstrating the sensor’s excellent electrocatalytic activity in PBS at pH 6.0. As the BPS concentration increases, the current response rises proportionally, indicating a robust and predictable sensor performance. The calibration curve (Figure 7B) reveals a linear dependence of the current response on BPS concentration within the tested range. The regression equation was determined as:
IBPS (µA) = (0.108 ± 0.003) CBPS (µM) + (1.642 ± 0.010), (R2 = 0.9921)
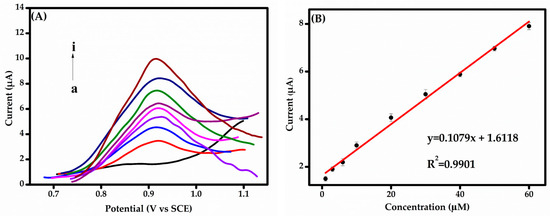
Figure 7.
(A) DPV responses obtained for different BPS concentrations: a–i: blank, 1.0, 3.0, 6.0, 10.0, 20.0, 30.0, 40.0, and 60.0 µM in 0.1 M PBS (pH 6.0). (B) Calibration curve of BPS concentration in the range of 1.0 to 60 µM.
This linearity, along with the relatively wide concentration range, ensures accurate quantification across diverse levels, making the detection method highly applicable to various analytical scenarios. The sensor exhibited a sensitivity of 0.108 µA/µM and achieved a limit of detection (LoD) of 2.00 µM (S/N = 3). An LoD was also determined (S/N = 3, confidence level = 95%). The LoD was calculated using the equation LoD = 3σ/S, where σ represents the standard deviation of the blank signal and S denotes the sensitivity, defined as the slope of the calibration curve.
Table 1 compares the analytical performance (LoD, sensitivity, and linear range) of the proposed MIP@f-MWCNT/GCE sensor with previously reported methods for BPS detection. The sensor demonstrates a broad linear range, competitive with other methods such as CTpPa-2/GCE and the platinum/poly-diamond powder hybrid. Unlike methods like AuAgPtPCD-GO or 3-D GO–C60 nano-assembly, which rely on complex synthesis processes involving multi-metallic nanostructures and graphene oxide composites, the proposed sensor offers significant advantages in simplicity, cost-effectiveness, and scalability. The estimated fabrication cost is approximately only USD 0.08 per sensor (see Table S1, Supporting Information), primarily due to the affordability of the key materials used. This is significantly cheaper than noble-metal-based sensors or chromatographic methods. Scalable synthesis because of the facile fabrication process further enhances cost efficiency. Some of these other reported methods often require multiple chemical reactions, precise control of conditions, and expensive precursors, making them less practical for large-scale or on-site applications.

Table 1.
Comparison of key parameters for BPS determination between this work with those previously reported.
Further, as shown in Table 1, the sensor’s linear range (1–60 μM) and LoD of 2.00 μM are consistent with BPS concentrations typically reported in various sample types, which often fall between 0.1 and 100 μM. The European Food Safety Authority (EFSA) recently established a tolerable daily intake (TDI) for BPA of 0.2 ng per kilogram of body weight per day. This translates to approximately 0.0307 μM in drinking water for an average adult weighing 70 kg and consuming 2 L of water daily [55,56]. While our sensor’s LOD is higher than this drinking water threshold, it is still well-suited for the rapid screening of more contaminated samples, such as wastewater or plastic leachates, where BPS levels often exceed 1 μM. Future work will aim to improve the sensor’s sensitivity to meet more stringent regulatory limits and support broader environmental and food safety applications.
The MIP@f-MWCNT/GCE sensor achieves comparable performance in LoD, sensitivity, and linear range while being budget-friendly, eco-friendly, and easy to fabricate. Its straightforward design makes it well-suited for on-site use and scalable production. However, optimizing the MIP synthesis process could further enhance its performance, making it even more competitive with advanced but less scalable techniques like AuAgPtPCD-GO and 3-D GO–C60 nano-assembly. These results confirm the sensitivity and reliability of the MIP@f-MWCNT-modified GCE for BPS detection, showcasing its potential for advanced electrochemical sensing applications.
