µ-NMR Technology for Biomedical Applications: A Review
Abstract
1. Introduction
2. Principles of NMR
3. µ-NMR System
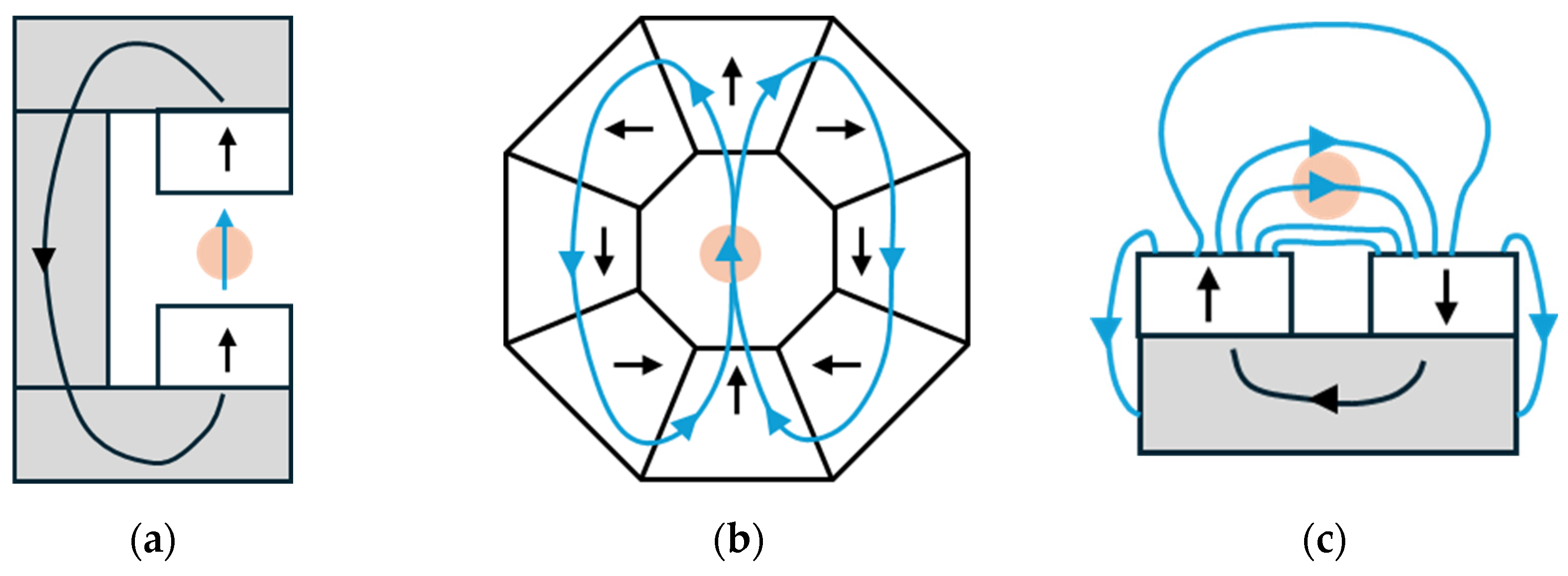
3.1. Permanent Magnet
3.2. Types of Microcoils
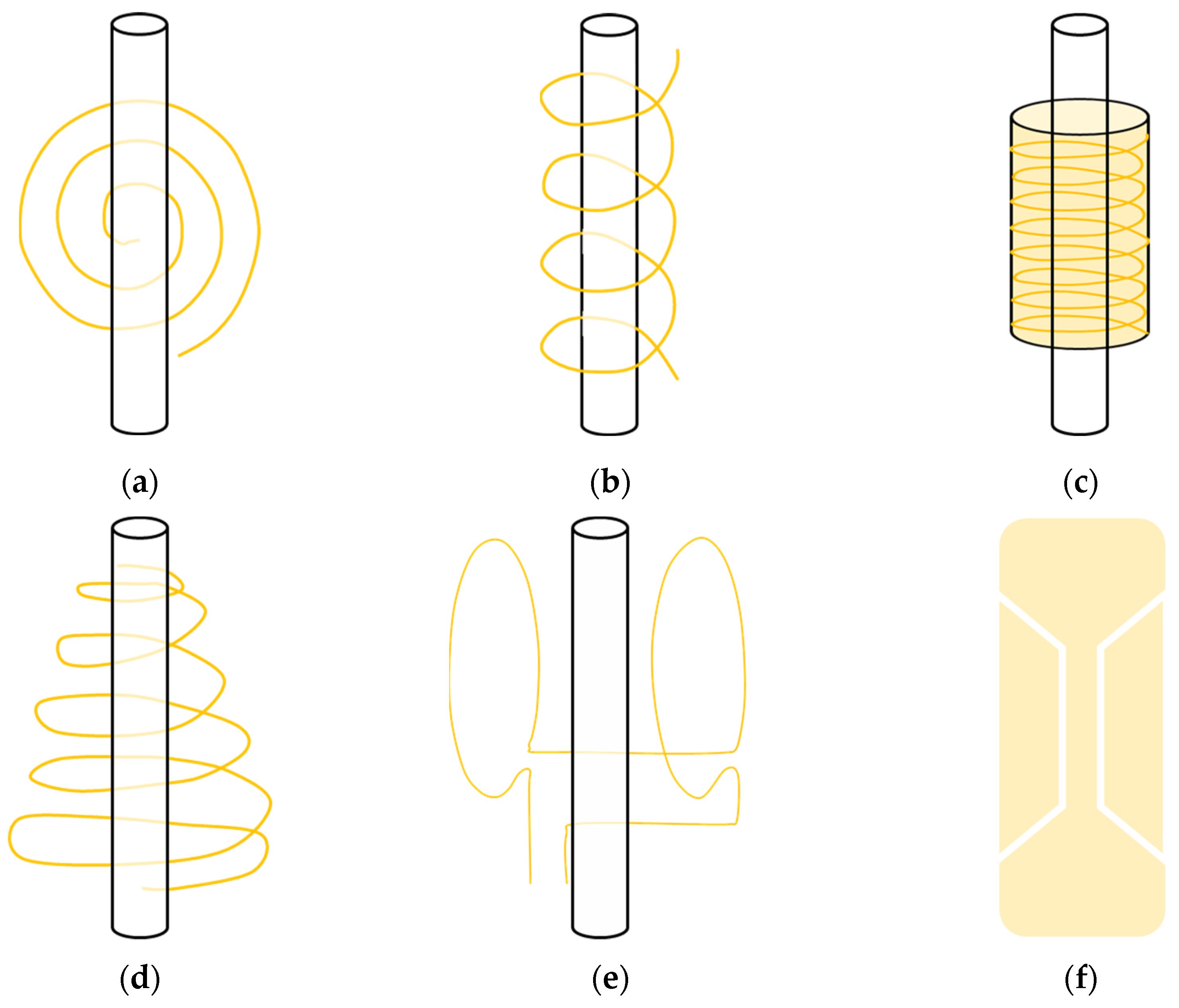
3.2.1. Planar Microcoils
3.2.2. Solenoid Microcoils
3.2.3. Scroll Microcoils
3.2.4. Cone-Shaped Microcoils
3.2.5. Helmholtz Microcoils
3.2.6. Stripline Microcoils
3.3. NMR Chip: Transceiver Electronics
4. Point-Of-Care Applications Based on μ-NMR Systems
5. Challenges and Limitations
5.1. Sensitivity and SNR
5.2. Resolution and Spectral Overlap
5.3. Integration with Other Technologies
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Jiang, D.; Feng, S.; Ren, C.; Guo, J. µ-NMR at the point of care testing. Electrophoresis 2020, 41, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Dupré, A.; Lei, K.-M.; Mak, P.-I.; Martins, R.P.; Peng, W.K. Micro-and nanofabrication NMR technologies for point-of-care medical applications—A review. Microelectron. Eng. 2019, 209, 66–74. [Google Scholar] [CrossRef]
- Raman, S.; Lange, O.F.; Rossi, P.; Tyka, M.; Wang, X.; Aramini, J.; Liu, G.; Ramelot, T.A.; Eletsky, A.; Szyperski, T. NMR structure determination for larger proteins using backbone-only data. Science 2010, 327, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Carballal, R.; Fernandez-Megia, E.; Jimenez, C.; Riguera, R. NMR methods for unravelling the spectra of complex mixtures. Nat. Prod. Rep. 2011, 28, 78–98. [Google Scholar] [CrossRef]
- Casarini, D.; Lunazzi, L.; Mazzanti, A. Recent advances in stereodynamics and conformational analysis by dynamic NMR and theoretical calculations. Eur. J. Org. Chem. 2010, 2010, 2035–2056. [Google Scholar] [CrossRef]
- Robinette, S.L.; Brüschweiler, R.; Schroeder, F.C.; Edison, A.S. NMR in metabolomics and natural products research: Two sides of the same coin. Acc. Chem. Res. 2012, 45, 288–297. [Google Scholar] [CrossRef]
- Gowda, G.N.; Raftery, D. Advances in NMR-based metabolomics. Compr. Anal. Chem. 2014, 63, 187–211. [Google Scholar]
- Price, K.E.; Vandaveer, S.S.; Lunte, C.E.; Larive, C.K. Tissue targeted metabonomics: Metabolic profiling by microdialysis sampling and microcoil NMR. J. Pharm. Biomed. Anal. 2005, 38, 904–909. [Google Scholar] [CrossRef]
- Felli, I.C.; Pierattelli, R. Recent progress in NMR spectroscopy: Toward the study of intrinsically disordered proteins of increasing size and complexity. IUBMB Life 2012, 64, 473–481. [Google Scholar] [CrossRef]
- Markwick, P.R.; Malliavin, T.; Nilges, M. Structural biology by NMR: Structure, dynamics, and interactions. PLoS Comput. Biol. 2008, 4, e1000168. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Danieli, E.; Blumich, B.; Ananikov, V.P. Miniaturization of NMR systems: Desktop spectrometers, microcoil spectroscopy, and “NMR on a chip” for chemistry, biochemistry, and industry. Chem. Rev. 2014, 114, 5641–5694. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.M.; Skrtic, S.; Sharer, K.; Hill, J.M.; Dunbar, C.E.; Koretsky, A.P. MRI detection of single particles for cellular imaging. Proc. Natl. Acad. Sci. USA 2004, 101, 10901–10906. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liu, Y.; Qin, L.; Lee, H.; Weissleder, R.; Ham, D. Small NMR biomolecular sensors. Solid-State Electron. 2013, 84, 13–21. [Google Scholar] [CrossRef]
- Tilgner, M.; Vater, T.S.; Habbel, P.; Cheng, L.L. High-resolution magic angle spinning (HRMAS) NMR methods in metabolomics. In NMR-Based Metabolomics Methods Protocols; Humana: New York, NY, USA, 2019; pp. 49–67. [Google Scholar]
