Biosensors for Micro- and Nanoplastics Detection: A Review
Abstract
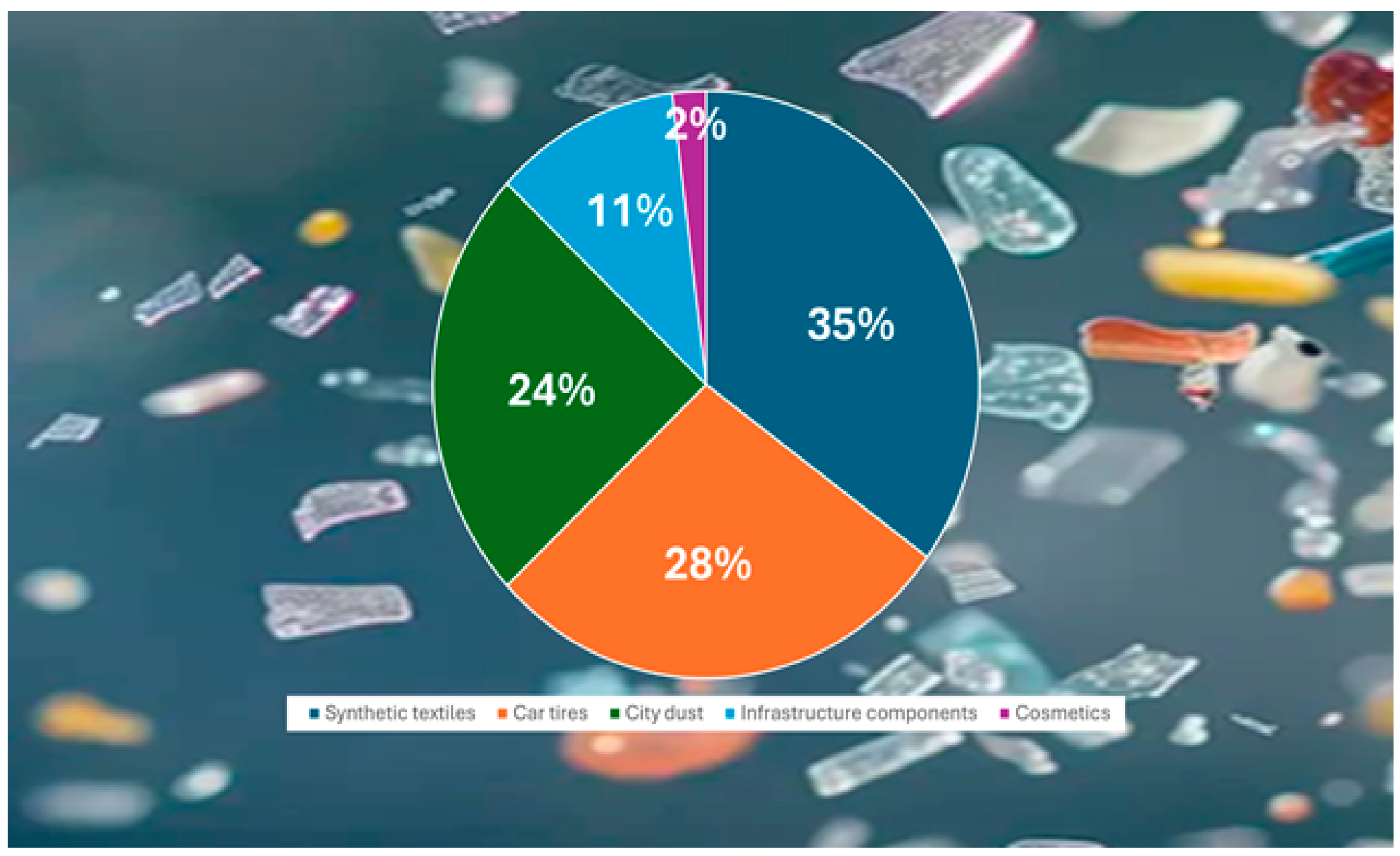
1. Introduction
| Species | Impact Assessment | Reference |
|---|---|---|
| Invertebrates: | ||
| Daphnia magna (water flea) | Frequently used in toxicity studies due to showing reduced growth, reproduction, and physiological changes | [23] |
| Chironomus riparius (non-biting midge) | Exhibits morphological deformities and reduced emergence rates | [24] |
| Gammarus pulex (amphipod) | Shows reduced assimilation efficiency and avoidance behavior toward microplastics | [13] |
| Fish: | ||
| Danio rerio (zebrafish) | Accumulates microplastics in the gills, gut, and liver, leading toinflammation and oxidative stress | [25,26] |
| Rutilus rutilus (roach) | Ingests microplastics, with larger females showing higher ingestion rates | [27] |
| Micropterus salmoides (largemouth bass) | Found to contain high concentrations of microplastics in closed lake ecosystems | [28] |
| Amphibians: | ||
| Alytes obstetricans (common midwife toad) | Tadpoles show bioaccumulation and mortality when exposed to microplastics | [29] |
| Physalaemus cuvieri (South American frog) | Tadpoles exhibit significant morphological and cytological changes due to microplastics exposure | [30] |
| Microalgae and Cyanobacteria: | ||
| Chlamydomonas reinhardtii | Shows reduced growth and photosynthetic efficiency when exposed to microplastics | [31] |
| Microcystis aeruginosa | Exhibits increased toxin production and cell membrane damage | [32] |
| Chlorella vulgaris | Shows reduced photosynthetic efficiency when exposed to MP leachates | [33] |
2. Conventional Methods for the Analysis of MPs and NPs
2.1. Visual Inspection and Microscopy
2.2. Spectroscopic Techniques
2.3. Chromatographic Methods
3. Biosensor-Based Methods for Microplastic Detection
- Development of Specific Recognition Elements: Creating antibodies, aptamers, and molecular imprinting polymers can enhance the specificity and rapid identification of MPs based on their shape, size, and polymer type.