3.5. Selectivity and Interference Studies
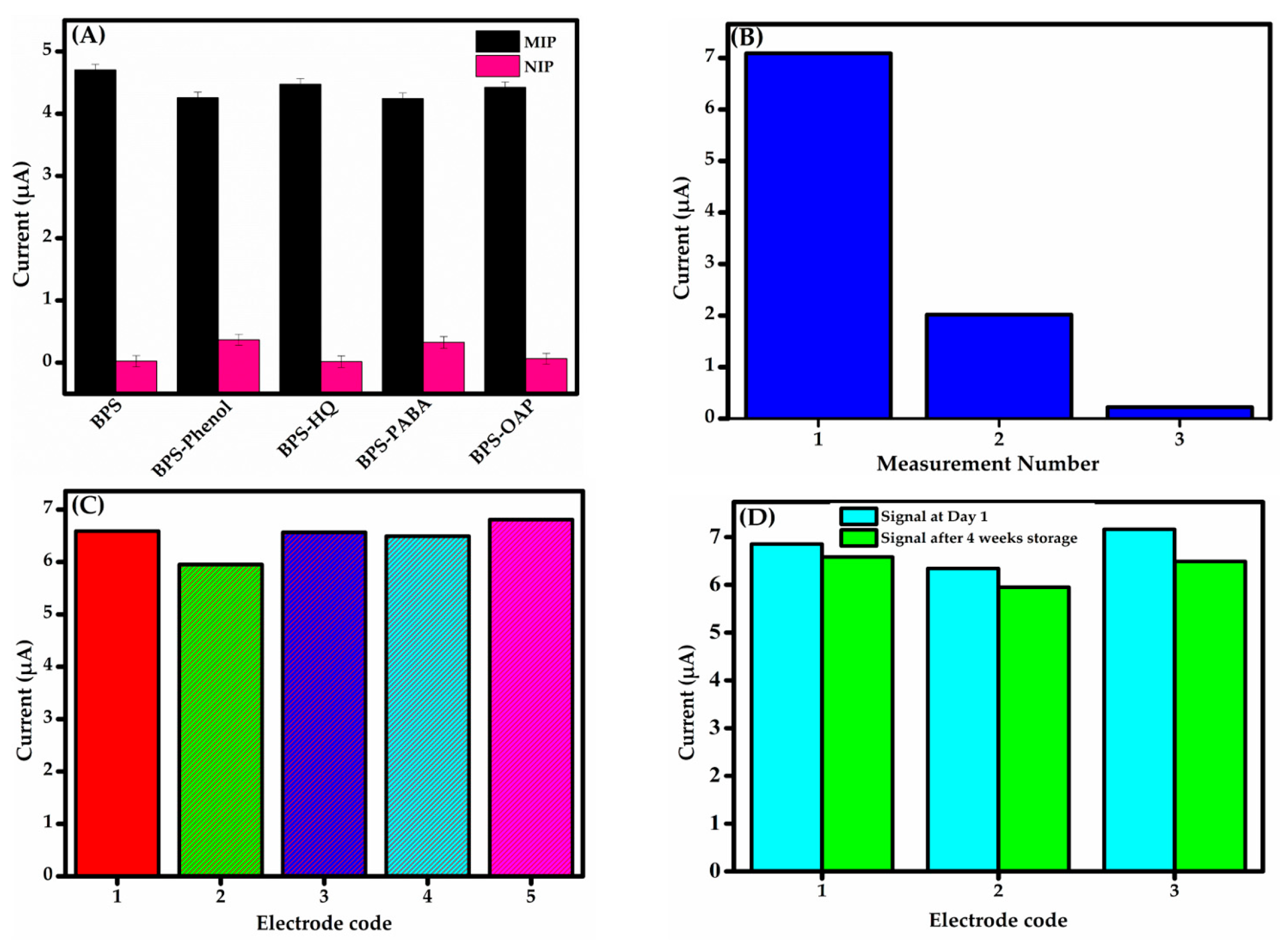
Due to the matrix’s complexity and the presence of interfering substances, fabricating a highly selective sensor is essential for the accurate detection of BPS. To evaluate the selectivity of the fabricated sensor, phenolic analogue molecules, namely phenol, para-aminobenzoic acid (PABA), o-aminophenol (OAP), and hydroquinone (HQ), were selected for testing to conduct competitive adsorption studies. Specifically, all these phenolic compounds share the hydroxyl group (–OH) as a common functional group with the analyte, contributing to their resemblance at a molecular level [50]. The sensor was tested in 0.1 M PBS (pH 6) with 25 μM BPS alone and in a mixture containing BPS (25 μM) plus 10-fold higher concentrations of structural analogues. The results confirm its high selectivity for BPS, with minimal interference from competing species. As shown in Figure 8A, anti-interference experiments confirm that the tested analogues did not significantly impact sensor performance. Therefore, the oxidation potential of each of these compounds is not located in the studied potential range and does not restrict the detection of BPS. This is attributed to the precise fit between the size, conformation, and functional groups of the BPS molecule and the imprinted cavities in the MIP film.
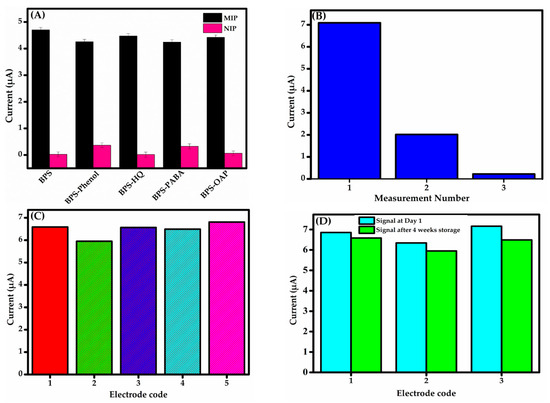
Figure 8.
(A) Selectivity of GCE-MIP and GCE-NIP sensors for BPS (25 μM) alone versus BPS (25 μM) mixed with 10-fold higher concentrations of phenol, HQ, PABA, and OAP. (B) Electrode repeatability analysis: reduction in peak current during subsequent BPS measurements. (C) Reproducibility of the DPV responses. (D) Stability tests after 4 weeks storage.
3.6. Repeatability, Reproducibility, and Stability of the Fabricated Sensor