- Polishchuk, D.; Gardeniers, H. A compact permanent magnet for microflow NMR relaxometry. J. Magn. Reson. 2023, 347, 107364. [Google Scholar] [CrossRef]
- Plata, M. Protein NMR on a Chip: Development of an Integrated Microfluidic Platform for Studying Protein-Ligand Interactions by Nuclear Magnetic Resonance. Ph.D. Thesis, University of Southampton, Southampton, UK, 2023. [Google Scholar]
- Fan, S.; Zhou, Q.; Lei, K.-M.; Mak, P.-I.; Martins, R.P. Miniaturization of a nuclear magnetic resonance system: Architecture and design considerations of transceiver integrated circuits. IEEE Trans. Circuits Syst. I Regul. Pap. 2022, 69, 3049–3060. [Google Scholar] [CrossRef]
- Andrew, E.; Szczesniak, E. A historical account of NMR in the solid state. Prog. Nucl. Magn. Reson. Spectrosc. 1995, 28, 11–36. [Google Scholar] [CrossRef]
- Mlynárik, V. Introduction to nuclear magnetic resonance. Anal. Biochem. 2017, 529, 4–9. [Google Scholar] [CrossRef]
- Storey, P. Introduction to magnetic resonance imaging and spectroscopy. Magn. Reson. Imaging Methods Biol. Appl. 2006, 124, 3–57. [Google Scholar]
- Edwards, J.C. Principles of NMR; Process NMR Associates LLC: Danbury, CT, USA, 2009. [Google Scholar]
- Bank, S. Some principles of NMR spectroscopy and their novel application. Concepts Magn. Reson. Educ. J. 1997, 9, 83–93. [Google Scholar] [CrossRef]
- Danieli, E.; Perlo, J.; Blümich, B.; Casanova, F. Small magnets for portable NMR spectrometers. Angew. Chem. Int. Ed. 2010, 49, 4133–4135. [Google Scholar] [CrossRef]
- Moresi, G.; Magin, R. Miniature permanent magnet for table-top NMR. Concepts Magn. Reson. Part B Magn. Reson. Eng. Educ. J. 2003, 19, 35–43. [Google Scholar] [CrossRef]
- Blümich, B.; Rehorn, C.; Zia, W. Magnets for Small-Scale and Portable NMR; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Blümich, B.; Haber-Pohlmeier, S.; Zia, W. Compact NMR; Walter de Gruyter: Berlin, Germany, 2014. [Google Scholar]
- Trout, S. Material selection of permanent magnets, considering thermal properties correctly. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing and Coil Winding Conference (Cat. No. 01CH37264), Cincinnati, OH, USA, 28 October 2001; pp. 365–370. [Google Scholar]
- Kassen, A.G.; White, E.M.; Hu, L.; Tang, W.; Zhou, L.; Kramer, M.J.; Anderson, I.E. Novel mechanisms for solid-state processing and grain growth with microstructure alignment in alnico-8 based permanent magnets. AIP Adv. 2018, 8, 056206. [Google Scholar] [CrossRef]
- Crozier-Bioud, T.; Momeni, V.; Gonzalez-Gutierrez, J.; Kukla, C.; Luca, S.; Rolere, S. Current challenges in NdFeB permanent magnets manufacturing by Powder Injection Molding (PIM): A review. Mater. Today Phys. 2023, 34, 101082. [Google Scholar] [CrossRef]
- Cui, J.; Kramer, M.; Zhou, L.; Liu, F.; Gabay, A.; Hadjipanayis, G.; Balasubramanian, B.; Sellmyer, D. Current progress and future challenges in rare-earth-free permanent magnets. Acta Mater. 2018, 158, 118–137. [Google Scholar] [CrossRef]
- Calin, M.-D.; Helerea, E. Temperature influence on magnetic characteristics of NdFeB permanent magnets. In Proceedings of the 2011 7th International Symposium on Advanced Topics in Electrical Engineering (ATEE), Bucharest, Romania, 12–14 May 2011; pp. 1–6. [Google Scholar]
- Yan, M.; Jin, J.; Ma, T. Grain boundary restructuring and La/Ce/Y application in Nd–Fe–B magnets. Chin. Phys. B 2019, 28, 077507. [Google Scholar] [CrossRef]
- Cui, B.; Liu, X.; Nlebedim, I.C.; Cui, J. Mechanically robust high magnetic-performance Sm-Co sintered magnets through microstructure engineering. J. Alloys Compd. 2022, 926, 166869. [Google Scholar] [CrossRef]
