Abstract
The electrochemical polymerization of guaiacol was studied in different organic solvents. Significant electrode blocking was observed in dichloromethane. Microscopic studies verified the formation of a coherent deposit on the platinum electrode. In acetonitrile, the insulating deposit formation proceeded above 20 mM monomer concentration. The differences in layer formation performed in acetic acid or ethyl acetate only allowed us to make estimations in a narrow range of the composition of their binary solvent mixtures utilizing the shape of curves related to guaiacol electropolymerization. Guaiacol was therefore not reliable in solvent composition estimations within the entire range. Due to its apolar nature, a poly (guaiacol) modified platinum macroelectrode was assessed for analyses in solutions prepared with organic solvents. The analytical performance of the modified electrode was tested with butylhydroxyanisole and butylhydroxytoluene. Linear sweep voltammetry was applied under stirred conditions, and the noise of stirring diminished compared with the bare electrode, although lower sensitivity was noticed.
1. Introduction
Due to their wide applicability, electrochemical polymerizations have been the focus of research for several decades. The monomers themselves, or a moiety within the molecule, are possible candidates for layer growth during polarization, which is anodic in almost every case. The derivatives of phenols make up one group of the most popular compounds, and the electrochemical polymerization of these compounds is the most widely studied. Several investigations have confirmed that when monohydroxy phenols undergo oxidation, the unoccupied para position is the most favored site for coupling the monomers. In general, the application of basic solutions leads to very efficient coating of the conducting surfaces when phenolate ions are the reactants [1]. During electrochemical oxidation of phenols, several processes happen simultaneously, including initiation (formation of the corresponding phenoxyl radical), propagation, chain growth termination, and tail-to-tail coupling. With respect to layer growth, the head-to-tail couplings are important where the corresponding poly (phenyleneoxides) form [2].
There are many deposits of polyphenols, most of which can serve as protective coatings against corrosion in both acidic and basic conditions, while methoxyphenol polymers exhibit excellent properties in metal protection [3]. Due to the carbon–carbon and ether linkages, these coatings are resistant to hydrolysis. The reaction medium influences the composition of the organic film, while the surface state of the metal offers protection against aggressive media [4].
In the literature, guaiacol derivatives have been the main subject of extensive study for decades for the preparation of coatings and for electroanalytical applications. Electrocatalytic properties can be boosted by electrode modification with respect to both sensitivity and selectivity. Many deposits have permselective properties, especially towards neurotransmitters, whose molecular sizes are smaller than other frequently occurring interfering compounds. In the work of Milczarek et al., using flow injection analysis, guaiacol and other derivatives proved their excellent selectivity properties in the presence of ascorbic acid, uric acid, and acetaminophen [5]. The polyeugenol-modified platinum electrode proved to be an excellent choice for the attainment of low interference of ascorbic acid in the determination of dopamine [6]. On the other hand, several modes exist for the modification of electrodes with polyeugenol films. These are summarized in [7] and are based on o-quinone moieties in the film. It is worth mentioning that in vivo oxygen sensing was possible with polyeugenol film as a permselective layer used in the brain for oxygen levels [8]. Curcumin’s electrocatalytic properties were tested successfully in the simultaneous determination of some neurotransmitters [9]. Polycurcumin nanospheres proved their usefulness in the quantification of mercury ions due to the formation of functional groups of modification layer complexes [10]. Coniferyl alcohol is also a guaiacol derivative, and artificial lignin builds up during its electrooxidation [11]. This process was carried out both in aqueous and organic (CH2Cl2/methanol) systems and in accordance with expectations, the polymerization took place in both types of solutions. Vanillin and vanillic acid are also popular in electrode modification because of their anodic polymerization. Poly (vanillic acid) showed excellent properties on a carbon nanotube-modified glassy carbon electrode for simultaneous determination of ascorbic acid, dopamine, and uric acid [12]. Dopamine and ascorbic acid quantification was possible with a carbon paste electrode modified with polyvanillin [13]. This modified electrode exhibited signal enhancement in the analysis of adrenaline and uric acid [14]. When a platinum electrode was covered by a polyvanillin deposit, the electroanalysis of a-lipoic acid could be carried out [15]. Electropolymerized ferulic acid was examined both in aqueous and organic solvents, but the former was appropriate only for polymer formation [16]. When carbon nanotubes were modified with poly (ferulic acid), o-quinone moieties formed, and polymerization occurred through the carbon–carbon double bond, thus elevating the analysis of bioactive materials [17]. The functionalization of high-conductance carbon black with syringic acid through covalent immobilization made possible the detection of L-cysteine [18]. The preparation of poly (2-methoxy-4-vinylphenol) on multiwalled carbon nanotubes by free radical polymerization using glassy carbon as a template led to the anodic oxidation of NADH with low overpotential [19]. The molecularly imprinted film of poly (scopoletin) was capable of sensing human serum albumin [20].
As guaiacol has not been studied widely in nonaqueous solvents (unlike its natural and synthetic derivatives), it was one of the targets of the present work. In earlier works, deposition studies were mainly conducted in basic environments to enhance the quantity and compactness of deposit, so the utilization of polymers formed without acidic and basic additives had high interest in respect of possible applications. Compactness is a particularly undesired property in electroanalytical procedures. The role of this compound in electroanalysis was highlighted in two respects: its electropolymerization as an indicator of solvent composition and its polymer in the electroanalysis of butylhydroxytoluene under stirred conditions. Although pulsed voltammetric methods are very reliable and have excellent performance with respect to sensitivity and very often with respect to selectivity, the latter property may be destroyed if the peaks of different compounds are very close to each other due to the significant overlap.
2. Materials and Methods
All chemicals used were analytical reagent grade and used as received. The three-electrode cell used for the voltammetric experiments comprised a silver wire reference, a platinum rod counter, and a 1 mm diameter platinum disc working electrode. In some experiments, a 25 μm in diameter platinum microdisc was applied. The macrodisc was sealed in polyetheretherketone and the microdisc in glass. The active surfaces of the electrodes were cleaned by polishing them on a polishing cloth immersed in an aqueous suspension of alumina. Then, the electrodes were cleaned by thorough washing with distilled water, followed by ultrasonication in a bath to remove adsorbed species originating from the polishing. The potentiostat used during the experiments was supplied by Dropsens (Spain, Oviedo). The supporting electrolyte utilized throughout the experiments was tetrabutylammonium perchlorate (TBAP).
The micrographs of the deposits were prepared with a Jeol JSM-IT500HR scanning electron microscope (SEM) (Jeol, Tokyo, Japan). The equipment operated at an acceleration voltage of 5 kV. Before microscopic studies, the Pt electrodes were thoroughly cleaned with polishing, ultrasonicating, and finally rinsing with dry acetone to remove water and consequently prevent the adsorption of flue-dust particles. Water highly facilitates this process because it evaporates very slowly compared with acetone. At the end of electrolysis, the prepared layers were washed with the pure solvent used to remove the supporting electrolyte and unreacted monomers.
3. Results
In general, the position of substituents in phenols determines the susceptibility to electrode fouling, and para position is the most significantly favorable. Earlier investigations were conducted in basic solutions of guaiacol and its derivatives to obtain an adherent and compact layer. On the other hand, the solvent plays a critical role in the development of deposits as a result of monomer oxidation. A series of organic solvents were tested for guaiacol in 25 mM concentrations without additives. This enables us to see the differences when the supporting electrolyte is the only compound present with the studied compound in solution. In acetone, dimethylformamide, dimethyl sulfoxide, and 1-propanol, there was no current decline, indicating favorable solvation properties. Only a 12% decrease was observed in nitrobenzene by recording the fifth scan.