The sensor’s repeatability was tested via three consecutive measurements on a single electrode. Reuse proved ineffective, with peak current declining significantly in successive runs (Figure 8B), consistent with Maşide et al.’s findings [49] on similar BPS sensor limitations with the oxidation products adsorbing onto the electrode, reducing its active area and limiting reproducible responses—consistent with our CV data (Figure 4C and Figure 6A). These findings confirm the sensor’s non-reusability, arising from irreversible fouling or degradation of the MIP sensing layer. If the sensor cannot be developed for single-use applications, future work could address fouling via surface regeneration or alternative materials to enhance practicality or further optimization of the MIP structure by incorporating more robust and selective binding cavities to enhance tolerance against fouling.
Reproducibility was evaluated using five imprinted sensors fabricated under identical conditions (Figure 8C). This analysis was conducted to quantify the precision and consistency of the measurement outputs across the sensors. The sensors showed an RSD of 2.5% for BPS concentration measurements, indicating high reproducibility. This consistency ensures reliable results across analytical applications, confirming the sensor’s ability to produce accurate current responses under uniform conditions. To evaluate the long-term performance and reliability of the sensor, an initial DPV measurement established the baseline current response. Stability was calculated as per cent signal retention. As the same sensor could not be reused, three new sensors were refabricated, stored at 4 °C under dry, contamination-free conditions for four weeks, and then tested. As depicted in Figure 8D, the sensors retained a signal ranging from 90.6 to 96.0% of the original current response, demonstrating significant stability and suitability for extended use in detecting the target analyte.
