The Construction of Carbazole-Based Metal–Organic Frameworks as Fluorescent Probes for Picric Acid Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials, Synthetic Procedures, and Measurements
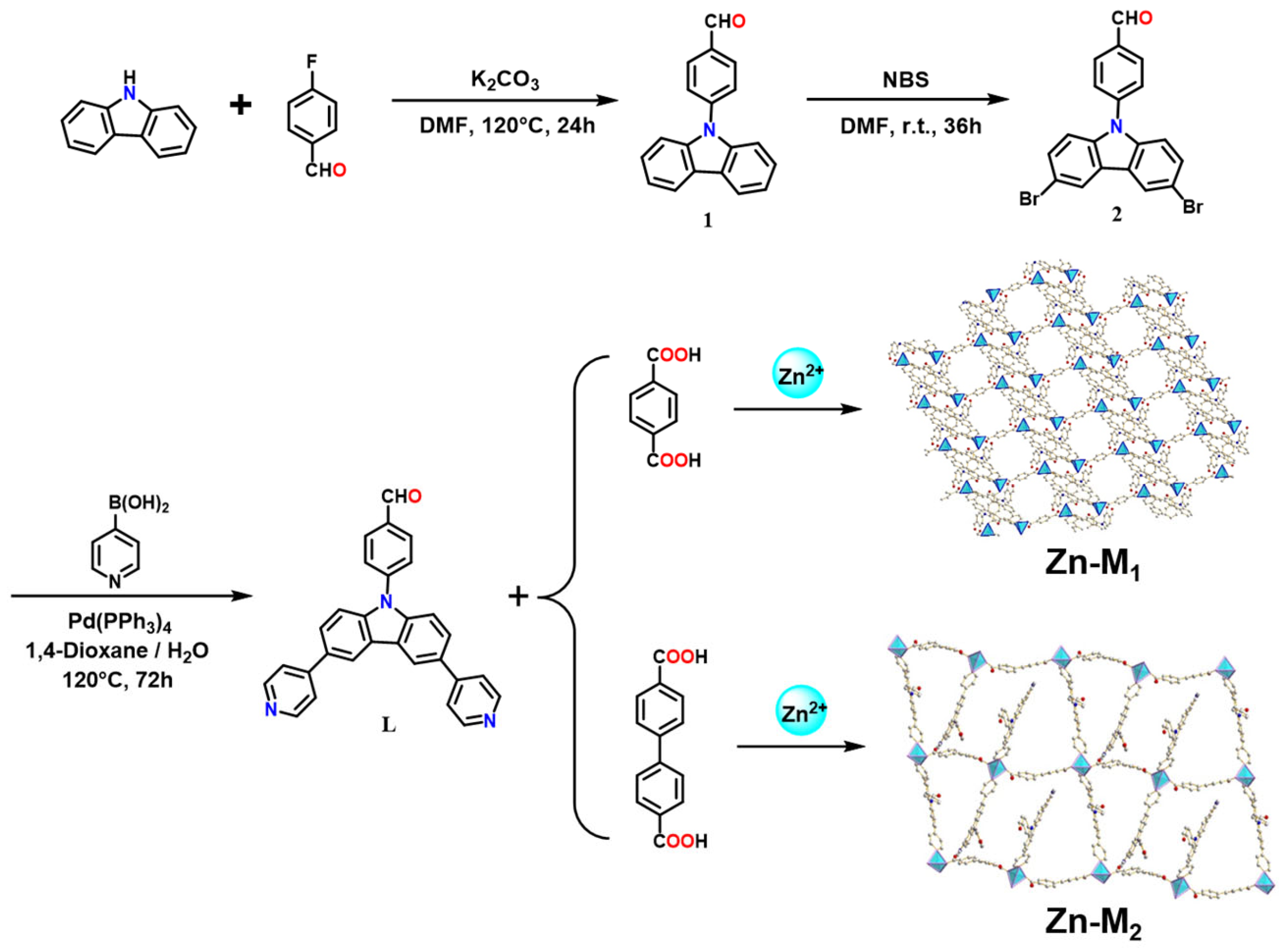
2.1.1. Synthesis of 4-(9H-Carbazol-9-yl) Benzaldehyde (1)
2.1.2. Synthesis of 4-(3,6-Dibromo-9H-Carbazol-9-yl) Benzaldehyde (2)
2.1.3. Synthesis of 4-(3,6-Dipyridyl-9H-Carbazol-9-yl) Benzaldehyde (L)
2.2. Synthesis of Zn-M1 and Zn-M2
2.3. Synthesis of Zn-M1-PSM
2.4. Zn-Ms Responses to Different NEs
2.4.1. Fluorescence Turn-Off Sensing of PA
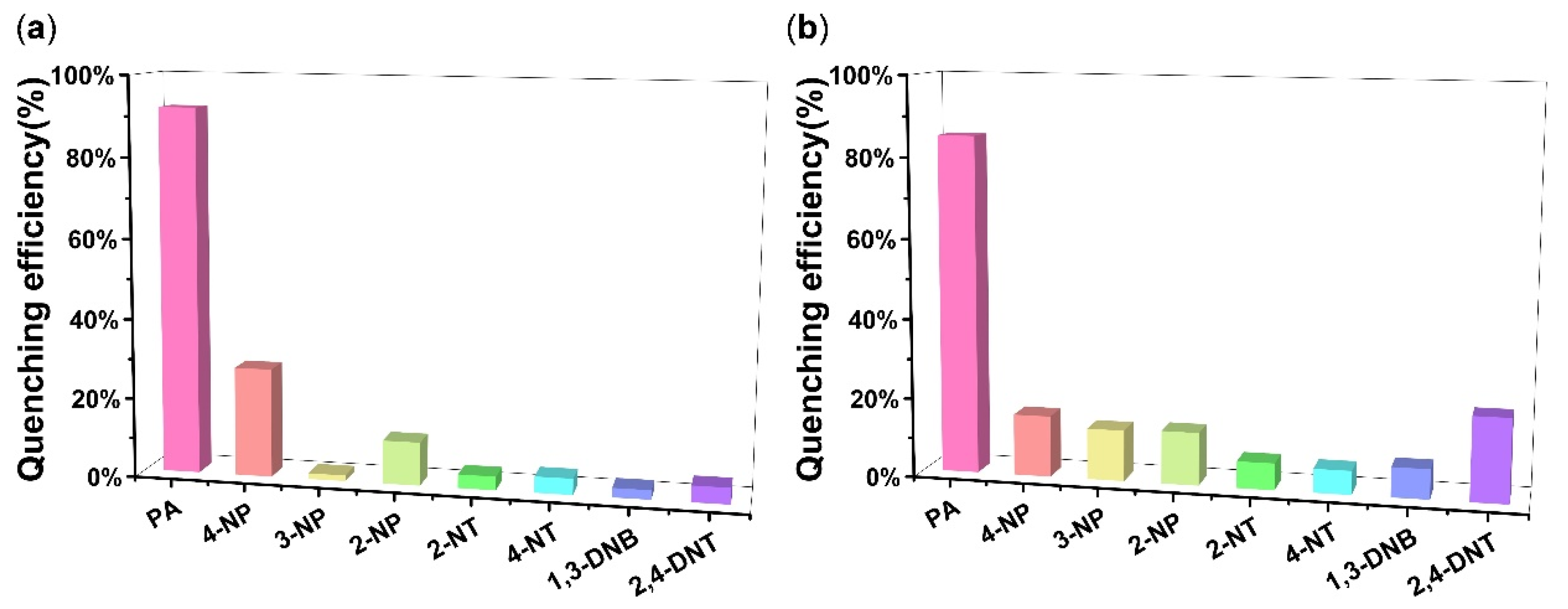
2.4.2. Selective Detection Experiment
3. Crystallography
4. Results and Discussion
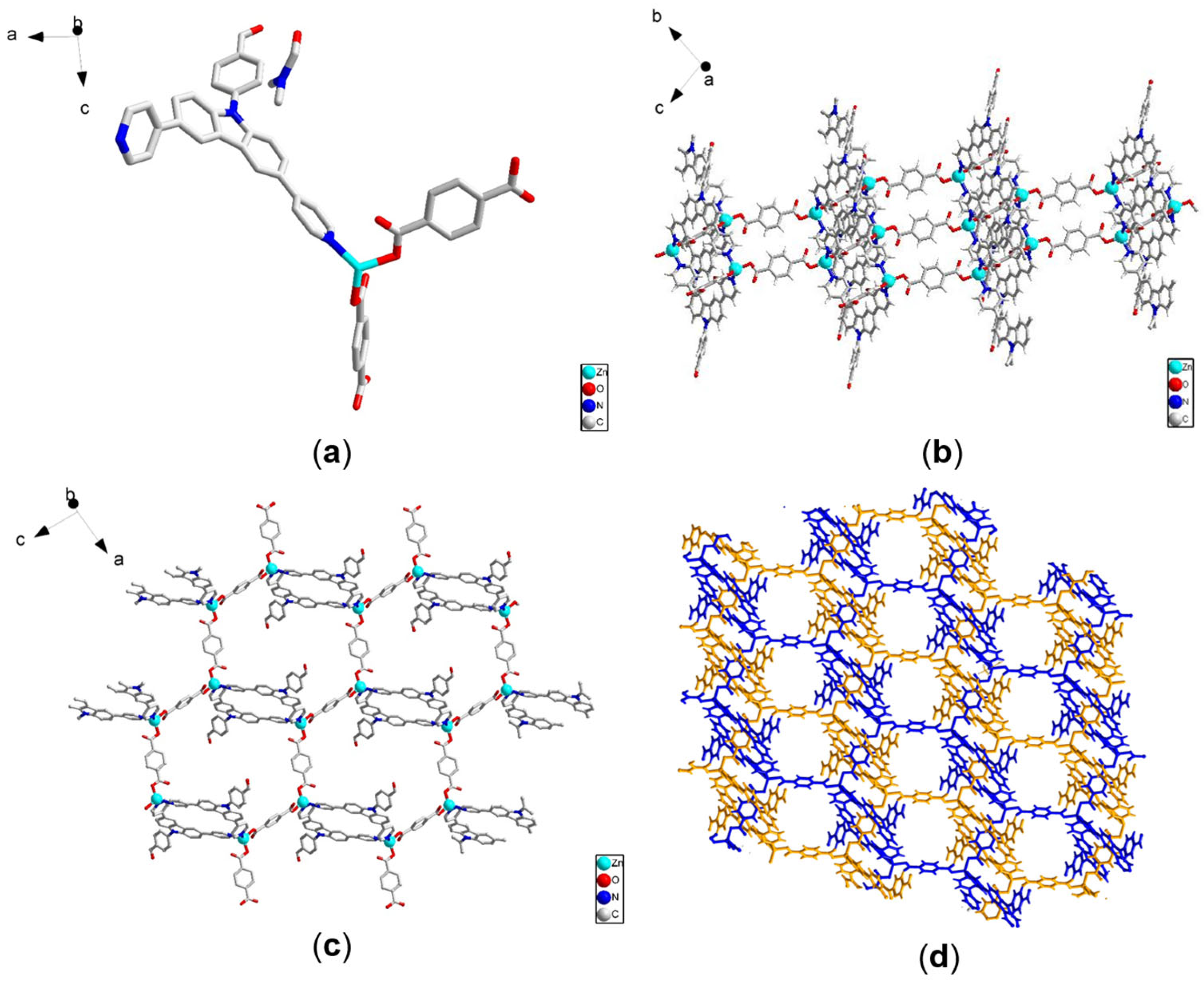
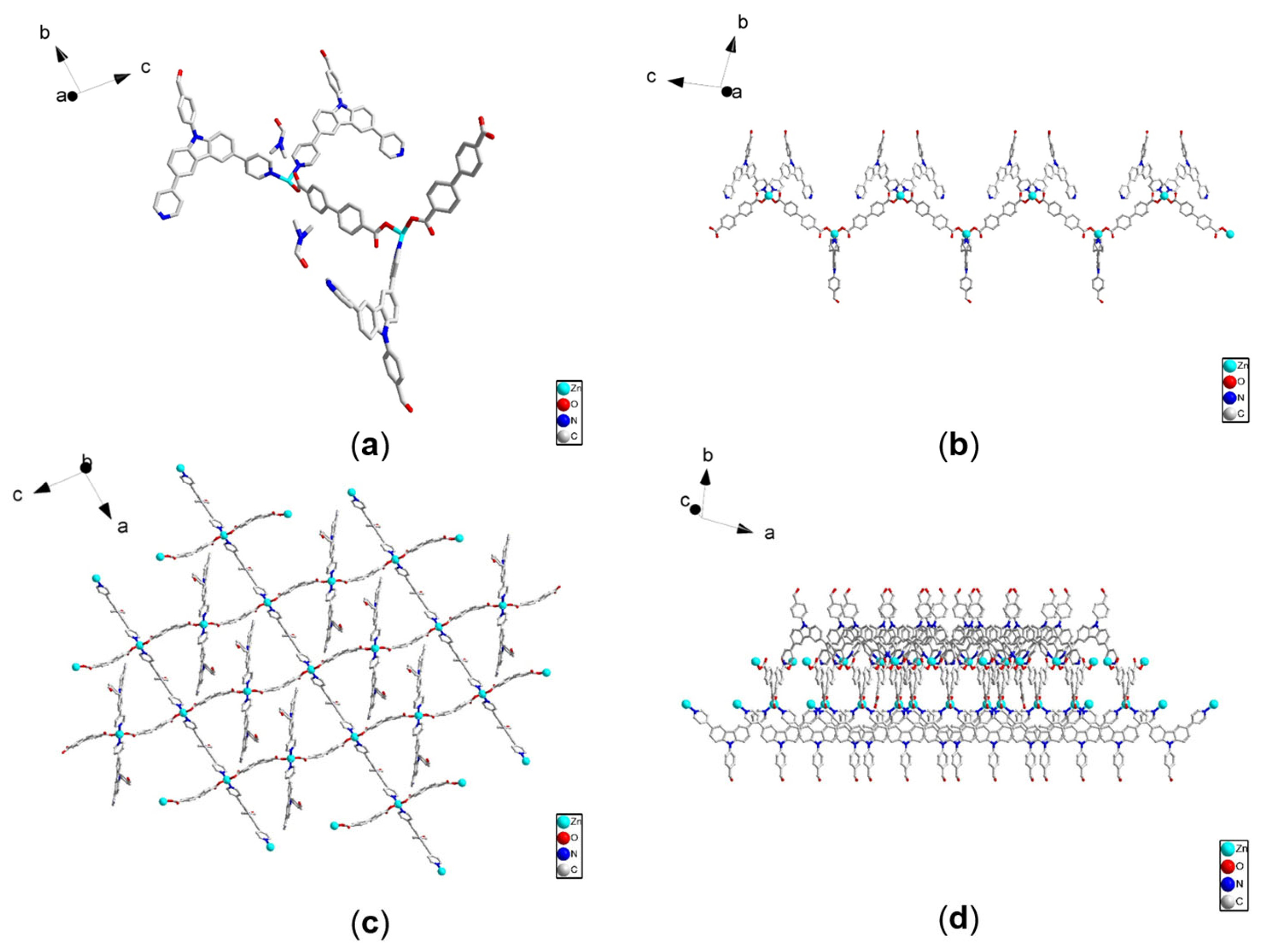
4.1. Structure of Two MOFs
4.2. PXRD, TGA, and FT-IR Characterization of Two MOFs
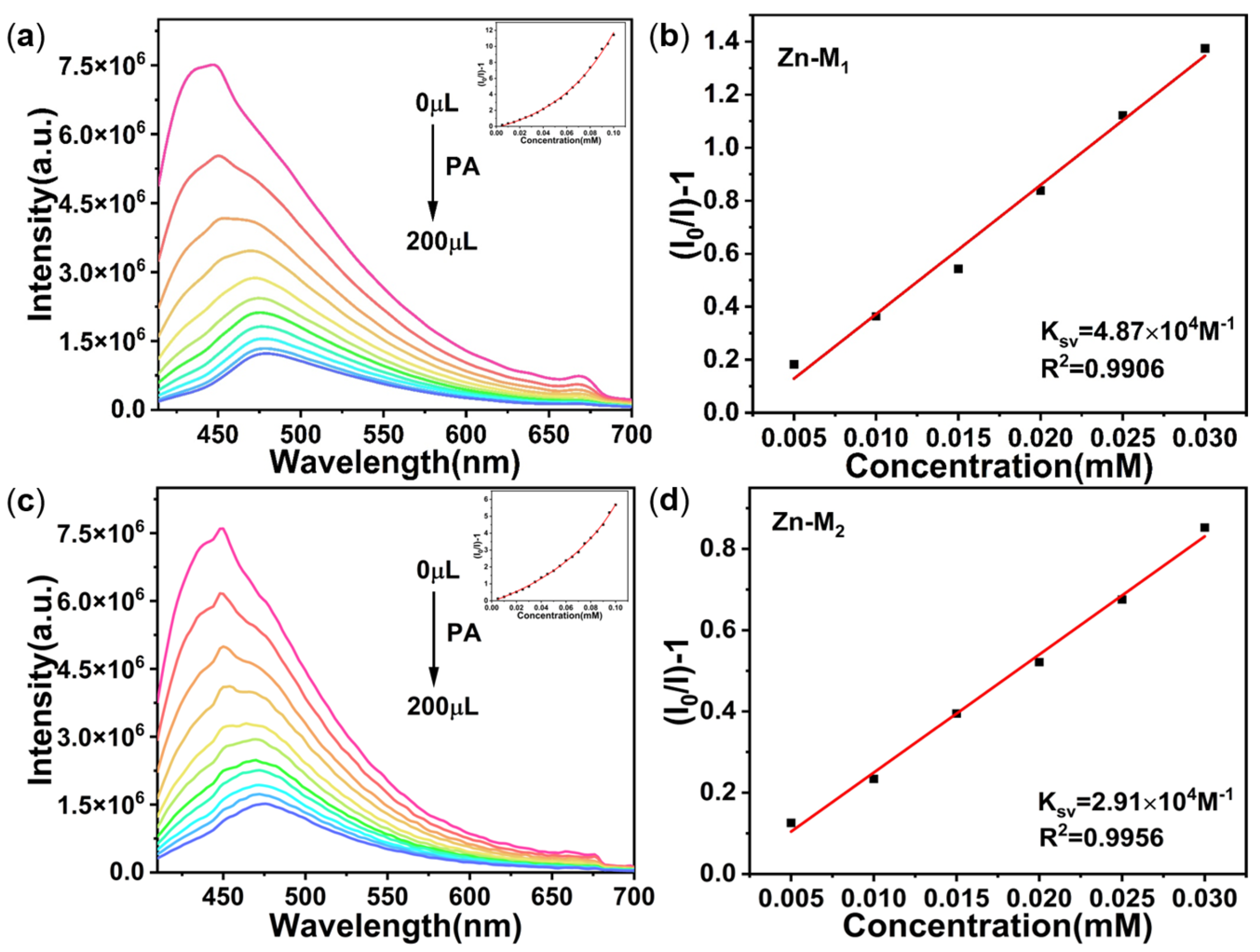
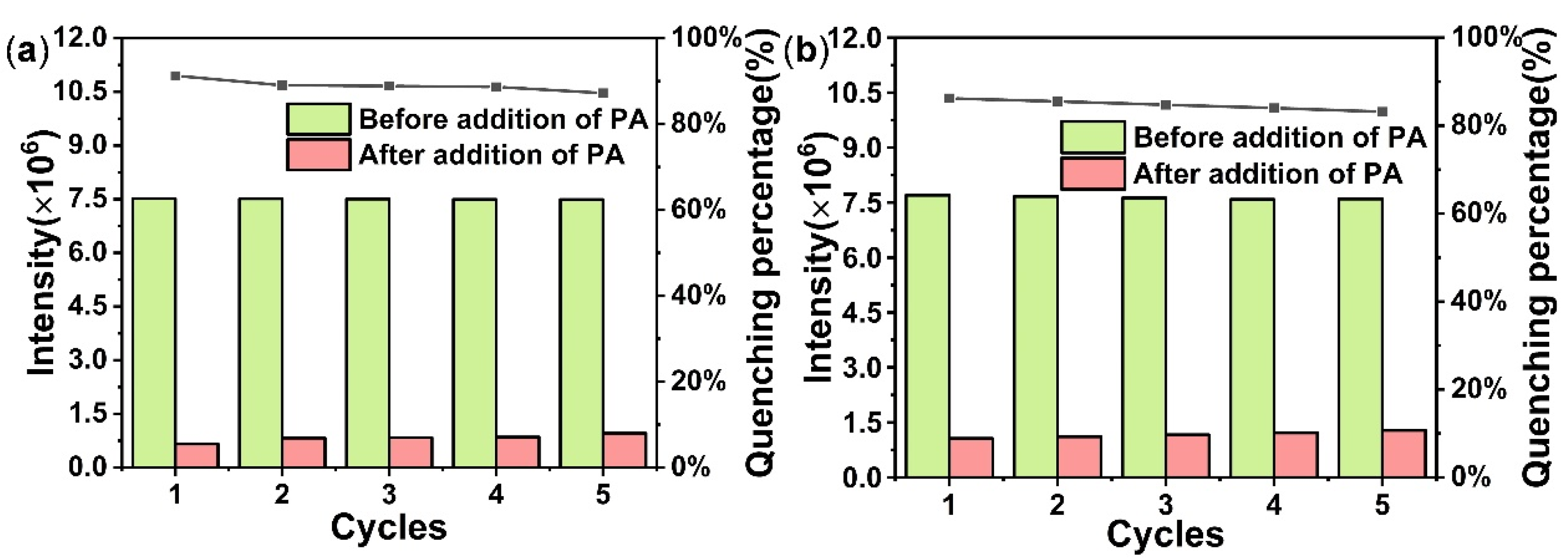
4.3. NEs Detection
4.4. Luminescence Quenching Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, L.J.; Yang, Z.; Li, Y.S.; Zu, B.Y.; Dou, X.C. Sensitive, real-time and anti-interfering detection of nitro-explosive vapors realized by ZnO/rGO core/shell micro-Schottky junction. Sens. Actuators B Chem. 2017, 239, 286–294. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.G.; Wang, G.F.; Zu, B.Y.; Dou, X.C. One-Step Instantaneous Detection of Multiple Military and Improvised Explosives Facilitated by Colorimetric Reagent Design. Anal. Chem. 2020, 92, 13980–13988. [Google Scholar] [CrossRef]
- Giannoukos, S.; Brkic, B.; Taylor, S.; Marshall, A.; Verbeck, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef]
- Goel, N.; Kumar, N. A stable nonanuclear Tb(III), cluster for selective sensing of picric acid. Inorg. Chim. Acta. 2017, 463, 14–19. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly Selective Detection of Nitro Explosives by a Luminescent Metal-Organic Framework. Angew. Chem. Int. Ed. 2013, 52, 2881–2885. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Lin, Z.; Song, L.; Wang, Z.H.; Chen, Q.; Yang, T.; Zhou, X.H.; Xiao, H.P.; Yin, X.J. Guest-induced reversible structural transitions and concomitant on/off luminescence switching of an Eu(III) metal-organic framework and its application in detecting picric acid. New J. Chem. 2015, 39, 2289–2295. [Google Scholar] [CrossRef]
- Desai, V.; Panchal, M.; Dey, S.; Panjwani, F.; Jain, V.K. Recent Advancements for the Recognization of Nitroaromatic Explosives Using Calixarene Based Fluorescent Probes. J. Fluoresc. 2022, 32, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Parmar, B.; Rachuri, Y.; Bisht, K.K.; Laiya, R.; Suresh, E. Mechanochemical and Conventional Synthesis of Zn(II)/Cd(II) Luminescent Coordination Polymers: Dual Sensing Probe for Selective Detection of Chromate Anions and TNP in Aqueous Phase. Inorg. Chem. 2017, 56, 2627–2638. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Wang, J.; Wei, X.; Lu, W. Conjugated Microporous Polymer-Based Fluorescent Probe for Selective Detection of Nitro-explosives and Metal Nitrates. ACS Appl. Mater. Interfaces 2025, 17, 4033–4043. [Google Scholar] [CrossRef]
- Tripathi, N.; Kumar, R.; Singh, P.; Kumar, S. Ratiometric fluorescence “Turn On” probe for fast and selective detection of TNT in solution, solid and vapour. Sens. Actuators B Chem. 2017, 246, 1001–1010. [Google Scholar] [CrossRef]
- He, W.; Lv, D.; Guan, Y.; Yu, S. Post-synthesis modification of metal–organic frameworks: Synthesis, characteristics, and applications. J. Mater. Chem. A 2023, 11, 24519–24550. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 31, 1450–1459. [Google Scholar] [CrossRef]
