1. Introduction
Hydrogen is a key factor for future energy solutions but there are safety-related issues which have to be addressed, due to the flammable nature of hydrogen in the 4–75% concentration range at atmospheric pressure. Thus, detecting hydrogen concentrations between 0.01% and 10% rapidly and accurately is crucial to alert to the formation of dangerous mixtures [
1]. Many groups are currently investigating the reliable detection of low concentrations of hydrogen using inexpensive sensors. As an active medium material, ZnO is widely investigated for gas sensors, but the particular case of hydrogen sensors has faced several limitations. For example, earlier devices suffered from insufficient sensitivity at low H
2 concentrations and poor selectivity in mixed-gas environments, often necessitating elevated operating temperatures [
2]. Recent efforts address these issues by leveraging nanoscale ZnO architectures and material modifications. Furthermore, previous studies have shown that a high surface-area-to-volume ratio is desirable for achieving a high sensitivity, by dramatically increasing the density of reactive sites, improving hydrogen adsorption and sensor response [
3], particularly in the case of Surface Acoustic Wave (SAW) sensors [
4]. Additionally, defect engineering (e.g., creating oxygen vacancies or other surface or sub-surface defects) and catalytic doping strategies have been shown to enhance performance. Introducing selected dopants (such as Pd or rare-earth elements) into or onto ZnO can promote H
2 dissociation and provide additional active sites, yielding higher response and even helping tailor the sensor’s selectivity [
3,
5]. However, ZnO nanowire surface is easily contaminated under ambient conditions (left in contact with air at room temperature) and, apart from the water vapors (composed of hydrogen and oxygen, both relevant to the hydrogen detection process), the most common element are carbon and nitrogen. Carbon contamination of a surface is known to have a slightly negative impact on hydrogen adsorption, since carbon sites interact weakly with hydrogen molecules and cannot dissociate it on their own [
6,
7]. On the other hand, studies have shown that the presence of nitrogen in carbon materials such as graphene, carbon nanotubes, or carbon xerogels enhances hydrogen adsorption [
8,
9,
10] by lowering the dissociation energy of the hydrogen molecule [
6,
11]. Furthermore, the combined effect of metals and N-doped carbon materials on hydrogen sorption has been studied [
9,
12]. Both ZnO and nitrogen substituted carbon materials can dissociatively adsorb hydrogen, so both surfaces can contribute to hydrogen sorption, and, consequently, to the sensing process.
In order to study and possibly overcome past limitations in ZnO hydrogen sensing, in the present studies we employ two types of ZnO nanowires: one with a tailored hexagonal cross-section and a limited number of crystal defects enabling a dual adsorption–absorption sensing mechanism and another one with cylindrical cross-section single-crystal nanowires with a dominant surface adsorption-based mechanism. Based on SAW sensors with such different ZnO nanostructure morphologies, the present study experimentally investigates the influence of carbon and nitrogen surface contamination of the active surface on hydrogen sensing processes and performance.
2. Materials and Methods
ZnO nanowires were grown by Vapor–Liquid–Solid (VLS) technique (
Figure 1b) on a quartz substrate, using laser ablation as a particle source (
Figure 1a). Sintered ZnO powder targets were used. The third harmonic (355 nm) of an Nd:YAG pulsed laser with 400 kHz repetition rate was used to ablate the target in an atmosphere of oxygen (1 Pa). Gold was pre-deposited on the quartz substrate. The VLS growth proceeds through the nucleation of nanowires from liquid gold catalyst drops (
Figure 1b), formed on the substrate upon heating. Impinging particles from the ablation plume are collected into the gold catalyst and when a critical particle concentration is achieved, ZnO nucleates underneath the catalyst drop, forming a nanowire. This approach is known to provide a reasonable control over the growth morphology [
13]. We should, however, emphasize that plasma reflection techniques were also used in order to filter out relatively large particles produced by the laser ablation process, particles which could potentially inhibit the catalyst growing process [
13]. Details on this plasma filtering technique have been described elsewhere [
14].
In order to detect and monitor the hydrogen sorption of the structures, these nanowires were grown on the (quartz) active area of a Surface Acoustic Wave (SAW). Sensor is placed in an ambient controlled atmosphere chamber (with constant temperature and humidity) where a gas controller is regulating hydrogen concentration into the chamber, and a detailed scheme of the sensor measurement setup is presented elsewhere [
4]. Theoretical [
15] and experimental investigations [
16,
17] show that the concentration of molecules adsorbed by the sensing film (and, respectively, sorbed mass) is proportional to the resulting frequency shift of the sensor oscillator circuit. At reasonably low gas concentrations (of hydrogen in our case), a SAW sensor should provide a linear response (frequency shift recorded by the frequency counter) with the monitored gas concentration. Thus, the sorbed hydrogen mass (relative) variation could be monitored by simply monitoring the frequency shift of the sensor oscillator circuit.
To monitor influence of surface contamination on sensor sensitivity, measurements of the sensor frequency shift variation with hydrogen concentration were performed in three cases: (i) for a 5-week air-contaminated ZnO grown surface, (ii) after a thermal treatment in air of the surface, and (iii) after treating the surface in hydrogen (cold) plasma. In the first case, the as-deposited samples were stored in ambient condition (and consequently contaminated) for several weeks before measurement was performed. For the second case the sample was heated at 600 °C for about 3 h and then cooled down at room temperature before performing the measurements, to desorb (part of) the contaminants. For the third case, the sample was exposed to a low-temperature plasma jet in Argon/Hydrogen [
18], exposure performed at room temperature. Plasma treatments conditions were 100 W RF power, duration 30 min, working pressure at about 7 Pa, ambient gas of Ar/H
2 mixture (gases mass flow 100/50 sccm), and a weak air flow. Optical Emission Spectroscopy (OES) investigation has confirmed [
19] the excited species of argon, H
β line of hydrogen and many nitrogen-based species that come from the air presence in our Ar/H
2 plasma. Furthermore, in order to confirm the nitrogen lines’ presence, we additionally introduced 5 more sccm of nitrogen in the chamber, and the amplification of nitrogen bands is visible (See
Supplementary Materials in
Figure S1) by comparing extra nitrogen and treatment condition spectra.
