Recent Advances on Fluorescent Sensors for Detection of Pathogenic Bacteria
Abstract
1. Introduction
2. Fluorescent Sensing Systems for Bacteria Detection
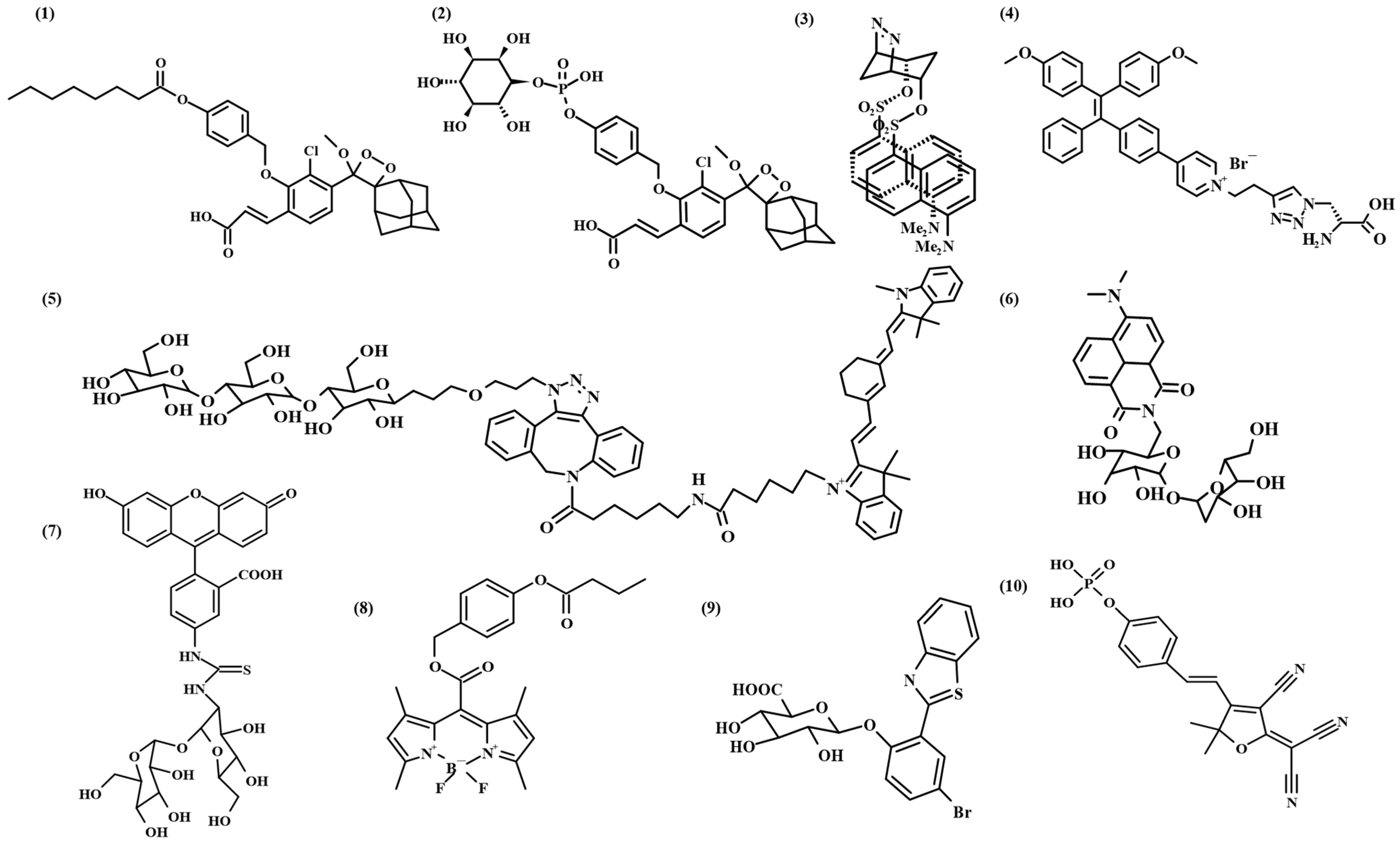
2.1. Small Molecule-Based Fluorescent Probes
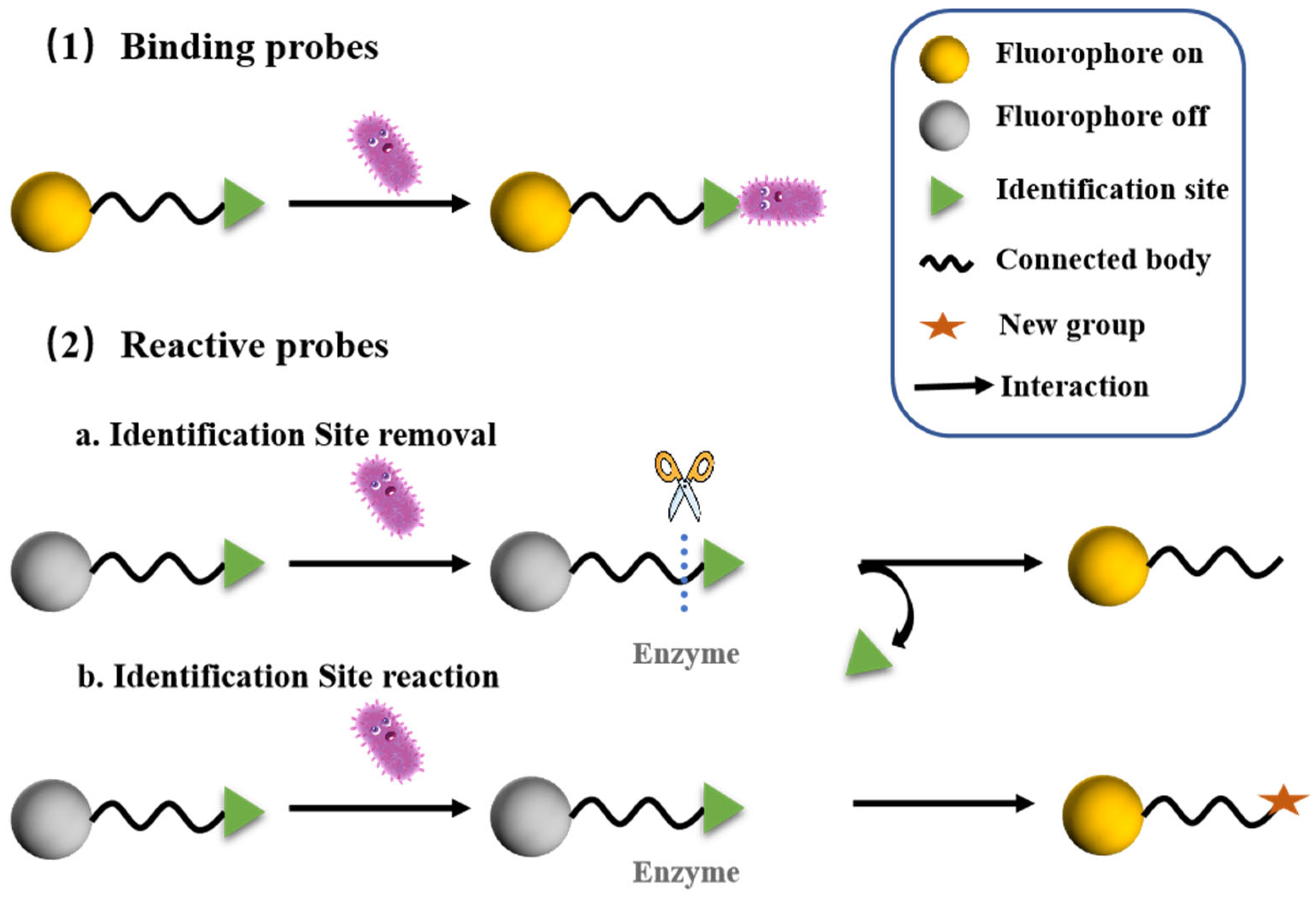
2.1.1. General Concepts
2.1.2. Reactive Probes
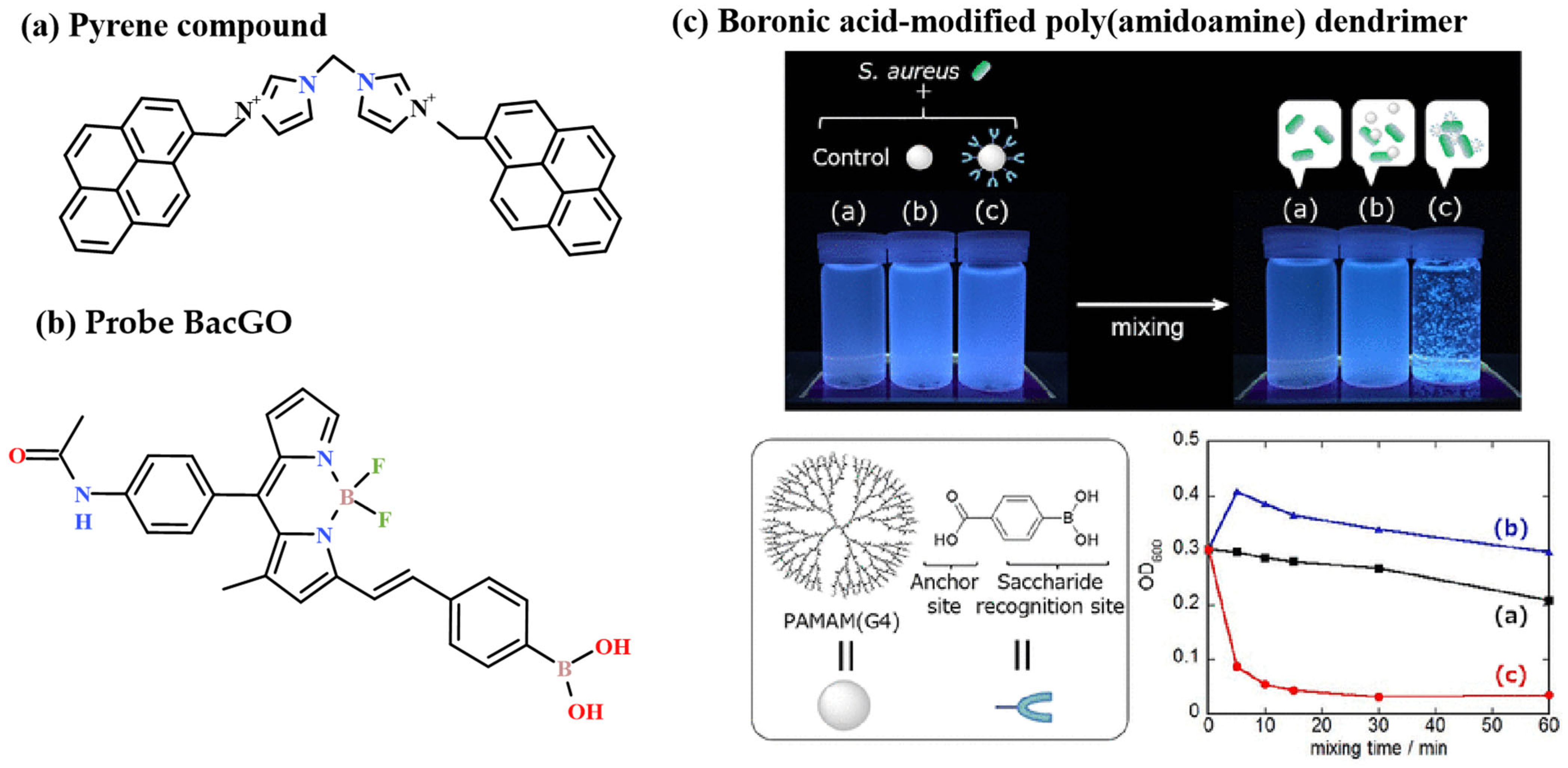
2.1.3. Binding Probes
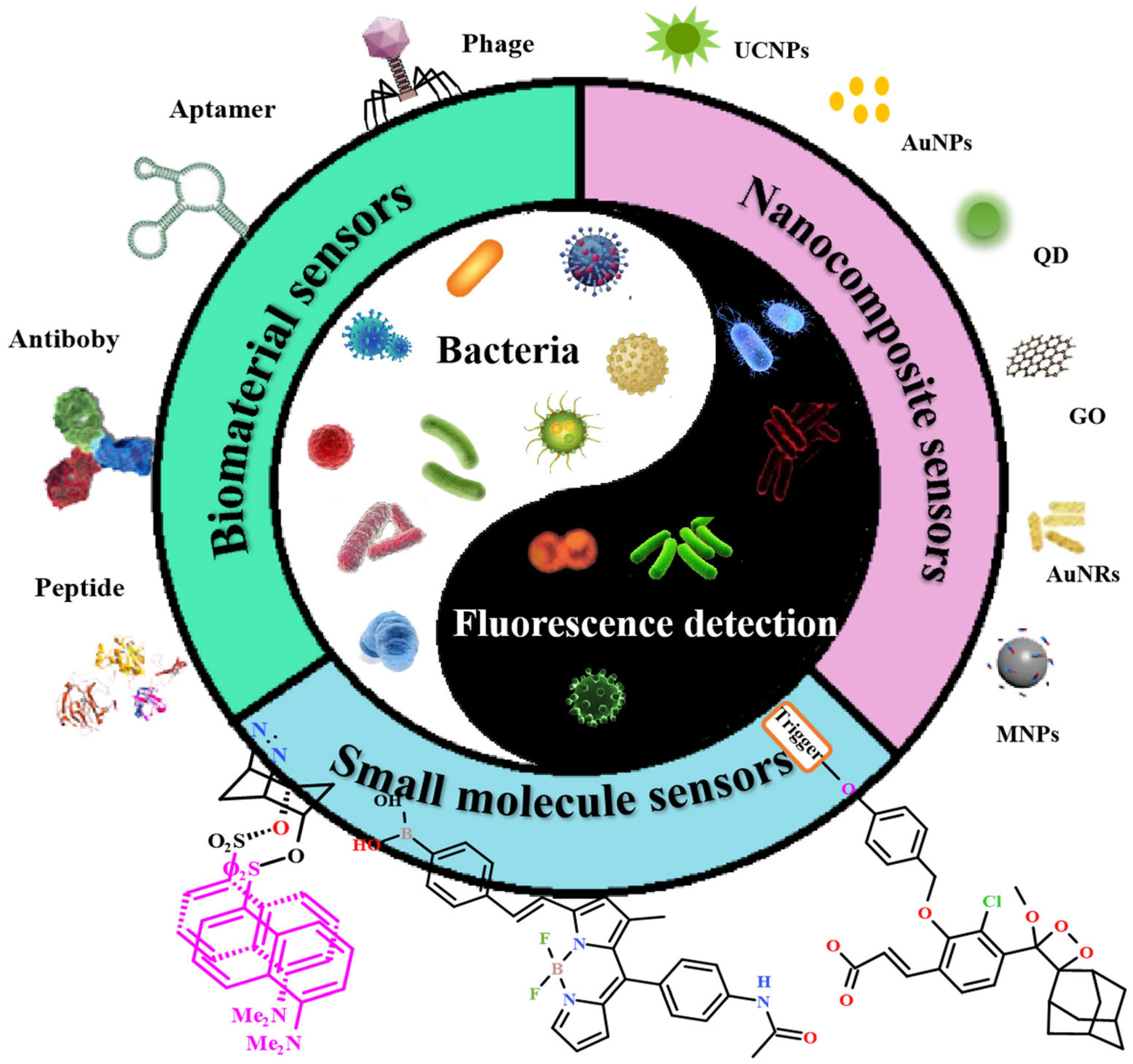
2.2. Biomaterial Sensors
2.2.1. Phage-Based Fluorescence
2.2.2. Aptamer-Based Fluorescence
2.2.3. Peptide-Based Fluorescence
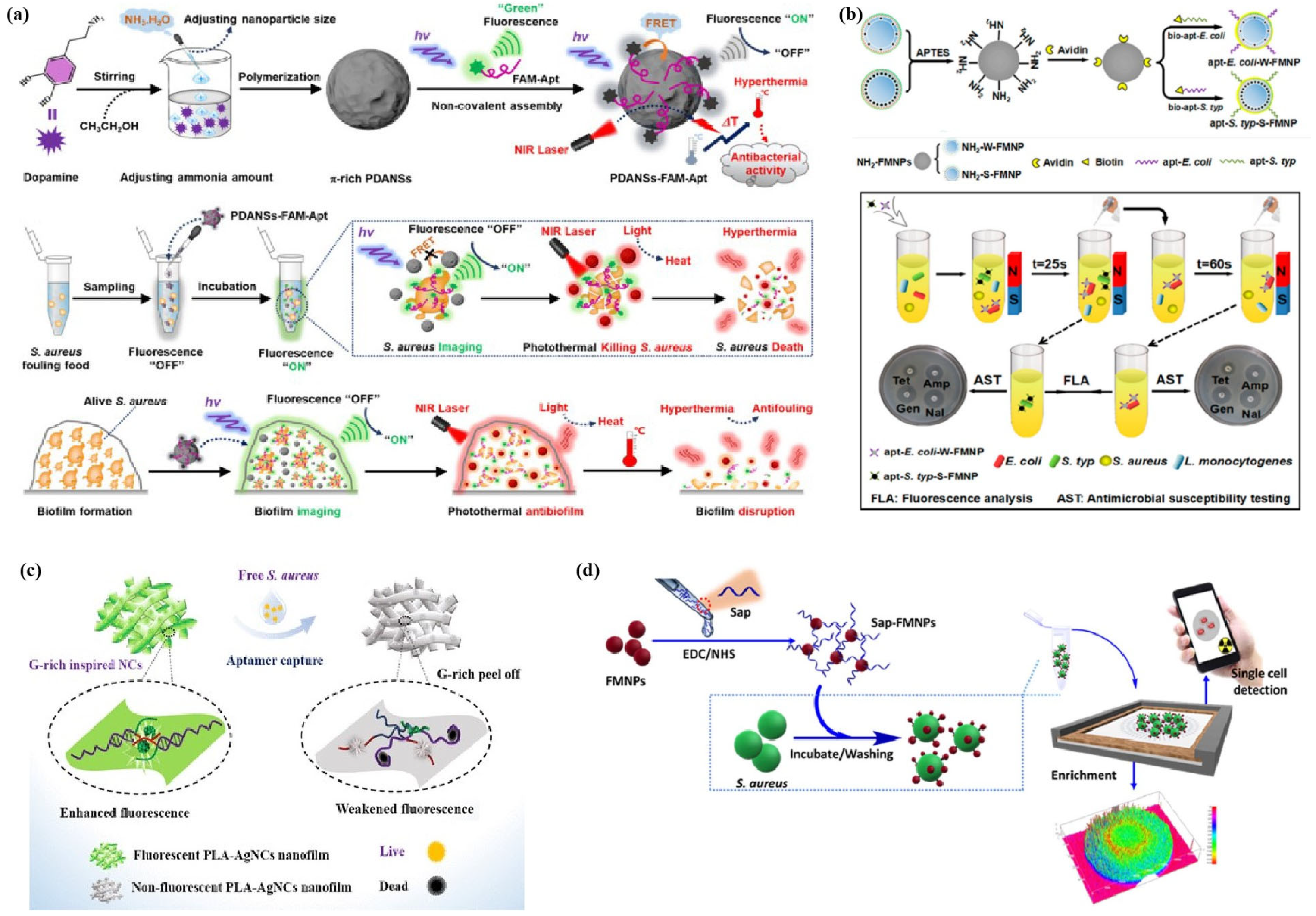
2.3. Nanocomposite Fluorescent Sensing System
2.3.1. UCNP-Based Nanoprobes
2.3.2. QD-Based Nanoprobes
2.3.3. Other Types of Nanoprobes
2.4. Mechanisms of Bacteria Detection
3. Conclusions and Prospects
- Designing high-performance probes that enable specific bacterial recognition while minimizing background interference and improving trace-level detection accuracy.
- Optimizing detection conditions to ensure mild operation, cost-effective preparation and scalability for real-world applications in food safety, clinical diagnostics and environmental monitoring.
