Quantitative and Qualitative Evaluation of Microplastic Contamination of Shrimp Using Visible Near-Infrared Multispectral Imaging Technology Combined with Supervised Self-Organizing Map †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.2.1. Qualitative Sample Preparation
2.2.2. Quantitative Sample Preparation
2.2.3. Size-Based Sample Preparation
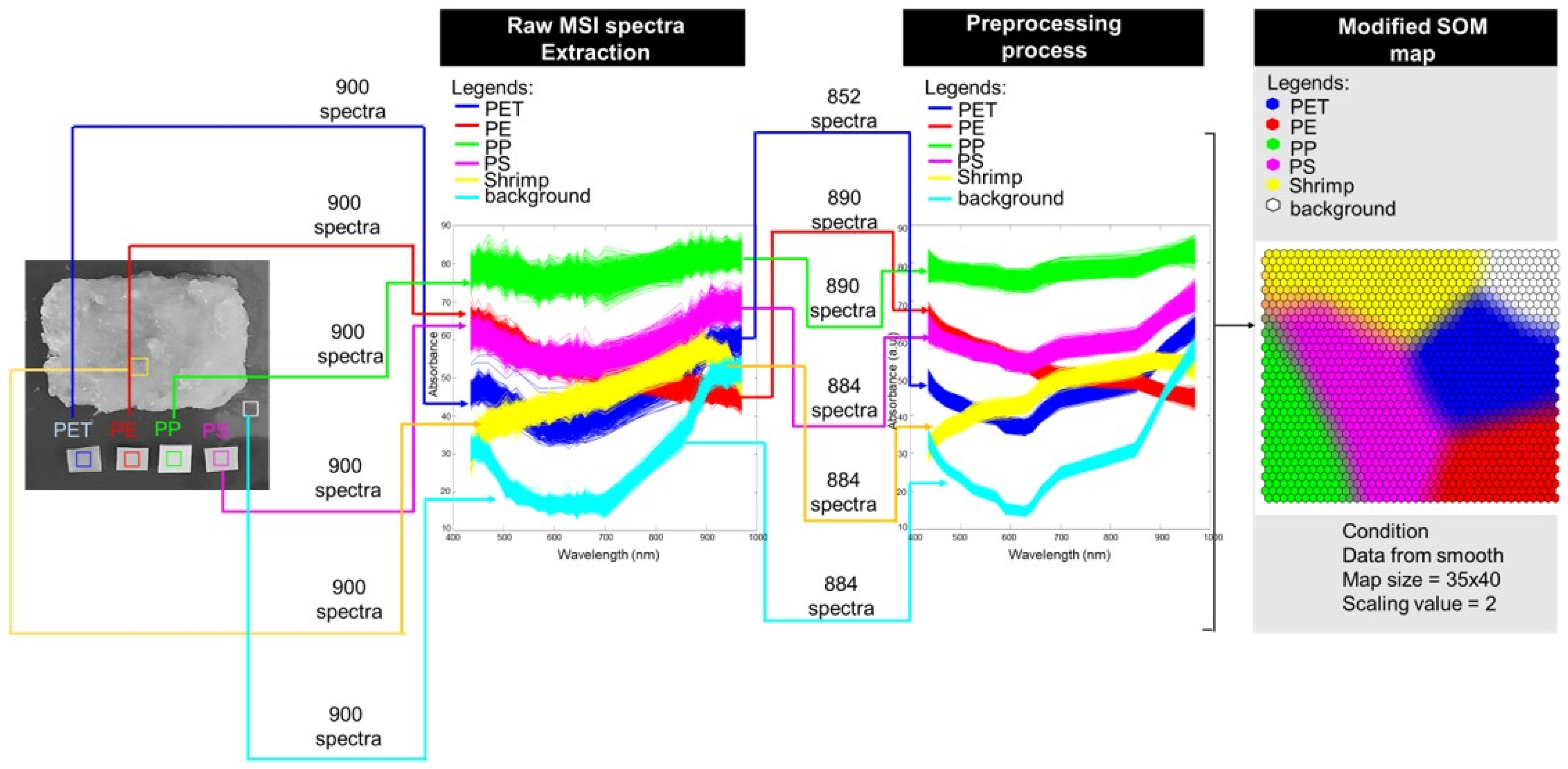
2.3. Vis-NIR Multispectral Imaging
2.4. Preprocessing
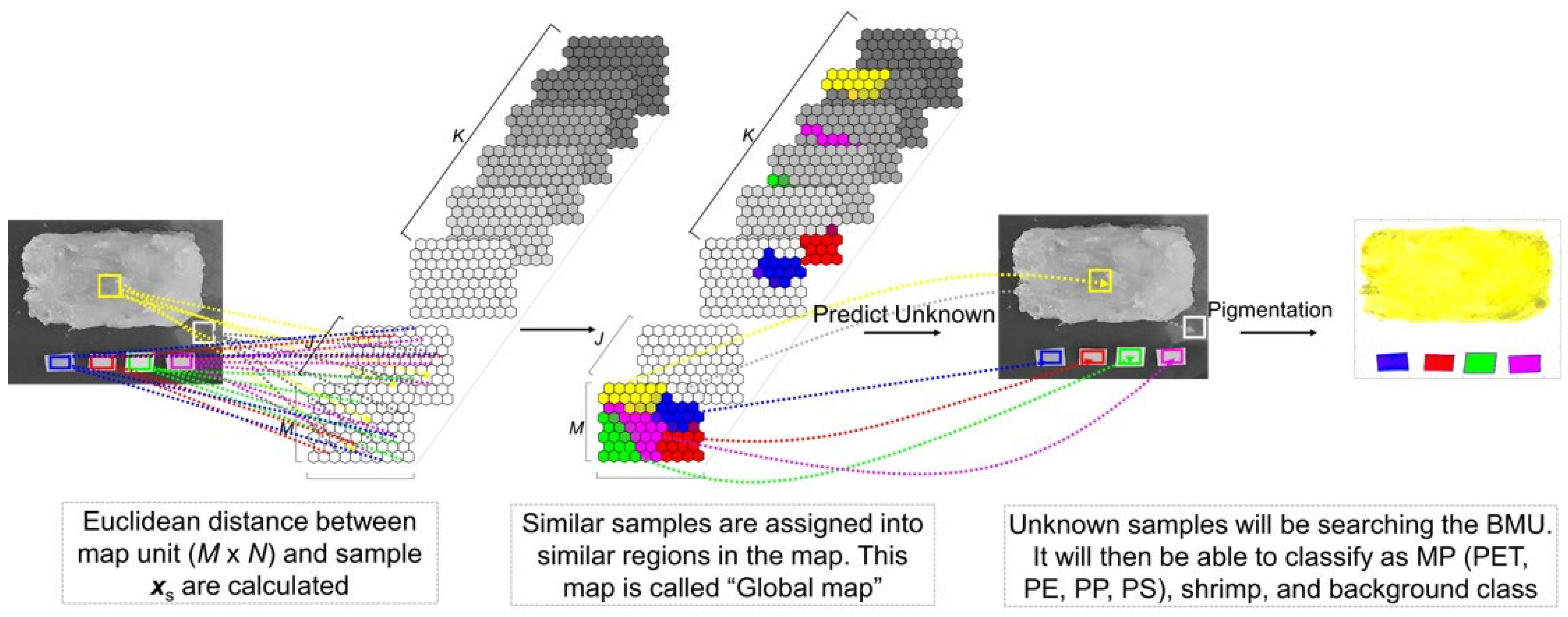
2.5. Modified Self-Organizing Map (SOM)
2.6. Evaluation of the SOM Model Performance
2.6.1. Qualitative Method
2.6.2. Quantitative Method
2.7. Evaluation of the Size Limit Detection for Microplastics
2.8. Principal Component Analysis (PCA)
3. Results and Discussion
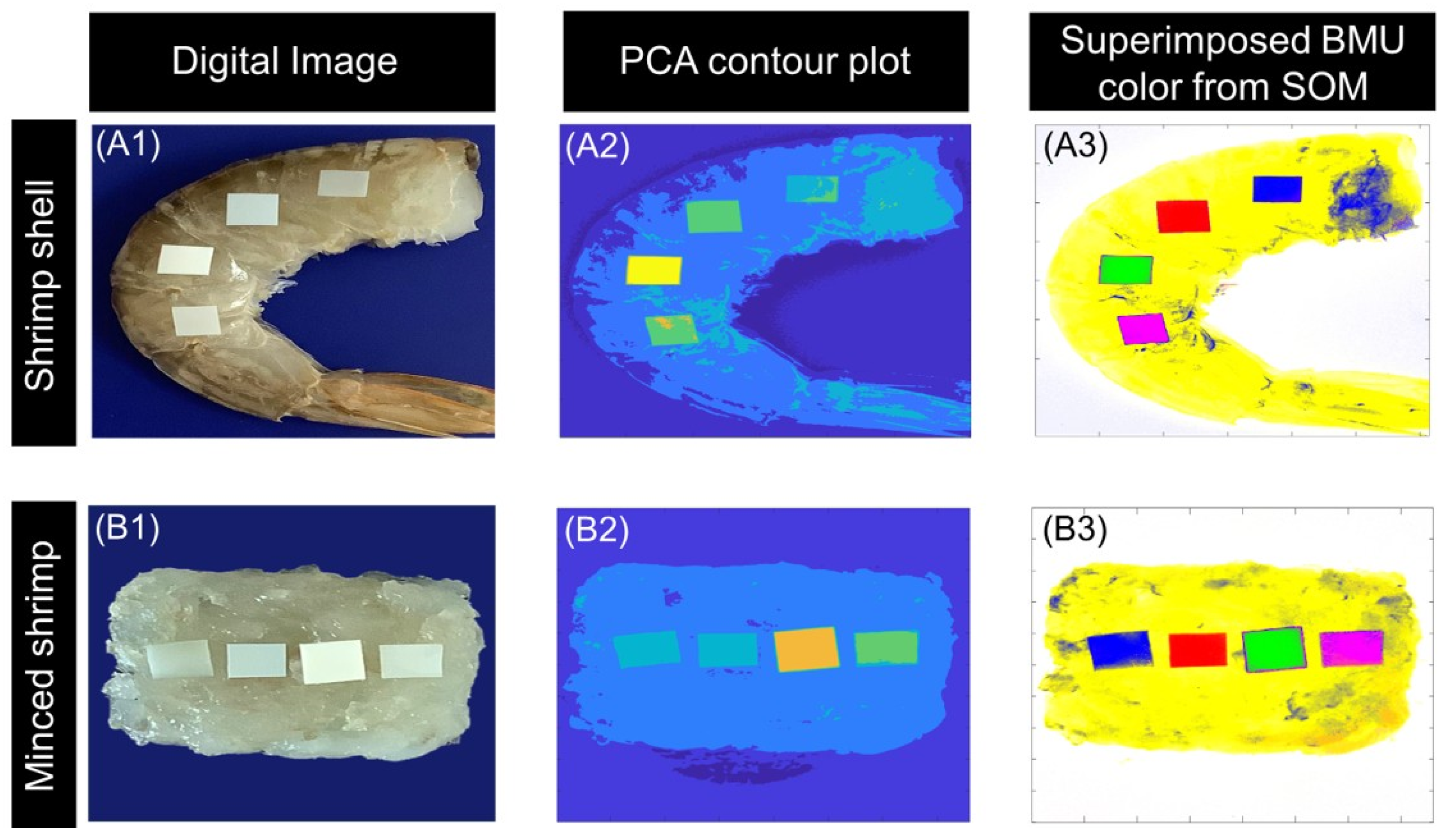
3.1. Qualitative Analysis
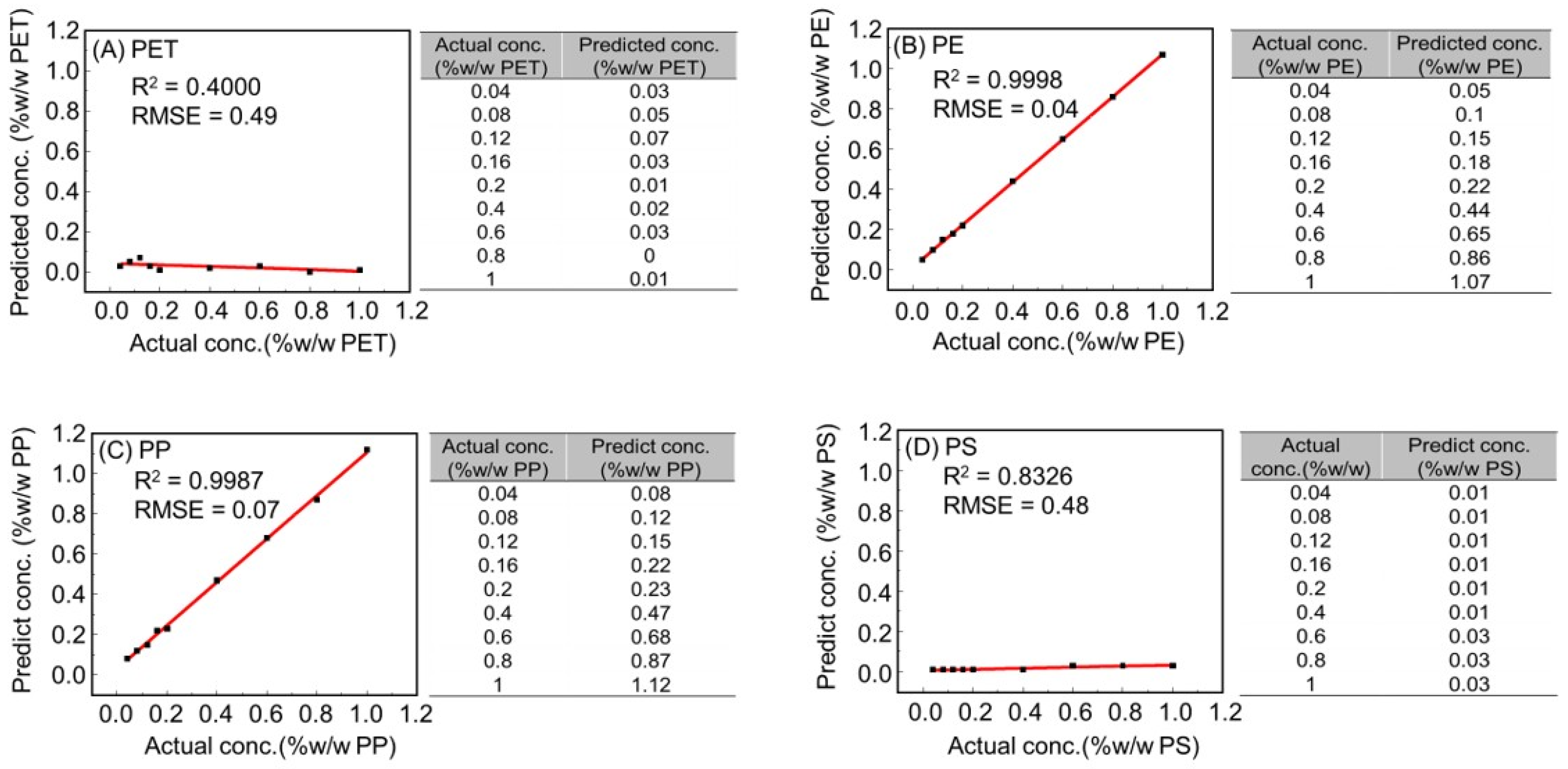
3.2. Quantitative Analysis
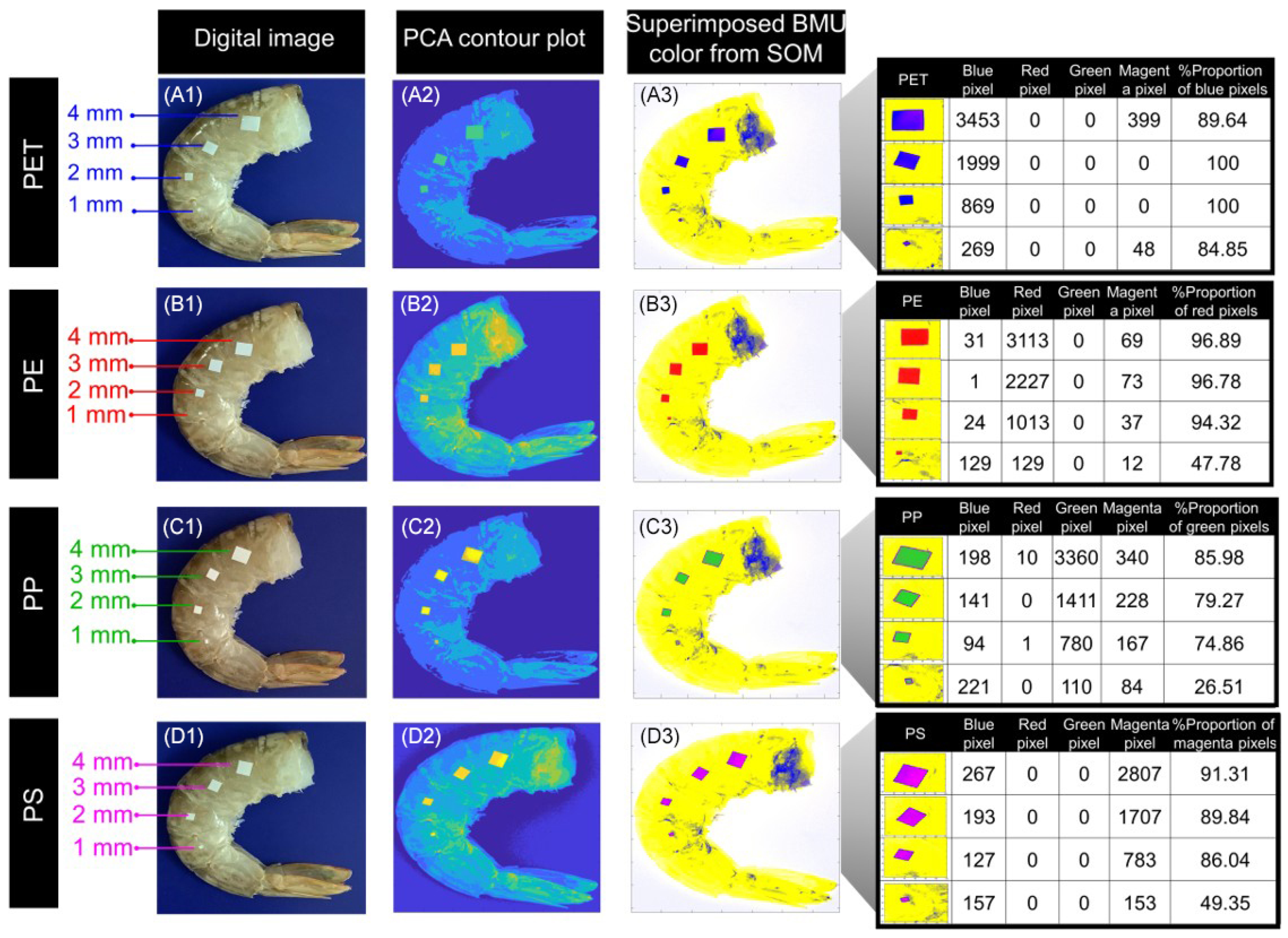
3.3. Size-Based Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchesi, C.; Rani, M.; Federici, S.; Alessandri, I.; Vassalini, I.; Ducoli, S.; Borgese, L.; Zacco, A.; Núñez-Delgado, A.; Bontempi, E.; et al. Quantification of Ternary Microplastic Mixtures through an Ultra-Compact Near-Infrared Spectrometer Coupled with Chemometric Tools. Environ. Res. 2023, 216, 114632. [Google Scholar] [CrossRef] [PubMed]
- Perumpully, S.J.; Kumar, R.P.; Gautam, S.; Ambade, B.; Gautam, A.S. An Inclusive Trend Study of Evaluation and Scientometric Analysis of Microplastics. Phys. Chem. Earth Parts A/B/C 2023, 132, 103455. [Google Scholar] [CrossRef]
- Sravya, G.C.; Gautam, S.; Kumar, K.U.; Poonguzhali, R.S.; Manuel, R.I. Assessing LDPE Microplastics’ Impact on Green Gram (Vigna radiata L. Wilczek) Cultivation: A Greenhouse Pot Experiment. Phys. Chem. Earth Parts A/B/C 2024, 136, 103710. [Google Scholar] [CrossRef]
- Khan, M.B.; Urmy, S.Y.; Setu, S.; Kanta, A.H.; Gautam, S.; Eti, S.A.; Rahman, M.M.; Sultana, N.; Mahmud, S.; Baten, M.A. Abundance, Distribution and Composition of Microplastics in Sediment and Fish Species from an Urban River of Bangladesh. Sci. Total Environ. 2023, 885, 163876. [Google Scholar] [CrossRef]