3. Results
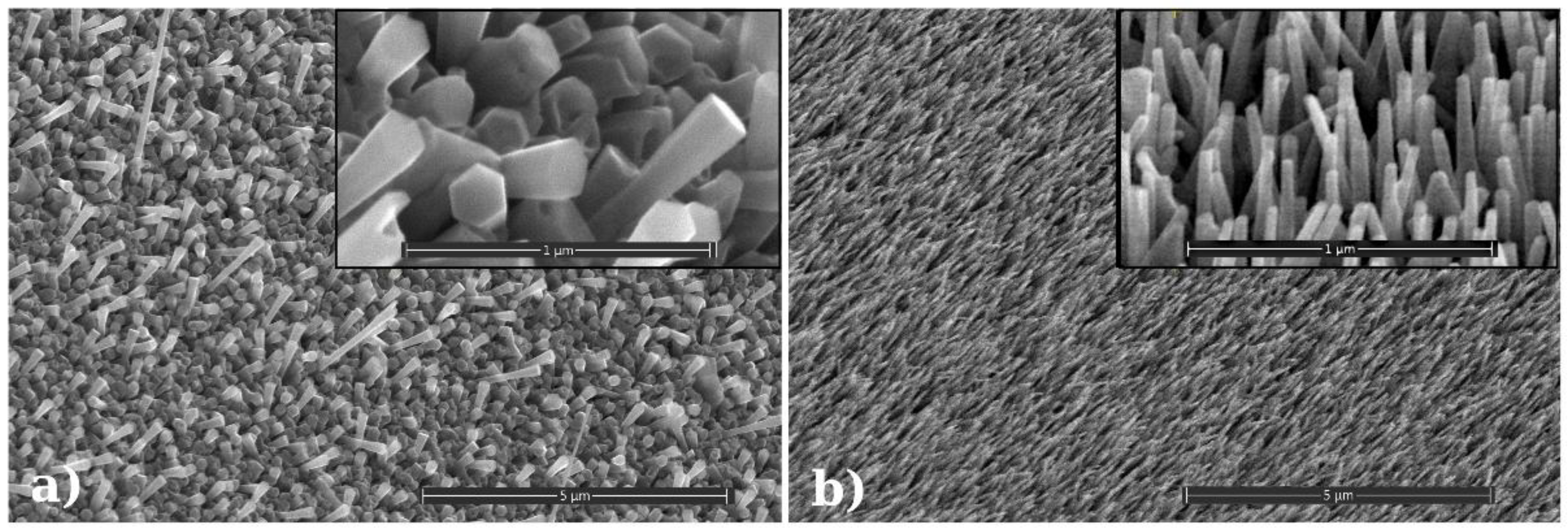
An SEM image of the grown nanowire morphology is presented in
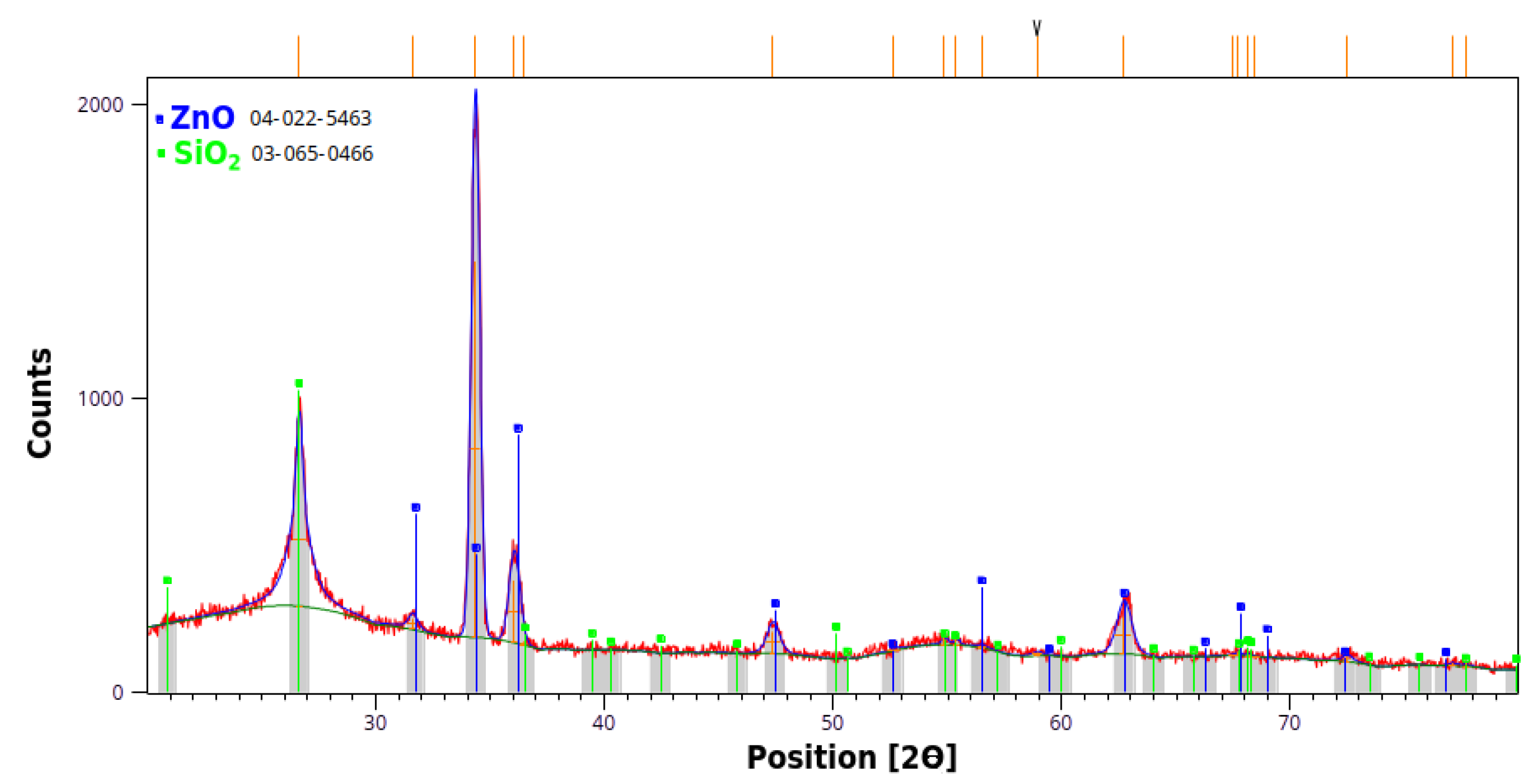
Figure 2, and the inset shows that the grown nanowires have a hexagonal cross-section. An X-ray diffraction investigation of the sample has shown that ZnO grows along the (001) direction, while no other crystalline structure (beside the Quartz substrate) has been noticed here (
Figure 3). Photoluminescence investigations found a peak centered on about 376 nm, slightly below the theoretical one (of ~380 nm), which is suggesting the presence of a limited number of potential structural defects.