3.7. Analytical Applications of the Sensor in Real Samples
To evaluate the practical feasibility of the developed sensor, it was used to detect BPS in extraction solutions obtained from plastic bottle samples. Recovery tests were carried out using the standard addition method to validate the accuracy of the sensor. The sample extracts were prepared as described in Section 2.6 of the experimental section. Known amounts of BPS were spiked into the sample solutions at three different concentration levels, and the concentrations were measured using DPV with the MIP@f-MWCNT/GCE sensor. Each measurement was performed in triplicate. The BPS concentrations obtained from the calibration curve were compared with the spiked values to calculate the recovery. As illustrated in Table 2, the sensor achieved excellent recoveries ranging from 95.6% to 105.0%, with relative standard deviation (RSD) values between 5.5% and 7.93%. These results confirm that the imprinted electrode offers a reliable and accurate method for detecting BPS in plastic bottle extracts.

Table 2.
Detection of BPS in three plastic bottle extract samples using the MIP@f-MWCNT-GCE sensor (n = 3).
4. Conclusions
In this study, we addressed the pressing need for efficient BPS detection by developing a novel electrochemical sensor that combines surface-imprinted MIPs with functionalized MWCNTs. This approach overcomes key challenges found in conventional detection methods, such as complex fabrication steps, reliance on precious metals, and the need for advanced instrumentation. The resulting MIP@f-MWCNT/chitosan/GCE sensor exhibited excellent analytical performance, achieving a low detection limit of 2.00 µM and a sensitivity of 0.108 µA/µM, with a wide linear detection range from 1 to 60 μM. These features, along with its demonstrated stability and successful validation using extracts from commercial plastic water bottles, support its potential for real-world applications. The sensor’s design is highly scalable due to its simplified fabrication process and the stable, reproducible nature of the MIP@f-MWCNT composite. Unlike more complex approaches that rely on batch-dependent procedures, expensive reagents, or harsh chemicals—such as those needed for GO-based composites or metal nanoparticles—this method uses a surface imprinting strategy that is cost-effective and practical. It holds promise for broader applications in environmental monitoring, food safety, and the electrochemical detection of other electroactive compounds. Future directions could include multi-analyte detection, real-time monitoring, the use of greener reagents to minimize environmental impact, and investigations into BPS migration under different storage and environmental conditions. Such studies would help assess human exposure risks and inform improvements in material safety and regulatory standards. Overall, this work contributes to advancements in scalable and economical platforms for detecting a wide range of electroactive species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13070236/s1. Figure S1: Monitoring using UV–Vis spectroscopy of solvent extraction of BPS template from MIPs, Figure S2: Carboxylation of pristine MWCNTs at room temperature for varying durations. Table S1: Estimated material costs for the fabrication of 100 electrochemical sensors based on MIP-modified f-MWCNTs.
Author Contributions
Conceptualization, Q.Z. and S.-N.D.; methodology, Q.Z. and C.M.; validation, C.M. and S.-N.D.; investigation, Q.Z., L.Z. and C.M.; writing—original draft preparation, C.M. and L.Z.; supervision, Q.Z. and S.-N.D.; writing—review and editing, L.Z. and S.-N.D.; project administration, Q.Z. and S.-N.D.; and acquisition of funding, S.-N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22174015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets and materials underpinning the findings of this study are openly accessible in the manuscript and its attached Supplementary Materials. However, for extended access or clarifications regarding the presented data, interested parties may contact the corresponding author directly.
