Heterostructures of CdSe Quantum Dots and g-C3N4 Applied as Electrochemiluminescent Probes for the Detection of Hydrogen Peroxide in Human Serum
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Synthesis of g-C3N4
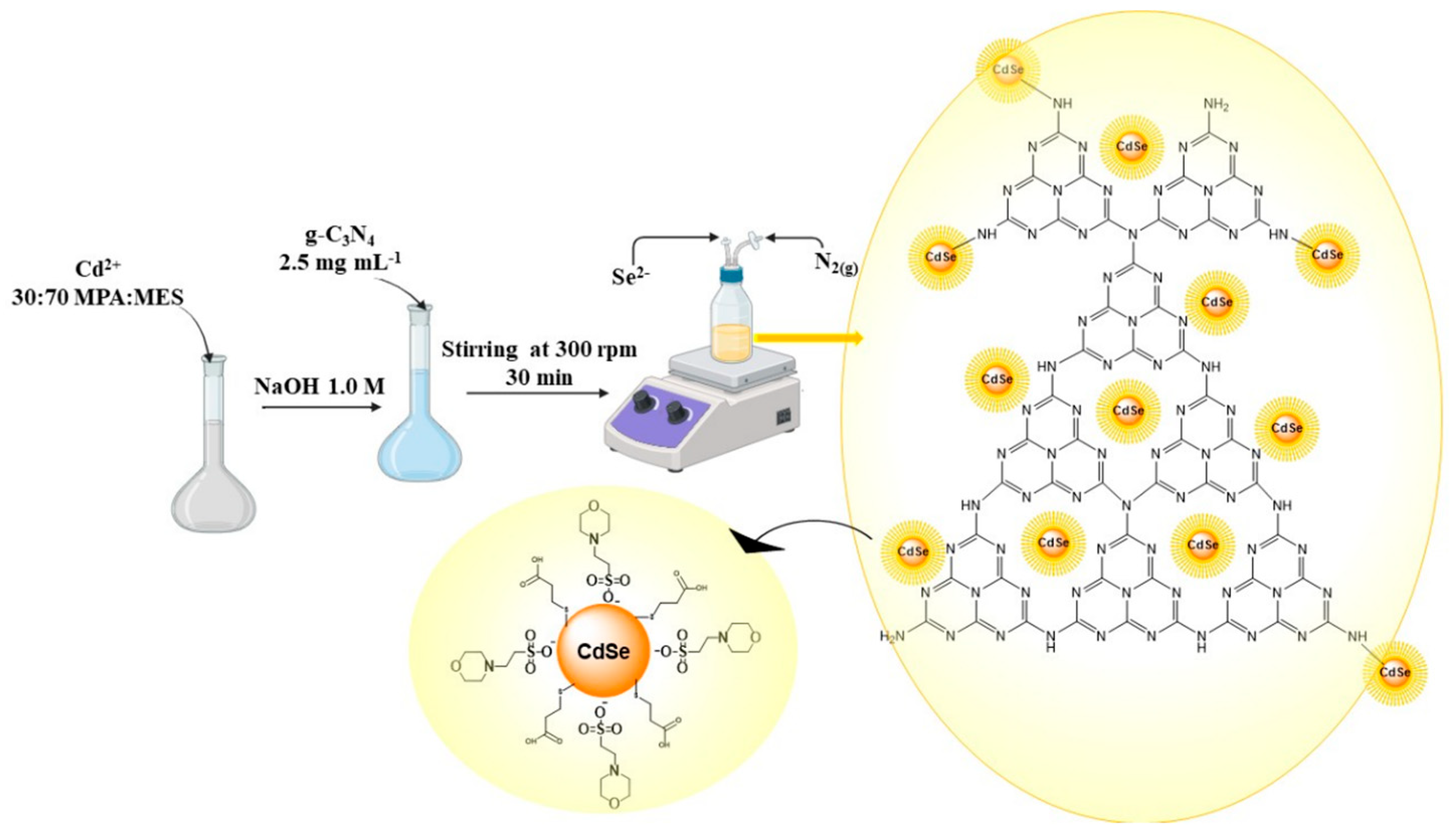
2.4. Synthesis of Heterostructures of g-C3N4 and QDs CdSe
3. Results
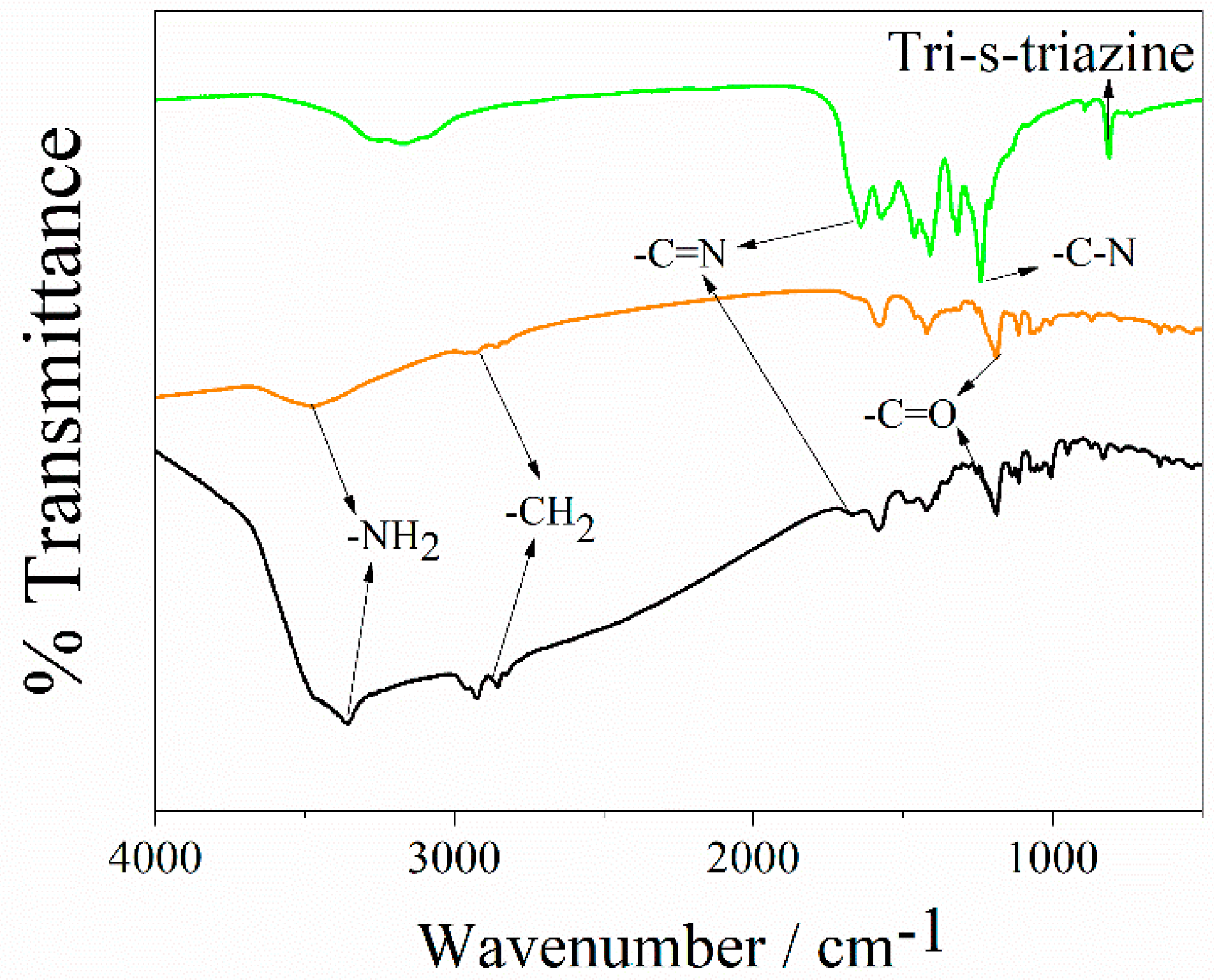
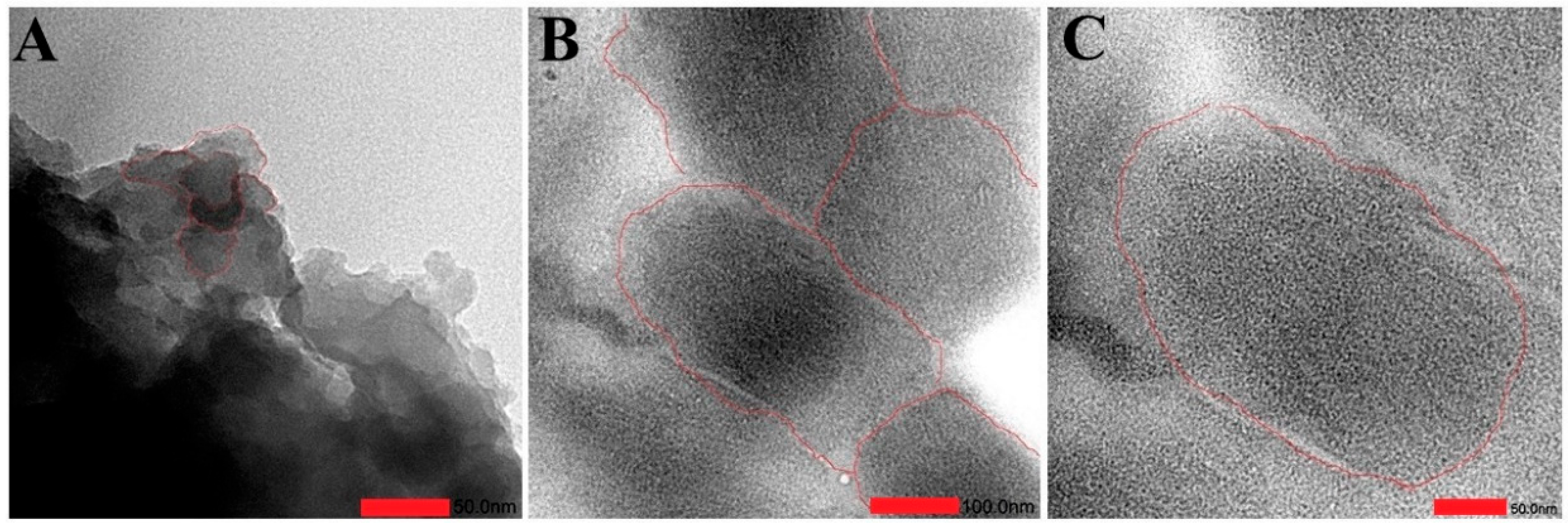
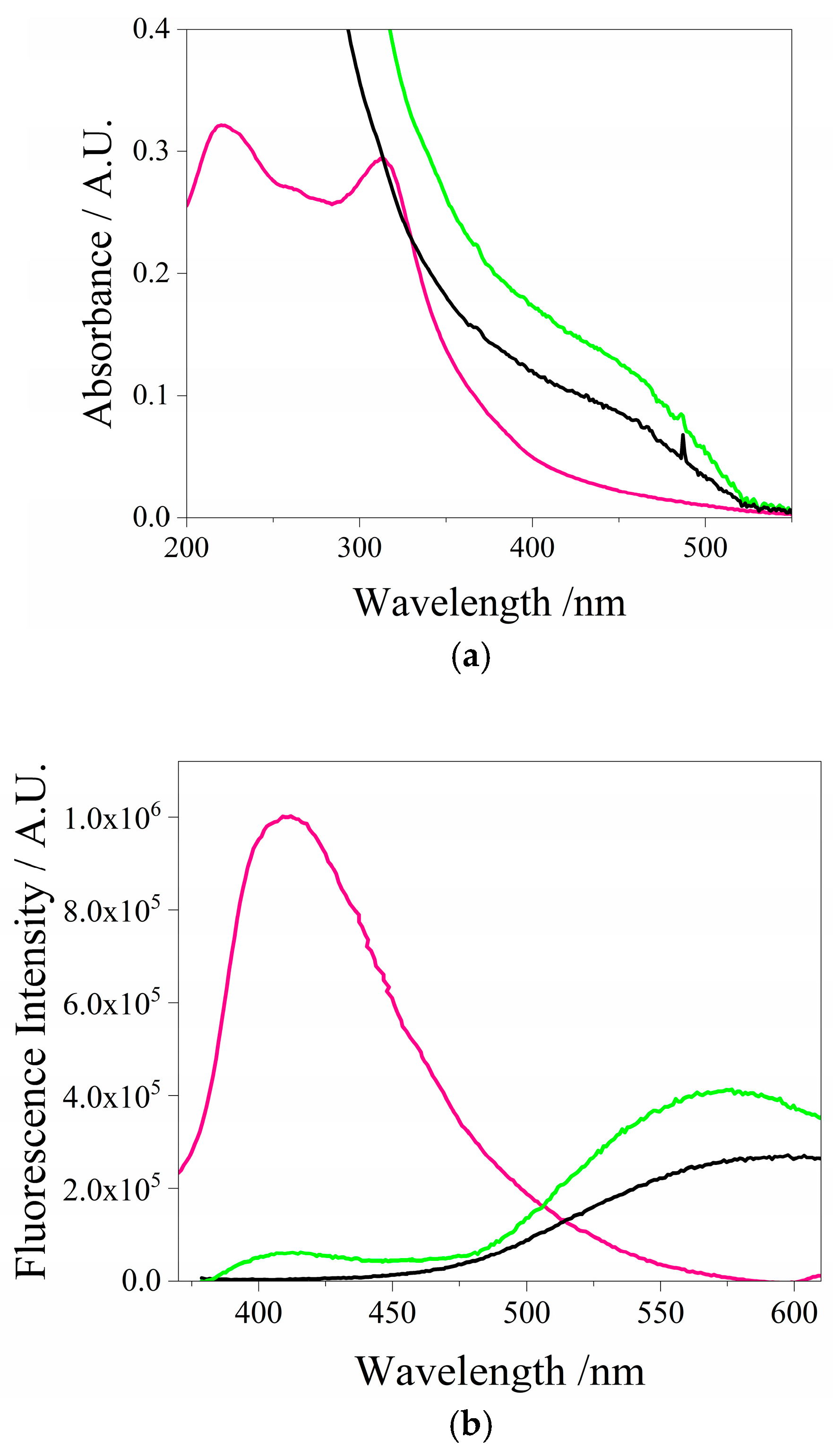
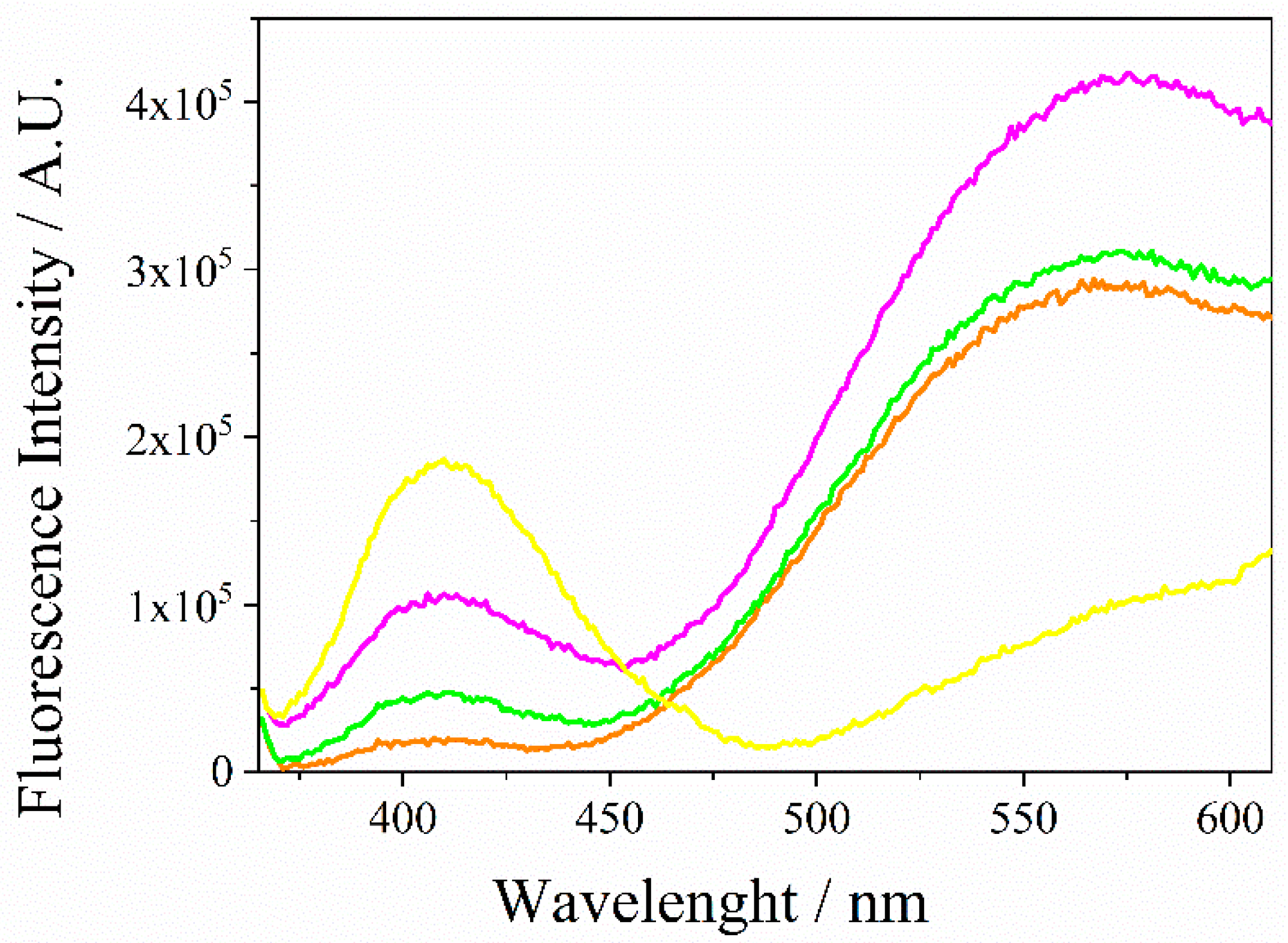
3.1. Spectroscopic Characterization of QDs CdSe@g-C3N4
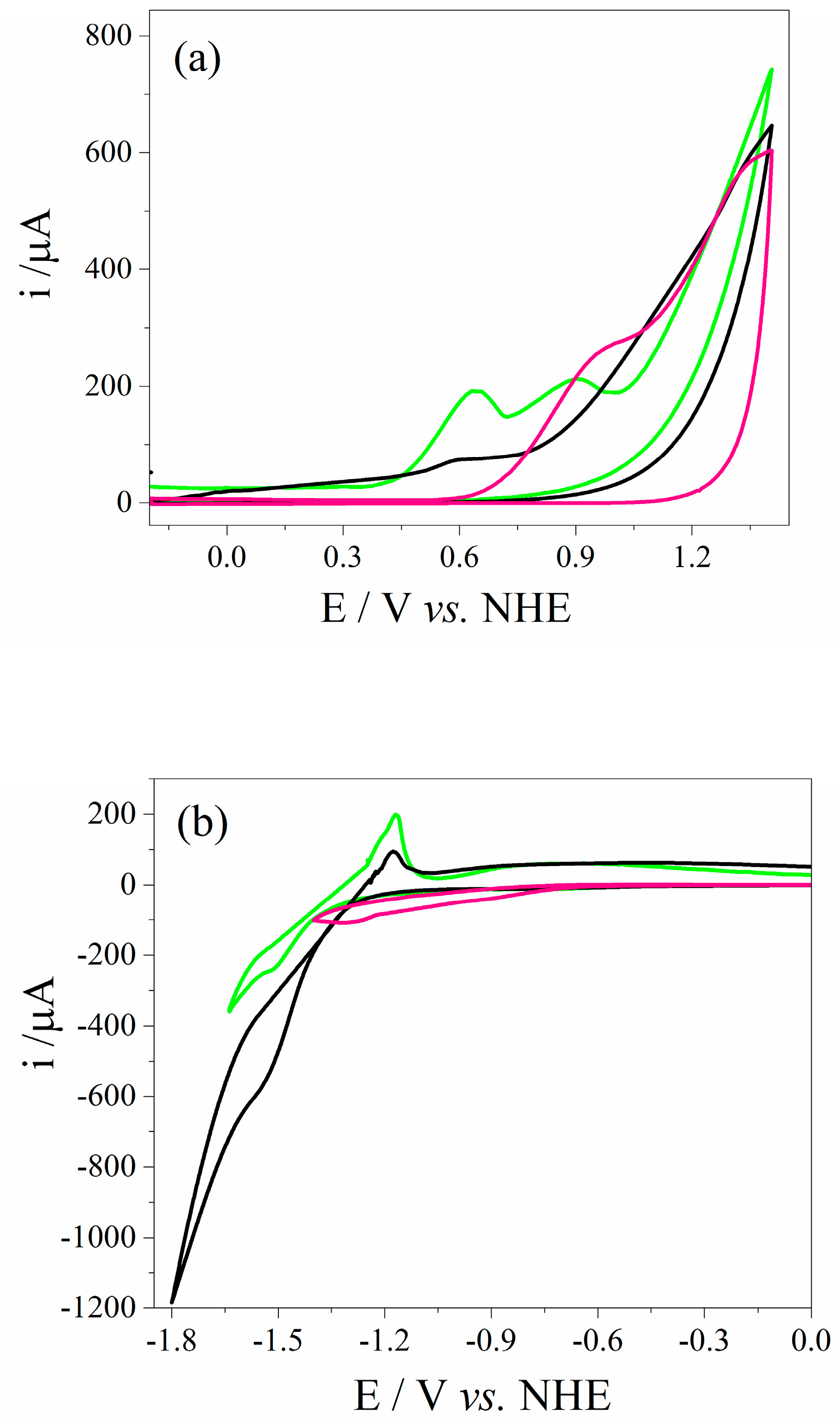
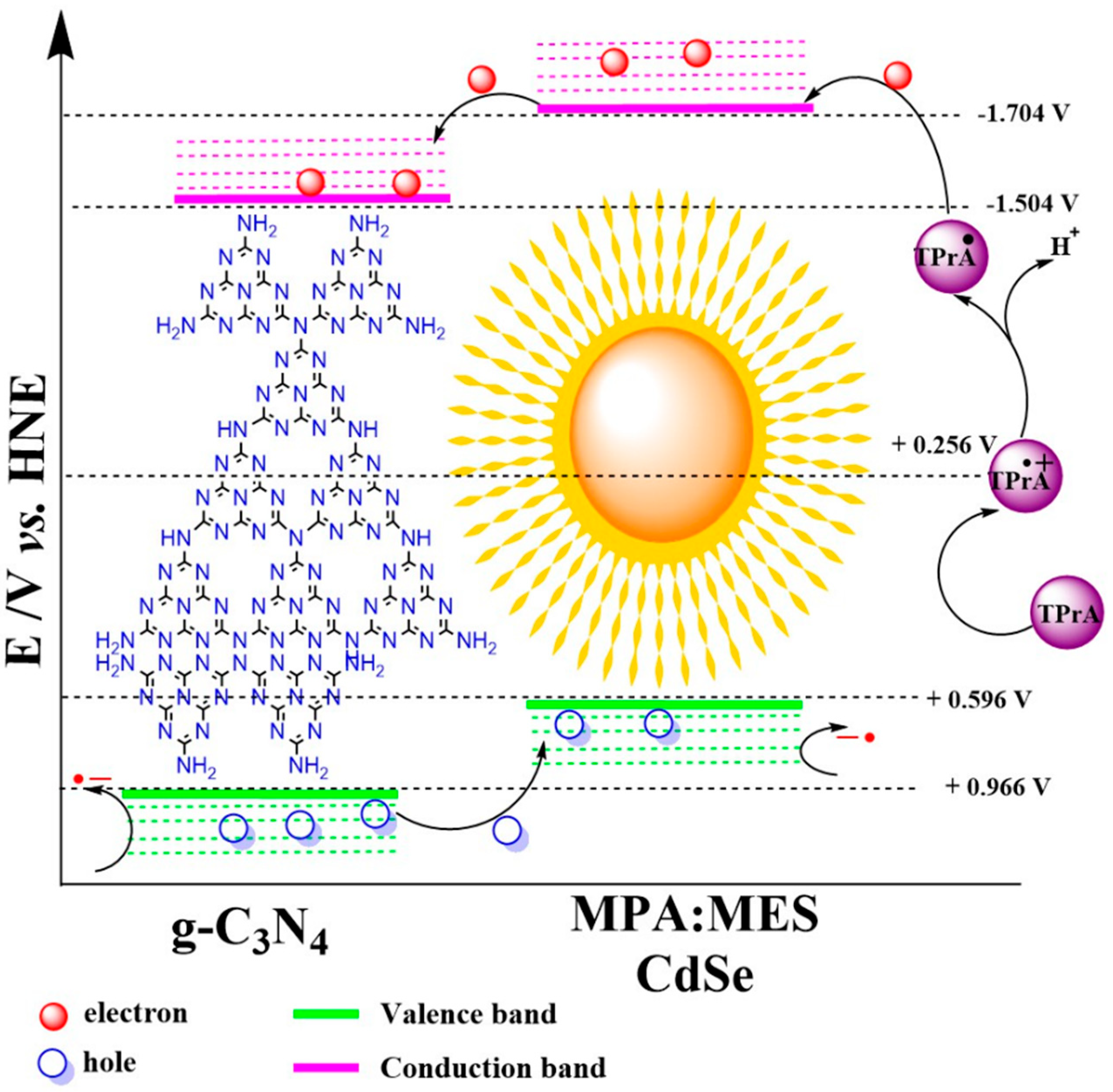
3.2. Electrochemical Characterization of QD CdSe@g-C3N4
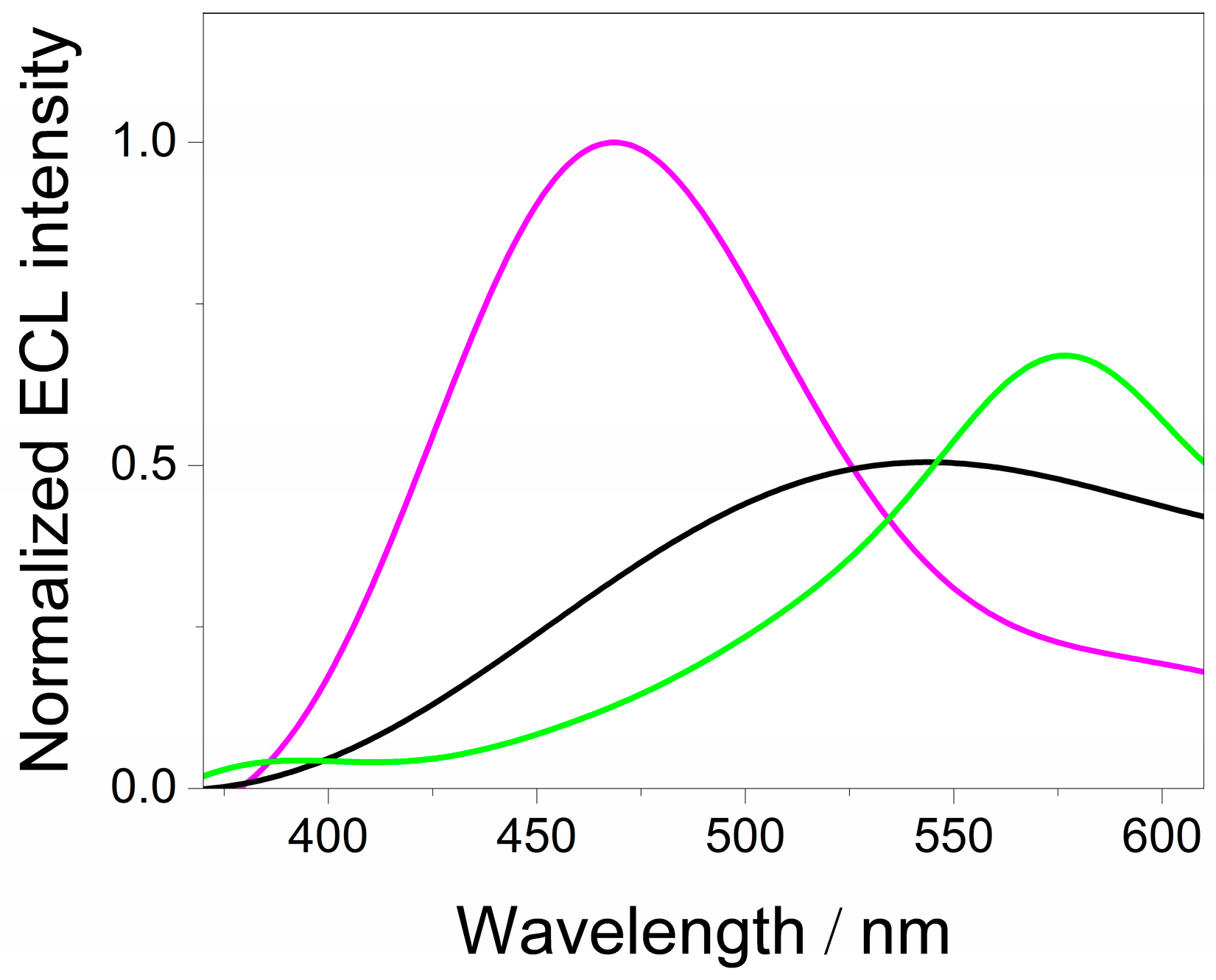
3.3. Electrochemiluminescence Analysis of QDs CdSe@g-C3N4
3.4. ECL Measurements of QDs CdSe@g-C3N4 Solution
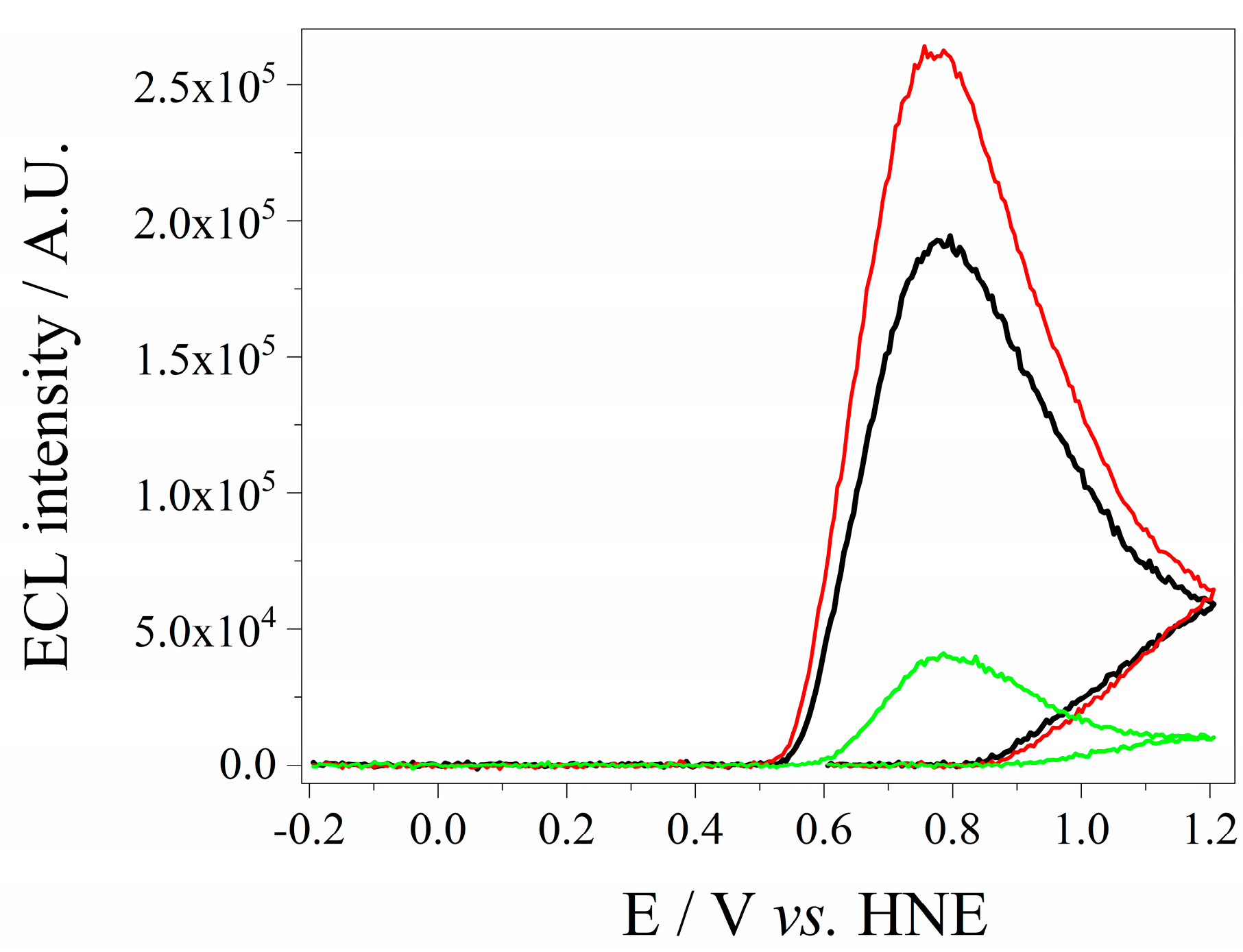
3.5. Determination of Optimal Ratio of QDs CdSe and g-C3N4
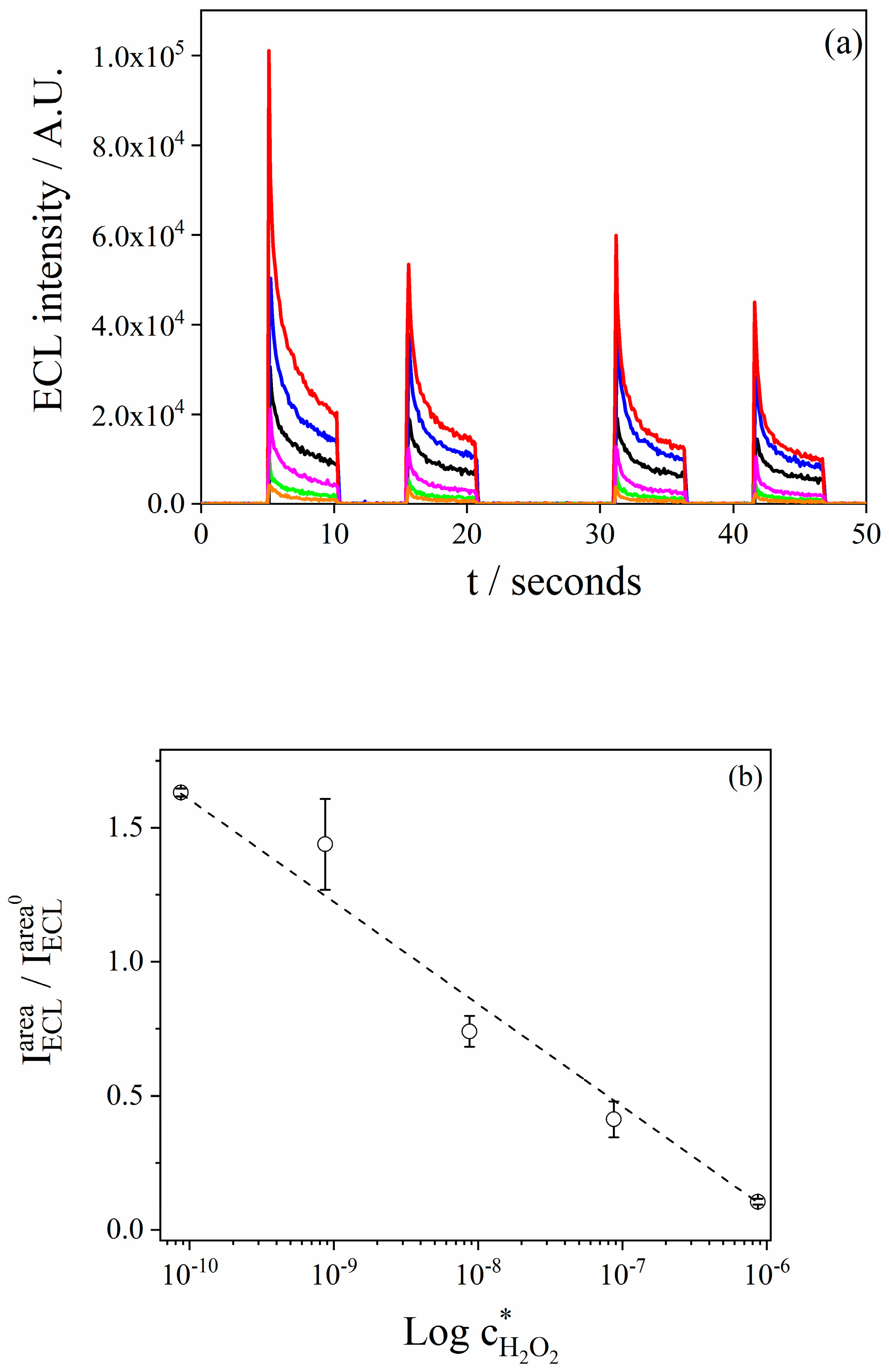
3.6. Analytical Performance of the Electrochemiluminiscence Sensor for H2O2
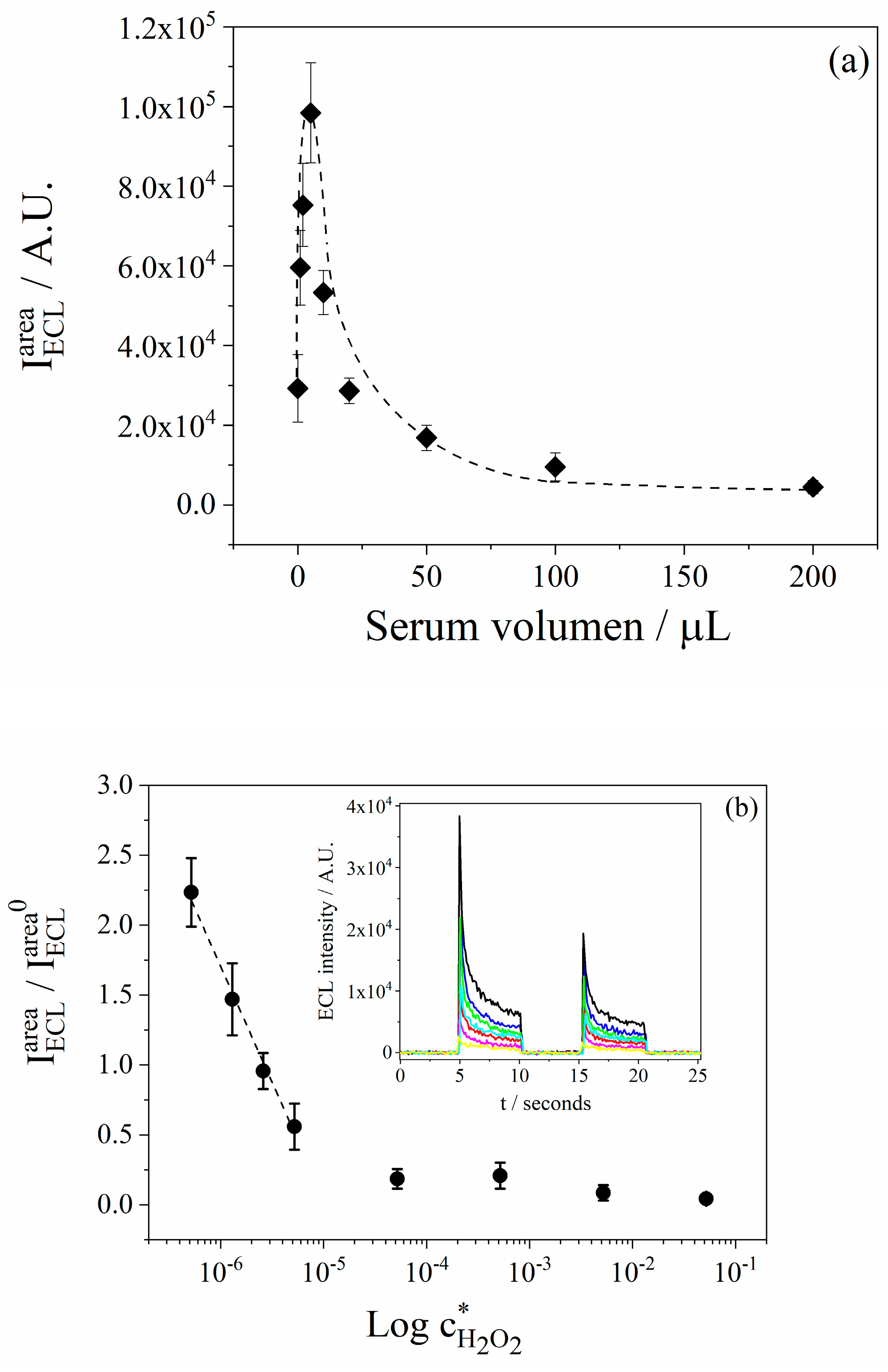
3.7. Quantification of H2O2 in Lyophilized Serum Samples
3.8. Comparison of the Performance of the ECL Sensor with Other Sensors for the Detection of H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QDs CdSe | cadmium selenide quantum dots |
| g-C3N4 | graphitic carbon nitride |
| ECL | electrochemiluminescence |
| RET | resonance energy transfer |
| LOD | limit of detection |
| LOQ | limit of quantification |
| Ea,p | anodic potential peak |
| Ec,p | cathodic potential peak |
| Eg | bandgap |
| MPA | mercaptopropionic acid |
| MES | 3-morpholinoethanesulfonic acid |
| TPrA | tripropylamine |
References
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Mol. Med. 2020, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zheng, L.; Wang, Z.; Xu, K.; Chen, W.; Liu, Z.; Chang, Z.; Zhou, Y.; Zhu, X.; Zhang, Y.; et al. Dual-mode ratiometric electrochemical and turn-on fluorescent probe for reliably detecting H2O2 in Parkinson’s disease serum. Sens. Actuators Rep. 2025, 9, 100305. [Google Scholar] [CrossRef]
- Wei, S.; Chang, S.; Li, H.; Fang, Z.; Zhu, L.; Xu, Y. Polymer photocatalysts for photocatalytic hydrogen peroxide production. Green Chem. 2024, 26, 6382–6403. [Google Scholar] [CrossRef]
- Karunakaran, C.; Madasamy, T.; Sethy, N.K. Chapter 3—Enzymatic Biosensors. Biosens. Bioelectron. 2015, 133–204. [Google Scholar] [CrossRef]