- Liu, S. Sm–Co high-temperature permanent magnet materials. Chin. Phys. B 2019, 28, 017501. [Google Scholar] [CrossRef]
- Minard, K.R.; Wind, R.A. Solenoidal microcoil design—Part II: Optimizing winding parameters for maximum signal-to-noise performance. Concepts Magn. Reson. 2001, 13, 190–210. [Google Scholar] [CrossRef]
- Hoult, D.I.; Richards, R. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. 1969 1976, 24, 71–85. [Google Scholar] [CrossRef]
- Wu, N.; Peck, T.L.; Webb, A.G.; Magin, R.L.; Sweedler, J.V. 1H-NMR spectroscopy on the nanoliter scale for static and online measurements. Anal. Chem. 1994, 66, 3849–3857. [Google Scholar] [CrossRef]
- Van Bentum, P.; Janssen, J.; Kentgens, A. Towards nuclear magnetic resonance μ-spectroscopy and μ-imaging. Analyst 2004, 129, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Badilita, V.; Meier, R.C.; Spengler, N.; Wallrabe, U.; Utz, M.; Korvink, J.G. Microscale nuclear magnetic resonance: A tool for soft matter research. Soft Matter 2012, 8, 10583–10597. [Google Scholar] [CrossRef]
- Krishnapriya, S.; Komaragiri, R.S.; Suja, K. Performance Evaluation of Via-free Non-spiral Planar Microcoils. In Proceedings of the 2020 IEEE 15th International Conference on Nano/Micro Engineered and Molecular System (NEMS), San Diego, CA, USA, 30 September 2020; pp. 247–250. [Google Scholar]
- Krishnapriya, S.; Chandrakar, H.; Komaragiri, R.S.; Suja, K. Performance analysis of planar microcoils for biomedical wireless power transfer links. Sādhanā 2019, 44, 187. [Google Scholar] [CrossRef]
- Bastawrous, M.; Gruschke, O.; Soong, R.; Jenne, A.; Gross, D.; Busse, F.; Nashman, B.; Lacerda, A.; Simpson, A.J. Comparing the potential of Helmholtz and planar NMR microcoils for analysis of intact biological samples. Anal. Chem. 2022, 94, 8523–8532. [Google Scholar] [CrossRef]
- Eroglu, S.; Gimi, B.; Roman, B.; Friedman, G.; Magin, R.L. NMR spiral surface microcoils: Design, fabrication, and imaging. Concepts Magn. Reson. Part B Magn. Reson.Eng. Educ. J. 2003, 17, 1–10. [Google Scholar] [CrossRef]
- Fratila, R.M.; Velders, A.H. Small-volume nuclear magnetic resonance spectroscopy. Annu. Rev. Anal. Chem. 2011, 4, 227–249. [Google Scholar] [CrossRef]
- Meier, R.C.; Höfflin, J.; Badilita, V.; Wallrabe, U.; Korvink, J.G. Microfluidic integration of wirebonded microcoils for on-chip applications in nuclear magnetic resonance. J. Micromech. Microeng. 2014, 24, 045021. [Google Scholar] [CrossRef]
- Jolic, K.; Ghantasala, M.; Hayes, J.; Jin, H. Fabrication of three-dimensional inductor coil using excimer laser micromachining. J. Micromech. Microeng. 2003, 13, 782. [Google Scholar] [CrossRef]
- Ehrmann, K.; Saillen, N.; Vincent, F.; Stettler, M.; Jordan, M.; Wurm, F.M.; Besse, P.-A.; Popovic, R. Microfabricated solenoids and Helmholtz coils for NMR spectroscopy of mammalian cells. Lab Chip 2007, 7, 373–380. [Google Scholar] [CrossRef]
- Kentgens, A.; Bart, J.; Van Bentum, P.; Brinkmann, A.; Van Eck, E.; Gardeniers, J.G.; Janssen, J.; Knijn, P.; Vasa, S.; Verkuijlen, M. High-resolution liquid-and solid-state nuclear magnetic resonance of nanoliter sample volumes using microcoil detectors. J. Chem. Phys. 2008, 128, 052202. [Google Scholar] [CrossRef]
- Barne, A.; Malhari, S.; Lakhani, T.; Mehendale, N. A Review on Types of NMR Micro-Coil Designs. SSRN 2022. [Google Scholar] [CrossRef]
- Grant, S.; Murphy, L.; Magin, R.; Friedman, G. Analysis of multilayer radio frequency microcoils for nuclear magnetic resonance spectroscopy. IEEE Trans. Magn. 2001, 37, 2989–2998. [Google Scholar] [CrossRef]