Author Contributions
Funding
Conflicts of Interest
References
- Yeni, F.; Yavaş, S.; Alpas, H.; Soyer, Y. Most common foodborne pathogens and mycotoxins on fresh produce: A review of recent outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Martinović, T.; Andjelković, U.; Gajdošik, M.Š.; Rešetar, D.; Josić, D. Foodborne pathogens and their toxins. J. Proteom. 2016, 147, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, Y.; Wu, M.; Ma, F.; Xu, Y.; Deng, B.; Zhang, J.; Zhu, G.; Lu, Y. Role of long polar fimbriae type 1 and 2 in pathogenesis of mammary pathogenic Escherichia coli. J. Dairy Sci. 2021, 104, 8243–8255. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; Tobin, C.; Ross, R.P.; Stanton, C.; Hill, C. Foodborne pathogens and zoonotic diseases. In Raw Milk Balance Between Hazards and Benefits; Academic Press: New York, NY, USA, 2019; pp. 259–272. [Google Scholar]
- Dai, J.; Bai, M.; Li, C.; Cui, H.; Lin, L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020, 105, 211–222. [Google Scholar] [CrossRef]
- Bonah, E.; Huan, X.; Yi, R.; Aheto, J.H.; Osae, R.; Golly, M. Electronic nose classification and differentiation of bacterial foodborne pathogens based on support vector machine optimized with particle swarm optimization algorithm. J. Food Process Eng. 2019, 42, e13236. [Google Scholar] [CrossRef]
- Chen, H.; Kang, L.; Li, K.; Feng, T.; Ni, Z.; Gao, E.; Fang, Z. Accurate quantification on the change of some live pathogenic microbial flora in fermented feed. Int. J. Food Sci. Technol. 2023, 58, 3552–3559. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol. 2019, 57, 1977–1990. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of hyperspectral imaging as a nondestructive technique for foodborne pathogen detection and characterization. Foodborne Pathog. Dis. 2019, 16, 712–722. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Yang, H.; Aheto, J.H.; Ren, Y.; Yu, S.; Tu, H. Detection of Salmonella Typhimuriumcontamination levels in fresh pork samples using electronic nose smellprints in tandem with support vector machine regression and metaheuristic optimization algorithms. J. Food Sci. Technol. 2020, 58, 3861–3870. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, B.; Wang, J.; Li, B.; He, K.; Zhang, X. Rapid detection of Escherichia coli O157:H7 by loop-mediated isothermal amplification coupled with a lateral flow assay targeting the z3276 genetic marker. Food Anal. Methods 2021, 15, 908–916. [Google Scholar] [CrossRef]
- Long, L.; Cao, S.; Jin, B.; Yuan, X.; Han, Y.; Wang, K. Construction of a novel fluorescent probe for on-site measuring hydrogen sulfide levels in food samples. Food Anal. Methods 2019, 12, 852–858. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman spectroscopy for food quality assurance and safety monitoring: A review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Babadi, A.A.; Sun, C.; Ariffin, N.M.; Khatib, A.; Selamat, J.; Zou, X. Application, challenges and future prospects of recent nondestructive techniques based on the electromagnetic spectrum in food quality and safety. Food Chem. 2024, 441, 138402. [Google Scholar] [CrossRef]
- Gorsuch, J.P.; Jones, Z.; Le Saint, D.; Kitts, C.L. Enumeration of industrial Bacillus assemblages in commercial products with customized plate-counting assays. J. Microbiol. Methods 2019, 164, 105682. [Google Scholar] [CrossRef]
- Van Nevel, S.; Koetzsch, S.; Proctor, C.R.; Besmer, M.D.; Prest, E.I.; Vrouwenvelder, J.S.; Knezevg, A.; Boon, N.; Hammes, F. Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring. Water Res. 2017, 113, 191–206. [Google Scholar] [CrossRef]
- Ou, F.; Mcgoverin, C.; Swift, S.; Vanholsbeeck, F. Bacterial cell enumeration using flow cytometry. In Proceedings of the Asia-pacific Optical Sensors Conference, Shanghai, China, 11–14 October 2016; p. 18. [Google Scholar]
- Chiang, E.L.C.; Lee, S.; Medriano, C.A.; Li, L.; Bae, S. Assessment of physiological responses of bacteria to chlorine and UV disinfection using a plate count method, flow cytometry and viability PCR. J. Appl. Microbiol. 2022, 132, 1788–1801. [Google Scholar] [CrossRef]
- Lee, W.I.; Park, Y.; Shrivastava, S.; Jung, T.; Meeseepong, M.; Lee, J.; Jeon, B.; Yang, S.; Lee, N.E. A fully integrated bacterial pathogen detection system based on count-on-a-cartridge platform for rapid, ultrasensitive, highly accurate and culture-free assay. Biosens. Bioelectron. 2020, 152, 112007. [Google Scholar] [CrossRef]
- Santiago-Felipe, S.; Tortajada-Genaro, L.A.; Puchades, R.; Maquieira, A. Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis. Anal. Chim. Acta 2014, 811, 81–87. [Google Scholar] [CrossRef]
- Umrao, P.D.; Kumar, V.; Kaistha, S.D. Enzyme-Linked Immunosorbent Assay Detection of Bacterial Wilt–Causing Ralstonia Solanacearum. In Experimental Protocols in Biotechnology; Humana: New York, NY, USA, 2020; pp. 1–18. [Google Scholar]
- Akkaya, O.; Guvenc, H.I.; Yuksekkaya, S.; Opus, A.; Guzelant, A.; Kaya, M.; Kurtoglu, M.G.; Kaya, N. Real-Time PCR detection of the most common bacteria and viruses causing meningitis. Clin. Lab. 2017, 63, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, P.D.; Evander, M.; Petersson, K.; Mellhammar, L.; Lehmusvuori, A.; Karhunen, U.; Soikkeli, M.; Seppä, T.; Tuunainen, E.; Spangar, A.; et al. Integrated acoustic separation, enrichment, and microchip polymerase chain reaction detection of bacteria from blood for rapid sepsis diagnostics. Anal. Chem. 2016, 88, 9403–9411. [Google Scholar] [CrossRef] [PubMed]
- Athamanolap, P.; Hsieh, K.; O’Keefe, C.M.; Zhang, Y.; Yang, S.; Wang, T.H. Nanoarray digital PCR with high-resolution melt enables broad bacteria identification and pheno-molecular antimicrobial susceptibility test. Anal. Chem. 2019, 91, 12784–12792. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Talley, N.J.; Koloski, N.; Macdonald, G.A.; Kendall, B.J.; Shanahan, E.R.; Walker, M.M.; Keely, S.; Jones, M.P.; Morrison, M.; et al. Duodenal bacterial load as determined by quantitative polymerase chain reaction in asymptomatic controls, functional gastrointestinal disorders and inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 155–167. [Google Scholar] [CrossRef]
- Pandey, C.M.; Tiwari, I.; Singh, V.N.; Sood, K.N.; Sumana, G.; Malhotra, B.D. Highly sensitive electrochemical immunosensor based on graphene-wrapped copper oxide-cysteine hierarchical structure for detection of pathogenic bacteria. Sens. Actuators B Chem. 2017, 238, 1060–1069. [Google Scholar] [CrossRef]
- Liébana, S.; Brandão, D.; Alegret, S.; Pividori, M.I. Electrochemical immunosensors, genosensors and phagosensors for Salmonella detection. Anal. Methods 2014, 6, 8858–8873. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors–a powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Soares, R.R.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-induced graphene electrochemical immunosensors for rapid and label-free monitoring of Salmonella enterica in chicken broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]
- Zhao, S.; Jiao, T.; Wang, Z.; Adade, S.Y.S.S.; Wu, X.; Qin, O.; Chen, Q. On-line detecting soluble sugar, total acids, and bacterial concentration during kombucha fermentation based on the visible/near infrared combined meta-heuristic algorithm. J. Food Compos. Anal. 2023, 123, 105653. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, L.; Wang, J.; Pan, J.; Yan, Y.; Zhang, X. A precise and efficient detection of Seta-Cyfluthrin via fluorescent molecularly imprinted polymers with ally fluorescein as functional monomer in agricultural products. Food Chem. 2017, 217, 620–627. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Sun, L.; Kong, D.; Sheng, J.; Wang, K.; Dong, C. A green, simple, and rapid detection for amaranth in candy samples based on the fluorescence quenching of nitrogen-doped graphene quantum dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Zhao, S.; Jiao, T.; Adade, S.Y.S.S.; Wang, Z.; Wu, X. Based on vis-NIR combined with ANN for on-line detection of bacterial concentration during kombucha fermentation. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Z.; Zou, X.; Shi, J.; Mao, H.; Zhao, J.; Hao, L.; Holmes, M. Detection of meat-borne trimethylamine based on nanoporous colorimetric sensor arrays. Food Chem. 2016, 197, 930–936. [Google Scholar]