- United Nations Environment Assembly. Plastic Waste Causes Financial Damage of US$13 Billion to Marine Ecosystems Each Year as Concern Grows over Microplastics. Available online: https://www.unep.org/news-and-stories/press-release/plastic-waste-causes-financial-damage-us13-billion-marine-ecosystems (accessed on 15 June 2025).
- Ogunola, O.S.; Onada, O.A.; Falaye, A.E. Mitigation Measures to Avert the Impacts of Plastics and Microplastics in the Marine Environment (A Review). Environ. Sci. Pollut. Res. 2018, 25, 9293–9310. [Google Scholar] [CrossRef]
- Mouat, J.; Lozano, R.L.; Bateson, H. Economic Impacts of Marine Litter; KIMO: Shetland, UK, 2010. [Google Scholar]
- Meng, X.; Chen, S.; Li, D.; Song, Y.; Sun, L. Identification of Marine Microplastics Based on Laser-Induced Fluorescence and Principal Component Analysis. J. Hazard. Mater. 2024, 465, 133352. [Google Scholar] [CrossRef]
- Jin, N.; Song, Y.; Ma, R.; Li, J.; Li, G.; Zhang, D. Characterization and Identification of Microplastics Using Raman Spectroscopy Coupled with Multivariate Analysis. Anal. Chim. Acta 2022, 1197, 339519. [Google Scholar] [CrossRef]
- Adeogun, A.O.; Ibor, O.R.; Khan, E.A.; Chukwuka, A.V.; Omogbemi, E.D.; Arukwe, A. Detection and Occurrence of Microplastics in the Stomach of Commercial Fish Species from a Municipal Water Supply Lake in Southwestern Nigeria. Environ. Sci. Pollut. Res. 2020, 27, 31035–31045. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red Tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low Levels of Microplastics (MP) in Wild Mussels Indicate That MP Ingestion by Humans Is Minimal Compared to Exposure via Household Fibres Fallout during a Meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Li, J.Y.Q.; Nankervis, L.; Dawson, A.L. Digesting the Indigestible: Microplastic Extraction from Prawn Digestive Tracts. Front. Environ. Chem. 2022, 3, 903314. [Google Scholar] [CrossRef]
