Abstract
Although traditional drug delivery methods are widely used in clinical practice, their inherent limitations often compromise therapeutic efficacy. Therefore, the development of more precise and efficient drug delivery systems is essential to enhance treatment outcomes and reduce adverse effects. Implantable drug delivery systems (IDDSs) represent intelligent platforms capable of autonomously regulating drug release in response to a patient’s physiological state. By enabling controlled release and personalized dosing, IDDSs have been widely applied in the management of chronic conditions such as diabetes and cancer. With ongoing technological advancements, modern IDDSs must meet increasing demands for both precision delivery and real-time physiological monitoring. In this context, organic electrochemical transistor (OECT)-based biosensors, known for their high sensitivity and excellent real-time signal processing capabilities, have demonstrated significant advantages in early diagnosis and continuous pathological monitoring. While both IDDS and OECT technologies have shown promising progress individually, challenges remain in achieving long-term stability, biocompatibility, scalable manufacturing, and system-level integration. This review systematically summarizes recent advances in IDDSs and functional OECT-based biosensors across various application domains. Furthermore, it explores potential future directions for their combined development, focusing on technological convergence, materials innovation, interdisciplinary collaboration, and the design of intelligent control systems. Looking ahead, the seamless integration of OECT-based biosensors with IDDSs holds the potential to create more precise and efficient closed-loop therapeutic platforms, accelerating progress in the fields of personalized and precision medicine.
1. Introduction
The efficacy of clinical treatments largely depends on the optimization of drug delivery strategies. Conventional approaches, such as oral and intravenous administration, remain the most widely used methods in clinical practice. However, their inherent limitations often compromise therapeutic effectiveness. Oral delivery is subject to the first-pass effect, which significantly reduces bioavailability, while an intravenous injection enables rapid drug distribution but is frequently associated with systemic side effects. Moreover, these methods lack the ability to precisely regulate dosing in real time and cannot dynamically adapt to an individual patient’s physiological status, ultimately affecting both treatment efficacy and safety [1]. To address these limitations, the development of more accurate and efficient drug delivery systems is essential. Notably, the emergence of closed-loop IDDSs offers new hope for patients with chronic conditions, enabling them to maintain normal daily lives without the burden of frequent injections or long-term medication regimens. IDDSs are intelligent platforms that autonomously regulate drug release based on physiological signals. These systems can be implanted directly at the disease site or in accessible regions such as subcutaneous tissue, with delivery to target lesions achieved via catheters [2]. Furthermore, the implant’s geometry, drug formulation, and release profile can be tailored to the patient’s specific needs, thereby supporting personalized medicine.
Modern medical technology is increasingly shifting toward precision, personalization, and real-time dynamic management. However, significant challenges remain in current approaches to disease diagnosis and treatment [3]. Chronic diseases such as diabetes, cancer, and neurodegenerative disorders require long-term monitoring of physiological parameters. However, conventional in vitro diagnostic methods, such as blood analysis, magnetic resonance imaging (MRI), and positron emission tomography–computed tomography (PET-CT), are limited by long sampling intervals, poor real-time responsiveness, and operational complexity. Moreover, traditional drug delivery typically involves fixed dosing regimens that fail to dynamically adapt to a patient’s changing physiological state, often leading to inconsistent therapeutic efficacy, increased drug resistance, or undesirable side effects. These limitations have motivated researchers to develop advanced technologies capable of early and accurate disease detection, along with intelligent, feedback-responsive drug delivery systems [4].
A biosensor is a device that integrates a biological recognition element with a physical or chemical transducer to detect and quantitatively analyze specific substances in biological samples, based on selective interactions between biomolecules and target analytes [5,6,7]. With rapid technological advancements, biosensors have expanded their role beyond diagnostics to include therapeutic monitoring, drug management, and personalized medicine [8,9]. Their core advantage lies in the ability to deliver rapid, accurate, and cost-effective real-time monitoring, making them particularly well suited for high-demand health applications such as chronic disease surveillance, cancer screening, and diabetes management [10,11,12,13].
In medical diagnostics, biosensors facilitate early disease detection by identifying biomarkers such as proteins, DNA, RNA, and metabolites. For instance, sensor platforms incorporating nanomaterials can efficiently detect cancer-associated biomarkers, significantly improving the sensitivity and accuracy of early cancer screening [14,15]. Additionally, portable biosensing devices, such as blood glucose monitors, offer patients with diabetes a convenient means of real-time condition tracking, thereby supporting more effective disease self-management [16].
With the advancement of modern science and technology, a wide range of biosensors with diverse working principles has emerged. One of the primary classification approaches is based on the signal transduction mechanism—that is, how biochemical recognition events are converted into measurable output signals such as physical, chemical, thermal, or biological changes [5]. Among these, electrochemical biosensors have been extensively applied in disease detection and clinical diagnostics due to their high sensitivity, selectivity, and real-time detection capabilities [17]. These sensors operate by translating biological interactions into changes in electrical parameters, such as current, voltage, or conductance, thereby allowing for the quantitative analysis of target analyte concentrations.
Traditional electrochemical biosensors (EBSs) typically operate through a two- or three-electrode configuration, including a working electrode, a reference electrode, and a counter electrode. These sensors detect analytes by measuring the current or voltage generated through redox reactions at the working electrode, often mediated by biological recognition elements such as enzymes or antibodies. While EBSs offer high selectivity and relatively simple fabrication, they usually lack inherent signal amplification, require external circuitry for processing, and are limited in their capacity for miniaturization and long-term stability in physiological environments.
In contrast, OECTs represent an evolution in biosensing design, and they are built on a three-terminal architecture comprising source, drain, and gate electrodes. The combination of transistors with organic small-molecule materials and conjugated polymers makes up the OECT, which is widely used today. Among them, the organic small-molecule materials have the advantages of high selectivity, high sensitivity and a fast response, while the stability and tunability of conjugated polymers enable them to maintain sensing properties and precisely regulate their structures and properties under different environments [18,19]. The combination of the two promotes the design of electrochemical transistor structure biosensors and realizes multifunctional applications. The conductive channel—typically composed of mixed ionic–electronic conducting polymers such as PEDOT:PSS—responds to gate-induced ionic changes in the surrounding electrolyte. The result is an amplified electronic response that directly correlates with the analyte concentration. Unlike EBSs, OECTs offer built-in signal amplification, low-voltage operation, and high compatibility with flexible and conformal substrates, which are essential features for real-time, implantable, and closed-loop therapeutic applications [20,21].
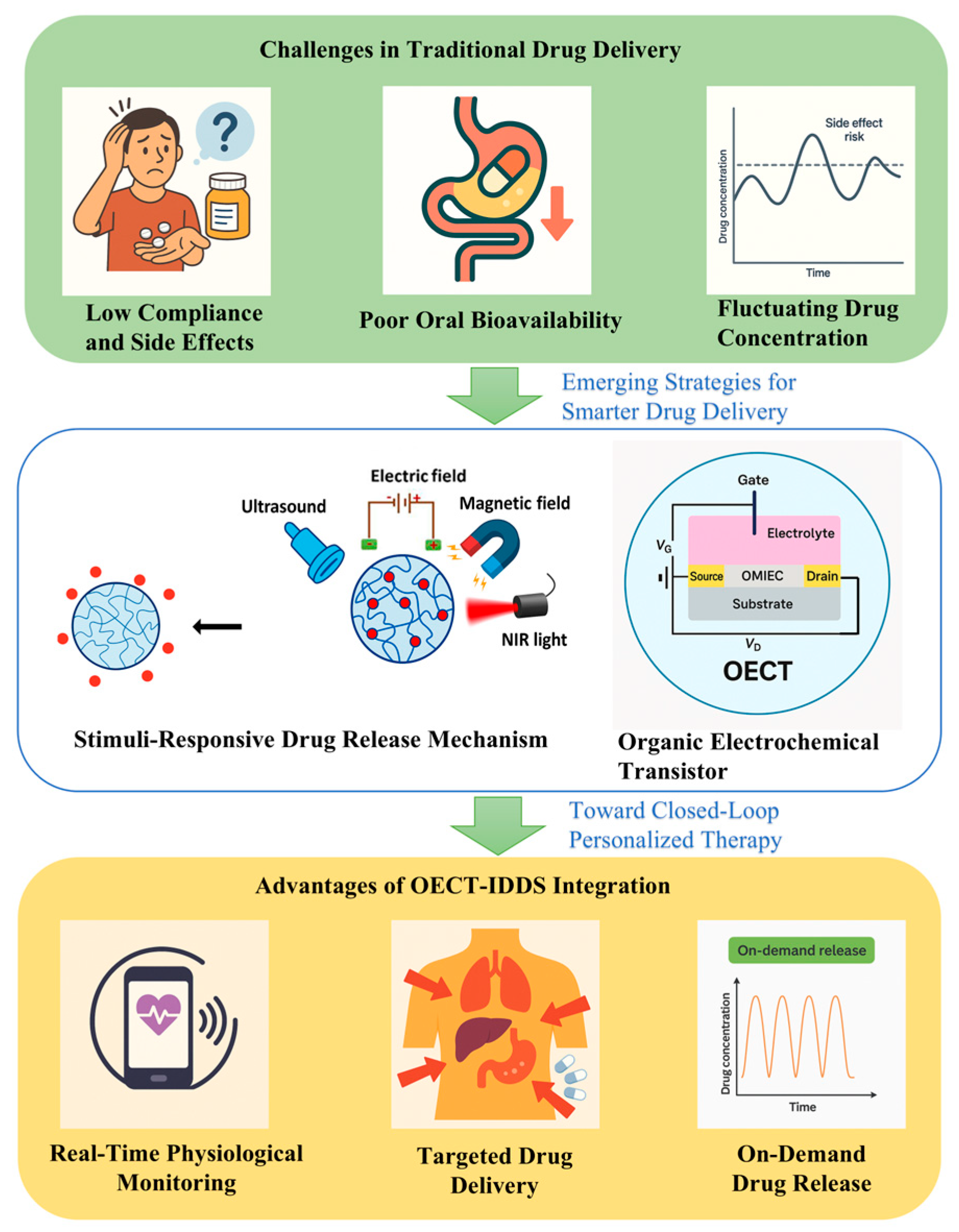
Furthermore, the gate terminal of an OECT can be selectively functionalized with biorecognition elements. Upon target binding, ion exchange at the gate alters the doping level of the channel, modulating the drain current in a highly sensitive and reversible manner. This capability enables continuous monitoring of biomarkers and real-time triggering of controlled drug release, forming a tightly coupled biosensing–actuation interface suitable for smart drug delivery systems. To further advance the precision of personalized medicine, smart closed-loop therapeutic systems have been developed. OECTs offer real-time, highly sensitive detection of biological signals, while IDDSs enable precise, responsive drug administration based on these signals. The integration of OECTs with IDDSs allows for the real-time monitoring, feedback control, and closed-loop regulation of intelligent drug delivery. Their complementary strengths enhance both the biocompatibility and application flexibility of biosensing platforms, facilitating the accurate detection of diverse biomolecules in wearable and implantable systems (Figure 1).
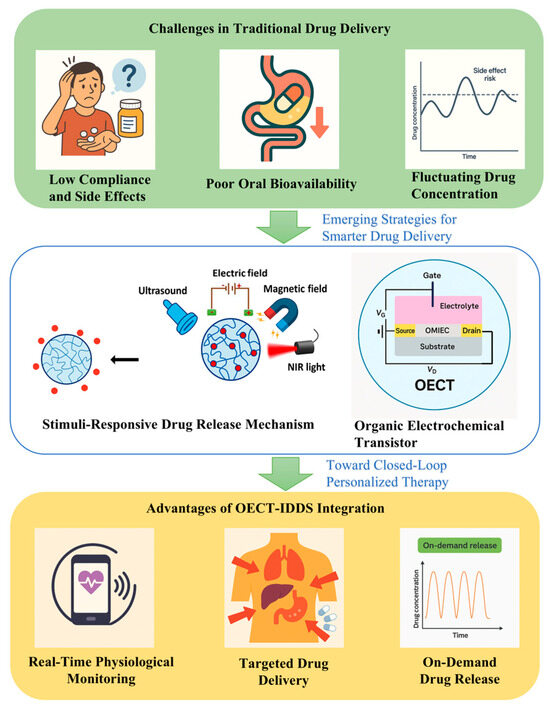
Figure 1.
Schematic illustration of the clinical need, enabling technologies, and therapeutic outcomes of integrating OECT-based biosensors with IDDSs.
This paper first introduces the innovative advancements of IDDSs in precision drug delivery, with a focus on intelligent controlled-release materials and the associated technical challenges. Next, it outlines the working principles of OECT-based biosensors and highlights their advantages in disease detection and real-time physiological monitoring, with a particular emphasis on applications in chronic disease management, food safety, and environmental surveillance. Finally, the feasibility of combining OECTs with IDDSs to construct smart therapeutic systems is discussed, along with future prospects for their use in personalized medicine, implantable medical devices, and remote health monitoring.
2. IDDS Technology Review
IDDSs are surgically implanted medical devices designed to achieve targeted, localized, and controlled drug release by integrating drug reservoirs with biosensing or programmable control mechanisms. These systems typically consist of miniature, programmable pumps or drug reservoirs implanted within subcutaneous tissue and connected to target sites via catheters or other delivery conduits, thereby enabling precise dosing and sustained drug release [22]. The IDDS has demonstrated significant potential in the treatment of chronic diseases and the advancement of personalized medicine, particularly in the long-term management of conditions such as diabetes [23,24], infection [25], epilepsy [26], and osteoporosis [27].
2.1. Basic Working Principle of IDDSs
IDDS-integrated biosensors are designed to monitor real-time physiological signals and regulate drug delivery accordingly. These biosensors are embedded within implantable devices to continuously assess patient-specific biological markers, such as glucose levels, hormone concentrations, or disease-related biomarkers, in order to guide controlled and responsive drug release [28,29]. By delivering drugs directly to targeted tissues or organs, these systems minimize off-target exposure and effectively reduce the risk of adverse side effects [30].
The working principle of IDDS biosensors typically relies on electrochemical, optical, or piezoelectric sensors that detect specific biochemical markers in bodily fluids, such as blood or interstitial fluid. When fluctuations in biomarker levels are detected, the sensor activates the drug delivery module to release an appropriate dose of therapeutic agents. Technologies enabling precise control include microchip-based systems for programmable dosing and osmotic pumps for continuous, controlled release [31,32]. These platforms frequently incorporate micro-electromechanical systems (MEMSs) and biocompatible materials to ensure precise, sustained, and safe drug administration over time [33].