- Wang, Y.H.; Fin, H.; Ma, Q.; Mo, K.; Mao, H.Z.; Feldhoff, A.; Cao, X.Z.; Li, Y.S.; Pan, F.S.; Jiang, Z.Y. A MOF Glass Membrane for Gas Separation. Angew. Chem. Int. Ed. 2020, 59, 4365–4369. [Google Scholar] [CrossRef]
- Qian, Q.H.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Sun, Y.W.; Yan, J.H.; Gao, Y.L.; Ji, T.T.; Chen, S.X.; Wang, C.; Lu, P.; Li, Y.S.; Liu, Y. Fabrication of Highly Oriented Ultrathin Zirconium Metal-Organic Framework Membrane from Nanosheets towards Unprecedented Gas Separation. Angew. Chem. Int. Ed. 2023, 62, e202216697. [Google Scholar]
- Li, S.; Han, W.Y.; An, Q.F.; Yong, K.T.; Yin, M.J. Defect Engineering of MOF-Based Membrane for Gas Separation. Adv. Funct. Mater. 2023, 33, 2303447. [Google Scholar] [CrossRef]
- Wang, C.; An, B.; Lin, W.B. Metal-Organic Frameworks in Solid-Gas Phase Catalysis. ACS Catal. 2019, 9, 130–146. [Google Scholar] [CrossRef]
- Sepehrmansourie, H.; Alamgholiloo, H.; Pesyan, N.N.; Zolfigol, M.A. A MOF-on-MOF strategy to construct double Z-scheme heterojunction for high-performance photocatalytic degradation. Appl. Catal. B Environ. Energy 2023, 321, 122082. [Google Scholar] [CrossRef]
