Study on the Geographic Traceability and Growth Age of Panax ginseng C. A. Meyer Base on an Electronic Nose and Fourier Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Selection and Preparation
2.2. FTIR
2.3. E-Nose
2.4. Data Processing and Analysis
3. Results and Discussion
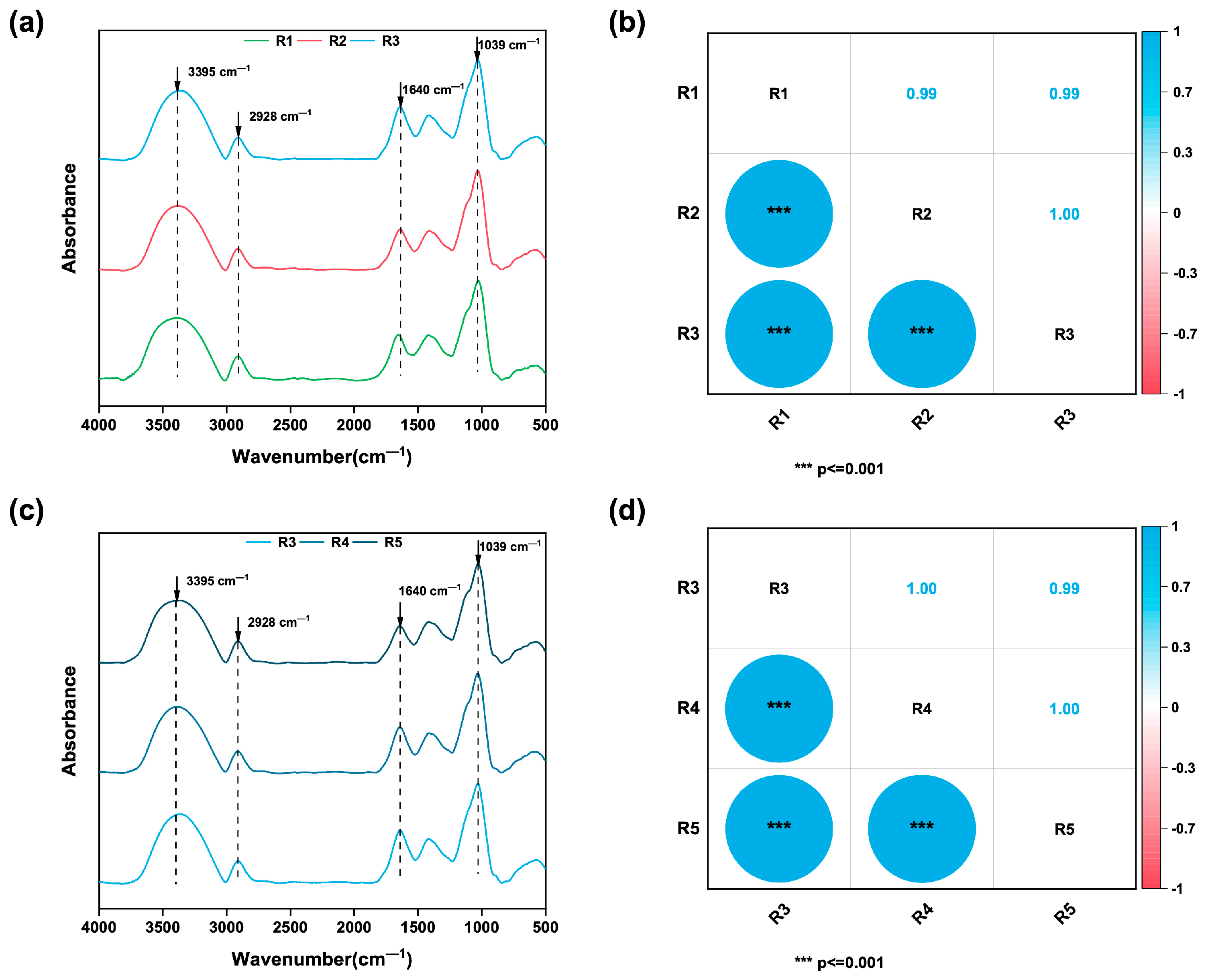
3.1. FTIR Spectra Analsis
3.2. E-Nose Singal Analysis
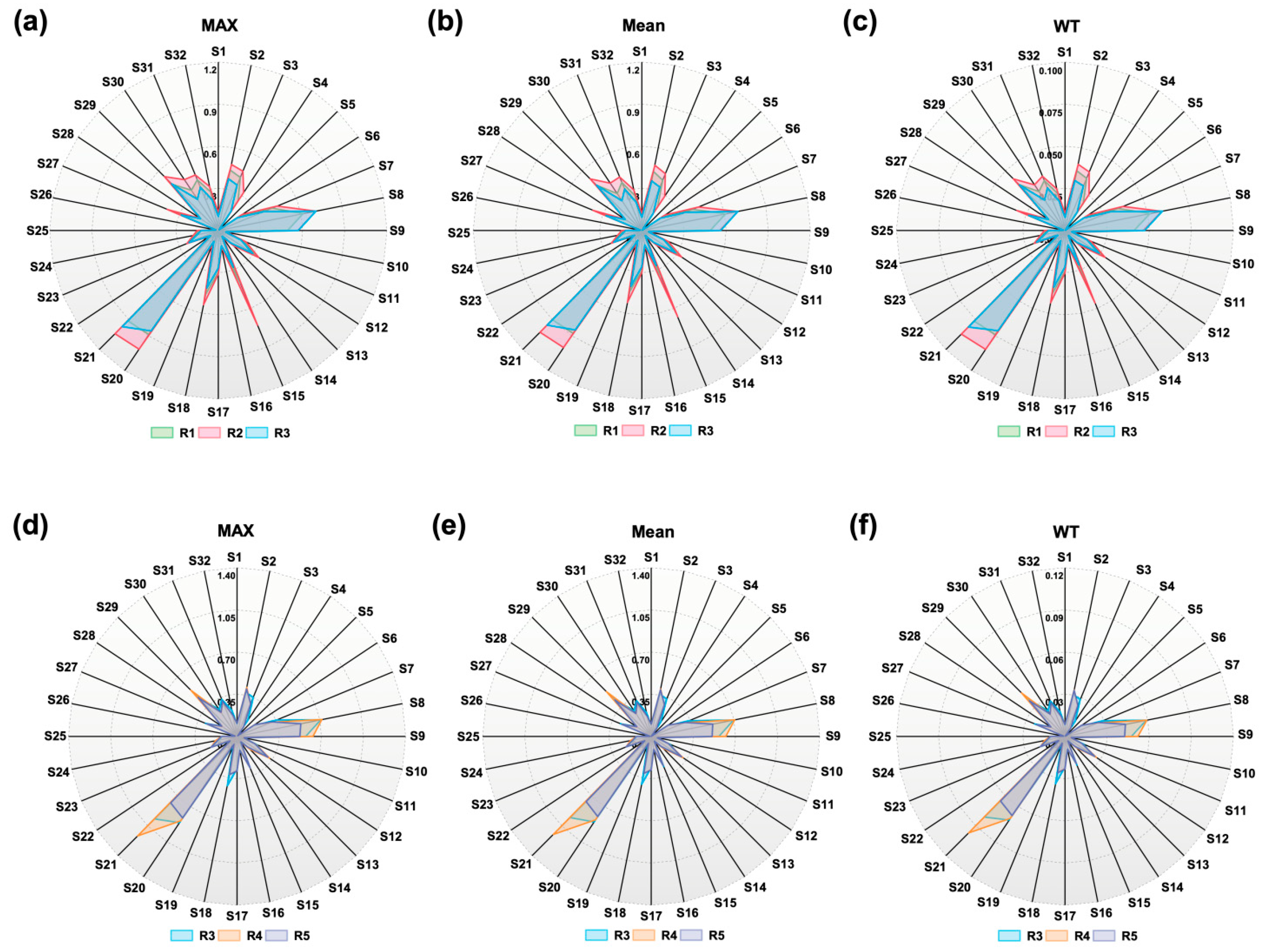
3.2.1. E-Nose Response Data Analysis
3.2.2. Pattern Recognition Analysis
- Support vector machine (SVM): constructs optimal separation of hyperplanes by maximizing inter-class margins [33]
- K-nearest neighbor (KNN): classifies samples based on majority voting among K most similar training instances (Euclidean distance metric) [34]
- Artificial neural network (ANN): nonlinear statistical model mimicking biological neural networks to capture complex input–output relationships [35]
4. Conclusions
- (1)
- FTIR spectroscopic analysis revealed significant differences in secondary metabolites among ginseng samples from different geographic origins and growth years, validating our sample selection rationale;
- (2)
- E-nose radar plots visually distinguished sensor response patterns across ginseng varieties;
- (3)
- Classification models achieved optimal performance with the following:
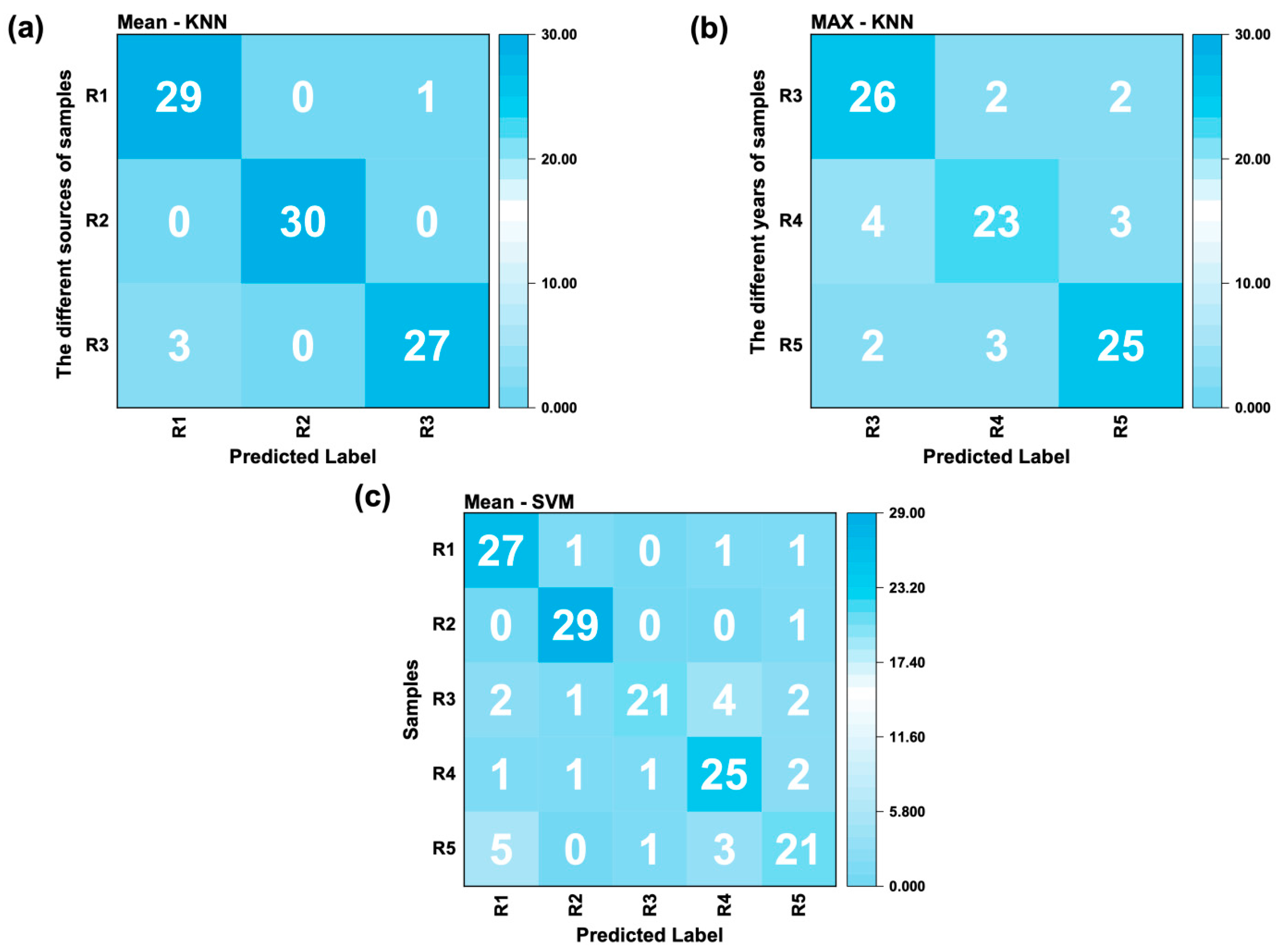
- Mean-KNN (96% accuracy) for geographic origin differentiation
- Max-KNN (82% accuracy) for growth year discrimination
- Mean-SVM (82% accuracy) for combined geographic traceability/year analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, R.; Lu, K.; Zong, G.; Xia, Y.; Han, H.; Zhao, Y.; Wei, Z.; Lu, Y. Ginseng polysaccharides: Potential antitumor agents. J. Ginseng Res. 2023, 47, 9–22. [Google Scholar] [CrossRef]
- Siegel, R.K. Ginseng Abuse Syndrome: Problems with the Panacea. JAMA 1979, 241, 1614–1615. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.P. The chemical constituents of ginseng plants. Comp. Med. East. West. 1977, 5, 123–145. [Google Scholar] [CrossRef]
