Using BiOI/BiOCl Composite-Enhanced Cathodic Photocurrent and Amplifying Signal Variation in AgI for Developing a Highly Sensitive Photoelectrochemical Immunosensing Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BiOI/BiOCl Composites
2.2. Preparation of Ab2-AgI Conjugates
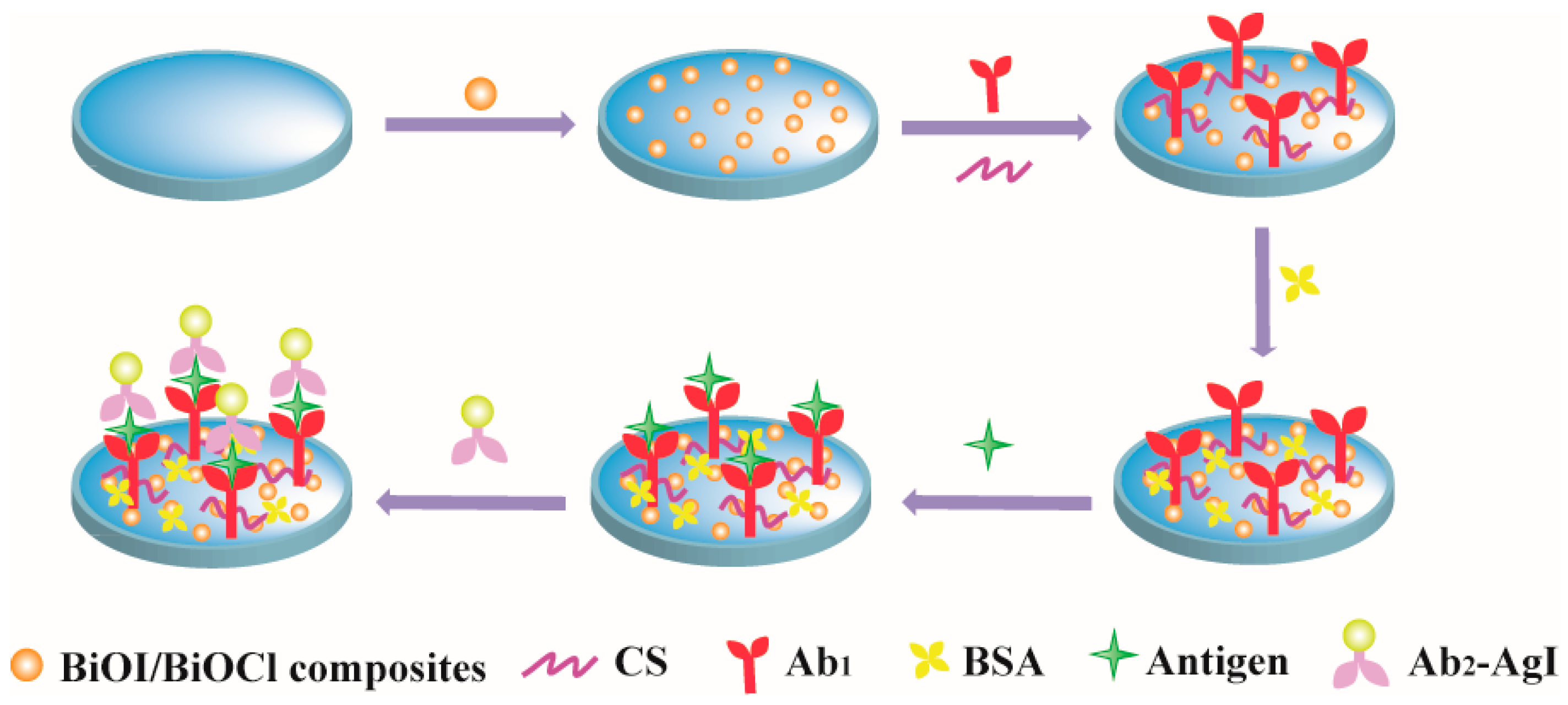
2.3. Fabrication of PEC Immunosensor
2.4. PEC Measurement
3. Results and Discussion
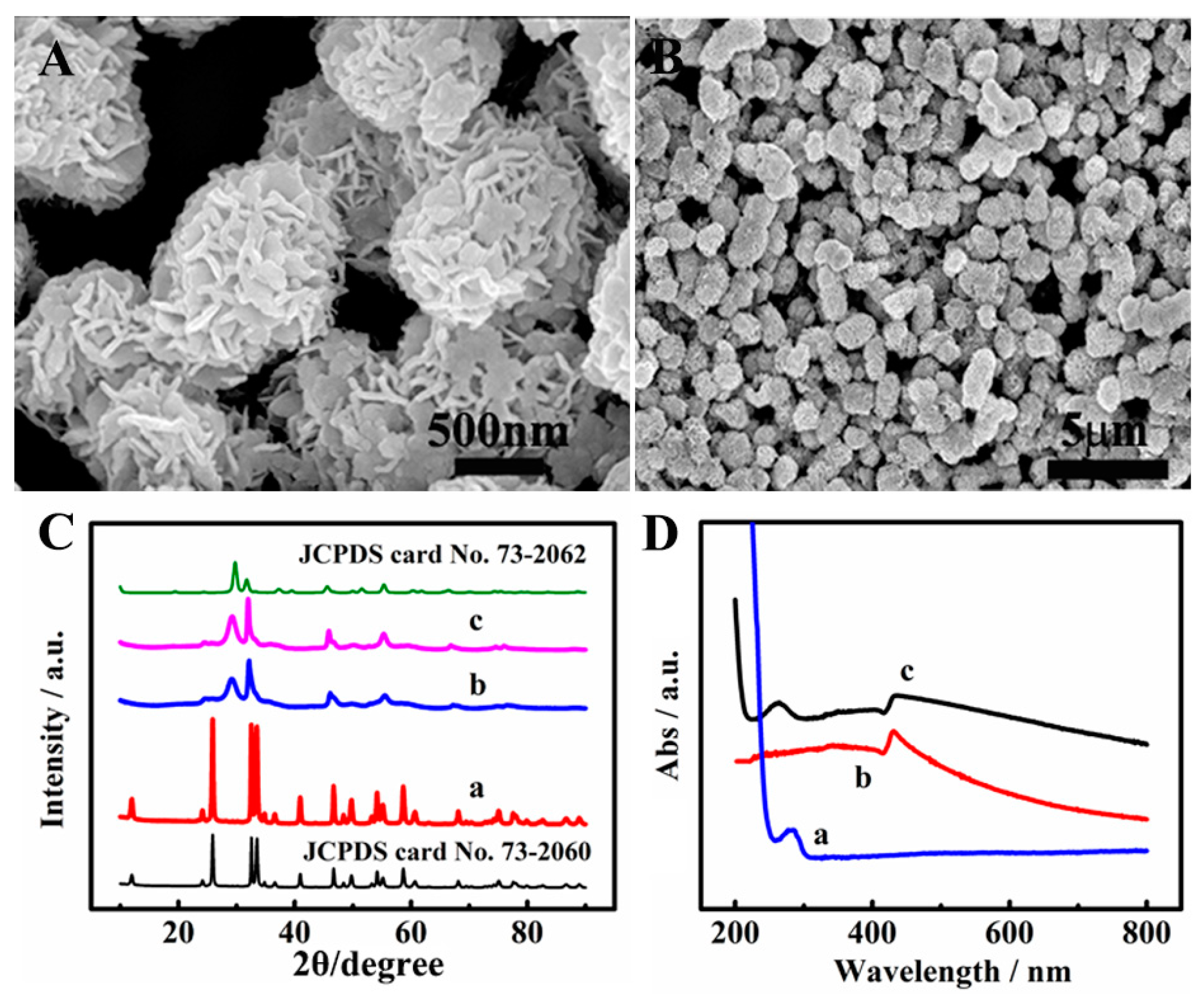
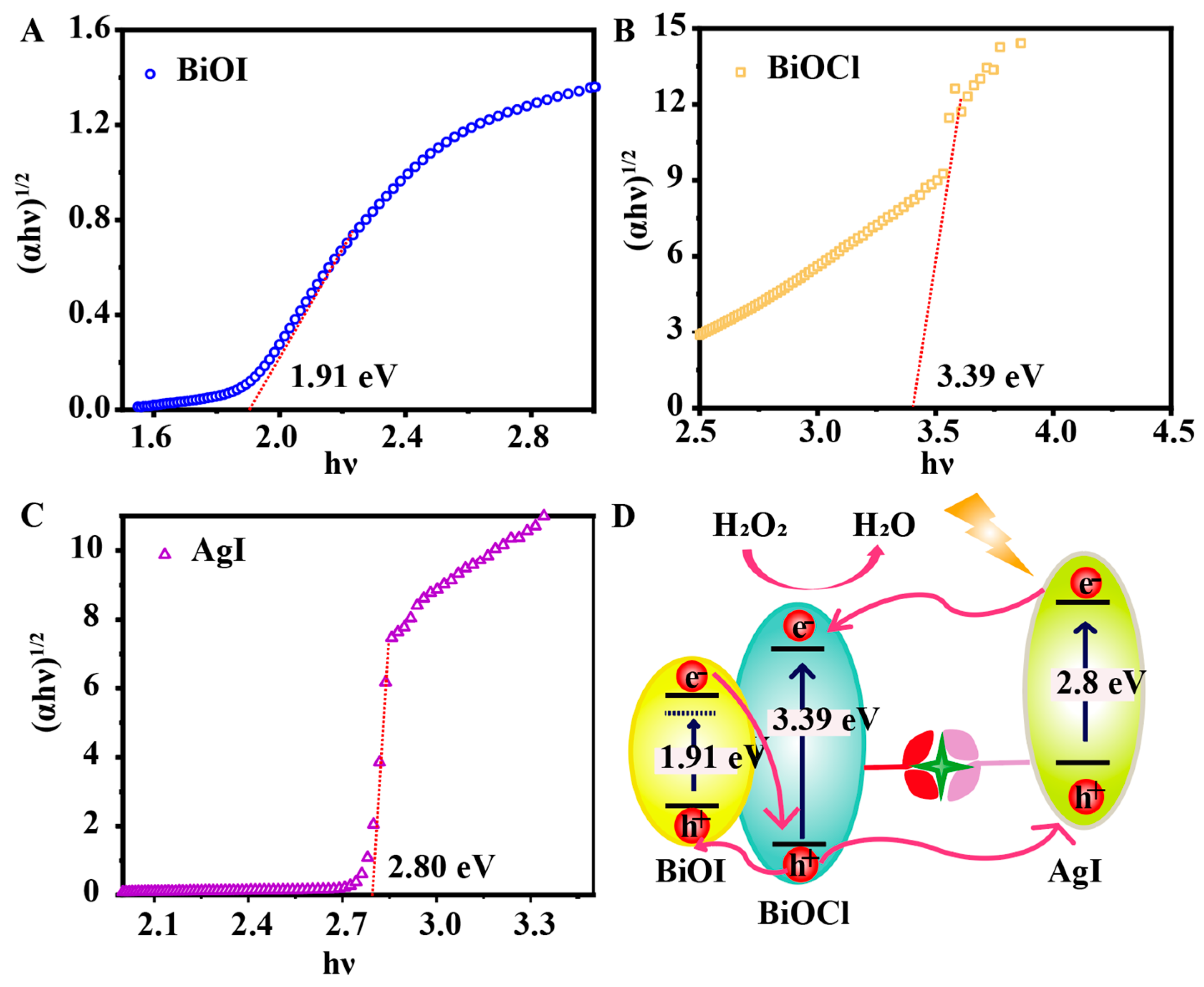
3.1. Morphological Characterization of BiOI/BiOCl
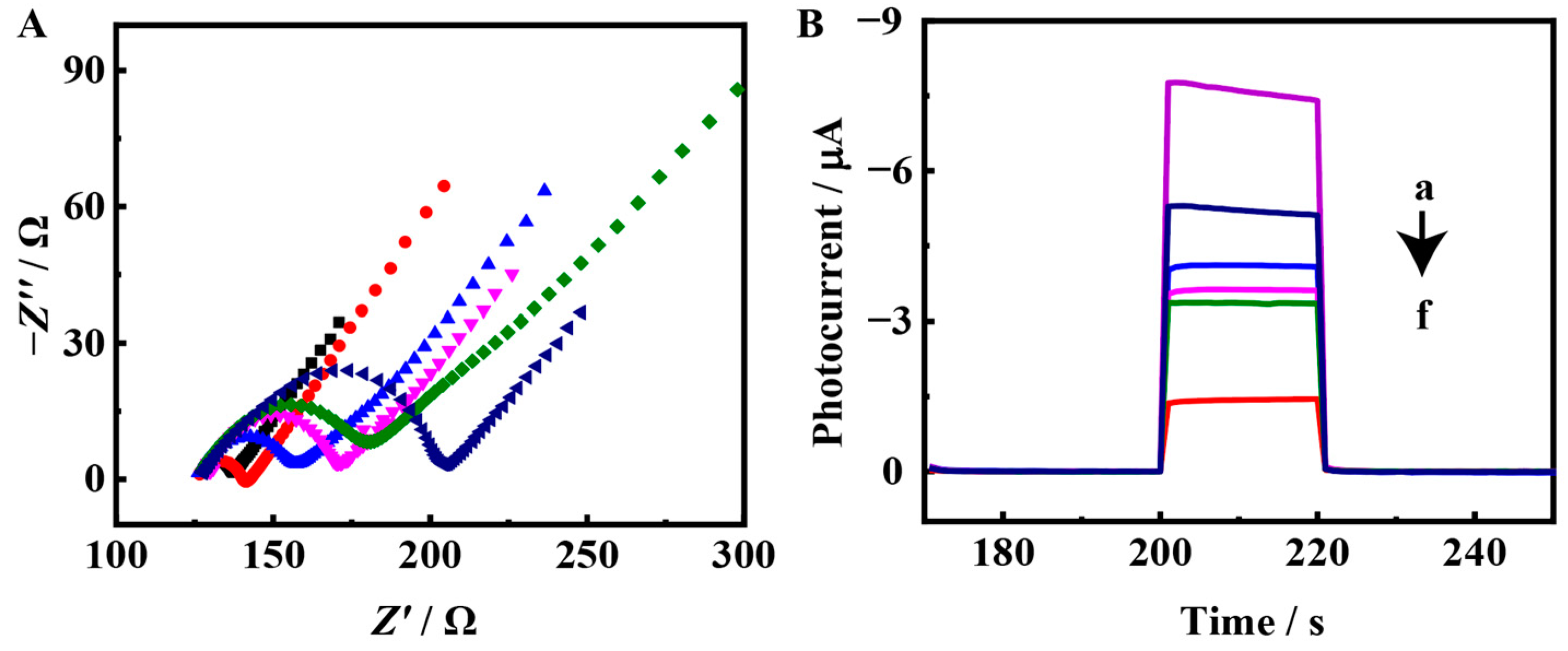
3.2. Characterization of the PEC Biosensor
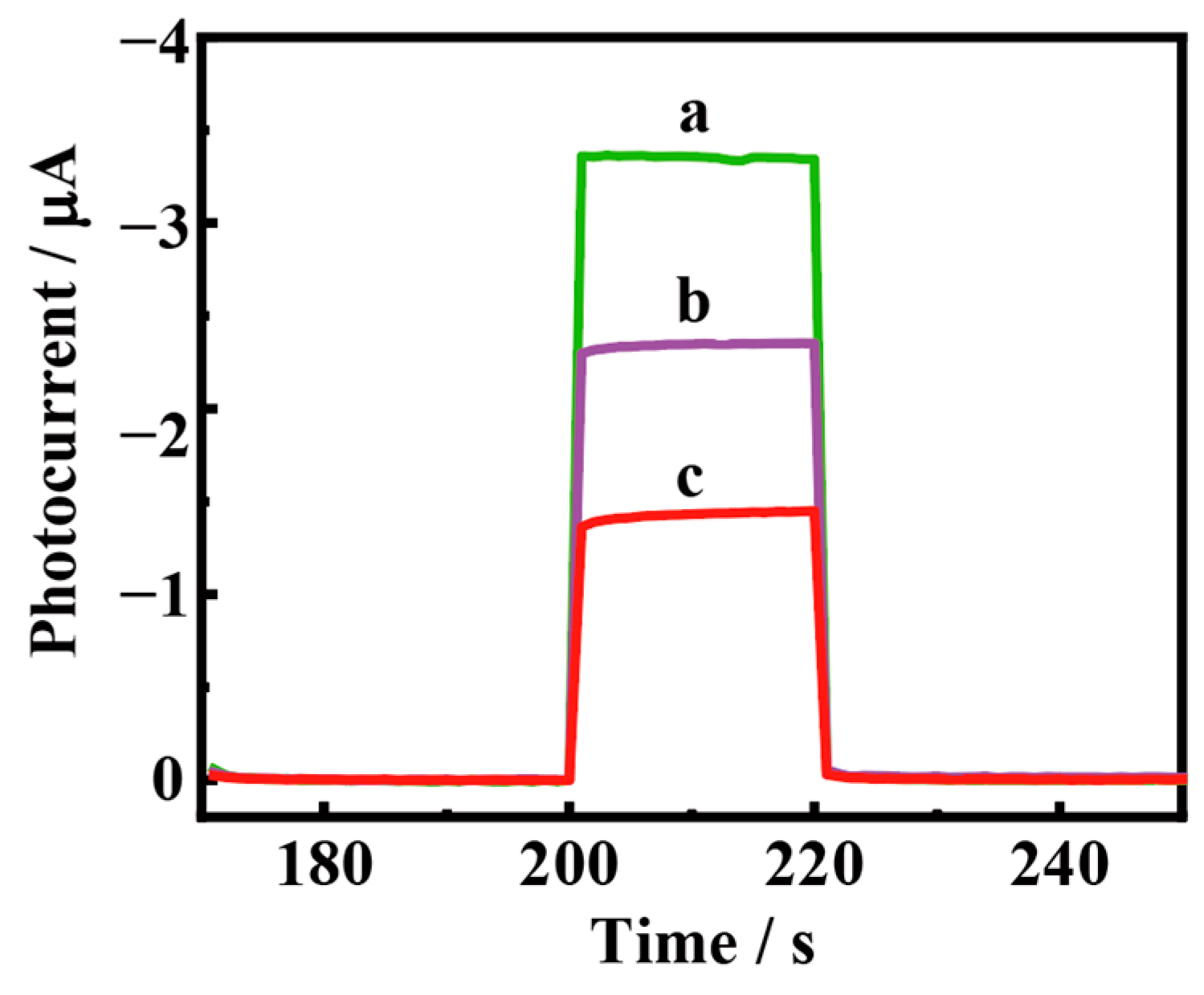
3.3. PEC Response of the Biosensing Platform
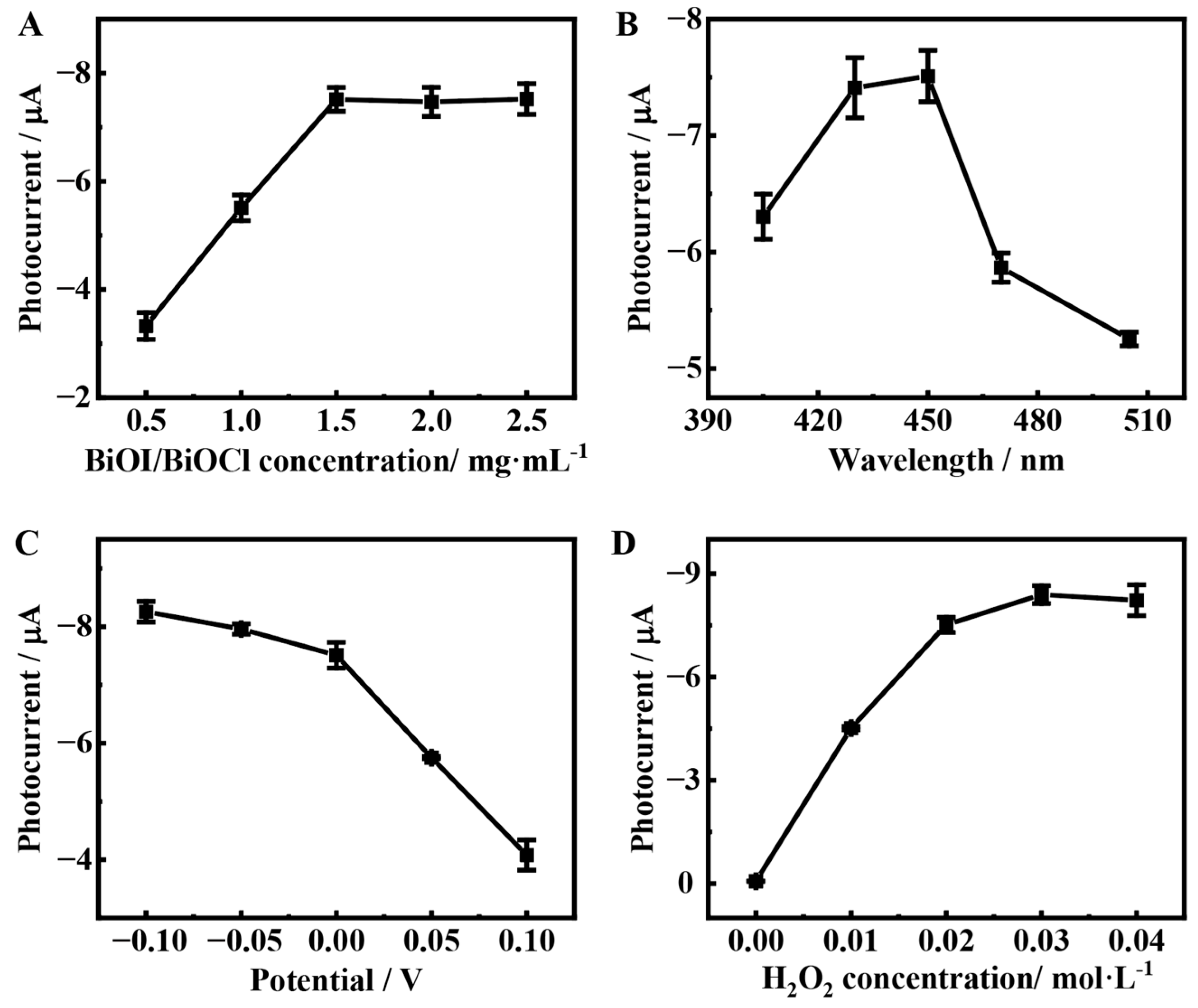
3.4. Optimization of the Detection Conditions
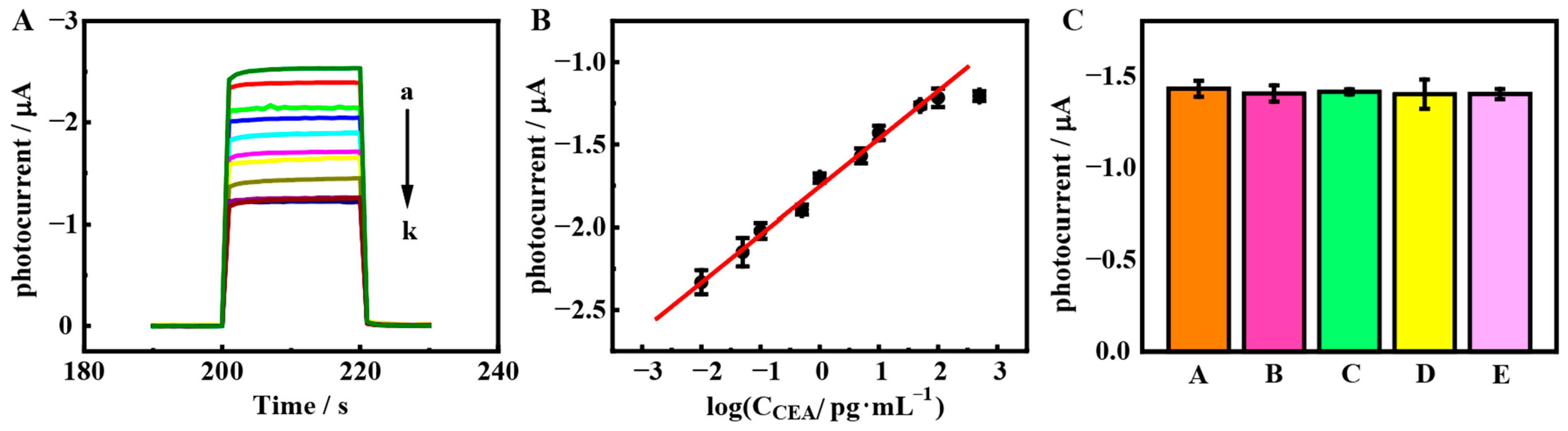
3.5. PEC Bioanalysis
3.6. PEC Assay of Human Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, X.; Wang, X.; Kong, Y.; Dai, M.; Han, D.; Liu, Z. FRET modulated signaling: A versatile strategy to construct photoelectrochemical microsensors for in vivo analysis. Angew. Chem. Int. Ed. 2021, 60, 11774–11778. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Wu, F.; Tao, M.; Wei, W.; Wang, Z.; Wang, Z.; Dai, Z. Polarity conversion of the Ag2S/AgInS2 heterojunction by radical-induced positive feedback polydopamine adhesion for signal-switchable photoelectrochemical biosensing. Anal. Chem. 2023, 95, 15008–15016. [Google Scholar] [CrossRef] [PubMed]
- Petruleviciene, M.; Savickaja, I.; Juodkazyte, J.; Ramanavicius, A. Investigation of WO3 and BiVO4 photoanodes for photoelectrochemical sensing of xylene, toluene and methanol. Chemosensors 2023, 11, 552. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Zhang, Q.; Zhang, Z. Synergistic modulation of BiOI by atomic-level vacancies and dominant facets for efficient photocatalytic degradation of bisphenol A. J. Mater. Chem. C 2024, 12, 17676–17686. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Teng, Z.; Liu, Y.; Liu, X.; Cong, C.; Lai, K.; Li, L.; Dong, J.; Li, N.; et al. Gas-liquid interface derived lightweight and twistable BiOI/In2O3/tape assembly for photocatalytic NO removal. Chem. Eng. J. 2024, 497, 154629. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Lee, L.C.; Kurchin, R.C.; Huq, T.N.; Zhang, K.H.L.; Sponseller, M.; Nienhaus, L.; Brandt, R.E.; Jean, J.; Polizzotti, J.A.; et al. Strongly enhanced photovoltaic performance and defect physics of air-stable bismuth oxyiodide (BiOI). Adv. Mater. 2017, 29, 1702176. [Google Scholar] [CrossRef]
- Huq, T.N.; Lee, L.C.; Eyre, L.; Li, W.; Jagt, R.A.; Kim, C.; Fearn, S.; Pecunia, V.; Deschler, F.; MacManus-Driscoll, J.L.; et al. Electronic structure and optoelectronic properties of bismuth oxyiodide robust against percent-level Iodine-, oxygen-, and bismuth-related surface defects. Adv. Funct. Mater. 2020, 30, 1909983. [Google Scholar] [CrossRef]
- Yu, S.; Mei, L.; Xu, Y.; Xue, T.; Fan, G.; Han, D.; Chen, G.; Zhao, W. Liposome-mediated in situ formation of AgI/Ag/BiOI Z-Scheme heterojunction on foamed nickel electrode: A proof-of-concept study for cathodic liposomal photoelectrochemical bioanalysis. Anal. Chem. 2019, 91, 3800–3804. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, J.; Xu, J.; Shan, L.; Lv, J.; Wu, C.; Zhang, L.; Li, L.; Yu, J. Ultrasensitive paper-based photoelectrochemical biosensor for acetamiprid detection enabled by spin-state manipulation and polarity-switching. Anal. Chem. 2024, 96, 12262–12269. [Google Scholar] [CrossRef]
- Yosefi, L.; Haghighi, M. Fabrication of nanostructured flowerlike p-BiOI/p-NiO heterostructure and its efficient photocatalytic performance in water treatment under visible-light irradiation. Appl. Catal. B 2018, 220, 367–378. [Google Scholar] [CrossRef]
