Recent Progress in Nanomaterials for Electrochemical Sensing of Natural Bioactive Compounds
Abstract
1. Introduction
2. Classes of Nanomaterials for Electrochemical Sensing
2.1. Carbon-Based Materials
2.2. Metal and Metal Oxide Nanoparticles
2.3. Two-Dimensional Noncarbon Materials
2.4. Polymer-Based Materials and Crystal Frameworks
2.5. Thermolysis-Derived Electrode Modifiers
| Synthesis Method | Modified Electrode | Analyte | Method | LOD (μM) | Linear Range (µM) | Sensitivity (μA μM–1) | Reproducibility (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Carbon-based materials | - | graphite powder/paraffin wax paste | DA IL-6 | Amp. | 62.5 75 pg | - | - | - | [23] |
| - | GR-MWCNT/GCE | AA DA UA | DPV | 6.71 0.58 7.30 | 100–1000 5–50 50–500 | 0.076 1.38 0.181 | 1.83 1.92 2.17 | [24] | |
| Chemical oxidation | CB-GO/SPCE | UA | FI-Amp. | 0.01 | 0.05–2000 | 0.0191 | 3.10 | [25] | |
| Thermal decomposition | GQD/SPCE | Codeine | CV/DP | 0.21 | 0.73–12 | - | 3.9 | [26] | |
| Electrochemical exfoliation | GLDH/rGO/NiF | Glutamate | CV/DPV | 0.1 | 5–300 | 4.8 µA/(µM·cm2) | 3 | [27] | |
| Solvothermal | C-CoCP@GO/GCE | HQ CC | DPV | 0.46 0.27 | 3–600 3–1750 | - | - 2.3 | [28] | |
| - | CNTs/Au MEA | Glucose | EIS | 0.2 | 0.2–27.5 | 168.03 kΩ−1 M−1 | 1.28 | [29] | |
| Metal and metal oxide nanoparticles | - | AuNPs/SPE | B1 | DPV | 0.08 ng L−1 | 1 ng L−1–1 mg L−1 | - | 3.8 | [30] |
| - | AuNPs/rGO/SPE | Pyocyanin | DPV | 0.27 (PBS) | 1–100 | - | - | [31] | |
| Bioreduction | rGO-AuNPs/CNT/SPE | Estradiol | DPV | 0.003 | 0.05–1.0 | - | 5.3 | [32] | |
| Surface functionalization | Au-surface | CRP | EIS | 0.7 Μg mL−1 | 0.7–10 Μg mL−1 | - | - | [33] | |
| Two-step chemical synthesis | AgNPs-MWCNT/GCE | H2O2 | Amp. | 0.38 | 1–1000 | 2556 µA/(µM·cm2) | 1.2 | [34] | |
| Chemical reduction | rGO-AgNPs/GCE | Estriol | DPV | 0.021 | 0.1–3 | - | 1.5 | [35] | |
| Two-step chemical reduction | GC/rGO-CuNPs | Estriol | DPV | 0.17 | 0.5–3.0 | - | - | [36] | |
| - | CPE/Fe3O4 NPs | sinapic acid syringic acid rutin | DPV | 0.22 0.26 0.08 | 0.9–8 1–9.1 0.3–3 | - | 4.2 3.6 4.6 | [37] | |
| Hydrothermal | FeWO4/CPE | Morphine | SWV | 0.58 | 5–85 | - | 2.56 | [38] | |
| Sol–gel | PB–fCNT/GC HRP–TiO2/fCNT/GC | H2O2 | Chronoamp. | 0.015 0.81 | 0.05–0.8 0.5–7.5 | 163.01 963 | - | [39] | |
| Hydrothermal | ZnO/CNS/MCPE | Quercetin | DPV | 0.04 | 0.166–3.63 | - | 1.76 | [40] | |
| Hydrothermal | NiO/ZnO/GCE | Dopamine | LSV | 0.036 | 0.01–4 | - | 3 | [41] | |
| Co-precipitation | NiO nano/CPE | GA | SWV | 0.04 | 0.2–100, 100–200 | - | 3.1 | [42] | |
| Solution combustion | Bi2O3(n)@SWCNT/CP | Honokiol | SWV | 0.17 | 0.1–50 | 4.96 | [43] | ||
| Hydrothermal | BiOCl/CPE | Quinine | DPV | 0.14 | 10–140 | 1.995 | 5.7 | [44] | |
| Mechanochemical | MgO-MWCNTs-MCPE | Guanine Adenine Epinephrine | CV | 0.92 1.49 0.83 | 10–80 | - | 6.23 7.28 8.32 | [45] | |
| - | CeO2 NPs/CPE | Diethylstilbestrol 17β-estradiol | SWV | 1.3 12.1 | 10–100 100–1200 | - | <4 <3 | [46] | |
| Hard-templating | ZnO-CeO2/GCE | DA UA | DPV | 0.39 0.49 | 5–800 10–1000 | 1122.86 908.53 | 6.2 | [47] | |
| Two-dimensional noncarbonmaterials | Green synthesis | MoS2/rGO/Ag/GCE | AA DA UA | CV | 10.41 0.009 5.94 | 0.75–75 2.5–12.5 0.125–12.5 | - | - | [48] |
| Hydrothermal | MoS2/ILCPE | AA | DPV | 0.2 | 1–1000 | 0.1011 | - | [49] | |
| Atomic layer deposition | CNTs-WS2/SPCE | B2 | DPV | 1.24 | 0–45 | 9 μA μM−1 cm−2 | - | [50] | |
| Hydrothermal | WS2/CS/SPE | Histamine | DPV | 0.0844 | 1–100 | 1.44 × 10–4 mA/μM cm2 | 6.03 | [51] | |
| Etching and stirred electrolysis | Ti3C2Tx MXene/CuxO/CFP | Glucose | Chronoamp. | 0.065 | 0.001–4.655, 5.155–16.155 | 361, 133 mA/μM cm2 | 4% | [52] | |
| Etching | S-MXene/HG/GCE | DA | Chronoamp. | 0.058 | 0.1–255 | - | 2.08 | [53] | |
| Polymer-based materials | Polymerization | Chitosan-modified CMWCNT | Quercetin | - | 0.23 | 1–245.5 245.5–630.5 | - | - | [54] |
| Polymerization | poly(cytosine)/GCE | Guanine | SWV | 0.0061 | 0.1–200 | - | 3.4 | [55] | |
| Solvothermal | Ppy/MoO3/ITO | DA | SWV | 0.0022 | 0.005–0.25 | - | - | [57] | |