- He, Q.; Zang, S.; Zeng, Y.; Wang, B.; Song, X. A bifunctional fluorescent probe for dual-channel detection of H2O2 and HOCl in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 328, 125464. [Google Scholar] [CrossRef]
- Hong, C.; Wu, S.; Chen, S.; Lin, Z.; Chen, Q. Highly sensitive colorimetric method for the detection of organophosphorus pesticides based on H2O2 etching of silver-coated gold nanostars. Sens. Actuators B Chem. 2025, 425, 137004. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, A.; Halim, S.A.; Uddin, J.; Khan, A.; El Deeb, S.; Al-Harrasi, A. Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials 2022, 12, 1475. [Google Scholar] [CrossRef]
- Ghaedamini, H.; Kim, D.-S. A non-enzymatic hydrogen peroxide biosensor based on cerium metal-organic frameworks, hemin, and graphene oxide composite. Bioelectrochemistry 2025, 161, 108823. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Wang, W.; Wang, X.; Liu, C.; Xie, Q.; Li, Z.; Zhang, Z. Enhanced photoelectrochemical and photocatalytic performance of TiO2 nanorod arrays/CdS quantum dots by coating TiO2 through atomic layer deposition. Nano Energy 2015, 11, 400–408. [Google Scholar] [CrossRef]
- Wang, P.; Cao, L.; Chen, Y.; Wu, Y.; Di, J. Photoelectrochemical Biosensor Based on Co3O4 Nanoenzyme Coupled with PbS Quantum Dots for Hydrogen Peroxide Detection. ACS Appl. Nano Mater. 2019, 2, 2204–2211. [Google Scholar] [CrossRef]
- Quyen, T.T.B.; My, N.N.T.; Tran, N.T.Q.; Khang, T.M.; Thanh, L.H.V.; Le Anh Tuan, B.; Pham, D.T.; Le Thanh Huynh, N. Rapidly synthesis of AuM (M = Pt, Pd) hexagonals/graphene quantum dots nanostructures and their application for non-enzyme hydrogen peroxide detection. J. Mater. Res. 2024, 39, 2947–2961. [Google Scholar] [CrossRef]
- Kaplan, S.; Suna Karatekin, R.; Avci, G.; Kahya Düdükcü, M. A highly efficient catalyst consisting of Ni and S double-doped reduced graphene modified with ZnO nanoparticles for hydrogen peroxide electroreduction. Mater. Sci. Eng. B 2024, 308, 117581. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Cirone, J.; Chen, A. Fluorescent Fe3O4 Quantum Dots for H2O2 Detection. ACS Appl. Nano Mater. 2019, 2, 2076–2085. [Google Scholar] [CrossRef]
- Ruiyi, L.; Qingqing, X.; Zaijun, L.; Ruiling, Z.; Yongqiang, Y.; Xiaohao, L. Synthesis of histidine, serine and folic acid-functionalized and boron and iron-doped graphene quantum dot with excellent optical behavior and peroxidase-like activity for colorimetric and fluorescence detection of H2O2 in food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 324, 124950. [Google Scholar] [CrossRef]