- Fluorescent Probes: Utilizing advanced fluorescent probes like Nile red and conjugated polymer nanoparticles (CPNs) can help in the rapid and selective detection of MPs. Improving the dyeing efficiency, reducing false positives through co-staining approaches, and a better understanding of fluorophore-plastic interactions are essential [51].
- Electrochemical Methods: Developing sensors based on electrochemical impedance spectroscopy (EIS) and other electrochemical techniques can provide the rapid and selective detection of MPs in various food matrices [54].
- Artificial Intelligence (AI): Leveraging AI for automatic image processing, classification, and analysis of MPs can significantly enhance the efficiency and accuracy of detection methods [55].
3.1. Receptor-Based Approaches
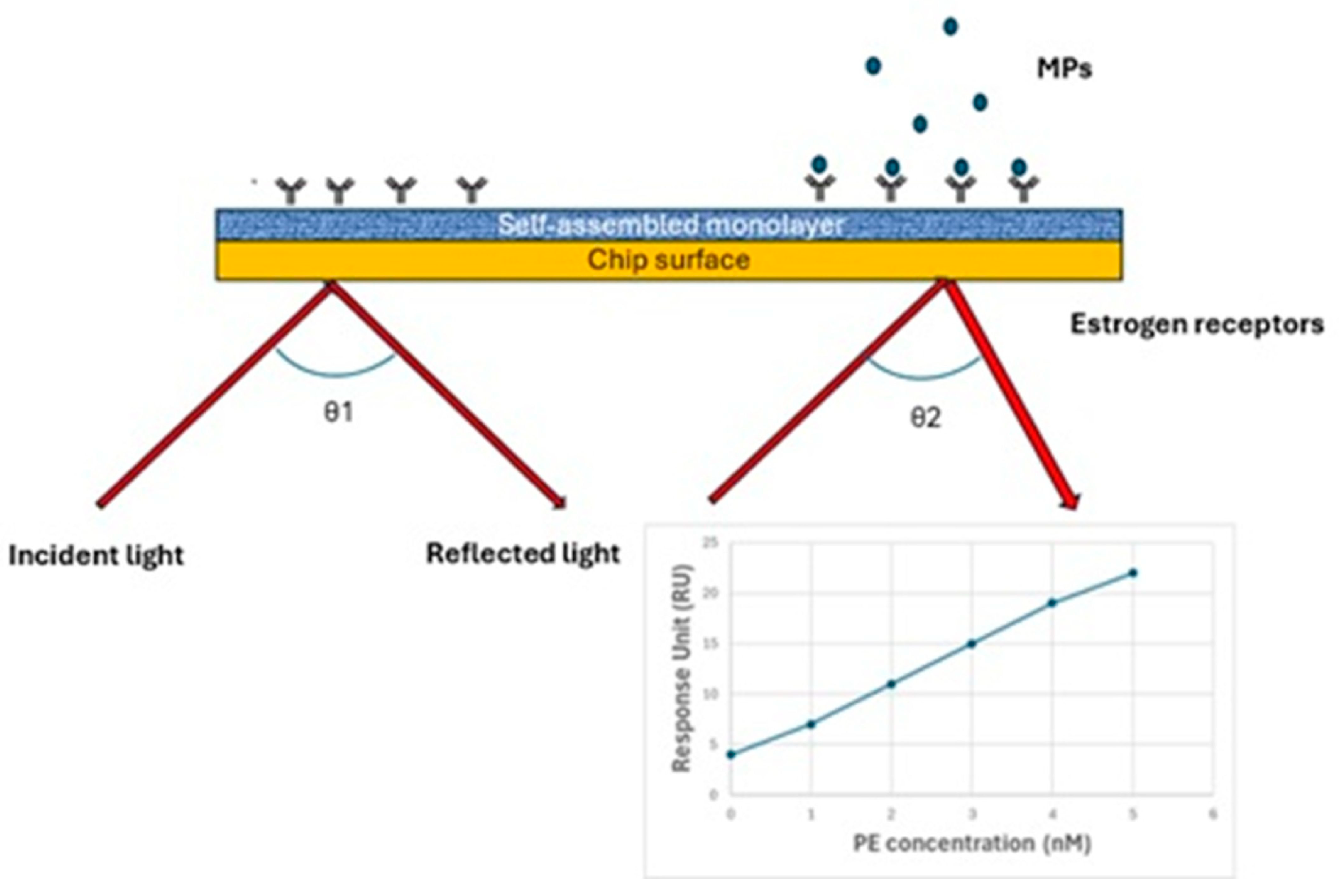
3.2. Optical Biosensors
- High Sensitivity: SAW sensors exhibit high sensitivity, due to the strong localization of mechanical excitation in the surface region, making them more responsive to surrounding variations such as biorecognition events [72].
- Miniaturization: These sensors can be miniaturized, allowing for portable and on-field applications; this is beneficial for environmental monitoring and precision agriculture [73].
- Quick and Cheap Detection: The sensors provide rapid and cost-effective nanoplastic detection, which is useful for early diagnosis and monitoring campaigns [74].