- My, T.T.A.; Dat, N.D.; Hung, N.Q. Occurrence and Characteristics of Microplastics in Wild and Farmed Shrimps Collected from Cau Hai Lagoon, Central Vietnam. Molecules 2023, 28, 4634. [Google Scholar] [CrossRef]
- Gurjar, U.R.; Xavier, M.; Nayak, B.B.; Ramteke, K.; Deshmukhe, G.; Jaiswar, A.K.; Shukla, S.P. Microplastics in Shrimps: A Study from the Trawling Grounds of North Eastern Part of Arabian Sea. Environ. Sci. Pollut. Res. 2021, 28, 48494–48504. [Google Scholar] [CrossRef]
- Niemcharoen, S.; Haetrakul, T.; Palić, D.; Chansue, N. Microplastic-Contaminated Feed Interferes with Antioxidant Enzyme and Lysozyme Gene Expression of Pacific White Shrimp (Litopenaeus vannamei) Leading to Hepatopancreas Damage and Increased Mortality. Animals 2022, 12, 3308. [Google Scholar] [CrossRef]
- Hossain, M.S.; Rahman, M.S.; Uddin, M.N.; Sharifuzzaman, S.M.; Chowdhury, S.R.; Sarker, S.; Nawaz Chowdhury, M.S. Microplastic Contamination in Penaeid Shrimp from the Northern Bay of Bengal. Chemosphere 2020, 238, 124688. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, G.E. Microplastics: An Emerging Threat to Food Security and Human Health. J. Food Sci. Technol. 2020, 57, 1601–1608. [Google Scholar] [CrossRef]
- Huang, H.; Qureshi, J.U.; Liu, S.; Sun, Z.; Zhang, C.; Wang, H. Hyperspectral Imaging as a Potential Online Detection Method of Microplastics. Bull. Environ. Contam. Toxicol. 2021, 107, 754–763. [Google Scholar] [CrossRef]
- Gautam, S.; Rathikannu, S.; Katharine, S.P.; Marak, L.K.R.; Alshehri, M. Beyond the Surface: Microplastic Pollution Its Hidden Impact on Insects and Agriculture. Phys. Chem. Earth Parts A/B/C 2024, 135, 103663. [Google Scholar] [CrossRef]
- Gebejes, A.; Hrovat, B.; Semenov, D.; Kanyathare, B.; Itkonen, T.; Keinänen, M.; Koistinen, A.; Peiponen, K.E.; Roussey, M. Hyperspectral Imaging for Identification of Irregular-Shaped Microplastics in Water. Sci. Total Environ. 2024, 944, 173811. [Google Scholar] [CrossRef]
