BiVO4-Based Photoelectrochemical Sensors for the Detection of Diclofenac: The Role of Doping, Electrolytes and Applied Potentials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
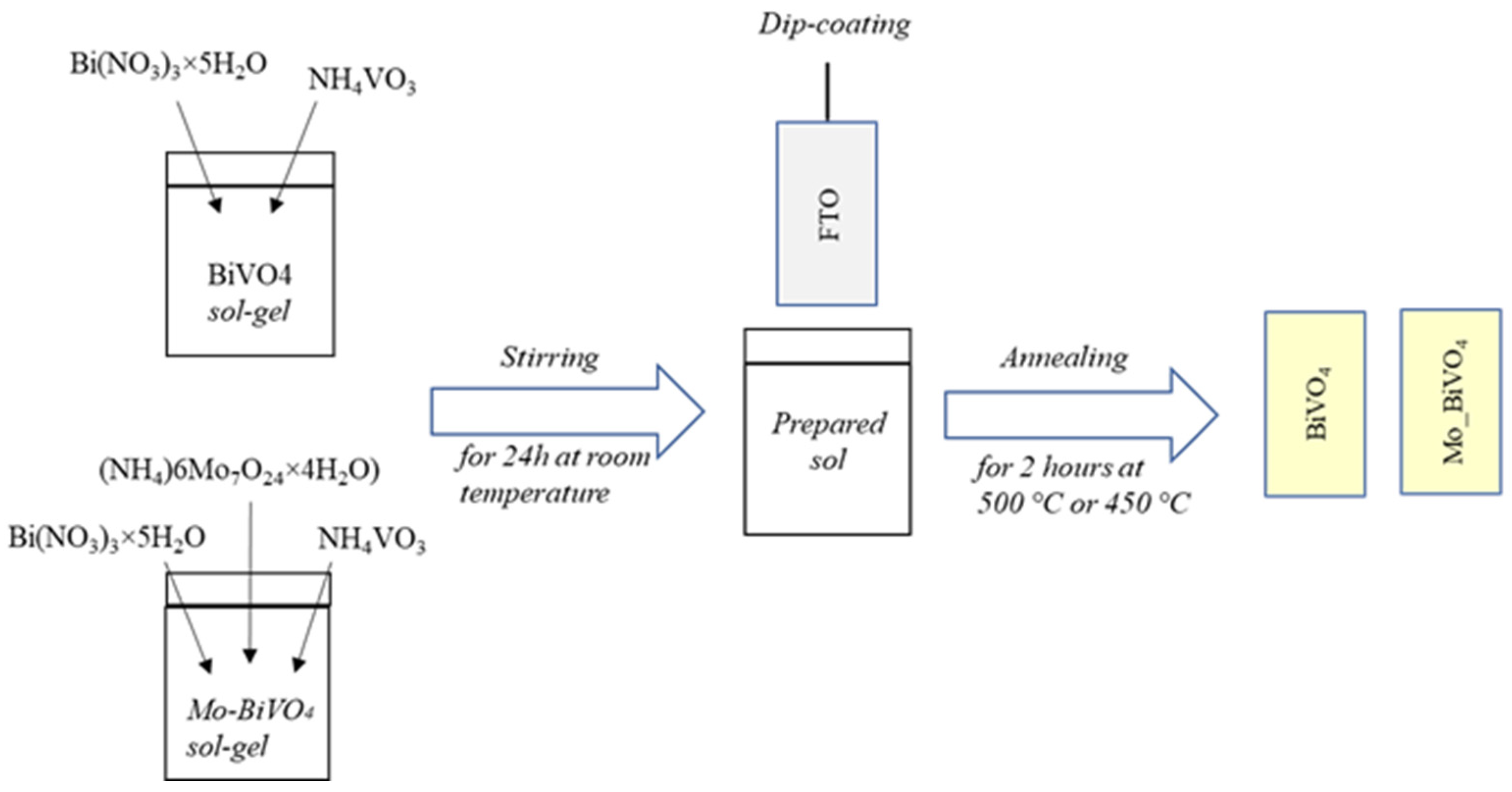
2.2. Synthesis of BiVO4 and Mo_BiVO4 Coatings
2.3. Structural and Morphological Analysis
2.4. Photoelectrochemical Investigations
3. Results and Discussion
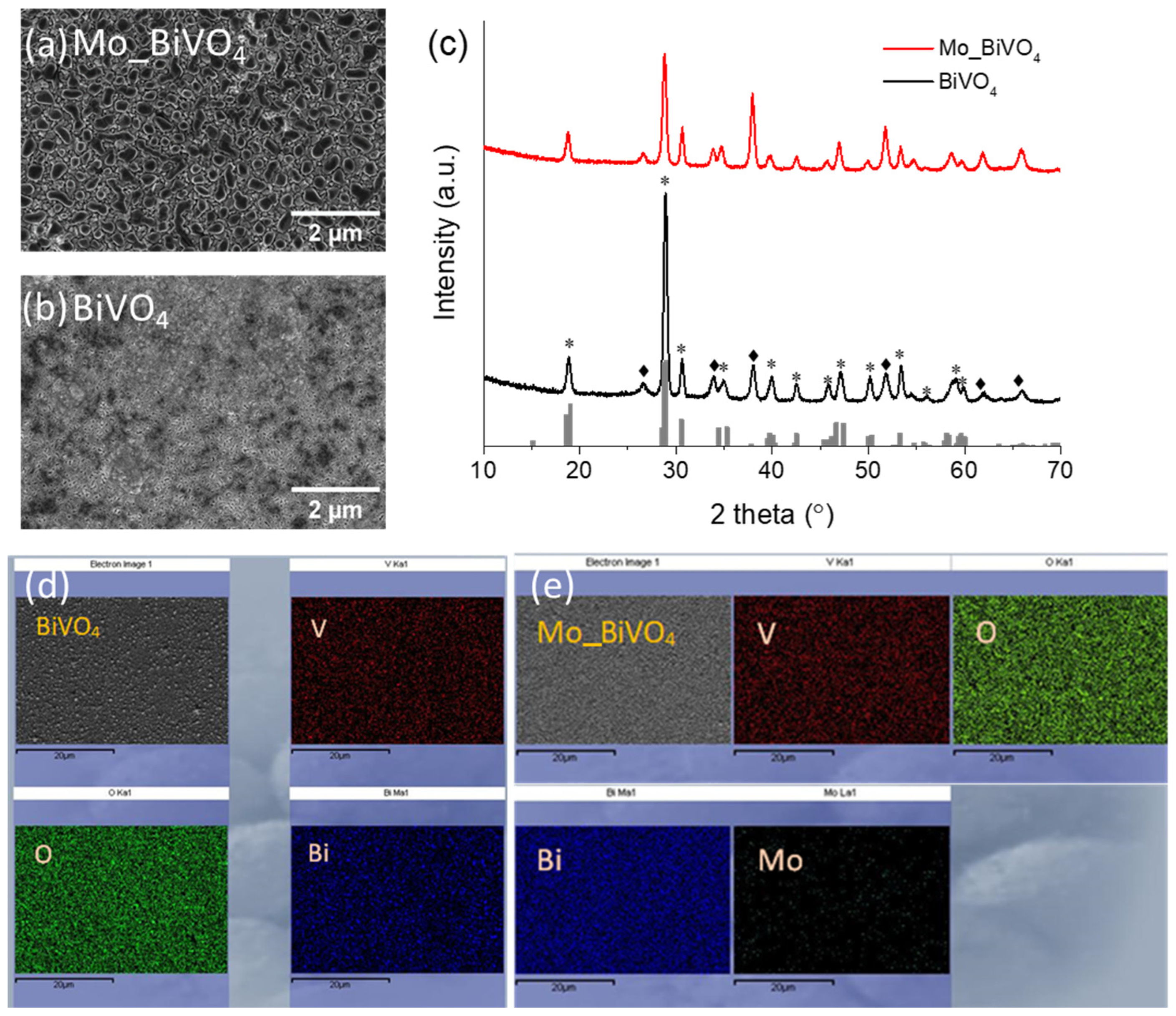
3.1. Structural and Morphological Properties of BiVO4 and Mo_BiVO4 Coatings
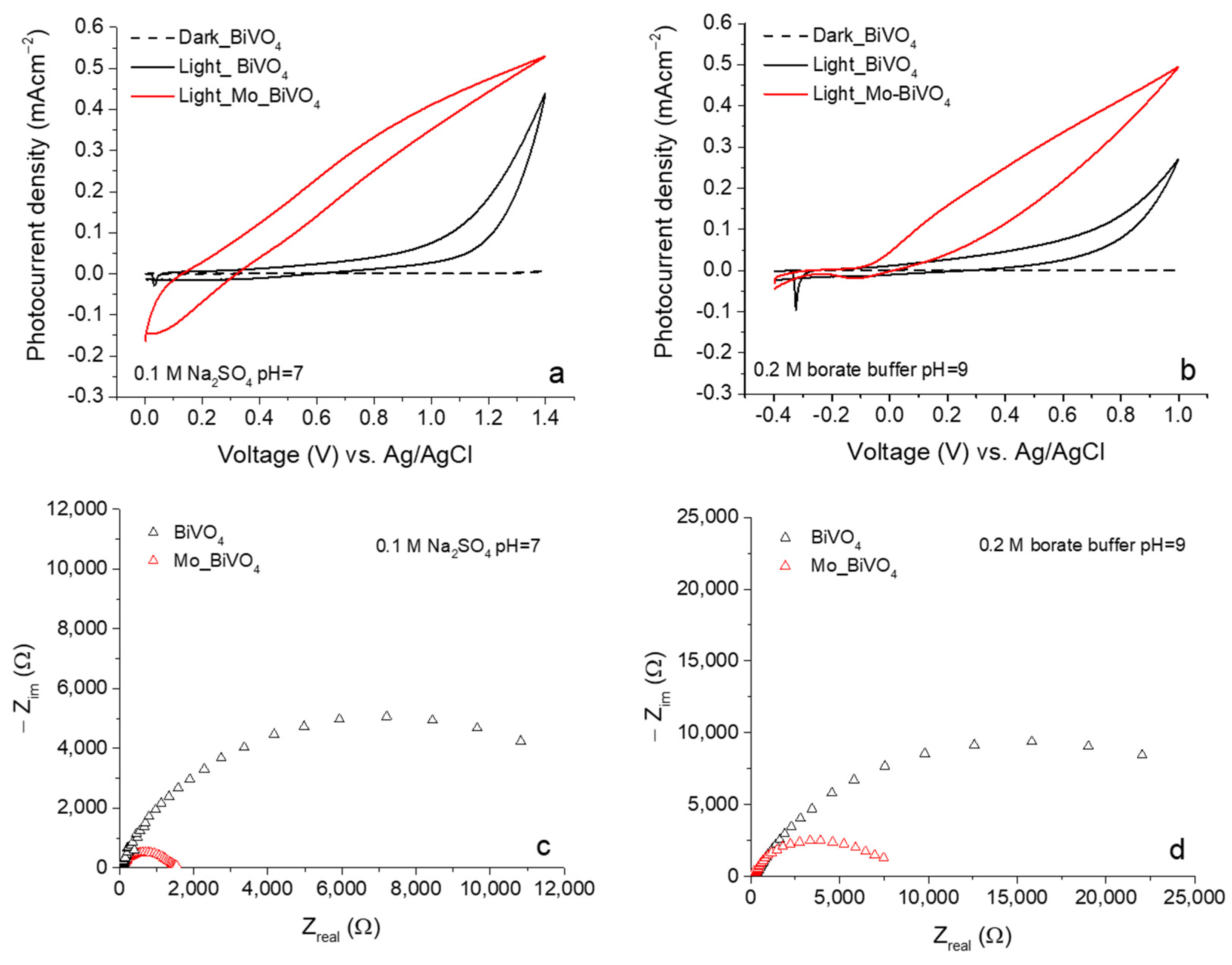
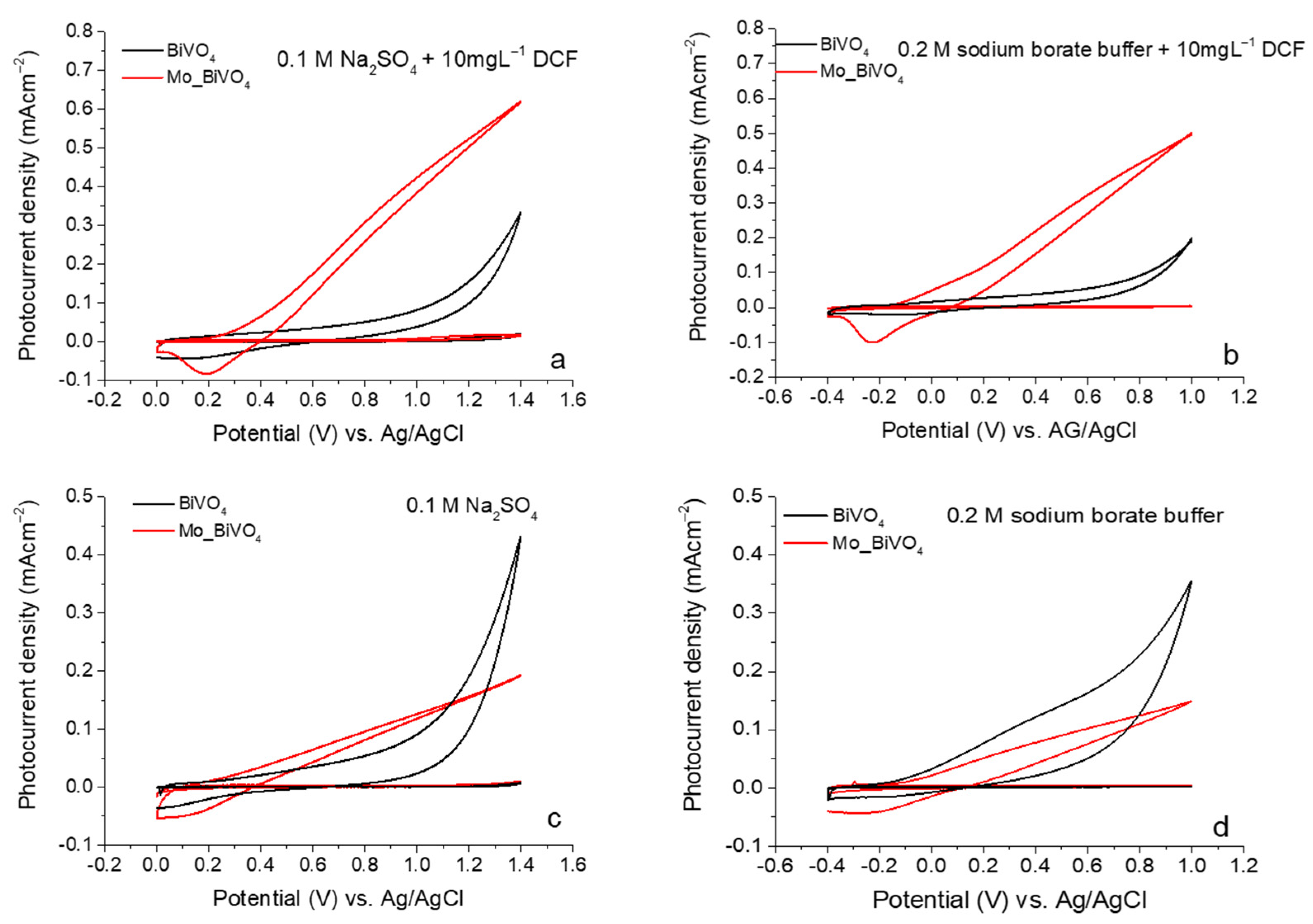
3.2. Photoelectrochemical Investigations of BiVO4 and Mo_BiVO4 Coatings
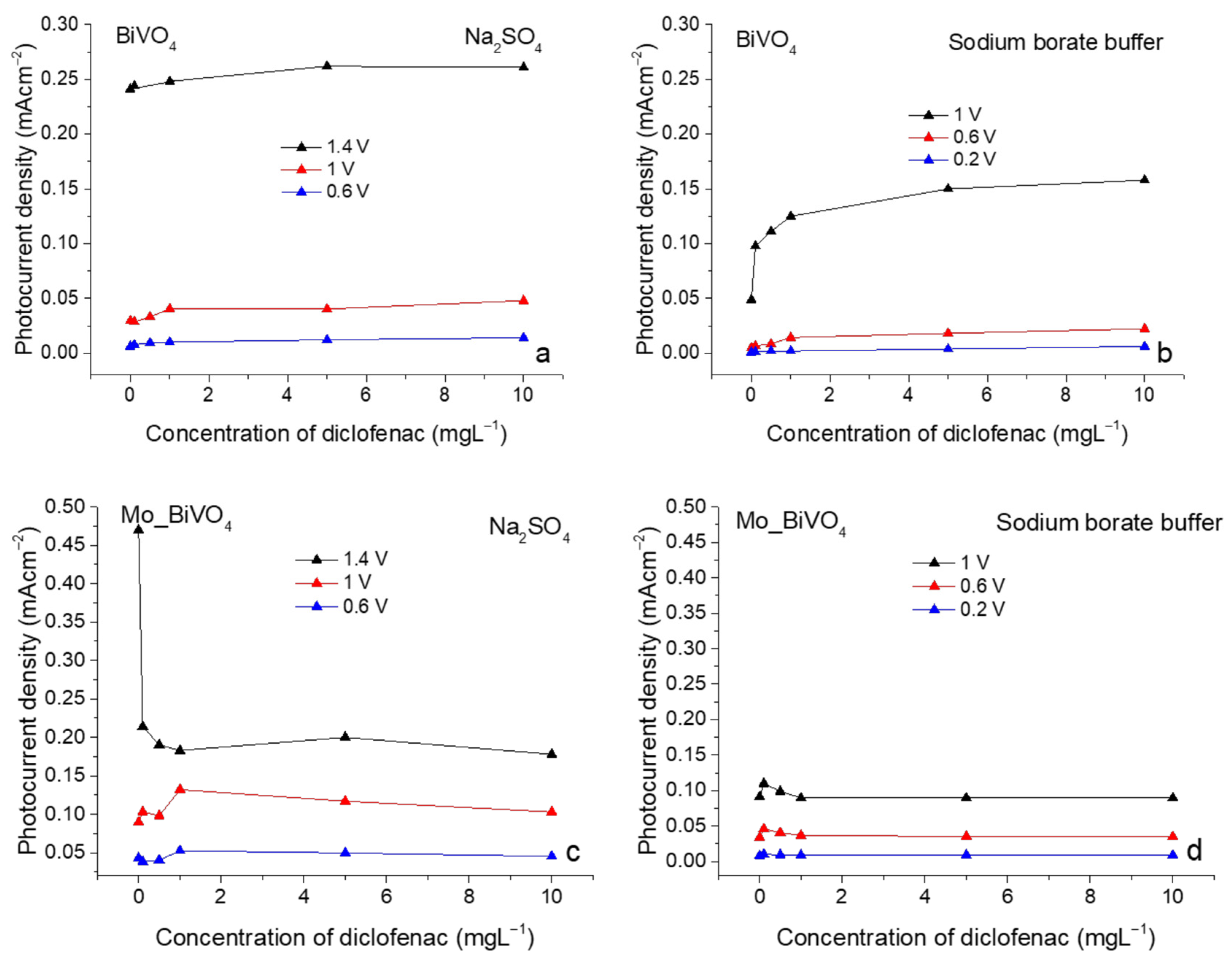
3.3. Photoelectrochemical Sensing of Diclofenac
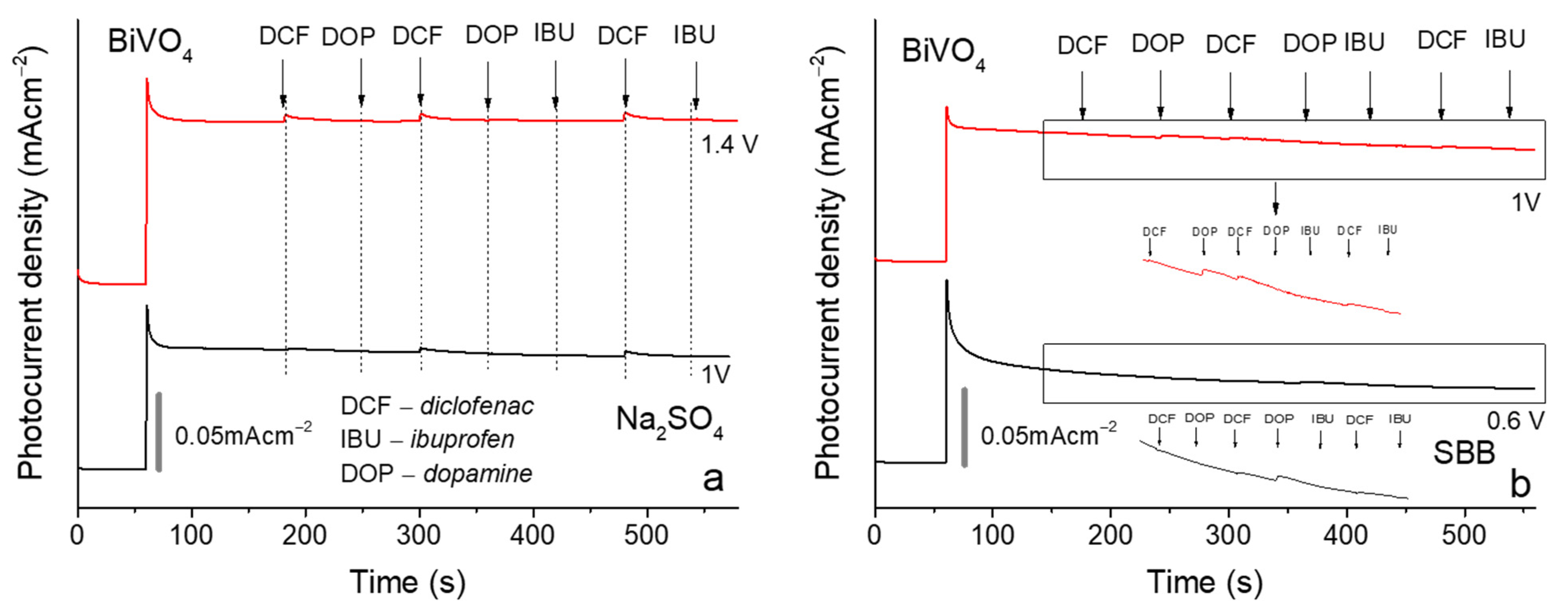
3.4. Selectivity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.K.; Choi, K.S. Enhancing Long-Term Photostability of BiVO4 Photoanodes for Solar Water Splitting by Tuning Electrolyte Composition. Nat. Energy 2018, 3, 53–60. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Barros, R. Pharmaceuticals in Water: Risks to Aquatic Life and Remediation Strategies. Hydrobiology 2023, 2, 395–409. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A Review on Pharmaceuticals Removal from Waters by Single and Combined Biological, Membrane Filtration and Ultrasound Systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Nguyen, H.L.; Hung, N.T.Q.; La, D.D.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Occurrence, Fate, and Potential Risk of Pharmaceutical Pollutants in Agriculture: Challenges and Environmentally Friendly Solutions. Sci. Total Environ. 2023, 899, 165323. [Google Scholar] [CrossRef]
- dos Santos, D.F.; Moreira, W.M.; de Araújo, T.P.; Bergamasco, R.; Ostroski, I.C.; de Barros, M.A.S.D. Non-Conventional Processes Applied for the Removal of Pharmaceutics Compounds in Waters: A Review. Process Saf. Environ. Prot. 2022, 167, 527–542. [Google Scholar] [CrossRef]