- Sharma, S.A.; Marimuthu, M.; Varghese, A.W.; Wu, J.; Xu, J.; Luo, X.; Devaraj, S.; Lan, Y.; Li, H.; Chen, Q. A review of biomolecules conjugated lanthanide up-conversion nanoparticles-based fluorescence probes in food safety and quality monitoring applications. Crit. Rev. Food Sci. Nutr. 2022, 64, 6129–6159. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Liu, G.; Xiao, Y.; Tian, Y.; Fang, S. Plasmonic colorimetry and G-quadruplex fluorescence-based aptasensor: A dual-mode, protein-free and label-free detection for OTA. Food Chem. 2024, 448, 139115. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J.; et al. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2024, 463, 141288. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Li, D.; Wu, J.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Sulfadiazine detection in aquatic products using upconversion nanosensor based on photo-induced electron transfer with imidazole ligands and copper ions. Food Chem. 2024, 456, 139992. [Google Scholar] [CrossRef]
- Li, H.; Bei, Q.; Hassan, M.M.; Marimuthu, M.; Adade, S.Y.S.S.; Chen, Q.; Zareef, M. A Cu2+-modulated UCNPs@RBD sensor for sensitive detection of tetracyclines in food based on the spirolactam open-loop reaction. J. Food Compos. Anal. 2024, 133, 106374. [Google Scholar] [CrossRef]
- Liang, N.; Shi, B.; Hu, X.; Li, W.; Huang, X.; Li, Z.; Zhang, X.; Zou, X. A ternary heterostructure aptasensor based on metal-organic framework and polydopamine nanoparticles for fluorescent detection of sulfamethazine. Food Chem. 2024, 460, 140570. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Murugesan, A.; Shoaib, M.; Sheng, W.; Chen, Q.S. Functionalized metal-organic frameworks with biomolecules for sensing and detection applications of food contaminants. Crit. Rev. Food Sci. Nutr. 2024. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2021, 63, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, H.; Pan, W.; Chen, Q.; Ouyang, Q.; Zhao, J. Dual-color upconversion nanoparticles (ucnps)-based fluorescent immunoassay probes for sensitive sensing foodborne pathogens. Food Anal. Methods 2017, 10, 2036–2045. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Haruna, S.A.; Hassan, M.; Chen, Q. Recent advances in rare earth ion-doped upconversion nanomaterials: From design to their applications in food safety analysis. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3732–3764. [Google Scholar] [CrossRef]
- Song, S.H.; Gao, Z.F.; Guo, X.; Chen, G.H. Aptamer-based detection methodology studies in food safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Shao, X.; Feng, Y.; Xie, P.; Luo, Y.; Huang, K.; Xu, W. Colorimetric detection and typing of E. coli lipopolysaccharides based on a dual aptamer-functionalized gold nanoparticle probe. Microchim. Acta 2019, 186, 111. [Google Scholar] [CrossRef]
- Wilson, D.; Materon, E.M.; Ibanez-Redin, G.; Faria, R.C.; Correa, D.S.; Oliveira, O.N., Jr. Electrical detection of pathogenic bacteria in food samples using information visualization methods with a sensor based on magnetic nanoparticles functionalized with antimicrobial peptides. Talanta 2019, 194, 611–618. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Li, M.; Sun, C.; Ren, D.; Li, Y. Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGO. Biosens. Bioelectron. 2018, 117, 347–353. [Google Scholar] [CrossRef]
- Richter, Ł.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Paczesny, J.; Hołyst, R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov. Today 2018, 23, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hinkley, T.; Chen, J.; Talbert, J.N.; Nugen, S.R. Phage based electrochemical detection of Escherichia coli in drinking water using affinity reporter probes. Analyst 2019, 144, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhang, X.; Wu, F.G.; Chen, Y. Colorimetric and test stripe-based assay of bacteria by using vancomycin-modified gold nanoparticles. Sens. Actuators B Chem. 2019, 281, 408–414. [Google Scholar] [CrossRef]
- Chen, J.; Andler, S.M.; Goddard, J.M.; Nugen, S.R.; Rotello, V.M. Integrating recognition elements with nanomaterials for bacteria sensing. Chem. Soc. Rev. 2017, 46, 1272–1283. [Google Scholar] [CrossRef]
- Li, H.; Ahmad, W.; Rong, Y.; Chen, Q.; Zuo, M.; Ouyang, Q.; Guo, Z. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food. Food Control 2020, 107, 106761. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Z.; Wang, Y.; Han, J.; Ni, L.; Wang, L.; Zhang, H.; Li, J.; Qiu, Y. A dual site-controlled probe for fluorescent monitoring of intracellular pH and colorimetric monitoring of Cu2+. Sens. Actuators B Chem. 2018, 270, 35–44. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.; Han, J.; Ni, L.; Zhang, H.; Li, C.; Li, J.; Qiu, Y. A novel fluorescent probe based on biphenyl and rhodamine for multi-metal ion recognition and its application. Dalton Trans. 2018, 47, 3378–3387. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Z.; Liu, R.; Ni, L.; Qiu, Y.; Han, J.; Wang, Y. A novel OFF-ON-OFF fluorescence probe based on coumarin for Al3+ and F− detection and bioimaging in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 299–305. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Corres, J.M.; Arregui, F.J. Fluorescent sensors for the detection of heavy metal ions in aqueous media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef]
- Sekar, A.; Yadav, R.; Basavaraj, N. Fluorescence quenching mechanism and the application of green carbon nanodots in the detection of heavy metal ions: A review. New J. Chem. 2021, 45, 2326–2360. [Google Scholar] [CrossRef]
- Guo, W.Y.; Fu, Y.X.; Liu, S.Y.; Mei, L.C.; Sun, Y.; Yin, J.; Yang, W.C.; Yang, G.F. Multienzyme-targeted fluorescent probe as a biosensing platform for broad detection of pesticide residues. Anal. Chem. 2021, 93, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, C.; Guo, X.; Gao, J.; Zhang, P.; Ding, C. Fluorescent determination of butyrylcholinesterase activity and its application in biological imaging and pesticide residue detection. ACS Sens. 2021, 6, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ji, R.; Wang, X.; Yu, C.; Yu, Y.; Yang, X. Fluorescence detection of boscalid pesticide residues in grape juice. Optik 2019, 180, 236–239. [Google Scholar] [CrossRef]
- Chen, L.; Lu, J.; Luo, M.; Yu, H.; Chen, X.; Deng, J.; Hou, X.; Hao, E.; Wei, J.; Li, P. A ratiometric fluorescent sensing system for the selective and ultrasensitive detection of pesticide residues via the synergetic effects of copper nanoclusters and carbon quantum dots. Food Chem. 2022, 379, 132139. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, J.; Hu, Z.; Meng, Q.; Wang, Y.; Zhang, R.; Zhang, Z. A near-infrared fluorescence probe for the detection of bisulfite in vivo and food samples. Dye. Pigment. 2022, 200, 110119. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Z.; Wang, Y.; Han, J.; Ni, L.; Zhang, H.; Li, J.; Yanli, M. A cyanobiphenyl based fluorescent probe for rapid and specific detection of hypochlorite and its bio-imaging applications. Sens. Actuators B Chem. 2018, 262, 57–63. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, T.; Song, E.; Fan, Y.; Min, D.; Zeng, L.; Bao, G.M. High-performance near-infrared fluorescence probe for fast and specific visualization of harmful sulfite in food, living cells, and zebrafish. Chem. Eng. J. 2022, 427, 131563. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Bai, X.; Li, P.; Su, D.; Zhang, W.; Zhang, W.; Tang, B. Highly specific cys fluorescence probe for living mouse brain imaging via evading reaction with other biothiols. Anal. Chem. 2019, 91, 8591–8594. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, M.; Wu, T.; Zhang, X.; Shen, Y.; Ernest, U.; Gui, L.; Wang, F.; He, Q.; Chen, H. Design and synthesis of NQO1 responsive fluorescence probe and its application in bio-imaging for cancer diagnosis. Talanta 2019, 198, 323–329. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Li, Y.; Yan, S.; Zhou, Q.; Gao, B.; Peng, J.; Du, J.; Fu, Q.; Jia, S.; et al. A novel, universal and sensitive lateral-flow based method for the detection of multiple bacterial contamination in platelet concentrations. Anal. Sci. 2012, 28, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.; Ozimok, C.; Sicard, C.; Aguirre, S.D.; Ali, M.M.; Li, Y.; Brennan, J.D. Multiplexed paper test strip for quantitative bacterial detection. Anal. Bioanal. Chem. 2012, 403, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Brown, C.L.; Jahanshahi-Anbuhi, S.; Kannan, B.; Li, Y.; Filipe, C.D.; Brennan, J.D. A printed multicomponent paper sensor for bacterial detection. Sci. Rep. 2017, 7, 12335. [Google Scholar] [CrossRef]