- de Jourdan, B.; Philibert, D.; Asnicar, D.; Davis, C.W. Microplastic Biomonitoring Studies in Aquatic Species: A Review & Quality Assessment Framework. Sci. Total Environ. 2024, 957, 177541. [Google Scholar] [CrossRef]
- Marcuello, C. Present and Future Opportunities in the Use of Atomic Force Microscopy to Address the Physico-Chemical Properties of Aquatic Ecosystems at the Nanoscale Level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Vidal, C.; Pasquini, C. A Comprehensive and Fast Microplastics Identification Based on Near-Infrared Hyperspectral Imaging (HSI-NIR) and Chemometrics. Environ. Pollut. 2021, 285, 117251. [Google Scholar] [CrossRef]
- Piarulli, S.; Malegori, C.; Grasselli, F.; Airoldi, L.; Prati, S.; Mazzeo, R.; Sciutto, G.; Oliveri, P. An Effective Strategy for the Monitoring of Microplastics in Complex Aquatic Matrices: Exploiting the Potential of Near Infrared Hyperspectral Imaging (NIR-HSI). Chemosphere 2022, 286, 131861. [Google Scholar] [CrossRef] [PubMed]
- Faltynkova, A.; Wagner, M. Developing and Testing a Workflow to Identify Microplastics Using Near Infrared Hyperspectral Imaging. Chemosphere 2023, 336, 139186. [Google Scholar] [CrossRef] [PubMed]
- Makmuang, S.; Aït-Kaddour, A. Assessment of Microplastic Contamination in Shrimp Utilizing Multispectral Imaging, Fluorescence, and Infrared Spectroscopy. J. Food Compos. Anal. 2025, 146, 107864. [Google Scholar] [CrossRef]
- Manley, M. Near-Infrared Spectroscopy and Hyperspectral Imaging: Non-Destructive Analysis of Biological Materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef]
- Okere, E.E.; Ambaw, A.; Perold, W.J.; Opara, U.L. Vis-NIR and SWIR Hyperspectral Imaging Method to Detect Bruises in Pomegranate Fruit. Front. Plant Sci. 2023, 14, 1151697. [Google Scholar] [CrossRef]
- Lanjewar, M.G.; Asolkar, S.; Parab, J.S.; Morajkar, P.P. Detecting Starch-Adulterated Turmeric Using Vis-NIR Spectroscopy and Multispectral Imaging with Machine Learning. J. Food Compos. Anal. 2024, 136, 106700. [Google Scholar] [CrossRef]
- Kohonen, T. The Self-Organizing Map. Proc. IEEE 1990, 78, 1464–1480. [Google Scholar] [CrossRef]