2.2. Main Advantages of IDDS Biosensors
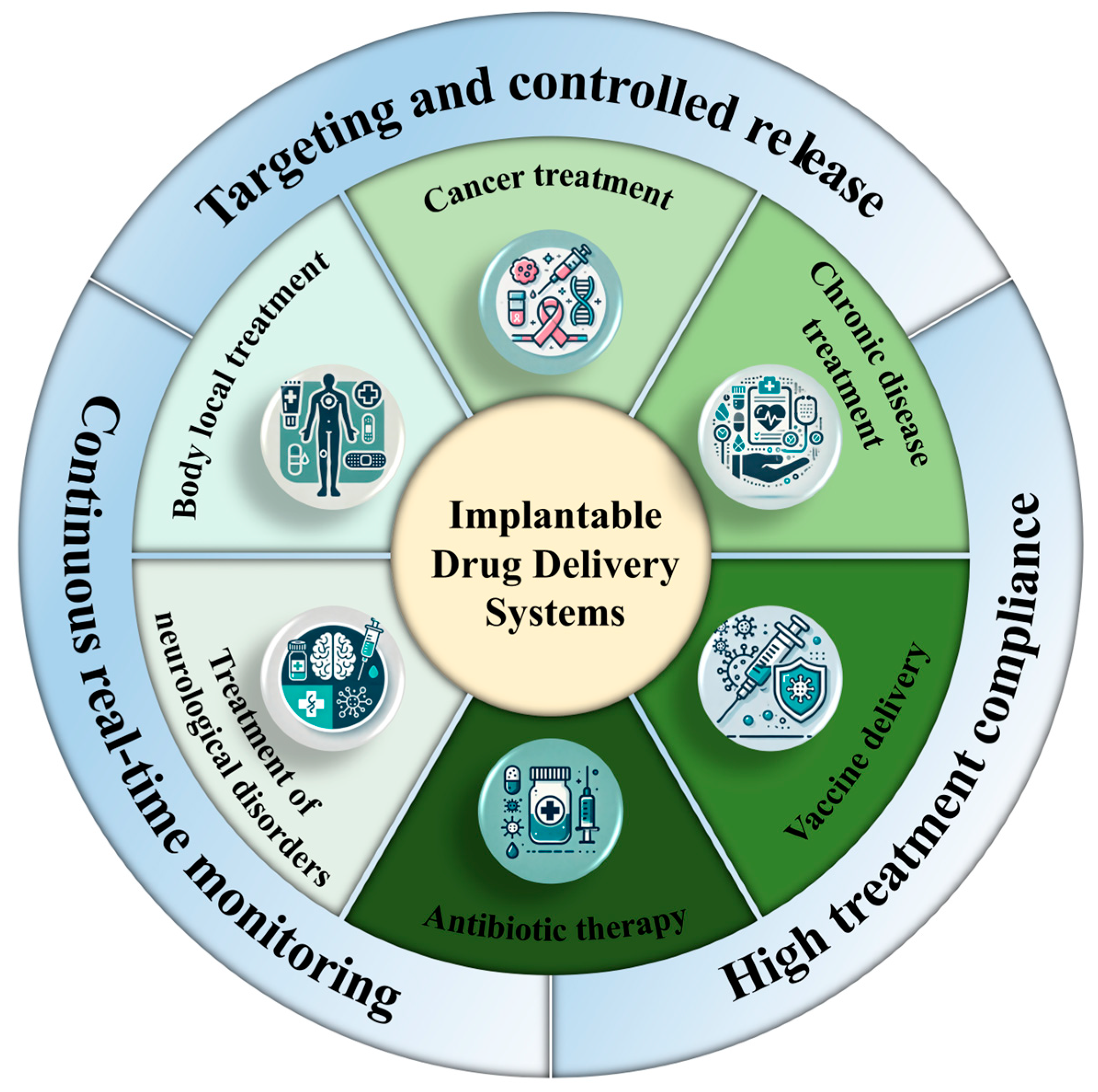
One of the most significant advantages of IDDSs is their ability to provide continuous drug administration while simultaneously monitoring physiological conditions in real time. This enables immediate adjustments in dosing, thereby optimizing therapeutic outcomes. By dynamically modulating drug release based on a patient’s current physiological state, the IDDS facilitates personalized treatment regimens—particularly beneficial for patients managing conditions such as diabetes, cancer, or chronic pain, where frequent medication adjustments are required [33].
Typically implanted subcutaneously or in other biocompatible locations, IDDSs are designed to be minimally invasive and are capable of delivering drugs over extended periods without the need for repeated medical visits or additional interventions. By eliminating the burden of frequent or manual dosing, the IDDS improves patient adherence, especially among those with chronic illnesses who may face challenges with complex medication schedules [34]. Furthermore, through the precise control of drug release, these systems help reduce the risk of overdosing or underdosing, thereby minimizing side effects and enhancing overall treatment safety (Figure 2) [35].
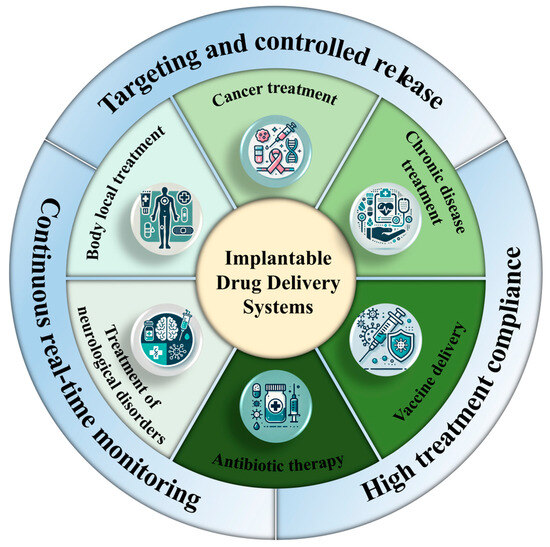
Figure 2.
Advantages and application diagram of IDDSs.
2.3. Practical Application and Technical Progress of IDDSs
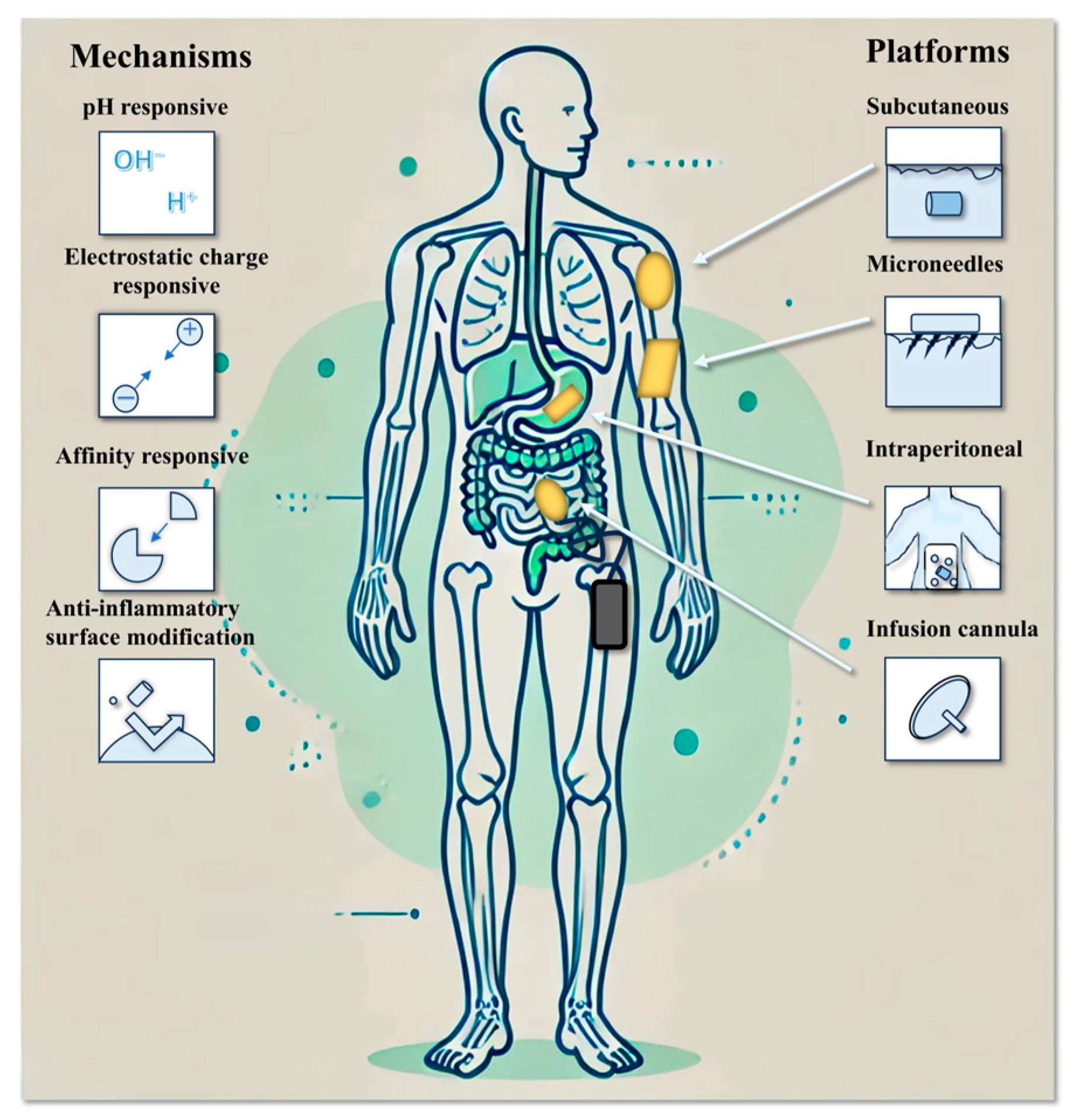
The IDDS enables long-term, controlled, and continuous drug release by maintaining drug concentrations within the therapeutic window, thereby minimizing fluctuations in treatment efficacy caused by poor patient compliance. Moreover, the direct implantation of an IDDS into diseased tissues or targeted sites significantly increases local drug concentrations, enhances therapeutic effectiveness, and reduces systemic exposure and associated adverse effects. This approach ultimately improves both the safety and precision of drug delivery (Figure 3).
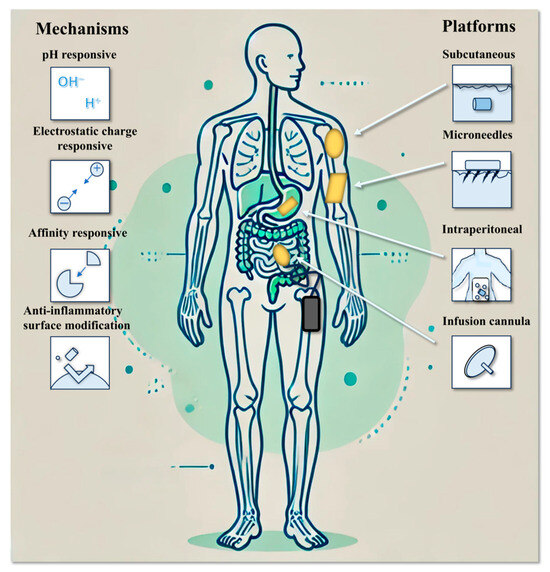
Figure 3.
Trends and technologies for implantable drug delivery systems.
Subcutaneous implantation is the most commonly used approach for IDDS placement, typically performed in the abdominal area or upper arm to ensure ease of access and environmental protection. Subcutaneous implants are widely employed for the delivery of hormone therapies, such as etonogestrel (Implanon®), and local anesthetics like bupivacaine (Exparel®) [36,37]. These systems are capable of sustaining drug release over periods ranging from 3 to 12 months, offering prolonged therapeutic benefits.
Despite their promising clinical potential, subcutaneous implant systems still present certain risks, including implantation site infections, local discomfort, and the necessity for removal by healthcare professionals upon completion of the treatment cycle [38]. Therefore, in both the design and clinical application of such systems, key considerations must include biocompatibility, patient comfort, and long-term safety to optimize therapeutic outcomes and minimize the risk of complications.
2.3.1. Diabetes Management
The treatment of chronic diseases represents a primary focus of IDDS research. Integrating biosensors with insulin pumps enables dynamic insulin delivery based on real-time glucose monitoring, significantly improving patient outcomes and reducing the risks of hypoglycemia and hyperglycemia. By continuously tracking blood glucose levels and administering insulin or other therapeutics in real time, the IDDS helps patients maintain optimal glycemic control [39]. A representative example is the ITCA 650, the first implantable, injectable glucagon-like peptide-1 (GLP-1) receptor agonist delivery system based on the Medici Drug Delivery System™ developed by Intarcia Therapeutics (Boston, MA, USA). This device, comprising a micro-pump and a drug reservoir, provides continuous and stable exenatide release for over six months, effectively controlling blood glucose levels. It significantly reduces dosing frequency, enhances treatment adherence, and lowers the risk of systemic side effects associated with fluctuating drug levels [40].
2.3.2. Neurological Disorder Therapy
In patients with Parkinson’s disease, an IDDS can be utilized to monitor dopamine or other neurotransmitters associated with disease progression and drug metabolism. Based on these real-time biochemical signals, the system can deliver neuroprotective agents or dopamine precursors to correct neurotransmitter imbalances, support motor function, and slow disease progression.
A notable example is ProNeura™ (Titan Pharmaceuticals, Inc., San Francisco, CA, USA), a non-biodegradable implantable drug delivery system composed of an ethylene-vinyl acetate (EVA) matrix embedded with therapeutic agents. This platform has been successfully applied for the long-term delivery of dopamine receptor agonists, such as ropinirole, for managing Parkinson’s disease, as well as for the sustained release of triiodothyronine (T3) in the treatment of hypothyroidism. By maintaining stable plasma drug levels through a zero-order release profile, ProNeura™ reduces the dosing frequency, enhances treatment adherence, and minimizes adverse effects associated with fluctuations in drug levels [41].
2.3.3. Pain Management
IDDSs can also continuously monitor pain-related biomarkers and deliver analgesic or anti-inflammatory drugs directly into the bloodstream or targeted tissues as needed [42]. For patients suffering from chronic pain conditions, such as arthritis or neuropathy, IDDS biosensors can enable on-demand drug release, improving therapeutic precision and responsiveness [43]. By continuously tracking inflammatory markers associated with pain, the system is capable of administering accurate doses of analgesics or anti-inflammatory agents, thereby enhancing pain management while minimizing the risks of overmedication and adverse side effects.
In a large-scale study, David et al. utilized product surveillance registry data to evaluate the safety and efficacy of an intrathecal IDDS for managing chronic non-malignant pain. The study followed 4646 patients who received IDDS treatment between August 2003 and October 2019, with an average follow-up duration of 44 months. The most common reasons for study discontinuation were site closures and patient deaths (46.2%), whereas adverse events and device-related issues accounted for only 10.2% of cases. These findings indicate that IDDSs may offer a safer and more effective long-term alternative to systemic opioid therapy, improving both patient adherence and satisfaction while reducing opioid-related complications [44].
2.3.4. Cancer Therapy
IDDSs can also play a critical role in cancer therapy by monitoring specific tumor markers or metabolites and delivering chemotherapeutic agents or immunotherapies directly to the tumor site. This targeted delivery approach minimizes systemic toxicity, enhances drug efficacy, and enables the continuous assessment of tumor progression [45].
Gliadel® wafers (Arbor Pharmaceuticals, Inc., Atlanta, GA, USA) represent a biodegradable implantable drug delivery system specifically designed for the localized delivery of the chemotherapeutic agent carmustine. While carmustine is highly effective against malignant cells, it is also associated with significant toxicity to healthy tissues. By implanting Gliadel® wafers directly into the brain tumor resection cavity, the system enables localized, controlled release of carmustine, thereby reducing systemic exposure and improving therapeutic precision. Clinical studies have demonstrated that the use of Gliadel® wafers significantly improves survival in patients with recurrent glioblastoma multiforme—a finding validated in Phase III clinical trials—further supporting its clinical value in brain tumor treatment [46,47].