- Asif, S.; Das, D.; Basu, S.; Morgan, D.; Datta, A.; Sen, K. Generating luminescent Graphene quantum Dots from Tryptophan: Fluorosensors for hydrogen peroxide in cancer cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 323, 124887. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Ma, Q. Recent progress and challenges in ECL biosensors for the identification of small extracellular vesicle and clinical diagnosis. TrAC Trends Anal. Chem. 2025, 189, 118253. [Google Scholar] [CrossRef]
- Atacan, K.; Özacar, M. Construction of a non-enzymatic electrochemical sensor based on CuO/g-C3N4 composite for selective detection of hydrogen peroxide. Mater. Chem. Phys. 2021, 266, 124527. [Google Scholar] [CrossRef]
- Majdoub, M.; Sengottuvelu, D.; Nouranian, S.; Al-Ostaz, A. Graphitic Carbon Nitride Quantum Dots (g-C3N4 QDs): From Chemistry to Applications. ChemSusChem 2024, 17, e202301462. [Google Scholar] [CrossRef]
- Low, S.S.; Chen, Z.; Li, Y.; Lu, Y.; Liu, Q. Design principle in biosensing: Critical analysis based on graphitic carbon nitride (g-C3N4) photoelectrochemical biosensor. TrAC Trends Anal. Chem. 2021, 145, 116454. [Google Scholar] [CrossRef]
- Roy, R.; Chacko, A.R.; Abraham, T.; Korah, B.K.; John, B.K.; Punnoose, M.S.; Mohan, C.; Mathew, B. Recent Advances in Graphitic Carbon Nitrides (g-C3N4) as Photoluminescence Sensing Probe: A Review. ChemistrySelect 2022, 7, e202200876. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, M.; Zheng, X.; Zhong, Y.; Zhu, X. Strategies to improve the electrochemiluminescence performance of graphitic carbon nitride nanomaterials. J. Electroanal. Chem. 2023, 947, 117784. [Google Scholar] [CrossRef]
- Liu, J.-W.; Luo, Y.; Wang, Y.-M.; Duan, L.-Y.; Jiang, J.-H.; Yu, R.-Q. Graphitic Carbon Nitride Nanosheets-Based Ratiometric Fluorescent Probe for Highly Sensitive Detection of H2O2 and Glucose. ACS Appl. Mater. Interfaces 2016, 8, 33439–33445. [Google Scholar] [CrossRef] [PubMed]
- Ju, P.; He, Y.; Wang, M.; Han, X.; Jiang, F.; Sun, C.; Wu, C. Enhanced Peroxidase-Like Activity of MoS2 Quantum Dots Functionalized g-C3N4 Nanosheets towards Colorimetric Detection of H2O2. Nanomaterials 2018, 8, 976. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Zhao, J.; Chen, S.; Yuan, R. An ultrasensitive sensing platform for microRNA-155 based on H2O2 quenched hydroxide-dependent ECL emission of PFO Pdots. Biosens. Bioelectron. 2020, 150, 111872. [Google Scholar] [CrossRef]