- Tominaga, T. Rapid detection of coliform bacteria using a lateral flow test strip assay. J. Microbiol. Methods 2019, 160, 29–35. [Google Scholar] [CrossRef]
- Roth-Konforti, M.; Green, O.; Hupfeld, M.; Fieseler, L.; Heinrich, N.; Ihssen, J.; Vorberg, R.; Wick, L.; Spitz, U.; Shabat, D. Ultrasensitive detection of Salmonella and Listeria monocytogenes by small-molecule chemiluminescence probes. Angew. Chem. Int. Ed. Engl. 2019, 131, 10469–10475. [Google Scholar] [CrossRef]
- Green, O.; Eilon, T.; Hananya, N.; Gutkin, S.; Bauer, C.R.; Shabat, D. Opening a gateway for chemiluminescence cell imaging: Distinctive methodology for design of bright chemiluminescent dioxetane probes. ACS Cent. Sci. 2017, 3, 349–358. [Google Scholar] [CrossRef]
- Rattray, N.J.; Zalloum, W.A.; Mansell, D.; Latimer, J.; Schwalbe, C.H.; Blake, A.J.; Bichenkova, E.V.; Freeman, S. Fluorescent probe for detection of bacteria: Conformational trigger upon bacterial reduction of an azo bridge. Chem. Commun. 2012, 48, 6393–6395. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Tamai, H.; Logviniuk, D.; Jones, P.G.; Fridman, M.; Werz, D.B. Azide-functionalized derivatives of the virulence-associated sugar pseudaminic acid: Chiral pool synthesis and labeling of bacteria. Chemistry 2021, 27, 10595–10600. [Google Scholar] [CrossRef]
- Hu, F.; Qi, G.; Mao, D.; Zhou, S.; Wu, M.; Wu, W.; Liu, B. Visualization and In Situ Ablation of Intracellular Pathogenic bacteria through Metabolic Labeling. Angew. Chem. Int. Ed. Engl. 2020, 59, 9288–9292. [Google Scholar] [CrossRef]
- Zlitni, A.; Gowrishankar, G.; Steinberg, I.; Haywood, T.; Sam Gambhir, S. Maltotriose-based probes for fluorescence and photoacoustic imaging of bacterial infections. Nat. Commun. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Sahile, H.A.; Rens, C.; Shapira, T.; Andersen, R.J.; Av-Gay, Y. DMN-Tre Labeling for Detection and High-Content Screening of Compounds against Intracellular Mycobacteria. ACS Omega 2020, 5, 3661–3669. [Google Scholar] [CrossRef]
- Backus, K.M.; Boshoff, H.I.; Barry, C.S.; Boutureira, O.; Patel, M.K.; D’Hooge, F.; Lee, S.S.; Via, L.E.; Tahlan, K.; Barry, C.E. Uptake of unnatural trehalose analogs as a reporter for Mycobacterium tuberculosis. Nat. Chem. Biol. 2011, 7, 228–235. [Google Scholar] [CrossRef]
- Lee, U.; Kim, Y.H.; Yoon, K.S.; Kim, Y. Selective butyrate esterase probe for the rapid colorimetric and fluorogenic identification of moraxella catarrhalis. Anal. Chem. 2020, 92, 16051–16057. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, Q.; Feng, Y.; Chen, M.; Zhang, S.; Chen, M.; Zhang, J.; Yang, G.; Ding, Y.; Yang, X.; et al. Off-on fluorogenic substrate harnessing ESIPT and AIE features for in situ and long-term tracking of β-glucuronidase in Escherichia coli. Sens. Actuators B Chem. 2020, 304, 127242. [Google Scholar] [CrossRef]
- Gwynne, L.; Williams, G.T.; Yan, K.C.; Patenall, B.L.; Gardiner, J.E.; He, X.P.; Maillard, J.Y.; James, T.D.; Sedgwick, A.C.; Jenkins, A.T.A. TCF-ALP: A fluorescent probe for the selective detection of Staphylococcus bacteria and application in “smart” wound dressings. Biomater. Sci. 2021, 9, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, H.; Xu, S.; Liu, J.; Chen, Y.; Gui, L.; Li, H.; Li, R.; Yuan, Z.; Li, B. β-Lactamase-Responsive Probe for Efficient Photodynamic Therapy of Drug-Resistant Bacterial Infection. ACS Sens. 2022, 7, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Peukert, M.S.C.; Gholap, S.P.; Green, O.; Pinkert, L.; Heuvel, J.; Doron Shabat, M.H.; Brönstrup, M. Enzyme-activated, chemiluminescent siderophore-dioxetane probes enable the selective and highly sensitive detection of bacterial pathogens. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201423. [Google Scholar] [CrossRef]
- Moore, C.; Cheng, Y.; Tjokro, N.; Zhang, B.; Kerr, M.; Hayati, M.; Chang, K.C.J.; Shah, N.; Chen, C.; Jokerst, J.V. A Photoacoustic-fluorescent imaging probe for proteolytic gingipains expressed by porphyromonas gingivalis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201843. [Google Scholar] [CrossRef]
- Xin, E.Y.H.; Kwek, G.; An, X.; Sun, C.; Liu, S.; Qing, N.S.; Lingesh, S.; Jiang, L.; Liu, G.; Xing, B. Enzymes in Synergy: Bacteria Specific Molecular Probe for Locoregional Imaging of Urinary Tract Infection in vivo. Angew. Chem. Int. Ed. Engl. 2024, 63, e202406843. [Google Scholar] [CrossRef]
- Jantarug, K.; Tripathi, V.; Morin, B.; Iizuka, A.; Kuehl, R.; Morgenstern, M.; Clauss, M.; Khanna, N.; Bumann, D.; Rivera-Fuentes, P. A Far-Red Fluorescent Probe to Visualize Gram-Positive Bacteria in Patient Samples. ACS Infect. Dis. 2024, 10, 1545–1551. [Google Scholar] [CrossRef]
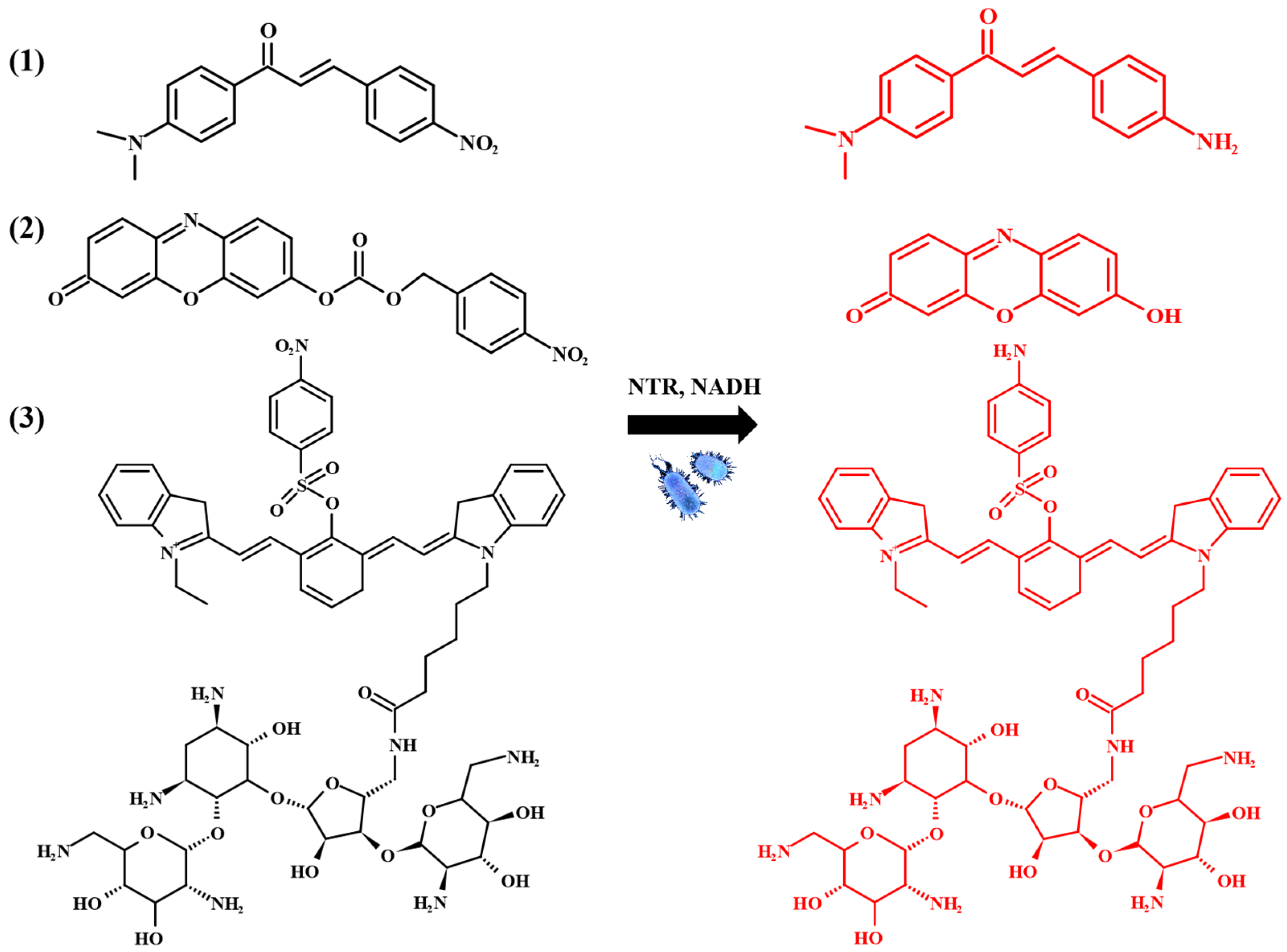
- Qin, W.; Xu, C.; Zhao, Y.; Yu, C.; Shen, S.; Li, L.; Huang, W. Recent progress in small molecule fluorescent probes for nitroreductase. Chin. Chem. Lett. 2018, 29, 1451–1455. [Google Scholar] [CrossRef]
- Wangngae, S.; Pewklang, T.; Chansaenpak, K.; Ganta, P.; Worakaensai, S.; Siwawannapong, K.; Kluaiphanngam, S.; Nantapong, N.; Lai, R.-Y.; Kamkaew, A. A chalcone-based fluorescent responsive probe for selective detection of nitroreductase activity in bacteria. New J. Chem. 2021, 45, 11566–11573. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, S.; Yoon, Y.; Lee, M.H. A resorufin-based fluorescent turn-on probe responsive to nitroreductase activity and its application to bacterial detection. Dye. Pigment. 2019, 171, 107779. [Google Scholar] [CrossRef]
- Wu, L.L.; Wang, Q.; Wang, Y.; Zhang, N.; Zhang, Q.; Hu, H.Y. Rapid differentiation between bacterial infections and cancer using a near-infrared fluorogenic probe. Chem. Sci. 2020, 11, 3141–3145. [Google Scholar] [CrossRef]
- Austrian, R. The Gram stain and the etiology of lobar pneumonia: An historical note. Bact. Rev. 1960, 24, 261–265. [Google Scholar] [CrossRef]
- Hucker, G.J.; Conn, H.J. Methods of Gram staining. N. Y. State Agric. Exp. Stn. 1923, 93, 3–37. [Google Scholar]
- Kass, E.D. History of the specialty of infectious diseases in the United States. Ann. Intern. Med. 1987, 106, 745–756. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Wen, G.; Zhang, X.; Luo, Y.; Qin, A.; Liang, A.; Jiang, Z. A sensitive fluorescence method for detection of E. coli using rhodamine 6G dyeing. Luminescence 2016, 31, 972–977. [Google Scholar] [CrossRef]
- Long, S.; Qiao, Q.; Miao, L.; Xu, Z. A self-assembly/disassembly two-photo ratiometric fluorogenic probe for bacteria imaging. Chin. Chem. Lett. 2019, 30, 573–576. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Wang, D.; Cui, Q.; Li, Z.; Wang, L.; Yang, H. Unique fluorescence properties of a self-assembling bis-pyrene molecule. Chin. Chem. Lett. 2018, 29, 1645–1647. [Google Scholar] [CrossRef]
- Leng, S.; Qiao, Q.L.; Gao, Y.; Miao, L.; Deng, W.G.; Xu, Z.C. SNAP-tag fluorogenic probes for wash free protein labeling. Chin. Chem. Lett. 2017, 28, 1911–1915. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Y.; Zou, P. Genetically-encoded voltage indicators. Chin. Chem. Lett. 2017, 28, 1925–1928. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Hu, L.L.; Tan, Y.; Liu, S.H.; Yin, J. Recent advances in formaldehyde-responsive fluorescent probes. Chin. Chem. Lett. 2017, 28, 1935–1942. [Google Scholar] [CrossRef]
- Ning, P.; Wang, W.; Chen, M.; Feng, Y.; Meng, X. Recent advances in mitochondria-and lysosomes-targeted small-molecule two-photon fluorescent probes. Chin. Chem. Lett. 2017, 28, 1943–1951. [Google Scholar] [CrossRef]
- Wu, D.; Shen, Y.; Chen, J.; Liu, G.; Chen, H.; Yin, J. Naphthalimide-modified near-infrared cyanine dye with a large stokes shift and its application in bioimaging. Chin. Chem. Lett. 2017, 28, 1979–1982. [Google Scholar] [CrossRef]