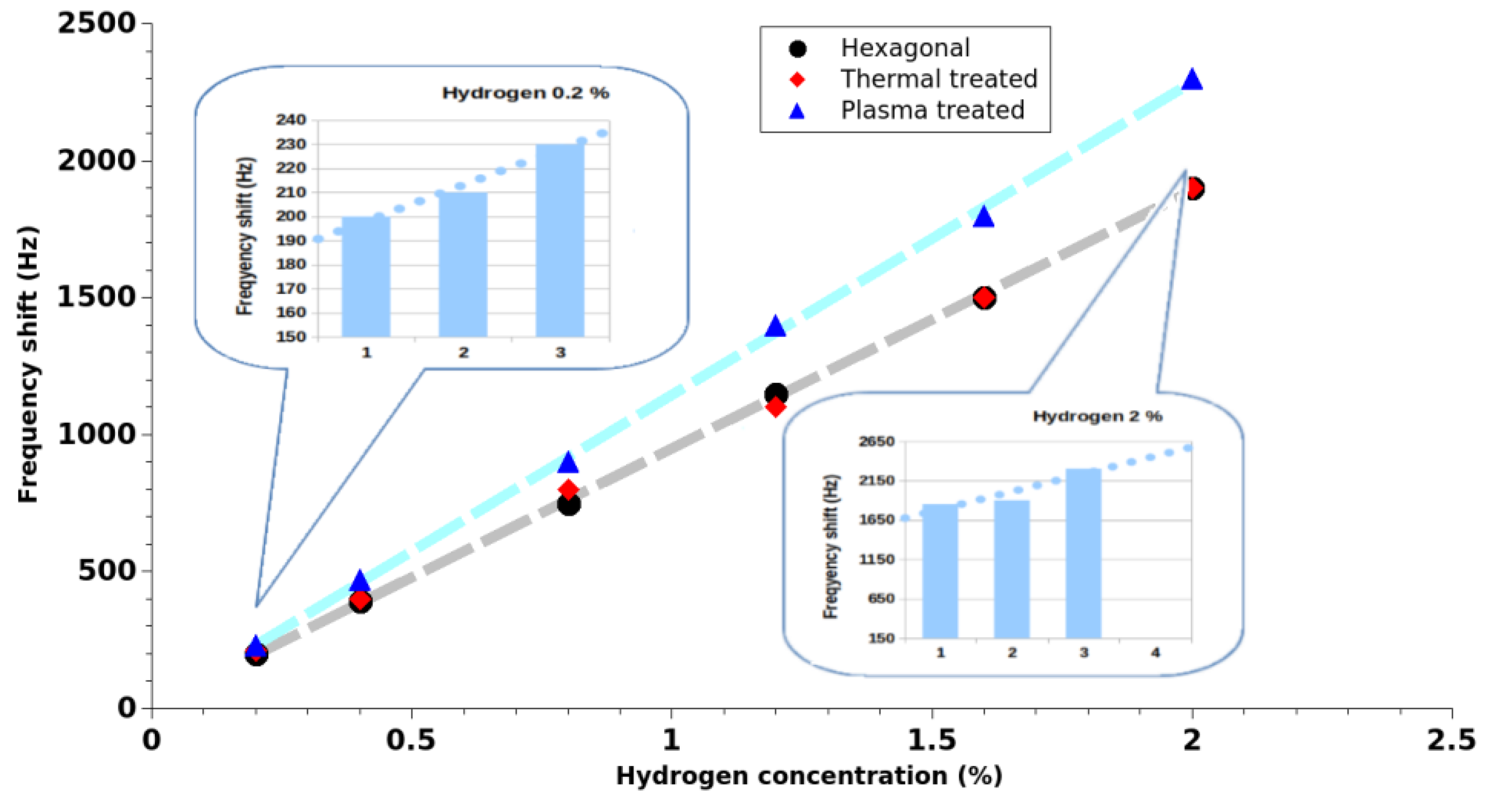
Frequency shift of the sensor with the hydrogen concentration for the three cases are presented in
Figure 4. As it can be seen, while a small improvement of the sensor response (increase of the frequency shift) was obtained after the thermal treatment, a significant improvement (up to 25%) of the sensor response was obtained just after the plasma treatment case. While surface morphology was similar to the original one after both treatments, we focused our investigation on the nanostructure surface treatment-induced changes.