2.3.5. Infectious Disease Therapy
In the context of infectious diseases, IDDS biosensors can continuously monitor pathogen-associated biomarkers, such as bacterial DNA or viral load, and trigger the release of appropriate antibiotics or antiviral agents. By tailoring drug administration to the type and severity of infection, these systems help prevent overtreatment and mitigate the risk of antibiotic resistance. Antiretroviral drugs, for example, can be delivered at precisely timed intervals based on real-time biomarker data, ensuring optimal therapeutic efficacy while minimizing resistance development [48].
Several studies have demonstrated the feasibility of implantable antiviral delivery platforms. The dolutegravir implant, formulated with poly(lactic-co-glycolic acid)/N-methyl-pyrrolidone, achieved sustained drug release for five months in humanized BLT mouse models [49]. Entecavir implants, fabricated through hot-melt extrusion and coated polymer tablets, maintained drug release for 87 days in rats [50]. Similarly, tenofovir alafenamide was delivered in beagles for 40 days using platinum microperforated silicon tubes coated with polyvinyl alcohol (PVA) [51]. These findings highlight the potential of implantable systems to extend drug release duration, reduce patient compliance challenges, and improve therapeutic outcomes. Moreover, variations in implant materials and configurations can be tailored to different animal models to optimize in vivo bioavailability and safety. Table 1 summarizes the different IDDSs listed in the text.

Table 1.
Representative examples of IDDSs discussed in this review.
2.4. Limitations and Challenges of IDDS Biosensors
One of the primary challenges facing IDDSs is ensuring long-term biocompatibility while minimizing immune responses. Prolonged exposure to implanted devices can trigger inflammation, fibrosis, or other adverse tissue reactions, which may compromise system functionality and patient safety. Additionally, although IDDS biosensors are designed for continuous operation, they require regular monitoring and maintenance over time to ensure stable performance [52]. Both the drug delivery components and sensing modules must maintain functional integrity over months or even years without degradation—a goal complicated by factors such as material fatigue, biofouling, and sensor drift [53,54].
Accurate monitoring and dosing depend on the precise calibration of the system to align sensor outputs with the patient’s physiological state. In vivo calibration, particularly for dynamic or patient-specific biomarkers, presents significant technical challenges for both device development and clinical application [35]. Furthermore, implantable drug reservoirs are inherently limited by size and material constraints, restricting the number and volume of therapeutics that can be stored and delivered. While increasing the reservoir size may prolong dosing intervals, it also raises concerns regarding implant safety and invasiveness. Additionally, drugs with poor chemical stability, high molecular weight, or high potency and toxicity may not be suitable for incorporation into implantable systems [55].
The manufacturing of IDDS biosensors involves the integration of sensors, drug delivery components, and biocompatible materials, often requiring sophisticated fabrication processes that drive up production costs. This can limit accessibility, particularly in low-resource settings. Therefore, ensuring both cost-effectiveness and high manufacturing quality remains a major concern [56].
Finally, the deployment of implantable drug delivery systems introduces ethical considerations related to patient privacy (e.g., physiological data monitoring), autonomy (control over drug administration), and long-term safety. Moreover, regulatory approval for such systems involves complex and time-consuming validation procedures to ensure compliance with clinical standards.
2.5. Conclusion of IDDSs
By enabling localized, sustained, and programmable drug administration, IDDSs overcome many limitations of traditional systemic therapies, such as poor patient adherence, fluctuating drug concentrations, and systemic side effects. Through their ability to maintain therapeutic drug levels within the optimal window over extended periods, IDDSs have demonstrated significant benefits in the treatment of chronic diseases, pain management, cancer, and infectious conditions. Furthermore, when integrated with biosensing technologies, IDDSs can form intelligent closed-loop systems capable of real-time monitoring and adaptive drug release. Despite challenges related to biocompatibility, long-term stability, limited drug reservoir capacity, and manufacturing costs, continued progress in biomaterials, microfabrication, and implantable electronics is expected to drive the clinical translation and broader adoption of IDDSs in personalized healthcare.
3. Overview of Functional OECT Biosensors
OECT-based biosensors represent a rapidly emerging technology with broad potential in biomedical applications. By harnessing the unique advantages of organic semiconductors and electrochemical transistor architectures, these biosensors offer high sensitivity, low-voltage operation, and real-time monitoring of biological signals. As the demand for non-invasive, responsive, and efficient diagnostic tools continues to rise, OECT biosensors are attracting increasing attention for their adaptability in disease detection, wearable health monitoring, and clinical diagnostics. Their compatibility with flexible substrates and capability for on-site signal amplification positions them as a promising platform for next-generation biosensing systems [57,58].
3.1. Basic Working Principle of OECT-Based Biosensors
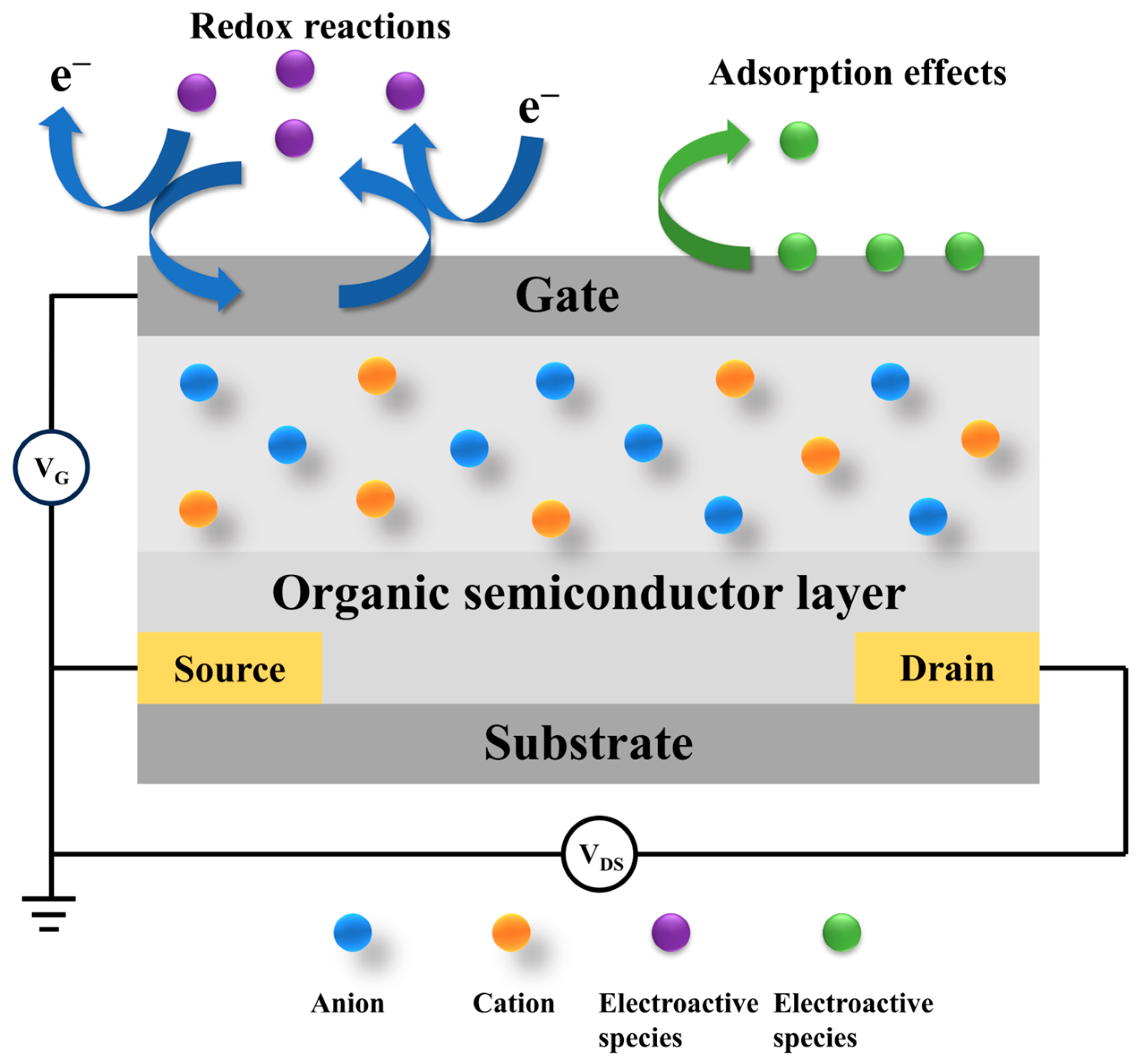
Based on the type of recognition elements used, OECT-based biosensors can be broadly classified into two categories: electroactive and non-electroactive systems [59]. Electroactive biosensors rely on redox-active materials, where the interaction between the target analyte and electroactive species on the device surface induces measurable changes in current or potential [60]. In contrast, non-electroactive biosensors employ bioelectrochemical transduction mechanisms, where the presence or concentration of target analytes is detected through specific biomolecular recognition events, without direct redox activity (Figure 4) [61].
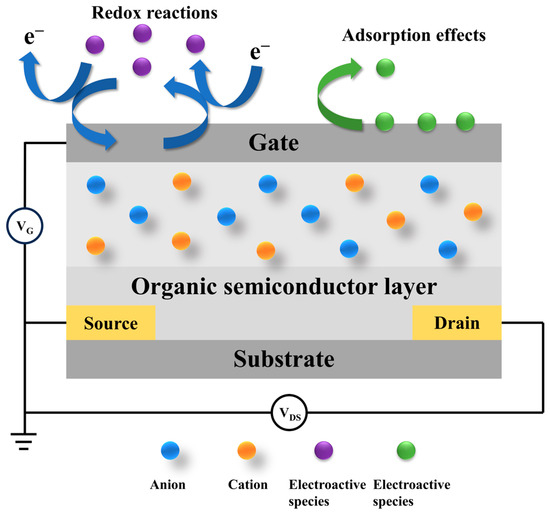
Figure 4.
Schematic diagram of the basic structure and working principle of an OECT biosensor.
The operation of an OECT biosensor relies on the interaction between an organic semiconductor and a biological sample. Structurally, the device includes source, drain, and gate electrodes, similar to a conventional transistor; however, the semiconductor layer is composed of organic materials rather than inorganic ones [62]. When a biological target interacts with the sensor, it induces the modulation of the ionic and electronic currents through the organic semiconductor, which is subsequently detected and quantified. The bioactive sensing layer is typically functionalized with enzymes or receptors that selectively bind to specific biomolecules, such as glucose or cancer-related biomarkers, thereby triggering a measurable electrochemical response [58].
Organic semiconductors offer excellent mechanical flexibility, making OECT biosensors well suited for integration into wearable devices and flexible electronic platforms. Among them, the most widely studied materials are conjugated polymer composites, particularly poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonate) (PEDOT:PSS). The PEDOT backbone facilitates hole transport, while the PSS component imparts hydrophilicity, allowing the material to be readily dispersed in water. This enables simple solution-based processing and film deposition, forming efficient ion-conductive pathways into and out of the material [63,64].
The ionic transport capability of the organic semiconductor layer allows OECT biosensors to effectively respond to changes in biological and chemical environments. This unique feature enables the design of novel device architectures with enhanced performance and adaptability. Furthermore, a wide range of deposition and patterning techniques are compatible with various substrates, including flexible and stretchable materials. The ability to fabricate OECT devices on such substrates opens up new possibilities for non-invasive, continuous health monitoring systems that can be comfortably worn throughout the day [65,66,67].
Sensor stability and reliability are critical considerations for IDDS applications, particularly given the challenges posed by long-term implantation. The materials used in biosensors integrated within IDDS must exhibit high durability and resistance to biofouling to ensure consistent performance in the physiological environment. In this context, the use of PEDOT and other conjugated polymers as organic semiconductor materials in OECT-based biosensors enhances both electrochemical responsiveness and biocompatibility when interfaced with biological samples. Additionally, incorporating nanomaterials such as carbon nanotubes and graphene has shown excellent potential for further improving the biological and electronic compatibility of the sensors [68]. In addition, flexible and stretchable materials are widely utilized in OECT device fabrication to enhance mechanical adaptability [69]. Examples include carbon cloth grid electrodes, paper-based flexible substrates, and textile-based wearable patches, all of which have been employed to develop OECT-based biosignal detection systems [70,71,72]. These materials not only improve the mechanical durability of the devices but also enhance their compatibility and comfort when in contact with human tissue, making them well suited for long-term wearable and implantable applications [73].
3.2. Biomolecule Immobilization in OECT Biosensors
The performance and selectivity of OECT-based biosensors are highly dependent on the immobilization strategies used for biological recognition elements such as enzymes, antibodies, aptamers, and nucleic acids. Effective immobilization not only preserves the bioactivity of these functional components but also enhances signal stability and target specificity at the device interface.
Physical adsorption, a straightforward and non-destructive method, relies on weak interactions like electrostatic forces and hydrogen bonding to anchor biomolecules onto the transducer surface. While convenient, this approach often suffers from limited stability and susceptibility to desorption, particularly under physiological conditions [74]. In contrast, covalent binding forms robust chemical linkages between functional groups on the biomolecule and the modified surface of the OECT. Techniques such as carbodiimide (EDC/NHS) coupling or silanization chemistry enable durable attachment, which has been widely applied to immobilize glucose oxidase and various antibodies on PEDOT:PSS-based transducers [75]. Entrapment strategies provide another effective route, where biorecognition elements are embedded within a conductive polymer matrix that is often composed of hydrogel-formulated PEDOT:PSS or hybrid organic materials. This configuration offers high ionic–electronic conductivity and maintains functional biomolecular activity, making it particularly attractive for flexible and implantable devices [76].
Selecting an appropriate immobilization strategy requires balancing biocompatibility, signal stability, fabrication complexity, and compatibility with real-time feedback, particularly in closed-loop therapeutic applications.