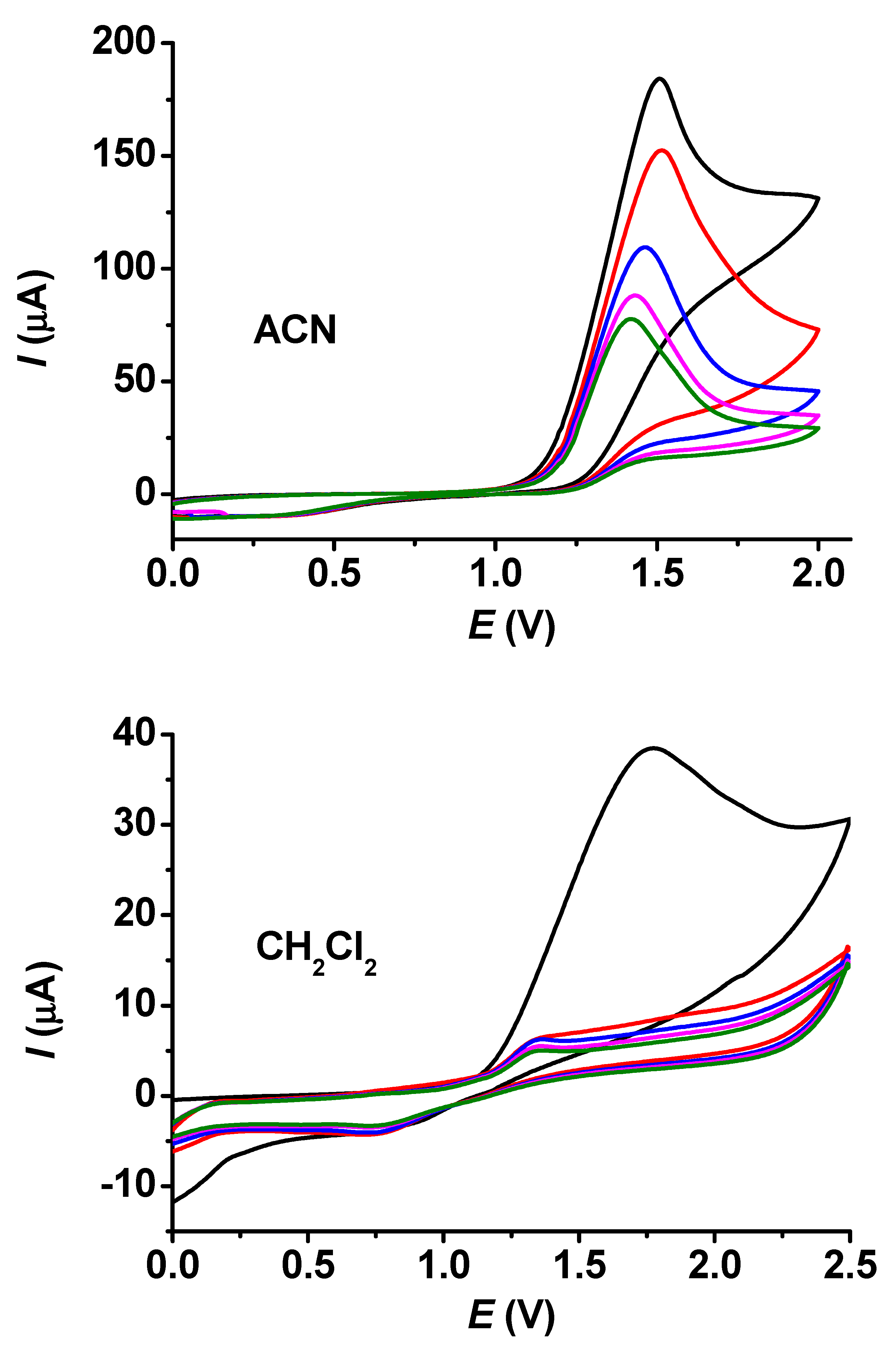
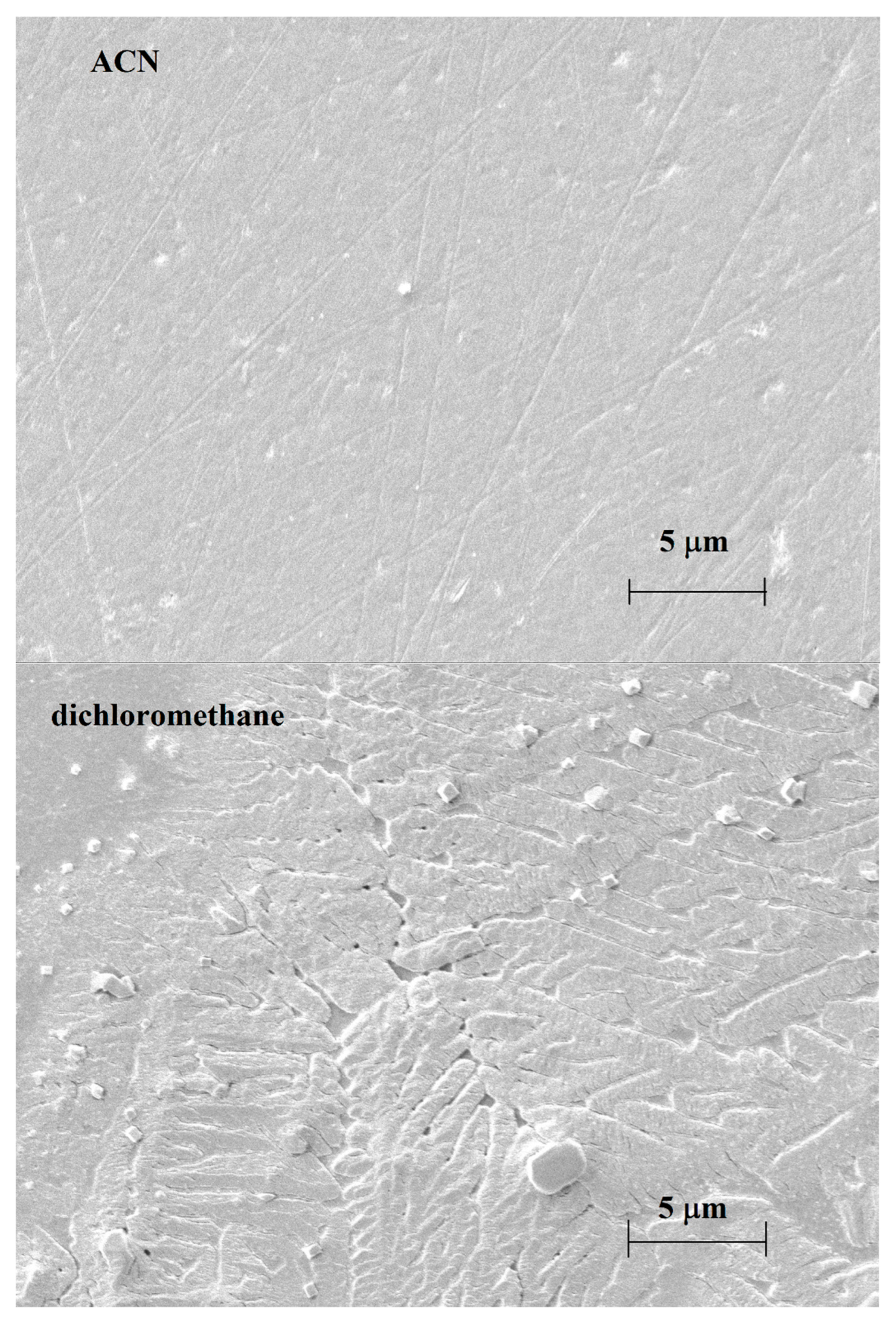
In Figure 1, the voltammograms taken in acetonitrile and dichloromethane for 25 mM guaiacol are displayed. The currents diminished continuously in acetonitrile, but they decreased gradually in dichloromethane. The related micrographs, highlighting the significantly different results for these two solvents, are shown in Figure 2. The micrograph of the layer grown in acetonitrile seems to be very thin, as scratches of the platinum surface are clearly seen, but the deposit formed in dichloromethane has identical characteristics. The polymerized guaiacol is concentrated mainly in bundles and cubic forms. These configurations developed continuously, but the electrode became almost completely fouled during the first cycle in comparison with the voltammetric experiments; this can also be seen from the diminished currents compared with acetonitrile.
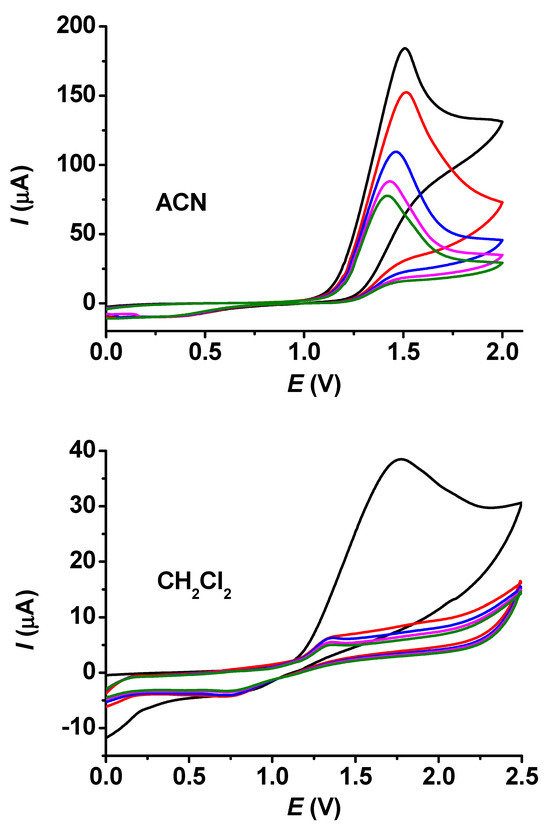
Figure 1.
Repetitive cyclic voltammograms of guaiacol in acetonitrile (ACN) and in CH2Cl2 (c = 25 mM, supporting electrolyte 0.05 M TBAP, scan rate 0.1 V/s. The colors from black to green denote the subsequent curves with continuously decreasing currents due to the growth of insulating film.

Figure 2.
Scanning electron micrographs of deposits grown in solutions prepared with acetonitrile and dichloromethane containing guaiacol in 25 mM concentration.
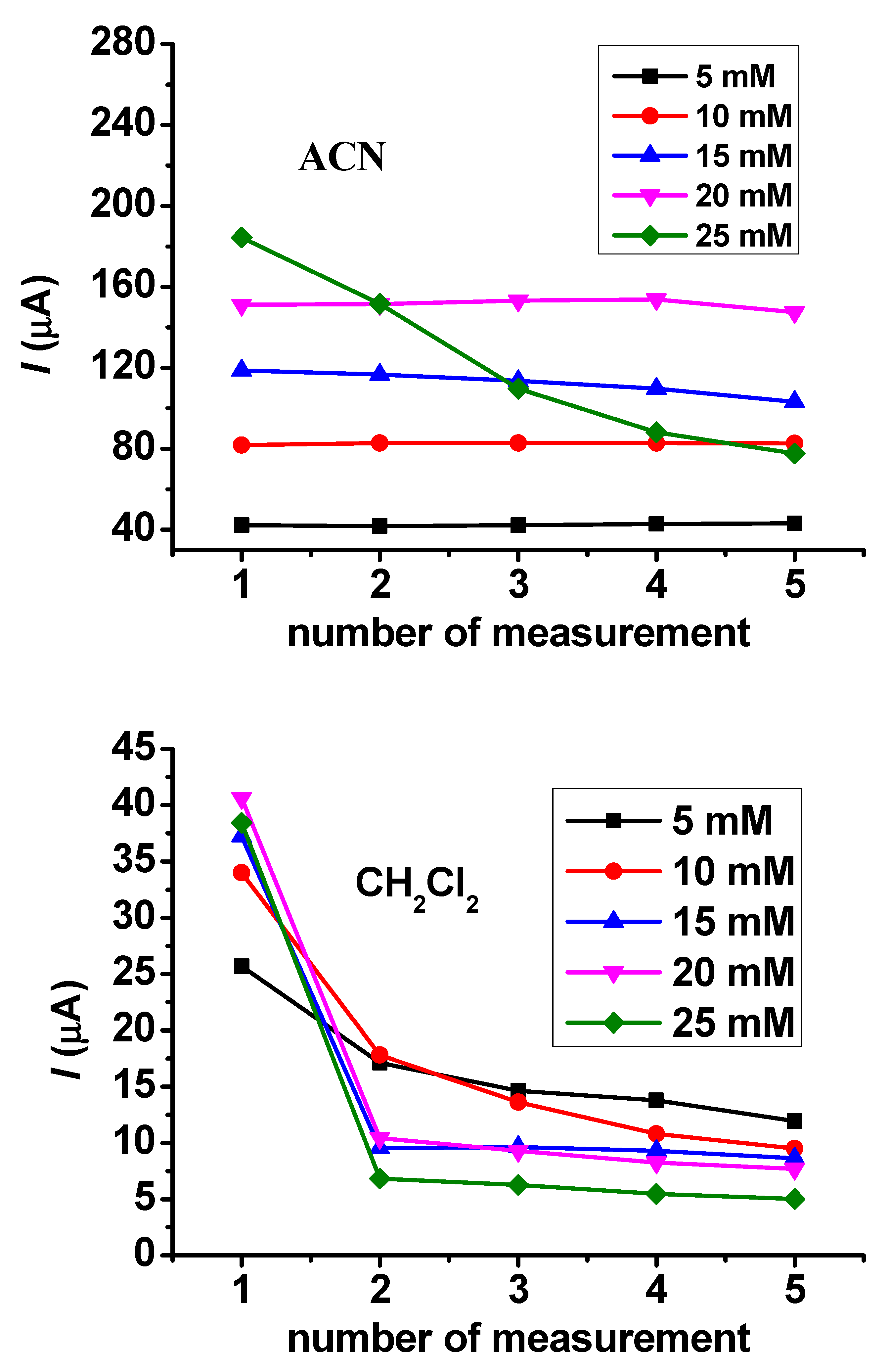
The dependence of reproducibility on the concentration also provides useful information about the susceptibility of a compound to polymerization. These experiments were conducted in guaiacol concentrations between 5 and 25 mM. In acetonitrile solvent, there was weak fouling above 10 mM concentration, but at 25 mM concentration it became very visible (Figure 3). In dichloromethane, the deactivation was rapid at 5 mM concentration, and this process accelerated at higher concentrations.