- Kang, K.S.; Ham, J.; Kim, Y.J.; Park, J.H.; Cho, E.J.; Yamabe, N. Heat-processed Panax ginseng and diabetic renal damage: Active components and action mechanism. J. Ginseng Res. 2013, 37, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef]
- Alinaghi, M.; Mokarram, P.; Ahmadi, M.; Bozorg-Ghalati, F. Biosynthesis of palladium, platinum, and their bimetallic nanoparticles using rosemary and ginseng herbal plants: Evaluation of anticancer activity. Sci. Rep. 2024, 14, 5798. [Google Scholar] [CrossRef]
- Ming, H.L.; Ming, H.L.; Rui, Y.H.; Shan, T.; Ke, K.L.; Xiao, J.G.; Yin, S.S.; Ying, P.W.; Zi, W.; Wei, L. Exploring the mechanism of active components from ginseng to manage diabetes mellitus based on network pharmacology and molecular docking. Sci. Rep. 2023, 13, 793. [Google Scholar]
- Sathya, S.; Zhang, J.; Feng, H.; Jing, P.; Lan, Y.; Cao, X.; Liu, Q. Comparison of chemical components and quality evaluation of Panax ginseng and its processed products from different habitats in Northeastern China. Biocatal. Agric. Biotechnol. 2024, 61, 103375. [Google Scholar] [CrossRef]
- Dai, Y.L.; Qiao, M.D.; Yu, P.; Zheng, F.; Yue, H.; Liu, S.Y. Comparing eight types of ginsenosides in ginseng of different plant ages and regions using RRLC-Q-TOF MS/MS. J. Ginseng Res. 2020, 44, 205–214. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Analysis of Ginsenoside Content (Panax ginseng) from Different Regions. Molecules 2019, 24, 3491. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Song, X.; Li, G.; Hou, J.; Han, Y.; Ye, P.; Zhang, H.; Chen, C.; Chen, Y.; Wang, E. Profiling and discovery of volatile marker compounds for Ginseng berries with different growth years by HS-SPME-GC-MS. Chin. J. Anal. Chem. 2022, 51, 100208. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, C.; Cha, B.; Choi, S.H.; Kwon, K. Component analysis of cultivated ginseng, cultivated wild ginseng, and wild ginseng and the change of ginsenoside components in the process of red ginseng. J. Pharmacopunct. 2010, 13, 63–77. [Google Scholar] [CrossRef]