- Khelifa, M.; Mounier, D.; Yaakoubi, N. Design of high performance scroll microcoils for nuclear magnetic resonance spectroscopy of nanoliter and subnanoliter samples. Sensors 2020, 21, 170. [Google Scholar] [CrossRef]
- Inamura, T.; Dohi, T. Cone-shaped micro coil for magnetic resonance imaging. In Proceedings of the 2013 IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 24 January 2013; pp. 335–338. [Google Scholar]
- Saqib, M.; Francis, S.; Francis, J. Design and development of Helmholtz coils for magnetic field. In Proceedings of the 2020 International Youth Conference on Radio Electronics, Electrical and Power Engineering (REEPE), Moscow, Russia, 12–14 March 2020; pp. 1–5. [Google Scholar]
- Li, Z.; Bao, Q.; Liu, C.; Li, Y.; Yang, Y.; Liu, M. Recent advances in microfluidics-based bioNMR analysis. Lab Chip 2023, 23, 1213–1225. [Google Scholar] [CrossRef]
- Jasiński, K.; Młynarczyk, A.; Latta, P.; Volotovskyy, V.; Węglarz, W.P.; Tomanek, B. A volume microstrip RF coil for MRI microscopy. Magn. Reson. Imaging 2012, 30, 70–77. [Google Scholar] [CrossRef]
- Jasinski, K.; Mlynarczyk, A.; Weglarz, W.; Latta, P.; Volotovskyy, V.; Tomanek, B. Application of microstrip RF coils to MRI microscopy. In Proceedings of the 37 Polish Seminar on Nuclear Magnetic Resonance and Its Applications, Cracow, Poland, 1–2 December 2010. [Google Scholar]
- Bart, J. Stripline-Based Microfluidic Devices for High-Resolution NMR Spectroscopy. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2009; pp. 12–20. [Google Scholar]
- van Meerten, S.; van Zelst, F.; Tijssen, K.; Kentgens, A. An optimized NMR stripline for sensitive supercritical fluid chromatography-nuclear magnetic resonance of microliter sample volumes. Anal. Chem. 2020, 92, 13010–13016. [Google Scholar] [CrossRef]
- Lei, K.-M.; Mak, P.-I.; Law, M.-K.; Martins, R.P. A µ NMR CMOS Transceiver Using a Butterfly-Coil Input for Integration with a Digital Microfluidic Device Inside a Portable Magnet. IEEE J. Solid-State Circuits 2016, 51, 2274–2286. [Google Scholar] [CrossRef]
- Grisi, M.; Gualco, G.; Boero, G. A broadband single-chip transceiver for multi-nuclear NMR probes. Rev. Sci. Instrum. 2015, 86, 044703. [Google Scholar] [CrossRef]
- Olson, D.L.; Norcross, J.A.; O’Neil-Johnson, M.; Molitor, P.F.; Detlefsen, D.J.; Wilson, A.G.; Peck, T.L. Microflow NMR: Concepts and capabilities. Anal. Chem. 2004, 76, 2966–2974. [Google Scholar] [CrossRef]
- Schroeder, F.C.; Gronquist, M. Extending the scope of NMR spectroscopy with microcoil probes. Angew. Chem. Int. Ed. 2006, 45, 7122–7131. [Google Scholar] [CrossRef]
- Grimes, J.H.; O’Connell, T.M. The application of micro-coil NMR probe technology to metabolomics of urine and serum. J. Biomol. NMR 2011, 49, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Andrews, C.; Hua, J.; Kim, H.L.; Sukumaran, D.K.; Szyperski, T.; Odunsi, K. Diagnosis of early stage ovarian cancer by 1H NMR metabonomics of serum explored by use of a microflow NMR probe. J. Proteome Res. 2011, 10, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Ryan, H.; Song, S.-H.; Zaß, A.; Korvink, J.; Utz, M. Contactless NMR spectroscopy on a chip. Anal. Chem. 2012, 84, 3696–3702. [Google Scholar] [CrossRef] [PubMed]
- Finch, G.; Yilmaz, A.; Utz, M. An optimised detector for in-situ high-resolution NMR in microfluidic devices. J. Magn. Reson. 2016, 262, 73–80. [Google Scholar] [CrossRef]
- Anders, J.; Chiaramonte, G.; SanGiorgio, P.; Boero, G. A single-chip array of NMR receivers. J. Magn. Reson. 2009, 201, 239–249. [Google Scholar] [CrossRef]
