Integrated Perspective on Functional Organic Electrochemical Transistors and Biosensors in Implantable Drug Delivery Systems
Abstract
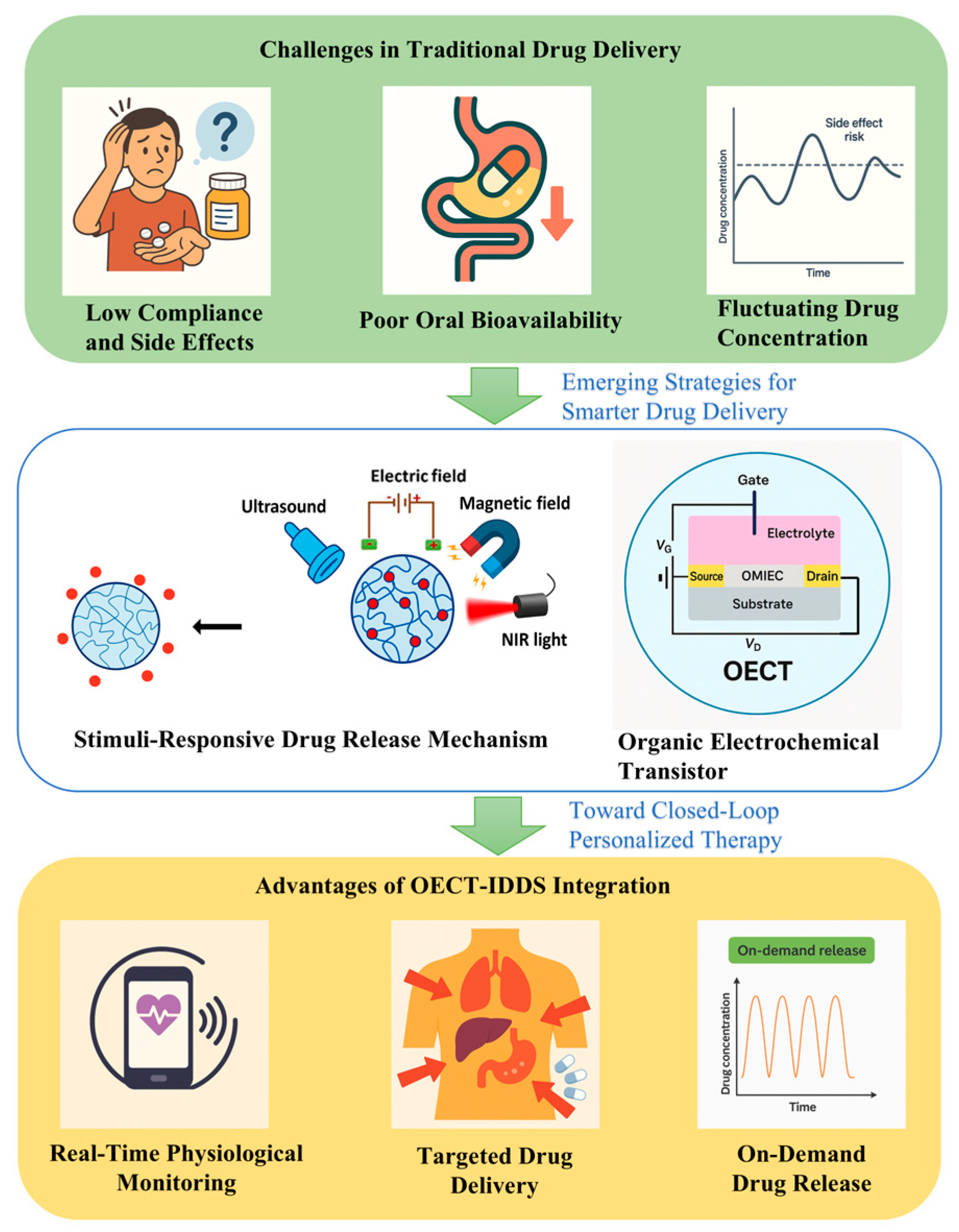
1. Introduction
2. IDDS Technology Review
2.1. Basic Working Principle of IDDSs
2.2. Main Advantages of IDDS Biosensors
2.3. Practical Application and Technical Progress of IDDSs
2.3.1. Diabetes Management
2.3.2. Neurological Disorder Therapy
2.3.3. Pain Management
2.3.4. Cancer Therapy
2.3.5. Infectious Disease Therapy
2.4. Limitations and Challenges of IDDS Biosensors
2.5. Conclusion of IDDSs
3. Overview of Functional OECT Biosensors
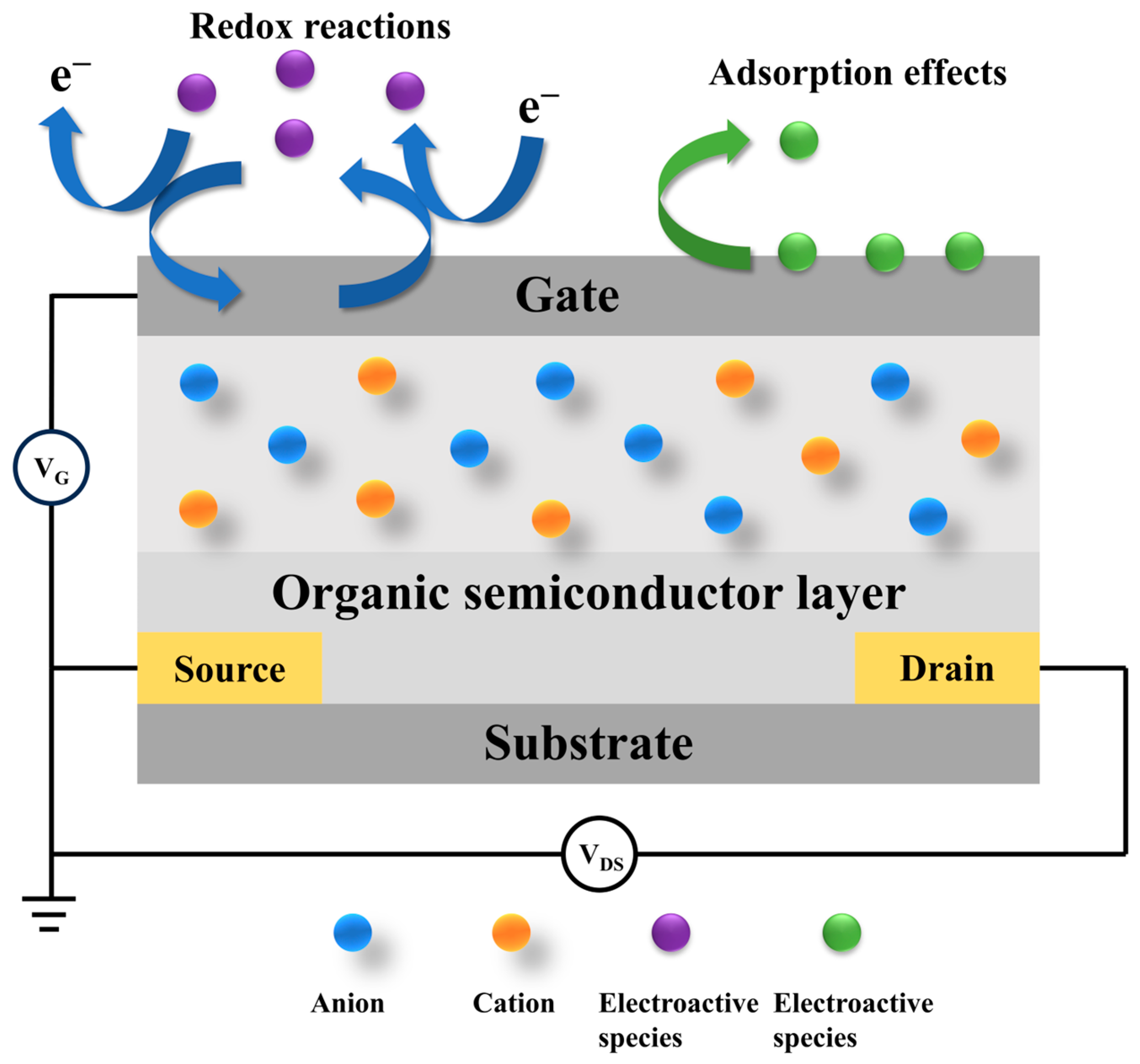
3.1. Basic Working Principle of OECT-Based Biosensors
3.2. Biomolecule Immobilization in OECT Biosensors
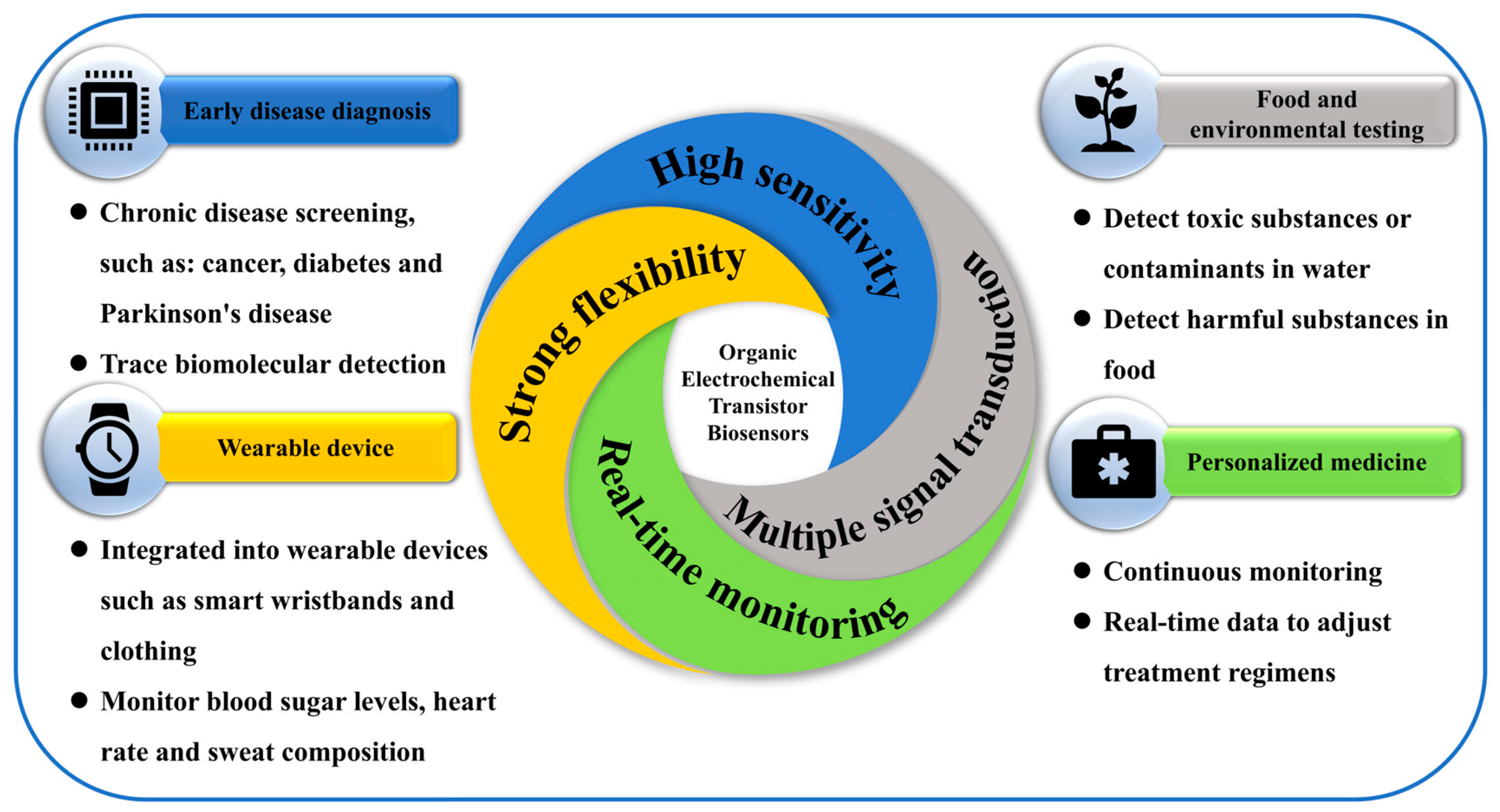
3.3. Advantages of OECT-Based Biosensors
3.4. Research and Application of a Functional Biosensor Based on OECTs
3.4.1. Glucose Sensing
3.4.2. Cancer Biomarker Detection
3.4.3. Nucleic Acid Detection
3.4.4. Environmental Monitoring
3.4.5. Body Fluid Detection
3.4.6. Personalized Medical Care
3.5. Limitations and Challenges of OECT-Based Biosensors
3.6. Conclusions of OECT-Based Biosensors
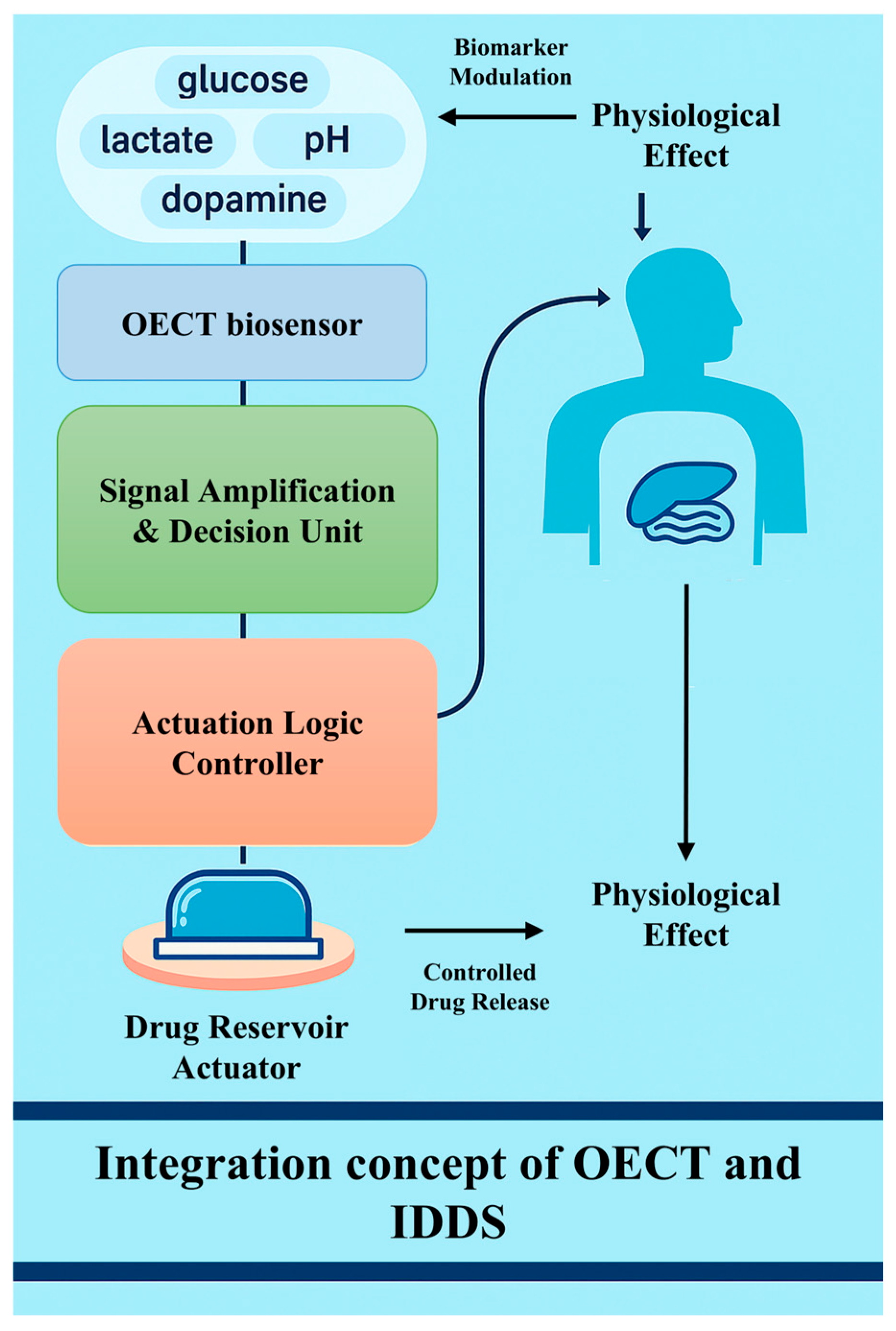
4. Combination of OECT-Based Biosensors and IDDSs
4.1. Closed-Loop Integration Mechanism
4.2. Representative Applications and Case Studies
4.3. Organic Electronic Ion Pump and Its Relevance
4.4. Comparative Evaluation of OECT-Integrated IDDSs and CMOS-Based Therapeutic Platforms
4.5. Comparative Evaluation of OECT-Integrated IDDSs and Optical -Based Therapeutic Platforms
4.6. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, C.; Bhardwaj, K.; Marculescu, R. Towards cell-based therapeutics: A bio-inspired autonomous drug delivery system. Nano Commun. Networks 2017, 12, 25–33. [Google Scholar] [CrossRef]
- Song, P.Y.; Jian, D.; Tng, H.; Hu, R.; Lin, G.M.; Meng, E.; Yong, K.T. An Electrochemically Actuated MEMS Device for Individualized Drug Delivery: An In Vitro Study. Adv. Healthc. Mater. 2013, 2, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- McKeating, K.S.; Aube, A.; Masson, J.F. Biosensors and nanobiosensors for therapeutic drug and response monitoring. Analyst 2016, 141, 429–449. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, S.; Wang, Q.; Li, J. Recent progress in biosensor regeneration techniques. Nanoscale 2024, 16, 2834–2846. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Sardini, E.; Serpelloni, M.; Tonello, S. Printed Electrochemical Biosensors: Opportunities and Metrological Challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. In Biosensor Technologies for Detection of Biomolecules; Estrela, P., Ed.; Portland Press Ltd.: London, UK, 2016; Volume 60, pp. 1–8. [Google Scholar]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Merkoci, A. Advanced Materials for Biosensors—Special Issue of SMALL. Small 2023, 19, e2308049. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037525. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Raston, N.H.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [CrossRef]