- Brereton, R.G. Self Organising Maps for Visualising and Modelling. Chem. Cent. J. 2012, 6, S1. [Google Scholar] [CrossRef]
- Lloyd, G.R.; Wongravee, K.; Silwood, C.J.L.; Grootveld, M.; Brereton, R.G. Self Organising Maps for Variable Selection: Application to Human Saliva Analysed by Nuclear Magnetic Resonance Spectroscopy to Investigate the Effect of an Oral Healthcare Product. Chemom. Intell. Lab. Syst. 2009, 98, 149–161. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic Contamination in Brown Shrimp (Crangon crangon, Linnaeus 1758) from Coastal Waters of the Southern North Sea and Channel Area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic Contamination in the Decapod Crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Braid, H.E.; Deeds, J.; DeGrasse, S.L.; Wilson, J.J.; Osborne, J.; Hanner, R.H. Preying on Commercial Fisheries and Accumulating Paralytic Shellfish Toxins: A Dietary Analysis of Invasive Dosidicus gigas (Cephalopoda Ommastrephidae) Stranded in Pacific Canada. Mar. Biol. 2012, 159, 25–31. [Google Scholar] [CrossRef]
- dos Anjos Guimarães, G.; de Moraes, B.R.; Ando, R.A.; Sant’Anna, B.S.; Perotti, G.F.; Hattori, G.Y. Microplastic Contamination in the Freshwater Shrimp Macrobrachium amazonicum in Itacoatiara, Amazonas, Brazil. Environ. Monit. Assess. 2023, 195, 434. [Google Scholar] [CrossRef]
- Saha, G.; Saha, S.C. Tiny Particles, Big Problems: The Threat of Microplastics to Marine Life and Human Health. Processes 2024, 12, 1401. [Google Scholar] [CrossRef]
- Makmuang, S.; Terdwongworakul, A.; Vilaivan, T.; Maher, S.; Ekgasit, S.; Wongravee, K. Mapping Hyperspectral NIR Images Using Supervised Self-Organizing Maps: Discrimination of Weedy Rice Seeds. Microchem. J. 2023, 190, 108599. [Google Scholar] [CrossRef]
- Makmuang, S.; Vilaivan, T.; Maher, S.; Ekgasit, S.; Wongravee, K. Discrimination of Thai Melon Seeds Using Near-Infrared Spectroscopy and Adaptive Self-Organizing Maps. Chemom. Intell. Lab. Syst. 2024, 245, 105060. [Google Scholar] [CrossRef]