- Ge, S.; Liu, J.; Liu, Y.; Song, J.; Wu, H.; Li, L.; Zhu, H.; Feng, B. Chemical Profiling, Quantitation, and Bioactivities of Ginseng Residue. Molecules 2023, 28, 7854. [Google Scholar] [CrossRef]
- Ma, F.; Huang, A.; Zhang, S.; Zhou, Q.; Zhang, Q. Identification of three Diospysros species using FT-IR and 2DCOS-IR. J. Mol. Struct. 2020, 1220, 128709. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Lei, Y.; Zhou, Q.; Sun, S.; Noda, I. Evaluation of different grades of ginseng using Fourier-transform infrared and two-dimensional infrared correlation spectroscopy. J. Mol. Struct. 2010, 974, 94–102. [Google Scholar] [CrossRef]
- Han, H.; Zhang, H.; Wang, Z.; Song, Y.; Zhou, H.; Qu, X. Rapidly Identification of Ginseng from Different Origins Using Three-step Infrared Macro-Fingerprinting Technique. J. Mol. Struct. 2024, 1310, 138332. [Google Scholar] [CrossRef]
- Cheng, L.; Meng, Q.; Lilienthal, A.J.; Qi, P. Development of compact electronic noses: A review. Meas. Sci. Technol. 2021, 32, 062002. [Google Scholar] [CrossRef]
- Aunsa-Ard, W.; Kerdcharoen, T. Electronic Nose for Analysis of Coffee Beans Obtained from Different Altitudes and Origin. In Proceedings of the KST 2022—2022 14th International Conference on Knowledge and Smart Technology, Chon buri, Thailand, 26–29 January 2022; pp. 147–151. [Google Scholar]
- Li, D.; Lei, T.; Zhang, S.; Shao, X.; Xie, C. A novel headspace integrated E-nose and its application in discrimination of Chinese medical herbs. Sens. Actuators B-Chem. 2015, 221, 556–563. [Google Scholar] [CrossRef]