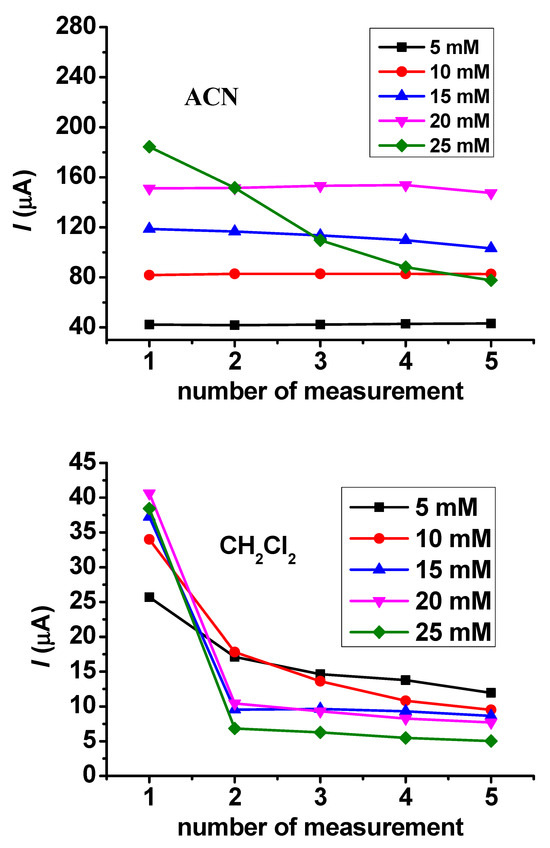
Figure 3.
Peak current values of repetitive cyclic voltammetric curves for guaiacol in acetonitrile and CH2Cl2 with a Pt macroelectrode.
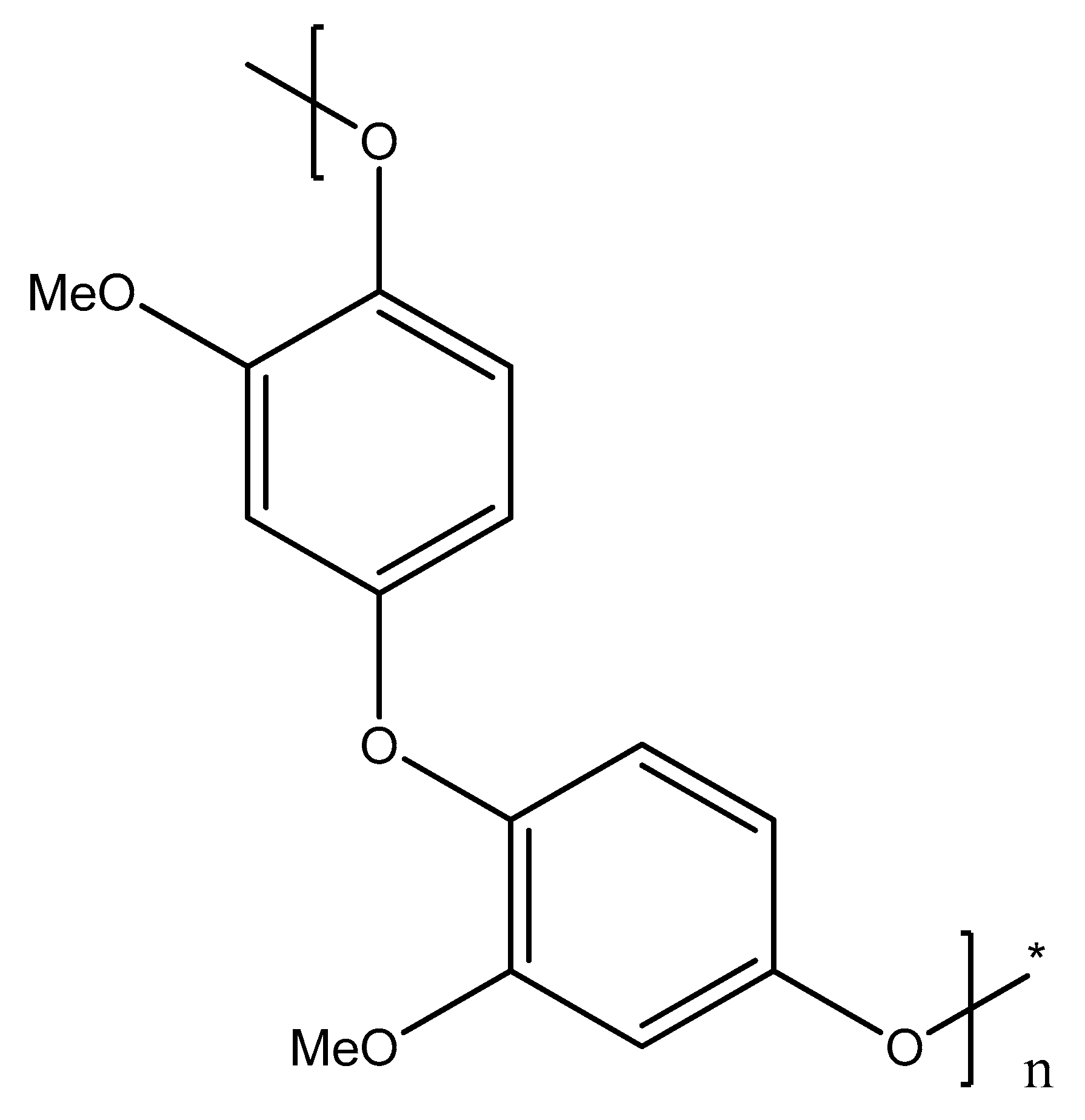
Figure 4 shows the possible structure of poly (guaiacol) that results from the para-coupling of electrochemically generated radicals. Earlier works have shown this [3], offering detailed descriptions of the mechanism of guaiacol electrooxidation in aqueous solutions; due to the presence of water, more dimeric forms appear, including more oxygen atoms than the monomer itself. In this work, the majority of solvents are aprotic, and the dimeric forms that appear include two attached cyclohexadienone units, as is usual in the case of phenols. These dimers and lower molecular weight oligomers are removed from the vicinity of the anode due to the favorable solvation properties of the organic solvents used. The polymer chain growth is elevated through the couplings via ether linkages.
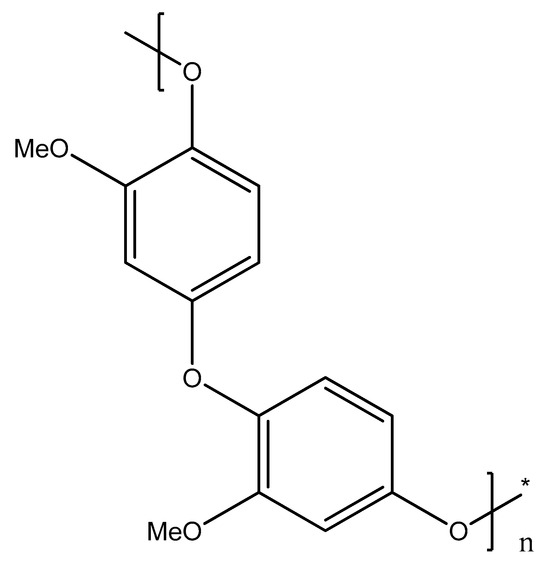
Figure 4.
Structure of the electrochemically prepared poly (guaiacol) deposit (asterisk denotes the end of polymer chain detail).
Using a macroelectrode in acetic acid and ethyl acetate, peaks do not usually show up for an electroactive material; only continuous current enhancements with the increase of potential appear, which can possibly be attributed to a significant ohmic drop due to the relatively low solvent permittivities. The heavy distortion of the voltammograms suggests that these observations are attributable to ohmic effects. Studies involving more compounds susceptible to anodic polymerization have highlighted that the current originating from the anodic oxidation of the corresponding material makes a high contribution to the currents [21]. This consequence was based on scans that followed the first scan because in several cases peaks showed up as a result of polymer formation residing in front of the electrode. This phenomenon was not observed in the case of guaiacol when the voltammetric experiments were conducted with a 25 μm Pt microelectrode. The repeatable curves verified that the adhesion of poly (guaiacol) was not satisfactory for a platinum surface, as it would enable current declines. In a recent work with 4-methoxyphenol, an isomer different from guaiacol, the microelectrode voltammograms were different in acetic acid and ethyl acetate, and the current magnitude depended on the solvent composition, showing a continuously decreasing tendency with an enhanced acetic acid content. The curves’ shapes were very similar in acetic acid and ethyl acetate, independent of concentration, but for kinetic reasons, the potentials reflected by the plateaus shifted to higher potentials in ethyl acetate.