- Ryan, H.; Song, S.-H.; Lamson, K.; Elliot, J.; Zaß, A.; Wang, K.; Korvink, J.; Reed, M.; Landers, J.; Utz, M. Metabolomic NMR on a chip via inductive coupling. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemsitry and Life Sciences, Seattle, WA, USA, 1–5 October 2011. [Google Scholar]
- Sharma, M.; Utz, M. Modular transmission line probes for microfluidic nuclear magnetic resonance spectroscopy and imaging. J. Magn. Reson. 2019, 303, 75–81. [Google Scholar] [CrossRef]
- Spengler, N.; Moazenzadeh, A.; Meier, R.C.; Badilita, V.; Korvink, J.; Wallrabe, U. Micro-fabricated Helmholtz coil featuring disposable microfluidic sample inserts for applications in nuclear magnetic resonance. J. Micromech. Microeng. 2014, 24, 034004. [Google Scholar] [CrossRef]
- Wensink, H.; Benito-Lopez, F.; Hermes, D.C.; Verboom, W.; Gardeniers, H.J.; Reinhoudt, D.N.; van den Berg, A. Measuring reaction kinetics in a lab-on-a-chip by microcoil NMR. Lab Chip 2005, 5, 280–284. [Google Scholar] [CrossRef]
- Haun, J.B.; Castro, C.M.; Wang, R.; Peterson, V.M.; Marinelli, B.S.; Lee, H.; Weissleder, R. Micro-NMR for rapid molecular analysis of human tumor samples. Sci. Transl. Med. 2011, 3, ra16–ra71. [Google Scholar] [CrossRef]
- Gee, M.S.; Ghazani, A.A.; Haq, R.; Wargo, J.A.; Sebas, M.; Sullivan, R.J.; Lee, H.; Weissleder, R. Point of care assessment of melanoma tumor signaling and metastatic burden from μNMR analysis of tumor fine needle aspirates and peripheral blood. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 821–828. [Google Scholar] [CrossRef]
- Lee, H.; Sun, E.; Ham, D.; Weissleder, R. Chip–NMR biosensor for detection and molecular analysis of cells. Nat. Med. 2008, 14, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, T.-J.; Figueiredo, J.-L.; Swirski, F.K.; Weissleder, R. Rapid detection and profiling of cancer cells in fine-needle aspirates. Proc. Natl. Acad. Sci. USA 2009, 106, 12459–12464. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.M.; Ghazani, A.A.; Chung, J.; Shao, H.; Issadore, D.; Yoon, T.-J.; Weissleder, R.; Lee, H. Miniaturized nuclear magnetic resonance platform for detection and profiling of circulating tumor cells. Lab Chip 2014, 14, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liu, Y.; Lee, H.; Weissleder, R.; Ham, D. CMOS RF biosensor utilizing nuclear magnetic resonance. IEEE J. Solid-State Circuits 2009, 44, 1629–1643. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, N.; Lee, H.; Weissleder, R.; Ham, D. CMOS mini nuclear magnetic resonance system and its application for biomolecular sensing. In Proceedings of the 2008 IEEE International Solid-State Circuits Conference-Digest of Technical Papers, San Francisco, CA, USA, 7 February 2008; pp. 140–602. [Google Scholar]
- Sun, N.; Yoon, T.-J.; Lee, H.; Andress, W.; Weissleder, R.; Ham, D. Palm NMR and 1-chip NMR. IEEE J. Solid-State Circuits 2010, 46, 342–352. [Google Scholar] [CrossRef]
- Dreyer, F.; Yang, Q.; Alnajjar, B.; Krüger, D.; Blümich, B.; Anders, J. A portable chip-based NMR relaxometry system with arbitrary phase control for point-of-care blood analysis. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 831–842. [Google Scholar] [CrossRef]
- Lei, K.-M.; Heidari, H.; Mak, P.-I.; Law, M.-K.; Maloberti, F.; Martins, R.P. A handheld high-sensitivity micro-NMR CMOS platform with B-field stabilization for multi-type biological/chemical assays. IEEE J. Solid-State Circuits 2016, 52, 284–297. [Google Scholar] [CrossRef]
- Lei, K.-M.; Ha, D.; Song, Y.-Q.; Westervelt, R.M.; Martins, R.; Mak, P.-I.; Ham, D. Portable NMR with parallelism. Anal. Chem. 2020, 92, 2112–2120. [Google Scholar] [CrossRef]