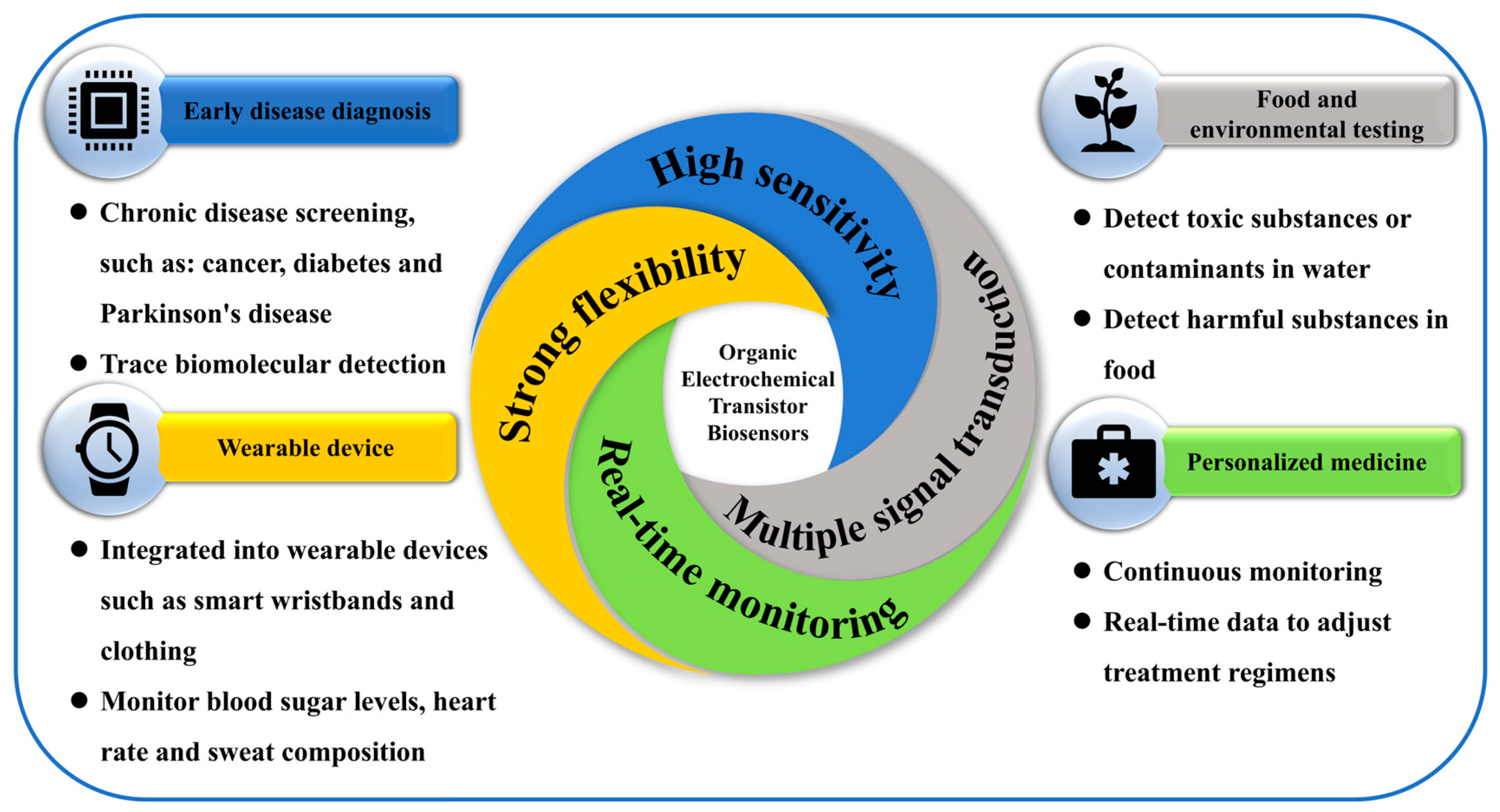
3.3. Advantages of OECT-Based Biosensors
OECTs exhibit exceptional sensitivity, enabling the detection of ultra-low concentrations of biological molecules. This capability makes them particularly well suited for early disease diagnosis, where low-abundance biomarkers are of critical importance. Unlike traditional biosensors that often require extensive sample preparation, OECT-based sensors offer real-time, continuous monitoring of biological signals [65,77,78,79]. This feature is especially advantageous for tracking dynamic physiological changes, such as glucose fluctuations in diabetic patients [80].
Moreover, OECTs are compatible with various signal transduction mechanisms, including electrochemical and optical modalities, enhancing their adaptability across a broad range of biomedical applications [81,82]. Importantly, OECTs operate under low-voltage conditions, which improves safety for in vivo use. This property facilitates their seamless integration into implantable or wearable medical devices that come into direct contact with biological tissues [81,83,84].
In practical applications, OECT-based biosensors demonstrate substantial clinical potential, particularly in continuous monitoring of vital signs and blood glucose levels [85,86]. They have also shown promise in neuromodulation therapies, offering innovative solutions for chronic pain management and personalized treatment strategies for patients with Parkinson’s disease [87]. As OECT biosensors are increasingly implemented in wearable and implantable formats, data security and patient privacy become critical concerns. These devices must ensure encrypted data transmission, secure wireless communication, and long-term operational reliability to be viable in clinical settings [88]. In summary, OECT-based biosensors meet the core requirements of IDDSs in terms of signal acquisition, data security, and remote monitoring capabilities. The integration of these technologies is expected to further enhance their value in precision medicine and enable more intelligent, responsive therapeutic systems.
Given the long-term operation of implantable devices, the IDDS requires biosensors to exhibit low power consumption to ensure sustainable energy supply and management. OECT-based biosensors are well suited for such applications, as they can be powered by in vivo or external energy harvesting technologies, including biofuel cells and wireless charging systems. Furthermore, the implementation of low-power circuit designs can significantly extend the lifespan of a device, reducing the need for frequent maintenance or replacement and enhancing the practicality of long-term therapeutic use [89] (Figure 5).
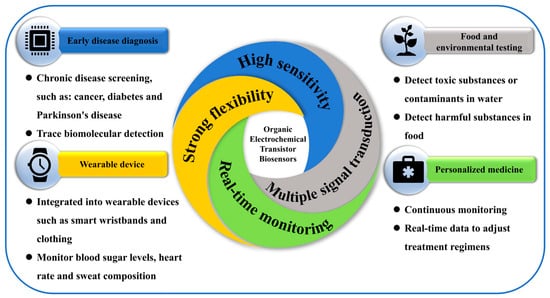
Figure 5.
Advantages and application diagram of OECT biosensors.
3.4. Research and Application of a Functional Biosensor Based on OECTs
One of the most prominent applications of OECT-based biosensors lies in early disease diagnosis, particularly for chronic conditions such as cancer and diabetes. Their high sensitivity and real-time monitoring capabilities enable the reliable detection of low-abundance biomarkers at early disease stages, providing critical support for timely interventions and improved clinical outcomes.
3.4.1. Glucose Sensing
The ability to regulate glucose metabolism can reflect the health level of the human body to a certain extent [90,91]. In the case of glucose detection, for example, glucose oxidase (Gox) is commonly used as the functional enzyme. The glucose molecules interact with Gox, leading to the oxidation of glucose, which generates a change in current at the gate of the transistor in the sample, allowing for real-time monitoring of glucose levels. This change in current is directly proportional to the concentration [10,92]. For the selective detection of glucose, the OECT gate electrode was modified with Pt NPs and Gox, which are biocompatible polymers [93]. The modification of Pt NPs and Gox significantly improved the sensitivity of the electrodes to glucose, showing a linear response to the logarithm of glucose concentration in the range of 100 nM to 5 mM [94]. The incorporation of chitosan and Nafion helps fix Gox on the gate electrode and improves the selectivity of the device. At the same time, graphene and graphene oxide increase the charge transfer and surface area of the gate to improve the sensitivity of the device, and the detection range can be as low as 10 nM to 1 μM.
3.4.2. Cancer Biomarker Detection
The OECT sensors can detect trace amounts of biomolecules in blood or other bodily fluids in real time, enabling the early diagnosis of diseases [95]. Furthermore, because OECT sensors can directly undergo electrochemical reactions with biological molecules such as tumor-associated proteins and small-molecular compounds, they are highly effective in detecting cancer-related biomarkers [96,97,98]. In the field of oncology research, proteins are widely used as tumor markers [99,100]. However, due to the generally weak interaction between proteins and organic semiconductors, traditional protein sensors may face the challenge of insufficient sensitivity when detecting specific biomarkers at ultra-low concentrations in physiological environments. In contrast, OECT sensors, with their inherent signal amplification capabilities, can effectively improve the detection sensitivity to overcome this limitation. Studies have shown that the OECT has demonstrated exceptional performance in specifically detecting cancer-related markers, such as human epidermal growth factor receptor 2 (HER2), with detection limits as low as 10 fg/mL [101]. In addition, PEDOT: PSS was used to bind Au NPs to the secondary antibody, and the detection limit of the prostate-specific antigen–anticoagulant trypsin complex was 1 pg/mL [102]. Glycoproteins, as protein–sugar complexes, are key components on the surface of eukaryotic cells and play an important role in cancer progression [103]. The PEDOT-PSS channel and the polydimethyl diallyl ammonium chloride/multi-walled carbon nanotube-modified gate have been demonstrated to specifically recognize mannose and detect glycan molecules on the surface of cancer cells. Even under the condition of low concentrations of cancer, it can still produce significant current responses [104]. By modifying the lectin and aptamer sequences, the sensor can be widely used in the analysis of various glycans and cancer cells.
3.4.3. Nucleic Acid Detection
OECT-based biosensors are often used in food safety monitoring to detect harmful substances in food, such as nucleic acid diagnosis of bacteria and viruses or allergen screening [105,106]. Nucleic acid diagnosis has important research value in many fields such as gene expression monitoring and virus and bacteria identification [107,108,109]. Conventional high-sensitivity OECT sensors typically rely on materials, such as carbon nanotubes, graphene, and polyelectrolyte layers, and involve complex synthesis and modification steps that limit their application. In recent years, DNA amplification techniques, such as loop-mediated isothermal amplification (LAMP) and hybridization chain reaction (HCR), have been developed as alternative strategies [96]. After HCR self-assembly is integrated into OECTs, the synthesis of long DNA can be triggered by a small number of target molecules to achieve efficient biometrics. Electrochemically deposited gold nanoparticles increase the gate surface area and promote the specific hybridization of HCR products (double-stranded DNA with long negative charge) with the target DNA, thus enhancing grid voltage deviation. This strategy enables the OECT to maintain high sensitivity at target DNA concentrations as low as 0.1 pM and, at the same time, has excellent selectivity, as it can effectively distinguish the target DNA from mismatched DNA and improve its application potential in nucleic acid detection and precision medicine. This approach offers greater real-time responsiveness and flexibility compared to traditional laboratory testing, making it well suited for quality control in large-scale food production.
3.4.4. Environmental Monitoring
In addition to biomedical applications, OECT biosensors are widely used in environmental monitoring and can be used to detect toxic substances or contaminants in water [21,110]. Traditional subsea oil spill detection is slow and limited in deployment. An alternative method is available to prepare OECT using poly (3,4-ethylenedioxythiophene)–poly (styrene sulfonate) as a channel. Coating the channel with a polystyrene film causes the OECT to have a large and measurable response to the oil. The oil in contact with the device will adsorb onto the polystyrene film and increase the impedance at the electrolyte interface. This method of detecting oil spills in subsea environments highlights the simplicity, effectiveness, and speed with which it provides accurate monitoring data even in complex environments [111]. Pesticides are persistent in the environment, and their residues can lead to the deterioration of soil and water quality. A graphene-based OECT sensor has been developed to detect trichlorphon based on its inhibition of acetylcholinesterase (AChE) activity [112]. The device uses AChE as a biometric element to achieve sensitive detection at trichlorfon concentrations as low as 10 nM, covering a detection range from 10 nM to 3 μM. The experimental results show that the current change (ΔI) of the device response can reach about 0.23 μA even at the 10 nM trichlorfon concentration. The detection mechanism is attributed to the inhibition of AChE activity induced by trichloramine, which affects the catalytic process of acetylcholine chloride (AChCl) and leads to the change in thiocholine (TCh) production. Notably, the OECT sensor shows excellent selection for potentially interfering substances such as metal ions, glucose, and other pesticides. In addition, the sensor can accurately determine the content of trichlorfon in rice samples at 300 nM and 3 μM, which shows good practical detection ability and application prospect [113].
3.4.5. Body Fluid Detection
Another major application of OECTs is in wearable devices, particularly for monitoring biological signals such as blood glucose levels, heart rate, and sweat composition. Due to their flexible and stretchable nature, OECT biosensors can be integrated into wearable devices like smart wristbands, clothing, and more, enabling continuous health monitoring around the clock. For example, OECT sensors have been used to develop real-time blood glucose monitoring systems, helping diabetic patients better manage their blood sugar levels. A wearable continuous glucose monitoring (CGM) device based on OECT technology with compact, coin-sized, fully integrated, wireless sensing features has been developed. An OECT was used as a glucose biosensor, and a microneedle array was used as a minimally invasive bridge for ISF sampling. A robust, adhesive, enzyme-laden hydrogel is used to improve the skin–device interface and enhance sensing reliability. Compared to traditional CGM systems based on electrochemical sensing technology, OECT-CGM can provide better noise resistance and on-demand sensitivity and resolution, which are critical for wearable applications [114]. Additionally, OECT sensors can monitor the chemical composition of sweat, allowing for the assessment of hydration levels, lactate concentrations, and other health indicators, thus further supporting long-term health monitoring [115,116,117]. A textile-based OECT structure has been developed for uric acid monitoring in wound exudates. The structure is made of poly (3,4-ethylenedioxythiophene)–polystyrene sulfonate (PEDOT-PSS) [118]. This structure has been shown to reliably and reversibly detect uric acid concentrations in synthetic wound exudates in the biologically relevant 220–750 μM range. In addition, it shows great potential for non-invasive human detection applications.
OECT-based biosensors are known for their high sensitivity, especially when it comes to detecting biomarkers at low concentrations. Their electrochemical reaction to biological samples allows them to detect small fluctuations in analyte concentrations, making them ideal for applications requiring precise monitoring. A typical study used a template-free electro polymerization process to construct a nanostructured poly (3,4-ethylenedioxythiophene) (PEDOT) derivative in the channel layer of the OECT for highly sensitive detection of cortisol in sweat [119]. The introduction of the nanostructure significantly enhanced the fixation ability of the cortisol antibody on the polymer layer, thus improving the biometric performance and signal transmission efficiency of the sensor. The constructed cortisol immune sensing system showed excellent linear detection ability in the concentration range of 1 fg/mL to 1 μg/mL, and the lowest detection limit (LOD) was up to 0.0088 fg/mL, showing extremely high sensitivity. In addition, the sensor has demonstrated excellent stability, repeatability, and reliability over a long period of use. In the artificial sweat test environment, the system can still maintain excellent detection accuracy, demonstrating its great potential for clinical diagnosis and wearable biosensors. The high sensitivity of OECT-based biosensors fills the gap of IDDSs lacking real-time detection and precise control mechanisms.
3.4.6. Personalized Medical Care
OECT-based biosensors also hold significant potential in the field of personalized medicine. When integrated with microfluidic chips, these sensors can continuously monitor multiple biomarkers in real time, enabling the dynamic adjustment of treatment plans based on patient-specific physiological data [95,120]. This individualized approach not only improves therapeutic efficacy but also minimizes unnecessary side effects.
As technology continues to evolve, OECT biosensors are expected to become essential tools in precision medicine—particularly in advanced therapeutic areas such as cancer immunotherapy and gene therapy—by providing continuous, high-resolution biological information to guide clinical decision-making. Table 2 summarizes the OECT-based biosensors listed in this article.

Table 2.
Representative applications of OECT-based biosensors.
3.5. Limitations and Challenges of OECT-Based Biosensors
Despite their promising potential, OECT-based biosensors still face several critical challenges. Key issues include improving the electrochemical stability of organic semiconductors, optimizing interfacial charge transport characteristics, and enhancing the recognition specificity and selectivity toward target biomolecules. The long-term stability and durability of organic semiconductors remain major concerns for practical biosensing applications. Current strategies to address these issues involve chemical modification of the semiconductor backbone and the application of protective coatings to improve material robustness.
Another important challenge lies in reproducibility and scalability. While OECT biosensors have demonstrated excellent performance at the laboratory scale, achieving consistent sensor behavior across large-scale manufacturing remains difficult. Variations in fabrication processes can lead to inconsistencies in device performance, which in turn limit their clinical reliability and standardization.
Finally, in in vivo applications, OECT sensors are susceptible to biofouling—an accumulation of proteins and other biological molecules on the sensor surface—which can interfere with signal transduction and compromise sensor function. To overcome this, ongoing research is focused on developing anti-fouling surface coatings and enzyme-free detection strategies to maintain sensing fidelity over extended periods [121].