- Chen, C.; Lu, S. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef]
- Kurnik, M.; Pang, E.Z.; Plaxco, K.W. An Electrochemical Biosensor Architecture Based on Protein Folding Supports Direct Real-Time Measurements in Whole Blood. Angew. Chem. Int. Ed. 2020, 59, 18442–18445. [Google Scholar] [CrossRef]
- Marks, A.; Griggs, S.; Gasparini, N.; Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 2022, 9, 2102039. [Google Scholar] [CrossRef]
- Friedlein, J.T.; McLeod, R.R.; Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 2018, 63, 398–414. [Google Scholar] [CrossRef]
- Rashid, R.B.; Ji, X.D.; Rivnay, J. Organic electrochemical transistors in bioelectronic circuits. Biosens. Bioelectron. 2021, 190, 13461. [Google Scholar] [CrossRef]
- Ravariu, C. From Enzymatic Dopamine Biosensors to OECT Biosensors of Dopamine. Biosensors 2023, 13, 806. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Meshkinfam, F.; Rizvi, G. A MEMS-Based Drug Delivery Device With Integrated Microneedle Array-Design and Simulation. J. Biomech. Eng. Trans. Asme 2021, 143, 081010. [Google Scholar] [CrossRef] [PubMed]
- Timko, B.P.; Arruebo, M.; Shankarappa, S.A.; McAlvin, J.B.; Okonkwo, O.S.; Mizrahi, B.; Stefanescu, C.F.; Gomez, L.; Zhu, J.; Zhu, A.; et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 2014, 111, 1349–1354. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.L.; Zhang, L.L.; Miron, R.J.; Liang, J.F.; Shi, M.S.; Mo, W.T.; Zheng, S.H.; Zhao, Y.B.; Zhang, Y.F. Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef]
- Wu, D.; Fei, F.; Zhang, Q.; Wang, X.; Gong, Y.W.; Chen, X.J.; Zheng, Y.Y.; Tan, B.; Xu, C.L.; Xie, H.J.; et al. Nanoengineered on-demand drug delivery system improves efficacy of pharmacotherapy for epilepsy. Sci. Adv. 2022, 8, abm3381. [Google Scholar] [CrossRef]
- Cui, Y.T.; Wang, Z.H.; Li, Z.H.; Ji, X.; Yuan, B.M.; Sun, Y.; Peng, C.G.; Leng, Y.; Dou, M.H.; Wang, J.C.; et al. Functionalized anti-osteoporosis drug delivery system enhances osseointegration of an inorganic-organic bioactive interface in osteoporotic microenvironment. Mater. Des. 2021, 206, 109753. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- He, G.Q.; Li, H.M.; Liu, J.Y.; Hu, Y.L.; Liu, Y.; Wang, Z.L.; Jiang, P. Recent Progress in Implantable Drug Delivery Systems. Adv. Mater. 2024, 36, 2312530. [Google Scholar] [CrossRef]
- Barik, A.; Chakravorty, N. Targeted Drug Delivery from Titanium Implants: A Review of Challenges and Approaches. In Trends in Biomedical Research; Pokorski, M., Ed.; Springer International Publishing Ag: Cham, Switzerland, 2020; Volume 1251, pp. 1–17. [Google Scholar]