An X-ray Photoelectron Spectroscopy (XPS) investigation on the active area surface after each detection measurement was performed and the results are presented for such a hexagonal nanowire morphology in
Figure 5. The peaks in the survey spectrum correspond to four chemical elements: Zn, O, N, and C. While the Zn and O are the structure elements, C corresponds to contamination from the air and plasma treatment; the presence of nitrogen only on the plasma-treated nanowires can be explained by the reaction between activated nitrogen species and the surface of the nanowires. Hydrogen plasma can activate nitrogen molecules by collisions, leading to their dissociation [
20]. The dissociated nitrogen atoms can then react with the adsorbed carbon species or with the ZnO, where they may occupy oxygen lattice positions [
21], thus forming the C-N and Zn-N bonds found by XPS analysis. Atomic film surface composition is summarized in
Table 1.
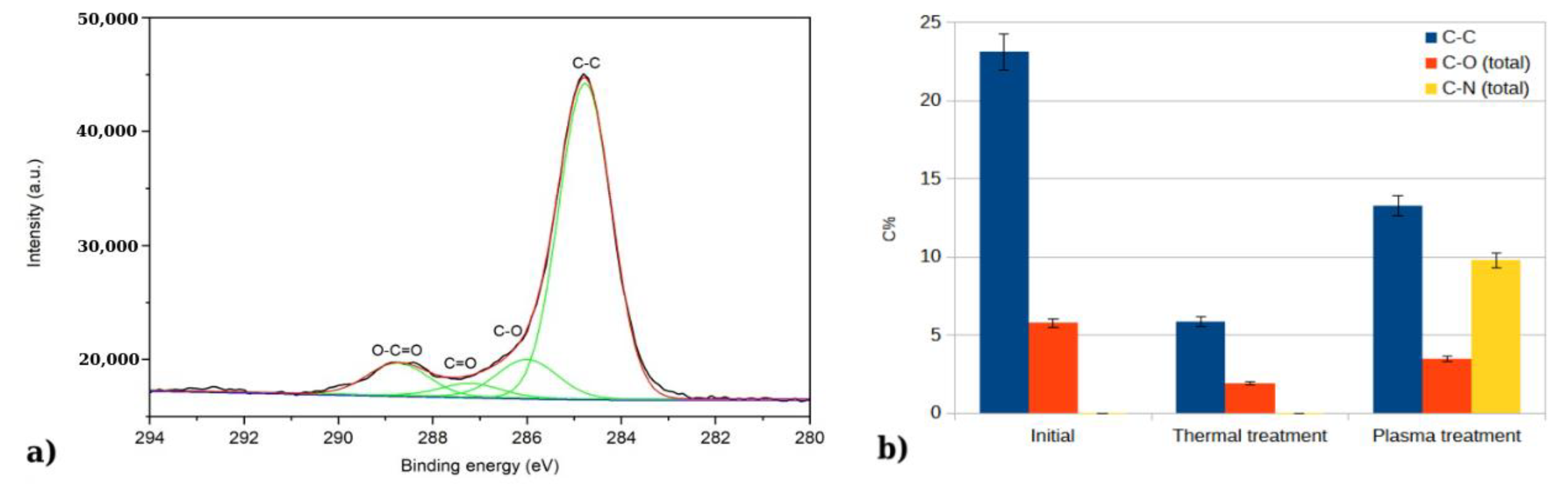
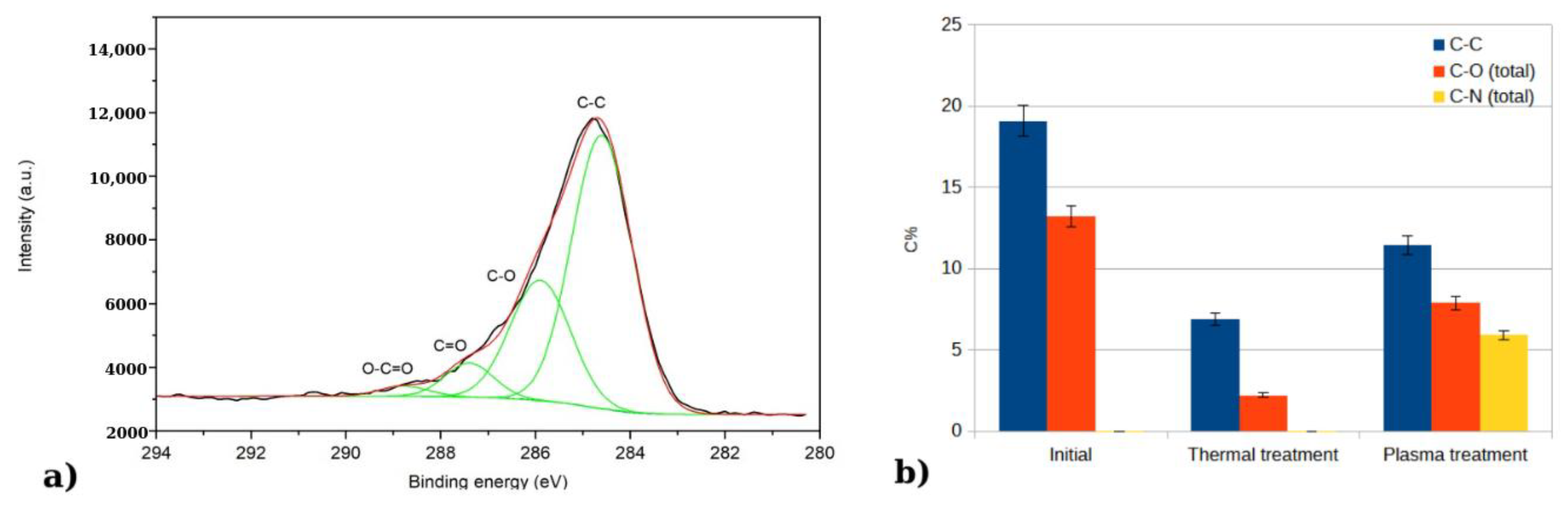
In a more detailed investigation of each element, we could notice that C 1s region could be de-convoluted in four peaks: one located at 284.7 eV and attributed to C in C-C bonds, one at 286 eV attributed to C in C-O bonds, one at 287.2.7 eV attributed to C in C=O bonds, and one at 288.7 eV attributed to C in O-C=O bonds [
22]. In
Figure 6a, the deconvolution is exemplified for the initial sample. After the thermal treatment, a decrease in carbon species is observed from the C 1s spectra, followed by an increase after the plasma treatment. In the case of the plasma-treated sample, the peaks at 286 eV and 287 eV could be attributed to both C-N and C-O bonds, as the values reported in literature for C-N bonds overlap with those for C-O bonds [
9,
23]. Thus, C-N content of the sample was estimated from the N 1s deconvolution, while the C-O content was estimated as the difference between the total C-N + C-O content minus the C-N content, and the evolution of chemical states after each treatment is summarized in
Figure 6b.