3.3. Electrochemical Biosensors
3.4. Bioinformatic Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, M.; Liu, P.; Zhang, Z.; Peng, J.; Guo, X. A systematic review on the aging of microplastics and the effects of typical factors in various environmental media. TrAC Trends Anal. Chem. 2023, 162, 117025. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent advances on ecological effects of microplastics on soil environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef]
- Kacprzak, S.; Tijing, L.D. Microplastics in indoor environment: Sources, mitigation and fate. J. Environ. Chem. Eng. 2022, 10, 107359. [Google Scholar] [CrossRef]
- Kefer, S.; Friedenauer, T.; Langowski, H.C. Characterisation of different manufactured plastic microparticles and their comparison to environmental microplastics. Powder Technol. 2022, 412, 117960. [Google Scholar] [CrossRef]
- Bajt, O. From plastics to microplastics and organisms. FEBS Open 2021, 11, 954–966. [Google Scholar] [CrossRef]
- Lim, X.Z. Microplastics are everywhere—But are they harmful. Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yang, C.; Zhang, T.; Tang, C.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Akhdhar, A.; Elwakeel, K.Z. Microplastics prevalence, interactions, and remediation in the aquatic environment: A critical review. J. Environ. Chem. Eng. 2021, 9, 106224. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y.J. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Yardy, L.; Callaghan, A. What the fluff is this?—Gammarus pulex prefer food sources without plastic microfibers. Sci. Total Environ. 2020, 715, 136815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Fackelmann, G.; Sommer, S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Chen, Y.; Song, G.; Zhang, H.; Huang, K.; Luo, Y.; Cheng, N. Discovery and solution for microplastics: New risk carriers in food. Food Chem. 2025, 471, 142784. [Google Scholar] [CrossRef]
- Deng, H.; Fu, Q.; Li, D.; Zhang, Y.; He, J.; Feng, D.; Zhao, Y.; Du, G.; Yu, H.; Ge, C. Microplastic-associated biofilm in an intensive mariculture pond: Temporal dynamics of microbial communities, extracellular polymeric substances and impacts on microplastics properties. J. Clean. Prod. 2021, 319, 128774. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Pang, W.; Tian, C.; Zhao, Y. Nanoplastic-induced genotoxicity and intestinal damage in freshwater benthic clams (Corbicula fluminea): Comparison with microplastics. ACS Nano 2021, 15, 9469–9481. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- El Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barcelὁ, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Jin, B.; Wünsch, U.J.; Su, Y.; Zhang, Y. Electrochemical and microbiological response of exoelectrogenic biofilm to polyethylene microplastics in water. Water Res. 2022, 211, 118046. [Google Scholar] [CrossRef] [PubMed]
- Aljaibachi, R.; Laird, W.B.; Stevens, F.; Callaghan, A. Impacts of polystyrene microplastics on Daphnia magna: A laboratory and a mesocosm study. Sci. Total Environ. 2020, 705, 135800. [Google Scholar] [CrossRef] [PubMed]
- Stanković, J.; Milošević, D.; Savić-Zdraković, D.; Yalçın, G.; Yildiz, D.; Beklioğlu, M.; Jovanović, B. Exposure to a microplastic mixture is altering the life traits and is causing deformities in the non-biting midge Chironomus riparius Meigen (1804). Environ. Pollut. 2020, 262, 114248. [Google Scholar] [CrossRef]