- Sutradhar, K.B.; Sumi, C.D. Implantable microchip: The futuristic controlled drug delivery system. Drug Deliv. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Almoshari, Y. Osmotic Pump Drug Delivery Systems-A Comprehensive Review. Pharmaceuticals 2022, 15, 430. [Google Scholar] [CrossRef]
- Hu, J.R.; Zhang, J.B.; Hou, Y.F.; Li, C.S.; Yang, W.N.; Fu, J.Z.; Hu, S.Y.; Liu, A.; He, Y. Digital electronics-free implantable drug delivery system for on-demand therapy. Chem. Eng. J. 2025, 504, 158763. [Google Scholar] [CrossRef]
- Santos, A.; Aw, M.S.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef]
- Vyas, K.S.; Rajendran, S.; Morrison, S.D.; Shakir, A.; Mardini, S.; Lemaine, V.; Nahabedian, M.Y.; Baker, S.B.; Rinker, B.D.; Vasconez, H.C. Systematic Review of Liposomal Bupivacaine (Exparel) for Postoperative Analgesia. Plast. Reconstr. Surg. 2016, 138, 748E–756E. [Google Scholar] [CrossRef]
- Funk, S.; Miller, M.M.; Mishell, D.R., Jr.; Archer, D.F.; Poindexter, A.; Schmidt, J.; Zampaglione, E.; Implanon, U.S.S.G. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception 2005, 71, 319–326. [Google Scholar] [CrossRef]
- Rael, C.T.; Lentz, C.; Carballo-Diéguez, A.; Giguere, R.; Dolezal, C.; Feller, D.; D’Aquila, R.T.; Hope, T.J. Understanding the Acceptability of Subdermal Implants as a Possible New HIV Prevention Method: Multi-Stage Mixed Methods Study. J. Med. Internet Res. 2020, 22, e16904. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xu, J.; Lim, J.; Nolan, J.K.; Lee, H.; Lee, C.H. Wearable Glucose Monitoring and Implantable Drug Delivery Systems for Diabetes Management. Adv. Healthc. Mater. 2021, 10, 100194. [Google Scholar] [CrossRef]
- Bertsch, T.; McKeirnan, K. ITCA 650. Clin. Diabetes 2018, 36, 265–267. [Google Scholar] [CrossRef][Green Version]
- Sreedharan, S.; Bankiewicz, K.; Patel, R. Continuous delivery of ropinirole by subdermal ProNeura™ implants. Mov. Disord. 2015, 30, S429. [Google Scholar]
- Brigham, N.C.; Ji, R.R.; Becker, M.L. Degradable polymeric vehicles for postoperative pain management. Nat. Commun. 2021, 12, 1367. [Google Scholar] [CrossRef]
- Bourge, R.C.; Waxman, A.B.; Gomberg-Maitland, M.; Shapiro, S.M.; Tarver, J.H.; Zwicke, D.L.; Feldman, J.P.; Chakinala, M.M.; Frantz, R.P.; Torres, F.; et al. Treprostinil Administered to Treat Pulmonary Arterial Hypertension Using a Fully Implantable Programmable Intravascular Delivery System Results of the DelIVery for PAH Trial. Chest 2016, 150, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Abd-Elsayed, A.; Calodney, A.; Stromberg, K.; Weaver, T.; Spencer, R.J. Targeted Drug Delivery for Chronic Nonmalignant Pain: Longitudinal Data From the Product Surveillance Registry. Neuromodulation 2021, 24, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Inoue, A.; Hirose, Y.; Morioka, M.; Horiguchi, K.; Natsume, A.; Arakawa, Y.; Iwasaki, K.; Fujiki, M.; Kumabe, T.; et al. Long-term effectiveness of Gliadel implant for malignant glioma and prognostic factors for survival: 3-year results of a postmarketing surveillance in Japan. Neurooncol. Adv. 2022, 4, vdab189. [Google Scholar] [CrossRef]