| - | [Cu2tpmc](ClO4)4/PVC/GCE | GA | DPV | 0.148 | 0.25–1 5–100 | - | - | [58] | |
| Co-precipitation electropolymerization | poly (glutamic acid)/ZnO NPs/CPE | B2 | SWV | 0.0007 | 0.005–10 | 21.53 | 1.2 | [59] | |
| Polymerization | LIG/G-PANI | Cortisol | CV EIS Chronoamp. | 0.0813 0.0577 0.105 | 0.1–100 0.1–100 0.25–100 | - | 5.85 | [60] | |
| Solution-phase assembly | GN@Ag/g-C3N4/GCE | Estradiol | CV | 0.002 | 0.005–8.0 | 0.07699 | 0.96 | [64] | |
| Thermal polymerization followed by thermal oxidation etching | C3N4–GO/GCE | AA DA UA | DPV | 3.7 0.07 0.43 | 30–3000 0.25–320 2.5–1100 | 0.038 0.46 0.049 μA μM−1 cm−2 | 2.94 1.83 2.65 | [65] | |
| Thermal polycondensation | BP-gCN/SPCE | Glucose | DPV | - | 0.2–1 | 1.1 µA mM−1 cm−2 | - | [66] | |
| In situ growth | COF/cCFE | DA | DPV | 0.0082 | 0–20 | 0.00076 | - | [67] | |
| Electrospinning Stabilization Carbonization | Mn-Co(2-MeIm)MOF@CNF//GCE | Histamine | DPV | 0.0896 | 10–1500 | 107.3 µA mM−1 cm−2 | 3.63 | [68] | |
| Solvothermal | Cu-MOF/CPE | Quercetin | DPV | 0.043 0.008 | 0.01–1 1–70 | - | 0.8743 | [69] | |
| - | SynFe + Ti/UF-TP@CPE | Caffeic acids | DPV | 0.046 | 0.5–100 | - | 2.8–3.4 | [70] | |
| Solid-state thermal decomposition | TPCeO2&MWCNT@CPE | DA | SWV | 0.14 | 0.5–100 | - | - | [71] | |
| Thermolysis-derived modifiers | Thermolysis | TPCo3O4&SWCNT@CPE | α-lipoic acid | SWV | 0.37 | 2–100 | - | - | [72] |
| Thermal decomposition in air | CuO/C-400 °C | Glucose | Amp. | 1.0 | 5.0–25.325 | 244.71 µA mM− 1 cm− 2 | - | [73] | |
| Pyrolisis | CeO2/siloxene/GCE | DA | DPV | 0.292 | 0.292–7.8 | - | - | [74] | |
| Hydrothermal decomposition | CeO2/Co3O4–4 | DA | DPV | 0.13 | 0.13–15 15–60 | 2.632 0.728 µA mM− 1 cm− 2 | - | [75] | |
| Thermolysis | MOFdNC/AuNPs&CPE | UA | SWV | 0.011 | 0.05–1 1–50 | - | 4.4 | [76] | |
| Thermolysis | MOFdCeO2/g-C3N4/CPE | GU | SWV | 0.12 | 0.5–100 | - | 1.6 | [77] |
3. Strategies for the Development of Nanostructures for Electrochemical Sensing
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Generalić Mekinić, I.; Šimat, V. Bioactive Compounds in Foods: New and Novel Sources, Characterization, Strategies, and Applications. Foods 2025, 14, 1617. [Google Scholar] [CrossRef]
- Robotics, O. Bioactive Compounds: Types, Biological Activities and Health Effects; Nova Science Publishers: Hauppauge, NK, USA, 2012. [Google Scholar]
- Alvarez-Leite, J.I. The Role of Bioactive Compounds in Human Health and Disease. Nutrients 2025, 17, 1170. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Ksouri, R.; Hamdi, S. Nanoemulsions as Potential Delivery Systems for Bioactive Compounds in Food Systems: Preparation, Characterization, and Applications in Food Industry. In Emulsions; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–403. ISBN 978-0-12-804306-6. [Google Scholar]
- Pai, S.; Hebbar, A.; Selvaraj, S. A Critical Look at Challenges and Future Scopes of Bioactive Compounds and Their Incorporations in the Food, Energy, and Pharmaceutical Sector. Environ. Sci. Pollut. Res. 2022, 29, 35518–35541. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu Ohiagu, F.; Chikezie, P.C.; Chikezie, C.M. Toxicological Significance of Bioactive Compounds of Plant Origin. Pharmacogn. Commun. 2021, 11, 67–77. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Zúñiga-Miranda, J.; Arias-Almeida, B.; Guamán, L.P. Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods. Molecules 2022, 27, 4490. [Google Scholar] [CrossRef] [PubMed]
- Madej, K.; Piekoszewski, W. Modern Approaches to Preparation of Body Fluids for Determination of Bioactive Compounds. Separations 2019, 6, 53. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Ramakrishna, A. (Eds.) Analytical Methods in the Determination of Bioactive Compounds and Elements in Food; Food Bioactive Ingredients; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-61878-0. [Google Scholar]