The O 1s spectra has been deconvoluted in three peaks: one at 530.1 eV, attributed to lattice oxygen (oxygen bound to Zn) [
24,
25], one at 531.4 eV, attributed to adsorbed oxygen or oxygen species, such as carbon oxides, hydroxides [
21], or lattice oxygen near stoichiometric defects [
26], and one at 532.5 eV, attributed to adsorbed water [
27]. Chemical states for the initial sample are presented in
Figure 7a. Similar processing of the oxygen peak performed after the treatments are showing that after thermal treatment, there is an increase in the total oxygen present on the surface, (
Figure 7b) and the oxygen present as adsorbed species amounts to 54% of the total atomic concentration, compared to the 23% present in the initial sample. Since there is a reduction in carbon content after the thermal treatment, the increase in the adsorbed oxygen species cannot be attributed to carbon oxides, but to an increase in adsorbed oxygen or water vapors. Plasma treatment results in a decrease in adsorbed oxygen species, from 54% to 35% atomic concentration.
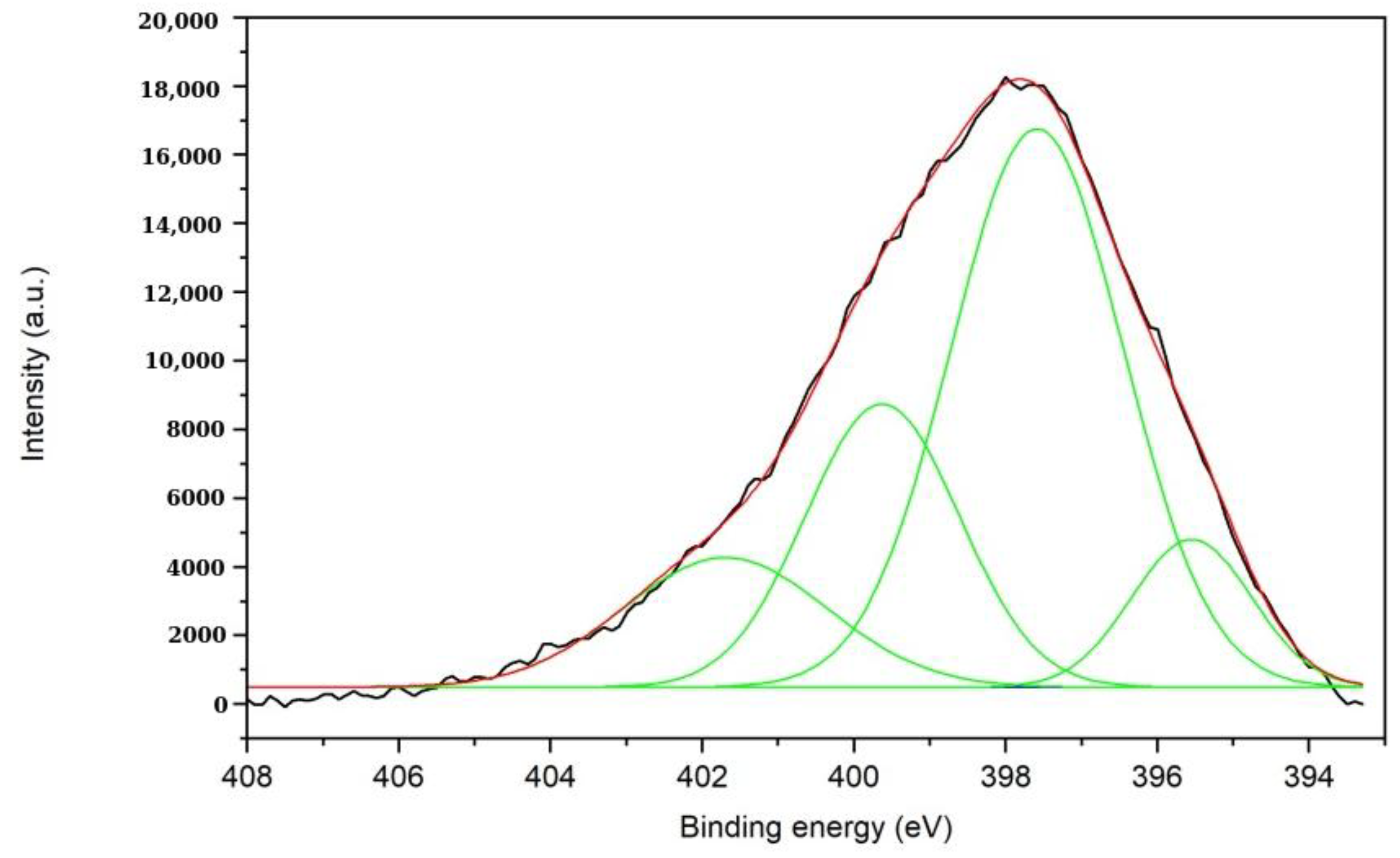
The N 1s region (
Figure 8) is only significantly present in the XPS spectrum of the plasma-treated sample. This region has been fitted with four peaks at 395.9 eV, 397.7 eV and 399.5 eV, and 401 eV corresponding to N in N-Zn bonds [
21,
26], N in pyridinic N-C bonds, N in pyrrolic N-C bonds, and N in graphitic N-C bonds [
6].