- Haim, O.; Agur, A.; Efrat, O.T.; Valdes, P.; Ram, Z.; Grossman, R. The clinical significance of radiological changes associated with gliadel implantation in patients with recurrent high grade glioma. Sci. Rep. 2023, 13, 11. [Google Scholar] [CrossRef]
- Flexner, C. Antiretroviral implants for treatment and prevention of HIV infection. Curr. Opin. HIV Aids 2018, 13, 374–380. [Google Scholar] [CrossRef]
- Kovarova, M.; Benhabbour, S.R.; Massud, I.; Spagnuolo, R.A.; Skinner, B.; Baker, C.E.; Sykes, C.; Mollan, K.R.; Kashuba, A.D.M.; García-Lerma, J.G.; et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 2018, 9, 4156. [Google Scholar] [CrossRef]
- Henry, S.J.; Barrett, S.E.; Forster, S.P.; Teller, R.S.; Yang, Z.; Li, L.; Mackey, M.A.; Doto, G.J.; Ruth, M.P.; Tsuchiya, T.; et al. Exploration of long-acting implant formulations of hepatitis B drug entecavir. Eur. J. Pharm. Sci. 2019, 136, 104958. [Google Scholar] [CrossRef]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J.; et al. Pharmacokinetics of Long-Acting Tenofovir Alafenamide (GS-7340) Subdermal Implant for HIV Prophylaxis. Antimicrob. Agents Chemother. 2015, 59, 3913–3919. [Google Scholar] [CrossRef]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and implantable devices for drug delivery: Applications and challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef]
- Joo, H.; Lee, Y.; Kim, J.; Yoo, J.S.; Yoo, S.; Kim, S.; Arya, A.K.; Kim, S.; Choi, S.H.; Lu, N.; et al. Soft implantable drug delivery device integrated wirelessly with wearable devices to treat fatal seizures. Sci. Adv. 2021, 7, eabd4639. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, F.; Zhu, X.X.; Alfadhel, A.; Ding, B.; Walton, J.P.; Cormier, D.; Frisina, R.D.; Borkholder, D.A. A nanoliter resolution implantable micropump for murine inner ear drug delivery. J. Control. Release 2019, 298, 27–37. [Google Scholar] [CrossRef]
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview, in: Grumezescu. In Nanostructures for the Engineering of Cells, Tissues and Organs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–511. [Google Scholar] [CrossRef]
- Long, G.; Mortimer, R.; Sanzenbacher, G. Recent Average Price Trends for Implantable Medical Devices, 2007–2011. 2013. Available online: https://www.analysisgroup.com/globalassets/content/insights/publishing/implantable_medical_device_price_trends.pdf (accessed on 3 April 2025).