- Apak, R.; Çapanoğlu, E.; Shahidi, F. (Eds.) Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications, 1st ed.; Functional Food Science and Technology Series; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-13538-8. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, R.; Bansal, D.; Bhateria, R.; Sharma, M. A Review on Recent Trends and Future Developments in Electrochemical Sensing. ACS Omega 2024, 9, 7336–7356. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Garcia-Miranda Ferrari, A.; Dempsey, N.C.; Banks, C.E. Electroanalytical Overview: Screen-Printed Electrochemical Sensing Platforms for the Detection of Vital Cardiac, Cancer and Inflammatory Biomarkers. Sens. Diagn. 2022, 1, 405–428. [Google Scholar] [CrossRef]
- Kamalasekaran, K.; Sundramoorthy, A.K. Applications of Chemically Modified Screen-Printed Electrodes in Food Analysis and Quality Monitoring: A Review. RSC Adv. 2024, 14, 27957–27971. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Screen-Printed Electrodes: Promising Paper and Wearable Transducers for (Bio)Sensing. Biosensors 2020, 10, 76. [Google Scholar] [CrossRef]
- Silva, R.M.; Da Silva, A.D.; Camargo, J.R.; De Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Imran, A.B.; Foyez, T. Voltammetric Sensors Modified with Nanomaterials: Applications in Rapid Detection of Bioactive Compounds for Health and Safety Monitoring. Discov. Electrochem. 2025, 2, 14. [Google Scholar] [CrossRef]
- Berkel, C.; Özbek, O. Green Electrochemical Sensors, Their Applications and Greenness Metrics Used: A Review. Electroanalysis 2024, 36, e202400286. [Google Scholar] [CrossRef]
- Adhikari, B.-R.; Govindhan, M.; Chen, A. Carbon Nanomaterials Based Electrochemical Sensors/Biosensors for the Sensitive Detection of Pharmaceutical and Biological Compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef]
- Pandey, R.R.; Chusuei, C.C. Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules 2021, 26, 6674. [Google Scholar] [CrossRef]
- Milne, S.A.; Lasserre, P.; Corrigan, D.K. Fabrication of a Graphite-Paraffin Carbon Paste Electrode and Demonstration of Its Use in Electrochemical Detection Strategies. Analyst 2024, 149, 4736–4746. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-H.; Xu, J.-Y.; Lin, J.-T.; Chiang, Y.-T.; Weng, Y.-C. Graphene–Multiwalled Carbon Nanotubes Modified Glassy Carbon Electrodes for Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. ACS Omega 2025, 10, 8160–8171. [Google Scholar] [CrossRef] [PubMed]
- Reanpang, P.; Mool-am-kha, P.; Upan, J.; Jakmunee, J. A Novel Flow Injection Amperometric Sensor Based on Carbon Black and Graphene Oxide Modified Screen-Printed Carbon Electrode for Highly Sensitive Determination of Uric Acid. Talanta 2021, 232, 122493. [Google Scholar] [CrossRef]
- Ferrer-Biechy, L.; Soriano, M.L.; Lucena, R.; Cárdenas, S. Graphene Quantum Dots Modified Electrodes as Electrochemical Sensing Tool towards the Detection of Codeine in Biological Fluids and Soft Drinks. Microchim. Acta 2024, 191, 722. [Google Scholar] [CrossRef]
- Alsharabi, R.M.; Pandey, S.K.; Singh, J.; Kayastha, A.M.; Saxena, P.S.; Srivastava, A. Ultra-Sensitive Electrochemical Detection of Glutamate Based on Reduced Graphene Oxide/Ni Foam Nanocomposite Film Fabricated via Electrochemical Exfoliation Technique Using Waste Batteries Graphite Rods. Microchem. J. 2024, 199, 110055. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Yao, W.; Kang, L.; Gao, E.; Fedin, V.P. An Electrochemical Sensor Based on Carbon Composites Derived from Bisbenzimidazole Biphenyl Coordination Polymers for Dihydroxybenzene Isomers Detection. Microchim. Acta 2024, 191, 20. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jaiswal, N.; Tiwari, I.; Ahmad, M.; Silva, S.R.P. Electrochemical Biosensors Based on in Situ Grown Carbon Nanotubes on Gold Microelectrode Array Fabricated on Glass Substrate for Glucose Determination. Microchim. Acta 2023, 190, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, L.; Dansou, D.M.; Tang, C.; Zhang, J.; Qin, Y.; Yu, Y. Development of an Electrochemical Sensor Modified with Gold Nanoparticles for Detecting Fumonisin B1 in Packaged Foods. RSC Adv. 2024, 14, 29254–29259. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Kannan, V.; Ahmad, M.H.; Mon, A.A.; Taufik, S.; Miskon, A.; Ong, K.K.; Yusof, N.A. An Electrochemical Sensor Based on Gold Nanoparticles-Functionalized Reduced Graphene Oxide Screen Printed Electrode for the Detection of Pyocyanin Biomarker in Pseudomonas Aeruginosa Infection. Mater. Sci. Eng. C 2021, 120, 111625. [Google Scholar] [CrossRef]