3.6. Conclusions of OECT-Based Biosensors
OECT-based biosensors hold strong promise for next-generation biomedical diagnostics due to their compatibility with miniaturized, flexible systems. Moving forward, integrating OECTs with complementary technologies such as microfluidics, wireless communication, and imaging will be essential for their translation into clinical applications.
4. Combination of OECT-Based Biosensors and IDDSs
This section explores how OECT-based biosensors and IDDS technologies can be integrated into a closed-loop therapeutic system. We discuss the synergistic mechanisms, real-world case studies, foundational technologies like OEIP, and some existing problems in the current technology.
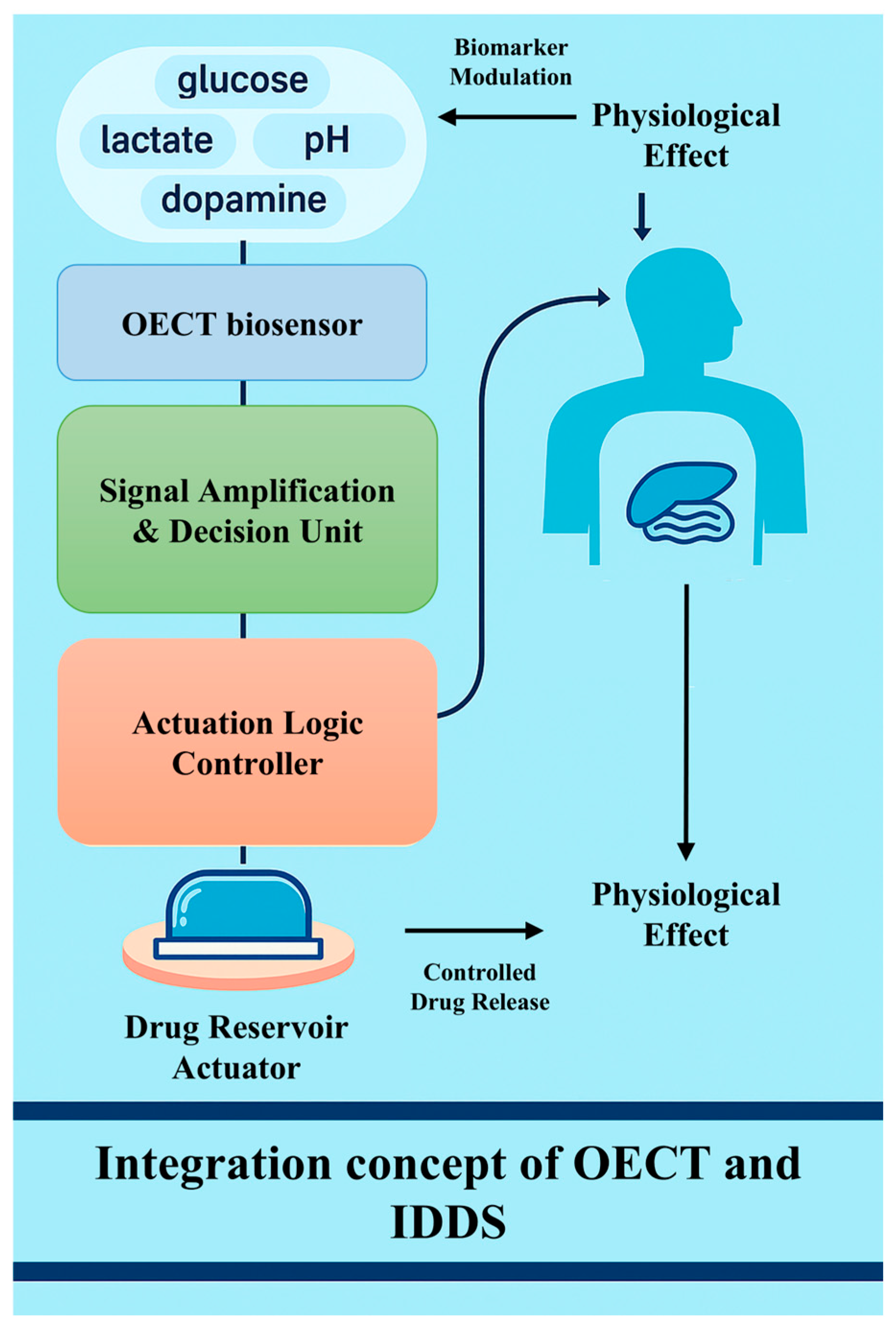
4.1. Closed-Loop Integration Mechanism
Biosensors have revolutionized modern medicine by providing real-time monitoring and precise interventions for a range of medical conditions. Today’s healthcare systems still lack highly sensitive real-time monitoring and precision drug delivery mechanisms. Among the many biosensor technologies, OECT-based biosensors and IDDSs represent two of the key innovations. OECT-based biosensors enable continuous physiological signal monitoring, which supports closed-loop regulation within IDDSs. This mechanism allows for highly sensitive and continuous monitoring, especially in detecting biomarkers such as glucose, lactic acid, or proteins associated with diseases such as cancer or diabetes. The IDDS, on the other hand, uses sensors implanted in the body to monitor biomarkers or drug concentrations and adjusts the rate of drug release based on the monitoring results. But the IDDS relies on the synchronization of long-term monitoring and dosing, so its work involves not only the detection of biological signals but also the precise administration of drugs. Because feedback loops are at the heart of their mechanism, biosensors are needed to continuously monitor a patient’s condition and trigger the drug delivery system to release precise doses of drugs. When the two are combined, the OECT provides high-sensitivity physiological monitoring signals, while the IDDS relies on sensor data for automated drug release regulation, forming a closed-loop control system (Figure 6).
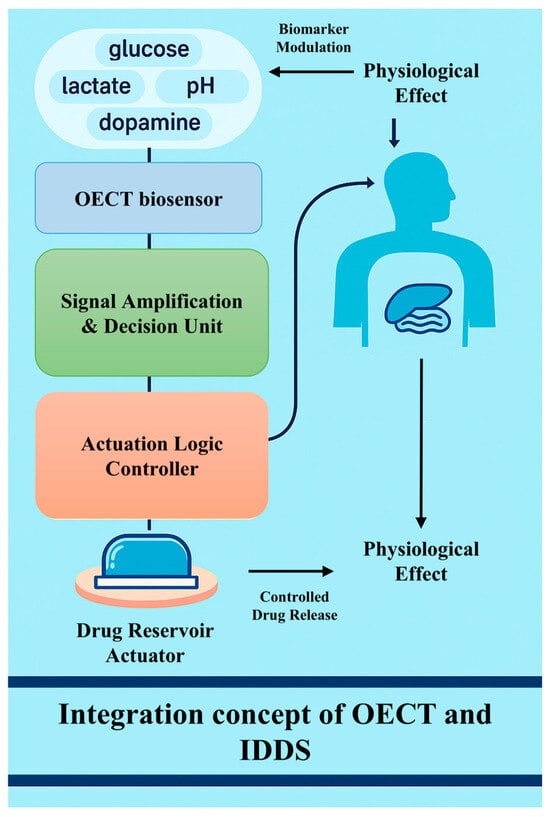
Figure 6.
Schematic representation of the integration concept between OECT biosensors and IDDSs.
4.2. Representative Applications and Case Studies
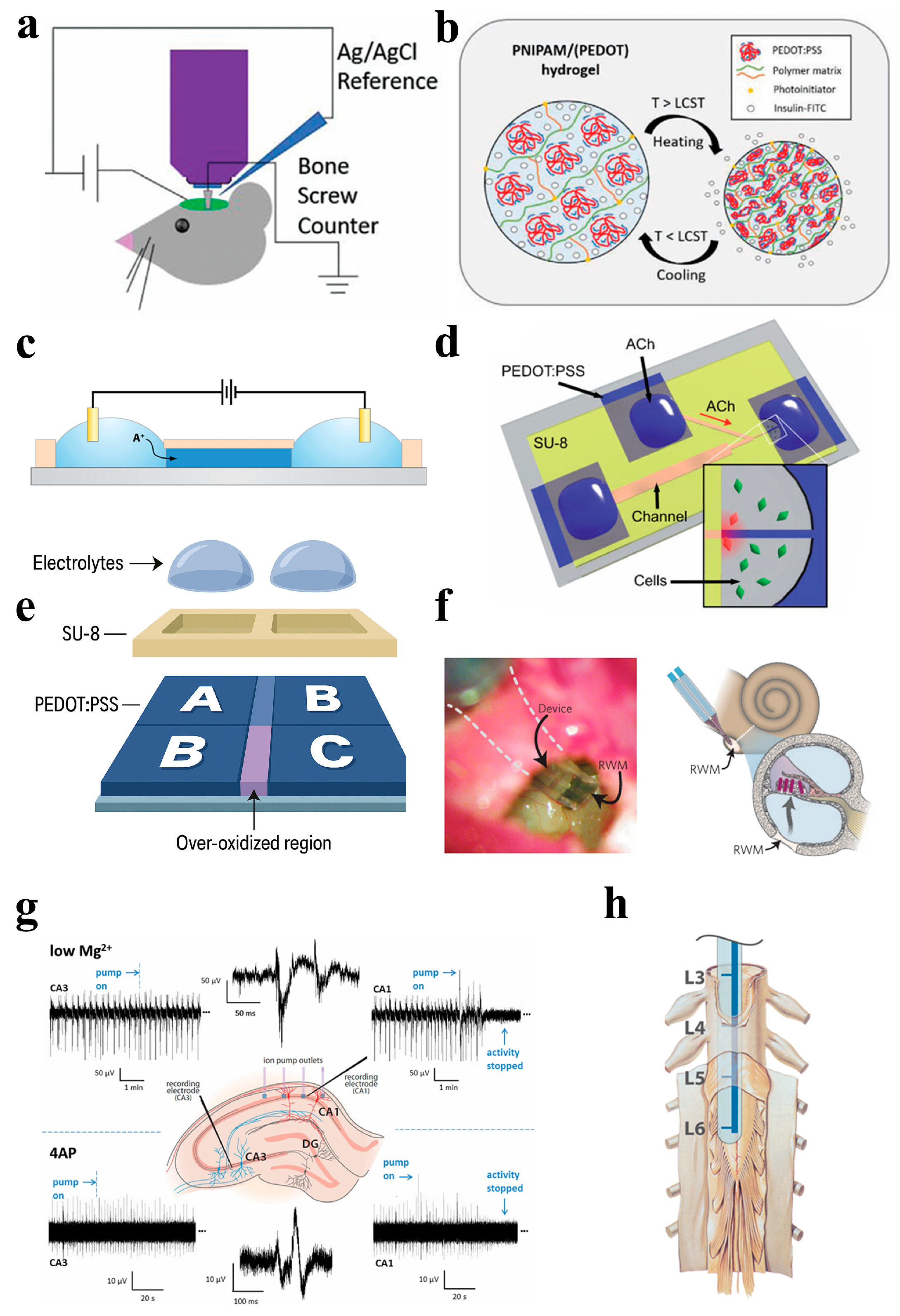
Examples of OECT-based biosensors applied to IDDSs also continue to emerge (Figure 7). Among them, Woeppel et al. doped sulfonated silica nanoparticles (SNPs) in PEDOT films, which can simultaneously load positively and negatively charged drugs. When electrical stimulation was applied, the drug release efficiency increased by 6.4 times and 16.8 times, respectively. In addition, PEDOT/SNP films containing α-amino-3-hydroxy-5-methyl-4-isoxazolpropionic acid (AMPA)/the alginate receptor antagonist DNQX were coated on carbon fiber electrodes and implanted in mouse brains. DNQX can be released by electrical stimulation, which effectively inhibits the action potential of mouse neurons, demonstrating the feasibility of OECTs for precision implantable drug delivery [122]. Although this platform demonstrates promising electrochemical properties and effective multi-drug release in both in vitro and in vivo models, it is currently positioned at the preclinical proof-of-concept stage, with no clinical trials reported to date. The ability to co-load oppositely charged drugs represents a significant step forward; however, achieving precise and selective release under complex physiological conditions remains a technical challenge. Furthermore, the long-term stability and biocompatibility of the conducting polymer materials under repeated electrical stimulation require further investigation. Addressing these aspects will be critical for advancing toward clinical translation and underscores the broader need to integrate sensing accuracy, implant robustness, and regulatory readiness in the design of next-generation implantable therapeutic systems.
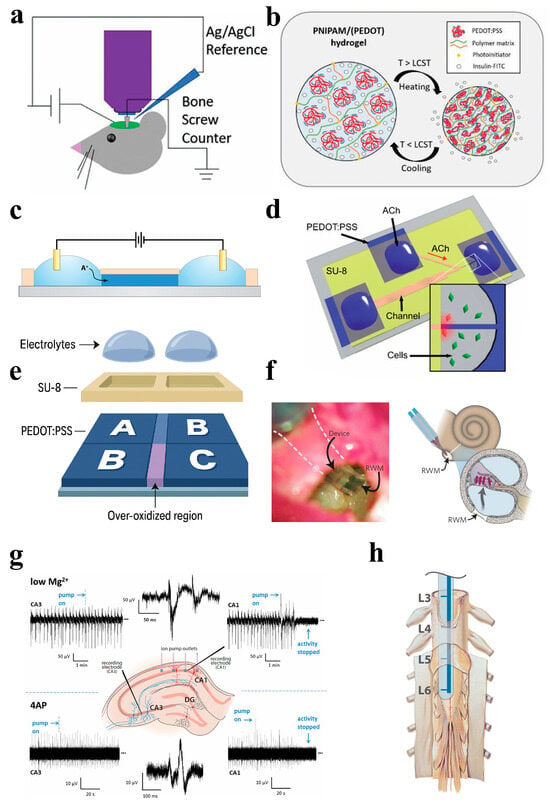
Figure 7.
(a) Schematic diagram of the experimental setup for intracranial delivery of DNQX in mice [122]. (b) PNIPAM/(PEDOT) hydrogel general mechanism for drug delivery [123]. (c) Device structure of ion-selective resistors for organic electron ion pumps [124]. (d) OEIP structure diagram for ACh delivery [125]. (e) Schematic of the organic electronic ion pump (OEIP) device. Ions (e.g., K+ and Ca2+) are transported from the source electrolyte across a cation-selective polyelectrolyte membrane via an electric field, without bulk fluid flow [126]. (f) In vivo application of OEIPs for selective stimulation of auditory neurons via glutamate delivery in the guinea pig cochlea. Photograph of an ion pump located on the circular window membrane (RWM) and diagram of the diffusion of neurotransmitters through the cochlear structure [124]. (g) Suppression of epileptiform activity in mouse hippocampal slices using OEIP-based GABA delivery [127]. (h) For neuropathic pain treatment based on OEIPs, the four exits are aligned with the location of the nerve bundle associated with the pain model into the spinal cord [128].