- White, H.S.; Kittlesen, G.P.; Wrighton, M.S. Chemical derivatization of an array of three gold microelectrodes with polypyr-role: Fabrication of a molecule-based transistor. J. Am. Chem. Soc. 2002, 106, 5375–5377. [Google Scholar] [CrossRef]
- Sun, C.F.; Wang, X.; Auwalu, M.A.; Cheng, S.S.; Hu, W.P. Organic thin film transistors-based biosensors. Ecomat 2021, 3, e12094. [Google Scholar] [CrossRef]
- Bai, L.M.; Elósegui, C.G.; Li, W.Q.; Yu, P.; Fei, J.J.; Mao, L.Q. Biological Applications of Organic Electrochemical Transistors: Electrochemical Biosensors and Electrophysiology Recording. Front. Chem. 2019, 7, 313. [Google Scholar] [CrossRef]
- Wallace, G.G.; Smyth, M.; Zhao, H. Conducting Electroactive Polymer-based Biosensors, Trac-Tend. Anal. Chem. 1999, 18, 245–251. [Google Scholar]
- Strakosas, X.; Bongo, M.; Owens, R.M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 2015, 132, 41735. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Watanabe, S.; Matsumoto, M.; Muto, T.; Aoyama, T. Majority carrier type conversion in solution-processed organic transistors and flexible complementary logic circuits. Appl. Phys. Lett. 2010, 96, 083303. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.C.; Jiang, Q.L.; Xu, J.K. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef]
- Ji, W.; Wu, D.Q.; Tang, W.; Xi, X.; Su, Y.Z.; Guo, X.J.; Liu, R.L. Carbonized silk fabric-based flexible organic electrochemical transistors for highly sensitive and selective dopamine detection. Sens. Actuators B Chem. 2020, 304, 127414. [Google Scholar] [CrossRef]
- Pierre, A.; Doris, S.E.; Lujan, R.; Street, R.A. Monolithic Integration of Ion-Selective Organic Electrochemical Transistors with Thin Film Transistors on Flexible Substrates. Adv. Mater. Technol. 2019, 4, 1800577. [Google Scholar] [CrossRef]
- Keene, S.T.; Fogarty, D.; Cooke, R.; Casadevall, C.D.; Salleo, A.; Parlak, O. Wearable Organic Electrochemical Transistor Patch for Multiplexed Sensing of Calcium and Ammonium Ions from Human Perspiration. Adv. Healthc. Mater. 2019, 8, e1901321. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, M.G.; Zhao, Y.D.; Chen, Y.T.; Noureen, B.; Du, L.P.; Wu, C.S. Functional Organic Electrochemical Transistor-Based Biosensors for Biomedical Applications. Chemosensors 2024, 12, 236. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavalli, V.K. The design, fabrication, and applications of flexible biosensing devices. Biosens. Bioelectron. 2019, 124, 96–114. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Y.F.; Xu, W.T. Recent Process of Flexible Transistor-Structured Memory. Small 2021, 17, e1905332. [Google Scholar] [CrossRef]
- Spanu, A.; Martines, L.; Bonfiglio, A. Interfacing cells with organic transistors: A review of in vitro and in vivo applications. Lab Chip 2021, 21, 795–820. [Google Scholar] [CrossRef]
- Saha, T.; Fang, J.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Wearable Osmotic-Capillary Patch for Prolonged Sweat Harvesting and Sensing. ACS Appl. Mater. Interfaces 2021, 13, 8071–8081. [Google Scholar] [CrossRef]
- Bocchetta, P.; Frattini, D.; Ghosh, S.; Mohan, A.M.V.; Kumar, Y.; Kwon, Y. Soft Materials for Wearable/Flexible Electrochemical Energy Conversion, Storage, and Biosensor Devices. Materials 2020, 13, 2733. [Google Scholar] [CrossRef]
- Rabe, M. Verdes, D. Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Li, D.X.; He, Q.; Cui, Y.; Duan, L.; Li, J.B. Immobilization of glucose oxidase onto gold nanoparticles with enhanced thermostability. Biochem. Biophys. Res. Commun. 2007, 355, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, E.; Huynh, M.; GhavamiNejad, P.; Zheng, H.; Saini, A.; Bakhshandeh, F.; Keyvani, F.; Mantaila, D.; Rahman, F.A.; Quadrilatero, J.; et al. A PEDOT:PSS-based composite hydrogel as a versatile electrode for wearable microneedle sensing platforms. Adv. Sens. Res. 2024, 3, 2300122. [Google Scholar] [CrossRef]
- Tarabella, G.; CoppedÃ, N.; Mosca, R.; Cicoira, F.; Iannotta, S. Organic electrochemical transistors operating with electrolytes of increasing complexity for (Bio)sensing. AIP Conf. Proc. 2012, 1479, 1880–1883. [Google Scholar]
- Xi, X.; Wu, D.Q.; Ji, W.; Zhang, S.N.; Tang, W.; Su, Y.Z.; Guo, X.J.; Liu, R.L. Manipulating the Sensitivity and Selectivity of OECT-Based Biosensors via the Surface Engineering of Carbon Cloth Gate Electrodes. Adv. Funct. Mater. 2020, 30, 1905361. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Nguyen, Q.H.; Ko, J.; Lee, H.; Kim, D.; Kim, Y.H.; Kim, D.Y.; Joo, Y. Conjugated Radical Polymer-Based Organic Electrochemical Transistors for Biosensing Devices. Chem. Mater. 2024, 36, 7897–7908. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Nawaz, A.; Liu, Q.; Leong, W.L.; Fairfull-Smith, K.E.; Sonar, P. Organic Electrochemical Transistors for In Vivo Bioelectronics. Adv. Mater. 2021, 33, 2101874. [Google Scholar] [CrossRef]
- Cea, C.; Spyropoulos, G.D.; Jastrzebska-Perfect, P.; Ferrero, J.J.; Gelinas, J.N.; Khodagholy, D. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat. Mater. 2020, 19, 679–686. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 7086. [Google Scholar] [CrossRef]