- Castro-Castellon, A.T.; Horton, A.A.; Hughes, J.M.R.; Rampley, C.; Jeffers, E.S.; Bussi, G.; Whitehead, P. Ecotoxicity of microplastics to freshwater biota: Considering exposure and hazard across trophic levels. Sci. Total Environ. 2022, 816, 151638. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the river Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, X.; Wang, Z.; Gun, L. Alleviating effect of EMs on oxidative stress and inflammation of Micropterus salmoides after microplastics exposure. Aquac. Int. 2024, 32, 3719–3732. [Google Scholar] [CrossRef]
- Boyero, L.; López-Rojo, N.; Bosch, J.; Alonso, A.; Correa-Araneda, F.; Pérez, J. Microplastics impair amphibian survival, body condition and function. Chemosphere 2020, 244, 125500. [Google Scholar] [CrossRef]
- Da Costa Araújo, A.P.; de Melo, N.F.S.; de Oliveira Junior, A.G.; Rodrigues, F.P.; Fernandes, T.; de Andrade Vieira, J.E.; Rocha, T.L.; Malafaia, G. How much are microplastics harmful to the health of amphibians? A study with pristine polyethylene microplastics and Physalaemus cuvieri. J. Hazard. Mater. 2020, 382, 121066. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, W.; Yuan, Y.; Li, Y.; Liu, X.; Wang, X.; Fan, Z. Growth inhibition, toxin production and oxidative stress caused by three microplastics in Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2021, 208, 111575. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiang, Y.; He, D.; Li, Y.; Zhao, Y.; Wang, S.; Pan, X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total Environ. 2019, 678, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Kalaronis, D.; Ainali, N.M.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microscopic techniques as means for the determination of microplastics and nanoplastics in the aquatic environment: A concise review. Green Anal. Chem. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Caputo, F.; Vogel, R.; Savage, J.; Vella, G.; Law, A.; Della Camera, G.; Hannon, G.; Peacock, B.; Mehn, D.; Ponti, J.; et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: Addressing some analytical challenges in the sub-micron size range. J. Coll. Interface Sci. 2021, 588, 401–417. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Govindu, D.; Tippani, R.; Porika, M.; Sura, S.P. Methodology of Assessing Microplastics and Nanoplastics in the Environment: Recent Advances in the Practical Approaches. In Micro and Nanoplastics in Soil: Threats to Plant-Based Food; Maddela, N.R., Reddy, K.V., Ranjit, P., Eds.; Springer: Cham, Switzerland, 2023; pp. 59–95. [Google Scholar]
- Enyoh, C.E.; Wang, Q.; Chowdhury, T.; Wang, W.; Lu, S.; Xiao, K.; Chowdhury, M.A.H. New analytical approaches for effective quantification and identification of nanoplastics in environmental samples. Processes 2021, 9, 2086. [Google Scholar] [CrossRef]
- Cao, K.; Sun, Y.; Zhang, J.; Su, H.; Wang, F.; Ji, N.; Ye, M.; Lu, H.; Zhao, W.; Liu, X.; et al. High-resolution characterization technology for micro-/nano-plastics. J. Phys. D Appl. Phys. 2024, 57, 223001. [Google Scholar] [CrossRef]