- Du, Y.; Ma, R.; Wang, L.; Qian, J.; Wang, Q. 2D/1D BiOI/g-C3N4 nanotubes heterostructure for photoelectrochemical overall water splitting. Sci. Total Environ. 2022, 838, 156166. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, Y.; Hao, L.; Cao, J.; Zhai, X.; Dou, K.; Jiang, F.; Sun, C. 1D Bi2S3 nanorods modified 2D BiOI nanoplates for highly efficient photocatalytic activity: Pivotal roles of oxygen vacancies and Z-scheme heterojunction. JMST 2023, 142, 45–59. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Yang, W.; Wang, F.; Liu, M.; Zhu, X.; Zhang, Y.; Yao, S. Exploitation of a turn-on photoelectrochemical sensing platform based on Au/BiOI for determination of copper(II) ions in food samples. J. Electroanal. Chem. 2021, 895, 115536. [Google Scholar] [CrossRef]
- Zheng, X.; Chu, Y.; Miao, B.; Fan, J. Ag-doped Bi2WO6/BiOI heterojunction used as photocatalyst for the enhanced degradation of tetracycline under visible-light and biodegradability improvement. J. Alloys Compd. 2022, 893, 162382. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Decomposition of highly persistent perfluorooctanoic acid by hollow Bi/BiOI1−xFx: Synergistic effects of surface plasmon resonance and modified band structures. J. Hazard. Mater. 2021, 402, 123459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Y.-C.; Zhao, W.-W. Recent advances of nanostructured materials for photoelectrochemical bioanalysis. Chemosensors 2021, 10, 14. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, D.; Xiao, R.; Wang, T.; Liang, Z.; Wu, Z.; Han, F.; Han, D.; Ma, Y.; Niu, L. An enzyme-free photoelectrochemical sensor platform for ascorbic acid detection in human urine. Chemosensors 2022, 10, 268. [Google Scholar] [CrossRef]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-free electrochemical immunosensors: A practical guide. Trac-Trends Anal. Chem. 2024, 180, 117949. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, W.; Xu, L.; Li, G.; Wan, Y.; Li, H. Nanobody-based label-free photoelectrochemical immunoassay for highly sensitive detection of SARS-CoV-2 spike protein. Anal. Chim. Acta 2022, 1211, 339904. [Google Scholar] [CrossRef]