- Ramesh, A.; Ajith, A.; Gudipati, N.S.; Vanjari, S.R.; John, S.A.; Biju, V.; Subrahmanyam, C. Hybridization of Co3S4 and Graphitic Carbon Nitride Nanosheets for High-performance Nonenzymatic Sensing of H2O2. Biosensors 2023, 13, 108. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X. A Review on Quantum Dots Modified g-C3N4-Based Photocatalysts with Improved Photocatalytic Activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef]
- Xian, J.; Weng, Y.; Guo, H.; Li, Y.; Yao, B.; Weng, W. One-pot fabrication of Fe-doped carbon nitride nanoparticles as peroxidase mimetics for H2O2 and glucose detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 218–224. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Wang, L.; Liu, G.; Cheng, H.M. Selective Breaking of Hydrogen Bonds of Layered Carbon Nitride for Visible Light Photocatalysis. Adv. Mater. (Deerfield Beach Fla.) 2016, 28, 6471–6477. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Lu, J.; Guo, Z.; Zhang, M.; Li, D.; Zhao, Z.; Song, S.; Liu, Y.; Qin, L. A CQD/CdS/g-C3N4 photocatalyst for dye and antibiotic degradation: Dual carrier driving force and tunable electron transfer pathway. Sep. Purif. Technol. 2023, 305, 122333. [Google Scholar] [CrossRef]
- Salazar-Marín, D.; Oza, G.; Real, J.A.D.; Cervantes-Uribe, A.; Pérez-Vidal, H.; Kesarla, M.K.; Torres, J.G.T.; Godavarthi, S. Distinguishing between type II and S-scheme heterojunction materials: A comprehensive review. Appl. Surf. Sci. Adv. 2024, 19, 100536. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Sha, H.; Xiong, X.; Jia, N. Design and Biosensing of a Ratiometric Electrochemiluminescence Resonance Energy Transfer Aptasensor between a g-C3N4 Nanosheet and Ru@MOF for Amyloid-β Protein. ACS Appl. Mater. Interfaces 2019, 11, 36299–36306. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Li, J.; Wang, F.; Yang, H.; Jiang, Y.; Zhang, P.; Cai, R.; Tan, W. Novel Ultralow-Potential Electrochemiluminescence Aptasensor for the Highly Sensitive Detection of Zearalenone Using a Resonance Energy Transfer System. Anal. Chem. 2023, 95, 15125–15132. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.; Zhang, Y.; Yu, H.; Rong, S.; Meng, L.; Song, S.; Mei, Y.; Pan, H.; Chang, D. Electrochemiluminescence immunosensor for cancer antigen 125 detection based on novel resonance energy transfer between graphitic carbon nitride and NIR CdTe/CdS QDs. J. Electroanal. Chem. 2021, 886, 115104. [Google Scholar] [CrossRef]