Conflicts of Interest
The authors declare no competing financial interests or personal relationships that could have influenced this work.
References
- Hamed, E.M.; Li, S.F.Y. Molecularly imprinted polymers-based sensors for bisphenol-A: Recent developments and applications in environmental, food and biomedical analysis. Trends Environ. Anal. Chem. 2022, 35, e00167. [Google Scholar] [CrossRef]
- Guida, M.; Troisi, J.; Ciccone, C.; Granozio, G.; Cosimato, C.; Sardo, A.D.S.; Ferrara, C.; Guida, M.; Nappi, C.; Zullo, F.; et al. Bisphenol A and congenital developmental defects in humans. Mutat. Res. 2015, 774, 33–39. (In English) [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermejo, M.; Mas-Pérez, I.; Murillo-Llorente, M.T. The Role of the Bisphenol A in Diabetes and Obesity. Biomedicines 2021, 9, 666. (In English) [Google Scholar] [CrossRef] [PubMed]
- Stillwater, B.J.; Bull, A.C.; Romagnolo, D.F.; Neumayer, L.A.; Donovan, M.G.; Selmin, O.I. Bisphenols and Risk of Breast Cancer: A Narrative Review of the Impact of Diet and Bioactive Food Components. Front. Nutr. 2020, 7, 581388. (In English) [Google Scholar] [CrossRef] [PubMed]
- Canada, G.O. Order amending schedule I to the hazardous products act (Bisphenol A). Can. Gaz. Part II 2010, 144, 413–426. [Google Scholar]
- Cheng, R.; Ding, Y.; Wang, Y.; Wang, H.; Zhang, Y.; Wei, Q. A novel molecularly imprinted electrochemiluminescence sensor based on cobalt nitride nanoarray electrode for the sensitive detection of bisphenol S. RSC Adv. 2021, 11, 11011–11019. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, X.-M.; Wang, F.; Gao, C.-J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly imprinted polymers (MIPs) combined with nanomaterials as electrochemical sensing applications for environmental pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Huang, Y.-Y.; Wang, L.; Shen, X.-F.; Wang, Y.-Y. Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor. Environ. Pollut. 2020, 263, 114616. [Google Scholar] [CrossRef]
- Zahedi, P.; Ziaee, M.; Abdouss, M.; Farazin, A.; Mizaikoff, B. Biomacromolecule template-based molecularly imprinted polymers with an emphasis on their synthesis strategies: A review. Polym. Adv. Technol. 2016, 27, 1124–1142. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Athira, V.S.; Sekhar, V.C. Electrochemical sensing and nano molar level detection of Bisphenol-A with molecularly imprinted polymer tailored on multiwalled carbon nanotubes. Polymer 2018, 146, 312–320. [Google Scholar] [CrossRef]
- Rao, H.; Chen, M.; Ge, H.; Lu, Z.; Liu, X.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. A novel electrochemical sensor based on Au@ PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2017, 87, 1029–1035. [Google Scholar] [CrossRef]
- Mohanan, V.M.A.; Kunnummal, A.K.; Biju, V.M.N. Selective electrochemical detection of dopamine based on molecularly imprinted poly (5-amino 8-hydroxy quinoline) immobilized reduced graphene oxide. J. Mater. Sci. 2018, 53, 10627–10639. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Kadhem, A.J.; Gentile, G.J.; de Cortalezzi, M.M.F. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef]