- Ren, J.C.; Huang, Y.L.; Zhu, H.; Zhang, B.H.; Zhu, H.K.; Shen, S.H.; Tan, G.Q.; Wu, F.; He, H.; Lan, S.; et al. Recent progress on MOF-derived carbon materials for energy storage. Carbon Energy 2020, 2, 176–202. [Google Scholar] [CrossRef]
- Lázaro, I.A.; Wells, C.; Forgan, R.S. Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery. Angew. Chem. Int. Ed. 2020, 59, 5211–5217. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Cheng, T.; Hu, J.; Zhou, C.; Wang, Y.; Zhang, M. Luminescent metal-organic frameworks for nitro explosives detection. Sci. China Chem. 2016, 59, 929–947. [Google Scholar] [CrossRef]
- Asad, M.; Anwar, M.I.; Miao, B.; Abbas, A.; Majeed, S.; Mir, I.A.; Rabbani, M.S.; Hussain, S.; Xu, S.; Al-Tahan, M.A.; et al. Recent advances in luminescent metal-organic frameworks (L-MOFs) as sustainable materials for sensing of potentially toxic environmental ubiquitous explosive contaminants. Sustain. Mater. Technol. 2024, 42, e01155. [Google Scholar] [CrossRef]
- Cong, C.; Ma, H. Photonic Metal-Organic Frameworks. Adv. Opt. Mater. 2021, 9, 2100733. [Google Scholar] [CrossRef]
- Peng, D.; Lin, W.; Pan, H.; Zhi, X.; Zhou, Q.; Yi, L.; Wang, F.; Ren, G.; Pan, Q. Fluorescence detection of Al3+ and mechanism exploration based on La-MOF. J. Solid State Chem. 2024, 339, 124946. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Yan, R.; Young, D.J.; Mi, Y.; Hu, F. Fabrication of ultrathin 2D MOF nanosheets for folic acid detection. Chin. J. Anal. Chem. 2023, 51, 100251. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Kou, Y.; Yang, X.; Ju, Z.; Liu, W. Multifunctional lanthanide MOF luminescent sensor built by structural designing and energy level regulation of a ligand. Inorg. Chem. Front. 2022, 9, 4065–4074. [Google Scholar] [CrossRef]
- Sun, B.; Tao, T.; Liu, L.; Ding, R.; Mao, Y. Electron Transfer Facilitated by π–π Stacking during the Nitrobenzene Recognition Process of an MOF Sensor. J. Phys. Chem. C. 2021, 125, 22, 12433–12440. [Google Scholar] [CrossRef]
- Liu, L.; Ding, R.; Mao, Y.; Sun, B. Theoretical investigations on the nitro-explosive sensing process of a MOF sensor: Roles of hydrogen bond and π-π stacking. Chem. Phys. Lett. 2022, 793, 139393. [Google Scholar] [CrossRef]
- Chongdar, S.; Mondal, U.; Chakraborty, T.; Banerjee, P.; Bhaumik, A. A Ni-MOF as Fluorescent/Electrochemical Dual Probe for Ultrasensitive Detection of Picric Acid from Aqueous Media. ACS Appl. Mater. Interfaces 2023, 15, 14575–14586. [Google Scholar] [CrossRef]
