Differentiation of Species and Provenance of Palm-Leaf Manuscripts Using Fourier Transform Infrared Spectroscopy and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Manuscript Samples
2.2. Preparation of Oil-Treated Palm-Leaf Samples
2.3. DRIFTS Analysis
2.4. Chemometric Analysis
3. Results and Discussion
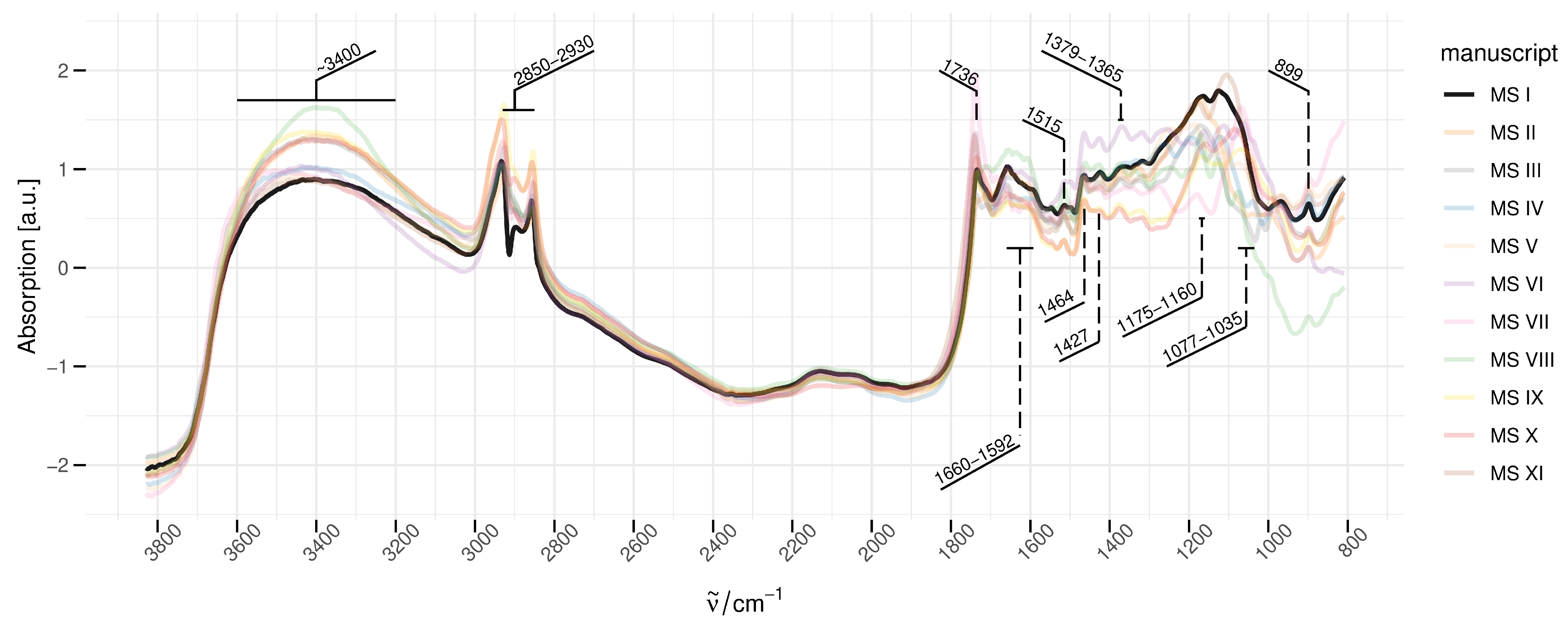
3.1. DRIFTS Spectra
3.2. Evaluation of DRIFTS as Fingerprinting Method for Palm-Leaf Manuscripts
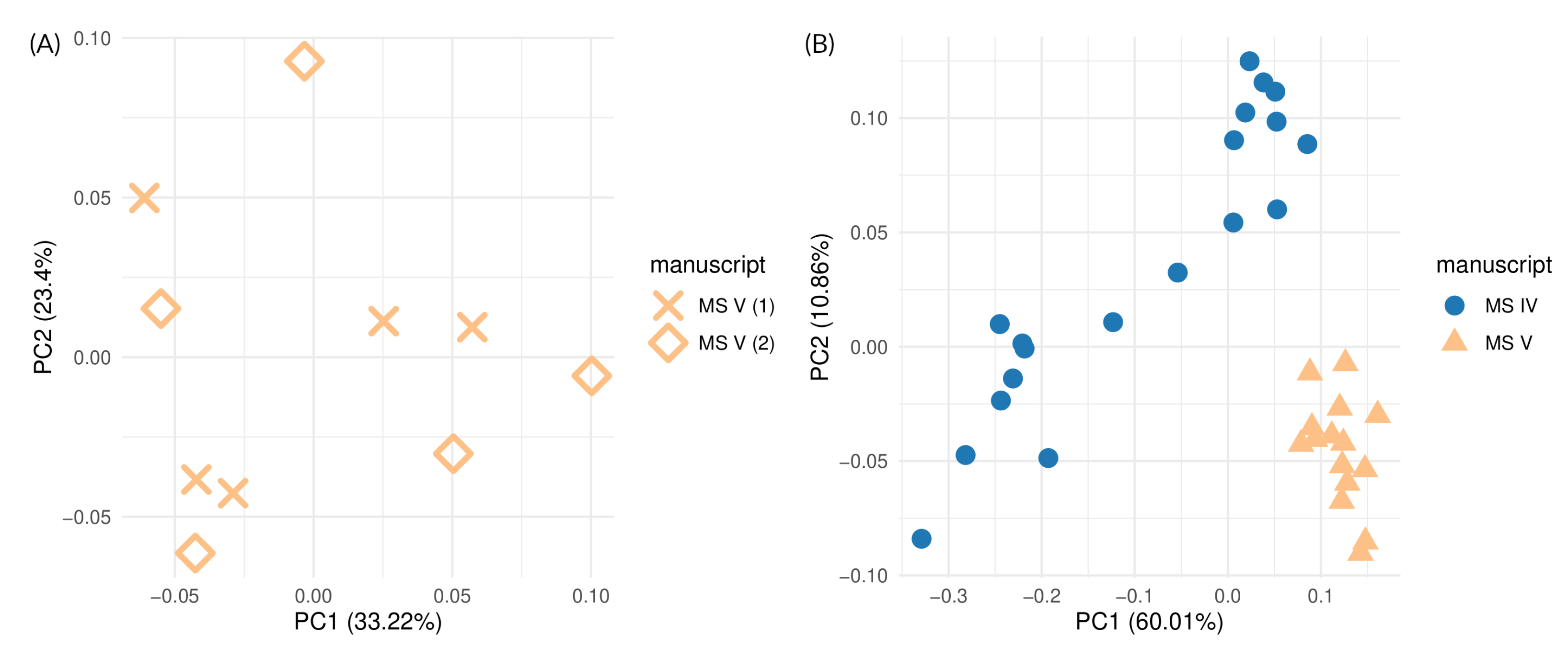
3.2.1. Reproducibility of Manuscript Analysis
3.2.2. Analysis of Different Locations on the Manuscript
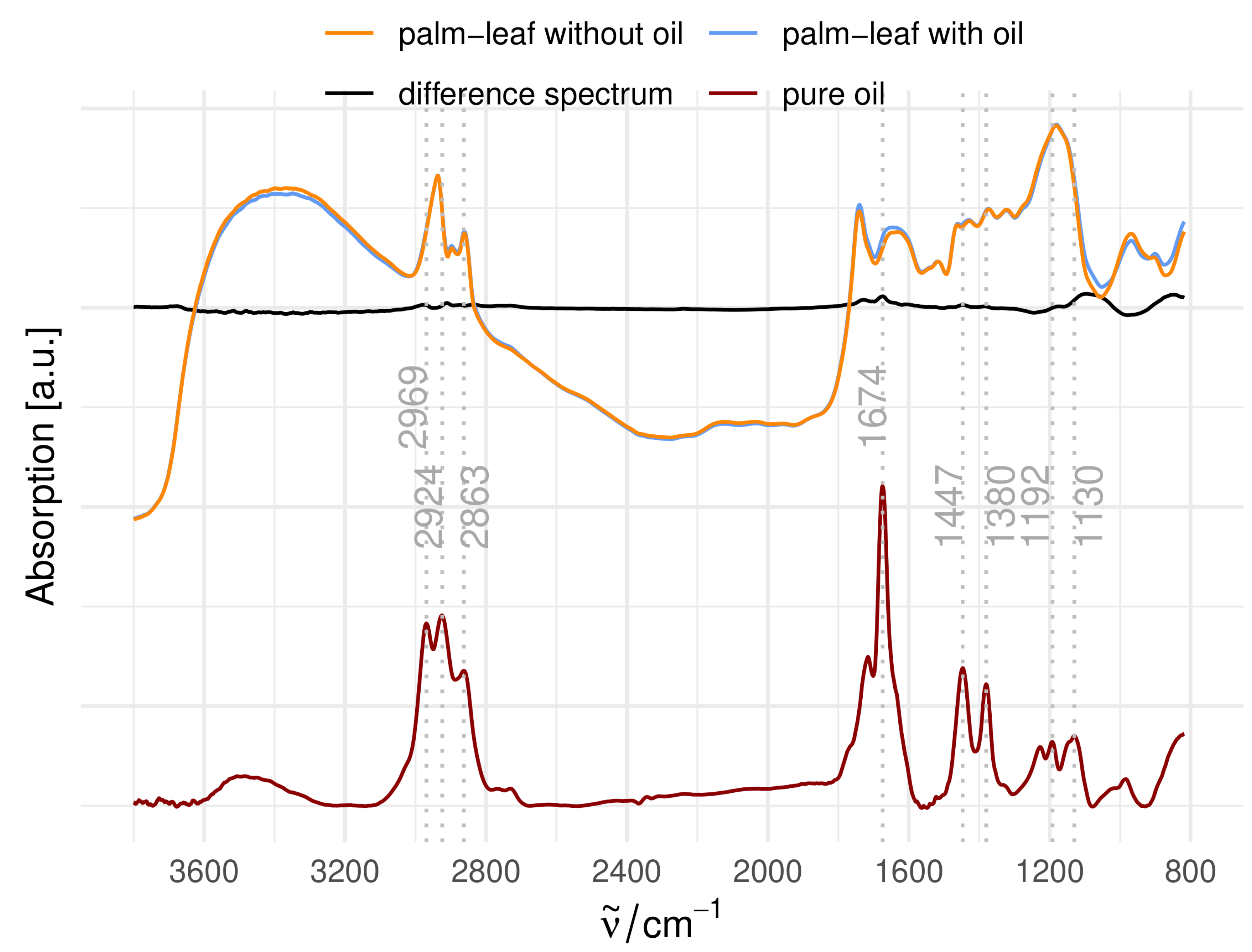
3.2.3. Influence of Oil
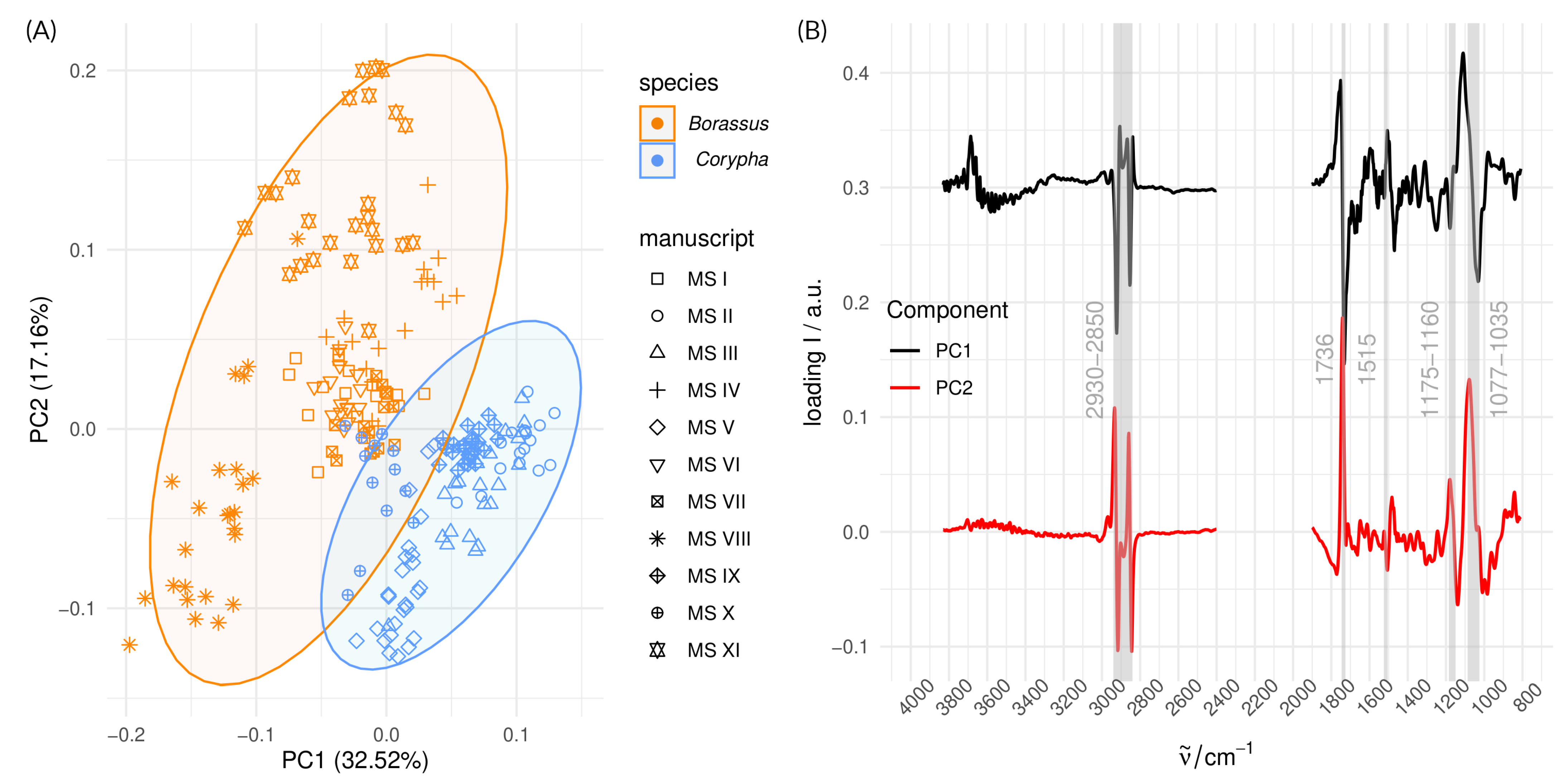
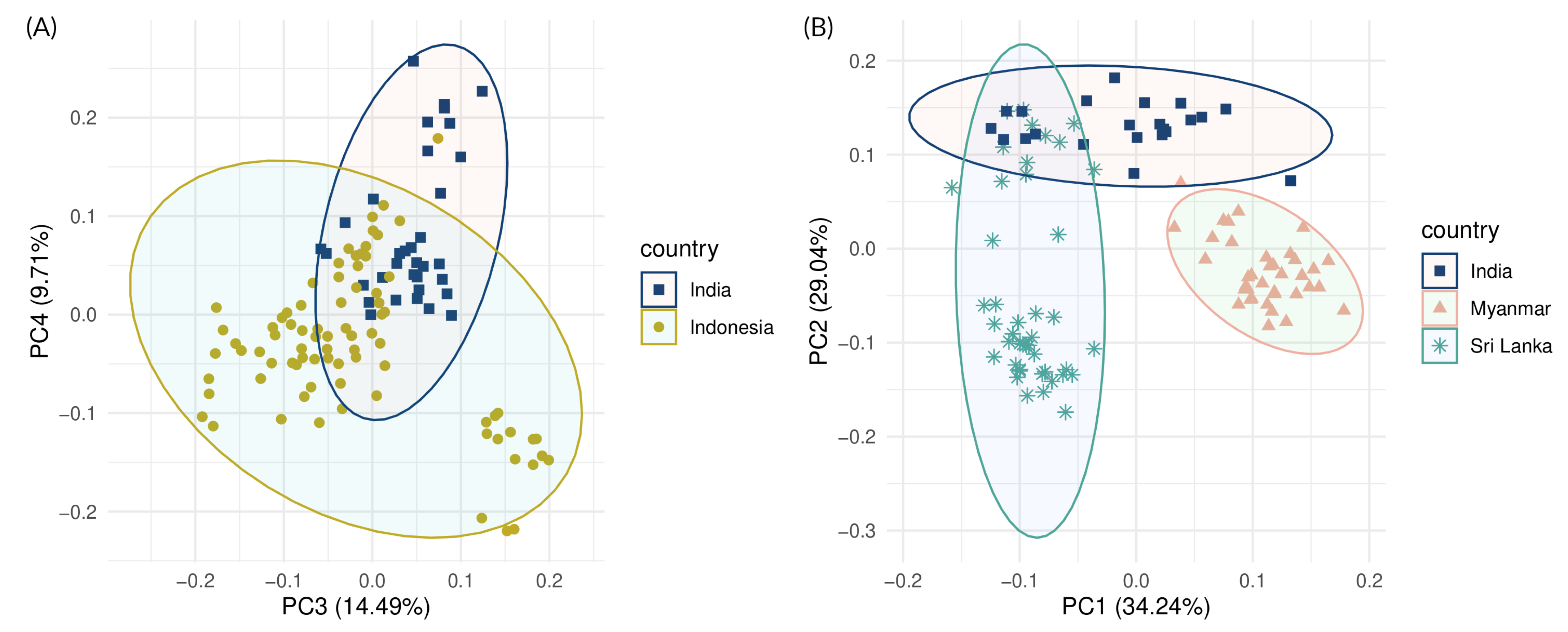
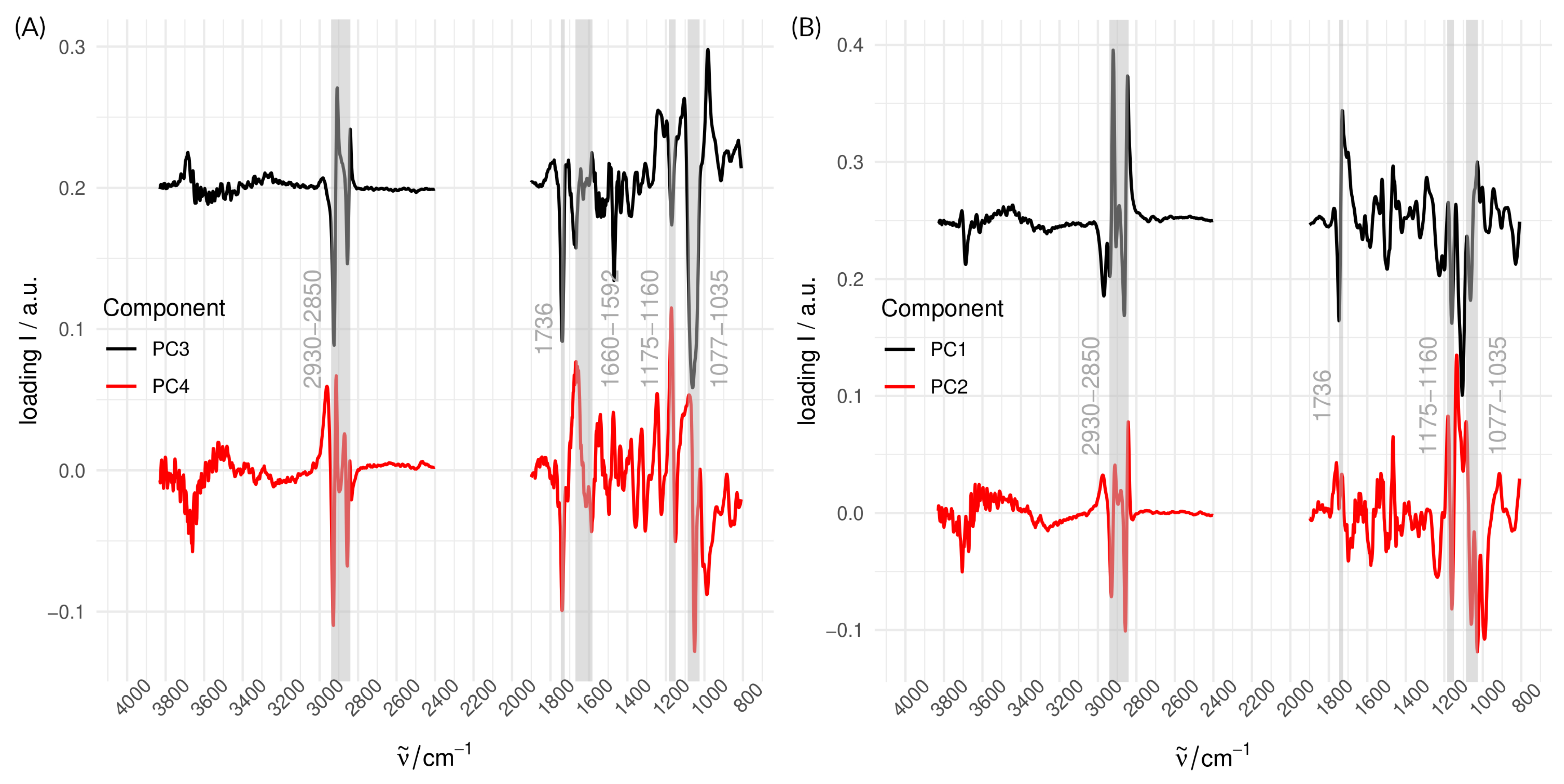
3.3. Analysis of Species and Provenance