- Dourbash, F.A.; Shestopalov, A.A.; Rothberg, L.J. Label-free immunoassay using droplet-based brewster’s angle straddle interferometry. Anal. Chem. 2021, 93, 4456–4462. [Google Scholar] [CrossRef]
- Han, Q.; Wang, R.; Xing, B.; Zhang, T.; Khan, M.S.; Wu, D.; Wei, Q. Label-free photoelectrochemical immunoassay for CEA detection based on CdS sensitized WO3@BiOI heterostructure nanocomposite. Biosens. Bioelectron. 2018, 99, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Esaulova, E.; Ward, J.P.; Malkova, O.N.; Runci, D.; Wong, P.; Noguchi, T.; Arthur, C.D.; Meng, W.; Alspach, E.; et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint cancer therapy. Cell 2018, 175, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Alan Saghatelian, K.M.G.; Desiree, A.T.; Ghadiri, M.R. DNA detection and signal amplification via an engineered allosteric enzyme. J. Am. Chem. Soc. 2003, 125, 344–345. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Qin, W. Enhanced selectivity in microdroplet-mediated enzyme catalysis. J. Am. Chem. Soc. 2024, 146, 24389–24397. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Liu, G.; Qiu, Q.; Zhang, J.; Hao, M.; Ren, H.; Zhang, Y. A pH-sensitive glucose oxidase and Hemin coordination micelle for multi-enzyme cascade and amplified cancer chemodynamic therapy. Small 2024, 20, 2407674. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, W.; Wang, P.; Song, G.; Song, Z.L.; Yang, Y.; Wang, Y.; Yin, B.; Rong, P.; Huan, S.; et al. Tumor-specific multipath nucleic acid damages strategy by symbiosed nanozyme@enzyme with synergistic self-cyclic catalysis. Small 2021, 17, 2100766. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Li, H.; Shi, J.; Sun, C.; Lu, G.; Yan, X. Dual-enhanced enzyme cascade hybrid hydrogel for the construction of optical biosensor. Biosens. Bioelectron. 2024, 263, 116613. [Google Scholar] [CrossRef]