- Feng, T.; Li, X.; Wu, J.; He, C.; Duan, C. Cavity-directed nitroaromatics sensing within a carbazole-based luminescent supramolecular M2L3 cage. Chin. Chem. Lett. 2020, 31, 95–98. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, J.; Wang, J.; Li, L.; Liu, Z.; Zhou, X.; You, Y.; Huang, W. A fluorescent Eu(III) MOF for highly selective and sensitive sensing of picric acid. Sci. China Chem. 2019, 62, 205–211. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Z.; Zhang, H.; Ye, Y.; Yang, Y.; Xiang, F.; Cai, K.; Xiang, S.; Chen, B.; Zhang, Z. A Flexible Hydrogen-Bonded Organic Framework Constructed from a Tetrabenzaldehyde with a Carbazole N−H Binding Site for the Highly Selective Recognition and Separation of Acetone. Angew. Chem. Int. Ed. 2022, 61, e202213959. [Google Scholar] [CrossRef]
- Xi, F.; Liu, H.; Yang, N.; Gao, E. Aldehyde-Tagged Zirconium Metal–Organic Frameworks: A Versatile Platform for Postsynthetic Modification. Inorg. Chem. 2016, 55, 4701–4703. [Google Scholar] [CrossRef]
- GB 5749-2006; Standards for Drinking Water Quality. National Standard of the People’s Republic of China: Beijing, China, 2006. Available online: https://www.dgav.pt/wp-content/uploads/2022/06/China-GB-5749-2006_Standards-for-Drinking-Water-Quality.pdf (accessed on 7 February 2025).