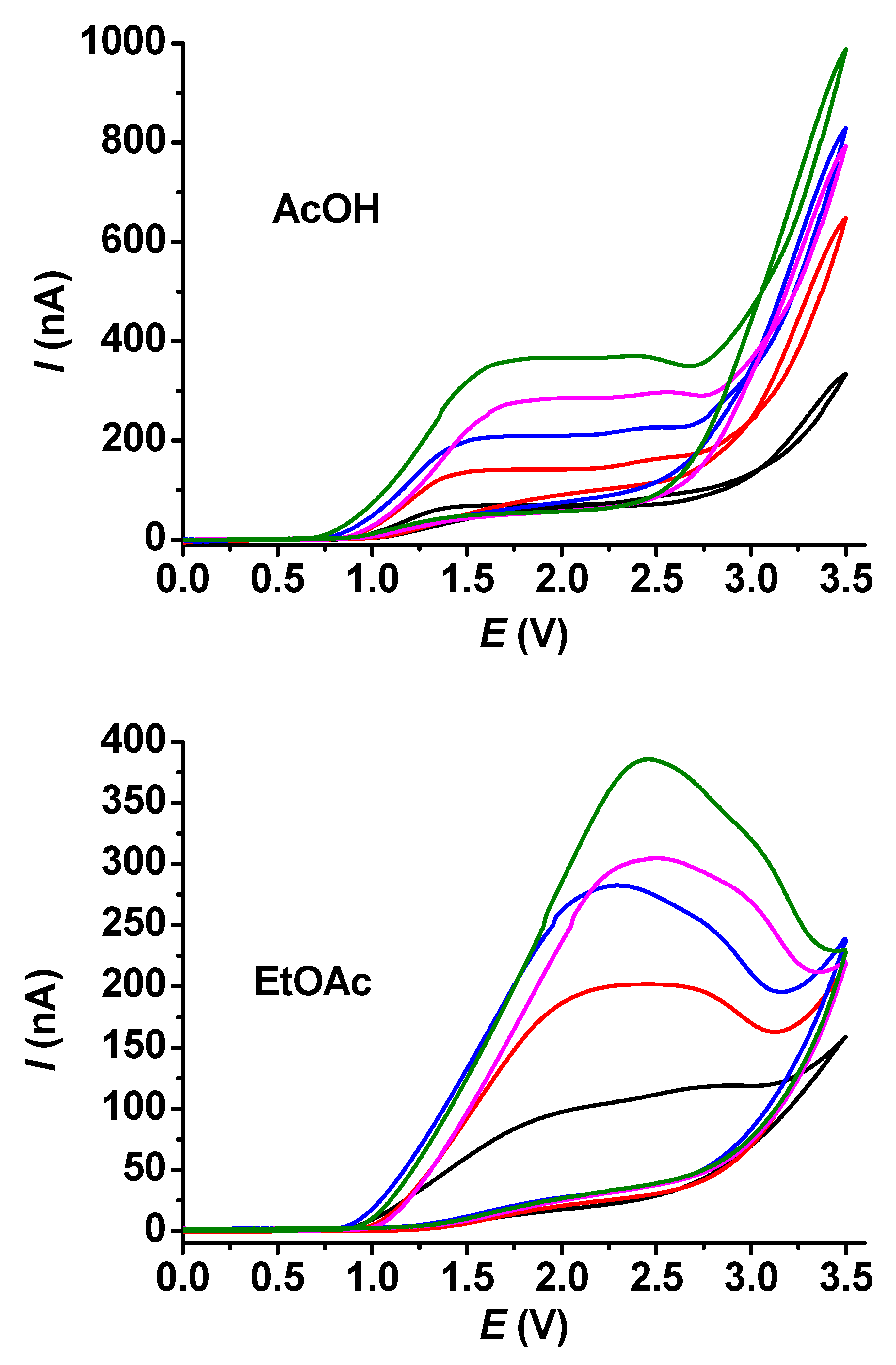
In the case of guaiacol, the shapes of the voltammograms were very similar to those obtained for acetic acid. This was also true for ethyl acetate, but peaks showed up even at 10 mM concentrations (Figure 5). This indicates that the products of guaiacol electrooxidation block the surface of the electrode more efficiently.
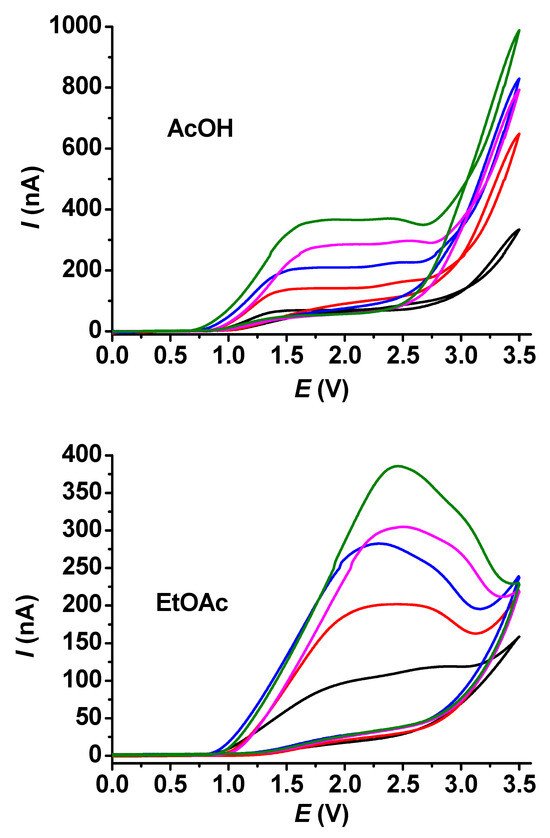
Figure 5.
Cyclic voltammetric curves for different concentrations of guaiacol in acetic acid (AcOH) and ethyl acetate (EtOAc) with 25 μm microelectrode (supporting electrolyte 50 mM TBAP, scan rate 0.1 V/s, black: 5 mM, red: 10 mM, blue: 15 mM, magenta: 20 mM, green: 25 mM guaiacol).
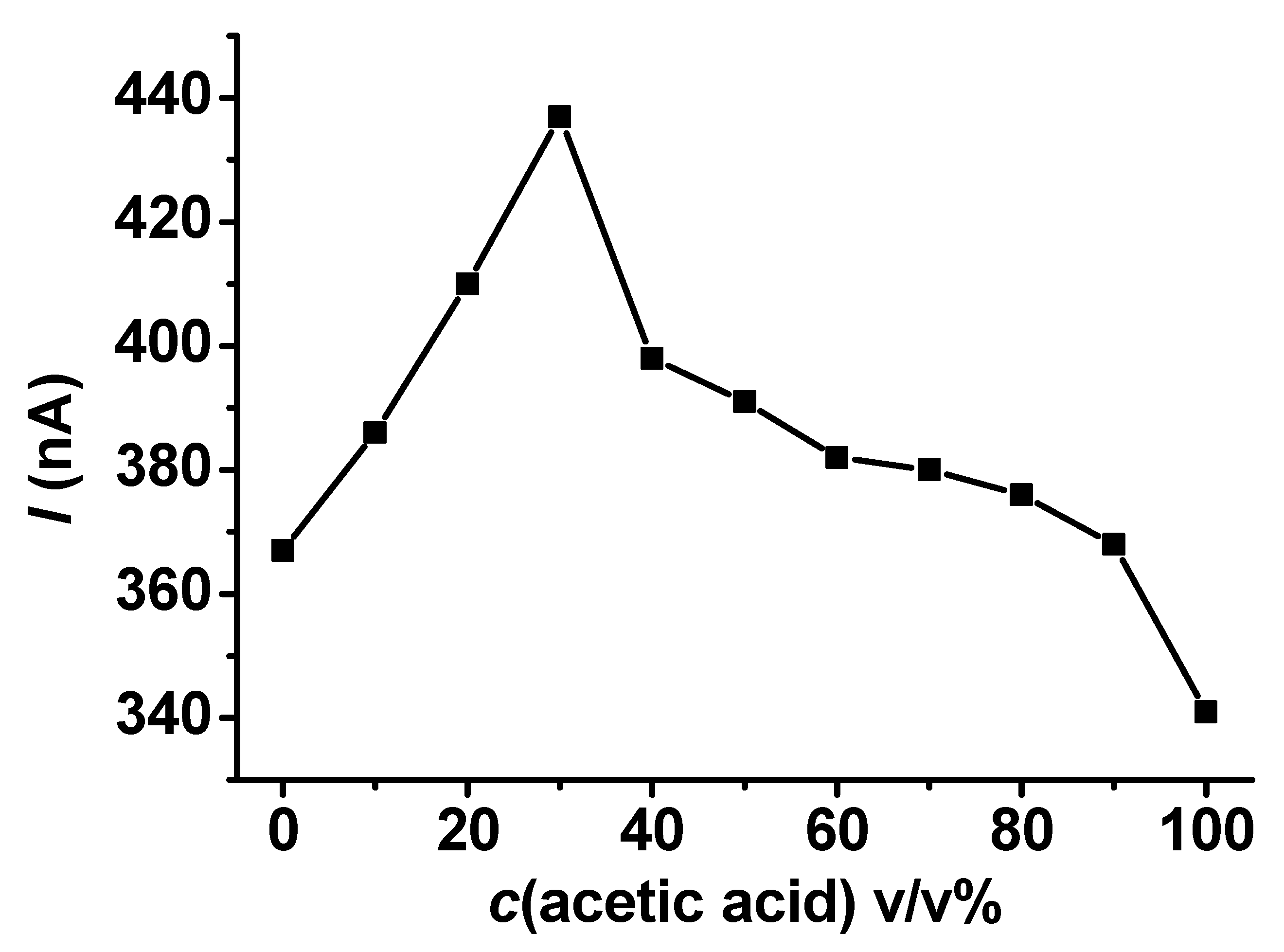
The electrochemical polymerization of a compound is an option for estimating the solvent composition. The shapes of curves and the magnitude of currents observed using a preset uniform concentration make this type of analysis possible. If the difference between solvent viscosities is high, the mass transport limited currents serve as analytical information. Dependence on the acetic acid–ethyl acetate binary solvent composition in the full range is revealed in Figure 6. The data are based mainly on the peak currents, where the quantity of ethyl acetate was significant. This is similar to the behavior of 4-methoxyphenol, but oxidation peaks appeared in more compositions, while the investigated compositions were the same, just as it was in the case of 4-methoxyphenol used in our earlier work. The current plateaus showed up at higher acetic acid contents. Figure 6 also shows that guaiacol cannot serve as an electroactive compound that would allow the estimation of the solvent composition of the binary systems of the outlined solvents. Surprisingly, the peak currents increased up to 40 v/v% acetic acid content and then declined, leading to a nonreliable calibration curve. The explanation for the current increase at higher ethyl acetate contents might be the better solvation properties of acetic acid for poly (guaiacol) and suppressed film propagation on the electrode. This phenomenon allowed the measurement of elevated current peaks that kept the shape characteristics experienced with ethyl acetate. This statement is based on the curves for acetic acid seen in the corresponding part of Figure 4.