- Fan, G.-C.; Han, L.; Zhu, H.; Zhang, J.-R.; Zhu, J.-J. Ultrasensitive photoelectrochemical immunoassay for matrix metalloproteinase-2 detection based on CdS:Mn/CdTe cosensitized TiO2 nanotubes and signal amplification of SiO2@Ab2 conjugates. Anal. Chem. 2014, 86, 12398–12405. [Google Scholar] [CrossRef]
- Wei, J.; Dong, H.; Gao, Y.; Su, X.; Tan, H.; Li, J.; Zhao, Q.; Guan, X.; Lu, Z.; Ouyang, J.; et al. Synergism of oxygen-iodine binary vacancies with the interfacial electric field: Enhancing CO2 photoreduction over VO–I-BiOCl/BiOI atomic-thin nanosheets. J. Mater. Chem. A 2023, 11, 4057–4066. [Google Scholar] [CrossRef]
- Sun, L.; Xiang, L.; Zhao, X.; Jia, C.-J.; Yang, J.; Jin, Z.; Cheng, X.; Fan, W. Enhanced visible-light photocatalytic activity of BiOI/BiOCl heterojunctions: Key role of crystal facet combination. ACS Catal. 2015, 5, 3540–3551. [Google Scholar] [CrossRef]
- Ren, H.; Han, Q.; Zhang, S.; Huang, Y.; Chen, Y.; Dai, H.; Yan, J.; Lin, Y. A photothermal assisted in situ signal-amplified electrochemical immunoassay based on multifunctional probe for detecting autoimmune hepatitis marker. Sens. Actuators B Chem. 2020, 309, 127823. [Google Scholar] [CrossRef]
- Sun, X.; Lu, J.; Wu, J.; Guan, D.; Liu, Q.; Yan, N. Enhancing photocatalytic activity on gas-phase heavy metal oxidation with self-assembled BiOI/BiOCl microflowers. J. Colloid Interface Sci. 2019, 546, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hallock, C.D.; Rose, M.J. Electrochemical impedance of well-passivated semiconductors reveals bandgaps, fermi levels, and interfacial density of states. J. Am. Chem. Soc. 2024, 146, 18989–18998. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Sun, D.-E.; Li, P.; Liu, Z. Fluorescence resonance energy transfer biosensor between upconverting nanoparticles and palladium nanoparticles for ultrasensitive CEA detection. Biosens. Bioelectron. 