- Kousheh, S.; Hajikhani, M.; Asgari, S.; Lin, M. Detection of micro-and nanoplastic particles in leafy green vegetables by SERS coupled with gold-silver core–shell nanoparticles. Microchim. Acta 2024, 191, 755. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Kontogiannis, A.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microplastics in the environment: Sampling, pretreatment, analysis and occurrence based on current and newly-exploited chromatographic approaches. Sci. Total Environ. 2021, 794, 148725. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Skrzypek, G.; Ortega-Zamora, C.; González-Sálamo, J.; Hernández-Sánchez, C.; Hernández-Borges, J. The current role of chromatography in microplastic research: Plastics chemical characterization and sorption of contaminants. J. Chromatogr. Open 2021, 1, 100001. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Fabbri, D.; Modugno, F. A review on challenges and developments of analytical pyrolysis and other thermoanalytical techniques for the quali-quantitative determination of microplastics. J. Anal. Appl. Pyrolysis 2020, 149, 104841. [Google Scholar] [CrossRef]
- González-González, R.B.; Flores-Contreras, E.A.; Gonzalez-Gonzalez, E.; Torres Castillo, N.E.; Parra-Saldivar, R.; Iqbal, H.M. Biosensor constructs for the monitoring of persistent emerging pollutants in environmental matrices. Ind. Eng. Chem. Res. 2022, 62, 4503–4520. [Google Scholar] [CrossRef]
- Tang, Y.; Hardy, T.J.; Yoon, J.Y. Receptor-based detection of microplastics and nanoplastics: Current and future. Biosens. Bioelectron. 2023, 234, 115361. [Google Scholar] [CrossRef]
- Popenda, A.; Wiśniowska, E.; Manuel, C. Biosensors in environmental analysis of microplastics and heavy metal compounds—A review on current status and challenges. Desal. Water Treat. 2024, 319, 100456. [Google Scholar] [CrossRef]
- Islam, S.; Abu Nayem, S.M.; Awal, A.; Aziz, M.A.; Saleh Ahammad, A.J. Microplastic Detection and Quantification with Biosensing Techniques. In Biosensing Technology for Human Health: Eco-friendly Materials and Real-world Applications; Manjunatha, J.G., Ed.; Royal Society of Chemistry: London, UK, 2024; pp. 193–213. [Google Scholar]
- Jebril, S.; Fredj, Z.; Saeed, A.A.; Gonçalves, A.M.; Kaur, M.; Kumar, A.; Singh, B. Nanomaterial-based electrochemical chemo (bio) sensors for the detection of nanoplastic residues: Trends and future prospects. RSC Sustain. 2024, 2, 832–851. [Google Scholar] [CrossRef]
- Sen, K.; Mondal, N.K. Insights: Review of Electrochemical Sensors for Microplastics Monitoring and Their Remediation. In Microplastics in the Terrestrial Environment, 1st ed.; Mondal, S., Das, P., Mondal, A., Chakraborty, P., Eds.; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–34. [Google Scholar]
- Mansouri, S. Recent developments of (bio)-sensors for detection of main microbiological and non-biological pollutants in plastic bottled water samples: A critical review. Talanta 2024, 274, 125962. [Google Scholar] [CrossRef]
- Awada, A.; Potter, M.; Wijerathne, D.; Gauld, J.W.; Mutus, B.; Rondeau-Gagne, S. Conjugated polymer nanoparticles as a universal high-affinity probe for the selective detection of microplastics. ACS Appl. Mater. Interfaces 2022, 14, 46562–46568. [Google Scholar] [CrossRef]
- Xu, D.; Su, W.; Luo, Y.; Wang, Z.; Yin, C.; Chen, B.; Zhang, Y. Cellulose nanofiber films with gold nanoparticles electrostatically adsorbed for facile surface- enhanced Raman scattering detection. ACS Appl. Mater. Interfaces 2024, 16, 23352–23361. [Google Scholar] [CrossRef]
- Luo, Y.; Su, W.; Xu, D.; Wang, Z.; Wu, H.; Chen, B.; Wu, J. Component identification for the SERS spectra of microplastics mixture with convolutional neural network. Sci. Total Environ. 2023, 895, 165138. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, G.; Wang, J. Highly selective electrochemical impedance spectroscopy-based graphene electrode for rapid detection of microplastics. Sci. Total Environ. 2023, 862, 160873. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.; Moghaddamfard, P.; Meng, W.; Wu, S.; Bao, Y. Artificial intelligence-empowered collection and characterization of microplastics: A review. J. Hazard. Mater. 2024, 471, 134405. [Google Scholar] [CrossRef]