In order to understand sensor response to different hydrogen concentrations after each treatment, we must consider hydrogen interaction with the surface contaminants. First, hydrogen adsorption on ZnO must be considered. Hydrogen is adsorbed on ZnO at room temperature through dissociation, forming O-H and Zn-H bonds [
28,
29]. The dissociated hydrogen can then migrate inside the ZnO structure through subsurface diffusion, in interstitial positions or occupying lattice vacancies [
30]. Migration of hydrogen inside the ZnO structure is also supported by the high mobility of intersitital hydrogen [
31]. Therefore, hydrogen can be both adsorbed and absorbed in ZnO. The sum of adsorbed and absorbed hydrogen creates a positive mass difference that gives rise to a frequency shift of the surface acoustic wave detected by the sensor.
Surface contaminants can cause changes and can even impede the adsorption of hydrogen on ZnO. For example, carbon impurities have a negative influence on hydrogen adsorption since carbon sites are unable to activate the hydrogen molecule towards dissociation on their own [
6]. This is because the hydrogen molecule interacts weakly with C-C bonds [
7]. This adsorption is weaker than the hydrogen sorption on ZnO, since ZnO can dissociate the hydrogen molecule [
32]. This explains the slight increase in frequency shift (correspondent to an increase in hydrogen sorption) after the surface carbon desorption caused by the thermal treatment. When it comes to nitrogen, it has been shown [
6,
8,
10] that the presence of nitrogen in different carbon materials actually enhances hydrogen sorption by lowering the dissociation energy of the hydrogen molecule [
6,
11]. XPS confirmed the presence of such nitrogen atoms (pyrrolic, pyridinic, and graphitic nitrogen) on the surface of our sensors. Hydrogen molecules activated at the nitrogen sites can further be transferred to the neighboring carbon sites [
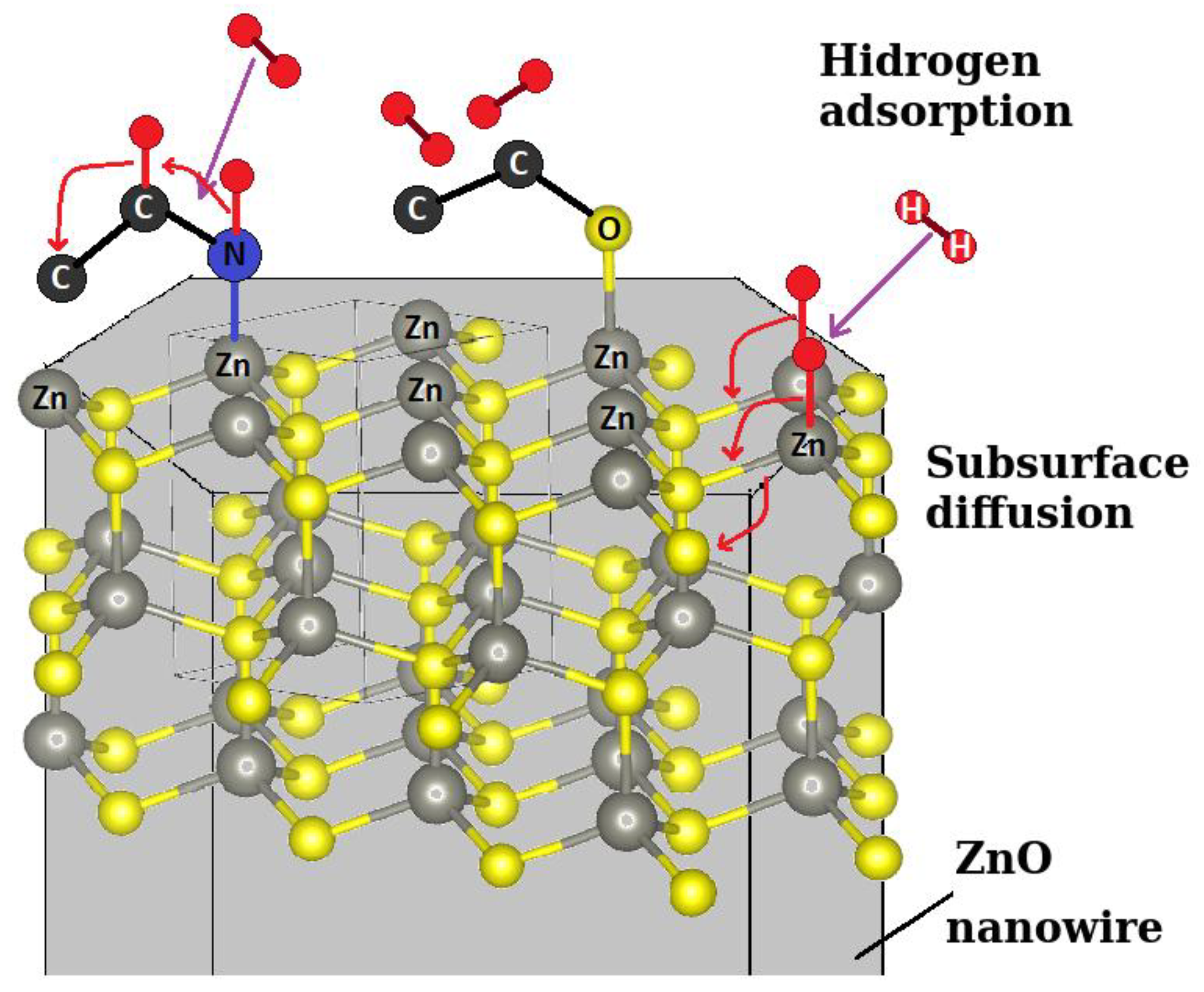
6]. A general scheme of the adsorption and migration processes of hydrogen on the ZnO surface is presented in
Figure 9. The presence of surface nitrogen has a clearly positive impact on the sensitivity of our SAW sensor, as it can be seen in the case of the plasma-treated sensor (
Figure 4) with a 12.91% surface nitrogen content (
Table 1).