- Windsor, F.M.; Ormerod, S.J.; Tyler, C.R. Endocrine Disruption in Aquatic Systems: Up-Scaling Research to Address Ecological Consequences. Biol. Rev. 2018, 93, 626–641. [Google Scholar] [CrossRef]
- Deng, H.; Hui, Y.; Zhang, C.; Zhou, Q.; Li, Q.; Du, H.; Hao, D.; Yang, G.; Wang, Q. MXene−derived Quantum Dots Based Photocatalysts: Synthesis, Application, Prospects, and Challenges. Chinese Chem. Lett. 2024, 35, 109078. [Google Scholar] [CrossRef]
- Chander, V.; Sharma, B.; Negi, V.; Aswal, R.S.; Singh, P.; Singh, R.; Dobhal, R. Pharmaceutical Compounds in Drinking Water. J. Xenobiot. 2016, 6, 5774. [Google Scholar] [CrossRef]
- Bruce, G.M.; Pleus, R.C.; Snyder, S.A. Toxicological Relevance of Pharmaceuticals in Drinking Water. Environ. Sci. Technol. 2010, 44, 5619–5626. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.; Li, L.; Jiang, X.; Wang, P.; Fang, K.; Li, J.; Hao, D.; Zhu, H.; Wang, Q.; et al. Synergistic Mechanisms for Efficient and Safe Antibiotic Removal: Effective Adsorption and Photocatalytic Degradation Using Aerogels. Sep. Purif. Technol. 2025, 354, 129455. [Google Scholar] [CrossRef]
- Ternes, T.A. Analytical Methods for the Determination of Pharmaceuticals in Aqueous Environmental Samples. TrAC Trends Anal. Chem. 2001, 20, 419–434. [Google Scholar] [CrossRef]
- Neven, L.; Shanmugam, S.T.; Rahemi, V.; Trashin, S.; Sleegers, N.; Carrión, E.N.; Gorun, S.M.; De Wael, K. Optimized Photoelectrochemical Detection of Essential Drugs Bearing Phenolic Groups. Anal. Chem. 2019, 91, 9962–9969. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xie, Z.; Xiao, G.; Sun, X.; Diao, H.; Zhou, X.; Yue, Z. Photoelectrochemical Sensors Based on Heterogeneous Nanostructures for in Vitro Diagnostics. Biosens. Bioelectron. X 2022, 11, 100200. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, X.; Bao, Y.; Niu, L. Recent Advances in Photoelectrochemical Sensors for Analysis of Toxins and Abused Drugs in the Environment. Chemosensors 2023, 11, 412. [Google Scholar] [CrossRef]
- Lv, J.; Chen, X.; Chen, S.; Li, H.; Deng, H. A Visible Light Induced Ultrasensitive Photoelectrochemical Sensor Based on Cu3Mo2O9/BaTiO3 P–n Heterojunction for Detecting Oxytetracycline. J. Electroanal. Chem. 2019, 842, 161–167. [Google Scholar] [CrossRef]
- Zarei, M. Sensitive Visible Light-Driven Photoelectrochemical Aptasensor for Detection of Tetracycline Using ZrO2/g-C3N4 Nanocomposite. Sens. Int. 2020, 1, 100029. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z. Development of a Novel Photosensing Method for the Detection of Ciprofloxacin Residues in Athlete’s Biological Samples. Alex. Eng. J. 2024, 107, 675–682. [Google Scholar] [CrossRef]
- da Silva Araújo, M.; Barretto, T.R.; Galvão, J.C.R.; Tarley, C.R.T.; Dall’Antônia, L.H.; de Matos, R.; Medeiros, R.A. Visible Light Photoelectrochemical Sensor for Acetaminophen Determination Using a Glassy Carbon Electrode Modified with BiVO4 Nanoparticles. Electroanalysis 2021, 33, 663–671. [Google Scholar] [CrossRef]
- Murugan, E.; Poongan, A. A New Sensitive Electrochemical Sensor Based on BiVO4/ZrO2@graphene Modified GCE for Concurrent Sensing of Acetaminophen, Phenylephrine Hydrochloride and Cytosine in Medications and Human Serum Samples. Diam. Relat. Mater. 2022, 126, 109117. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Feng, J.; Zhang, J. Label-Free Photoelectrochemical Aptasensing of Diclofenac Based on Gold Nanoparticles and Graphene-Doped CdS. Sens. Actuators B Chem. 2018, 256, 334–341. [Google Scholar] [CrossRef]