- Belkin, S. Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar] [CrossRef]
- Huang, C.-J.; Narasimha, G.V.; Chen, Y.-C.; Chen, J.-K.; Dong, G.-C. Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors. Biosensors 2021, 11, 219. [Google Scholar] [CrossRef]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-Binding Peptides: Smart Tools for Nanobiotechnology. Trends Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef]
- Liu, S.; Shang, E.; Liu, J.; Wang, Y.; Bolan, N.; Kirkham, M.B.; Li, Y. Microplastic Pollution and Its Potential Correlation with Environmental Factors in Daya Bay, South China Sea. Front. Environ. Sci. Eng. 2021, 16, 8. [Google Scholar]
- Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423, 127171. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Yousefi, S.R.; Jasim, L.S.; Salavati-Niasari, M. Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: Photocatalytic and antibacterial activities. Int. J. Hydrogen Energy 2022, 47, 14319–14330. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Novotný, F.; Pumera, M. Trapping and detecting nanoplastics by MXene-derived oxide microrobots. Nat. Commun. 2022, 13, 3573. [Google Scholar] [CrossRef]
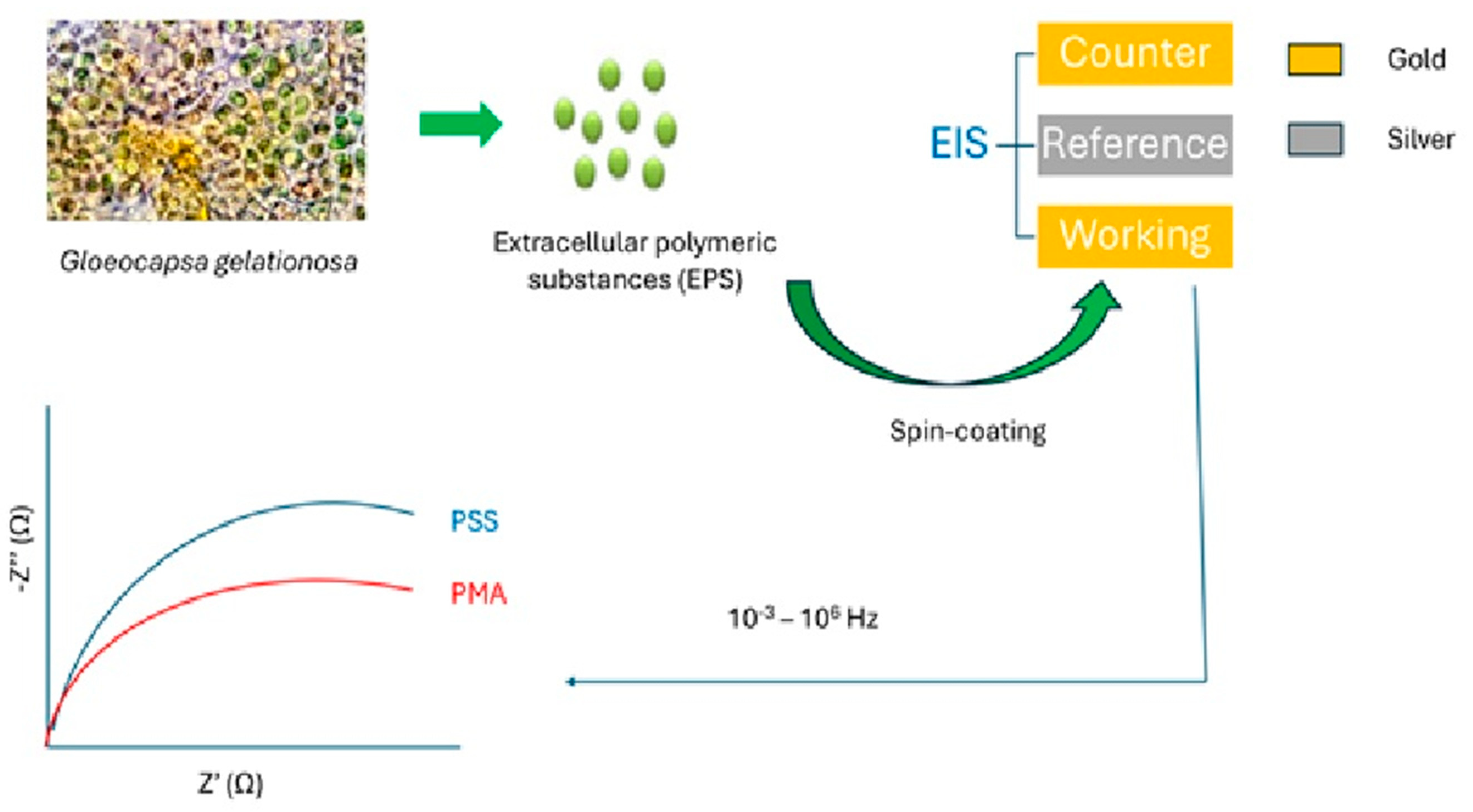
- Gongi, W.; Touzi, H.; Sadly, I.; Ben Ouada, H.; Tamarin, O.; Ben Ouada, H. A novel impedimetric sensor based on cyanobacterial extracellular polymeric substances for microplastics detection. J. Polym. Environ. 2022, 30, 4738–4748. [Google Scholar] [CrossRef] [PubMed]
- Seggio, M.; Arcadio, F.; Cennamo, N.; Zeni, L.; Bossi, A.M. A plasmonic gold nano-surface functionalized with the estrogen receptor for fast and highly sensitive detection of nanoplastics. Talanta 2024, 267, 125211. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, X.; Hong, S.; Zhang, H.; Zhang, Y. A platform for microplastic assessment in aquatic environments based on the protein corona-induced aggregation effect. Biosens. Bioelectron. 2024, 249, 116037. [Google Scholar] [CrossRef]
- Iri, A.H.; Shahrah, M.H.A.; Ali, A.M.; Qadri, S.A.; Erdem, T.; Ozdur, I.T.; Icoz, K. Optical detection of microplastics in water. Environ. Sci. Pollut. Res. 2021, 28, 63860–63866. [Google Scholar] [CrossRef]
- Lee, C.-H.; Fang, J.K.-H. The onset of surface-enhanced Raman scattering for single-particle detection of submicroplastics. J. Environ. Sci. 2022, 121, 58–64. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, N.; Choi, Y.; Kim, J.; Hwang, H.; Kim, C.; Lee, H.-Y.; Kim, S.; Kim, J.S.; Lee, H.H.; et al. Peptide-Decorated Microneedles for the Detection of Microplastics. Biosensors 2024, 14, 140. [Google Scholar] [CrossRef]
- Woo, H.; Kang, S.H.; Kwon, Y.; Choi, Y.; Kim, J.; Ha, D.-H.; Tanaka, M.; Okochi, M.; Kim, J.S.; Kim, H.K. Sensitive and specific capture of polystyrene and polypropylene microplastics using engineered peptide biosensors. RSC Adv. 2022, 12, 7680–7688. [Google Scholar] [CrossRef]
- Nozdriukhin, D.; Kalva, S.K.; Li, W.; Yashchenok, A.; Gorin, D.; Razansky, D.; Deán-Ben, X.L. Rapid Volumetric Optoacoustic Tracking of Individual Microparticles In Vivo Enabled by a NIR-Absorbing Gold−Carbon Shell. ACS Appl. Mater. Interfaces 2021, 13, 48423–48432. [Google Scholar] [CrossRef]
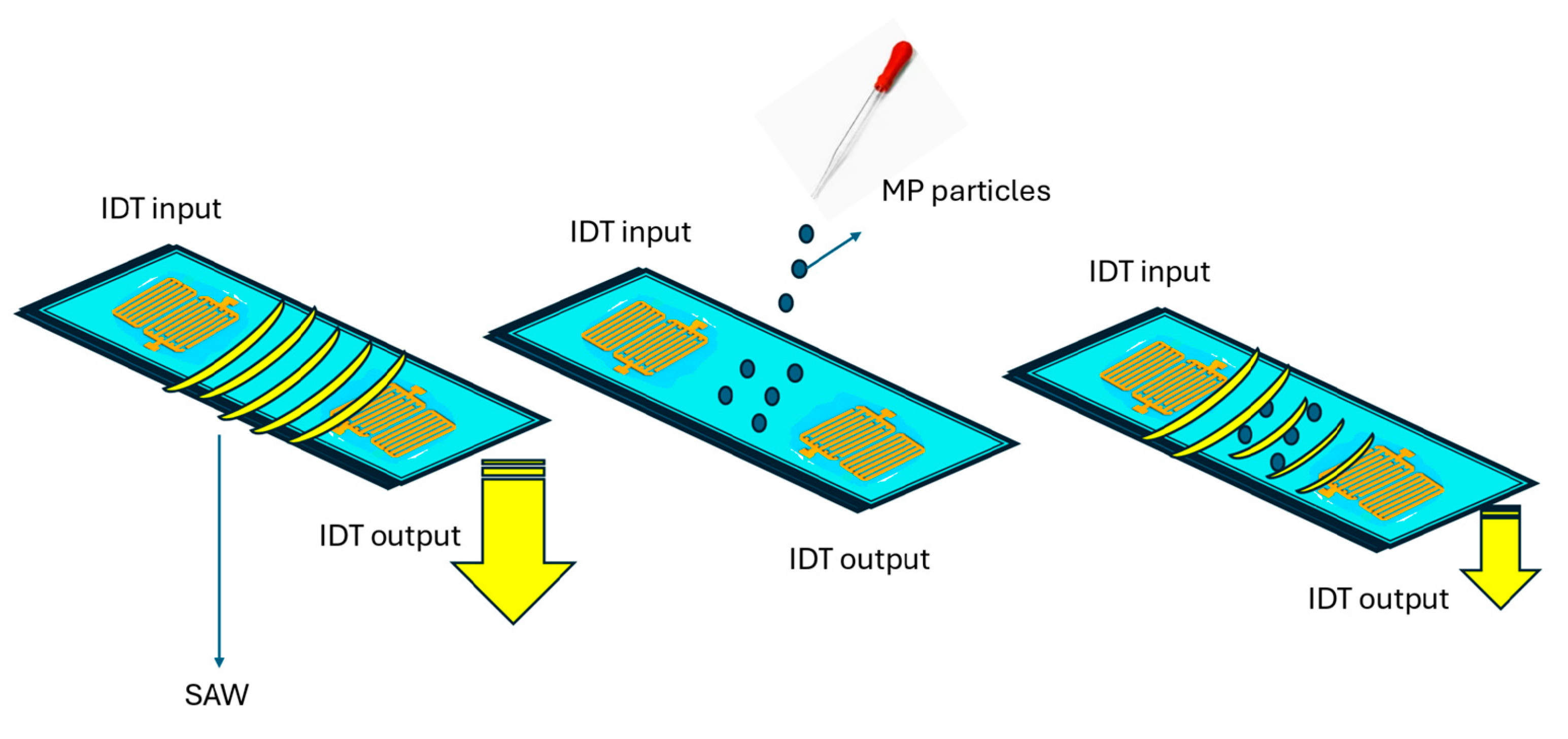