- Lavagna, L.; Bartoli, M.; Suarez-Riera, D.; Cagliero, D.; Musso, S.; Pavese, M. Oxidation of Carbon Nanotubes for Improving the Mechanical and Electrical Properties of Oil-Well Cement-Based Composites. ACS Appl. Nano Mater. 2022, 5, 6671–6678. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhang, Y.; Yin, X.-F.; Du, B.-B.; Zheng, C.; Yang, H.-H. A facile approach for preparation of molecularly imprinted polymers layer on the surface of carbon nanotubes. Talanta 2013, 105, 403–408. [Google Scholar] [CrossRef]
- Qazi, R.A.; Khattak, R.; Shah, L.A.; Ullah, R.; Khan, M.S.; Sadiq, M.; Hessien, M.M.; El-Bahy, Z.M. Effect of MWCNTs Functionalization on Thermal, Electrical, and Ammonia-Sensing Properties of MWCNTs/PMMA and PHB/MWCNTs/PMMA Thin Films Nanocomposites. Nanomaterials 2021, 11, 2625. (In English) [Google Scholar] [CrossRef]
- Meng, X.; Xiao, Z.; Scott, S.K. Preparation and Application of Electrochemical Sensor Based on Molecularly Imprinted Polymer Coated Multi-Walled Carbon Nanotubes for Nitrocellulose Detection. Propellants Explos. Pyrotech. 2019, 44, 1337–1346. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of functionalized multiwalled carbon nanotubes and comparison of their cellular toxicity between HEK 293 cells and zebra fish in vivo. Heliyon 2019, 5, e02605. (In English) [Google Scholar] [CrossRef]
- Hole, R.; Munde, A.; Jaybhaye, S. Functionalization of multiwalled carbon nanotubes with active pharmaceutical ingredient via carboxylation. Mater. Today Proc. 2021, 45, 3860–3862. [Google Scholar] [CrossRef]
- Sharipov, M.; Ju, T.J.; Azizov, S.; Turaev, A.; Lee, Y.-I. Novel molecularly imprinted nanogel modified microfluidic paper-based SERS substrate for simultaneous detection of bisphenol A and bisphenol S traces in plastics. J. Hazard. Mater. 2024, 461, 132561. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.B.; Li, J.; Sina, A.I.; Wuethrich, A.; Trau, M. Toward precision oncology: SERS microfluidic systems for multiplex biomarker analysis in liquid biopsy. Mater. Adv. 2022, 3, 1459–1471. [Google Scholar] [CrossRef]
- Hyder, A.; Ali, A.; Buledi, J.A.; Memon, R.; Al-Anzi, B.S.; Memon, A.A.; Kazi, M.; Solangi, A.R.; Yang, J.; Thebo, K.H. A NiO-nanostructure-based electrochemical sensor functionalized with supramolecular structures for the ultra-sensitive detection of the endocrine disruptor bisphenol S in an aquatic environment. Phys. Chem. Chem. Phys. 2024, 26, 10940–10950. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Zhao, X.; Liu, X.; Zhong, J.; Zhang, Z.; Zou, P.; Jiang, Y.; Wang, X.; Wang, Y. A novel molecularly imprinted electrochemical sensor based on graphene quantum dots coated on hollow nickel nanospheres with high sensitivity and selectivity for the rapid determination of bisphenol S. Biosens. Bioelectron. 2018, 100, 341–347. (In English) [Google Scholar] [CrossRef]
- Ansari, S.A. Graphene Quantum Dots: Novel Properties and Their Applications for Energy Storage Devices. Nanomaterials 2022, 12, 3814. (In English) [Google Scholar] [CrossRef]
- Sreelakshmi, T.R.; Sajini, T.; Mathew, B. Fabrication of Vinyl Functionalised Multiwalled Carbon Nanotubes for the Removal of Organic Pollutant. Adv. Mater. Res. 2023, 1175, 63–72. [Google Scholar] [CrossRef]
- Gorla, F.A.; Ferreira, M.D.P.; Santos, C.S.D.; de Matos, R.; Segatelli, M.G.; Tarley, C.R.T. Highly sensitive and selective dopamine determination using a photoelectrochemical sensor based on the BiVO4 and surface molecularly imprinted poly(acrylic acid-co-TRIM) on vinyl functionalized carbon nanotubes. J. Electroanal. Chem. 2024, 953, 117988. [Google Scholar] [CrossRef]