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated Total Reflectance Spectroscopy |
| CE | Common Era |
| CSMC | Centre for the Study of Manuscript Cultures |
| DRIFTS | Diffuse Reflectance Infrared Fourier Transform Spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| MS | Manuscript |
| PCA | Principal Component Analysis |
| SNV | Standard Normal Variate |
References
- Ciotti, G. Notes for an Ontological Approach within Manuscript Studies: Object Oriented Ontology and the Pothi Manuscript Culture. In Exploring Written Artefacts: Objects, Methods, and Concepts; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; pp. 865–888. [Google Scholar] [CrossRef]
- Agrawal, O.P. Conservation of Manuscripts and Paintings of South-East Asia; Butterworths Series in Conservation and Museology; Butterworths: London, UK; Boston, MA, USA, 1984. [Google Scholar]
- Freeman, R. Turning Over Old Leaves: Palm Leaves Used in South Asian Manuscripts. Book Pap. Group Annu. 2005, 24, 99–102. [Google Scholar]
- Sharma, D.; Singh, M.R.; Dighe, B. Chromatographic Study on Traditional Natural Preservatives Used for Palm Leaf Manuscripts in India. Restaurator. Int. J. Preserv. Library Arch. Mater. 2018, 39, 249–264. [Google Scholar] [CrossRef]
- Liu, G.L.; Kazarian, S.G. Recent Advances and Applications to Cultural Heritage Using ATR-FTIR Spectroscopy and ATR-FTIR Spectroscopic Imaging. Analyst 2022, 147, 1777–1797. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Sulé-Suso, J.; Millett, J.; Roach, P. Fourier Transform Infrared Spectroscopy as a Non-Destructive Method for Analysing Herbarium Specimens. Biol. Lett. 2023, 19, 20220546. [Google