- Rizzato, S.; Monteduro, A.G.; Buja, I.; Maruccio, C.; Sabella, E.; De Bellis, L.; Luvisi, A.; Maruccio, G. Optimization of SAW Sensors for Nanoplastics and Grapevine Virus Detection. Biosensors 2023, 13, 197. [Google Scholar] [CrossRef]
- Wen, W.; He, S.T.; Li, S.Z.; Liu, M.H.; Yong, P. Enhanced sensitivity of SAW gas sensor coated molecularly imprinted polymer incorporating high frequency stability oscillator. Sens. Actuator B-Chem. 2007, 125, 422–427. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Cai, G.N.; Tong, P.; Tang, D.P. Saw-Toothed Microstructure-Based Flexible Pressure Sensor as the Signal Readout for Point-of-Care Immunoassay. ACS Sens. 2019, 4, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Rizzato, S.; Bellis, L.D.; Elicio, V.; Formica, L.; Luvisi, A.; Maruccio, G. Detection of Ampelovirus and Nepovirus by Lab-on-a-Chip: A Promising Alternative to ELISA Test for Large Scale Health Screening of Grapevine. Biosensors 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Detzner, J.; Steil, D.; Pohlentz, G.; Legros, N.; Müthing, J. Surface acoustic wave (SAW) real-time interaction analysis of influenza Avirus hemagglutinins with sialylated neoglycolipids. Glycobiology 2021, 31, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Arcadio, F.; Seggio, M.; Pitruzzella, R.; Zeni, L.; Bossi, A.M.; Cennamo, N. An efficient bio-receptor layer combined with a plasmonic plastic optical fiber probe for cortisol detection in saliva. Biosensors 2024, 14, 351. [Google Scholar] [CrossRef]
- de Sousa, L.R.; Susmel, S.; Mosquera-Ortega, M.; Figueredo, F.; Corton, E. When microplastics meet electroanalysis: Future analytical trends for an emerging threat. Anal. Methods 2023, 15, 5978. [Google Scholar]
- Sowerby, S.J.; Broom, M.F.; Petersen, G.B. Dynamically resizable nanometre-scale apertures for molecular sensing. Sens. Actuators B 2007, 123, 325–330. [Google Scholar] [CrossRef]
- Yang, R.J.; Fuand, L.M.; Hou, H.H. Review and perspectives on microfluidic flow cytometers. Sens. Actuators B 2018, 266, 26–45. [Google Scholar] [CrossRef]
- Baumgarten, L.G.; Freitas, A.A.; Santana, E.R.; Winiarski, J.P.; Dreyer, J.P.; Vieira, I.C. Graphene and gold nanoparticle-based bionanocomposite for the voltammetric determination of bisphenol A in (micro)plastics. Chemosphere 2023, 334, 139016. [Google Scholar] [CrossRef]
- Bergman, M.T.; Xiao, X.; Hall, C.K. In silico design and analysis of plastic-binding peptides. J. Phys. Chem. B 2023, 127, 8370–8381. [Google Scholar] [CrossRef]
- Xiao, X.; Agris, P.F.; Hall, C.K. Introducing folding stability into the score function for computational design of RNA-binding peptides boosts the probability of success. Proteins 2016, 84, 700–711. [Google Scholar] [CrossRef]
- Jeon, J.W.; Choi, J.W.; Shin, Y.; Kang, T.; Chung, B.G. Machine learning-integrated droplet microfluidic system for accurate quantification and classification of microplastics. Water Res. 2025, 274, 123161. [Google Scholar] [CrossRef] [PubMed]
- Kŏsir, A.B.; Divieto, C.; Pavšič, J.; Pavarelli, S.; Dobnik, D.; Dreo, T.; Bellotti, R.; Sassi, M.P.; Žel, J. Droplet volume variability as a critical factor for accuracy of absolute quantification using droplet digital PCR. Anal. Bioanal. Chem. 2017, 409, 6689–6697. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Takhistov, P.; Alocilja, E.; de Corcuera, J.R.; Frey, M.W.; Gomes, C.L.; Mao, Y.J.; McLamore, E.S.; Lin, M.; Tsyusko, O.V.; et al. Bioanalytical approaches for the detection, characterization, and risk assessment of micro/nanoplastics in agriculture and food systems. Anal. Bioanal. Chem. 2022, 414, 4591–4612. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Jalili Ghazizadeh, A.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