- Feng, J.; Li, F.; Qian, Y.; Sun, X.; Fan, D.; Wang, H.; Ma, H.; Wei, Q. Mo-Doped Porous BiVO4/Bi2S3 Nanoarray to Enhance Photoelectrochemical Efficiency for Quantitative Detection of 17β-Estradiol. Sens. Actuators B Chem. 2020, 305, 127443. [Google Scholar] [CrossRef]
- Radić, G.; Perović, K.; Sharifi, T.; Kušić, H.; Kovačić, M.; Kraljić Roković, M. Electrochemical Characterisation of the Photoanode Containing TiO2 and SnS2 in the Presence of Various Pharmaceuticals. Catalysts 2023, 13, 909. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Ogundare, S.A.; Opoku, F.; Arotiba, O.A.; Mabuba, N. Theoretical and Experimental Insight into the Construction of FTO/NiSe2/BiVO4 Photoanode towards an Efficient Charge Separation for the Degradation of Pharmaceuticals in Water. J. Environ. Chem. Eng. 2023, 11, 110711. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Liu, H.; Li, M.; Wang, B. Photoelectrochemical Sensing of Hydrogen Peroxide Using TiO2 Nanotube Arrays Decorated with RGO/CdS. J. Alloys Compd. 2020, 815, 152241. [Google Scholar] [CrossRef]
- Han, F.; Song, Z.; Nawaz, M.H.; Dai, M.; Han, D.; Han, L.; Fan, Y.; Xu, J.; Han, D.; Niu, L. MoS2/ZnO-Heterostructures-Based Label-Free, Visible-Light-Excited Photoelectrochemical Sensor for Sensitive and Selective Determination of Synthetic Antioxidant Propyl Gallate. Anal. Chem. 2019, 91, 10657–10662. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sangili, A.; Chen, S.M.; Abinaya, M. Additive-Free Synthesis of BiVO4 Microspheres as an Electrochemical Sensor for Determination of Antituberculosis Drug Rifampicin. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 624, 126849. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Savickaja, I.; Juodkazyte, J.; Ramanavicius, A. Investigation of WO3 and BiVO4 Photoanodes for Photoelectrochemical Sensing of Xylene, Toluene and Methanol. Chemosensors 2023, 11, 552. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Zhang, J. Mo-Doped BiVO4 and Graphene Nanocomposites with Enhanced Photoelectrochemical Performance for Aptasensing of Streptomycin. Carbon 2017, 120, 194–202. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Devi, P. 2D Nanomaterial Photoelectrodes for Photoelectrochemical Degradation of Pollutants and Hydrogen Generation. In Two-Dimensional Materials for Environmental Applications; Springer: Cham, Switzerland, 2023; Volume 332, pp. 299–325. [Google Scholar]
- Vicenteno-Vera, A.G.; Campos-Hernandez, T.; Ramirez-Silva, M.T.; Galano, A.; Rojas-Hernandez, A. Determination of pKa Values of Diclofenac and Ibuprofen in Aqueous Solutions by Capillary Zone Electrophoresis. ECS Trans. 2010, 29, 443–448. [Google Scholar] [CrossRef]
- Chang, E.D.; Town, R.M.; Owen, S.F.; Hogstrand, C.; Bury, N.R. Effect of Water pH on the Uptake of Acidic (Ibuprofen) and Basic (Propranolol) Drugs in a Fish Gill Cell Culture Model. Environ. Sci. Technol. 2021, 55, 6848–6856. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Chen, Y.L.; Chen, S.W.; Yang, T.C.K. Rational Synthesis of BixFe1−xVO4 Heterostructures Impregnated Sulfur-Doped G-C3N4: A Visible-Light-Driven Type-II Heterojunction Photo(electro)catalyst for Efficient Photodegradation of Roxarsone and Photoelectrochemical OER Reactions. Appl. Catal. B Environ. 2022, 304, 120852. [Google Scholar] [CrossRef]
- Monfort, O.; Plesch, G. Bismuth Vanadate-Based Semiconductor Photocatalysts: A Short Critical Review on the Efficiency and the Mechanism of Photodegradation of Organic Pollutants. Environ. Sci. Pollut. Res. 2018, 25, 19362–19379. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswal, S.K.; Singhal, R.; Panda, P.k.; Dash, S.K. Green Nanoarchitectonics Ce-Co3O4/BiVO4 (P-N) Heterojunction Nanocomposite: Dual Functionality for Photodegradation of Congo Red and Supercapacitor Applications. Surf. Interfaces 2024, 52, 104954. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Wang, K.; Dai, Q.; Lei, C.; Yang, B.; Zhang, Q.; Lei, L.; Leung, M.K.H.; Hou, Y. Tuning D-Band Center of Tungsten Carbide via Mo Doping for Efficient Hydrogen Evolution and Zn–H2O Cell over a Wide pH Range. Nano Energy 2020, 74, 104850. [Google Scholar] [CrossRef]
- Xu, H.; Fan, W.; Zhao, Y.; Chen, B.; Gao, Y.; Chen, X.; Xu, D.; Shi, W. Amorphous Iron (III)-Borate Decorated Electrochemically Treated-BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. Chem. Eng. J. 2021, 411, 128480. [Google Scholar] [CrossRef]
- Ruan, G.; Ghosh, P.; Fridman, N.; Maayan, G. A Di-Copper-Peptoid in a Noninnocent Borate Buffer as a Fast Electrocatalyst for Homogeneous Water Oxidation with Low Overpotential. J. Am. Chem. Soc. 2021, 143, 10614–10623. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Savickaja, I.; Juodkazyte, J.; Grinciene, G.; Ramanavicius, A. Investigation of BiVO4-Based Advanced Oxidation System for Decomposition of Organic Compounds and Production of Reactive Sulfate Species. Sci. Total Environ. 2023, 875, 162574. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Parvin, M.; Savickaja, I.; Gece, G.; Naujokaitis, A.; Pakstas, V.; Pilipavicius, J.; Gegeckas, A.; Gaigalas, G.; Juodkazyte, J. WO3 Coatings for Photoelectrochemical Synthesis of Persulfate: Efficiency, Stability and Applicability. J. Solid State Electrochem. 2022, 26, 1021–1035. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Turuta, K.; Savickaja, I.; Juodkazyte, J.; Ramanavicius, A. Photoelectrochemical Degradation of Organic Compounds via Formed Reactive Chlorine and Sulfate Species by WO3-Based Photoanodes. J. Electroanal. Chem. 2023, 951, 117954. [Google Scholar] [CrossRef]