- Hu, L.; Shi, T.; Chen, J.; Cui, Q.; Yu, H.; Wu, D.; Ma, H.; Wei, Q.; Ju, H. Dual-quenching electrochemiluminescence resonance energy transfer system from CoPd nanoparticles enhanced porous g-C3N4 to FeMOFs-sCuO for neuron-specific enolase immunosensing. Biosens. Bioelectron. 2023, 226, 115132. [Google Scholar] [CrossRef]
- Shi, T.; Hu, L.; Chen, J.; Cui, Q.; Yu, H.; Li, Y.; Wu, D.; Ma, H.; Wei, Q.; Ju, H. Dual-quenching electrochemiluminescence system based on resonance energy transfer from gold dendrite@polypyrrole core–shell nanoparticles enhanced g-C3N4 to ZnONFs@PDA-sCuO for procalcitonin immunosensing. Sens. Actuators B Chem. 2022, 371, 132591. [Google Scholar] [CrossRef]
- Feng, Q.-M.; Shen, Y.-Z.; Li, M.-X.; Zhang, Z.-L.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. Dual-Wavelength Electrochemiluminescence Ratiometry Based on Resonance Energy Transfer between Au Nanoparticles Functionalized g-C3N4 Nanosheet and Ru(bpy)32+ for microRNA Detection. Anal. Chem. 2016, 88, 937–944. [Google Scholar] [CrossRef]
- Surana, K.; Singh, P.K.; Rhee, H.-W.; Bhattacharya, B. Synthesis, characterization and application of CdSe quantum dots. J. Ind. Eng. Chem. 2014, 20, 4188–4193. [Google Scholar] [CrossRef]
- Cheng, J.-l.; Liu, X.-P.; Chen, J.-S.; Mao, C.-j.; Jin, B.-K. Highly sensitive electrochemiluminescence biosensor for VEGF165 detection based on a g-C3N4/PDDA/CdSe nanocomposite. Anal. Bioanal. Chem. 2020, 412, 3073–3081. [Google Scholar] [CrossRef]
- Palomino, R.A.C.; Zon, M.A.; Fernández, H.; Porcal, G.V.; Arévalo, F.J. Influence of a dual-stabiliser formed by 3-morpholinoethanesulfonic and 3-mercaptopropionic acids on the photophysical, electrochemical and electrochemiluminescent properties of aqueous soluble CdSe quantum dots. J. Electroanal. Chem. 2024, 958, 118156. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Guru, S.; Rao, G.R. Review—Strategic Design of Layered Double Hydroxides and Graphitic Carbon Nitride Heterostructures for Photoelectrocatalytic Water Splitting Applications. J. Electrochem. Soc. 2022, 169, 046515. [Google Scholar] [CrossRef]
- Tong, J.; Zhang, L.; Li, F.; Wang, K.; Han, L.; Cao, S. Rapid and high-yield production of g-C3N4 nanosheets via chemical exfoliation for photocatalytic H2 evolution. RSC Adv. 2015, 5, 88149–88153. [Google Scholar] [CrossRef]