Scholar] [CrossRef] [PubMed]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy-Thread and Single Fibre Analysis by Attenuated Total Reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR Spectroscopy as a Viable Option for Textile Fiber Identification. Herit. Sci. 2019, 7, 93. [Google Scholar] [CrossRef]
- Manfredi, M.; Barberis, E.; Rava, A.; Robotti, E.; Gosetti, F.; Marengo, E. Portable Diffuse Reflectance Infrared Fourier Transform (DRIFT) Technique for the Non-Invasive Identification of Canvas Ground: IR Spectra Reference Collection. Anal. Methods 2015, 7, 2313–2322. [Google Scholar] [CrossRef]
- Manfredi, M.; Barberis, E.; Aceto, M.; Marengo, E. Non-Invasive Characterization of Colorants by Portable Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopy and Chemometrics. Spectrochim. Acta Part A Mol. Biomol. 2017, 181, 171–179. [Google Scholar] [CrossRef]
- Grzelec, M. Application of Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy—(ATR-FTIR) and Principal Component Analysis (PCA) in Identification of Copying Pencils on Different Paper Substrates. Herit. Sci. 2024, 12, 269. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, M.; Song, X. Analysis of Two Different Inks and Application Techniques on Palm Leaf Manuscripts Through Non-Invasive Analysis. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 2024, 45, 237–256. [Google Scholar] [CrossRef]
- Morais, C.L.M.; Lima, K.M.G.; Singh, M.; Martin, F.L. Tutorial: Multivariate Classification for Vibrational Spectroscopy in Biological Samples. Nat. Protoc. 2020, 15, 2143–2162. [Google Scholar] [CrossRef] [PubMed]
- Kokot, S.; Gilbert, C. Application of Drift Spectroscopy and Chemometrics to the Discrimination of Dye Mixtures Extracted from Fibres from Worn Clothing. Analyst 1994, 119, 671–676. [Google Scholar] [CrossRef]
- Workman, J. A Review of the Latest Research Applications Using FT-IR Spectroscopy. Adv. Infrared Spectrosc. Today’s Spectrosc. 2024, 39, 22–28. [Google Scholar] [CrossRef]
- Xu, W.; Xia, J.; Min, S.; Xiong, Y. Fourier Transform Infrared Spectroscopy and Chemometrics for the Discrimination of Animal Fur Types. Spectrochim. Acta Part A Mol. Biomol. 2022, 274, 121034. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Beleites, C.; Bonifacio, A.; Dahms, M.; Egert, B.; Fuller, S.; Gegzna, V.; Guliev, R.; Hanson, B.A.; Hermes, M.; Kammer, M.; et al. hyperSpec: A Package to Handle Hyperspectral Data Sets in R. 2024. Available online: https://r-hyperspec.github.io/hyperSpec (accessed on 23 May 2025).
