Organophosphorus Pesticide Photoelectrochemical/Electrochemical Dual-Mode Smartsensors Derived from Synergistic Co,N-TiO2@ZrO2/3DGH Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Co, N-TiO2 Nanoparticles
2.3. Preparation of ZrO2 Nanoparticles
2.4. Preparation of Co, N-TiO2@ZrO2/3DGH Nanocomposites
2.5. Photoelectrochemical and Electrochemical Detection of OPs
2.6. Sample Preparation, Extraction, and Matrix Study
2.7. Characterization
3. Results and Discussion
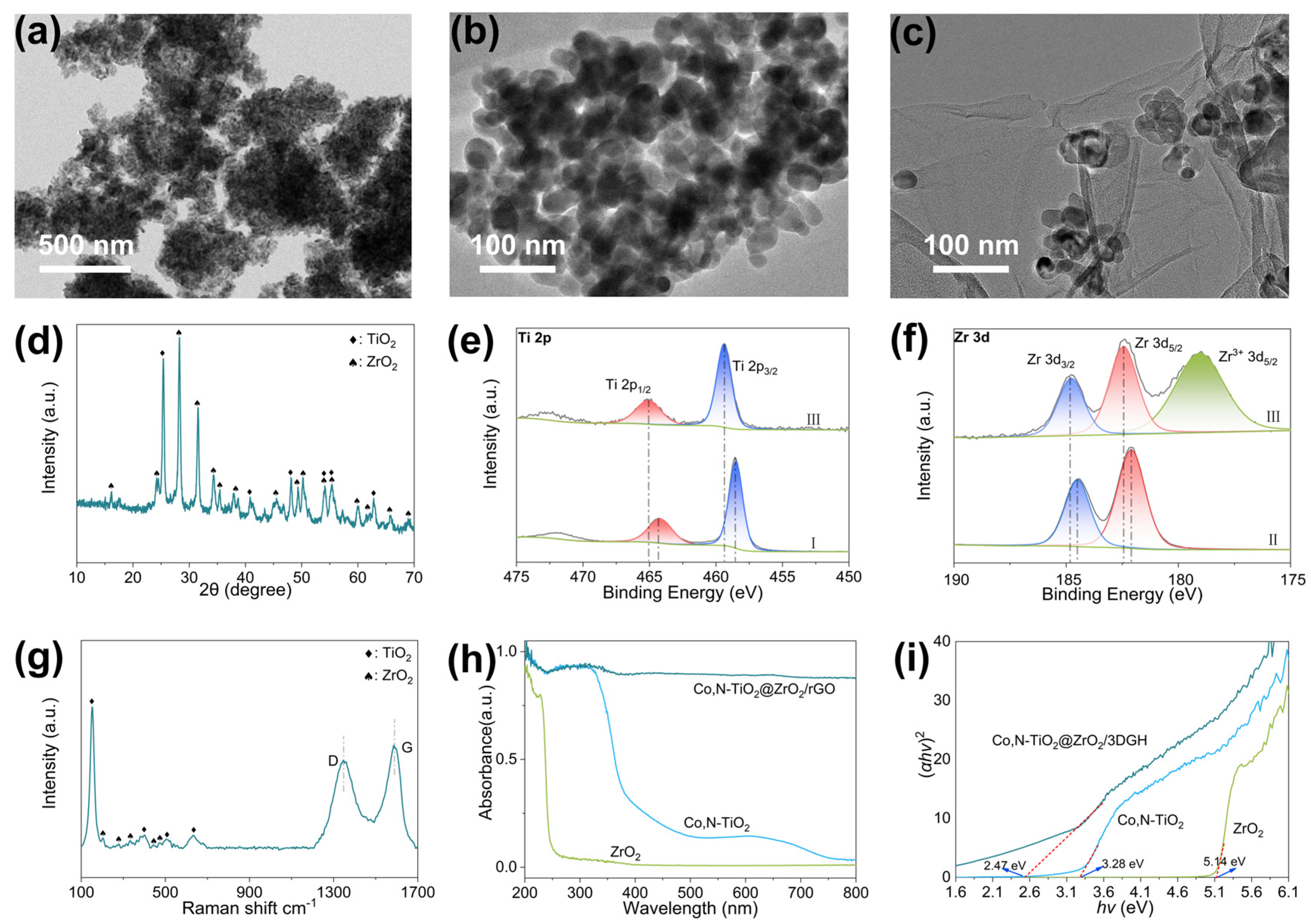
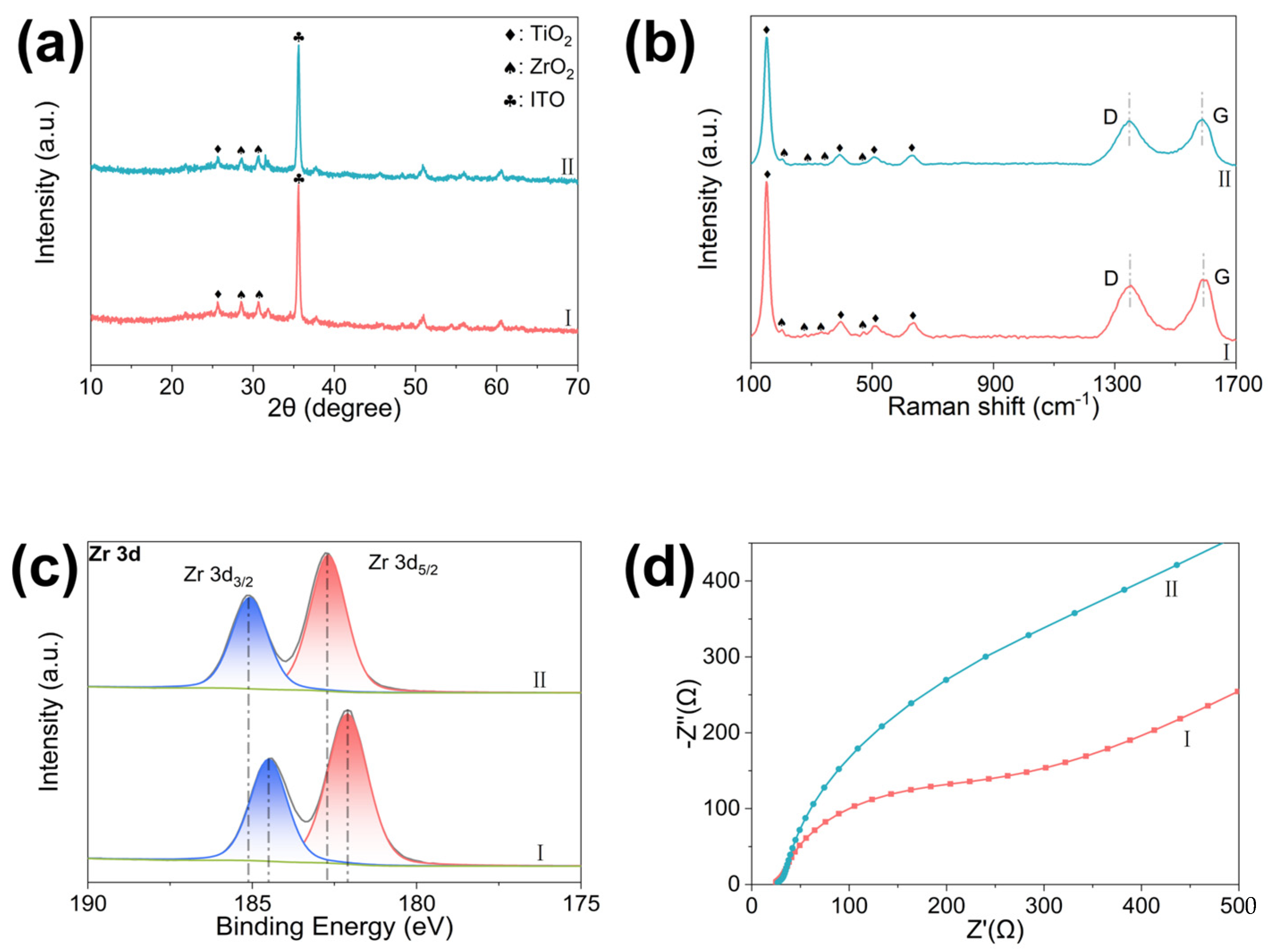
3.1. Characterization of Co,N-TiO2@ZrO2/3DGH Nanostructure
3.2. Sensing Mechanism of Co,N-TiO2@ZrO2/3DGH Nanostructure
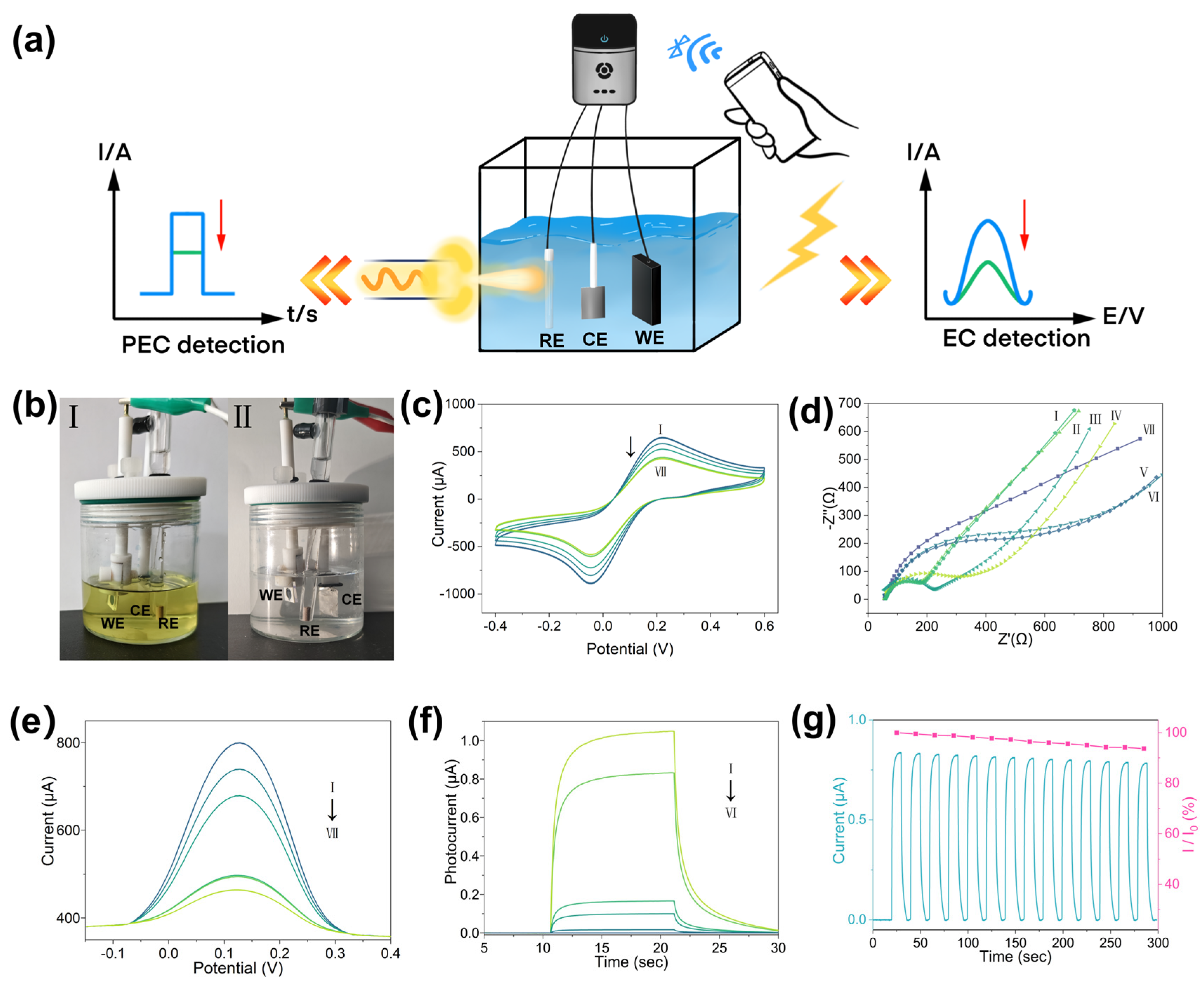
3.3. Testing and Optimization of the PEC-EC Dual-Mode Sensor
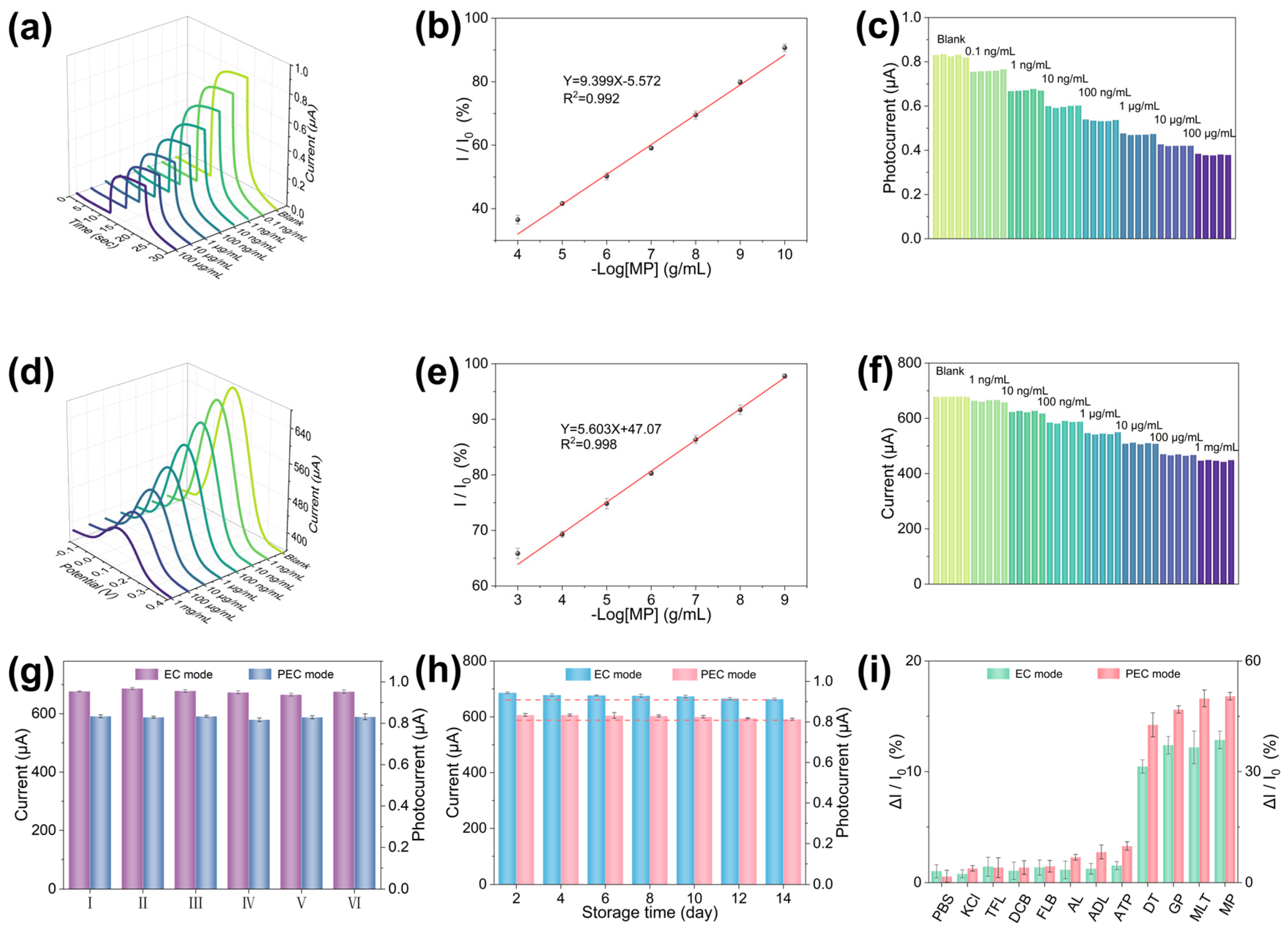
3.4. Performance Analysis of the PEC-EC Dual-Mode Sensor
3.5. Application of PEC-EC Dual-Mode Sensor in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wee, S.Y.; Aris, A.Z. Ecological risk estimation of organophosphorus pesticides in riverine ecosystems. Chemosphere 2017, 188, 575–581. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Liang, J.; Dong, Z.; Xu, N.; Chen, T.; Liang, J.; Xia, M.; Wang, F. A comprehensive review of multifunctional nanozymes for degradation and detection of organophosphorus pesticides in the environment. Toxics. 2024, 12, 926. [Google Scholar] [CrossRef]