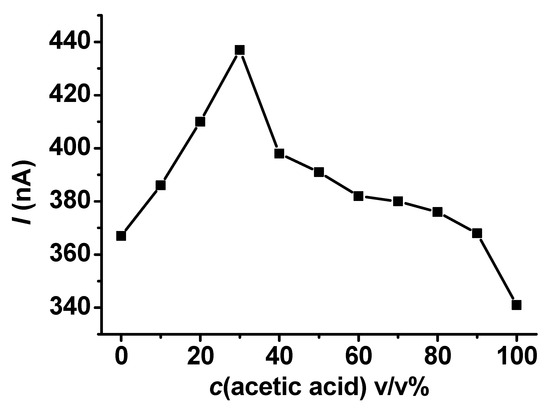
Figure 6.
Dependence of currents measured with 25 μm Pt microelectrode with a scan rate of 0.1 V/s on acetic acid content in its mixtures with ethyl acetate containing 25 mM guaiacol and 50 mM TBAP uniformly.
Performance of Deposits in Electroanalysis of Butylhydroxytoluene
There is a pressing need worldwide to carry out quantifications of a large number of organic compounds to control food safety. Butylhydroxytoluene (BHT) is one of the most commonly used synthetic antioxidants in many areas. In electrochemical procedures, many electrode materials proved appropriate choices, including platinum, gold, glassy carbon, and boron-doped diamond, and these were modified in many ways. For example, this compound is added to biodiesel, and the nature of the supporting electrolyte has a high influence on the reliability of quantification [22]. Differential pulse voltammetry has also proved useful in multicomponent analysis [23,24].
Procedures based on amperometric detection, such as hydrodynamic voltammetry, have also been developed. With these, multiple pulses are applied in combination with the flow-injection technique. This type of detection makes possible sensitive determinations under stirred conditions due to the significantly enhanced mass transport limited currents polarizing the electrode to a potential included in the diffusion-controlled range. Here, the contribution of the current of the studied compound to the overall current becomes constant, polarizing the electrode to a more positive (anodic process) or more negative (cathodic process) direction. Basically, when amperometric detection is used, the interference caused by coexisting electroactive compounds requires the subtraction of current signals. In these multicomponent systems, it is necessary to perform measurements at more potentials. The appropriate number of experiments depends on the number of analytes.
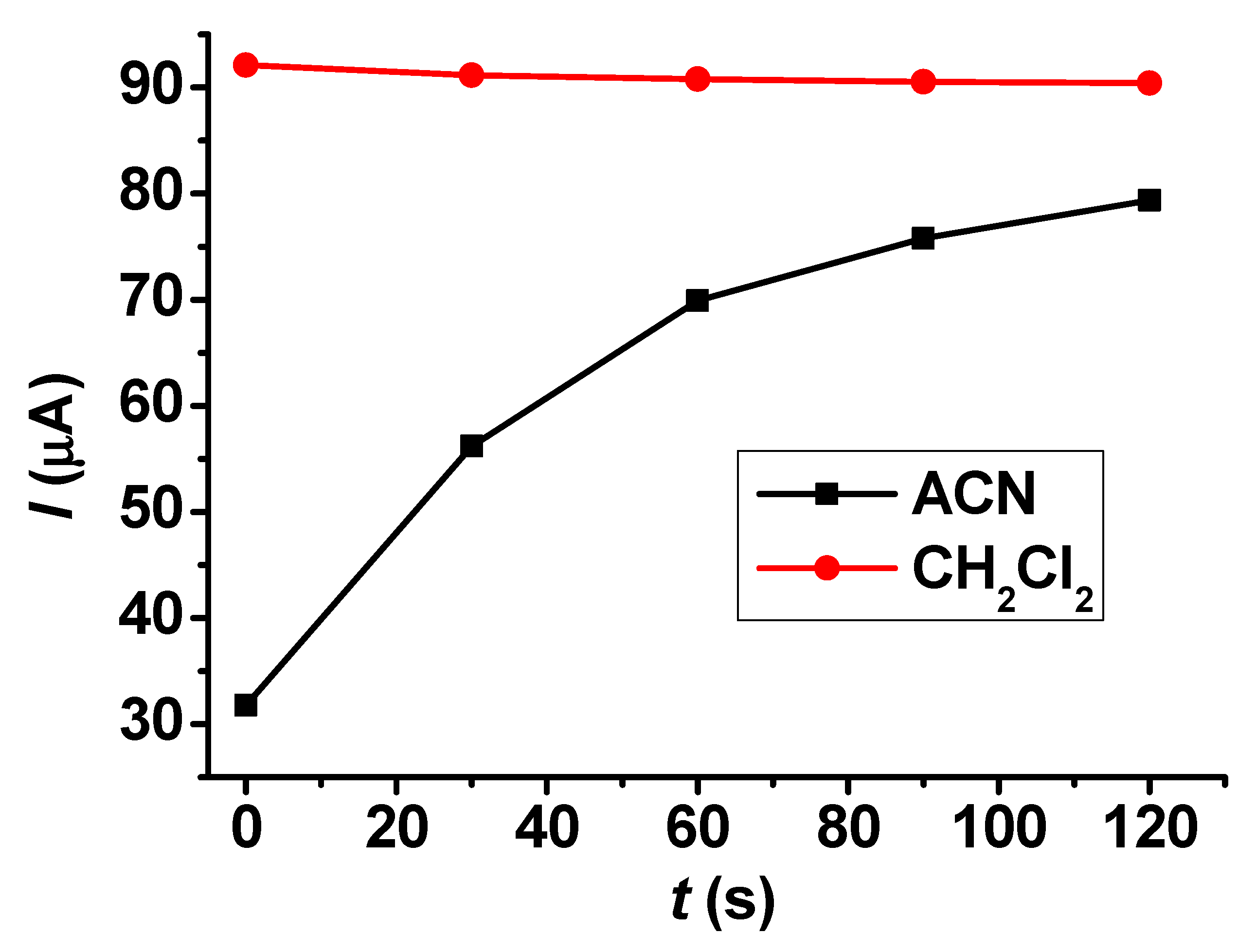
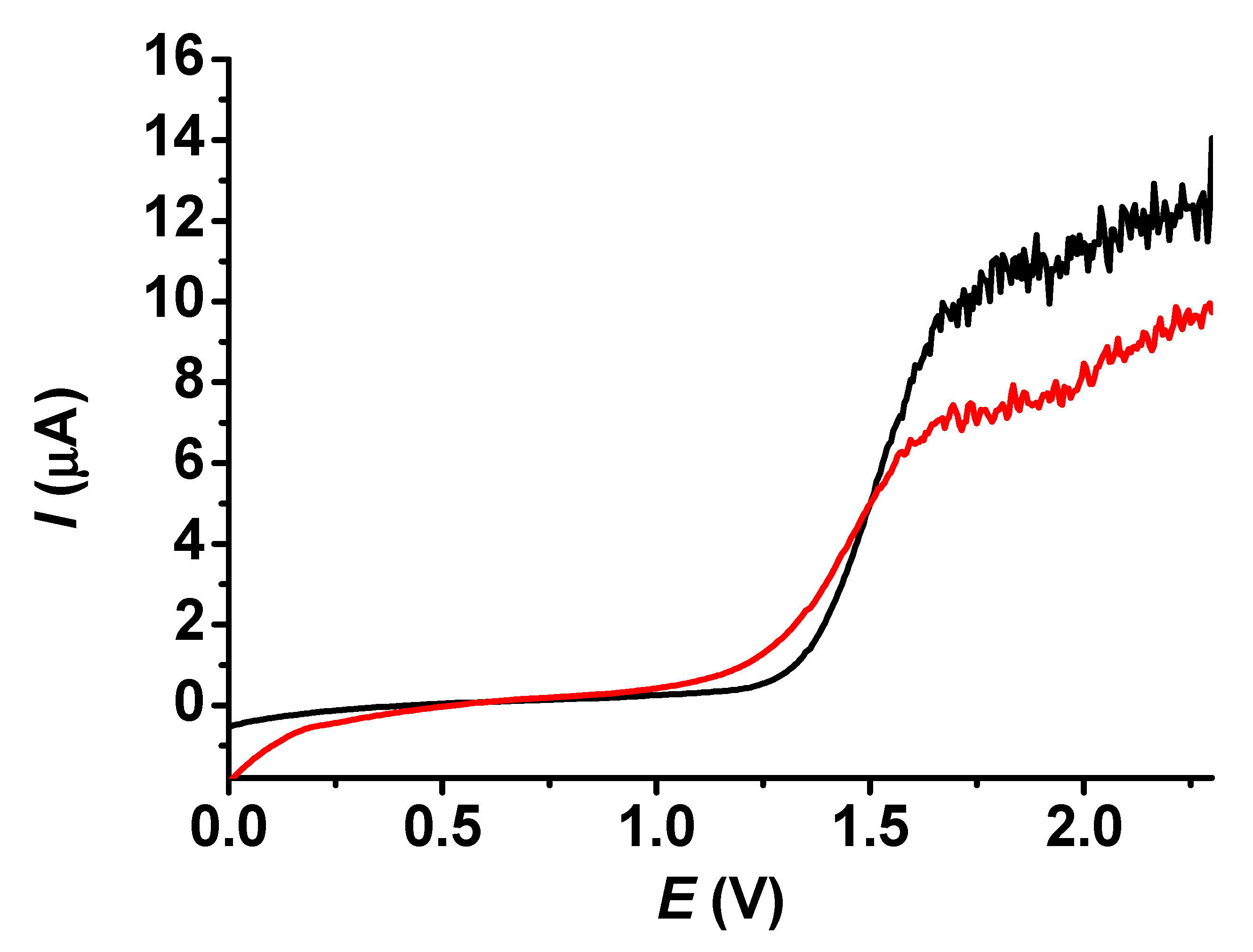
Figure 7 displays the results obtained with the electrochemically deposited modifying poly (guaiacol) layers deposited from acetonitrile and dichloromethane in a 5 mM acetonitrile solution of butylhydroxytoluene. The anodic peaks were taken into account at different times while the modified electrodes were immersed in the solution. The differences between the solvents are remarkable. Deposition from acetonitrile leads to a compact layer, whose permeability gets better in time. This observation confirms that a layer is present on the electrode, complementing the microscopic results, which do not show it clearly. The continuous increase of peak currents over time suggests a swelling of organic deposit that causes the improvement of porosity and, apparently, the partial recovery of the electroactive surface. On the other hand, the layer formed in dichloromethane has a highly permeable layer with a small contribution of other effects, as the peak currents recorded within the entire studied time interval are very close to the 88 μA value measured with the bare electrode. The results with the layer grown in dichloromethane in organic solvents seemed promising in the context of the analytes’ permeability, so this was also tested in aqueous solutions with redox probes; however, only ~70% of signals with the bare electrode were recovered, indicating that a smaller electrode surface is accessible to water due to the apolar nature of the film.