- Musa, A.; Kiely, J.; Luxton, R.; Honeychurch, K. An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors 2023, 13, 491. [Google Scholar] [CrossRef]
- Grammoustianou, A.; Saeidi, A.; Longo, J.; Risch, F.; Ionescu, A.M. Real Time Detection of C Reactive Protein in Interstitial Fluid Using Electrochemical Impedance Spectroscopy, towards Wearable Health Monitoring. arXiv 2024, arXiv:2407.16734. [Google Scholar] [CrossRef]
- Xu, D.; Hou, B.; Qian, L.; Zhang, X.; Liu, G. Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes. Molecules 2019, 24, 3411. [Google Scholar] [CrossRef]
- Donini, C.A.; Da Silva, M.K.L.; Simões, R.P.; Cesarino, I. Reduced Graphene Oxide Modified with Silver Nanoparticles for the Electrochemical Detection of Estriol. J. Electroanal. Chem. 2018, 809, 67–73. [Google Scholar] [CrossRef]
- Barreto, F.C.; Silva, M.K.L.; Cesarino, I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors 2022, 10, 395. [Google Scholar] [CrossRef]
- Pwavodi, P.C.; Ozyurt, V.H.; Asir, S.; Ozsoz, M. Electrochemical Sensor for Determination of Various Phenolic Compounds in Wine Samples Using Fe3O4 Nanoparticles Modified Carbon Paste Electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef]
- Ognjanović, M.; Nikolić, K.; Bošković, M.; Pastor, F.; Popov, N.; Marciuš, M.; Krehula, S.; Antić, B.; Stanković, D.M. Electrochemical Determination of Morphine in Urine Samples by Tailoring FeWO4/CPE Sensor. Biosensors 2022, 12, 932. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Fernández, L.; González, G.; Montero-Jiménez, M.; Uribe, R.; Díaz Barrios, A.; Espinoza-Montero, P.J. Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Nanomaterials 2019, 10, 64. [Google Scholar] [CrossRef]
- Saritha, D.; Koirala, A.R.; Venu, M.; Reddy, G.D.; Reddy, A.V.B.; Sitaram, B.; Madhavi, G.; Aruna, K. A Simple, Highly Sensitive and Stable Electrochemical Sensor for the Detection of Quercetin in Solution, Onion and Honey Buckwheat Using Zinc Oxide Supported on Carbon Nanosheet (ZnO/CNS/MCPE) Modified Carbon Paste Electrode. Electrochim. Acta 2019, 313, 523–531. [Google Scholar] [CrossRef]
- Naz, I.; Tahira, A.; Mallah, A.B.; Dawi, E.; Saleem, L.; Ibrahim, R.M.; Ibupoto, Z.H. Detection of Dopamine Using Hybrid Materials Based on NiO/ZnO for Electrochemical Sensor Applications. Catalysts 2025, 15, 116. [Google Scholar] [CrossRef]
- Mutić, T.; Ognjanović, M.; Ivković, D.; Nikolić, V.; Stanković, V.; Ristivojević, P.; Stanković, D. Improving Gallic Acid Detection in Plant Samples: Fabrication and Optimization of a Sensitive and Selective NiO-Supported Carbon Paste Electrode. J. Electroanal. Chem. 2024, 960, 118213. [Google Scholar] [CrossRef]
- Knežević, S.; Ognjanović, M.; Dojčinović, B.; Antić, B.; Vranješ-Đurić, S.; Manojlović, D.; Stanković, D.M. Sensing Platform Based on Carbon Paste Electrode Modified with Bismuth Oxide Nanoparticles and SWCNT for Submicromolar Quantification of Honokiol. Food Anal. Methods 2022, 15, 856–867. [Google Scholar] [CrossRef]
- Mutić, T.; Stanković, V.; Ognjanović, M.; Nikolić, V.B.; Gao, G.; Sojic, N.; Stanković, D. Pseudospherical Bismuth Oxychloride-Modified Carbon Paste Electrode for the Determination of Quinine in Beverages. Electrochem 2024, 5, 407–420. [Google Scholar] [CrossRef]
- Chetankumar, K.; Swamy, B.E.K.; Naik, H.S.B. MgO and MWCNTs Amplified Electrochemical Sensor for Guanine, Adenine and Epinephrine. Mater. Chem. Phys. 2021, 267, 124610. [Google Scholar] [CrossRef]