- Ha, D.; Paulsen, J.; Sun, N.; Song, Y.-Q.; Ham, D. Scalable NMR spectroscopy with semiconductor chips. Proc. Natl. Acad. Sci. USA 2014, 111, 11955–11960. [Google Scholar] [CrossRef]
- Finch, G. Optimised Detectors for Integration of NMR with Microfluidic Devices. Ph.D. Thesis, University of Southampton, Southampton, UK, 2017. [Google Scholar]
- Jiang, Y.; Ono, T.; Esashi, M. High aspect ratio spiral microcoils fabricated by a silicon lost molding technique. J. Micromech. Microeng. 2006, 16, 1057. [Google Scholar] [CrossRef]
- Li, Y.; Ahmad, M.M.; Hand, J.W.; Syms, R.R.; Gilderdale, D.; Collins, D.J.; Young, I.R. Microcoils on structured silicon substrates for magnetic resonance detection. IEEE Sens. J. 2007, 7, 1362–1369. [Google Scholar] [CrossRef]
- Peck, T.L.; Magin, R.L.; Lauterbur, P.C. Design and analysis of microcoils for NMR microscopy. J. Magn. Reson. Ser. B 1995, 108, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Badilita, V.; Kratt, K.; Baxan, N.; Mohmmadzadeh, M.; Burger, T.; Weber, H.; Elverfeldt, D.v.; Hennig, J.; Korvink, J.G.; Wallrabe, U. On-chip three dimensional microcoils for MRI at the microscale. Lab Chip 2010, 10, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Goloshevsky, A.; Walton, J.; Shutov, M.; De Ropp, J.; Collins, S.; McCarthy, M. Development of low field nuclear magnetic resonance microcoils. Rev. Sci. Instrum. 2005, 76, 024101. [Google Scholar] [CrossRef]
- Renaud, L.; Armenean, M.; Berry, L.; Kleimann, P.; Morin, P.; Pitaval, M.; O’Brien, J.; Brunet, M.; Saint-Jalmes, H. Implantable planar rf microcoils for NMR microspectroscopy. Sens. Actuators A Phys. 2002, 99, 244–248. [Google Scholar] [CrossRef]
- Massin, C.; Boero, G.; Vincent, F.; Abenhaim, J.; Besse, P.-A.; Popovic, R. High-Q factor RF planar microcoils for micro-scale NMR spectroscopy. Sens. Actuators A Phys. 2002, 97, 280–288. [Google Scholar] [CrossRef]
- Evelhoch, J.L.; Crowley, M.G.; Ackerman, J.J. Signal-to-noise optimization and observed volume localization with circular surface coils. J. Magn. Reson. 1969 1984, 56, 110–124. [Google Scholar] [CrossRef]
- Noorbehesht, B.; Lee, H.; Enzmann, D.R. Signal-to-noise ratio enhancement in NMR spectroscopy using a vector-space technique. J. Magn. Reson. 1969 1987, 73, 423–435. [Google Scholar] [CrossRef]
- Taylor, H.; Haiges, R.; Kershaw, A. Increasing Sensitivity in Determining Chemical Shifts in One Dimensional Lorentzian NMR Spectra. J. Phys. Chem. A 2013, 117, 3319–3331. [Google Scholar] [CrossRef]
- Kolar, P.; Grbić, M.S.; Hrabar, S. Sensitivity enhancement of NMR spectroscopy receiving chain used in condensed matter physics. Sensors 2019, 19, 3064. [Google Scholar] [CrossRef]
- Eills, J.; Hale, W.; Sharma, M.; Rossetto, M.; Levitt, M.H.; Utz, M. High-resolution nuclear magnetic resonance spectroscopy with picomole sensitivity by hyperpolarization on a chip. J. Am. Chem. Soc. 2019, 141, 9955–9963. [Google Scholar] [CrossRef] [PubMed]
- Spengler, N.; Hoefflin, J.; Moazenzadeh, A.; Mager, D.; MacKinnon, N.; Badilita, V.; Wallrabe, U.; Korvink, J.G. Heteronuclear micro-Helmholtz coil facilitates µm-range spatial and sub-Hz spectral resolution NMR of nL-volume samples on customisable microfluidic chips. PLoS ONE 2016, 11, e0146384. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gu, H.; Wu, J.; Shi, Y. Resolution of the NMR spectrum using wavelet transform. Appl. Spectrosc. 2000, 54, 731–738. [Google Scholar] [CrossRef]