- She, X.; Xu, H.; Xu, Y.; Yan, J.; Xia, J.; Xu, L.; Song, Y.; Jiang, Y.; Zhang, Q.; Li, H. Exfoliated graphene-like carbon nitride in organic solvents: Enhanced photocatalytic activity and highly selective and sensitive sensor for the detection of trace amounts of Cu2+. J. Mater. Chem. A 2014, 2, 2563–2570. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Dai, K.; Liang, C. Amine-Modified S-Scheme Porous g-C3N4/CdSe–Diethylenetriamine Composite with Enhanced Photocatalytic CO2 Reduction Activity. ACS Appl. Energy Mater. 2021, 4, 956–968. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Chae, K.H.; Won, S.O. Mechanistic investigations on emission characteristics from g-C3N4, g-C3N4@Pt and g-C3N4@Ag nanostructures using X-ray absorption spectroscopy. Curr. Appl. Phys. 2018, 18, 1458–1464. [Google Scholar] [CrossRef]
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7, 100. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Gupta, N.; Todi, K.; Narayan, T.; Malhotra, B.D. Graphitic carbon nitride-based nanoplatforms for biosensors: Design strategies and applications. Mater. Today Chem. 2022, 24, 100770. [Google Scholar] [CrossRef]
- Li, C.; Zou, X.; Lin, W.; Mourad, H.; Meng, J.; Liu, Y.; Abdellah, M.; Guo, M.; Zheng, K.; Nordlander, E. Graphitic Carbon Nitride/CdSe Quantum Dot/Iron Carbonyl Cluster Composite for Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 6280–6289. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, X.; Ying, E.; Dong, S. Quantum Dot Electrochemiluminescence in Aqueous Solution at Lower Potential and Its Sensing Application. J. Phys. Chem. C 2008, 112, 4451–4454. [Google Scholar] [CrossRef]
- Daviddi, E.; Oleinick, A.; Svir, I.; Valenti, G.; Paolucci, F.; Amatore, C. Theory and Simulation for Optimising Electrogenerated Chemiluminescence from Tris(2,2′-bipyridine)-ruthenium(II)-Doped Silica Nanoparticles and Tripropylamine. ChemElectroChem 2017, 4, 1719–1730. [Google Scholar] [CrossRef]
- Miao, W.; Choi, J.P.; Bard, A.J. Electrogenerated chemiluminescence 69: The tris(2,2′-bipyridine)ruthenium(II), (Ru(bpy)3)2+/tri-n-propylamine (TPrA) system revisited-a new route involving TPrA*+ cation radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Gan, Y.; Qin, J.; Ma, L.; Liu, Q.; Huang, L.; Yang, Z.; Zang, G.; Zhu, S. An ultrasensitive solid-state ECL biosensor based on synergistic effect between Zn-NGQDs and porphyrin-based MOF as “on-off-on” platform. Colloids Surf. B Biointerfaces 2023, 226, 113322. [Google Scholar] [CrossRef]