- Souza, M.B.; Santos, J.S.; Pontes, M.S.; Nunes, L.R.; Oliveira, I.P.; Lopez Ayme, A.J.; Santiago, E.F.; Grillo, R.; Fiorucci, A.R.; Arruda, G.J. CeO2 Nanostructured Electrochemical Sensor for the Simultaneous Recognition of Diethylstilbestrol and 17β-Estradiol Hormones. Sci. Total Environ. 2022, 805, 150348. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Chen, Y.; Deng, D.; He, H.; Lei, Y.; Luo, L. ZnO-CeO2 Hollow Nanospheres for Selective Determination of Dopamine and Uric Acid. Molecules 2024, 29, 1786. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, B.; Al Mamun, H.; Konwar, M.; Patar, S.; Saikia, P.; Jyoti Borthakur, L. Non-Enzymatic Electrochemical Biosensor for Dopamine Detection Using MoS2/rGO/Ag Nanostructure. ChemistrySelect 2023, 8, e202205030. [Google Scholar] [CrossRef]
- Soltani-Nejad, H.; Nejad, F.G.; Beitollahi, H. Development of an Efficient Electrochemical Sensor Based on MoS2 Nanosheets and Ionic Liquid Modified Carbon Paste Electrode for Determination of Ascorbic Acid in the Presence of Vitamin B6. Food Meas. 2024, 18, 1318–1327. [Google Scholar] [CrossRef]
- Zribi, R.; Raza, M.H.; Pinna, N.; Neri, G. Electrochemical Performance of WS2-CNT Core–Shell Heterostructures for the Detection of Vitamin B2. Proceedings 2024, 97, 39. [Google Scholar]
- Singh, D.; Srivastava, A.; Chaturvedi, V.K.; Singh, J. Nanostructured WS2 @Chitosan-Modified Screen-Printed Carbon Electrodes for Efficient Amperometric Detection of Histamine. ACS Omega 2025, 10, 3153–3164. [Google Scholar] [CrossRef]
- Feng, L.; Chen, J.; Yang, M.; Wang, J.; Yin, S.; Zhang, D.; Qin, W.; Song, J. CuxO Decorated Ti3C2Tx MXene Composites for Non-Enzymatic Glucose Sensing with Large Linear Ranges. Microchim. Acta 2024, 191, 451. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Li, C.; Han, J.; Huang, W.; Zhou, J.; Yang, Y. Hydrophilic Antifouling 3D Porous MXene/Holey Graphene Nanocomposites for Electrochemical Determination of Dopamine. Microchem. J. 2022, 181, 107713. [Google Scholar] [CrossRef]
- Duan, M.; Sun, Y.; Ma, S.; Liu, X.; Li, Q.; Zhang, C.; Zhen, Q.; Pang, H. Efficient Quercetin Sensor Utilizing Chitosan-Modified Carboxylated MWCNTs for Fast and Accurate Analysis. React. Chem. Eng. 2025, 10, 2548–2559. [Google Scholar] [CrossRef]
- Metto, M.; Tesfaye, A.; Atlabachew, M.; Abebe, A.; Fentahun, T.; Munshea, A. A Novel Poly(Cytosine)-Based Electrochemical Biosensor for Sensitive and Selective Determination of Guanine in Biological Samples. ACS Omega 2024, 9, 26222–26234. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Crapnell, R.D.; Eersels, K.; Van Grinsven, B.; Kunhiraman, A.K.; Singla, P.; McClements, J.; Banks, C.E.; Novakovic, K.; Peeters, M. Reviewing the Use of Chitosan and Polydopamine for Electrochemical Sensing. Curr. Opin. Electrochem. 2022, 32, 100885. [Google Scholar] [CrossRef]
- Alahmadi, N.; El-Said, W.A. Electrochemical Sensing of Dopamine Using Polypyrrole/Molybdenum Oxide Bilayer-Modified ITO Electrode. Biosensors 2023, 13, 578. [Google Scholar] [CrossRef]
- Petković, B.B.; Stanković, D.; Milčić, M.; Sovilj, S.P.; Manojlović, D. Dinuclear Copper(II) Octaazamacrocyclic Complex in a PVC Coated GCE and Graphite as a Voltammetric Sensor for Determination of Gallic Acid and Antioxidant Capacity of Wine Samples. Talanta 2015, 132, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, G.; Negash, N.; Tessema, M. Sensitive and Selective Determination of Vitamin B2 in Non-Alcoholic Beverage and Milk Samples at Poly (Glutamic Acid)/Zinc Oxide Nanoparticles Modified Carbon Paste Electrode. BMC Chem. 2022, 16, 69. [Google Scholar] [CrossRef]
- Sarkar, P.P.; Ashraf, A.; Jalal, A.H.; Alam, F.; Islam, N. A Highly Sensitive Electrochemical Immunosensor for Cortisol Detection. Biosensors 2025, 15, 321. [Google Scholar] [CrossRef]
- Jiang, T.; Lin, B.; Yan, Y.; Lv, D.; Xu, Y.; Ren, X.; Wang, T.; Qiao, S.; Jiang, X. Fabrication of an Electrochemical Sensor Based on Molecular Imprinting Technology for Detecting Elemene. Int. J. Electrochem. Sci. 2023, 18, 100361. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Cabaj, J. Electrochemical Biosensor for Detection of 17β-Estradiol Using Semi-Conducting Polymer and Horseradish Peroxidase. RSC Adv. 2020, 10, 9079–9087. [Google Scholar] [CrossRef]