2016, 86, 791–798. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Hou, T.; Li, F. A versatile immobilization-free photoelectrochemical biosensor for ultrasensitive detection of cancer biomarker based on enzyme-free cascaded quadratic amplification strategy. Biosens. Bioelectron. 2016, 77, 220–226. [Google Scholar] [CrossRef]
- Wei, Y.-p.; Liu, X.-P.; Mao, C.-j.; Niu, H.-L.; Song, J.-M.; Jin, B.-K. Highly sensitive electrochemical biosensor for streptavidin detection based on CdSe quantum dots. Biosens. Bioelectron. 2018, 103, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, B.; Wang, Y.; Zhao, F.; Zeng, B. Electrochemiluminescence immunosensor for the detection of carcinoembryonic antigen based on oxygen vacancy-rich Co3O4 nanorods and luminol. ACS Appl. Nano Mater 2021, 4, 7264–7271. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, C.; Li, P.; Wu, T.; Yang, N.; Wang, X.; Wang, Y.; Li, C. ZnS/C/MoS2 nanocomposite derived from metal–organic framework for high-performance photo-electrochemical immunosensing of carcinoembryonic antigen. Small 2019, 15, 1902086. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wan, X.; Wei, Q.; Zeng, Y.; Tang, D. Smartphone-based point-of-care photoelectrochemical immunoassay coupling with ascorbic acid-triggered photocurrent-polarity conversion switching. Biosens. Bioelectron. 2025, 267, 116749. [Google Scholar] [CrossRef]
- Zhong, Z.; Ding, L.; Man, Z.; Zeng, Y.; Pan, B.; Zhu, J.-J.; Zhang, M.; Cheng, F. Versatile metal–organic framework incorporating Ag2S for constructing a photoelectrochemical immunosensor for two breast cancer markers. Anal. Chem. 2024, 96, 8837–8843. [Google Scholar] [CrossRef]