A recent multifunctional bioelectronic device that integrates temperature monitoring and drug release functions uses an electrically thermally responsive hydrogel (PEDOT:PSS/PNIPAM composite hydrogel) as a gate electrode for OECTs. The hydrogel-based OECT has excellent biocompatibility, can be prepared by digital light processing (DLP) 4D printing technology, and exhibits thermal response characteristics with a low critical solution temperature (LCST) of ≈35 °C. When the temperature increases beyond the LCST, the hydrogel shrinks, resulting in a significant change in resistance, enabling reversible detection over a temperature range of 25–45 °C and showing high sensitivity (0.05 °C−1). The experimental results showed that the release rate of insulin–FITC at 37 °C was 82 ± 4%, which realized the precise release of insulin at human temperature through a temperature-sensitive response. It is worth noting that the storage and release functions of hydrogels do not affect their thermal response characteristics and the overall performance of the OECT. This study demonstrates its potential as an intelligent drug delivery platform that combines implantable precision drug release with real-time monitoring of physiological signals, providing new ideas for the development of customizable and intelligent bioelectronic devices [123]. Although this hydrogel-based OECT platform shows excellent biocompatibility and thermo-triggered insulin release in vitro, it remains at the preclinical stage, with no reported in vivo data or clinical trials. Key challenges include the lack of long-term implantation studies and uncertainty in phase-transition stability under physiological conditions. Additionally, future work is needed to address the scalability of the fabrication and integration of real-time feedback control, which are essential for clinical translation of such smart drug delivery systems.
4.3. Organic Electronic Ion Pump and Its Relevance
OECT-based biosensors have great research space in the field of IDDS applications. Similarly, biocompatibility and ion–electron coupling properties provide important ideas for developing advanced precision drug delivery technologies. As a device with significant commonality in its mechanism, the organic electronic ion pump (OEIP) has attracted wide attention in the early years [124]. It uses an electric field drive mechanism to achieve precision drug delivery without liquid flow. The core structure of the OEIP usually uses the PEDOT:PSS conducting polymer as the electron conduction channel, which connects the drug storage bin to the target tissue through the polyelectrolyte ion exchange membrane. Under weak voltage drive, the directional migration of charged bioactive molecules can be controlled efficiently and precisely so as to achieve local accurate release. Compared to traditional mechanical pumps or diffusion-type release systems, this technology eliminates the need for liquid flow, significantly reducing side effects caused by fluid flow perturbations or nonspecific diffusion.
The core structure of the OEIP is usually composed of three parts: an electron conduction layer, an ion exchange membrane, and a drug storage layer. The electron conductive layer is generally made of conductive polymer materials such as PEDOT:PSS, which has good biocompatibility and flexibility, can effectively conduct electron conduction, and can closely fit with the target tissue interface. The ion exchange membrane is made of a polyelectrolyte material that allows for the selective passage of specific charged drug ions, enabling precise electronically controlled ion migration when driven by an applied external voltage. The drug storage layer is used to store charged bioactive molecules to be released, such as neurotransmitters or other drug ions. In the OEIP system, when a weak voltage is applied, the electric field generated by the electron-conducting layer causes the charged molecules in the drug storage layer to migrate towards the target tissue region through the ion exchange membrane, thus achieving spatially selective and precise drug release. This release mechanism eliminates the need for traditional mechanical pumping or liquid flow, greatly reducing the risk of drug diffusion to non-target tissues.
The results summarized by Gabrielsson et al. show that the OEIP system has successfully delivered acetylcholine (ACh) to in vitro neuron culture models, achieving rapid responses of less than 200 milliseconds and highly accurate spatial control of less than 50 micron. Thus, the selective activation of local neurons can be effectively realized, and the interference to the surrounding tissues can be avoided [125]. This ability of precise regulation is of great significance for constructing a controllable neural interface and restoring damaged neural function. In addition, the researchers also applied OEIP to the epilepsy model in mouse brain sections and effectively inhibited pathological neuro-abnormal discharge by electronically delivering the inhibitory neurotransmitter GABA (gamma-aminobutyric acid), demonstrating the preclinical application prospect of the OEIP for precise interventions in neuropathological states [127]. Further studies have also confirmed the potential of OEIP in live animal models. For example, by delivering glutamate (Glu) in the guinea pig cochlea, the auditory signal was successfully simulated and selective chemical stimulation of the auditory nerve was achieved, reflecting the great potential of OEIP in the field of auditory nerve repair and neuro prosthesis [124]. In addition, the four-channel OEIP device effectively raised the pain threshold level of the animals by simultaneously releasing GABA through multiple points in a rat model of spinal cord pain and significantly reduced the drug dose to only one percent of the traditional approach, highlighting the advantages of OEIP in improving drug delivery efficiency and reducing systemic side effects [128].
In addition, Isaksson et al. developed a conductive polymer OEIP based on PEDOT:PSS. The device consists of a conductive polymer electrode, an ion-selective polyelectrolyte bridge, an ion storage region, and a target tissue region. In experiments, by applying a weak applied voltage, the system succeeds in achieving precise delivery of bioactive ions such as K+ and Ca2+ in the absence of liquid flow, with a spatial localization accuracy of micrometers (about 50 µm) and a temporal response of milliseconds, thereby precisely regulating the depolarization of the neuron membrane and the activation of intracellular calcium signaling pathways. Real-time monitoring of changes in the concentration of calcium ions in neuronal cells using fluorescence microscopic imaging clearly demonstrated that an electric field-driven OEIP device can induce changes in the membrane potential and trigger a rapid response to intracellular Ca2+ signals [126].
OEIPs have shown significant promise in preclinical studies for achieving electronically controlled and spatially resolved drug delivery. For example, selective glutamate release in the cochlea of guinea pigs has demonstrated potential in modulating auditory signals, while GABA delivery via OEIPs has effectively reduced neuropathic pain in spinal cord models [129]. These results validate the core capabilities of OEIPs in neuromodulation and localized therapy. While current implementations remain in the preclinical stage, they have laid a strong foundation for further development. Key areas for advancement include enhancing long-term operational stability, expanding toward multi-analyte delivery, and improving integration with biosensors for real-time feedback control. Moreover, ensuring chronic biocompatibility and establishing scalable fabrication processes will be essential for clinical translation. Given their unique mechanism of ion-selective and non-fluidic delivery, OEIPs represent a highly promising platform for future closed-loop therapeutic systems, particularly when combined with flexible electronics and precision biointerfaces.
OEIP technology combines high spatio-temporal precision control ability, electrochemical driving advantages, and the biocompatibility of flexible materials and has demonstrated clear drug targeted delivery, disease treatment, and neuroregulation effects in a variety of in vivo and in vitro experimental models. Although OEIP technology itself is not a typical three-terminal structure of OECTs, its material system, ion–electron coupling mechanism, and interaction characteristics with the biological interface and the precise regulation mechanism of signals and substances driven by electric fields are highly similar to those of OECT technology. It provides important theoretical support and experimental basis for the collaborative integration of OECT biosensors and IDDSs in the future.
4.4. Comparative Evaluation of OECT-Integrated IDDSs and CMOS-Based Therapeutic Platforms
CMOS-based biosensor–drug delivery systems, built upon mature complementary metal–oxide–semiconductor (CMOS) technology, offer highly integrated solutions that combine sensing, signal processing, power management, and wireless communication within a single microchip. This integration allows for real-time data acquisition, embedded logic computation, and autonomous therapeutic decisions. Their proven reliability in implantable electronics, such as cardiac pacemakers, cochlear implants, and neural probes, makes CMOS platforms a powerful option for closed-loop biomedical devices [130,131].
However, mechanical rigidity remains a key limitation. CMOS chips are traditionally fabricated on brittle silicon wafers that are mechanically mismatched with the soft, curved, and mobile biological environment. This rigidity increases the likelihood of foreign body responses, such as inflammation or fibrotic encapsulation, which can impair both sensing fidelity and drug release performance over time [131]. Although efforts have been made to thin silicon dies or integrate them onto flexible substrates, these techniques often require complex packaging strategies (e.g., flexible printed circuit boards and stretchable interposers), adding bulk and fabrication complexity.
In terms of biocompatibility, the CMOS itself is not inherently bioinert, and the exposed metal traces and oxide layers must be fully encapsulated using biocompatible coatings such as parylene, epoxy, or silicone. Any failure in this encapsulation may lead to electrolyte leakage, corrosion, or cytotoxic reactions, compromising device stability in chronic applications [131].
From a systems integration perspective, coupling CMOS sensors with implantable drug delivery components (e.g., micromechanical pumps, electrothermal valves, and electrochemical actuators) is not straightforward. It requires heterogenous integration across electronic, fluidic, and mechanical domains, often involving additional interconnects, microfluidic routing, and custom circuit designs for actuator control. This not only increases system complexity but also raises the development cost and size of the device, potentially limiting its miniaturization for fully implantable use [132].
While CMOS platforms can achieve ultra-low power consumption through advanced circuit design, their active subsystems (e.g., ADCs, wireless transceivers, and processors) still require stable power supply regulation, and the inclusion of energy storage (e.g., microbatteries) or wireless power receivers may enlarge the implant footprint. OECT-based IDDSs, on the other hand, operate at much lower voltages (<1 V) and current levels and can be powered by energy harvesters or microcapacitors, enabling more compact and autonomous operation [83]
Finally, custom CMOS biosensor development involves high non-recurring engineering (NRE) costs, mask fabrication, and access to semiconductor foundries. This poses a barrier to rapid prototyping and personalization. In contrast, OECT devices can be fabricated via low-cost solution processing or printing techniques, allowing greater flexibility in design and easier adaptation to different sensing targets or patient-specific geometries [132].
In summary, while CMOS-based systems offer unmatched circuit integration and processing capabilities, their mechanical rigidity, integration complexity, and high customization costs make them less ideal for soft-tissue-conformal, highly personalized, and energy-efficient closed-loop drug delivery platforms. OECT-IDDSs provide a complementary alternative that excels in softness, sensitivity, power efficiency, and fabrication accessibility, especially in emerging minimally invasive therapeutic scenarios.
4.5. Comparative Evaluation of OECT-Integrated IDDSs and Optical -Based Therapeutic Platforms
Optical biosensor–drug delivery systems utilize light–matter interactions, such as fluorescence resonance energy transfer (FRET), absorbance, or surface plasmon resonance (SPR), to achieve highly sensitive and specific biochemical detection [133]. These platforms are valued for their ability to perform non-invasive and multiplexed monitoring and are widely applied in continuous glucose monitoring and tumor marker detection. However, despite their sensitivity, optical systems suffer from several critical limitations when considered for closed-loop implantable drug delivery.
First, optical sensor systems require complex component integration, including light sources (e.g., LEDs or lasers), detectors (e.g., photodiodes), and wavelength-specific filters. These components not only increase device size and rigidity, but also impose precise alignment requirements, which are difficult to maintain in dynamic or deforming tissue environments [134]. Misalignment or tissue scattering can rapidly degrade signal quality and measurement accuracy, especially in chronic implants where encapsulation or fibrosis occurs.
Second, optical methods consume more energy, particularly when active illumination (for excitation) or high-frequency sampling is required. The continuous operation of internal light sources can quickly drain battery resources or generate localized heating, raising concerns for thermal tissue damage. In contrast, OECTs operate at sub-1 V and microampere currents, offering a safer and energy-efficient alternative for long-term use [83].
Third, many optical-responsive drug delivery systems depend on external light triggering such as near-infrared (NIR) irradiation to activate photothermal hydrogels or photocleavable drug carriers. While this allows spatiotemporal control, it also requires transdermal light transmission or the surgical implantation of optical windows, which complicates implantation logistics and long-term usability. Tissue absorption and scattering further limit the depth and uniformity of light penetration, making deep-tissue optical control unreliable for consistent actuation [134].
From a fabrication perspective, optical systems often involve hybrid micro-assembly—combining rigid optical elements with soft polymers or drug reservoirs. Achieving a stable integration of these disparate materials at the microscale with biocompatibility and durability presents manufacturing challenges. Moreover, most optical devices lack true mechanical flexibility, restricting their conformity to tissue curvature and increasing the risk of mechanical irritation or migration over time.
By comparison, OECT-IDDS platforms offer fully integrated electronic sensing–actuation architectures with minimal external dependencies. Their soft, printed construction allows for conformal implantation, while their fast signal response and analog modulation capabilities make them well suited for continuous feedback-based drug control. Thus, while optical biosensors retain their niche in surface or wearable diagnostics, OECT-IDDSs are more viable for deeply implanted, autonomous, and long-term personalized therapies.
4.6. Challenges and Future Directions
With the advancement of biosensor technologies, the integration of OECT-based biosensors and IDDSs offers significant potential in real-time monitoring, disease prevention, and personalized therapy. By combining sensitive biomarker detection with responsive drug release, this synergy enables intelligent closed-loop treatment systems capable of automatically adjusting dosing based on physiological data.
However, several technical and practical challenges remain. Long-term implantation requires that devices maintain performance and biocompatibility under physiological conditions without inducing immune responses or biofouling. Organic semiconductors, though generally compatible, still demand deeper investigation regarding chronic tissue interaction [135]. Moreover, large-scale manufacturing with uniform quality, stable real-time control algorithms, and system complexity increases with the number of monitored biomarkers, posing barriers to clinical translation and commercialization [136,137].
In the future, smart closed-loop therapeutic systems will rely on the continuous collection and storage of large volumes of biological data, encompassing patient health metrics, treatment regimens, and even genetic information. As such, ensuring data security and protecting patient privacy will be of paramount importance. Encryption technologies and robust data protection protocols must be seamlessly integrated into the design of biosensors to safeguard sensitive patient information. Consequently, the deployment of biosensor technologies in clinical settings will require the establishment of comprehensive ethical frameworks and regulatory guidelines to uphold patient autonomy and ensure informed consent.
Moreover, the integration of telemedicine with artificial intelligence (AI) and machine learning (ML) algorithms offers a powerful path forward for intelligent therapy management. Through remote patient monitoring and algorithm-driven treatment optimization, healthcare providers can adjust drug release rates from implantable devices in real time, without requiring in-person consultations. These advancements have the potential to enhance treatment precision, improve patient outcomes, and expand access to personalized medicine on a global scale [138].
5. Conclusions
In our research on functional OECT-based biosensors and IDDSs, we see that both technologies have a lot of potential in the biomedical field. The IDDS offers a new solution for chronic disease management by enabling personalized and precise drug control, especially for diseases such as diabetes and cancer that require long-term monitoring and tailored treatment. Due to their high sensitivity, real-time monitoring capabilities, and applications in disease diagnosis, OECT-based biosensors are particularly suitable for diseases requiring early detection and rapid responses. However, while each technology has significant advantages, they still face many challenges, such as long-term stability, mass production issues, biocompatibility, device life, complex integration difficulties, etc. In the future, with technological progress and material innovation, the intelligent closed-loop treatment system built by the combination of the two is expected to overcome the current limitations and provide more accurate and efficient real-time monitoring and drug release support.
In summary, the integration of OECT biosensors and IDDS technology not only expands the application prospects of the two technologies but also provides a new idea for the construction of intelligent and personalized medical solutions. Future research should further focus on technology integration, material development, and overcoming clinical challenges and promote the maturity of relevant technologies through interdisciplinary collaboration so as to truly realize their wide application in the medical field.