- Da Silva, D.N.; Pereira, A.C. An Electrochemical Sensor Modified with a Molecularly Imprinted Polymer and Carbon Black for 17-β-Estradiol Detection. Anal. Methods 2022, 14, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Agrahari, S.; Gautam, R.K.; Tiwari, I. Fabrication of an Innovative Electrochemical Sensor Based on Graphene-Coated Silver Nanoparticles Decorated over Graphitic Carbon Nitride for Efficient Determination of Estradiol. Environ. Sci. Pollut. Res. 2022, 31, 38628–38644. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Wang, Q.; Wang, X.; Wang, S. Electrochemical Sensor Based on an Electrode Modified with Porous Graphitic Carbon Nitride Nanosheets (C3N4) Embedded in Graphene Oxide for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Microchim. Acta 2020, 187, 149. [Google Scholar] [CrossRef]
- Ozkahraman, E.E.; Eroglu, Z.; Efremov, V.; Maryyam, A.; Abbasiasl, T.; Das, R.; Mirzajani, H.; Hanedar, B.A.; Beker, L.; Metin, O. High Performance Black Phosphorus/Graphitic Carbon Nitride Heterostructure-Based Wearable Sensor for Real-Time Sweat Glucose Monitoring. Adv. Mater. Technol. 2024, 10, e00106. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, R.; Li, X.; Dong, N.; Zhu, B.; Wang, J.; Lin, X.; Su, B. COF-Coated Microelectrode for Space-Confined Electrochemical Sensing of Dopamine in Parkinson’s Disease Model Mouse Brain. J. Am. Chem. Soc. 2023, 145, 23727–23738. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.; Ahmad, M.d.W.; Kim, B.H.; Kamal, T.; Yang, D.-J.; Patra, C.N.; Hossain, S.S.; Choudhury, A. Manganese Cobalt-MOF@carbon Nanofiber-Based Non-Enzymatic Histamine Sensor for the Determination of Food Freshness. Anal. Bioanal. Chem. 2023, 415, 3487–3501. [Google Scholar] [CrossRef]
- Özyurt, V.H. Copper-Based Metal Organic Framework Modified Electrochemical Sensor for Detection of Quercetin in Food Samples. Eur. Food Res. Technol. 2023, 249, 2515–2528. [Google Scholar] [CrossRef]
- Petković, B.B.; Kostić, M.; Samaržija-Jovanović, S.; Ivanović, A.; Laban, B.; Veljović, D.; Stanković, D.M. An Efficient Electrochemical Sensing of Caffeic Acid at Thermolysis Prepared Urea-Formaldehyde Resin Modified with Fe(III) and Ti(IV) Oxide Particles. Int. J. Electrochem. Sci. 2022, 17, 221214. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Fabián, M.; Avdin, V.V.; Manojlović, D.D.; Đurić, S.V.r.a.n.j.e.š.; Petković, B.B. CeO2-Doped–Domestic Carbon Material Decorated with MWCNT as an Efficient Green Sensing Platform for Electrooxidation of Dopamine. Surf. Interfaces 2021, 25, 101211. [Google Scholar] [CrossRef]
- Petković, B.B.; Ognjanović, M.; Antić, B.; Viktorovich Avdin, V.; Manojlović, D.D.; Vranješ Đurić, S.; Stanković, D.M. Easily Prepared Co3O4 Doped Porous Carbon Material Decorated with Single-wall Carbon Nanotubes Applied in Voltammetric Sensing of Antioxidant A-lipoic Acid. Electroanalysis 2021, 33, 446–454. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, X.; Zhao, J.; Cao, J.; Jiang, Z.; Qin, J. MOF Derived Core-Shell CuO/C with Temperature-Controlled Oxygen-Vacancy for Real Time Analysis of Glucose. J. Nanobiotechnol. 2022, 20, 507. [Google Scholar] [CrossRef]
- Ge, C.; Ramachandran, R.; Wang, F. CeO2-Based Two-Dimensional Layered Nanocomposites Derived from a Metal–Organic Framework for Selective Electrochemical Dopamine Sensors. Sensors 2020, 20, 4880. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, X.; Wu, P.; Wang, R.; Xie, L.; Li, Z.; Zhu, Z.; Zhao, H.; Lan, M. ZIF-67 MOF Derived Co-Based CeO2 Electrochemical Sensor for Dopamine. Electrochim. Acta 2023, 463, 142802. [Google Scholar] [CrossRef]
- Ognjanović, M.; Marković, M.; Girman, V.; Nikolić, V.; Vranješ-Đurić, S.; Stanković, D.M.; Petković, B.B. Metal–Organic Framework-Derived CeO2/Gold Nanospheres in a Highly Sensitive Electrochemical Sensor for Uric Acid Quantification in Milk. Chemosensors 2024, 12, 231. [Google Scholar] [CrossRef]