- Olivieri, A.C. Practical guidelines for reporting results in single- and multi-component analytical calibration: A tutorial. Anal. Chim. Acta 2015, 868, 10–22. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, G.; Jiang, C.; Zheng, X.T.; Zheng, L.; Li, C.M. Highly sensitive detection of hydrogen peroxide at a carbon nanotube fiber microelectrode coated with palladium nanoparticles. Microchim. Acta 2014, 181, 63–70. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.-b.; Pan, H.-y.; Ding, Y.-y.; Wan, J.-g.; Wang, G.-h.; Han, M. Detection of hydrogen peroxide at a palladium nanoparticle-bilayer graphene hybrid-modified electrode. Sens. Actuators B Chem. 2016, 230, 690–696. [Google Scholar] [CrossRef]
- Zhou, T.; Yao, Q.; Zhao, T.; Chen, X. One-pot synthesis of fluorescent DHLA-stabilized Cu nanoclusters for the determination of H2O2. Talanta 2015, 141, 80–85. [Google Scholar] [CrossRef]
- Chang, H.-C.; Ho, J.-a.A. Gold Nanocluster-Assisted Fluorescent Detection for Hydrogen Peroxide and Cholesterol Based on the Inner Filter Effect of Gold Nanoparticles. Anal. Chem. 2015, 87, 10362–10367. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Zhang, X.; Chai, H.; Huang, Y. One-pot synthesis of the CuNCs/ZIF-8 nanocomposites for sensitively detecting H2O2 and screening of oxidase activity. Biosens. Bioelectron. 2018, 105, 65–70. [Google Scholar] [CrossRef]
- Chen, H.; Fang, A.; He, L.; Zhang, Y.; Yao, S. Sensitive fluorescent detection of H2O2 and glucose in human serum based on inner filter effect of squaric acid-iron(III) on the fluorescence of upconversion nanoparticle. Talanta 2017, 164, 580–587. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Ren, Y.; Fan, J. Ratiometric determination of hydrogen peroxide based on the size-dependent green and red fluorescence of CdTe quantum dots capped with 3-mercaptopropionic acid. Microchim. Acta 2019, 186, 277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Huang, L.; Liao, Y.; Liu, Y.; Xiao, Q. A novel fluorescent probe for H2O2 detection based on CdSe@ZnS quantum dots/Ag nanocluster hybrid. Anal. Chim. Acta 2020, 1106, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, H.; Fan, Z.; Li, M.; Du, P.; Liu, C.; Li, Y.; Li, H.; Cao, H. A Highly Sensitive and Selective Hydrogen Peroxide Biosensor Based on Gold Nanoparticles and Three-Dimensional Porous Carbonized Chicken Eggshell Membrane. PLoS ONE 2015, 10, e0130156. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef]
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Danzer, K.; Currie, L.A. Guidelines for calibration in analytical chemistry. Part I. Fundamentals and single component calibration (IUPAC Recommendations 1998). Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
| Sample | Lifetime/ns | % Population | Lifetime/ns | % Population |
|---|---|---|---|---|