- Henry, J.; Endres, J.L.; Sadykov, M.R.; Bayles, K.W.; Svechkarev, D. Fast and accurate identification of pathogenic bacteria using excitation–emission spectroscopy and machine learning. Sens. Diagn. 2024, 3, 1253–1262. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Liu, X.; Choi, E.G.; Lee, J.Y.; Choi, S.Y.; Kim, J.Y.; Wang, L.; Park, S.J.; Kim, B.; Lee, Y.A.; et al. Development of a universal fluorescent probe for gram-positive bacteria. Angew. Chem. Int. Ed. Engl. 2019, 131, 8514–8519. [Google Scholar] [CrossRef]
- Mader, H.S.; Wolfbeis, O.S. Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim. Acta 2008, 162, 1–34. [Google Scholar] [CrossRef]
- Saito, S.; Massie, T.L.; Maeda, T.; Nakazumi, H.; Colyer, C.L. On-column labeling of gram-positive bacteria with a boronic acid functionalized squarylium cyanine dye for analysis by polymer-enhanced capillary transient isotachophoresis. Anal. Chem. 2012, 84, 2452–2458. [Google Scholar] [CrossRef]
- Tsuchido, Y.; Horiuchi, R.; Hashimoto, T.; Ishihara, K.; Kanzawa, N.; Hayashita, T. Rapid and selective discrimination of gram-positive and gram-negative bacteria by boronic acid-modified poly(amidoamine) dendrimer. Anal. Chem. 2019, 91, 3929–3935. [Google Scholar] [CrossRef]
- Guan, D.; Chen, F.; Xiong, L.; Tang, F.; Faridoon; Qiu, Y.; Zhang, N.; Gong, L.; Li, J.; Lan, L.; et al. Extra sugar on vancomycin: New analogues for combating multidrug-resistant Staphylococcus aureus and vancomycin-resistant Enterococci. J. Med. Chem. 2018, 61, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.; Megia-Fernandez, A.; Norberg, D.; Duncan, S.; Marshall, A.; Akram, A.R.; Quinn, T.; Young, I.; Bruce, A.M.; Scholefield, E.; et al. Molecular detection of Gram-positive bacteria in the human lung through an optical fiber-based endoscope. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Andrew Wang, T.S.; Chen, P.L.; Sarah Chen, Y.C.; Hung, H.M.; Huang, J.Y. Selectively targeting and differentiating vancomycin-resistant staphylococcus aureus via dual synthetic fluorescent probes. ACS Infect. Dis. 2021, 7, 2584–2590. [Google Scholar] [CrossRef]
- Carmi, O.A.; Stewart, G.S.; Ulitzur, S.; Kuhn, J. Use of bacterial luciferase to establish a promoter probe vehicle capable of nondestructive real-time analysis of gene expression in Bacillus spp. J. Bacteriol. 1987, 169, 2165–2170. [Google Scholar] [CrossRef]
- He, Y.; Wang, M.; Fan, E.; Ouyang, H.; Yue, H.; Su, X.; Liao, G.; Wang, L.; Lu, S.; Fu, Z. Highly specific bacteriophage-affinity strategy for rapid separation and sensitive detection of viable Pseudomonas aeruginosa. Anal. Chem. 2017, 89, 1916–1921. [Google Scholar] [CrossRef]
- Wu, Y.; Brovko, L.; Griffiths, M.W. Influence of phage population on the phage-mediated bioluminescent adenylate kinase (AK) assay for detection of bacteria. Lett. Appl. Microbiol. 2001, 33, 311–315. [Google Scholar] [CrossRef]
- Yang, X.; Wisuthiphaet, N.; Young, G.M.; Nitin, N. Rapid detection of Escherichia coli using bacteriophage-induced lysis and image analysis. PLoS ONE 2020, 15, 0233853. [Google Scholar] [CrossRef]
- Franche, N.; Vinay, M.; Ansaldi, M. Substrate-independent luminescent phage-based biosensor to specifically detect enteric bacteria such as E. coli. Environ. Sci. Pollut. Res. 2017, 24, 42–51. [Google Scholar] [CrossRef]
- Goodridge, L.; Chen, J.; Griffiths, M. Development and characterization of a fluorescent-bacteriophage assay for detection of Escherichia coli O157: H7. Appl. Environ. Microbiol. 1999, 65, 1397–1404. [Google Scholar] [CrossRef]
- Goodridge, L.; Chen, J.R.; Griffiths, M. The use of a fluorescent bacteriophage assay for detection of Escherichia coli O157:H7 in inoculated ground beef and raw milk. Int. J. Food Microbiol. 1999, 47, 43–50. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Lieberman, S.H.; Andrews, J.M.; Rohwer, F.L.; Wegley, L.E.; Breitbart, M. Use of fluorescently labeled phage in the detection and identification of bacterial species. Appl. Spectrosc. 2003, 57, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Y.; Jiang, Q.; Huang, K.H.; Zhang, C.Y.; Tang, T.S. Rapid detection of Salmonella in food by using fluorescently labeled phage O-I. Acta Microbiol. Sin. 2009, 49, 372–377. [Google Scholar]
- Low, H.Z.; Böhnlein, C.; Sprotte, S.; Wagner, N.; Fiedler, G.; Kabisch, J.; Franz, C.M. Fast and easy phage-tagging and live/dead analysis for the rapid monitoring of bacteriophage infection. Front. Microbiol. 2020, 11, 602444. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal. Chem. 2018, 107, 60–77. [Google Scholar] [CrossRef]
- Robati, R.Y.; Arab, A.; Ramezani, M.; Langroodi, F.A.; Abnous, K.; Taghdisi, S.M. Aptasensors for quantitative detection of kanamycin. Biosens. Bioelectron. 2016, 82, 162–172. [Google Scholar] [CrossRef]
- Feng, C.; Dai, S.; Wang, L. Optical aptasensors for quantitative detection of small biomolecules: A review. Biosens. Bioelectron. 2014, 59, 64–74. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Huang, Q.; Tong, T.; Zhou, Y.; Li, Z.; Bai, Q.; Liang, H.; Chen, L. Recent advances on aptamer-based biosensors for detection of pathogenic bacteria. World J. Microbiol. Biotechnol. 2021, 37, 45. [Google Scholar] [CrossRef]
- Maeng, J.S.; Kim, N.; Kim, C.T.; Han, S.R.; Lee, Y.J.; Lee, S.W.; Lee, M.H.; Cho, Y.J. Rapid detection of food pathogens using RNA aptamers-immobilized slide. J. Nanosci. Nanotechnol. 2012, 12, 5138–5142. [Google Scholar] [CrossRef]
- Song, M.S.; Sekhon, S.S.; Shin, W.R.; Kim, H.C.; Min, J.; Ahn, J.Y.; Kim, Y.H. Detecting and discriminating Shigella sonnei using an aptamer-based fluorescent biosensor platform. Molecules 2017, 22, 825. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; Guardia, M. Peptide based biosensors. TrAC Trends Anal. Chem. 2018, 107, 1–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Li, S.; Wu, J.; Liu, Q. Peptide-based biosensors. Peptide Applications in Biomedicine. Biotechnol. Bioeng. 2018, 136, 565–601. [Google Scholar]
- Sun, H.; Gu, X.; Zhang, Q.; Xu, H.; Zhong, Z.; Deng, C. Cancer nanomedicines based on synthetic polypeptides. Biomacromolecules 2019, 20, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, W.; Li, R.Q.; Qiu, W.X.; Zhuang, Z.N.; Cheng, S.X.; Zhang, X.Z. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv. Funct. Mater. 2018, 28, 1804492–1804513. [Google Scholar] [CrossRef]
- Charron, C.L.; Hickey, J.L.; Nsiama, T.K.; Cruickshank, D.R.; Turnbull, W.L.; Luyt, L. GMolecular imaging probes derived from natural peptides. Nat. Prod. Rep. 2016, 33, 761–800. [Google Scholar] [CrossRef]
- Ahangarpour, M.; Kavianinia, I.; Harris, P.W.; Brimble, M.A. Photo-induced radical thiol–ene chemistry: A versatile toolbox for peptide-based drug design. Chem. Soc. Rev. 2021, 50, 898–944. [Google Scholar] [CrossRef]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, 1703444. [Google Scholar] [CrossRef]
- Li, K.; Liu, C.J.; Zhang, X.Z. Multifunctional peptides for tumor therapy. Adv. Drug Deliv. Rev. 2020, 160, 36–51. [Google Scholar] [CrossRef]
- Qi, G.; Hu, F.; Shi, L.; Wu, M.; Liu, B. An AIEgen-peptide conjugate as a phototheranostic agent for phagosome-entrapped bacteria. Angew. Chem. Int. Ed. Engl. 2019, 58, 16229–16235. [Google Scholar] [CrossRef]
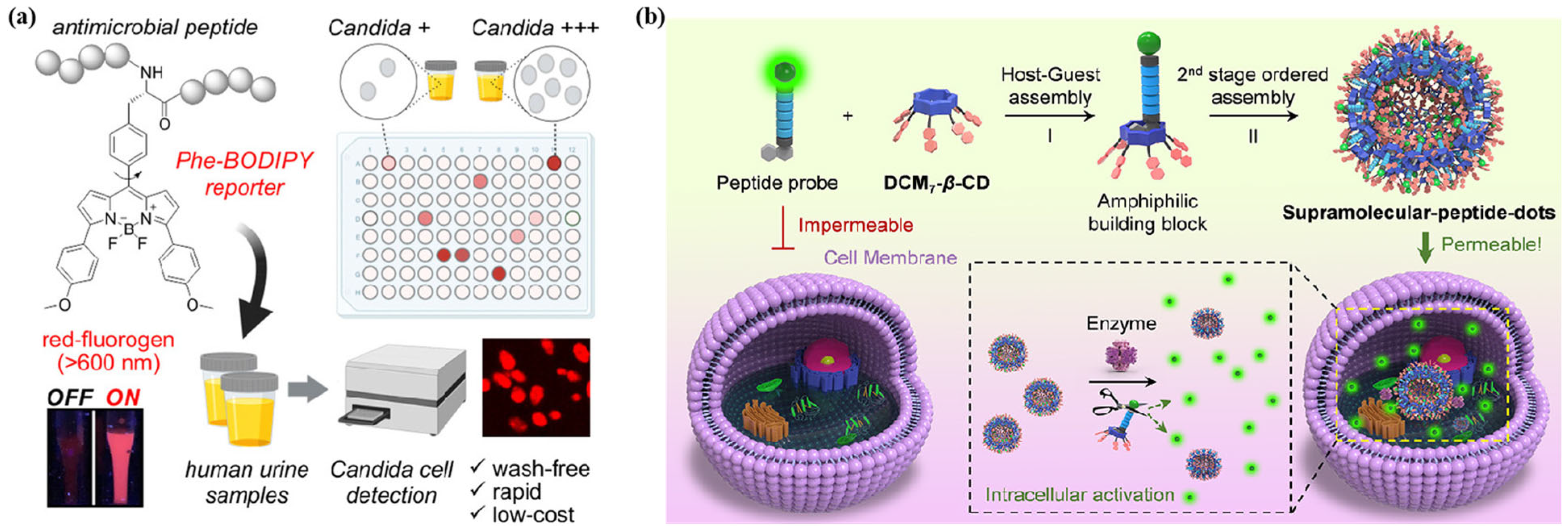
- Mendive-Tapia, L.; Mendive-Tapia, D.; Zhao, C.; Gordon, D.; Benson, S.; Bromley, M.J.; Wang, W.; Wu, J.; Kopp, A.; Ackermann, L. Rational design of phe-bodipy amino acids as fluorogenic building blocks for peptide-based detection of urinary tract candida infections. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117218. [Google Scholar] [CrossRef]
- Jiao, J.B.; Wang, G.Z.; Hu, X.L.; Zang, Y.; Maisonneuve, S.; Sedgwick, A.C.; Sessler, J.L.; Xie, J.; Li, J.; He, X.P.; et al. Cyclodextrin-based peptide self-assemblies (Spds) that enhance peptide-based fluorescence imaging and antimicrobial efficacy. J. Am. Chem. Soc. 2020, 142, 1925–1932. [Google Scholar] [CrossRef]