- Choong, Y.K.; Sun, S.; Zhou, Q.; Lan, J.; Lee, H.L.; Chen, X. Verification of Ganoderma (lingzhi) commercial products by Fourier Transform infrared spectroscopy and two-dimensional IR correlation spectroscopy. J. Mol. Struct. 2014, 1069, 60–72. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC-MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ying, K.; Bai, J. Savitzky-golay smoothing and differentiation filter for even number data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Bertie, J.E.; Keefe, C.D.; Jones, R.N. Infrared intensities of liquids viii. accurate baseline correction of transmission spectra of liquids for computation of absolute intensities, and the 1036 cm−1 band of benzene as a potential intensity standard. Can. J. Chem. 1991, 69, 1609–1618. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Sheng, D.; Huang, D.; Li, W.; Wang, X. Distinction of broken cellular wall ganoderma lucidum spores and g. lucidum spores using ftir microspectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Aranganathan, L.; Rajasree, S.R.R.; Suman, T.Y.; Remya, R.R.; Gobalakrishnan, M. Comparison of molecular characteristics of type a humic acids derived from fish waste and sugarcane bagasse co-compost influenced by various alkaline extraction protocols. Microchem. J. 2019, 149, 104038. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tang, L.; Li, L.; Zhou, Q.; Li, Y.J.; Li, J.; Wang, Y.Z. Geographic Authentication of Eucommia ulmoides Leaves Using Multivariate Analysis and Preliminary Study on the Compositional Response to Environment. Front. Plant Sci. 2020, 11, 79. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, D.; Liang, Y.; Zhou, H.; Wang, Y. Infrared spectral analysis and antioxidant activity of Dictamnus dasycarpus Turcz with different growth years. J. Mol. Struct. 2021, 1229, 129780. [Google Scholar] [CrossRef]