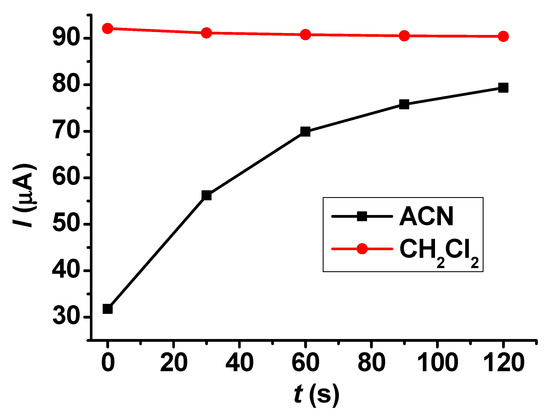
Figure 7.
Dependence on time of linear sweep voltammetric peak currents of 10 mM BHT in acetonitrile with modifying layers grown in acetonitrile and dichloromethane (supporting electrolyte 50 mM TBAP, scan rate 0.1 V/s).
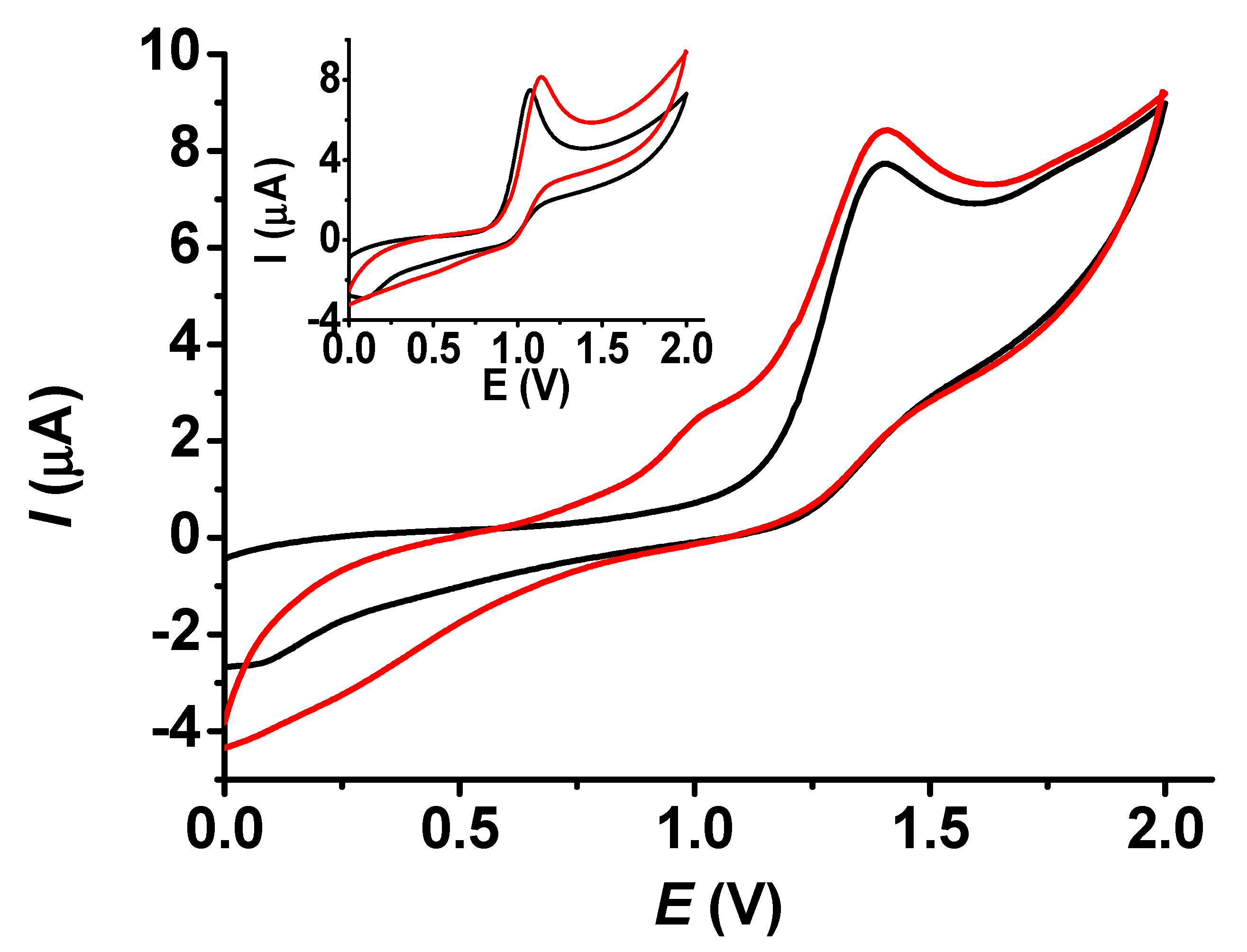
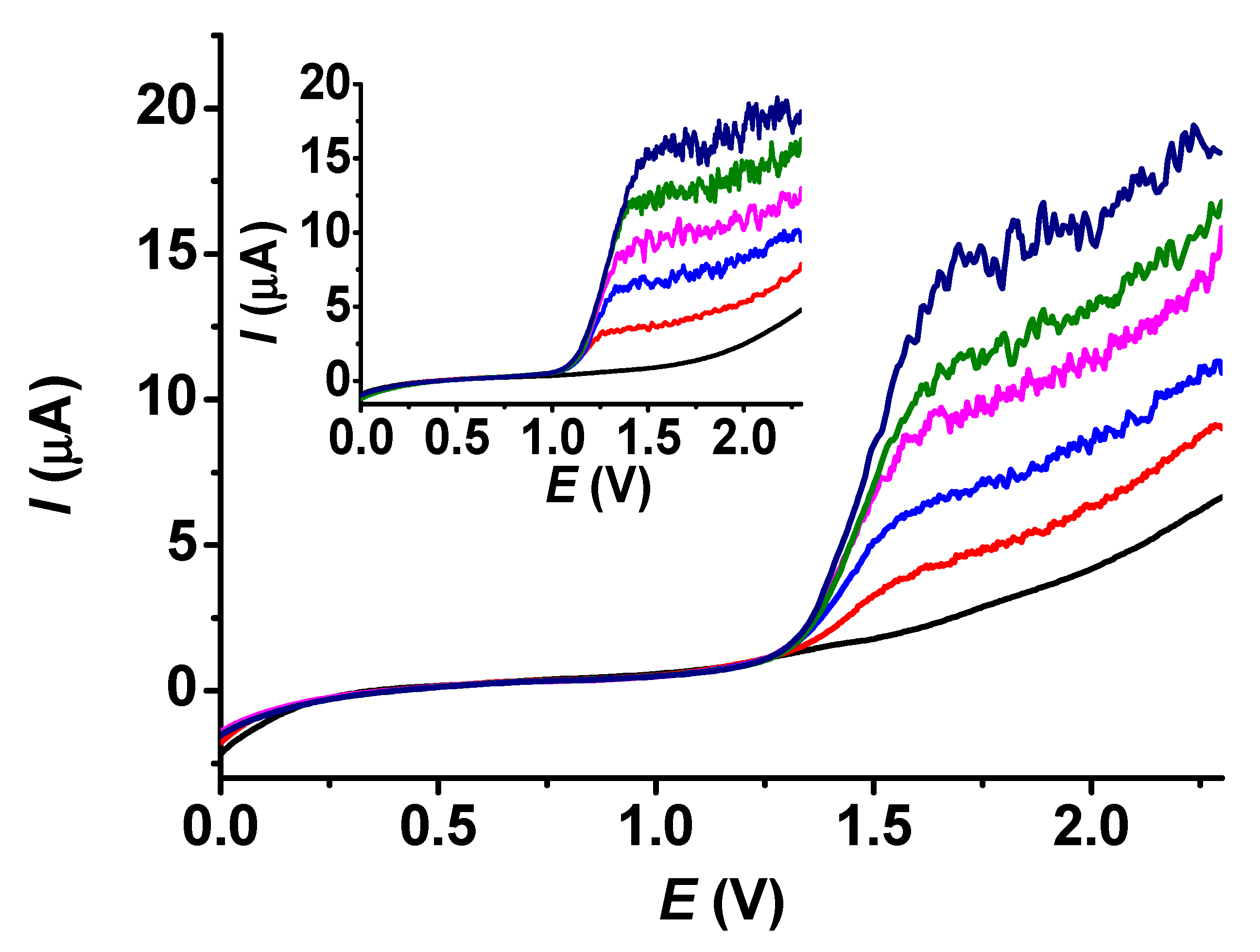
The comparison of bare and modified electrodes is an essential task to consider their performances and establish the goodness of the modifying layer towards selected compounds. These studies were carried out for both BHT and BHA (Figure 8). Similar to the results in Figure 7, the layer grown in dichloromethane allowed somewhat higher peak heights than those obtained with a bare platinum disc. This was a consequence of the appearance of a prepeak at around 1 V on the voltammogram of BHT with a modified electrode. This can be attributed to the poly (guaiacol) layer, as redox behavior was also observed during the depositions. As the oxidation peak of BHA appeared at the same potential as that of the prepeak, there was no difference in shape between the bare and modified electrodes.
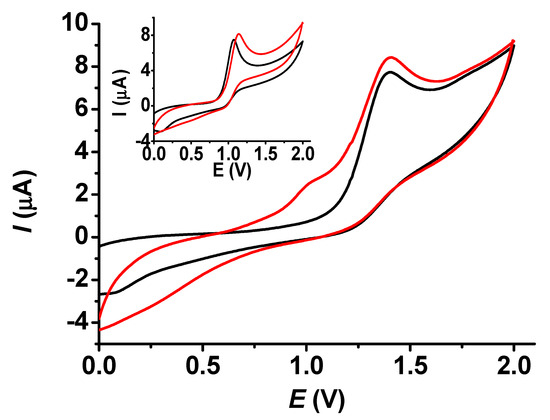
Figure 8.