- Xue, J.; Mao, K.; Cao, H.; Feng, R.; Chen, Z.; Du, W.; Zhang, H. Portable sensors equipped with smartphones for organophosphorus pesticides detection. Food Chem. 2024, 4, 1374563. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Helm, P.A.; Morse, D.; Reiner, E.J. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere 2018, 191, 288–295. [Google Scholar] [CrossRef]
- Wang, H.; Leeming, M.G.; Cochran, B.J.; Hook, J.M.; Ho, J.; Nguyen, G.T.H.; Zhong, L.; Supuran, C.T.; Donald, W.A. Nontargeted Identification of Plasma Proteins O-, N-, and S-Transmethylated by O-Methyl Organophosphates. Anal. Chem. 2020, 92, 15420–15428. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, M.; Li, X.; Jiang, Y.; Yu, L.; Zhao, Y.; Wen, L.; Xue, Q. Engineering an Ag/Au bimetallic nanoparticle-based acetylcholinesterase SERS biosensor for in situ sensitive detection of organophosphorus pesticide residues in food. Anal. Bioanal. Chem. 2023, 415, 203–210. [Google Scholar] [CrossRef]
- Li, T.; Wen, B.; Zhang, Y.; Zhang, L.; Li, J. Au@ZrO2 core-shell nanoparticles as a surface-enhanced Raman scattering substrate for organophosphorus compounds detection. J. Raman Spectrosc. 2022, 53, 1386–1393. [Google Scholar] [CrossRef]
- Wen, L.; Wang, J.; Liu, Z.; Tao, C.-A.; Rao, J.; Hang, J.; Li, Y. A portable acetylcholinesterase—based electrochemical sensor for field detection of organophosphorus. RSC Adv. 2023, 13, 6389–6395. [Google Scholar] [CrossRef]
- Kotagiri, Y.G.; Sandhu, S.S.; Morales, J.F.; Fernando, P.U.A.I.; Tostado, N.; Harvey, S.P.; Moores, L.C.; Wang, J. Sensor Array Chip for Real-Time Field Detection and Discrimination of Organophosphorus Neurotoxins. ChemElectroChem 2022, 9, e202200349. [Google Scholar] [CrossRef]