- Lorieau, J.L.; Malooley, A.; Banerjee, I. Super-Resolution NMR Spectroscopy using the Intersection of Non-Redundant Information on Resonance Groups. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, J.; Lin, Y.; Chen, Z. Boosting resolution in NMR spectroscopy by chemical shift upscaling. Anal. Chim. Acta 2020, 1110, 109–114. [Google Scholar] [CrossRef]
- Petrakis, L.; Sederholm, C.H. Temperature-Dependent Chemical Shifts in the NMR Spectra of Gases. J. Chem. Phys. 1961, 35, 1174–1178. [Google Scholar] [CrossRef]
- Muller, N.; Reiter, R.C. Temperature dependence of chemical shifts of protons in hydrogen bonds. J. Chem. Phys. 1965, 42, 3265–3269. [Google Scholar] [CrossRef]
- Miralles, V.; Huerre, A.; Malloggi, F.; Jullien, M.-C. A review of heating and temperature control in microfluidic systems: Techniques and applications. Diagnostics 2013, 3, 33–67. [Google Scholar] [CrossRef]
- Slomp, G. Temperature Effects in Nuclear Magnetic Resonance Spectroscopy. Rev. Sci. Instrum. 1959, 30, 1024–1027. [Google Scholar] [CrossRef]
- Bowyer, P.J.; Swanson, A.G.; Morris, G.A. Analyzing and correcting spectrometer temperature sensitivity. J. Magn. Reson. 2001, 152, 234–246. [Google Scholar] [CrossRef]
- Hiller, S.; Arthanari, H.; Wagner, G. The T-lock: Automated compensation of radio-frequency induced sample heating. J. Biomol. NMR 2009, 44, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kakar, S.; Mandal, D. Electromagnetic interference. In Proceedings of the 2011 3rd International Conference on Electronics Computer Technology, Kanyakumari, India, 8–11 April 2011; pp. 1–5. [Google Scholar]
- Chung, D. Materials for electromagnetic interference shielding. J. Mater. Eng. Perform. 2000, 9, 350–354. [Google Scholar] [CrossRef]
- Parsa, J.; O’Reilly, T.; Webb, A. A single-coil-based method for electromagnetic interference reduction in point-of-care low field MRI systems. J. Magn. Reson. 2023, 346, 107355. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, L.; Li, Z.; Yang, Z.; Qiu, B.; Wu, X.; Shi, F.; Du, J. Artificial intelligence enhanced two-dimensional nanoscale nuclear magnetic resonance spectroscopy. npj Quantum Inf. 2020, 6, 79. [Google Scholar] [CrossRef]
- Shukla, V.K.; Heller, G.T.; Hansen, D.F. Biomolecular NMR spectroscopy in the era of artificial intelligence. Structure 2023, 31, 1360–1374. [Google Scholar] [CrossRef]
- Kuhn, S. Applications of machine learning and artificial intelligence in NMR. Magn. Reson. Chem. 2022, 60, 1019–1020. [Google Scholar] [CrossRef]


| Magnet | Description | Advantages | Drawbacks |
|---|---|---|---|
| AlCiNo | One of the oldest types of permanent magnets, known for temperature stability. | Excellent temperature stability Resistant to corrosion | Lower magnetic field strength compared to NdFeB and SmCo Easily demagnetized if not handled properly |
| NdFeB | The most used and commercially available permanent magnets. | High magnetic field strength High remanence and coercivity Excellent performance in small sizes | Prone to corrosion Less effective at high temperatures |
| SmCo | High-performing magnet made from an alloy of samarium and cobalt. | High resistance to demagnetization Stable performance at elevated temperatures | Brittle and prone to cracking |
| Microcoil | Description | Advantages | Drawbacks |
|---|---|---|---|
| Planar | Flat coils fabricated on a substrate. Includes spiral and non-spiral designs. | Ease of fabrication Integration with microfluidics Miniaturization Open-access design | Relatively inhomogeneous fields Reduced NMR performance compared to other designs |