Author Contributions
T.Z. contributed to manuscript writing, literature research, and figure preparation. X.-L.H. and Y.L. contributed to project conceptualization, supervision, and manuscript revision. J.-M.X., S.-F.Z. and Y.-Z.H. assisted in manuscript revision and project supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32271538 to Xiao-Le Han, 22073070 to Yi Liu), the National Natural Science Foundation of China Joint Fund (U23A2089 to Yi Liu), and Hubei Provincial Natural Science Foundation Program (Excellent Research Team Project 2025AFA009 to Yi Liu).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lo, C.; Bhardwaj, K.; Marculescu, R. Towards cell-based therapeutics: A bio-inspired autonomous drug delivery system. Nano Commun. Networks 2017, 12, 25–33. [Google Scholar] [CrossRef]
- Song, P.Y.; Jian, D.; Tng, H.; Hu, R.; Lin, G.M.; Meng, E.; Yong, K.T. An Electrochemically Actuated MEMS Device for Individualized Drug Delivery: An In Vitro Study. Adv. Healthc. Mater. 2013, 2, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- McKeating, K.S.; Aube, A.; Masson, J.F. Biosensors and nanobiosensors for therapeutic drug and response monitoring. Analyst 2016, 141, 429–449. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, S.; Wang, Q.; Li, J. Recent progress in biosensor regeneration techniques. Nanoscale 2024, 16, 2834–2846. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Sardini, E.; Serpelloni, M.; Tonello, S. Printed Electrochemical Biosensors: Opportunities and Metrological Challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. In Biosensor Technologies for Detection of Biomolecules; Estrela, P., Ed.; Portland Press Ltd.: London, UK, 2016; Volume 60, pp. 1–8. [Google Scholar]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Merkoci, A. Advanced Materials for Biosensors—Special Issue of SMALL. Small 2023, 19, e2308049. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037525. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Raston, N.H.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [CrossRef]
- Chen, C.; Lu, S. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef]
- Kurnik, M.; Pang, E.Z.; Plaxco, K.W. An Electrochemical Biosensor Architecture Based on Protein Folding Supports Direct Real-Time Measurements in Whole Blood. Angew. Chem. Int. Ed. 2020, 59, 18442–18445. [Google Scholar] [CrossRef]
- Marks, A.; Griggs, S.; Gasparini, N.; Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 2022, 9, 2102039. [Google Scholar] [CrossRef]
- Friedlein, J.T.; McLeod, R.R.; Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 2018, 63, 398–414. [Google Scholar] [CrossRef]
- Rashid, R.B.; Ji, X.D.; Rivnay, J. Organic electrochemical transistors in bioelectronic circuits. Biosens. Bioelectron. 2021, 190, 13461. [Google Scholar] [CrossRef]
- Ravariu, C. From Enzymatic Dopamine Biosensors to OECT Biosensors of Dopamine. Biosensors 2023, 13, 806. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Meshkinfam, F.; Rizvi, G. A MEMS-Based Drug Delivery Device With Integrated Microneedle Array-Design and Simulation. J. Biomech. Eng. Trans. Asme 2021, 143, 081010. [Google Scholar] [CrossRef] [PubMed]
- Timko, B.P.; Arruebo, M.; Shankarappa, S.A.; McAlvin, J.B.; Okonkwo, O.S.; Mizrahi, B.; Stefanescu, C.F.; Gomez, L.; Zhu, J.; Zhu, A.; et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 2014, 111, 1349–1354. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.L.; Zhang, L.L.; Miron, R.J.; Liang, J.F.; Shi, M.S.; Mo, W.T.; Zheng, S.H.; Zhao, Y.B.; Zhang, Y.F. Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef]
- Wu, D.; Fei, F.; Zhang, Q.; Wang, X.; Gong, Y.W.; Chen, X.J.; Zheng, Y.Y.; Tan, B.; Xu, C.L.; Xie, H.J.; et al. Nanoengineered on-demand drug delivery system improves efficacy of pharmacotherapy for epilepsy. Sci. Adv. 2022, 8, abm3381. [Google Scholar] [CrossRef]
- Cui, Y.T.; Wang, Z.H.; Li, Z.H.; Ji, X.; Yuan, B.M.; Sun, Y.; Peng, C.G.; Leng, Y.; Dou, M.H.; Wang, J.C.; et al. Functionalized anti-osteoporosis drug delivery system enhances osseointegration of an inorganic-organic bioactive interface in osteoporotic microenvironment. Mater. Des. 2021, 206, 109753. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- He, G.Q.; Li, H.M.; Liu, J.Y.; Hu, Y.L.; Liu, Y.; Wang, Z.L.; Jiang, P. Recent Progress in Implantable Drug Delivery Systems. Adv. Mater. 2024, 36, 2312530. [Google Scholar] [CrossRef]
- Barik, A.; Chakravorty, N. Targeted Drug Delivery from Titanium Implants: A Review of Challenges and Approaches. In Trends in Biomedical Research; Pokorski, M., Ed.; Springer International Publishing Ag: Cham, Switzerland, 2020; Volume 1251, pp. 1–17. [Google Scholar]
- Sutradhar, K.B.; Sumi, C.D. Implantable microchip: The futuristic controlled drug delivery system. Drug Deliv. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Almoshari, Y. Osmotic Pump Drug Delivery Systems-A Comprehensive Review. Pharmaceuticals 2022, 15, 430. [Google Scholar] [CrossRef]
- Hu, J.R.; Zhang, J.B.; Hou, Y.F.; Li, C.S.; Yang, W.N.; Fu, J.Z.; Hu, S.Y.; Liu, A.; He, Y. Digital electronics-free implantable drug delivery system for on-demand therapy. Chem. Eng. J. 2025, 504, 158763. [Google Scholar] [CrossRef]
- Santos, A.; Aw, M.S.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef]
- Vyas, K.S.; Rajendran, S.; Morrison, S.D.; Shakir, A.; Mardini, S.; Lemaine, V.; Nahabedian, M.Y.; Baker, S.B.; Rinker, B.D.; Vasconez, H.C. Systematic Review of Liposomal Bupivacaine (Exparel) for Postoperative Analgesia. Plast. Reconstr. Surg. 2016, 138, 748E–756E. [Google Scholar] [CrossRef]
- Funk, S.; Miller, M.M.; Mishell, D.R., Jr.; Archer, D.F.; Poindexter, A.; Schmidt, J.; Zampaglione, E.; Implanon, U.S.S.G. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception 2005, 71, 319–326. [Google Scholar] [CrossRef]
- Rael, C.T.; Lentz, C.; Carballo-Diéguez, A.; Giguere, R.; Dolezal, C.; Feller, D.; D’Aquila, R.T.; Hope, T.J. Understanding the Acceptability of Subdermal Implants as a Possible New HIV Prevention Method: Multi-Stage Mixed Methods Study. J. Med. Internet Res. 2020, 22, e16904. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xu, J.; Lim, J.; Nolan, J.K.; Lee, H.; Lee, C.H. Wearable Glucose Monitoring and Implantable Drug Delivery Systems for Diabetes Management. Adv. Healthc. Mater. 2021, 10, 100194. [Google Scholar] [CrossRef]
- Bertsch, T.; McKeirnan, K. ITCA 650. Clin. Diabetes 2018, 36, 265–267. [Google Scholar] [CrossRef][Green Version]
- Sreedharan, S.; Bankiewicz, K.; Patel, R. Continuous delivery of ropinirole by subdermal ProNeura™ implants. Mov. Disord. 2015, 30, S429. [Google Scholar]
- Brigham, N.C.; Ji, R.R.; Becker, M.L. Degradable polymeric vehicles for postoperative pain management. Nat. Commun. 2021, 12, 1367. [Google Scholar] [CrossRef]
- Bourge, R.C.; Waxman, A.B.; Gomberg-Maitland, M.; Shapiro, S.M.; Tarver, J.H.; Zwicke, D.L.; Feldman, J.P.; Chakinala, M.M.; Frantz, R.P.; Torres, F.; et al. Treprostinil Administered to Treat Pulmonary Arterial Hypertension Using a Fully Implantable Programmable Intravascular Delivery System Results of the DelIVery for PAH Trial. Chest 2016, 150, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Abd-Elsayed, A.; Calodney, A.; Stromberg, K.; Weaver, T.; Spencer, R.J. Targeted Drug Delivery for Chronic Nonmalignant Pain: Longitudinal Data From the Product Surveillance Registry. Neuromodulation 2021, 24, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Inoue, A.; Hirose, Y.; Morioka, M.; Horiguchi, K.; Natsume, A.; Arakawa, Y.; Iwasaki, K.; Fujiki, M.; Kumabe, T.; et al. Long-term effectiveness of Gliadel implant for malignant glioma and prognostic factors for survival: 3-year results of a postmarketing surveillance in Japan. Neurooncol. Adv. 2022, 4, vdab189. [Google Scholar] [CrossRef]
- Haim, O.; Agur, A.; Efrat, O.T.; Valdes, P.; Ram, Z.; Grossman, R. The clinical significance of radiological changes associated with gliadel implantation in patients with recurrent high grade glioma. Sci. Rep. 2023, 13, 11. [Google Scholar] [CrossRef]
- Flexner, C. Antiretroviral implants for treatment and prevention of HIV infection. Curr. Opin. HIV Aids 2018, 13, 374–380. [Google Scholar] [CrossRef]
- Kovarova, M.; Benhabbour, S.R.; Massud, I.; Spagnuolo, R.A.; Skinner, B.; Baker, C.E.; Sykes, C.; Mollan, K.R.; Kashuba, A.D.M.; García-Lerma, J.G.; et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 2018, 9, 4156. [Google Scholar] [CrossRef]
- Henry, S.J.; Barrett, S.E.; Forster, S.P.; Teller, R.S.; Yang, Z.; Li, L.; Mackey, M.A.; Doto, G.J.; Ruth, M.P.; Tsuchiya, T.; et al. Exploration of long-acting implant formulations of hepatitis B drug entecavir. Eur. J. Pharm. Sci. 2019, 136, 104958. [Google Scholar] [CrossRef]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J.; et al. Pharmacokinetics of Long-Acting Tenofovir Alafenamide (GS-7340) Subdermal Implant for HIV Prophylaxis. Antimicrob. Agents Chemother. 2015, 59, 3913–3919. [Google Scholar] [CrossRef]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and implantable devices for drug delivery: Applications and challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef]
- Joo, H.; Lee, Y.; Kim, J.; Yoo, J.S.; Yoo, S.; Kim, S.; Arya, A.K.; Kim, S.; Choi, S.H.; Lu, N.; et al. Soft implantable drug delivery device integrated wirelessly with wearable devices to treat fatal seizures. Sci. Adv. 2021, 7, eabd4639. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, F.; Zhu, X.X.; Alfadhel, A.; Ding, B.; Walton, J.P.; Cormier, D.; Frisina, R.D.; Borkholder, D.A. A nanoliter resolution implantable micropump for murine inner ear drug delivery. J. Control. Release 2019, 298, 27–37. [Google Scholar] [CrossRef]
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview, in: Grumezescu. In Nanostructures for the Engineering of Cells, Tissues and Organs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–511. [Google Scholar] [CrossRef]
- Long, G.; Mortimer, R.; Sanzenbacher, G. Recent Average Price Trends for Implantable Medical Devices, 2007–2011. 2013. Available online: https://www.analysisgroup.com/globalassets/content/insights/publishing/implantable_medical_device_price_trends.pdf (accessed on 3 April 2025).
- White, H.S.; Kittlesen, G.P.; Wrighton, M.S. Chemical derivatization of an array of three gold microelectrodes with polypyr-role: Fabrication of a molecule-based transistor. J. Am. Chem. Soc. 2002, 106, 5375–5377. [Google Scholar] [CrossRef]
- Sun, C.F.; Wang, X.; Auwalu, M.A.; Cheng, S.S.; Hu, W.P. Organic thin film transistors-based biosensors. Ecomat 2021, 3, e12094. [Google Scholar] [CrossRef]
- Bai, L.M.; Elósegui, C.G.; Li, W.Q.; Yu, P.; Fei, J.J.; Mao, L.Q. Biological Applications of Organic Electrochemical Transistors: Electrochemical Biosensors and Electrophysiology Recording. Front. Chem. 2019, 7, 313. [Google Scholar] [CrossRef]
- Wallace, G.G.; Smyth, M.; Zhao, H. Conducting Electroactive Polymer-based Biosensors, Trac-Tend. Anal. Chem. 1999, 18, 245–251. [Google Scholar]
- Strakosas, X.; Bongo, M.; Owens, R.M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 2015, 132, 41735. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Watanabe, S.; Matsumoto, M.; Muto, T.; Aoyama, T. Majority carrier type conversion in solution-processed organic transistors and flexible complementary logic circuits. Appl. Phys. Lett. 2010, 96, 083303. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.C.; Jiang, Q.L.; Xu, J.K. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef]
- Ji, W.; Wu, D.Q.; Tang, W.; Xi, X.; Su, Y.Z.; Guo, X.J.; Liu, R.L. Carbonized silk fabric-based flexible organic electrochemical transistors for highly sensitive and selective dopamine detection. Sens. Actuators B Chem. 2020, 304, 127414. [Google Scholar] [CrossRef]
- Pierre, A.; Doris, S.E.; Lujan, R.; Street, R.A. Monolithic Integration of Ion-Selective Organic Electrochemical Transistors with Thin Film Transistors on Flexible Substrates. Adv. Mater. Technol. 2019, 4, 1800577. [Google Scholar] [CrossRef]
- Keene, S.T.; Fogarty, D.; Cooke, R.; Casadevall, C.D.; Salleo, A.; Parlak, O. Wearable Organic Electrochemical Transistor Patch for Multiplexed Sensing of Calcium and Ammonium Ions from Human Perspiration. Adv. Healthc. Mater. 2019, 8, e1901321. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, M.G.; Zhao, Y.D.; Chen, Y.T.; Noureen, B.; Du, L.P.; Wu, C.S. Functional Organic Electrochemical Transistor-Based Biosensors for Biomedical Applications. Chemosensors 2024, 12, 236. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavalli, V.K. The design, fabrication, and applications of flexible biosensing devices. Biosens. Bioelectron. 2019, 124, 96–114. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Y.F.; Xu, W.T. Recent Process of Flexible Transistor-Structured Memory. Small 2021, 17, e1905332. [Google Scholar] [CrossRef]
- Spanu, A.; Martines, L.; Bonfiglio, A. Interfacing cells with organic transistors: A review of in vitro and in vivo applications. Lab Chip 2021, 21, 795–820. [Google Scholar] [CrossRef]
- Saha, T.; Fang, J.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Wearable Osmotic-Capillary Patch for Prolonged Sweat Harvesting and Sensing. ACS Appl. Mater. Interfaces 2021, 13, 8071–8081. [Google Scholar] [CrossRef]
- Bocchetta, P.; Frattini, D.; Ghosh, S.; Mohan, A.M.V.; Kumar, Y.; Kwon, Y. Soft Materials for Wearable/Flexible Electrochemical Energy Conversion, Storage, and Biosensor Devices. Materials 2020, 13, 2733. [Google Scholar] [CrossRef]