- Chen, J.; Guo, B.; Yan, R.; Sun, S.; Zhou, Q. Rapid and automatic chemical identification of the medicinal flower buds of Lonicera plants by the benchtop and hand-held Fourier transform infrared spectroscopy. Spectrochimica acta. Part A Mol. Biomol. Spectrosc. 2017, 182, 81–86. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.Y.; Wang, Y.Z. FT-MIR and NIR spectral data fusion: A synergetic strategy for the geographical traceability of Panax notoginseng. Anal. Bioanal. Chem. 2018, 410, 91–103. [Google Scholar] [CrossRef]
- Ou, L.X.; Liu, M.Y.; Zhu, L.Y.; Zhang, D.W.; Lu, H.L. Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor. Nanomic. Lett. 2022, 14, 206. [Google Scholar] [CrossRef]
- Xiang, Y.; Zou, M.; Ou, F.; Zhu, L.; Xu, Y.; Zhou, Q.; Lei, C. A Comparison of the Impacts of Different Drying Methods on the Volatile Organic Compounds in Ginseng. Molecules 2024, 29, 5235. [Google Scholar] [CrossRef] [PubMed]
- Qingxia, L.; Tong, L.; Yunlong, C.; Xin, W.; DaWen, S. Predicting wheat gluten concentrations in potato starch using GPR and SVM models built by terahertz time-domain spectroscopy. Food Chem. 2024, 432, 137235. [Google Scholar]
- Madson, M.F.S.D.; Rodrigues, J.B.; Marry, E.M.S.D.; Helena, L.N.S.D.; Fabricio, F.S.D.; Lúcia, L.H.F.D.; Natácia, S.E.S.D. KNN algorithm and multivariate analysis to select and classify starch films. Food Packag. Shelf Life 2022, 34, 100976. [Google Scholar]
- Bhagya Raj, G.V.S.; Dash Kshirod, K. Comprehensive study on applications of artificial neural network in food process modeling. Crit. Rev. Food Sci. Nutr. 2020, 62, 21–28. [Google Scholar] [CrossRef]
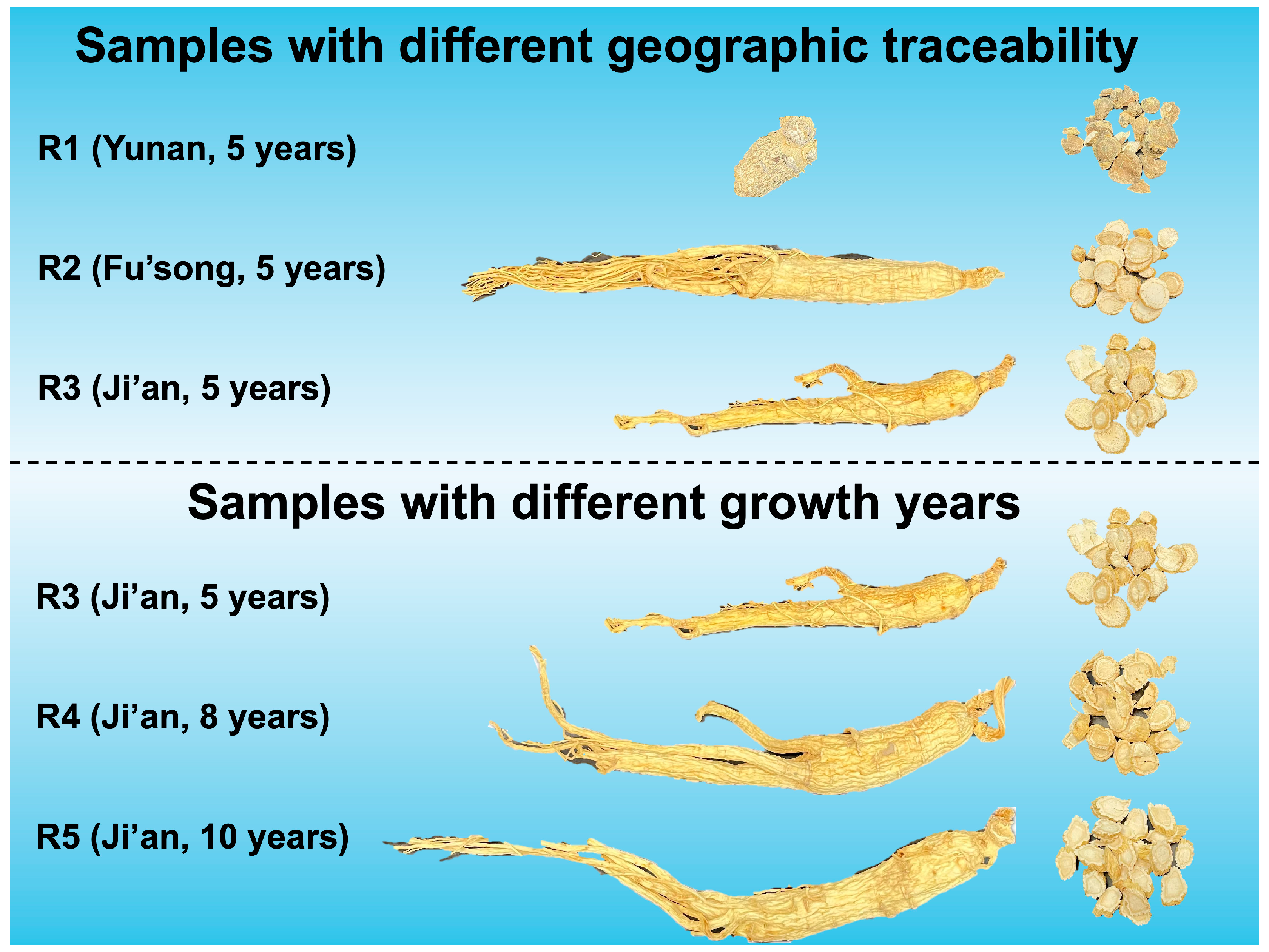
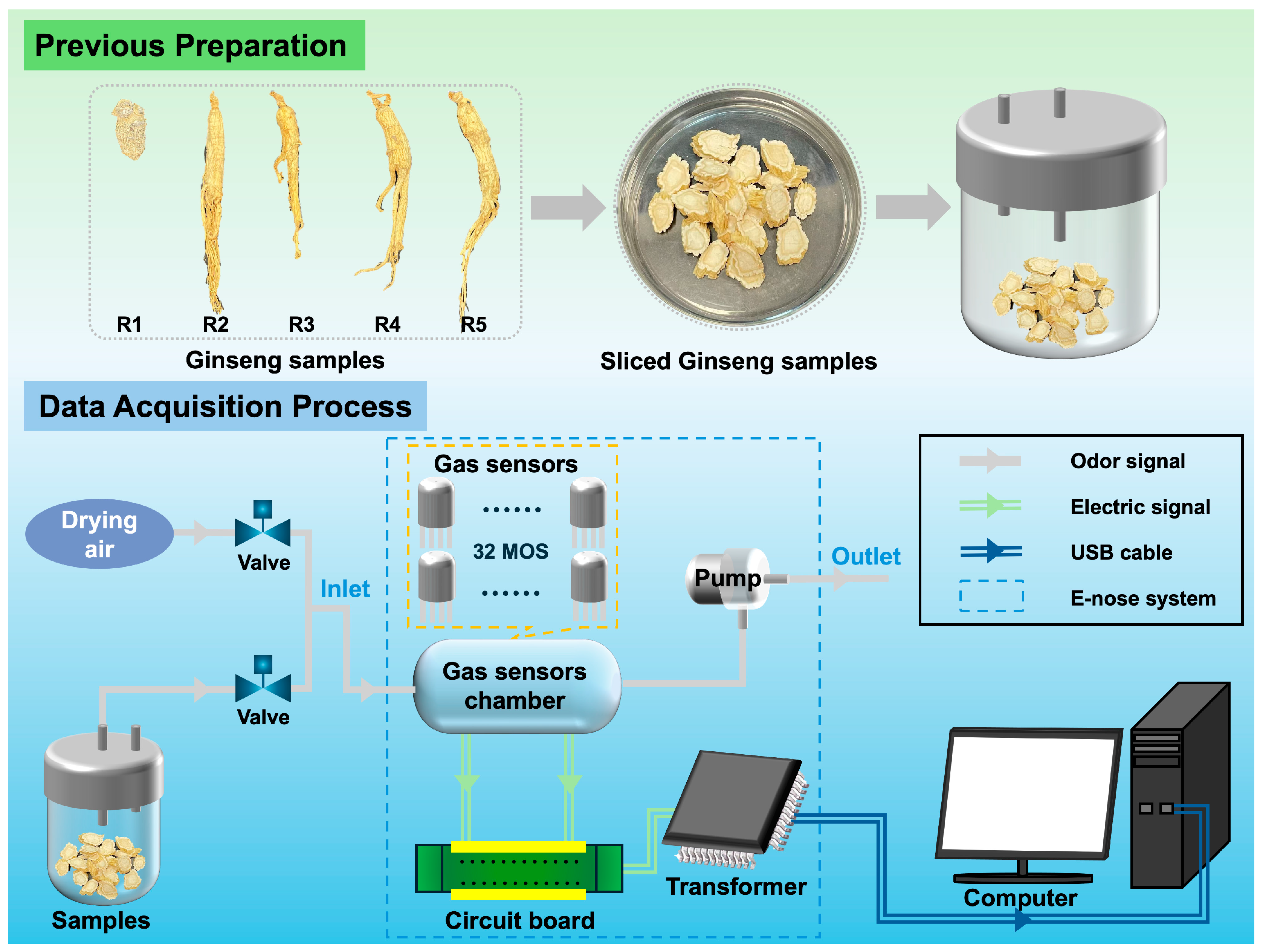
| Types | Yunnan | Fu’song | Ji’an |
|---|---|---|---|
| Longitude and latitude | 97°31′–106°11′ E 21°8′–29°15′ N | 127°01′–128°06′ E 41°42′–42°49′ N | 125°34′–126°32′ E 40°52′–41°35′ N |
| Soil (pH values) | Yellow–red soil (pH 4.2–5.7) | Dark-brown forest soil (pH 5.5–6.5) | Dark-brown forest soil (pH 5.5–6.5) |
| Soil organic matter | 55.40 g/Kg | 34.79–93.00 g/Kg | 26.33–58.17 g/Kg |
| Available phosphorus (AP) | 41.69 mg/Kg | 15.08–112.47 mg/Kg | 54.55–204.59 mg/Kg |