- Stevens, A.; Ramirez-Lopez, L. An Introduction to the Prospectr Package. R package Vignette. 2024. Available online: https://antoinestevens.github.io/prospectr/ (accessed on 23 May 2025).
- Reddy, K.O.; Guduri, B.R.; Rajulu, A.V. Structural Characterization and Tensile Properties of Borassus Fruit Fibers. J. Appl. Polym. Sci. 2009, 114, 603–611. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, M.; Cheng, L.; Li, X. Investigations on the Structure and Properties of Palm Leaf Sheath Fiber. Cellulose 2015, 22, 1039–1051. [Google Scholar] [CrossRef]
- Bouramdane, Y.; Fellak, S.; El Mansouri, F.; Boukir, A. Impact of Natural Degradation on the Aged Lignocellulose Fibers of Moroccan Cedar Softwood: Structural Elucidation by Infrared Spectroscopy (ATR-FTIR) and X-ray Diffraction (XRD). Fermentation 2022, 8, 698. [Google Scholar] [CrossRef]
- Chu, S.; Lin, L.; Tian, X. Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma. Forests 2023, 14, 1775. [Google Scholar] [CrossRef]
- Pandey, K.K. A Study of Chemical Structure of Soft and Hardwood and Wood Polymers by FTIR Spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Reddy, K.O.; Shukla, M.; Uma Maheswari, C.; Varada Rajulu, A. Mechanical and Physical Characterization of Sodium Hydroxide Treated Borassus Fruit Fibers. J. For. Res. 2012, 23, 667–674. [Google Scholar] [CrossRef]
- Spectral Database for Organic Compounds (SDBS). IR Spectrum. SDBS No.: 6790, RN 5392-40-5. Available online: https://sdbs.db.aist.go.jp/CompoundLanding.aspx?sdbsno=6790 (accessed on 11 March 2025).

| Manuscript | Object No. | Species | Country | Region | Meas. (No. Pages) |
|---|---|---|---|---|---|
| MS I | CSMC, 1/2014 | Borassus flabellifer | Indonesia | Bali | 16 (4) |
| MS II | CSMC, 1/2017 | Corypha umbraculifera | Sri Lanka | unknown | 18 (5) |
| MS III | CSMC, 1/2018 | Corypha umbraculifera | India | Kerala | 22 (5) |
| MS IV | Private Collection, MS Tamil 1 | Borassus flabellifer | India | Tamil Nadu | 19 (5) |
| MS V | CSMC, T 12/2021 | Borassus flabellifer | India | Odisha | 10 (1) ×2 |
| MS VI | CSMC, T 29/2021 | Borassus flabellifer | Indonesia | Bali | 15 (3) |
| MS VII | CSMC, T 32/2021 | Borassus flabellifer | Indonesia | Bali | 25 (5) |
| MS VIII | CSMC, T 33/2021 | Corypha umbraculifera | Sri Lanka | unknown | 25 (5) |
| MS IX | CSMC, T 34-1/2021 | Corypha umbraculifera | Myanmar | unknown | 25 (5) |
| MS X | CSMC, T 34-2/2021 | Corypha umbraculifera | Myanmar | unknown | 15 (3) |
| MS XI | CSMC, T add. 6/2021 | Borassus flabellifer | Indonesia | Bali | 25 (5) |
| Wavenumber (cm−1) | Tentative Assignment; Wavenumber, Reference (cm−1) | Method |
|---|---|---|
| ∼3400 | (OH) cellulosic fibres; 3335 [7] | ATR |
| (OH) cellulose; 3348 [25] | DRIFTS | |
| (OH) acid/methanol; 3600–3000 [21] | FTIR | |
| ∼2930–2850 | (CH2), (CHx) cellulosic fibres; 2850, 2900 [7] | ATR |