Now, the question is whether or not we can distinguish between contaminant contribution within analyte adsorption and the absorption process. In order to try to answer this question, by using the same nanowire growing techniques and same SAW sensor-based (relative) evaluation of the hydrogen mass, we have grown a slightly different ZnO nanowire morphology, with a smaller diameter and, respectively, a circular cross-section, in order to compare the contaminant influence on a different surface-to-volume ratio active area surface.
An SEM image of the SAW sensor active layer morphology for a circular morphology is presented in
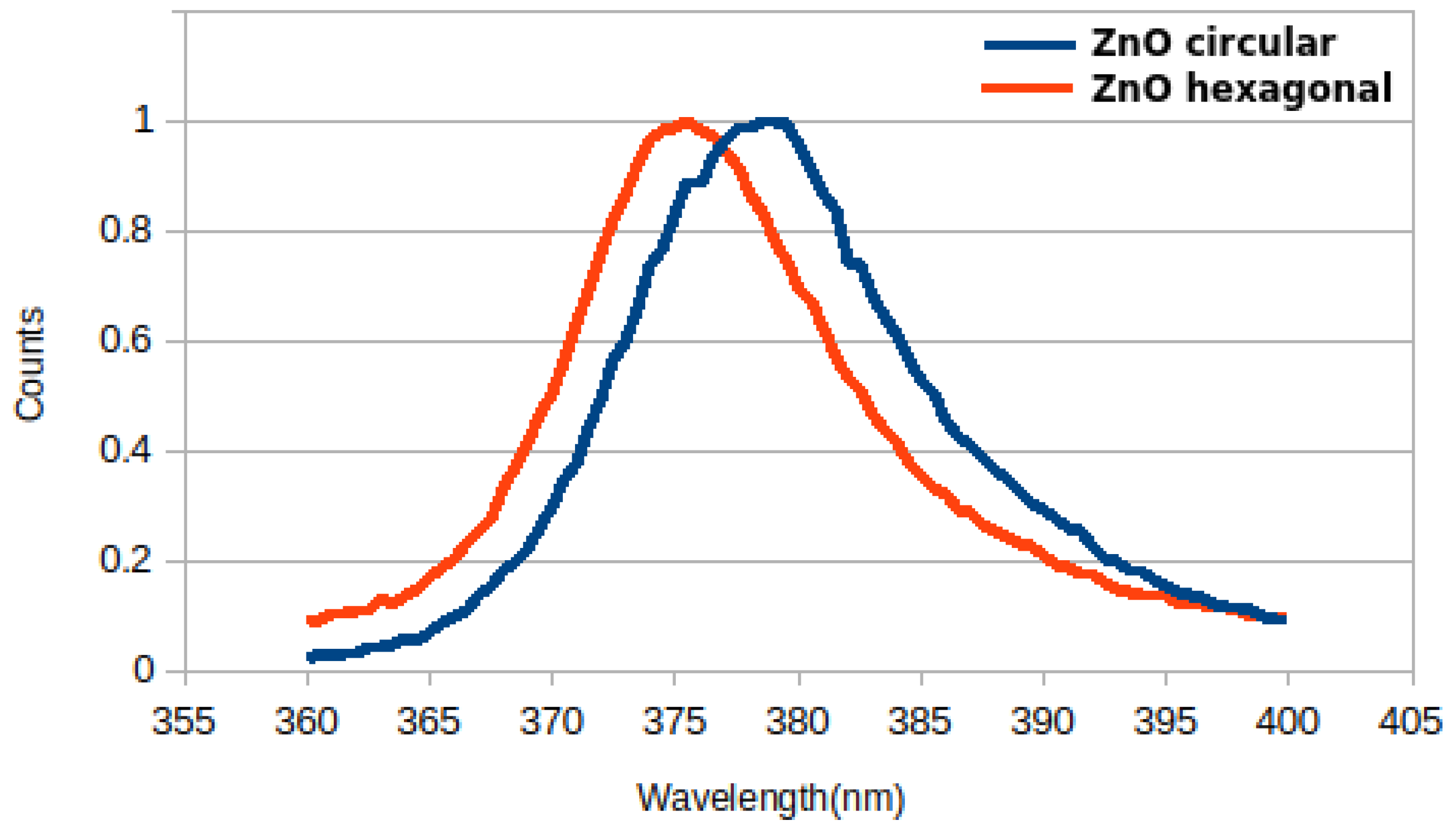
Figure 2b. As one could see, its diameter (between 30 and 60 nm, with an average of 47 nm) is about 6–7 times smaller than the hexagonal ZnO nanowire diameter (between 175 and 236 nm, with an average of 209 nm). Luminescence comparative investigations of both circular and hexagonal structures were also performed using an UV excitation source; a luminescence peak rather identical with the theoretical one (~380 nm—
Figure 10 suggests a ‘perfect’ ZnO crystal structure of the circular nanowires, while the hexagonal nanowires had their peak slightly deviated from the theoretical one, showing the presence of some structural defects.