- Makmuang, S.; Nootchanat, S.; Ekgasit, S.; Wongravee, K. Non-Destructive Method for Discrimination of Weedy Rice Using near Infrared Spectroscopy and Modified Self-Organizing Maps (SOMs). Comput. Electron. Agric. 2021, 191, 106522. [Google Scholar] [CrossRef]
- Corradini, F.; Bartholomeus, H.; Huerta Lwanga, E.; Gertsen, H.; Geissen, V. Predicting Soil Microplastic Concentration Using Vis-NIR Spectroscopy. Sci. Total Environ. 2019, 650, 922–932. [Google Scholar] [CrossRef]
- Rodionova, O.; Kucheryavskiy, S.; Pomerantsev, A. Efficient Tools for Principal Component Analysis of Complex Data—A Tutorial. Chemom. Intell. Lab. Syst. 2021, 213, 104304. [Google Scholar] [CrossRef]
- Boukria, O.; Boudalia, S.; Bhat, Z.F.; Hassoun, A.; Aït-Kaddour, A. Evaluation of the Adulteration of Camel Milk by Non-Camel Milk Using Multispectral Image, Fluorescence and Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 300, 122932. [Google Scholar] [CrossRef]
- Bonifazi, G.; Fiore, L.; Pelosi, C.; Serranti, S. Evaluation of Plastic Packaging Waste Degradation in Seawater and Simulated Solar Radiation by Spectroscopic Techniques. Polym. Degrad. Stab. 2023, 207, 110215. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Loudiyi, M.; Ferlay, A.; Gruffat, D. Performance of Fluorescence Spectroscopy for Beef Meat Authentication: Effect of Excitation Mode and Discriminant Algorithms. Meat Sci. 2018, 137, 58–66. [Google Scholar] [CrossRef]
- Pavillon, N.; Smith, N.I. Immune Cell Type, Cell Activation, and Single Cell Heterogeneity Revealed by Label-Free Optical Methods. Sci. Rep. 2019, 9, 17054. [Google Scholar] [CrossRef]
- Wongravee, K.; Ishigaki, M.; Ozaki, Y. Chemometrics as a Green Analytical Tool. RSC Green Chem. 2020, 66, 277–336. [Google Scholar] [CrossRef]
- Wongravee, K.; Lloyd, G.R.; Silwood, C.J.; Grootveld, M.; Brereton, R.G. Supervised Self Organizing Maps for Classification and Determination of Potentially Discriminatory Variables: Illustrated by Application to Nuclear Magnetic Resonance Metabolomic Profiling. Anal. Chem. 2010, 82, 628–638. [Google Scholar] [CrossRef]