- Cancelliere, R.; Rea, G.; Severini, L.; Cerri, L.; Leo, G.; Paialunga, E.; Mantegazza, P.; Mazzuca, C.; Micheli, L. Expanding the circularity of plastic and biochar materials by developing alternative low environmental footprint sensors. Green Chem. 2023, 25, 6774–6783. [Google Scholar] [CrossRef]
- Wondimu, K.T.; Geletu, A.K.; Kedir, W.M. Recent developments in monitoring of organophosphorus pesticides in food samples. J. Agric. Food Res. 2025, 19, 101709. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.-C.; Wang, S.; Sillanpää, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Yang, P.; Hou, X.; Gao, X.; Peng, Y.; Li, Q.; Niu, Q.; Liu, Q. Recent Trends in Self-Powered Photoelectrochemical Sensors: From the Perspective of Signal Output. ACS Sens. 2024, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, L.; Zhuang, X.; Tang, P.; Chen, G.; Wang, H. A visible-light-driven photoelectrochemical sensor for the sensitive and selective detection of chlorpyrifos via CoS2 quantum dots/CdS nanowires nanocomposites with 0D/1D heterostructure. Chem. Eng. J. 2023, 476, 146770. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Zhang, J.; Yang, Z.; Li, J.; Cao, Y.; Wu, K.; Liu, Z.; Hao, J.; Ye, X. NIR-excitable POM-encapsulated Yb-Bi2S3 decorated graphene for wearable photoelectrochemical sensing. Adv. Funct. Mater. 2024, 34, 2315917. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, W.; Li, J.; Yu, X.; Zhu, Q.; Dai, Z. Component reconstitution-driven photoelectrochemical sensor for sensitive detection of Cu2+ based on advanced CuS/CdS p-n junction. Sci. China Chem. 2019, 62, 1725–1731. [Google Scholar] [CrossRef]
- Peng, J.; Huang, Q.; Zhuge, W.; Liu, Y.; Zhang, C.; Yang, W.; Xiang, G. Blue-light photoelectrochemical sensor based on nickel tetra-amined phthalocyanine-graphene oxide covalent compound for ultrasensitive detection of erythromycin. Biosens. Bioelectron. 2018, 106, 212–218. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Li, B.; Liu, B.; Ma, H.; Li, D.; Dong, L.; Li, F.; Chen, X.; Yin, X. VXC-72R/ZrO2/GCE-based electrochemical sensor for the high-sensitivity detection of methyl parathion. Materials 2019, 12, 3637. [Google Scholar] [CrossRef]
- Hao, N.; Dai, Z.; Xiong, M.; Hua, R.; Lu, J.; Wang, K. A portable solar-driven ratiometric photo-electrochromic visualization biosensor for detection of ochratoxin A. Sens. Actuators B Chem. 2020, 306, 127594. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A. A review on self-modification of zirconium dioxide nanocatalysts with enhanced visible-light-driven photodegradation of organic pollutants. J. Hazard. Mater. 2022, 423, 126996. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, R.; Di Tinno, A.; Cataldo, A.; Bellucci, S.; Kumbhat, S.; Michelia, L. Nafion-based label-free immunosensor as a reliable warning system: The case of AFB1 detection in cattle feed. Microchem. J. 2023, 191, 108868. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc Plot Method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Huang, S.; Yao, J.; Li, B.; Ning, G.; Xiao, Q. Integrating target-responsive CD-CdTe QD-based ratiometric fluorescence hydrogel with smartphone for visual and on-site determination of dichlorvos. Microchim. Acta 2021, 188, 318. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, X.; Zhang, Y.; Chen, H.; Ye, Y.; Wu, Y. Polydopamine-based nanozyme with dual-recognition strategy-driven fluorescence-colorimetric dual-mode platform for Listeria monocytogenes detection. J. Hazard. Mater. 2022, 439, 129582. [Google Scholar] [CrossRef]
- Wei, D.; Li, M.; Wang, Y.; Zhu, N.; Hua, X.; Zhao, B.; Zhang, Z.; Yin, D. Encapsulating gold nanoclusters into metal-organic frameworks to boost luminescence for sensitive detection of copper ions and organophosphorus pesticides. J. Hazard. Mater. 2023, 441, 129890. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Kong, D.; Yan, X.; Zhao, X.; Li, H.; Liu, F.; Sun, P.; Lin, Y.; Lu, G. Integrating target-responsive hydrogels with smartphone for on-site ppb-Level quantitation of organophosphate pesticides. ACS Appl. Mater. Interfaces 2019, 11, 27605–27614. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Liu, Y.; Zhang, X.; Tang, Y.; Chai, H.; Huang, Y. Size-controllable Fe-N/C single-atom nanozyme with exceptional oxidase-like activity for sensitive detection of alkaline phosphatase. Sens. Actuators B Chem. 2020, 305, 127511. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Xu, X.; Niu, X.; Wang, M.; Zhu, H.; Pan, J. Analyte-triggered oxidase-mimetic activity loss of Ag3PO4/UiO-66 enables colorimetric detection of malathion completely free from bioenzymes. Sens. Actuators B Chem. 2021, 338, 129866. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Lu, L.; Ma, X.; Gong, L.; Huang, X.; Liu, G.; Yu, Y. CuO nanoparticles decorated 3D graphene nanocomposite as non-enzymatic electrochemical sensing platform for malathion detection. J. Electroanal. Chem. 2018, 812, 82–89. [Google Scholar] [CrossRef]
- Gao, P.; Hussain, M.Z.; Zhou, Z.; Warnan, J.; Elsner, M.; Fischer, R.A. Zr-based metalloporphyrin MOF probe for electrochemical detection of parathion-methyl. Biosens. Bioelectron. 2024, 261, 116515. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, Y.; Nie, Y.; Lu, L.; Yang, C. A Cu2+-triggered turn-on fluorescence non-enzymatic probe based on covalent organic framework for the detection of methyl parathion. Anal. Chim. Acta 2025, 1346, 343775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Elder, T.; Cheng, Z.; Zhan, K.; Peng, Y.; Li, M. Cellulose nanofiber-templated metal-organic frameworks for fluorescent detection of methyl parathion pesticides. J. Environ. Chem. Eng. 2024, 12, 112670. [Google Scholar] [CrossRef]