| Climate | Subtropical Monsoon | Warm temperate continental monsoon | Warm temperate continental monsoon |
| Annual sunshine duration | 1000–2800 h | 2021–2833 h | 2021–2833 h |
| Annual precipitation | 1085 mm | 800 mm | 800–1000 mm |
| Annual average temperature | 18.5 °C | 4.0 °C | 6.5 °C |
| Serial Number | Name | Geographic Traceability | Growth Years | Number of Servings | Servings per |
|---|---|---|---|---|---|
| R1 | Panaxnotoginseng(Burkill)F.H. Chen ex C. H. Chow | Yunnan | 5 years | 30 | 20 g |
| R2 | Panax quinquefolius L. | Fu’song | 5 years | 30 | 20 g |
| R3 | Panax ginseng C. A. Mey. | Ji’an | 5 years | 30 | 20 g |
| R4 | Panax ginseng C. A. Mey. | Ji’an | 8 years | 30 | 20 g |
| R5 | Panax ginseng C. A. Mey. | Ji’an | 10 years | 30 | 20 g |
| Serial Number | Sensor Model | Main Detection Object | Sensitivity | Marker |
|---|---|---|---|---|
| S1 | TGS2612 | Alkanes, propane, n-Butane | 400–1000 ppm | Figaro, Japan |
| S2 | TGS2611 | Alkanes | 500–10,000 ppm | Figaro, Japan |
| S3 | TGS2620 | Alkanols, organic solvent | 50–5000 ppm | Figaro, Japan |
| S4 | TGS2603 | Food odor (amines and sulfur-containing) | 1–10 ppm | Figaro, Japan |
| S5 | TGS2602 | VOCs, ammonia, hydrogen sulfide, etc. | 1–30 ppm | Figaro, Japan |
| S6 | TGS2610 | Propane, n-Butane | 500–10,000 ppm | Figaro, Japan |
| S7 | TGS2600 | Hydrogen, alcohol, etc. | 1–30 ppm | Figaro, Japan |
| S8 | GSBT11 | VOCs, HC, organic compounds | 0.1–10 ppm | Ogam, Korea |
| S9 | MS1100 | VOCs, aldehydes, benzene compound | 5–1000 ppm | Ogam, Korea |
| S10 | MP135 | Hydrogen, alcohol, carbon monoxide | 10–500 ppm | Winsen, China |
| S11 | MP901 | Alcohol, aldehydes, benzene compound, etc. | 10–1000 ppm | Winsen, China |
| S12 | MP-9 | Carbon monoxide, alkanes | 50–1000 ppm | Winsen, China |
| S13 | MP-3B | Alcohol | 0–500 ppm | Winsen, China |
| S14 | MP-4 | Alkanes, biogas | 300–10,000 ppm | Winsen, China |
| S15 | MP-5 | Combustible gas | 300–10,000 ppm | Winsen, China |