- Bragato, G.; Piccolo, G.; Sattier, G.; Sada, C. Identification of Spectral Responses of Different Plastic Materials by Means of Multispectral Imaging. Environ. Sci. Process. Impacts 2024, 26, 802–813. [Google Scholar] [CrossRef]
- Kanyathare, B.; Asamoah, B.O.; Ishaq, U.; Amoani, J.; Räty, J.; Peiponen, K.E. Optical Transmission Spectra Study in Visible and Near-Infrared Spectral Range for Identification of Rough Transparent Plastics in Aquatic Environments. Chemosphere 2020, 248, 126071. [Google Scholar] [CrossRef]
- Chen, H.; Shin, T.; Park, B.; Ro, K.; Jeong, C.; Jeon, H.J.; Tan, P.L. Coupling Hyperspectral Imaging with Machine Learning Algorithms for Detecting Polyethylene (PE) and Polyamide (PA) in Soils. J. Hazard. Mater. 2024, 471, 134346. [Google Scholar] [CrossRef] [PubMed]
- Krishna de Guzman, M.; Stanic-Vucinic, D.; Gligorijevic, N.; Wimmer, L.; Gasparyan, M.; Lujic, T.; Vasovic, T.; Dailey, L.A.; Van Haute, S.; Cirkovic Velickovic, T. Small Polystyrene Microplastics Interfere with the Breakdown of Milk Proteins during Static in Vitro Simulated Human Gastric Digestion. Environ. Pollut. 2023, 335, 122282. [Google Scholar] [CrossRef] [PubMed]
- Gligorijevic, N.; Lujic, T.; Mutic, T.; Vasovic, T.; de Guzman, M.K.; Acimovic, J.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Ovalbumin Interaction with Polystyrene and Polyethylene Terephthalate Microplastics Alters Its Structural Properties. Int. J. Biol. Macromol. 2024, 267, 131564. [Google Scholar] [CrossRef]
- Lorenzo-Navarro, J.; Castrillón-Santana, M.; Sánchez-Nielsen, E.; Zarco, B.; Herrera, A.; Martínez, I.; Gómez, M. Deep Learning Approach for Automatic Microplastics Counting and Classification. Sci. Total Environ. 2021, 765, 142728. [Google Scholar] [CrossRef]