- Lu, L.; Zeng, R.; Lin, Q.; Huang, X.; Tang, D. Cation exchange reaction-mediated photothermal and polarity-switchable photoelectrochemical dual-readout biosensor. Anal. Chem. 2023, 95, 16335–16342. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, Y.; Lian, H.; Cao, X.; Liu, B.; Wei, X. Self-assembled DNA/SG-I nanoflower: Versatile photocatalytic biosensors for disease-related markers. Anal. Chem. 2025, 97, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yu, Z.; Wu, D.; Li, M.; Tang, D. Target-induced oxygen vacancy on the etching WO3 photoanode for in-situ amplified photoelectrochemical immunoassay. Biosens. Bioelectron. 2025, 279, 117405. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, J.; Wan, X.; Wang, X.; Zeng, Y.; Liu, X.; Tang, D. Mechanism exploration of the photoelectrochemical immunoassay for the integration of radical generation with self-quenching. Anal. Chem. 2024, 96, 15503–15510. [Google Scholar] [CrossRef]
- Liang, H.; Luo, Y.; Li, Y.; Song, Y.; Wang, L. An immunosensor using electroactive COF as signal probe for electrochemical detection of carcinoembryonic antigen. Anal. Chem. 2022, 94, 5352–5358. [Google Scholar] [CrossRef]
- Yu, X.; Cao, Y.; Zhao, Y.; Xia, J.; Yang, J.; Xu, Y.; Zhao, J. Proximity amplification-enabled electrochemical analysis of tumor-associated glycoprotein biomarkers. Anal. Chem. 2023, 95, 15900–15907. [Google Scholar] [CrossRef]
- Chen, S.; Tian, S.; Wang, Y.; Li, M.; Tang, D. Harnessing bifunctional nanozyme with potent catalytic and signal amplification for innovating electrochemical immunoassay. Biosens. Bioelectron. 2025, 278, 117340. [Google Scholar] [CrossRef]
| Detection Methods | Linear Response Range | Limit of Detection | Refs. |
|---|---|---|---|
| Photoelectrochemistry | 0.02 to 80 ng·mL−1 | 8.47 pg·mL−1 | [39] |
| Photoelectrochemistry | 0.01 to 32 ng·mL−1 | 2.3 pg·mL−1 | [40] |
| Photoelectrochemistry | 0.5 to 100 ng·mL−1 | 0.21 ng·mL−1 | [41] |
| Photoelectrochemistry | 0.5 to 80.0 ng·mL−1 | 0.5 ng·mL−1 | [42] |
| Photoelectrochemistry | 0.02 to 80 ng·mL−1 | 12.9 pg·mL−1 | [43] |