- Kan, X.; Zhao, Y.; Geng, Z.; Wang, Z.; Zhu, J.-J. Composites of Multiwalled Carbon Nanotubes and Molecularly Imprinted Polymers for Dopamine Recognition. J. Phys. Chem. C 2008, 112, 4849–4854. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Alexander, S. Synthesis and characterization of vinyl-functionalized multiwalled carbon nanotubes based molecular imprinted polymer for the separation of chlorpyrifos from aqueous solutions. J. Chem. Technol. Biotechnol. 2013, 88, 1847–1858. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Z.; Hu, Y.; Li, J.; Fan, X. Multiple functionalization of multi-walled carbon nanotubes with carboxyl and amino groups. Appl. Surf. Sci. 2013, 276, 476–481. [Google Scholar] [CrossRef]
- Du, J.; Gao, R.; Mu, H. A Novel Molecularly Imprinted Polymer Based on Carbon Nanotubes for Selective Determination of Dioctyl Phthalate from Beverage Samples Coupled with GC/MS. Food Anal. Methods 2016, 9, 2026–2035. [Google Scholar] [CrossRef]
- Gao, R.; Su, X.; He, X.; Chen, L.; Zhang, Y. Preparation and characterisation of core–shell CNTs@MIPs nanocomposites and selective removal of estrone from water samples. Talanta 2011, 83, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Bakather, O.Y.; Al-Tawbini, B.; Bukhari, A.A.; Abuilaiwi, F.A.; Fettouhi, M.B.; Butler, I. Effect of carboxylic functional group functionalized on carbon nanotubes surface on the removal of lead from water. Bioinorg. Chem. Appl. 2010, 2010, 603978. (In English) [Google Scholar] [CrossRef]
- da Silva, N.A.; Haiduke, R.L.A. Infrared intensity analysis of hydroxyl stretching modes in carboxylic acid dimers by means of the charge–charge flux–dipole flux model. J. Comput. Chem. 2019, 40, 2482–2490. [Google Scholar] [CrossRef]
- Abdelkader, R.; Mohammed, B. Green Synthesis of Cationic Polyacrylamide Composite Catalyzed by An Ecologically Catalyst Clay Called Maghnite-H+ (Algerian MMT) Under Microwave Irradiation. Bull. Chem. React. Eng. Catal. 2016, 11, 170–175. [Google Scholar] [CrossRef]
- Singh, B.P.; Choudhary, V.; Teotia, S.; Gupta, T.K.; Singh, V.N.; Dhakate, S.R.; Mathur, R.B. Solvent Free, Efficient, Industrially Viable, Fast Dispersion Process Based Amine Modified MWCNT Reinforced Epoxy Composites Of Superior Mechanical Properties. Adv. Mater. Lett. 2015, 6, 104–113. [Google Scholar] [CrossRef]
- Tsague, L.F.; Ejuh, G.; Ngoupo, A.T.; Assatse, Y.T.; Kamsi, R.Y.; Abe, M.O.; Ndjaka, J. Ab-initio and density functional theory (DFT) computational study of the effect of fluorine on the electronic, optical, thermodynamic, hole and electron transport properties of the circumanthracene molecule. Heliyon 2023, 9, e19647. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Zalpour, N. Selective detection of Asulam with in-situ dopamine electropolymerization based electrochemical MIP sensor. React. Funct. Polym. 2021, 169, 105069. [Google Scholar] [CrossRef]
- Song, C.; Yang, Y.; Zhou, Y.; Wang, L.; Zhu, S.; Wang, J.; Zeng, R.; Zheng, Y.; Guan, S. Electrochemical polymerization of dopamine with/without subsequent PLLA coating on Mg-Zn-Y-Nd alloy. Mater. Lett. 2019, 252, 202–206. [Google Scholar] [CrossRef]