| (CHn) alkyl, aliphatic, aromatic; 2860–2970 [21] | FTIR | |
| (CH2) asymmetric, symmetric methylene groups in fats and waxes; 2927, 2855 [24] | ATR | |
| (CH) cellulose, 2902 [25] | DRIFTS | |
| ∼1736 | (C=O) ester cellulosic fibres; 1735 [7] | ATR |
| pectin/hemicellulose; 1735 [24] | ATR | |
| (C=O) hemicellulose/lignin/ferulate; 1738 [23] | ATR | |
| ∼1660–1592 | (O-H) adsorbed water/hydrogen bond hemicellulose-lignin; 1635–1621 [23] | ATR |
| (C=C) aromatic/cellulosic fibres; 1595 [7] | ATR | |
| adsorbed water; 1635 [7] | ATR | |
| conjugated carbonyl group of lignin; 1650 [24] | ATR | |
| ∼1515 | (C=C) aromatic/cellulosic fibres; 1505 [7] | ATR |
| aromatic skeletal vibration/lignin; 1509 [26] | FTIR | |
| ∼1464, 1427 | (CH) cellulose; 1430 [25] | DRIFTS |
| (O-CH3) Methoxyl-O-CH3; 1470–1430 [21] | FTIR | |
| (C-H) cellulosic fibres; 1420, 1455 [7] | ATR | |
| (C-OH) alcohol/cellulosic fibres; 1455 [7] | ATR | |
| (C-H2) scissoring/cellulosic fibres; 1475 [7] | ATR | |
| ∼1379–1365 | (CH) cellulose; 1372 [25] | DRIFTS |
| (C-H) cellulosic fibres; 1365 [7] | ATR | |
| ∼1175–1160 | (C-O-C) cellulose/hemicellulose; 1161 [26] | FTIR |
| (C-O-C) cellulose; 1163 [25] | DRIFTS | |
| (C-O-C) pyranose ring; 1170, 1082 [21] | FTIR | |
| (C-O-C) glycosidic/cellulosic fibres; 1105 [7] | ATR | |
| (C-C) ring breathing/cellulosic fibres; 1155 [7] | ATR | |
| (C-O) triglycerides; 1237 [24] | ATR | |
| ∼1077–1035 | (C-OH) alcohol/cellulosic fibres; 1050, 1025 [7] | ATR |
| (C-O) cellulose; 1059, 1033 [25] | DRIFTS | |
| ,(C-O) C-OH; 1060 [21] | FTIR | |
| (C-O) glycosidic bond of polysaccharides; 1031 [4] | ATR | |
| (C-O) triglycerides; 1086 [24] | ATR | |
| ∼899 | (CH), (glucose ring) cellulose; 897 [25] | DRIFTS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voges, L.F.; Horn, N.; Ciotti, G.; Seifert, S. Differentiation of Species and Provenance of Palm-Leaf Manuscripts Using Fourier Transform Infrared Spectroscopy and Chemometrics. Chemosensors 2025, 13, 196. https://doi.org/10.3390/chemosensors13060196
Voges LF, Horn N, Ciotti G, Seifert S. Differentiation of Species and Provenance of Palm-Leaf Manuscripts Using Fourier Transform Infrared Spectroscopy and Chemometrics. Chemosensors. 2025; 13(6):196. https://doi.org/10.3390/chemosensors13060196
Chicago/Turabian StyleVoges, Lucas F., Nils Horn, Giovanni Ciotti, and Stephan Seifert. 2025. "Differentiation of Species and Provenance of Palm-Leaf Manuscripts Using Fourier Transform Infrared Spectroscopy and Chemometrics" Chemosensors 13, no. 6: 196. https://doi.org/10.3390/chemosensors13060196
APA StyleVoges, L. F., Horn, N., Ciotti, G., & Seifert, S. (2025). Differentiation of Species and Provenance of Palm-Leaf Manuscripts Using Fourier Transform Infrared Spectroscopy and Chemometrics. Chemosensors, 13(6), 196. https://doi.org/10.3390/chemosensors13060196