- Han, W.S.; Lee, H.Y.; Jung, S.H.; Lee, S.J.; Jung, J.H. Silica-based chromogenic and fluorogenic hybrid chemosensor materials. Chem. Soc. Rev. 2009, 38, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- El-Safty, S.A.; Prabhakaran, D.; Ismail, A.A.; Matsunaga, H.; Mizukami, F. Nanosensor design packages: A smart and compact development for metal ions sensing responses. Adv. Funct. Mater. 2007, 17, 3731–3745. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.E.; Seo, J.; Jeong, I.Y.; Lee, S.S.; Jung, J.H. Optical sensor based on nanomaterial for the selective detection of toxic metal ions. Adv. Funct. Mater. 2007, 17, 3441–3446. [Google Scholar] [CrossRef]
- Wang, H.; He, Y. Recent advances in silicon nanomaterial-based fluorescent sensors. Sensors 2017, 17, 268. [Google Scholar] [CrossRef]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Kim, K.H.; Bhardwaj, N. Nanomaterial-based fluorescent sensors for the detection of lead ions. J. Hazard. Mater. 2021, 407, 124379. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Liu, H.; Jing, Y.; Zhou, D.; Ji, Y.; Widengren, J.; Bai, X.; Song, H. A multiband NIR upconversion core-shell design for enhanced light harvesting of silicon solar cells. Light Sci. Appl. 2024, 13, 312. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chen, S. Face-to-face integrated tandem quantum-dot LEDs with high performance and multifunctionality. Light Sci. Appl. 2025, 14, 171. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, R.; Zou, G.; Ge, Z.; Zhang, Q.; Qiao, Y.; Ding, X.; Jiang, G.; Lou, Y.; Guo, Y.; et al. Deterministic resonance fluorescence improvement of single quantum dots by optimized surface passivation. Light Sci. Appl. 2025, 14, 170. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Xing, B.; Yang, P.; Lin, J. Multifunctional UCNPs@PDA-ICG nanocomposites for upconversion imaging and combined photothermal/photodynamic therapy with enhanced antitumor efficacy. J. Mater. Chem. B 2016, 4, 4884–4894. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Q.; Qin, S.; Fu, Y.; Liu, R.; Zhi, J.; Shan, C. Nanodiamonds conjugated upconversion nanoparticles for bio-imaging and drug delivery. J. Colloid Interface Sci. 2019, 537, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.H.; Chen, L.; McCurdy, C.M.; Tarapacki, C.M.; Chronik, B.A.; Zhang, J. Development of biocompatible NaGdF4: Er3+, Yb3+ upconversion nanoparticles used as contrast agents for bio-imaging. Can. J. Chem. Eng. 2019, 97, 2678–2684. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, T.; Liu, P.; Ren, J.; Li, Y.; Jiang, H.; Zhang, L.; Zhu, J. NIR-light-activated ratiometric fluorescent hybrid micelles for high spatiotemporally controlled biological imaging and chemotherapy. Small 2020, 16, 2005667. [Google Scholar] [CrossRef] [PubMed]
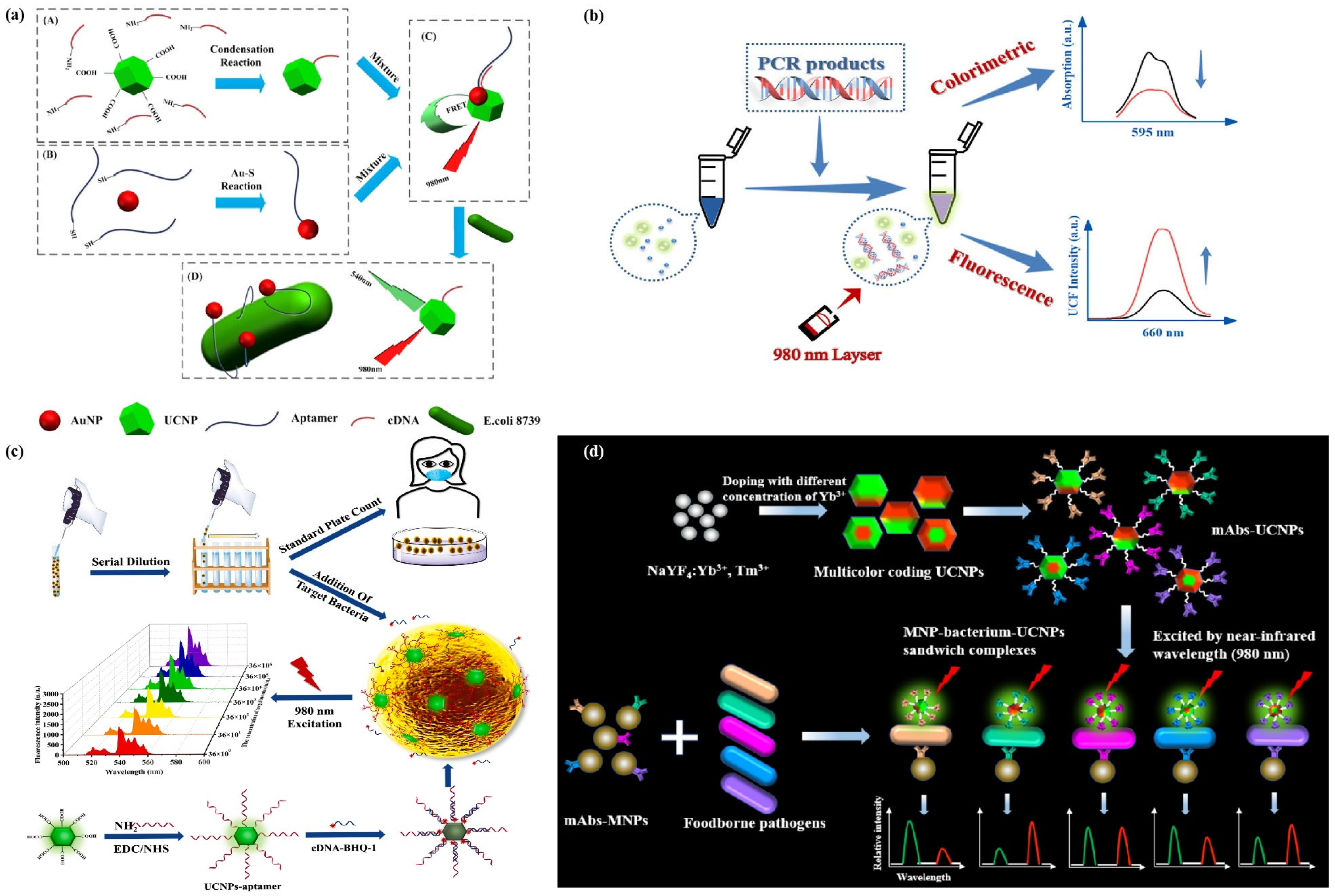
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef]
- Song, Y.; Chen, M.; Han, L.; Yan, Z.; Pan, L.; Tu, K. A novel ADA-coated UCNPs@NB sensing platform combined with nucleic acid amplification for rapid detection of Escherichia coli. Anal. Chim. Acta 2023, 1239, 340751. [Google Scholar] [CrossRef]
- Liu, R.; Ali, S.; Haruna, S.A.; Ouyang, Q.; Li, H.; Chen, Q. Development of a fluorescence sensing platform for specific and sensitive detection of pathogenic bacteria in food samples. Food Control 2022, 131, 108419. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Q.; Huang, F.; Xu, Z.; Zhou, L.; Zhao, S. Multicolor coding up-conversion nanoplatform for rapid screening of multiple foodborne pathogens. ACS Appl. Mater. Interfaces 2021, 13, 26782–26789. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; He, N.; Sun, Y.; Grimes, C.A.; Cai, Q. Tri-doped alkaline earth sulfide nanoparticles as a new class of highly efficient probe with near-IR stimulated fluorescence for in vivo and ultrasensitive bacteria targeted imaging. Sens. Actuators B Chem. 2020, 305, 127427. [Google Scholar] [CrossRef]
- Cheng, Y.; Da Ling, S.; Geng, Y.; Wang, Y.; Xu, J. Microfluidic synthesis of quantum dots and their applications in bio-sensing and bio-imaging. Nanoscale Adv. 2021, 3, 2180–2195. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, Z.; Liu, Q.; Huang, D.; Wei, Y.; Hu, Y.; Lian, X.; Hu, C. A facile and one-pot synthesis of fluorescent graphitic carbon nitride quantum dots for bio-imaging applications. New J. Chem. 2017, 41, 3930–3938. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Dai, S.; Miao, T.; Chen, J.; Wang, Z. Simultaneous detection of pathogenic bacteria using an aptamer based biosensor and dual fluorescence resonance energy transfer from quantum dots to carbon nanoparticles. Microchim. Acta 2015, 182, 917–923. [Google Scholar] [CrossRef]
- Fu, X.; Huang, K.; Liu, S. A rapid and universal bacteria-counting approach using CdSe/ZnS/SiO2 composite nanoparticles as fluorescence probe. Anal. Bioanal. Chem. 2010, 396, 1397–1404. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Jiang, Y.; Chuan, N.; Su, X.; Ji, J. Sensitive quantification and visual detection of bacteria using CdSe/ZnS@SiO2 nanoparticles as fluorescent probes. Anal. Methods 2014, 6, 6802–6808. [Google Scholar] [CrossRef]
- Xue, X.; Pan, J.; Xie, H.; Wang, J.; Zhang, S. Fluorescence detection of total count of Escherichia coli and Staphylococcus aureus on water-soluble CdSe quantum dots coupled with bacteria. Talanta 2009, 77, 1808–1813. [Google Scholar] [CrossRef]
- Duan, N.; Wu, S.; Yu, Y.; Ma, X.; Xia, Y.; Chen, X.; Huang, Y.; Wang, Z. A dual-color flow cytometry protocol for the simultaneous detection of Vibrio parahaemolyticus and Salmonella typhimurium using aptamer conjugated quantum dots as labels. Anal. Chim. Acta 2013, 804, 151–158. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Zheng, L.; Qi, P.; Zhang, D. Identification of bacteria by a fluorescence sensor array based on three kinds of receptors functionalized carbon dots. Sens. Actuators B Chem. 2019, 286, 206–213. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Zhang, T.; Jiang, Y. Rapid and sensitive detection of Salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe. Anal. Methods 2015, 7, 1701–1706. [Google Scholar] [CrossRef]
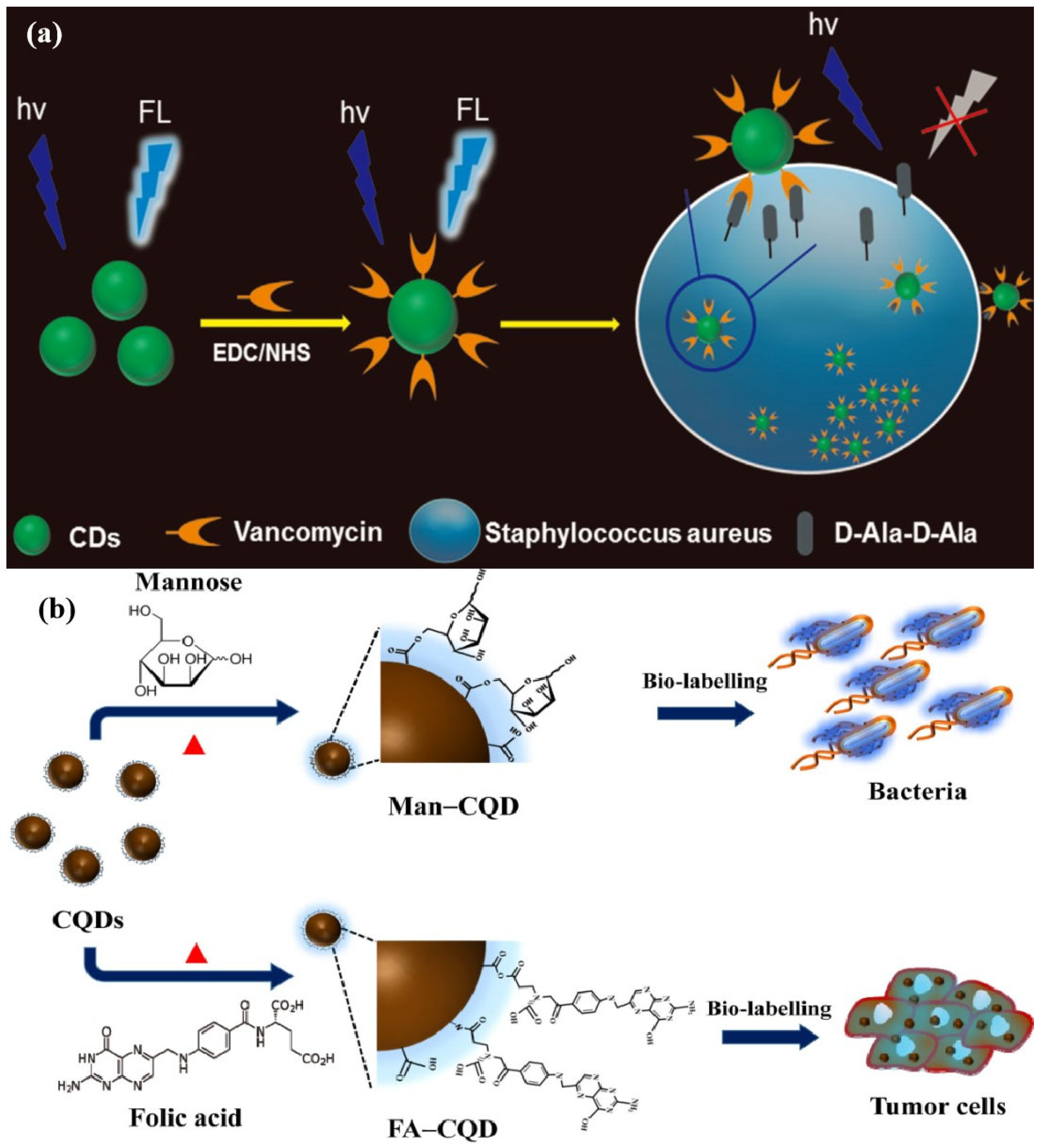
- Weng, C.I.; Chang, H.T.; Lin, C.H.; Shen, Y.W.; Unnikrishnan, B.; Li, Y.J.; Huang, C.C. One-step synthesis of biofunctional carbon quantum dots for bacterial labeling. Biosens. Bioelectron. 2015, 68, 1–6. [Google Scholar] [CrossRef]
- Zhong, D.; Zhuo, Y.; Feng, Y.; Yang, X. Employing carbon dots modified with vancomycin for assaying Gram-positive bacteria like Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chowdhuri, A.R.; Mahto, T.K.; Samui, A.; kumar Sahu, S. One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli. RSC Adv. 2016, 6, 72471–72478. [Google Scholar] [CrossRef]
- Pan, T.; Shan, X.; Jiang, D.; Qi, L.; Wang, W.; Chen, Z. Fluorometric aptasensor for determination of escherichia coli O157: H7 by FRET effect between aminated carbon quantum dots and graphene oxide. Anal. Sci. 2021, 37, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.P.J.; Harroun, S.G.; Chen, S.Y.; Unnikrishnan, B.; Li, Y.J.; Huang, C.C. Solid-state synthesis of self-functional carbon quantum dots for detection of bacteria and tumor cells. Sens. Actuators B 2016, 228, 465–470. [Google Scholar] [CrossRef]
- Duan, N.; Gong, W.; Wang, Z.; Wu, S. An aptasensor based on fluorescence resonance energy transfer for multiplexed pathogenic bacteria determination. Anal. Methods 2016, 8, 1390–1395. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Xu, Y.; Gan, Z.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Li, Y. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Tian, Z.Q.; Lu, D.; Yu, Y.; Cestellos-Blanco, S.; Sakimoto, K.K.; Yang, P.D. Bacteria photosensitized by intracellular goldnanoclusters for solar fuel production. Nat. Nanotechnol. 2018, 13, 900–905. [Google Scholar] [CrossRef]
- Liu, N.W.; Wang, Y.C.; Wang, Z.P.; He, Q.X.; Liu, Y.; Dou, X.Y.; Yin, Z.M.; Li, Y.; Zhu, H.G.; Yuan, X. Conjugating AIE-featured AuAg nanoclusters with highly luminescent carbon dots for improved visible-light-driven antibacterial activity. Nanoscale 2022, 14, 8183–8191. [Google Scholar] [CrossRef]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, T.; Swihart Song, E. Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Fu, F.; Li, L.; Li, J.; Li, G.; Song, Y.; Swihart, M.; Song, E. Dual-recognition förster resonance energy transfer based platform for one-step sensitive detection of pathogenic bacteria using fluorescent vancomycin–gold nanoclusters and aptamer–gold nanoparticles. Anal. Chem. 2017, 89, 4085–4090. [Google Scholar] [CrossRef]