| S16 | MP-2 | Propane | 200–10,000 ppm | Winsen, China |
| S17 | MP503 | Alcohol, aldehydes, benzene compound, etc. | 1–1000 ppm | Winsen, China |
| S18 | MP801 | Aldehydes, benzene compound, etc. | 0.5–1000 ppm | Winsen, China |
| S19 | MP905 | Aldehydes, benzene compound, etc. | 0.5–1000 ppm | Winsen, China |
| S20 | MP402 | Alkanes | 300–1000 ppm | Winsen, China |
| S21 | WSP1110 | NO2 | 0.1–10 ppm | Winsen, China |
| S22 | WSP2110 | Alcohol, aldehydes, benzene compound, etc. | 1–50 ppm | Winsen, China |
| S23 | WSP7110 | Hydrogen sulfide | 0–50 ppm | Winsen, China |
| S24 | MP-7 | Carbon monoxide | 50–1000 ppm | Winsen, China |
| S25 | TGS2612 | Alkanes, propane, n-Butane | 400–1000 ppm | Figaro, Japan |
| S26 | TGS2611 | Alkanes | 500–10,000 ppm | Figaro, Japan |
| S27 | TGS2620 | Alkanols, organic solvent | 50–5000 ppm | Figaro, Japan |
| S28 | MP-3B | Alcohol | 0–500 ppm | Winsen, China |
| S29 | MP702 | Ammonia gas | 0–100 ppm | Winsen, China |
| S30 | TGS2610 | Propane, n-Butane | 500–10,000 ppm | Figaro, Japan |
| S31 | TGS2600 | Hydrogen, alcohol, etc. | 1–30 ppm | Figaro, Japan |
| S32 | TGS2618 | Propane, n-Butane | 500–10,000 ppm | Figaro, Japan |
| Feature | Samples | S6 | S10 | S14 | S17 | S22 |
|---|---|---|---|---|---|---|
| Max | R3 | 0.16510 | 0.05524 | 0.05035 | 0.26764 | 0.06683 |
| R4 | 0.17487 | 0.05585 | 0.05463 | 0.28230 | 0.06775 | |
| R5 | 0.17639 | 0.05768 | 0.05493 | 0.28687 | 0.06805 | |
| Mean | R3 | 0.15815 | 0.49686 | 0.04483 | 0.25899 | 0.06202 |
| R4 | 0.16832 | 0.05061 | 0.04777 | 0.27470 | 0.06268 | |
| R5 | 0.16996 | 0.05245 | 0.04843 | 0.27677 | 0.06308 | |
| WT | R3 | 0.01334 | 0.00414 | 0.00376 | 0.02166 | 0.00514 |
| R4 | 0.01425 | 0.00423 | 0.00412 | 0.02287 | 0.00529 | |
| R5 | 0.01427 | 0.00441 | 0.00430 | 0.02315 | 0.00530 |
| Feature | Model | Train (ACC %) | Test (ACC %) |
|---|---|---|---|
| Max | SVM | 100 | 93 |
| KNN | 99 | 97 | |
| ANN | 58 | 56 | |