- Ma, J.; Huang, G.; Li, J.; Li, J.; Yan, L.; Wei, J.; Zhang, Q. Facile green preparation of highly fluorescent nitrogen-doped carbon dots from bagasse for sensitive methyl parathion detection. Food Chem. 2025, 466, 142183. [Google Scholar] [CrossRef] [PubMed]

| Test Solution | PEC Mode | EC Mode |
|---|---|---|
| SSWS | 0.17 ± 0.04 ng/mL | 0.21 ± 0.03 ng/mL |
| SSWS-P | 14.74 ± 0.15 ng/mL | 15.49 ± 0.22 ng/mL |
| SCS | 0.49 ± 0.03 ng/mL | 0.68 ± 0.05 ng/mL |
| SCS-P (10 ng/mL) | 9.78 ± 0.18 ng/mL | 10.45 ± 0.23 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ma, H.; Mo, H.; Zhu, N. Organophosphorus Pesticide Photoelectrochemical/Electrochemical Dual-Mode Smartsensors Derived from Synergistic Co,N-TiO2@ZrO2/3DGH Platform. Chemosensors 2025, 13, 167. https://doi.org/10.3390/chemosensors13050167
Zhang Z, Ma H, Mo H, Zhu N. Organophosphorus Pesticide Photoelectrochemical/Electrochemical Dual-Mode Smartsensors Derived from Synergistic Co,N-TiO2@ZrO2/3DGH Platform. Chemosensors. 2025; 13(5):167. https://doi.org/10.3390/chemosensors13050167
Chicago/Turabian StyleZhang, Zhouxiaolong, Hongting Ma, Hao Mo, and Nan Zhu. 2025. "Organophosphorus Pesticide Photoelectrochemical/Electrochemical Dual-Mode Smartsensors Derived from Synergistic Co,N-TiO2@ZrO2/3DGH Platform" Chemosensors 13, no. 5: 167. https://doi.org/10.3390/chemosensors13050167
APA StyleZhang, Z., Ma, H., Mo, H., & Zhu, N. (2025). Organophosphorus Pesticide Photoelectrochemical/Electrochemical Dual-Mode Smartsensors Derived from Synergistic Co,N-TiO2@ZrO2/3DGH Platform. Chemosensors, 13(5), 167. https://doi.org/10.3390/chemosensors13050167