- Yan, R.; Shou, Z.; Chen, J.; Wu, H.; Zhao, Y.; Qiu, L.; Jiang, P.; Mou, X.Z.; Wang, J.; Li, Y.Q. On-off-on gold nanocluster-based fluorescent probe for rapid Escherichia coli differentiation, detection and bactericide screening. ACS Sustain. Chem. Eng. 2018, 6, 4504. [Google Scholar] [CrossRef]
- Qi, X.; Ye, Y.; Wang, H.; Zhao, B.; Xu, L.; Zhang, Y.; Wang, X.; Zhou, N. An ultrasensitive and dual-recognition SERS biosensor based on Fe3O4@Au-Teicoplanin and aptamer functionalized Au@Ag nanoparticles for detection of Staphylococcus aureus. Talanta 2022, 250, 123648. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zheng, L.; Wu, T.; Ding, X.; Chen, F.; Yuan, Y.; Fan, G.; Shen, Y. Size-dependent modulation of polydopamine nanospheres on smart nanoprobes for detection of pathogenic bacteria at single-cell level and imaging-guided photothermal bactericidal activity. ACS Appl. Mater. Interfaces 2020, 12, 35626–35637. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Liao, Z.; Sun, Y.; Cheng, Q.; Song, Y.; Song, E.; Tan, W. Magnetism-resolved separation and fluorescence quantification for near-simultaneous detection of multiple pathogens. Anal. Chem. 2018, 90, 9621–9628. [Google Scholar] [CrossRef]
- Shrivastava, S.; Lee, W.I.; Lee, N.E. Culture-free, highly sensitive, quantitative detection of bacteria from minimally processed samples using fluorescence imaging by smartphone. Biosens. Bioelectron. 2018, 109, 90–97. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Zhu, L.J.; Lin, S.H.; Li, C.W.; Li, X.Y.; Huang, K.L.; Xu, W.T. Aptamer-functionalized DNA-silver nanocluster nanofilm for visual detection and elimination of bacteria. ACS Appl. Mater. Interfaces 2021, 13, 38647–38655. [Google Scholar] [CrossRef]
- Yin, P.; Wang, J.; Li, T.; Pan, Q.; Zhu, L.; Yu, F.; Zhao, Y.; Liu, H.B. A smartphone-based fluorescent sensor for rapid detection of multiple pathogenic bacteria. Biosens. Bioelectron. 2023, 242, 115744. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, J.; Mo, L.; Luo, Z.; Qin, Z.; Dai, Z.; Yi, C. Fluorescent on-site detection of multiple pathogens using smartphone-based portable device with paper-based isothermal amplification chip. Microchim. Acta 2022, 189, 333. [Google Scholar] [CrossRef]

| Categories | Bacterial Kinds | Morbid Substance | Clinical Disease |
|---|---|---|---|
| Pathogenic coccus | Staphylococcus | Coagulase, staphylococcal hemolytic toxin, leucovorin and enterotoxin | Suppurative inflammation, food poisoning and staphylococcal enteritis |
| Group A streptococcus | Lipoteichoic acid, M protein, invasive enzymes, pyrogenic exotoxin and hemolytic toxin | Suppurative infection, scarlet fever and allergic diseases (acute glomerulonephritis, rheumatism) | |
| Streptococcus pneumoniae | Capsule, hemolysin and neuraminidase | Lobar pneumonia | |
| Meningococcus | Capsule, pili and endotoxin | Epidemic meningitis | |
| Entericbacilli | Escherichia coli | Adhesin, endotoxin and enterotoxin | Sepsis, urinary tract infection, cholecystitis and diarrhoeal disease |
| Salmonella typhi | Highly toxic endotoxin, invasiveness and a few exotoxins | Intestinal fever includes typhoid and paratyphoid | |
| Shigella Castellani | Invasiveness, endotoxin and the production of a small number of exotoxins | Bacterial dysentery | |
| Anaerobic bacteria | Clostridium tetani | Highly toxic exotoxin | Tetanus |
| Clostridium botulinum | Highly toxic exotoxin (botulinum toxin) | Food poisoning, traumatic infection poisoning and infantile botulism | |
| ASPOROUS anaerobic bacteria | Pili, capsule, toxins and enzymes | Abdominal infection, oral infection, respiratory infection, septicemia, etc. | |
| Vibrio and Campylobacter | Vibrio cholerae | Flagella and pili, cholera enterotoxin | Cholera |
| Campylobacter | Unclear | Acute enteritis in infants | |
| Other | Mycoplasma | It has no cell wall and is highly polymorphic. It is the smallest prokaryotic cell type microorganism that can be cultured and proliferated | Mycoplasma |
| Rickettsia | Obligate living cell, a parasitic prokaryotic microbe | Epidemic typhus and tsutsugamushi | |
| Chlamydia | Prokaryotic microorganisms with small volume, specific living cell parasitism and unique development cycle | Trachoma and urinary tract infection | |
| Spirochete | Exotoxins and endotoxins similar to bacteria | Treponema pallidum can cause syphilis, and Leptospira can cause leptospirosis |
| Different Types | LOD (CFU/mL) | Advantages | Disadvantages |
|---|---|---|---|
| Small molecule sensors | 103–105 | a. Real-time detection enables dynamic monitoring of bacterial activity. b. Small molecular size facilitates penetration into tissues or biofilms. | a. Reaction-based probes rely on specific enzymes, resulting in limited species coverage. b. Binding-based probes are susceptible to environmental interference. c. Most probes exhibit poor water solubility and tend to aggregate in physiological environments. |
| Biomaterial-based sensors | 101–103 | a. Bio-recognition elements can precisely target bacterial surface biomarkers, significantly reducing cross-reactivity. b. Integration with fluorescence signal amplification techniques achieves a LOD at the single-bacterium level. c. Probes activated by the metabolic or enzymatic activity of live bacteria enable real-time tracking of bacterial proliferation or drug responses. d. Flexible selection of biological components or coupling with nanomaterials allows customizable sensor design. | a. Biomolecules are prone to degradation by temperature, pH or proteases, requiring stringent storage and handling conditions. b. Nonspecific adsorption or background fluorescence in complex samples may obscure the target signal. c. Reliance on specific biomarkers complicates detection of mutated bacterial strains or those lacking known surface targets. d. Antibody production is time-consuming and costly; aptamer screening requires SELEX technology; and nanomaterial synthesis involves complex processes. |
| Nanocomposite sensors | 1–10 | a. Nanomaterials exhibit large surface-to-volume ratios and quantum effects, enabling detection at ultra low bacterial concentrations. b. Nanocomposites can combine target recognition, signal amplification and self-cleaning properties. c. Fast electron transfer kinetics in nanomaterials allow real-time detection. d. Surface modifications reduce nonspecific binding in complex matrices. e. Nanocomposites are compatible with miniaturized devices (e.g., lab-on-a-chip, smartphone-based readers) for point-of-care testing. | a. Precise control of nanomaterial size, morphology and surface chemistry requires advanced techniques, increasing production costs. b. Some nanomaterials exhibit cytotoxicity, limiting their use in live-cell imaging or in vivo applications. c. Aggregation, oxidation or photobleaching of nanomaterials during storage or operation may degrade performance. d. Nanocomposites often rely on antibodies/aptamers, making them ineffective against bacteria lacking target biomarkers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Qi, Q.; Li, B.; Zhu, Z.; Lu, J.; Liu, L. Recent Advances on Fluorescent Sensors for Detection of Pathogenic Bacteria. Chemosensors 2025, 13, 182. https://doi.org/10.3390/chemosensors13050182
Tang X, Qi Q, Li B, Zhu Z, Lu J, Liu L. Recent Advances on Fluorescent Sensors for Detection of Pathogenic Bacteria. Chemosensors. 2025; 13(5):182. https://doi.org/10.3390/chemosensors13050182
Chicago/Turabian StyleTang, Xu, Qi Qi, Binrong Li, Zhi Zhu, Jian Lu, and Lei Liu. 2025. "Recent Advances on Fluorescent Sensors for Detection of Pathogenic Bacteria" Chemosensors 13, no. 5: 182. https://doi.org/10.3390/chemosensors13050182
APA StyleTang, X., Qi, Q., Li, B., Zhu, Z., Lu, J., & Liu, L. (2025). Recent Advances on Fluorescent Sensors for Detection of Pathogenic Bacteria. Chemosensors, 13(5), 182. https://doi.org/10.3390/chemosensors13050182