Cyclic voltammograms for 1 mM BHT (main graph) and for 1 mM BHA (inset graph) with bare (black curve) and poly (guaiacol) modified electrode (red curve) in acetonitrile (scan rate 0.1 V/s, supporting electrolyte 10 mM TBAP).
Linear sweep voltammetry (LSV) is a fast method that enables more or less selective determinations. Depending on the standard potentials of the coexisting analytes and on the procedure applied by electrode modification, LSV makes more or less reliable simultaneous determinations, as was possible for butylhydroxyanisol (BHA) and BHT. Since amperometric detection becomes lengthy in multicomponent systems, the performance of the LSV technique was assessed under stirred conditions. In many situations, due to the committing of experimental errors somewhere in the chosen procedure, a shift in anodic or cathodic potentials may appear. This can cause problems in a multicomponent system when these potentials are close to each other, leading to the detection of a signal that is not purely mass-transport-limited. In amperometry, experimenters work with preset potentials. As mass transport limited currents are reached with LSV, as they are with amperometry, the advantages of stirring are also exploited in the diffusion-controlled potential range. The use of LSV not only eliminates the problem arising from the shifts of peak potentials, but also, under stirred conditions, elevates the subtraction of signals in a multicomponent system, as we obtain current plateaus if the corresponding potentials are close to each other. We can obtain reliable results when the overlap does not lead to complications, unlike those obtained using pulsed techniques.
Since the noise caused by the stirring of solutions does not favor the reliability of determination, the effect of poly (guaiacol) coating needed to be established. Five cycles were taken in a 25 mM dichloromethane solution of guaiacol between 0 and 2.5 V. Figure 9 reveals the LSV curves taken under stirred conditions with a 700 rpm rotation speed in 0.5 mM acetonitrile solution of BHT. It is clearly seen that the noise of stirring decreased to approximately half that of the bare electrode. Usually, this type of noise has some fluctuations, so the data-reading reliability becomes unsatisfactory if the magnitudes of these fluctuations are high. If it is reduced, the analysis of the curves is rendered more comfortable. In an earlier work, a deposit of 2′,6′-dihydroxyacetophenone prepared in dimethyl sulfoxide exhibited a drastic alleviation of the noise of stirring [25], but this was not so significant in the case of poly (guaiacol). In accordance with the microscopic results, the deposit distribution throughout the whole electrode surface was not uniform, and the almost constant currents of BHT (see Figure 7) also reinforce the consequence that the organic layer could weakly exclude the solution flows within the pores of polymeric film. On the other hand, the application of the poly (guaiacol) modifying layer proved not very fruitful, as it resulted in lower mass transport limited currents, although the higher stability of the signal due to the reduced noise level compensated a lot.
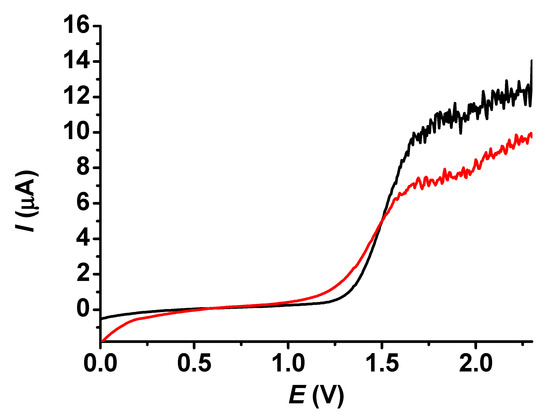
Figure 9.
Linear sweep voltammetric curves taken with bare platinum electrode (black curve) and with poly (guaiacol) modified electrode (red curve) in 0.3 mM acetonitrile solution of BHT containing 10 mM TBAP supporting electrolyte with scan rate of 0.1 V/s.
Comparing the above LSV results under the stirred conditions shown in Figure 8, the conclusion is that the presence of deposit renders the rotation speed at the electrode smaller and allows a reduced mass transport limited signal. The contribution of the previously observed slight contribution of the film to the signal becomes negligible due to the large enhancement of stirring. This property is more significant for the bare electrode. During stirring, a Prandtl boundary layer develops, and there is a thin layer in front of the electrode, in which mass transport can occur mainly by diffusion. Due to this relatively slow process, the signal decreases when the quiescent layer has a higher thickness. The reduction of this thickness has a limiting value when a layer is present on the electrode. This hinders the movement of the solution.
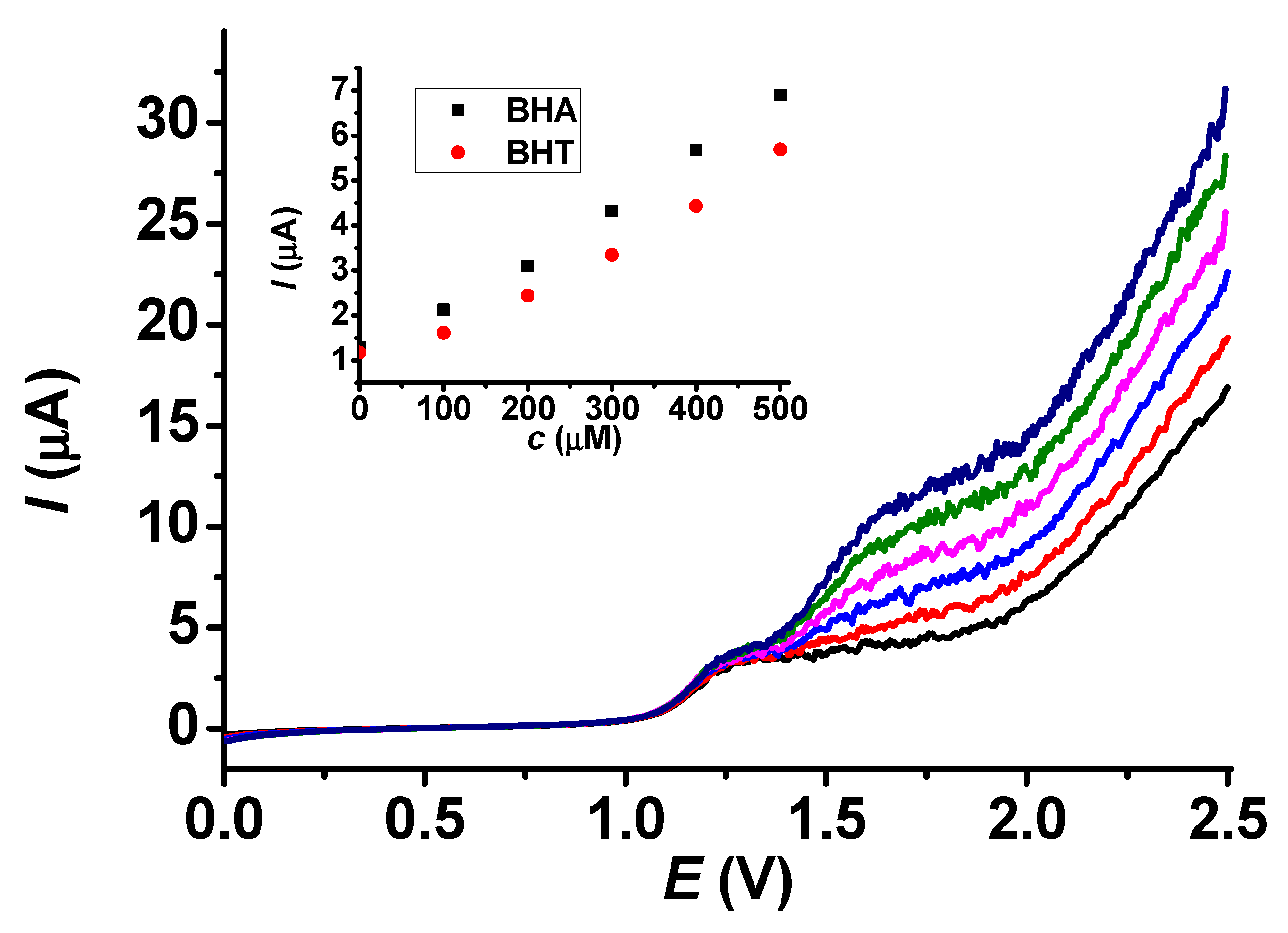
Since BHA is the most frequently occurring interfering compound of BHT, calibrations were carried out with the same concentrations of the poly (guaiacol) modified electrode. As expected, the related curves are stationary voltammograms (Figure 10). However, they do not exhibit horizontal lines due to background currents. The curve taken in a blank solution serves as a guide for establishing the contributions of the presenting analytes. The calibration curves were linear. The valid equation for BHA was I (μA) = 0.481 + 0.029c (μM), and for BHT, it was I (μA) = 1.553 + 0.022c (μM).
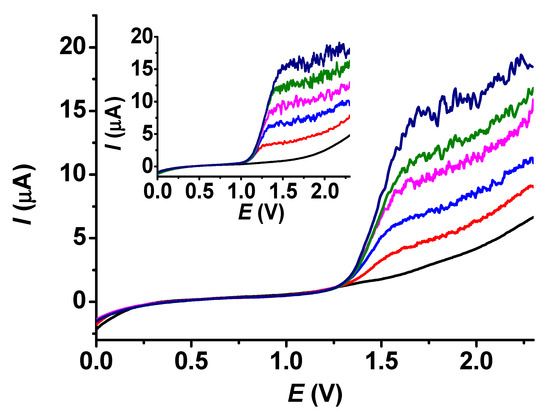
Figure 10.
LSV voltammograms in acetonitrile under stirred conditions with 700 rpm of BHT (main graph) and BHA (inset graph) (supporting electrolyte 10 mM TBAP, scan rate 0.1 V/s, black: 0 μM, red: 100 μm, blue: 200 μM, magenta: 300 μM, green: 400 μM, navy: 500 μM).
The voltammograms show that the extent of noise increases gradually with concentration, especially when the plateaus are reached. This suggests a proportionality of the noise to the magnitude of the signal. The advantage originating from the choice of linear sweep voltammetry paired with stirring against amperometry, also applying stirring continuously, is clearly seen in the curves of Figure 10. For each solution conductivity, there is a shift in potential with concentration where the plateaus begin; in our case, there is a small difference between the anodic potentials of BHA and BHT. A choice of constant potential would lead to mistakes in experimental design and by utilizing LSV, we can see the whole voltammogram. Simple subtraction yields the analytical signals when more analytes are present.