- Petković, B.B.; Kolev, H.; Veljović, D.; Stanković, D.M.; Antić, B.; Ognjanović, M. MOF-Derived Nanoceria/Graphitic Carbon Nitride as an Efficient Electrochemical Modifier for Guanine Sensor with Diffusional Response. J. Alloys Compd. 2025, 1011, 178471. [Google Scholar] [CrossRef]
- Silveri, F.; Della Pelle, F.; Compagnone, D. Recent Advances in Sustainable Strategies for the Integration of Nanostructured Sensing Surfaces in Electroanalytical Devices. TrAC Trends Anal. Chem. 2025, 185, 118175. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Lv, Y.; Zhang, R.; Zhao, S.; Karimi-Maleh, H. Cyclodextrin-Based Architectures for Electrochemical Sensing: From Molecular Recognition to Functional Hybrids. Anal. Methods 2025, 17, 4300–4320. [Google Scholar] [CrossRef]
- Singh, R.; Singh, J. Recent Advances in Nanostructured Cobalt Oxide (Co3O4): Addressing Methods and Design Strategies, Challenges, and Future Directions for Non-Enzymatic Sensor Applications. Sens. Actuators A Phys. 2025, 387, 116404. [Google Scholar] [CrossRef]
- Aidli, W.; Fumagalli, D.; Pifferi, V.; Falciola, L. From Dual-Mode to Multi-Modal Electrochemical Based Sensors: A Path toward Accurate Sensing. Curr. Opin. Electrochem. 2025, 50, 101655. [Google Scholar] [CrossRef]
- Yohan, R.K.; Jagannathan, M.; Sivalingam, G. The Role of Emerging Photoactive Nanostructures in Electrochemical Sensor Construction: Synthesis, Properties, Challenges, and Perspectives. J. Ind. Eng. Chem. 2025, 145, 234–254. [Google Scholar] [CrossRef]
- Nandhini, C.; Huang, C.-H.; Arul, P.; Huang, S.-T. Fabrication of Hybrid Nanocomposites for Electrochemical Evaluation of Food-Based Preservative and Bioactive Targets of Hydrogen Peroxide and Rutin in Real Fruit and Drug Samples. Food Chem. 2025, 469, 142502. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, J.; Zhao, Z.; Xie, Y.; Zhao, P.; Fei, J. Ultrathin Polypyrrole-Derived Porous Carbon Nanosheets Integrated with Fe3O4 Nanoparticles for Enhanced Electrochemical Detection of Catechin. Microchim. Acta 2025, 192, 353. [Google Scholar] [CrossRef]
- Ragumoorthy, C.; Felix, M.A.J.; Chen, S.-M.; Phang, X.-E.; Viveke, A.A. Electrochemical Detection and Specific Recognition of Anti-Bacterial Flavonoid in Food and Biological Samples Using NiMnCo-S/rGO Substrate. Food Chem. 2025, 492, 145308. [Google Scholar] [CrossRef]
- Qi, C.; Kong, Y.; Yang, Z. Constructing High-Accessibility Fe Single-Atoms via Directional Anchoring Strategy for Boosting Electrochemical Sensing. Chem. Eng. J. 2025, 505, 159479. [Google Scholar] [CrossRef]
- Guo, M.; Han, J.; Ran, Q.; Zhao, M.; Liu, Y.; Zhu, G.; Wang, Z.; Zhao, H. Multifunctional Integrated Electrochemical Sensing Platform Based on Mesoporous Silica Nanoparticles Decorated Single-Wall Carbon Nanotubes Network for Sensitive Determination of Gallic Acid. Ceram. Int. 2023, 49, 37549–37560. [Google Scholar] [CrossRef]
- Dos Santos, D.E.F.; Baumgarten, L.G.; Martins, E.C.; Dreyer, J.P.; Santana, E.R.; Winiarski, J.P.; Vieira, I.C. A Sustainable Nanomaterial Based on Gold Nanoparticles and Graphene for Highly Sensitive Electrochemical Sensing of Caffeic Acid in Coffees. Food Anal. Methods 2024, 17, 1348–1358. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Li, S.; Yang, J.; Dong, J. In-Situ Growth of Cerium-Based Metal Organic Framework on Multi-Walled Carbon Nanotubes for Electrochemical Detection of Gallic Acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129318. [Google Scholar] [CrossRef]
- Parasuraman, B.; Chinnapayan, S.; Rangaraju, H.; Paramasivam, S.; Sangaraju, S.; Thangavelu, P.; Huang, C.-H. Rapid Detection of Caffeic Acid in Food Beverages Using a Non-Enzymatic Electrochemical Sensor Based on a Bi2 S3/CNF Nanocomposite. Sustain. Food Technol. 2024, 2, 717–728. [Google Scholar] [CrossRef]
- Ognjanović, M.; Nikolić, K.; Radenković, M.; Lolić, A.; Stanković, D.; Živković, S. Picosecond Laser-Assisted Synthesis of Silver Nanoparticles with High Practical Application as Electroanalytical Sensor. Surf. Interfaces 2022, 35, 102464. [Google Scholar] [CrossRef]
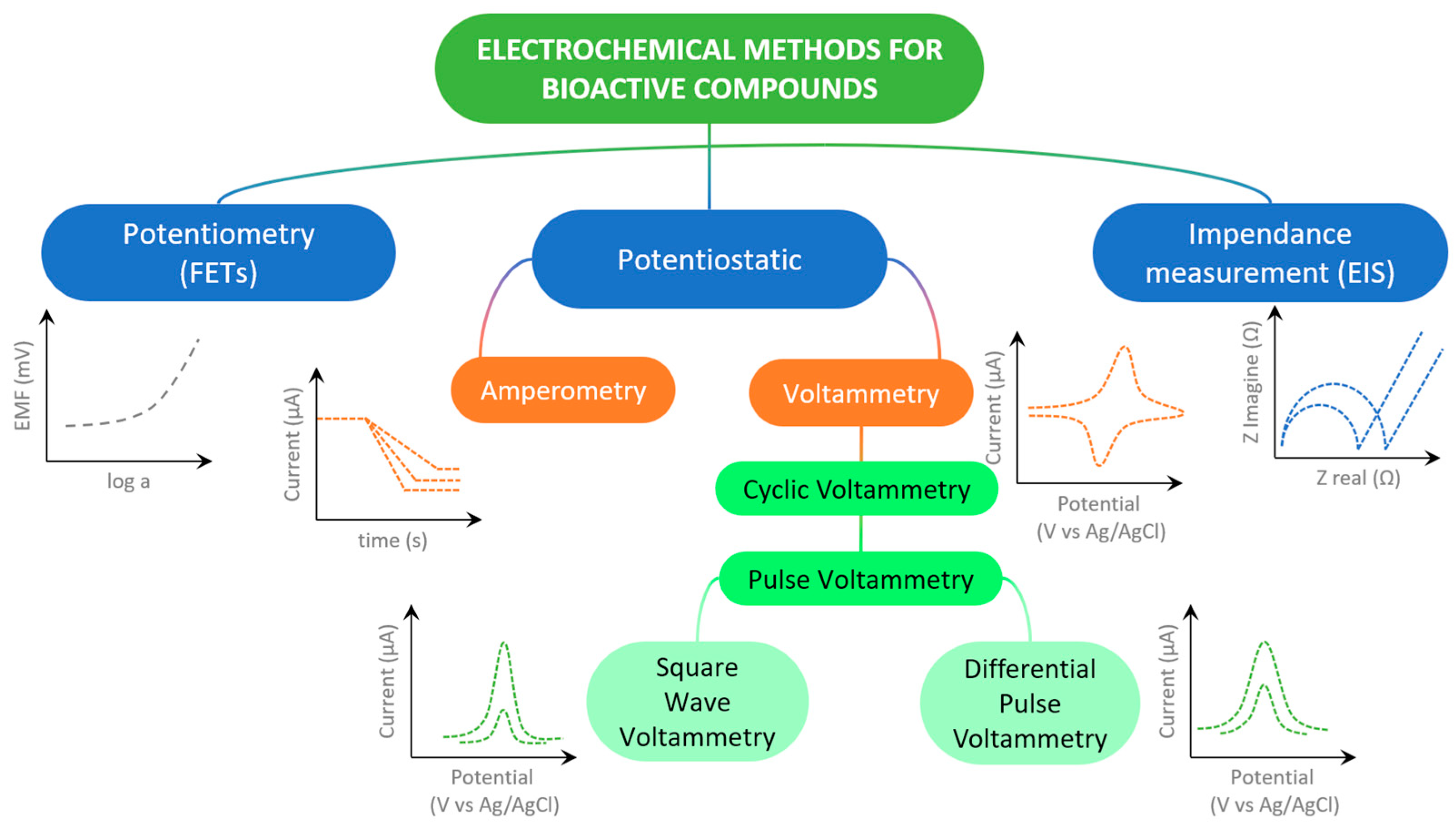
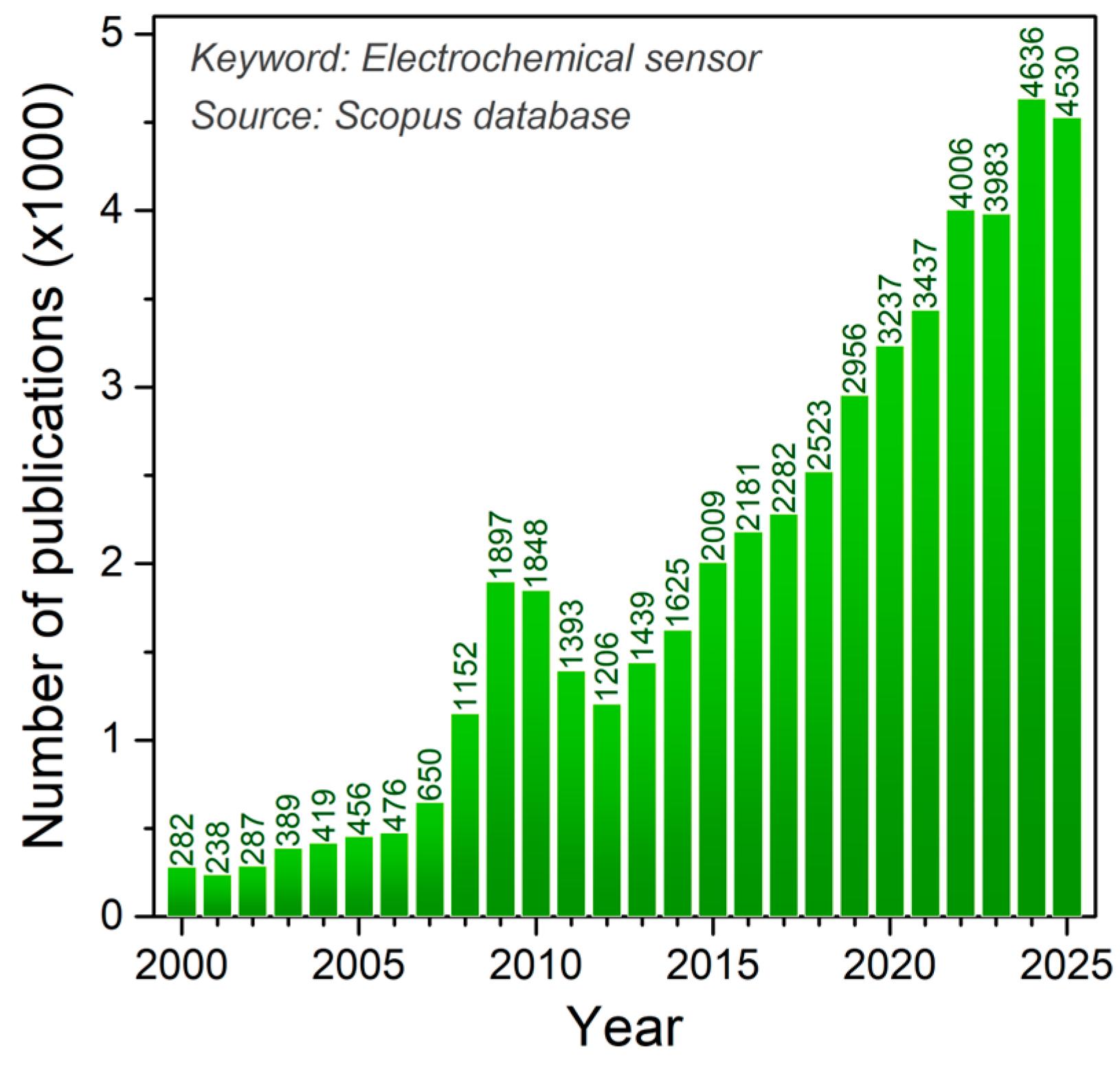


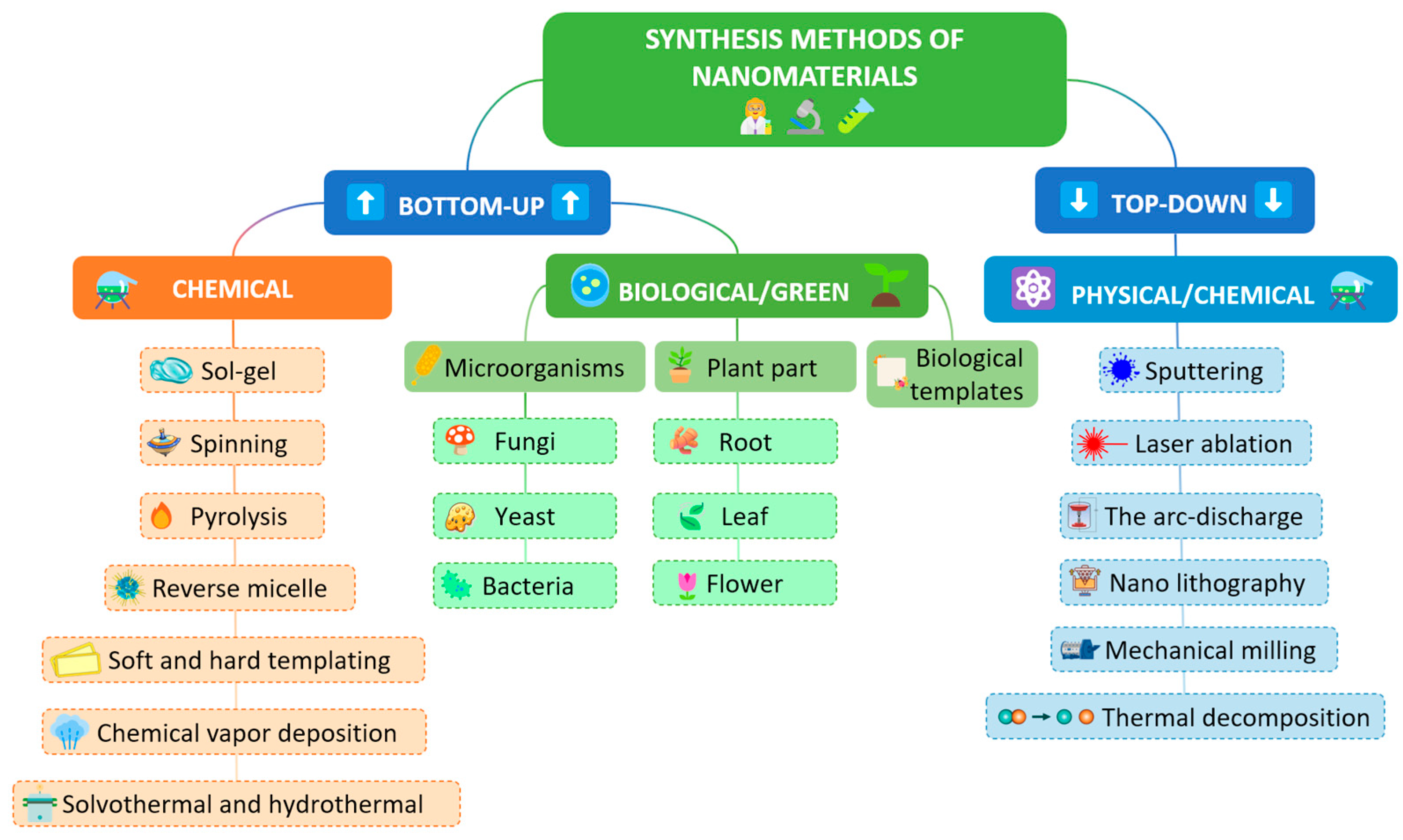