| Mean | SVM | 98 | 91 |
| KNN | 100 | 96 | |
| ANN | 86 | 79 | |
| WT | SVM | 100 | 88 |
| KNN | 100 | 92 | |
| ANN | 94 | 84 |
| Feature | Model | Train (ACC %) | Test (ACC %) |
|---|---|---|---|
| Max | SVM | 98 | 78 |
| KNN | 100 | 82 | |
| ANN | 36 | 33 | |
| Mean | SVM | 98 | 80 |
| KNN | 91 | 82 | |
| ANN | 84 | 71 | |
| WT | SVM | 85 | 71 |
| KNN | 100 | 78 | |
| ANN | 80 | 76 |
| Feature | Model | Train (ACC %) | Test (ACC %) |
|---|---|---|---|
| Max | SVM | 100 | 81 |
| KNN | 93 | 86 | |
| ANN | 96 | 76 | |
| Mean | SVM | 100 | 82 |
| KNN | 91 | 84 | |
| ANN | 98 | 80 | |
| WT | SVM | 100 | 75 |
| KNN | 100 | 78 | |
| ANN | 69 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Qiao, J.; Liu, C.; Zhou, Z.; Kong, C.; Chang, Z.; Weng, X.; Zhang, S. Study on the Geographic Traceability and Growth Age of Panax ginseng C. A. Meyer Base on an Electronic Nose and Fourier Infrared Spectroscopy. Chemosensors 2025, 13, 176. https://doi.org/10.3390/chemosensors13050176
Li J, Qiao J, Liu C, Zhou Z, Kong C, Chang Z, Weng X, Zhang S. Study on the Geographic Traceability and Growth Age of Panax ginseng C. A. Meyer Base on an Electronic Nose and Fourier Infrared Spectroscopy. Chemosensors. 2025; 13(5):176. https://doi.org/10.3390/chemosensors13050176
Chicago/Turabian StyleLi, Jinying, Jianlei Qiao, Chang Liu, Zhigang Zhou, Cheng Kong, Zhiyong Chang, Xiaohui Weng, and Shujun Zhang. 2025. "Study on the Geographic Traceability and Growth Age of Panax ginseng C. A. Meyer Base on an Electronic Nose and Fourier Infrared Spectroscopy" Chemosensors 13, no. 5: 176. https://doi.org/10.3390/chemosensors13050176
APA StyleLi, J., Qiao, J., Liu, C., Zhou, Z., Kong, C., Chang, Z., Weng, X., & Zhang, S. (2025). Study on the Geographic Traceability and Growth Age of Panax ginseng C. A. Meyer Base on an Electronic Nose and Fourier Infrared Spectroscopy. Chemosensors, 13(5), 176. https://doi.org/10.3390/chemosensors13050176