- Jayaweera, Y.U.; Hennayaka, H.M.A.I.; Herath, H.M.L.P.B.; Kumara, G.M.P.; Mahagamage, M.G.Y.L.; Rodrigo, U.D.; Manatunga, D.C. A Comprehensive Investigation of Microplastic Contamination and Polymer Toxicity in Farmed Shrimps; L. vannamei and P. monodon. Water Air Soil Pollut. 2025, 236, 168. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Uddin, S.; Lyons, B. Evidence of Microplastics (MP) in Gut Content of Major Consumed Marine Fish Species in the State of Kuwait (of the Arabian/Persian Gulf). Mar. Pollut. Bull. 2020, 154, 111052. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef]
- Lou, F.; Wang, J.; Sun, C.; Song, J.; Wang, W.; Pan, Y.; Huang, Q.; Yan, J. Influence of Interaction on Accuracy of Quantification of Mixed Microplastics Using Py-GC/MS. J. Environ. Chem. Eng. 2022, 10, 108012. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Rochman, C.M. Microwave-Assisted Extraction for Quantification of Microplastics Using Pyrolysis–Gas Chromatography/Mass Spectrometry. Environ. Toxicol. Chem. 2021, 40, 2733–2741. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makmuang, S.; Aït-Kaddour, A. Quantitative and Qualitative Evaluation of Microplastic Contamination of Shrimp Using Visible Near-Infrared Multispectral Imaging Technology Combined with Supervised Self-Organizing Map. Chemosensors 2025, 13, 237. https://doi.org/10.3390/chemosensors13070237
Makmuang S, Aït-Kaddour A. Quantitative and Qualitative Evaluation of Microplastic Contamination of Shrimp Using Visible Near-Infrared Multispectral Imaging Technology Combined with Supervised Self-Organizing Map. Chemosensors. 2025; 13(7):237. https://doi.org/10.3390/chemosensors13070237
Chicago/Turabian StyleMakmuang, Sureerat, and Abderrahmane Aït-Kaddour. 2025. "Quantitative and Qualitative Evaluation of Microplastic Contamination of Shrimp Using Visible Near-Infrared Multispectral Imaging Technology Combined with Supervised Self-Organizing Map" Chemosensors 13, no. 7: 237. https://doi.org/10.3390/chemosensors13070237
APA StyleMakmuang, S., & Aït-Kaddour, A. (2025). Quantitative and Qualitative Evaluation of Microplastic Contamination of Shrimp Using Visible Near-Infrared Multispectral Imaging Technology Combined with Supervised Self-Organizing Map. Chemosensors, 13(7), 237. https://doi.org/10.3390/chemosensors13070237