- Berggren, M.; Forchheimer, R.; Bobacka, J.; Svensson, P.O.; Nilsson, D.; Larsson, O.; Ivaska, A. PEDOT:PSS-Based Electrochemical Transistors for Ion-to-Electron Transduction and Sensor Signal Amplification. In Organic Semiconductors in Sensor Applications; Springer: Berlin, Germany, 2008; pp. 263–280. [Google Scholar]
- Lee, H.; Lee, S.; Lee, W.; Yokota, T.; Fukuda, K.; Someya, T. Ultrathin Organic Electrochemical Transistor with Nonvolatile and Thin Gel Electrolyte for Long-Term Electrophysiological Monitoring. Adv. Funct. Mater. 2019, 29, 1906982. [Google Scholar] [CrossRef]
- Rezali, F.A.M.; Soin, N.; Hatta, S.; Daut, M.H.M.; Nouxman, M.H.A.; Hussin, H. Design Strategies and Prospects in Developing Wearable Glucose Monitoring System Using Printable Organic Transistor and Microneedle: A Review. IEEE Sens. J. 2022, 22, 13785–13799. [Google Scholar] [CrossRef]
- Spooren, A.; Rondou, P.; Debowska, K.; Lintermans, B.; Vermeulen, L.; Samyn, B.; Skieterska, K.; Debyser, G.; Devreese, B.; Vanhoenacker, P.; et al. Resistance of the dopamine D4 receptor to agonist-induced internalization and degradation. Cell. Signal. 2010, 22, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Lee, H.; Yea, J.; Jang, K.-I. Wearable electrochemical sensors for real-time monitoring in diabetes mellitus and associated complications. Soft Sci. 2024, 4, 15. [Google Scholar] [CrossRef]
- Premanode, B.; Toumazou, C. A novel, low power biosensor for real time monitoring of creatinine and urea in peritoneal dialysis. Sens. Actuators B Chem. 2007, 120, 732–735. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Schwab, U.S. Relationship of Dietary Fat to Glucose Metabolism. Atherosclerosis 2000, 150, 227–243. [Google Scholar] [CrossRef]
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian desynchrony and glucose metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef]
- Karen, D.; Marine, B.; Alan, R. Single on-chip gold nanowires for electrochemical biosensing of glucose. Analyst 2011, 136, 4507–4513. [Google Scholar]
- Liao, J.J.; Lin, S.W.; Yang, Y.; Liu, K.; Du, W.C. Highly selective and sensitive glucose sensors based on organic electrochemical transistors using TiO2 nanotube arrays-based gate electrodes. Sens. Actuators B Chem. 2015, 208, 457–463. [Google Scholar] [CrossRef]
- Liao, C.Z.; Zhang, M.; Niu, L.Y.; Zheng, Z.J.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Hama, A.; Chen, X.X.; McCulloch, I.; Inal, S. Microfluidics integrated n-type organic electrochemical transistor for metabolite sensing. Sens. Actuators B Chem. 2021, 329, 129251. [Google Scholar] [CrossRef]
- Chen, C.H.; Song, Q.Y.; Lu, W.T.; Zhang, Z.T.; Yu, Y.H.; Liu, X.Y.; He, R.X. A sensitive platform for DNA detection based on organic electrochemical transistor and nucleic acid self-assembly signal amplification. RSC Adv. 2021, 11, 37917–37922. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.Y.; Gu, X.; Tsang, C.M.; Tsao, S.W.G.; Hsing, I.M. Organic electrochemical transistor array for monitoring barrier integrity of epithelial cells invaded by nasopharyngeal carcinoma. Sens. Actuators B Chem. 2019, 297, 126761. [Google Scholar] [CrossRef]
- Song, Q.Y.; Wang, W.Y.; Liang, J.J.; Chen, C.H.; Cao, Y.P.; Cai, B.; Chen, B.L.; He, R.X. Fabrication of PEDOT:PSS-based solution gated organic electrochemical transistor array for cancer cells detection. RSC Adv. 2023, 13, 36416–36423. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Keski-oja, J.; Vaheri, A. Extracellular Matrix Proteins Characterize Human Tumor Cell Lines. Int. J. Cancer 1981, 27, 755–761. [Google Scholar] [CrossRef]
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, N.X.; Yang, A.N.; Law, H.K.W.; Li, L.; Yan, F. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, N.E.; Park, J.S.; Park, I.J.; Kim, J.G.; Cho, H.J. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef]
- Chen, L.Z.; Fu, Y.; Wang, N.X.; Yang, A.N.; Li, Y.Z.; Wu, J.; Ju, H.X.; Yan, F. Organic Electrochemical Transistors for the Detection of Cell Surface Glycans. ACS Appl. Mater. Interfaces 2018, 10, 18470–18477. [Google Scholar] [CrossRef]
- He, R.X.; Zhang, M.; Tan, F.; Leung, P.H.M.; Zhao, X.Z.; Chan, H.L.W.; Yang, M.; Yan, F. Detection of bacteria with organic electrochemical transistors. J. Am. Chem. Soc. 2012, 22, 22072–22076. [Google Scholar] [CrossRef]
- Sophocleous, M.; Contat-Rodrigo, L.; García-Breijo, E.; Georgiou, J. Organic Electrochemical Transistors as an Emerging Platform for Bio-Sensing Applications: A Review. IEEE Sens. J. 2021, 21, 3977–4006. [Google Scholar] [CrossRef]
- O’Connor, L.; Glynn, B. Recent advances in the development of nucleic acid diagnostics. Expert Rev. Med. Devices 2010, 7, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Umek, R.; Lin, S.W.; Vielmetter, J.; Terbrueggen, R.; Irvine, B.; Yu, C.; Kayyem, J.; Yowanto, H.; Blackburn, G.; Farkas, D. Electronic Detection of Nucleic Acids: A Versatile Platform for Molecular Diagnostics. J. Mol. Diagn. 2001, 3, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.J.; Lu, J.M.; Yu, T.; Long, Y.; Liu, G.Z. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206, 114109. [Google Scholar] [CrossRef]