| Compound Class | Prominent Members | Physiological Role/Activity |
|---|---|---|
| Polyphenols | quercetin, caffeic acid, | Antioxidants, present in plants and food |
| gallic acid, syringic acid, | ||
| catechin | ||
| Alkaloids | caffeine, codeine, | Nitrogen heterocycles, often strongly pharmacologically active |
| quinine, nicotine, | ||
| morphine | ||
| Vitamins | A, C, B6, | Essential microelements with regulatory functions |
| B12, D | ||
| Amines and biogenic amines | dopamine, serotonin | Neurotransmitters and signaling molecules |
| adrenaline, histamine | ||
| Amino acids and peptides | guanine, adenine, | Basic building blocks of proteins and regulatory hormones |
| glutamate, proline | ||
| insulin | ||
| Proteins and enzymes | Thrombin, Albumin, | Catalytic, transport, or structural functions |
| Hemoglobin, C-reactive protein | ||
| Steroid hormones | estradiol, estriol, | Hormones with a steroid structure |
| testosterone, epinephrine, | ||
| cortisol | ||
| Carotenoids and terpenoids | β-carotene, | Plant pigments, antioxidants |
| lycopene, elemene | ||
| Flavonoids | Rutin | Powerful antioxidants and anti-inflammatory agents |
| luteolin | ||
| naringenin | ||
| Metabolites and bioactive small molecules | glucose, uric acid, creatinine | Source of energy, antioxidants, maintain organ functions |
| Material | Structure | Main Advantages | Main Limitations |
|---|---|---|---|
| Carbon Nanotubes (CNTs) (SWCNT, MWCNT) | 1D tubular structures | High electrical conductivity; Large surface area; Fast electron transfer kinetics; Easy chemical functionalization (–COOH, –OH); Excellent carriers for metal nanoparticles, enzymes | Poor dispersibility in water; Aggregation tendency; Quality varies (purity, length, defects); More expensive than carbon black |
| Graphene/Reduced Graphene Oxide (rGO) | 2D single-/few-layer sheets | Extremely high surface area; Excellent conductivity (especially rGO); Many active sites for adsorption; Easily functionalized; Relatively low cost | Restacking/aggregation of graphene sheets; Variable quality (layer number, defects); Requires proper reduction/processing to optimize conductivity |
| Carbon Black (CB) | Amorphous carbon nanoparticles | Very cheap and accessible; Good conductivity for cost; Easy mixing with polymers/pastes; Highly stable | Lower surface area than CNT/graphene; Limited functional groups; Not ideal for ultra-low LOD sensing |
| Carbon Quantum Dots (CQDs)/Graphene Quantum Dots (GQDs) | 0D nano-dots (2–10 nm) | Huge surface-to-mass ratio; Rich in surface functional groups; Excellent water solubility; Strong fluorescence (dual-mode sensing: electrochemical + optical); Highly biocompatible | More complex synthesis; Size and surface chemistry must be precisely controlled; Conductivity lower than graphene unless hybridized |
| Carbon Nanorods/Nanofibers | 1D rod/fiber structures | Good mechanical stability; High surface area;Improved porosity vs. CNTs; Good support for catalysts | Conductivity lower than CNT/graphene Less studied; Preparation may require templates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petković, B.B.; Ognjanović, M.; Stanković, D.M. Recent Progress in Nanomaterials for Electrochemical Sensing of Natural Bioactive Compounds. Chemosensors 2025, 13, 429. https://doi.org/10.3390/chemosensors13120429
Petković BB, Ognjanović M, Stanković DM. Recent Progress in Nanomaterials for Electrochemical Sensing of Natural Bioactive Compounds. Chemosensors. 2025; 13(12):429. https://doi.org/10.3390/chemosensors13120429
Chicago/Turabian StylePetković, Branka B., Miloš Ognjanović, and Dalibor M. Stanković. 2025. "Recent Progress in Nanomaterials for Electrochemical Sensing of Natural Bioactive Compounds" Chemosensors 13, no. 12: 429. https://doi.org/10.3390/chemosensors13120429
APA StylePetković, B. B., Ognjanović, M., & Stanković, D. M. (2025). Recent Progress in Nanomaterials for Electrochemical Sensing of Natural Bioactive Compounds. Chemosensors, 13(12), 429. https://doi.org/10.3390/chemosensors13120429