As biodiesel is one of the products most frequently spiked with antioxidants, we used it for real sample analysis. Biodiesels consist basically of methyl esters of fatty acids, and a sample was acquired without antioxidants. Standard addition serves as a good method in regard to matrix effects because, in our situation, the real sample could modify the viscosity properties of the sample, and we could also establish the background current. To improve the solubility, dichloromethane was used as a solvent. The prepared poly (guaiacol) deposit was conditioned in dichloromethane by taking LSV curves until they stabilized. We dissolved 1 g of the sample in the solvent mixture in a 10 mL volumetric flask and placed the solution in a glass beaker. The aliquots of stock solutions were added during stirring at 700 rpm. The related results are revealed in Figure 11. The current values at 1.75 V were utilized during the evaluation because they were within the diffusion-controlled ranges of both analytes. As Figure 11 reveals, the plateaus of BHA and BHT are well separated, and the current signal of BHA was recovered at 104%. It could be concluded from our results that the use of dichloromethane as a solvent and washing product increased the lifetime of the organic layer, probably because the solubility of poly (guaiacol) was low in dichloromethane.
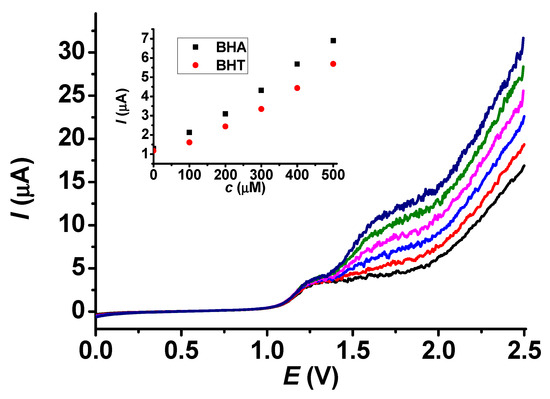
Figure 11.
Standard addition curves for BHT in presence of 300 mM BHA (main graph) and calibration plots of BHA and BHT in dichloromethane containing a biodiesel sample (scan rate 0.1 V/s, supporting electrolyte 20 mM TBAP, black: 0 μM, red: 100 μM, blue: 200 μM, magenta: 300 μM, green: 400 μM, navy: 500 μM).
4. Conclusions
The use of linear sweep voltammetry has benefits in electroanalysis because the simultaneous application of stirring continuously renews the concentration close to the electrode surface, ensuring a significantly enhanced concentration gradient. This special utilization of linear sweep voltammetry makes possible sensitive and relatively fast determinations of selected compounds by improving the signal-to-noise ratio due to the alleviation of stirring noise. Our studies can be a basis for the further improvement of modifying layers by optimizing the layer thickness and porosity, while stirring noise is reduced by finding the appropriate monomer and a method for porosity regulation. On the other hand, such findings may contribute to the development of electroanalytical methods that apply the flow of the solution. In summary, we utilized the benefits of bare electrodes while exploiting the advantages of stirring.
Author Contributions
Conceptualization, L.K.; methodology, L.K.; investigation, L.K.; writing—original draft preparation, L.K.; writing—review and editing, resources, funding acquisition S.K.-M.; visualization, software, data analysis P.S.; supervision, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hungarian National Research Development and Innovation Office (NKFI), grant number NKFI-137793, the Chinese-Hungarian Intergovernmental S&T Cooperation Programme (Project no.: CH-10-6/2024 and 2024-1.2.5-TÉT-2024-00006), and the New National Excellence Program of the Ministry for Innovation and Technology Project no. TKP2021-EGA-17. The financial support of the University of Pécs under Project 015_2024_PTE_RK/31 is highly appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maali, A.; Najoua, D.; Rihab, H.; Hasen, M.S.; Hechmi, S.; Emilia, M.; Salma, B. Electrodeposition of 4,4′-di-tert-butylbiphenyl peroxide from the anodic oxidation of p-tert-butylphenol in an alkaline acetonitrile solution. J. Appl. Electrochem. 2017, 47, 507–516. [Google Scholar]
- Bruno, F.; Pham, M.C.; Dubois, J.E. Polaromicrotribometric study of polyphenylene oxide film formation on metal electrodes by electrolysis of disubstituted phenols. Electrochim. Acta 1977, 22, 451–457. [Google Scholar] [CrossRef]
- Youssef, S.; Dalila, K.; Ridha, A. Electropolymerization of phenol, o-nitrophenol and o-methoxyphenol on gold and carbon steel materials and their corrosion protection effects. Prog. Org. Coat. 2010, 69, 335–343. [Google Scholar]
- Mengoli, G.; Marco, M.M. An overview of phenol electropolymerization for metal protection. J. Electrochem. Soc. 1987, 134, 643C–652C. [Google Scholar] [CrossRef]
- Milczarek, G.; Ciszewski, A. Permselective properties of electropolymerized guaiacol derivatives. Electroanalysis 2003, 15, 529–532. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G. Polyeugenol-modified platinum electrode for selective detection of dopamine in the presence of ascorbic acid. Anal. Chem. 1999, 71, 1055–1061. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G. Preparation and general properties of chemically modified electrodes based on electrosynthesized thin polymeric films derived from eugenol. Electroanalysis 2001, 13, 860–867. [Google Scholar] [CrossRef]
- Paul, D.W.; Prajapati, I.; Reed, M.L. Electropolymerized eugenol: Evaluation as a protective film for oxygen sensing. Sens. Actuators B Chem. 2013, 183, 129–135. [Google Scholar] [CrossRef]
- Devadas, B.; Rajkumar, M.; Chen, S.M. Electropolymerization of curcumin on glassy carbon electrode and its electrocatalytic application for the voltammetric determination of epinephrine and p-acetoaminophenol. Colloids Surf. B 2014, 116, 674–680. [Google Scholar] [CrossRef]
- Kumar, K.K.; Devendiran, M.; Kalaivani, R.A.; Narayanan, S.S. Polycurcumin nanospheres modified electrode for nanoscale detection of mercury ions in seawater. Chem. Phys. Lett. 2021, 781, 138974. [Google Scholar] [CrossRef]
- Matsushita, Y.; Sekiguchi, T.; Ichino, R.; Fukushima, K. Electropolymerization of coniferyl alcohol. J. Wood Sci. 2009, 55, 344–349. [Google Scholar] [CrossRef]
- Silva, L.V.D.; Silva, F.A.S.; Kubota, L.T.; Lopes, C.B.; Lima, P.R.; Costa, E.O.; Pinho Júnior, W.; Goulart, M.O.F. Amperometric sensor based on carbon nanotubes and electropolymerized vanillic acid for simultaneous determination of ascorbic acid, dopamine, and uric acid. J. Solid State Electrochem. 2016, 20, 2389–2393. [Google Scholar] [CrossRef]
- Manjunatha, J.G.; Swamy, B.E.K.; Deraman, M.; Mamatha, G.P. Simultaneous voltammetric measurement of ascorbic acid and dopamine at poly (vanillin) modified carbon paste electrode: A cyclic voltammetric study. Der. Pharm. Chem. 2012, 4, 2489–2497. [Google Scholar]
- Madhuchandra, H.D.; Swamy, B.E.K. Poly (vanillin) modified carbon paste electrode for the determination of adrenaline: A voltammetric study. Mater. Sci. Energy Technol. 2019, 2, 697–702. [Google Scholar] [CrossRef]
- Duran, S.T.; Hassine, C.B.A.; Burç, M.; Güngör, Ö. Voltammetric determination of a-lipoic acid using poly(vanillin) modified platinum electrode. Anal. Bioanal. Electrochem. 2020, 12, 857–869. [Google Scholar]
- Matsushita, Y.; Nakamura, A.; Aoki, D.; Yagami, S.; Fukushima, K. Bio-based polymer from ferulic acid by electropolymerization. BioResources 2016, 11, 9789–9802. [Google Scholar] [CrossRef]
- Da Silva, L.V.; Lopes, C.B.; da Silva, W.C.; de Paiva, Y.G.; dos Santos Silva, F.A.; Lima, P.R.; Kubota, L.T.; Goulart, M.O.F. Electropolymerization of ferulic acid on multi-walled carbon nanotubes modified glassy carbon electrode as a versatile platform for NADH, dopamine and epinephrine separate detection. Microchem. J. 2017, 133, 460–467. [Google Scholar] [CrossRef]
- Sundaram, S.; Kadir, M.R.A. A new highly conducting carbon black (CL-08) modified electrode functionalized with syringic acid for sensitive and selective L-cysteine electrocatalysis at low potential. Electrochim. Acta 2017, 224, 475–486. [Google Scholar] [CrossRef]
- Corrêa, C.C.; Santhiago, M.; e Silva, C.C.C.; Formiga, A.L.B.; Kubota, L.T. Synthesis and electrochemical characterization of poly(2-methoxy-4-vinylphenol) with MWCNTs. Electroanalysis 2011, 23, 2562–2568. [Google Scholar] [CrossRef]
- Stojanovic, Z.; Erdőssy, J.; Keltai, K.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polyscopoletin nanofilms for human serum albumin detection. Anal. Chim. Acta 2017, 977, 1–9. [Google Scholar] [CrossRef]
- Kiss, L.; Szabó, P. Acetic acid and ethyl acetate as solvents for electropolymerization reactions, considering 4-methoxyphenol and composition of solvent mixtures. Organics 2024, 5, 670–683. [Google Scholar] [CrossRef]
- Jaromira, C.; Markéta, T.; Tomás, M.; Renáta, S.; Jan, J. Voltammetric determination of BHT antioxidant at gold electrode in biodiesel. Electroanalysis 2012, 24, 1374–1379. [Google Scholar]
- Lívia, S.S.; Jonathan, W.V.D.; Pedro, R.B.F.; Mariliz, G.; Clarisse, M.S.P. Direct and simultaneous determination of four phenolic antioxidants in biodiesel using differential pulse voltammetry assisted by artificial neural networks and variable selection by decision trees. Fuel 2019, 236, 803–810. [Google Scholar]
- Lívia, S.S.; Pedro, R.B.F.; Clarisse, M.S.P.; Mariliz, G. A Chemometric-Assisted Voltammetric Method for Simultaneous Determination of Four Antioxidants in Biodiesel Samples. Energy Fuels 2020, 34, 412–418. [Google Scholar]
- Kiss, L.; Li, H.; Yan, H.; Kunsági-Máté, S. Comparison between electropolymers of 3,5-dihydroxybenzoic acid and 2′,6′-dihydroxyacetophenone in dimethyl sulfoxide and their analytical performance towards selected analytes with the role of the washing liquid. Molecules 2024, 29, 3972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).