Response of the circular ZnO nanowires SAW sensor to different hydrogen concentration after each treatment step (performed in similar conditions with the previous hexagonal sample) is presented in
Figure 11. It could be remarked here that sensor response has a clear saturation trend at least for initial and thermally treated cases, similar with previous [
4,
7] reports. Corroborating the significant increase in nanostructure surface to volume ratio and the increase in the circular nanowire crystallinity, we could roughly consider that the hydrogen sorption process in this case is mostly an adsorption process [
4], explaining the saturation trend at relatively low hydrogen concentrations. We have performed XPS surface investigations in a similar way as for the hexagonal nanowire case while focusing our investigations on the carbon and nitrogen states variations after each treatment step.
The C 1s region of the ZnO circular nanowire sample presents a similar four peaks structure after deconvolution and it has been assigned the same way as for the hexagonal nanowires. The deconvolution for the untreated sample is presented in
Figure 12a. Similarly to the hexagonal nanowires, carbon content decreases on the circular nanowires after thermal treatment, and increases after plasma treatment, as shown by the data obtained through deconvolution in
Figure 12b.
The N 1s region is again significant only in the case of the plasma-treated nanowires, and it has been deconvoluted (
Figure 13) into the same four peaks found for the plasma-treated hexagonal nanowires.
Elemental concentration variation of the circular ZnO nanowire sample after each treatment is summarized in
Table 2. It could be noticed that, after each processing step, circular nanowires have a similar carbon contamination concentration with the hexagonal nanowire. This should correspond to similar carbon desorption (in thermal treatment) and adsorbtion (in plasma treatment) of the two-nanowire morphology. However, a double nitrogen concentration after the plasma treatment has been obtained on the circular nanowire morphology in comparison with the hexagonal one.
4. Discussion
By comparing the sensor response for the two morphologies, we should start from the fact that the response of the circular nanowire SAW sensors tends to saturate to rather low hydrogen concentrations (
Figure 11), while the hexagonal morphology does not (
Figure 4). The hexagonal morphology nanowires have a considerably lower surface to volume ratio change (estimated to about 3000 times) and their structure has a higher potential number of structural defects compared to the circular ones. The higher response of the hexagonal nanowires sensor to hydrogen, along with their larger volume and larger number of structural defects, supports the presence of a hydrogen absorption process into the ZnO structure. Thus, if we approximate hydrogen sorption process of the circular nanowires as an adsorption process, then in the hexagonal case we will have to consider a combination of ‘adsorption’ and ‘absorption’ processes.
The second remark while comparing the sensor responses after different treatments is the fact that carbon presence has a comparable influence for both morphologies: Thus, a 21–23% carbon increase will generate a 3–5% increase of the sorbed hydrogen mass, regardless of the ZnO nanowire morphology section: circular or hexagonal. However, in the case of nitrogen, the influence is different; if for the hexagonal case a 12% increase will produce a 15–25% increase in the hydrogen mass sorption for the circular morphology case, a 24% increase of the nitrogen percent increase will produce just a 11–16% increase in the sorbed hydrogen mass. While approximating the sorption process of the ZnO circular nanowire as adsorption and approximating that the increase of the nitrogen sites will quasi-proportionally increase hydrogen molecules’ splitting probability and consequently the surface adsorption process, we could speculate that from the hexagonal nanowire 15–25% increase of the hydrogen sorbed mass, only about 5–7% will represent the enhancement of the adsorption process while the rest of 6–8% will represent an increase of the absorption process. This idea is supported on one side by the known catalytic effect of nitrogen and the higher potential number of absorption sites considered for the hexagonal structure in comparison with the circular one, and on the other hand is supported by the ‘more linear’ hexagonal nanowire sensor response to high hydrogen concentrations, behavior associated with the absorption process of the bulk (thin film) ZnO materials [
4]. Thus, nitrogen catalytic effect might actually be a way of extending the linear response of the ZnO nanowire-based SAW sensor response to higher concentrations of hydrogen and, respectively, the usability of ZnO nanowire-based sensors for a wider analyte concentration range.
The influence of nitrogen and carbon surface contamination on the sensing properties of ZnO-based hydrogen sensors has been widely explored, with previous reports providing insights into both beneficial and detrimental impacts of these contaminants. For instance, controlled nitrogen doping in ZnO was demonstrated to significantly enhance hydrogen detection capabilities due to increased chemical adsorption of hydrogen molecules [
33]. Conversely, carbon contamination, frequently encountered as an uncontrolled impurity, generally adsorbs onto the ZnO surface, interfering negatively by reducing the availability of active sites essential for hydrogen sensing [
21,
34]. Our experimental findings align with these previously reported phenomena and extend the current understanding by highlighting that simultaneous nitrogen and carbon contamination positively affects the hydrogen adsorption and chemisorption processes on ZnO nanowires, leading to increased sensitivity. These results corroborate findings by Zhang et al. [
21], which indicated that nitrogen atoms bonded with carbon contaminants combine their effectiveness as active acceptor sites. Thus, controlling the surface purity of ZnO nanostructures is critical for optimizing their performance in hydrogen sensing applications.