| τ1 | β1 | τ2 | β2 | |
| g-C3N4 | 0.41 | 12.62 | 2.84 | 87.38 |
| QD CdSe | 1.61 | 10.72 | 13.40 | 89.27 |
| QD CdSe@g-C3N4 | 1.39 | 8.98 | 14.07 | 91.02 |
| Sample | aEg/eV | Ea,p/V vs. HNE | Ec,p/V vs. HNE | ΔEp/V |
|---|---|---|---|---|
| g-C3N4 | 2.55 | 0.966 | −1.504 | 2.47 |
| QD CdSe | 2.23 | 0.594 | −1.724 | 2.32 |
| QD CdSe@g-C3N4 | 2.28 | 0.630 | −1.704 | 2.34 |
| Materials/Probe | Technique | Sample | Linear Range /μM | LOD /μM | Ref. |
|---|---|---|---|---|---|
| PdNP/CNT fibre | Amperometry | PBS, pH 7.4 | 2–1300 | 2 | [55] |
| Pd-NPs/BGFs/GCE | Amperometry | PBS, pH 7.4 | 4–13,500 | 1.5 | [56] |
| DHLA-CuNCs | Fluorescence sensing methods | Aqueous solution | 1–10 | 0.3 | [57] |
| AuNPs-AuNCs | PBS, pH 8 | 1–100 | 0.8 | [58] | |
| CuNCs/ZIF-8 | Acetate buffer, pH 7 | 0.5–30 | 0.5 | [59] | |
| Upconversion nanoparticles | PBS, pH 6.5 | 2.5–70 | 0.8 | [60] | |
| QDs CdTe | PBS, pH 7 | 10–125 | 0.3 | [61] | |
| CdSe@ZnS/AgNCs | Tris-HCl buffer | 0.5–60 | 0.3 | [62] | |
| CuO/g-C3N4/GCE | DPV | PBS, pH 7 | 0.5–50 | 0.31 | [17] |
| HRP/AuNPs/CESM | Amperometric | PBS, pH 7 | 10–2700 | 3 | [63] |
| QDs CdSe@g-C3N4 | ECL | Tris-HCl, pH 7.4 | 1 × 10−4–1 | 1.81 × 10−3 | This sensor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino, R.A.C.; Tocco, A.D.; Pierini, G.D.; Porcal, G.V.; Arévalo, F.J. Heterostructures of CdSe Quantum Dots and g-C3N4 Applied as Electrochemiluminescent Probes for the Detection of Hydrogen Peroxide in Human Serum. Chemosensors 2025, 13, 171. https://doi.org/10.3390/chemosensors13050171
Palomino RAC, Tocco AD, Pierini GD, Porcal GV, Arévalo FJ. Heterostructures of CdSe Quantum Dots and g-C3N4 Applied as Electrochemiluminescent Probes for the Detection of Hydrogen Peroxide in Human Serum. Chemosensors. 2025; 13(5):171. https://doi.org/10.3390/chemosensors13050171
Chicago/Turabian StylePalomino, Roodney Alberto Carrillo, Aylén Di Tocco, Gastón Darío Pierini, Gabriela Valeria Porcal, and Fernando Javier Arévalo. 2025. "Heterostructures of CdSe Quantum Dots and g-C3N4 Applied as Electrochemiluminescent Probes for the Detection of Hydrogen Peroxide in Human Serum" Chemosensors 13, no. 5: 171. https://doi.org/10.3390/chemosensors13050171
APA StylePalomino, R. A. C., Tocco, A. D., Pierini, G. D., Porcal, G. V., & Arévalo, F. J. (2025). Heterostructures of CdSe Quantum Dots and g-C3N4 Applied as Electrochemiluminescent Probes for the Detection of Hydrogen Peroxide in Human Serum. Chemosensors, 13(5), 171. https://doi.org/10.3390/chemosensors13050171