- Elli, G.; Hamed, S.; Petrelli, M.; Ibba, P.; Ciocca, M.; Lugli, P.; Petti, L. Field-Effect Transistor-Based Biosensors for Environmental and Agricultural Monitoring. Sensors 2022, 22, 4178. [Google Scholar] [CrossRef]
- Porter, E.B.; Adaryan, S.; Ardebili, H.; Biswal, S.L.; Verduzco, R. Detection of Crude Oil in Subsea Environments Using Organic Electrochemical Transistors. ACS Sens. 2024, 9, 3633–3640. [Google Scholar] [CrossRef]
- Guimarães, A.; De Assis, H.S.; Boeger, W. The effect of trichlorfon on acetylcholinesterase activity and histopathology of cultivated fish Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2007, 68, 57–62. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Qu, H.; Zheng, L. An Acetylcholinesterase-Functionalized Biosensor for Sensitive Detection of Organophosphorus Pesticides Based on Solution-Gated Graphene Transistors. ACS Agric. Sci. Technol. 2021, 1, 372–378. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.Y.; Tian, X.Y.; Wang, Y.; Cui, B.B.; Yang, Y.L.; Dai, S.L.; Lin, W.S.; Zhu, J.X.; Wang, J.Q.; et al. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, Q.W.; Li, Z.B.; Du, Z.F.; Hong, X.; Qiu, L.Z. An enzyme Biosensor Based on Organic Transistors for Recognizing α-Amino Acid Enantiomers. J. Electrochem. Soc. 2020, 167, 067517. [Google Scholar] [CrossRef]
- Pappa, A.M.; Ohayon, D.; Giovannitti, A.; Maria, L.P.; Savva, A.; Uguz, I.; Rivnay, J.; McCulloch, I.; Owens, R.M.; Inal, S. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 2018, 4, eaat0911. [Google Scholar] [CrossRef] [PubMed]
- Recky, J.R.N.; Montero-Jimenez, M.; Scotto, J.; Azzaroni, O.; Marmisollé, W.A. Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing. Chemosensors 2024, 12, 124. [Google Scholar] [CrossRef]
- Arcangeli, D.; Gualandi, I.; Mariani, F.; Tessarolo, M.; Ceccardi, F.; Decataldo, F.; Melandri, F.; Tonelli, D.; Fraboni, B.; Scavetta, E. Smart Bandaid Integrated with Fully Textile OECT for Uric Acid Real-Time Monitoring in Wound Exudate. ACS Sens. 2023, 8, 1593–1608. [Google Scholar] [CrossRef]
- Janardhanan, J.A.; Chen, Y.L.; Liu, C.T.; Tseng, H.S.; Wu, P.; She, J.W.; Hsiao, Y.S.; Yu, H.H. Sensitive Detection of Sweat Cortisol Using an Organic Electrochemical Transistor Featuring Nanostructured Poly(3,4-Ethylenedioxythiophene) Derivatives in the Channel Layer. Anal. Chem. 2022, 94, 7584–7593. [Google Scholar] [CrossRef]
- Curto, V.F.; Marchiori, B.; Hama, A.; Pappa, A.M.; Ferro, M.P.; Braendlein, M.; Rivnay, J.; Fiocchi, M.; Malliaras, G.G.; Ramuz, M.; et al. Organic transistor platform with integrated microfluidics for in-line multi-parametric in vitro cell monitoring. Microsyst. Nanoeng. 2017, 3, 17028. [Google Scholar] [CrossRef]
- Niu, Y.; Qin, Z.; Zhang, Y.; Chen, C.; Liu, S.; Chen, H. Expanding the potential of biosensors: A review on organic field effect transistor (OFET) and organic electrochemical transistor (OECT) biosensors. Mater. Futures 2023, 2, 042401. [Google Scholar] [CrossRef]
- Woeppel, K.M.; Zheng, X.S.; Schulte, Z.M.; Rosi, N.L.; Cui, X.Y.T. Nanoparticle Doped PEDOT for Enhanced Electrode Coatings and Drug Delivery. Adv. Healthc. Mater. 2019, 8, e1900622. [Google Scholar] [CrossRef]
- Lopez-Larrea, N.; Wustoni, S.; Penas, M.I.; Uribe, J.; Dominguez-Alfaro, A.; Gallastegui, A.; Inal, S.; Mecerreyes, D. PNIPAM/PEDOT:PSS Hydrogels for Multifunctional Organic Electrochemical Transistors. Adv. Funct. Mater. 2024, 34, 2403708. [Google Scholar] [CrossRef]
- Simon, D.T.; Kurup, S.; Larsson, K.C.; Hori, R.; Tybrandt, K.; Goiny, M.; Jager, E.H.; Berggren, M.; Canlon, B.; Richter-Dahlfors, A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat. Mater. 2009, 8, 742–746. [Google Scholar] [CrossRef]
- Tybrandt, K.; Larsson, K.C.; Kurup, S.; Simon, D.T.; Kjäll, P.; Isaksson, J.; Sandberg, M.; Jager, E.W.H.; Richter-Dahlfors, A.; Berggren, M. Translating Electronic Currents to Precise Acetylcholine-Induced Neuronal Signaling Using an Organic Electrophoretic Delivery Device. Adv. Mater. 2009, 21, 4442–4446. [Google Scholar] [CrossRef]
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Kjall, P.; Nilsson, D.; Robinson, N.D.; Berggren, M.; Richter-Dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar] [CrossRef]
- Williamson, A.; Rivnay, J.; Kergoat, L.; Jonsson, A.; Inal, S.; Uguz, I.; Ferro, M.; Ivanov, A.; Sjöström, T.A.; Simon, D.T.; et al. Controlling Epileptiform Activity with Organic Electronic Ion Pumps. Adv. Mater. 2015, 27, 3138–3144. [Google Scholar] [CrossRef]
- Berggren, M.; Glowacki, E.D.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K. In vivo organic bioelectronics for neuromodulation. Chem. Rev. 2022, 122, 4826–4846. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Xu, L.; Gutbrod, S.R.; Bonifas, A.P.; Su, Y.; Sulkin, M.S.; Lu, N.; Chung, H.-J.; Jang, K.-I.; Liu, Z.; Ying, M.; et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 2014, 5, 3329. [Google Scholar] [CrossRef]