| Technique | Working Principle | Strengths | Weaknesses |
|---|---|---|---|
| Fourier transform infrared spectroscopy (FTIR) | The basis of this technique is the Fourier-pair relationship between the interferogram (interference function) of a substance and its spectrum. Infrared light from the light source passes through a Michelson interferometer and is absorbed by the sample. The bonds between different atoms absorb light at different frequencies. | -Νon-destructive technique. -Simultaneous analysis can be performed for multiple compounds. -High sensitivity. -No calibration required. -High resolution. | -Only functional groups in a sample may be identified, not individual molecules. |
| Electron microscopy | A primary electron beam is brought into contact with the surface of the tested sample, creating several interactions (mainly X-ray), thereby generating information about the chemical and spatial distribution of MPs in the sample. | -High resolution. -Elemental analysis. -Wide scope of samples. | -Expensive and sophisticated instrumentation. -Need for an expert end-user. -Lack of portability. |
| Pyrolysis gas chromatography–mass spectrometry (Py-GC-MS) | MPs are pyrolyzed by controlled thermal degradation under an inert atmosphere. The resulting lower molecular-weight molecules are separated chromatographically by GC and detected through MS by their mass spectrum. | -Reduced interference by MP characteristics (e.g., color, size, and shape) and/or additives. -Small amount of sample required. | -Not suitable for highly complex and heterogeneous samples. -Expensive and sophisticated instrumentation. |
| Type of Assay | Biorecognition Element | Sample Type | LOD * | Reference |
|---|---|---|---|---|
| Electrochemical impedance spectrometry (EIS) | Extracellular polymeric substances from the cyanobacterium G. gelationosa | Standard solutions | 10−6 to 10−11 M | [63] |
| Graphene | Standard solutions | 15 nM | [81] | |
| Surface-plasmon resonance (SPR) | Estrogen receptor | Seawater | 1 ng/mL | [64,77] |
| Standard solutions | 0.09 nM | [76] | ||
| Raman scattering | - | Standard solutions | 0.015% w/v | [66] |
| MP-binding peptides | Mice tissue extracts | n/a | [68] | |
| Microfluidic CMOS sensor | - | Tap, river, and sea water | 0.01% w/v | [84] |
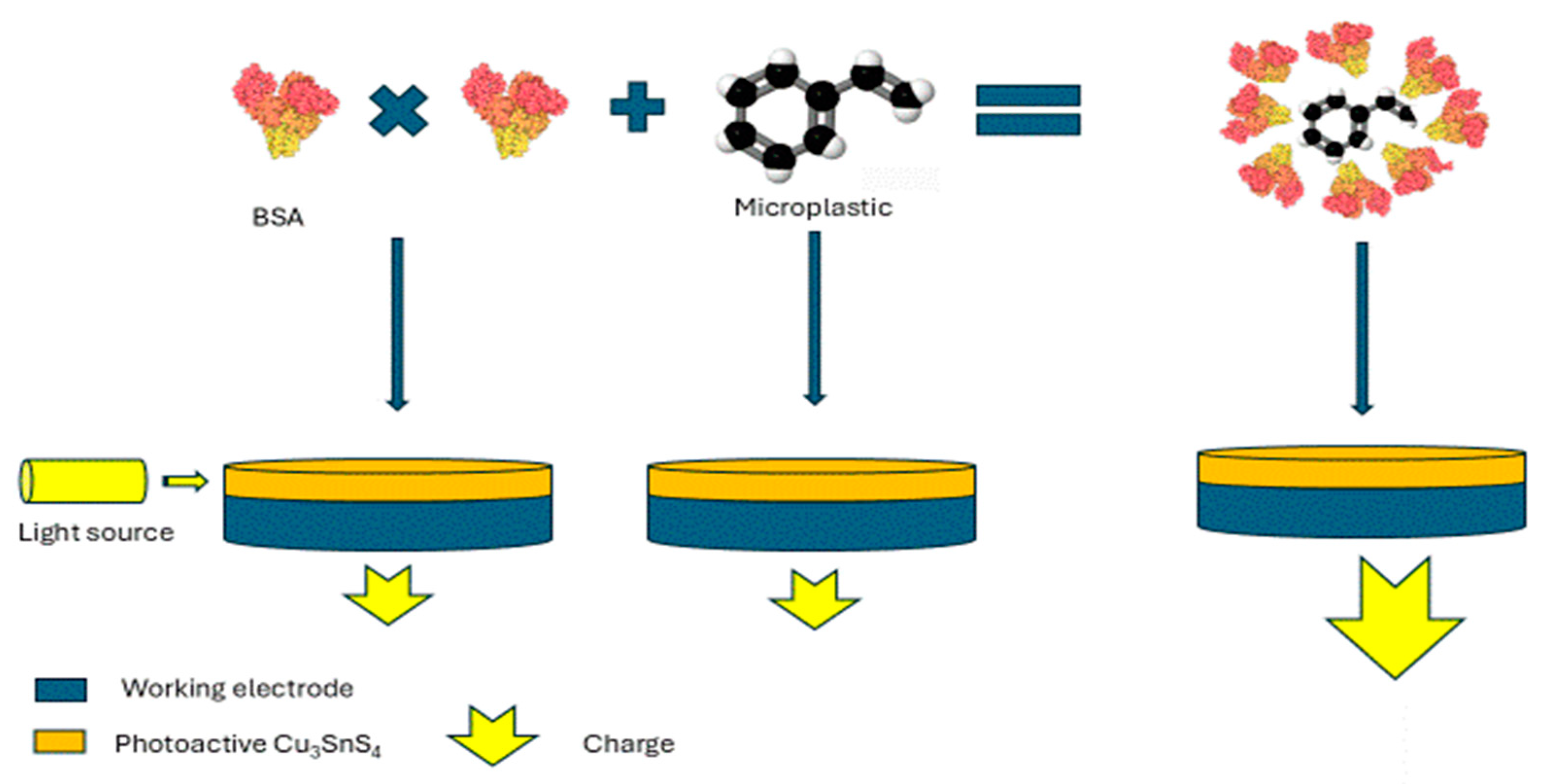
| Photoelectrochemical (PEC) | Protein | Tap and river water | 60 ng/mL | [65] |
| Surface acoustic wave (SAW) | Estrogen receptors | Standard solutions | 0.3 ng/mL | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoutakou, M.; Kintzios, S. Biosensors for Micro- and Nanoplastics Detection: A Review. Chemosensors 2025, 13, 143. https://doi.org/10.3390/chemosensors13040143
Daoutakou M, Kintzios S. Biosensors for Micro- and Nanoplastics Detection: A Review. Chemosensors. 2025; 13(4):143. https://doi.org/10.3390/chemosensors13040143
Chicago/Turabian StyleDaoutakou, Maria, and Spyridon Kintzios. 2025. "Biosensors for Micro- and Nanoplastics Detection: A Review" Chemosensors 13, no. 4: 143. https://doi.org/10.3390/chemosensors13040143
APA StyleDaoutakou, M., & Kintzios, S. (2025). Biosensors for Micro- and Nanoplastics Detection: A Review. Chemosensors, 13(4), 143. https://doi.org/10.3390/chemosensors13040143