- GB 3838-2002; Environmental Quality Standard for Surface Water. National Standard of the People’s Republic of China: Beijing, China, 2002. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/quality_standard/200710/t20071024_111792.shtml (accessed on 7 February 2025).
- Geng, T.; Ye, S.; Wang, Y.; Zhu, H.; Wang, X.; Liu, X. Conjugated microporous polymers-based fluorescein for fluorescence detection of 2,4,6-trinitrophenol. Talanta 2017, 165, 282–288. [Google Scholar] [CrossRef]
- Coutinho, A.; Prieto, M. Ribonuclease T1 and alcohol dehydrogenase fluorescence quenching by acrylamide: A laboratory experiment for undergraduate students. J. Chem. Educ. 1993, 70, 425. [Google Scholar] [CrossRef]
- Huang, R.; Yu, Z.; Li, Z.; Lin, X.; Hou, J.; Hu, Z.; Zou, J. Photoluminescent metal-organic frameworks and their composites: Fundamentals, applications, and outlooks. Coord. Chem. Rev. 2025, 526, 216358. [Google Scholar] [CrossRef]
- Xing, S.; Bing, Q.; Qi, H.; Liu, J.; Bai, T.; Li, G.; Shi, Z.; Feng, S.; Xu, R. Rational Design and Functionalization of a Zinc Metal–Organic Framework for Highly Selective Detection of 2,4,6-Trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9, 23828–23835. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A.; Piroozzadeh, M. A Dihydrotetrazine-Functionalized Metal–Organic Framework as a Highly Selective Luminescent Host–Guest Sensor for Detection of 2,4,6-Trinitrophenol. Inorg. Chem. 2022, 61, 7820–7834. [Google Scholar] [CrossRef]
- Gao, T.; Ma, Z.; Ren, Y.; Wang, Z.; Zhang, M.; Chen, X.; Wang, J. Highly sensitive fluorescent sensing and photocatalytic degradation performance of two-dimensional Tb-organic network. J. Rare Earths 2024, 42, 303–313. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Chen, F.; Bai, G.; Xu, H.; Xu, S. A Fluorescent Titanium-based Metal-Organic Framework Sensor for Nitro-aromatics Detection. Z. Anorg. Allg. Chem. 2021, 647, 759–763. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Chen, C.; Xu, C.; Ma, J.; Yang, L.; Wang, W.; Dou, W.; Liu, W. Function-Oriented: The Construction of Lanthanide MOF Luminescent Sensors Containing Dual-Function Urea Hydrogen-Bond Sites for Efficient Detection of Picric Acid. Chem. Eur. J. 2019, 25, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lei, M.; Zhang, T.; Zhang, Q.; Zhang, R.; Yang, M. A water-stable multi-responsive luminescent Zn-MOF sensor for detecting TNP, NZF and Cr2O72− in aqueous media. Dalton Trans. 2021, 50, 3816–3824. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Li, J.; Li, Q.; Guo, Q.; Ru, J.; Ma, C.; Han, Y. A Eu(iii) metal–organic framework based on anthracenyl and alkynyl conjugation as a fluorescence probe for the selective monitoring of Fe3+ and TNP. RSC Adv. 2022, 12, 26945–26952. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Wang, X.; Li, H.; He, C. The Construction of Carbazole-Based Metal–Organic Frameworks as Fluorescent Probes for Picric Acid Detection. Chemosensors 2025, 13, 105. https://doi.org/10.3390/chemosensors13030105
Jia Y, Wang X, Li H, He C. The Construction of Carbazole-Based Metal–Organic Frameworks as Fluorescent Probes for Picric Acid Detection. Chemosensors. 2025; 13(3):105. https://doi.org/10.3390/chemosensors13030105
Chicago/Turabian StyleJia, Yichang, Xin Wang, Hechuan Li, and Cheng He. 2025. "The Construction of Carbazole-Based Metal–Organic Frameworks as Fluorescent Probes for Picric Acid Detection" Chemosensors 13, no. 3: 105. https://doi.org/10.3390/chemosensors13030105
APA StyleJia, Y., Wang, X., Li, H., & He, C. (2025). The Construction of Carbazole-Based Metal–Organic Frameworks as Fluorescent Probes for Picric Acid Detection. Chemosensors, 13(3), 105. https://doi.org/10.3390/chemosensors13030105