- Sawan, M.; Yang, J.; Tarkhan, M.; Chen, J.; Wang, M.; Wang, C.; Xia, F.; Chen, Y.-H. Emerging Trends of Biomedical Circuits and Systems. Found. Trends® Integr. Circuits Syst. 2021, 1, 217–411. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Qiu, Y.; Wu, H.; Qin, W.; Liao, Y.; Yu, Q.; Cheng, H. Stretchable piezoelectric energy harvesters and self-powered sensors for wearable and implantable devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Li, Y.; Cui, B.; Zhang, S.M.; Li, B.X.; Li, J.M.; Liu, S.J.; Zhao, Q. Ion-Selective Organic Electrochemical Transistors: Recent Progress and Challenges. Small 2022, 18, 2107413. [Google Scholar] [CrossRef]
- Park, W.; Nguyen, V.P.; Jeon, Y.; Kim, B.; Li, Y.X.; Yi, J.; Kim, H.; Leem, J.W.; Kim, Y.L.; Kim, D.R.; et al. Biodegradable silicon nanoneedles for ocular drug delivery. Sci. Adv. 2022, 8, eabn1772. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.K.; Cen, D.; Zhang, T.; Jiang, S.; Wang, Y.F.; Cai, X.J.; Li, X.; Han, G.R. Implantable fibrous scaffold with hierarchical microstructure for the ‘on-site’ synergistic cancer therapy. Chem. Eng. J. 2020, 402, 126204. [Google Scholar] [CrossRef]
- Ross, J.S.; Bates, J.; Parzynski, C.S.; Akar, J.G.; Curtis, J.P.; Desai, N.R.; Freeman, J.V.; Gamble, G.M.; Kuntz, R.; Li, S.X.; et al. Can machine learning complement traditional medical device surveillance? A case study of dual-chamber implantable cardioverter-defibrillators. Med. Devices 2017, 10, 165–188. [Google Scholar] [CrossRef] [PubMed]



| Implant Example | Drug Type | Implant Material/Method | Model | Release Duration | Reference |
|---|---|---|---|---|---|
| Implanon® | Etonogestrel (hormone) | Biodegradable polymer rod | Human use | Up to 3 years | [36] |
| Exparel® | Bupivacaine (analgesic) | Liposomal encapsulation | Human use | Several days | [37] |
| ITCA 650 | Exenatide (GLP-1 receptor agonist) | Medici Drug Delivery System TM; osmotic micro-pump | Human (type 2 diabetes patients) | >6 months | [40] |
| ProNeura TM | Ropinirole (dopamine agonist) and T3 hormone (triiodothyronine) | Non-biodegradable EVA matrix with embedded drugs | Human (Parkinson’s disease and hypothyroidism) | Long-term | [41] |
| Gliadel® Wafers | Carmustine (chemotherapy) | Biodegradable polymer wafer (local brain implantation) | Human (glioblastoma multiforme patients) | short-to-medium term | [46,47] |
| Dolutegravir implant | Dolutegravir (antiviral) | PLGA/N-methyl-pyrrolidone | Humanized BLT mouse | 5 months | [49] |
| Entecavir implant | Entecavir (antiviral) | Hot-melt extrusion + polymer-coated tablets | Rat model | 87 days | [50] |
| Tenofovir alafenamide implant | Tenofovir alafenamide (antiviral) | Platinum microperforated silicon tube + PVA coating | Beagle dogs | 40 days | [51] |
| Application Target | Functionalization | Material | Detection Range | Reference |
|---|---|---|---|---|
| Glucose detection | GOx, Pt NPs, chitosan, and Nafion | Pt NP + PEDOT:PSS gate on polymer substrates | 100 nM–5 mM; 10 nM–1 μM (high selectivity) | [94] |
| Cancer biomarker detection (HER2, PSA) | Antibody-functionalized PEDOT:PSS and Au NPs | PEDOT:PSS channel + Au NP secondary antibodies | HER2: 10 fg/mL; PSA complex: 1 pg/mL | [101] |
| Glycoprotein/glycan detection | Lectin and aptamer-modified channels | PEDOT:PSS + PDDA/MWCNT gate modification | Significant response at low glycan levels | [104] |
| Nucleic acid detection | HCR, DNA probes, and Au NPs | Electrochemically deposited Au NPs + OECT array | 0.1 pM DNA; mismatch discrimination | [96] |
| Environmental pesticide detection(trichlorfon) | AChE enzyme on a graphene-based gate | Graphene OECT + AChE gate functionalization | 10 nM–3 μΜ; ΔΙ = 0.23 μA at 10 nM | [113] |
| Wound exudate uric acid monitoring | Textile-based PEDOT:PSS sensor | PEDOT:PSS on textile fibers | 220–750 μM in synthetic wound exudates | [118] |
| Cortisol detection in sweat | Cortisol antibody fixed on nanostructured PEDOT | Template-free electro-polymerized nanostructured PEDOT channel | 1 fg/mL–1 μg/mL; LOD = 0.0088 fg/mL | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.-L.; Zhou, T.; Xu, J.-M.; Zhang, S.-F.; Hu, Y.-Z.; Liu, Y. Integrated Perspective on Functional Organic Electrochemical Transistors and Biosensors in Implantable Drug Delivery Systems. Chemosensors 2025, 13, 215. https://doi.org/10.3390/chemosensors13060215
Han X-L, Zhou T, Xu J-M, Zhang S-F, Hu Y-Z, Liu Y. Integrated Perspective on Functional Organic Electrochemical Transistors and Biosensors in Implantable Drug Delivery Systems. Chemosensors. 2025; 13(6):215. https://doi.org/10.3390/chemosensors13060215
Chicago/Turabian StyleHan, Xiao-Le, Tao Zhou, Jian-Ming Xu, Shu-Feng Zhang, Ye-Zhou Hu, and Yi Liu. 2025. "Integrated Perspective on Functional Organic Electrochemical Transistors and Biosensors in Implantable Drug Delivery Systems" Chemosensors 13, no. 6: 215. https://doi.org/10.3390/chemosensors13060215
APA StyleHan, X.-L., Zhou, T., Xu, J.-M., Zhang, S.-F., Hu, Y.-Z., & Liu, Y. (2025). Integrated Perspective on Functional Organic Electrochemical Transistors and Biosensors in Implantable Drug Delivery Systems. Chemosensors, 13(6), 215. https://doi.org/10.3390/chemosensors13060215