- Rabe, M. Verdes, D. Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Li, D.X.; He, Q.; Cui, Y.; Duan, L.; Li, J.B. Immobilization of glucose oxidase onto gold nanoparticles with enhanced thermostability. Biochem. Biophys. Res. Commun. 2007, 355, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, E.; Huynh, M.; GhavamiNejad, P.; Zheng, H.; Saini, A.; Bakhshandeh, F.; Keyvani, F.; Mantaila, D.; Rahman, F.A.; Quadrilatero, J.; et al. A PEDOT:PSS-based composite hydrogel as a versatile electrode for wearable microneedle sensing platforms. Adv. Sens. Res. 2024, 3, 2300122. [Google Scholar] [CrossRef]
- Tarabella, G.; CoppedÃ, N.; Mosca, R.; Cicoira, F.; Iannotta, S. Organic electrochemical transistors operating with electrolytes of increasing complexity for (Bio)sensing. AIP Conf. Proc. 2012, 1479, 1880–1883. [Google Scholar]
- Xi, X.; Wu, D.Q.; Ji, W.; Zhang, S.N.; Tang, W.; Su, Y.Z.; Guo, X.J.; Liu, R.L. Manipulating the Sensitivity and Selectivity of OECT-Based Biosensors via the Surface Engineering of Carbon Cloth Gate Electrodes. Adv. Funct. Mater. 2020, 30, 1905361. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Nguyen, Q.H.; Ko, J.; Lee, H.; Kim, D.; Kim, Y.H.; Kim, D.Y.; Joo, Y. Conjugated Radical Polymer-Based Organic Electrochemical Transistors for Biosensing Devices. Chem. Mater. 2024, 36, 7897–7908. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Nawaz, A.; Liu, Q.; Leong, W.L.; Fairfull-Smith, K.E.; Sonar, P. Organic Electrochemical Transistors for In Vivo Bioelectronics. Adv. Mater. 2021, 33, 2101874. [Google Scholar] [CrossRef]
- Cea, C.; Spyropoulos, G.D.; Jastrzebska-Perfect, P.; Ferrero, J.J.; Gelinas, J.N.; Khodagholy, D. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat. Mater. 2020, 19, 679–686. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 7086. [Google Scholar] [CrossRef]
- Berggren, M.; Forchheimer, R.; Bobacka, J.; Svensson, P.O.; Nilsson, D.; Larsson, O.; Ivaska, A. PEDOT:PSS-Based Electrochemical Transistors for Ion-to-Electron Transduction and Sensor Signal Amplification. In Organic Semiconductors in Sensor Applications; Springer: Berlin, Germany, 2008; pp. 263–280. [Google Scholar]
- Lee, H.; Lee, S.; Lee, W.; Yokota, T.; Fukuda, K.; Someya, T. Ultrathin Organic Electrochemical Transistor with Nonvolatile and Thin Gel Electrolyte for Long-Term Electrophysiological Monitoring. Adv. Funct. Mater. 2019, 29, 1906982. [Google Scholar] [CrossRef]
- Rezali, F.A.M.; Soin, N.; Hatta, S.; Daut, M.H.M.; Nouxman, M.H.A.; Hussin, H. Design Strategies and Prospects in Developing Wearable Glucose Monitoring System Using Printable Organic Transistor and Microneedle: A Review. IEEE Sens. J. 2022, 22, 13785–13799. [Google Scholar] [CrossRef]
- Spooren, A.; Rondou, P.; Debowska, K.; Lintermans, B.; Vermeulen, L.; Samyn, B.; Skieterska, K.; Debyser, G.; Devreese, B.; Vanhoenacker, P.; et al. Resistance of the dopamine D4 receptor to agonist-induced internalization and degradation. Cell. Signal. 2010, 22, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Lee, H.; Yea, J.; Jang, K.-I. Wearable electrochemical sensors for real-time monitoring in diabetes mellitus and associated complications. Soft Sci. 2024, 4, 15. [Google Scholar] [CrossRef]
- Premanode, B.; Toumazou, C. A novel, low power biosensor for real time monitoring of creatinine and urea in peritoneal dialysis. Sens. Actuators B Chem. 2007, 120, 732–735. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Schwab, U.S. Relationship of Dietary Fat to Glucose Metabolism. Atherosclerosis 2000, 150, 227–243. [Google Scholar] [CrossRef]
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian desynchrony and glucose metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef]
- Karen, D.; Marine, B.; Alan, R. Single on-chip gold nanowires for electrochemical biosensing of glucose. Analyst 2011, 136, 4507–4513. [Google Scholar]
- Liao, J.J.; Lin, S.W.; Yang, Y.; Liu, K.; Du, W.C. Highly selective and sensitive glucose sensors based on organic electrochemical transistors using TiO2 nanotube arrays-based gate electrodes. Sens. Actuators B Chem. 2015, 208, 457–463. [Google Scholar] [CrossRef]
- Liao, C.Z.; Zhang, M.; Niu, L.Y.; Zheng, Z.J.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Hama, A.; Chen, X.X.; McCulloch, I.; Inal, S. Microfluidics integrated n-type organic electrochemical transistor for metabolite sensing. Sens. Actuators B Chem. 2021, 329, 129251. [Google Scholar] [CrossRef]
- Chen, C.H.; Song, Q.Y.; Lu, W.T.; Zhang, Z.T.; Yu, Y.H.; Liu, X.Y.; He, R.X. A sensitive platform for DNA detection based on organic electrochemical transistor and nucleic acid self-assembly signal amplification. RSC Adv. 2021, 11, 37917–37922. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.Y.; Gu, X.; Tsang, C.M.; Tsao, S.W.G.; Hsing, I.M. Organic electrochemical transistor array for monitoring barrier integrity of epithelial cells invaded by nasopharyngeal carcinoma. Sens. Actuators B Chem. 2019, 297, 126761. [Google Scholar] [CrossRef]
- Song, Q.Y.; Wang, W.Y.; Liang, J.J.; Chen, C.H.; Cao, Y.P.; Cai, B.; Chen, B.L.; He, R.X. Fabrication of PEDOT:PSS-based solution gated organic electrochemical transistor array for cancer cells detection. RSC Adv. 2023, 13, 36416–36423. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Keski-oja, J.; Vaheri, A. Extracellular Matrix Proteins Characterize Human Tumor Cell Lines. Int. J. Cancer 1981, 27, 755–761. [Google Scholar] [CrossRef]
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, N.X.; Yang, A.N.; Law, H.K.W.; Li, L.; Yan, F. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, N.E.; Park, J.S.; Park, I.J.; Kim, J.G.; Cho, H.J. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef]
- Chen, L.Z.; Fu, Y.; Wang, N.X.; Yang, A.N.; Li, Y.Z.; Wu, J.; Ju, H.X.; Yan, F. Organic Electrochemical Transistors for the Detection of Cell Surface Glycans. ACS Appl. Mater. Interfaces 2018, 10, 18470–18477. [Google Scholar] [CrossRef]
- He, R.X.; Zhang, M.; Tan, F.; Leung, P.H.M.; Zhao, X.Z.; Chan, H.L.W.; Yang, M.; Yan, F. Detection of bacteria with organic electrochemical transistors. J. Am. Chem. Soc. 2012, 22, 22072–22076. [Google Scholar] [CrossRef]
- Sophocleous, M.; Contat-Rodrigo, L.; García-Breijo, E.; Georgiou, J. Organic Electrochemical Transistors as an Emerging Platform for Bio-Sensing Applications: A Review. IEEE Sens. J. 2021, 21, 3977–4006. [Google Scholar] [CrossRef]
- O’Connor, L.; Glynn, B. Recent advances in the development of nucleic acid diagnostics. Expert Rev. Med. Devices 2010, 7, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Umek, R.; Lin, S.W.; Vielmetter, J.; Terbrueggen, R.; Irvine, B.; Yu, C.; Kayyem, J.; Yowanto, H.; Blackburn, G.; Farkas, D. Electronic Detection of Nucleic Acids: A Versatile Platform for Molecular Diagnostics. J. Mol. Diagn. 2001, 3, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.J.; Lu, J.M.; Yu, T.; Long, Y.; Liu, G.Z. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206, 114109. [Google Scholar] [CrossRef]
- Elli, G.; Hamed, S.; Petrelli, M.; Ibba, P.; Ciocca, M.; Lugli, P.; Petti, L. Field-Effect Transistor-Based Biosensors for Environmental and Agricultural Monitoring. Sensors 2022, 22, 4178. [Google Scholar] [CrossRef]
- Porter, E.B.; Adaryan, S.; Ardebili, H.; Biswal, S.L.; Verduzco, R. Detection of Crude Oil in Subsea Environments Using Organic Electrochemical Transistors. ACS Sens. 2024, 9, 3633–3640. [Google Scholar] [CrossRef]
- Guimarães, A.; De Assis, H.S.; Boeger, W. The effect of trichlorfon on acetylcholinesterase activity and histopathology of cultivated fish Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2007, 68, 57–62. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Qu, H.; Zheng, L. An Acetylcholinesterase-Functionalized Biosensor for Sensitive Detection of Organophosphorus Pesticides Based on Solution-Gated Graphene Transistors. ACS Agric. Sci. Technol. 2021, 1, 372–378. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.Y.; Tian, X.Y.; Wang, Y.; Cui, B.B.; Yang, Y.L.; Dai, S.L.; Lin, W.S.; Zhu, J.X.; Wang, J.Q.; et al. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, Q.W.; Li, Z.B.; Du, Z.F.; Hong, X.; Qiu, L.Z. An enzyme Biosensor Based on Organic Transistors for Recognizing α-Amino Acid Enantiomers. J. Electrochem. Soc. 2020, 167, 067517. [Google Scholar] [CrossRef]
- Pappa, A.M.; Ohayon, D.; Giovannitti, A.; Maria, L.P.; Savva, A.; Uguz, I.; Rivnay, J.; McCulloch, I.; Owens, R.M.; Inal, S. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 2018, 4, eaat0911. [Google Scholar] [CrossRef] [PubMed]
- Recky, J.R.N.; Montero-Jimenez, M.; Scotto, J.; Azzaroni, O.; Marmisollé, W.A. Urea Biosensing through Integration of Urease to the PEDOT-Polyamine Conducting Channels of Organic Electrochemical Transistors: pH-Change-Based Mechanism and Urine Sensing. Chemosensors 2024, 12, 124. [Google Scholar] [CrossRef]
- Arcangeli, D.; Gualandi, I.; Mariani, F.; Tessarolo, M.; Ceccardi, F.; Decataldo, F.; Melandri, F.; Tonelli, D.; Fraboni, B.; Scavetta, E. Smart Bandaid Integrated with Fully Textile OECT for Uric Acid Real-Time Monitoring in Wound Exudate. ACS Sens. 2023, 8, 1593–1608. [Google Scholar] [CrossRef]
- Janardhanan, J.A.; Chen, Y.L.; Liu, C.T.; Tseng, H.S.; Wu, P.; She, J.W.; Hsiao, Y.S.; Yu, H.H. Sensitive Detection of Sweat Cortisol Using an Organic Electrochemical Transistor Featuring Nanostructured Poly(3,4-Ethylenedioxythiophene) Derivatives in the Channel Layer. Anal. Chem. 2022, 94, 7584–7593. [Google Scholar] [CrossRef]
- Curto, V.F.; Marchiori, B.; Hama, A.; Pappa, A.M.; Ferro, M.P.; Braendlein, M.; Rivnay, J.; Fiocchi, M.; Malliaras, G.G.; Ramuz, M.; et al. Organic transistor platform with integrated microfluidics for in-line multi-parametric in vitro cell monitoring. Microsyst. Nanoeng. 2017, 3, 17028. [Google Scholar] [CrossRef]
- Niu, Y.; Qin, Z.; Zhang, Y.; Chen, C.; Liu, S.; Chen, H. Expanding the potential of biosensors: A review on organic field effect transistor (OFET) and organic electrochemical transistor (OECT) biosensors. Mater. Futures 2023, 2, 042401. [Google Scholar] [CrossRef]
- Woeppel, K.M.; Zheng, X.S.; Schulte, Z.M.; Rosi, N.L.; Cui, X.Y.T. Nanoparticle Doped PEDOT for Enhanced Electrode Coatings and Drug Delivery. Adv. Healthc. Mater. 2019, 8, e1900622. [Google Scholar] [CrossRef]
- Lopez-Larrea, N.; Wustoni, S.; Penas, M.I.; Uribe, J.; Dominguez-Alfaro, A.; Gallastegui, A.; Inal, S.; Mecerreyes, D. PNIPAM/PEDOT:PSS Hydrogels for Multifunctional Organic Electrochemical Transistors. Adv. Funct. Mater. 2024, 34, 2403708. [Google Scholar] [CrossRef]
- Simon, D.T.; Kurup, S.; Larsson, K.C.; Hori, R.; Tybrandt, K.; Goiny, M.; Jager, E.H.; Berggren, M.; Canlon, B.; Richter-Dahlfors, A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat. Mater. 2009, 8, 742–746. [Google Scholar] [CrossRef]
- Tybrandt, K.; Larsson, K.C.; Kurup, S.; Simon, D.T.; Kjäll, P.; Isaksson, J.; Sandberg, M.; Jager, E.W.H.; Richter-Dahlfors, A.; Berggren, M. Translating Electronic Currents to Precise Acetylcholine-Induced Neuronal Signaling Using an Organic Electrophoretic Delivery Device. Adv. Mater. 2009, 21, 4442–4446. [Google Scholar] [CrossRef]
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Kjall, P.; Nilsson, D.; Robinson, N.D.; Berggren, M.; Richter-Dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar] [CrossRef]
- Williamson, A.; Rivnay, J.; Kergoat, L.; Jonsson, A.; Inal, S.; Uguz, I.; Ferro, M.; Ivanov, A.; Sjöström, T.A.; Simon, D.T.; et al. Controlling Epileptiform Activity with Organic Electronic Ion Pumps. Adv. Mater. 2015, 27, 3138–3144. [Google Scholar] [CrossRef]
- Berggren, M.; Glowacki, E.D.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K. In vivo organic bioelectronics for neuromodulation. Chem. Rev. 2022, 122, 4826–4846. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Xu, L.; Gutbrod, S.R.; Bonifas, A.P.; Su, Y.; Sulkin, M.S.; Lu, N.; Chung, H.-J.; Jang, K.-I.; Liu, Z.; Ying, M.; et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 2014, 5, 3329. [Google Scholar] [CrossRef]
- Sawan, M.; Yang, J.; Tarkhan, M.; Chen, J.; Wang, M.; Wang, C.; Xia, F.; Chen, Y.-H. Emerging Trends of Biomedical Circuits and Systems. Found. Trends® Integr. Circuits Syst. 2021, 1, 217–411. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Qiu, Y.; Wu, H.; Qin, W.; Liao, Y.; Yu, Q.; Cheng, H. Stretchable piezoelectric energy harvesters and self-powered sensors for wearable and implantable devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Li, Y.; Cui, B.; Zhang, S.M.; Li, B.X.; Li, J.M.; Liu, S.J.; Zhao, Q. Ion-Selective Organic Electrochemical Transistors: Recent Progress and Challenges. Small 2022, 18, 2107413. [Google Scholar] [CrossRef]
- Park, W.; Nguyen, V.P.; Jeon, Y.; Kim, B.; Li, Y.X.; Yi, J.; Kim, H.; Leem, J.W.; Kim, Y.L.; Kim, D.R.; et al. Biodegradable silicon nanoneedles for ocular drug delivery. Sci. Adv. 2022, 8, eabn1772. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.K.; Cen, D.; Zhang, T.; Jiang, S.; Wang, Y.F.; Cai, X.J.; Li, X.; Han, G.R. Implantable fibrous scaffold with hierarchical microstructure for the ‘on-site’ synergistic cancer therapy. Chem. Eng. J. 2020, 402, 126204. [Google Scholar] [CrossRef]
- Ross, J.S.; Bates, J.; Parzynski, C.S.; Akar, J.G.; Curtis, J.P.; Desai, N.R.; Freeman, J.V.; Gamble, G.M.; Kuntz, R.; Li, S.X.; et al. Can machine learning complement traditional medical device surveillance? A case study of dual-chamber implantable cardioverter-defibrillators. Med. Devices 2017, 10, 165–188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).