| Solenoid | Three-dimensional helical coils resembling traditional NMR coils but on a smaller scale. | High magnetic field homogeneity High sensitivity | More complex fabrication Difficult to integrate with microfluidics |
| Scroll | Cylindrical coils with a spiraled conductor forming a scroll-like structure. | Compact design Good sensitivity and field homogeneity | Complex fabrication Difficult to integrate with microfluidics |
| Cone-shaped | Conical coils designed to fit specific sample geometries. | Focused magnetic field Adaptable to specific sample shapes | Complex fabrication Limited to specific applications |
| Helmholtz | Two parallel coils placed on opposite sides of the sample, generating a uniform magnetic field. | High magnetic field homogeneity Suitable for uniform field distribution | Bulky design More complex integration |
| Stripline | Two parallel microstrip elements conducting RF currents in opposite directions, creating a homogeneous RF field between them. | Homogeneous field Flexible design High sensitivity | Complex integration with microfluidics |
| Reference | Magnet | Microcoil | Sample Volume | Application | Target Biomarkers |
|---|---|---|---|---|---|
| Grimes et al. [63] | Varian INOVA spectrometer | Not available | 10 µL | Metabolomics | Urine (e.g.,): hippurate; lactate; creatinine; citrate Serum (e.g.,): citrate; creatinine; lactate; glucose |
| Ryan et al. [68] | Varian VNMR spectrometer | Planar | 1.2 µL | Lab-on-chip development | D-glucose |
| Finch et al. [66] | Bruker AVANCE III spectrometer | Double-stripline | 2 µL | Not available | Not available |
| Huan et al. [72] | Stray-field NdFeB magnet | Solenoid | Not available | Clinical cancer diagnosis | EpCAM; MUC-1; HER2; EGFR; B7-H3; Ki-67; p53; vimentin |
| Lee et al. [74] | NdFeB | Planar | 5 to 10 µL | Detection and profiling of circulating tumor cells | HER2; EGFR; EpCAM; VEGF; AFP; CA125; glucose; folic acid |
| Lee et al. [75] | NdFeB | Solenoid | 1 µL | Detection and profiling of circulating tumor cells | EpCAM; EGFR; HER2 |
| Castro et al. [76] | NdFeB | Planar | 10 µL | Detection and profiling of circulating tumor cells | EpCAM; EGFR; HER2; MUC-1; vimentin |
| Sun et al. [77] | Permanent magnet | Planar | 5 µL | Biomolecular sensing | VEGF; PSA; CEA; AFP |
| Sun et al. [79] | Permanent magnet | Solenoid | 2 µL | Biomolecular sensing | Not available |
| Dreyer et al. [80] | NdFeB H-shaped magnet | Solenoid | 90 µL | Point-of-care blood analysis | Not available |
| Lei et al. [81] | Permanent magnet | Planar | 2.5 µL | Biological/chemical assay | Avidin/CuSO4 |
| Lei et al. [82] | Halbach magnet | Solenoid | 0.8 µL | Analysis of small molecules for chemistry and biology | β-lactoglobulin |
| Ha et al. [83] | NdFeB Halbach magnet | Solenoid | 0.8 µL | Not available | Not available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeira-Antunes, B.; Ferreira, H.A. µ-NMR Technology for Biomedical Applications: A Review. Chemosensors 2024, 12, 248. https://doi.org/10.3390/chemosensors12120248
Sequeira-Antunes B, Ferreira HA. µ-NMR Technology for Biomedical Applications: A Review. Chemosensors. 2024; 12(12):248. https://doi.org/10.3390/chemosensors12120248
Chicago/Turabian StyleSequeira-Antunes, Beatriz, and Hugo Alexandre Ferreira. 2024. "µ-NMR Technology for Biomedical Applications: A Review" Chemosensors 12, no. 12: 248. https://doi.org/10.3390/chemosensors12120248
APA StyleSequeira-Antunes, B., & Ferreira, H. A. (2024). µ-NMR Technology for Biomedical Applications: A Review. Chemosensors, 12(12), 248. https://doi.org/10.3390/chemosensors12120248