- Jin, Y.-F.; Zhang, Y.-J.; Zhang, Y.-P.; Chen, J.; Zhou, X.-M.; Bai, L.-Y.; Curcio, M. Synthesis and evaluation of molecularly imprinted polymer for the determination of the phthalate esters in the bottled beverages by HPLC. J. Chem. 2013, 2013, 903210. [Google Scholar] [CrossRef]
- Caetano, F.R.; Felippe, L.B.; Zarbin, A.J.; Bergamini, M.F.; Marcolino-Junior, L.H. Gold nanoparticles supported on multi-walled carbon nanotubes produced by biphasic modified method and dopamine sensing application. Sens. Actuators B Chem. 2017, 243, 43–50. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, Q.; Qiao, J.; Ye, B.; Li, G. Simultaneous detection of bisphenol A and bisphenol S with high sensitivity based on a new electrochemical sensor. J. Electroanal. Chem. 2019, 854, 113541. [Google Scholar] [CrossRef]
- Wu, L.; Lin, Y.; Zhang, Y.; Wang, P.; Ding, M.; Nie, M.; Yan, C.; Chen, S. Ca(OH)2-mediated activation of peroxymonosulfate for the degradation of bisphenol S. RSC Adv. 2021, 11, 33626–33636. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Çakıcı, M.; Avan, A.A.; Filik, H.; Yetimoğlu, E.K. Individual and Simultaneous Electrochemical Detection of Bisphenol A and Bisphenol S in Food Samples Using Triethylenetetramine Functionalized Multi-Walled Carbon Nanotubes. Food Anal. Methods 2023, 16, 225–237. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Wu, M.; Shi, Y.; Yang, J.; Shan, J.; Liu, L. Simultaneous Determination of Bisphenol A and Bisphenol S Using Multi-Walled Carbon Nanotubes Modified Electrode. Int. J. Electrochem. Sci. 2018, 13, 11906–11922. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Wang, M.; Cao, J.; Li, L.; Wang, C.; Feng, N. Selective sensing of bisphenol A and bisphenol S on platinum/poly (diallyl dimethyl ammonium chloride)-diamond powder hybrid modified glassy carbon electrode. J. Electrochem. Soc. 2016, 163, B192. [Google Scholar] [CrossRef]
- Zhu, W.; Yue, X.; Duan, J.; Zhang, Y.; Zhang, W.; Yu, S.; Wang, Y.; Zhang, D.; Wang, J. Electrochemically co-reduced 3D GO-C60 nanoassembly as an efficient nanocatalyst for electrochemical detection of bisphenol S. Electrochim. Acta 2016, 188, 85–90. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Pang, Y.-H.; Shen, X.-F.; Jiang, R.; Wang, Y.-Y. Covalent organic framework DQTP modified pencil graphite electrode for simultaneous determination of bisphenol A and bisphenol S. Talanta 2022, 236, 122859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, D.; Yang, L.; Zhang, L. Constructed ILs@ hollow porous spherical Ni-loaded CdFe2O4 modified electrode for highly sensitive simultaneous electrochemical analysis of bisphenols. Sens. Actuators B Chem. 2017, 246, 800–808. [Google Scholar] [CrossRef]
- Prueitt, R.L.; Goodman, J.E. Evidence evaluated by European Food Safety Authority does not support lowering the temporary tolerable daily intake for bisphenol A. Toxicol. Sci. 2024, 198, 185–190. (In English) [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Antoniou, M.; Belcher, S.M.; Bergman, A.; Bhandari, R.K.; Birnbaum, L.S.; Cohen, A.; Collins, T.J.; Demeneix, B.; Fine, A.M.; et al. The Conflict between Regulatory Agencies over the 20,000-Fold Lowering of the Tolerable Daily Intake (TDI) for Bisphenol A (BPA) by the European Food Safety Authority (EFSA). Environ. Health Perspect. 2024, 132, 45001. (In English) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).