| Photoelectrochemistry | 0.02 to 50 ng·mL−1 | 8.9 pg·mL−1 | [44] |
| Electrochemistry | 0.11 to 80 ng·mL−1 | 0.034 ng·mL−1 | [45] |
| Electrochemistry | 0.001 to 100 ng·mL−1 | 0.419 pg·mL−1 | [46] |
| Electrochemistry | 0.020 to 100 ng·mL−1 | 0.013 ng·mL−1 | [47] |
| Photoelectrochemistry | 10 to 100 × 103 fg·mL−1 | 4.9 fg·mL−1 | this work |
| Simple Number | a | b | c | d | e |
|---|---|---|---|---|---|
| Developed method (ng·mL−1) | 2.06 | 0.81 | 7.05 | 14.35 | 27.63 |
| Reference method (ng·mL−1) | 2.27 | 0.778 | 6.78 | 15.89 | 31.34 |
| Relative error (%) | 9.25 | 2.61 | 3.98 | 9.69 | 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wan, W.; Wang, S.; Zeng, H.; Wu, Y.; Dai, Z.; Tu, W. Using BiOI/BiOCl Composite-Enhanced Cathodic Photocurrent and Amplifying Signal Variation in AgI for Developing a Highly Sensitive Photoelectrochemical Immunosensing Platform. Chemosensors 2025, 13, 164. https://doi.org/10.3390/chemosensors13050164
Zhang M, Wan W, Wang S, Zeng H, Wu Y, Dai Z, Tu W. Using BiOI/BiOCl Composite-Enhanced Cathodic Photocurrent and Amplifying Signal Variation in AgI for Developing a Highly Sensitive Photoelectrochemical Immunosensing Platform. Chemosensors. 2025; 13(5):164. https://doi.org/10.3390/chemosensors13050164
Chicago/Turabian StyleZhang, Mengyang, Weikang Wan, Shurui Wang, Huiyu Zeng, Yang Wu, Zhihui Dai, and Wenwen Tu. 2025. "Using BiOI/BiOCl Composite-Enhanced Cathodic Photocurrent and Amplifying Signal Variation in AgI for Developing a Highly Sensitive Photoelectrochemical Immunosensing Platform" Chemosensors 13, no. 5: 164. https://doi.org/10.3390/chemosensors13050164
APA StyleZhang, M., Wan, W., Wang, S., Zeng, H., Wu, Y., Dai, Z., & Tu, W. (2025). Using BiOI/BiOCl Composite-Enhanced Cathodic Photocurrent and Amplifying Signal Variation in AgI for Developing a Highly Sensitive Photoelectrochemical Immunosensing Platform. Chemosensors, 13(5), 164. https://doi.org/10.3390/chemosensors13050164