- Parvin, M.; Petrulevičienė, M.; Savickaja, I.; Šebeka, B.; Karpicz, R.; Grigucevičienė, A.; Ramanauskas, R.; Juodkazytė, J. Influence of Morphology on Photoanodic Behaviour of WO3 Films in Chloride and Sulphate Electrolytes. Electrochim. Acta 2022, 403, 139710. [Google Scholar] [CrossRef]
- Zhang, S.; Ahmet, I.; Kim, S.H.; Kasian, O.; Mingers, A.M.; Schnell, P.; Kölbach, M.; Lim, J.; Fischer, A.; Mayrhofer, K.J.J.; et al. Different Photostability of BiVO4 in near-pH-Neutral Electrolytes. ACS Appl. Energy Mater. 2020, 3, 9523–9527. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Fan, L.; Shuang, S.; Dong, C. Highly Sensitive Photoelectrochemical Sensing of Bisphenol a Based on Zinc phthalocyanine/TiO2 Nanorod Arrays. Talanta 2018, 189, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Tayyebi, A.; Soltani, T.; Lee, B.K. Effect of pH on Photocatalytic and Photoelectrochemical (PEC) Properties of Monoclinic Bismuth Vanadate. J. Colloid Interface Sci. 2019, 534, 37–46. [Google Scholar] [CrossRef]
- Das, P.K.; Arunachalam, M.; Seo, Y.J.; Ahn, K.S.; Ha, J.S.; Kang, S.H. Electrolyte Effects on Undoped and Mo-Doped BiVO4 Film for Photoelectrochemical Water Splitting. J. Electroanal. Chem. 2019, 842, 41–49. [Google Scholar] [CrossRef]
- Bai, X.; Gao, W.; Zhou, C.; Zhao, D.; Zhang, Y.; Jia, N. Photoelectrochemical Determination of Diclofenac Using Oriented Single-Crystalline TiO2 Nanoarray Modified with Molecularly Imprinted Polypyrrole. Microchim. Acta 2022, 189, 90. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Li, F.; Zheng, H.; Li, T.; Liu, X.; Zhu, J.; Zhou, Y.; Alwarappan, S. Ultrasensitive Photoelectrochemical Aptasensor for Diclofenac Sodium Based on Surface-Modified TiO2-FeVO4 Composite. Anal. Bioanal. Chem. 2021, 413, 193–203. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 nanoparticles/IL Nanocomposite Modified Glassy Carbon Electrode as a Voltammetric Sensor for Determination of the Non-Steroidal Anti-Inflammatory Drug Diclofenac. Mater. Sci. Eng. C 2012, 32, 1682–1689. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, M.; Zhang, L. Label-Free and Visible-Light Driven Photoelectrochemical Sensor with CuCo2O4@CoO Core-Shell Hybrid Rod as Photoanode for Selective Sensing Diclofenac. Electrochim. Acta 2021, 397, 139239. [Google Scholar] [CrossRef]
- do Prado, T.M.; Cincotto, F.H.; Fatibello-Filho, O.; Cruz de Moraes, F. Bismuth Vanadate/Reduced Graphene Oxide Nanocomposite Electrode for Photoelectrochemical Determination of Diclofenac in Urine. Electroanalysis 2018, 30, 2704–2711. [Google Scholar] [CrossRef]
- Feng, F.; Mitoraj, D.; Gong, R.; Gao, D.; Elnagar, M.M.; Liu, R.; Beranek, R.; Streb, C. High-Performance BiVO4 Photoanodes: Elucidating the Combined Effects of Mo-Doping and Modification with Cobalt Polyoxometalate. Mater. Adv. 2024, 5, 4932–4944. [Google Scholar] [CrossRef]

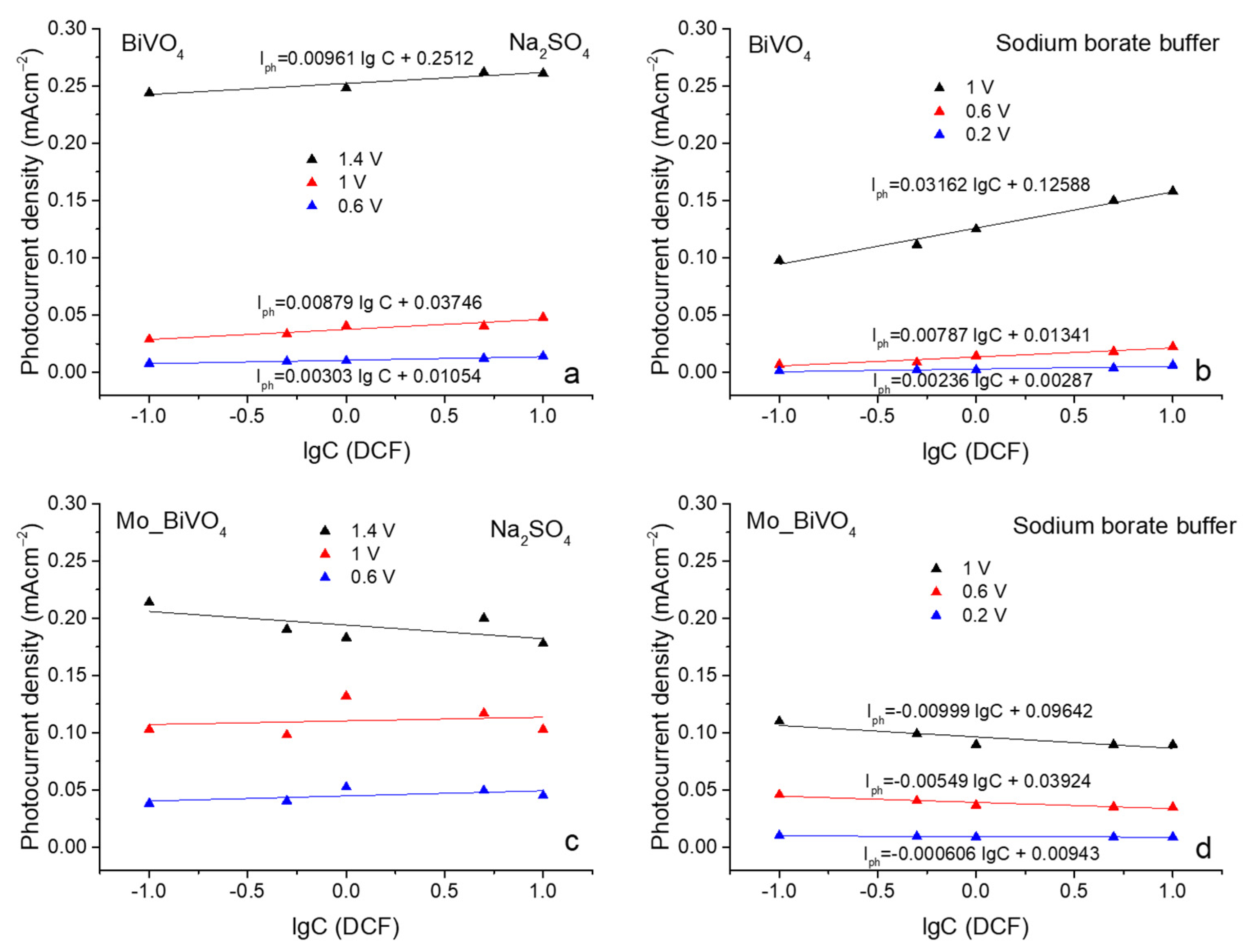
| Photoanode, Electrolyte | Potential, V | Regression Equation | R2 |
|---|---|---|---|
| BiVO4, 0.1 M SS | 1.4 | Iph = 0.00961 lgC + 0.2512 | 0.83 |
| 1 | Iph = 0.00879 lgC + 0.03746 | 0.85 | |
| 0.6 | Iph = 0.00303 lgC + 0.01054 | 0.96 | |
| BiVO4, 0.2 M SBB | 1 | Iph = 0.03162 lgC + 0.12588 | 0.98 |
| 0.6 | Iph = 0.00787 lgC + 0.01341 | 0.92 | |
| 0.2 | Iph = 0.00236 lgC + 0.00287 | 0.79 | |
| Mo_BiVO4, 0.1 M SS | 1.4 | Iph = −0.0119 lgC + 0.19401 | 0.25 |
| 1 | Iph = 0.00323 lgC + 0.11036 | −0.29 | |
| 0.6 | Iph = 0.0045 lgC + 0.0449 | 0.15 | |
| Mo_BiVO4, 0.2 M SBB | 1 | Iph = −0.00999 lgC + 0.09642 | 0.72 |
| 0.6 | Iph = −0.00549 lgC + 0.03924 | 0.85 | |
| 0.2 | Iph = −0.0006062 lgC + 0.00943 | 0.71 |
| Photoanode | Solution | Detection Range, μM | LOD, μM | Reference |
|---|---|---|---|---|
| TiO2 | 0.1 M Na2SO4 | 5.0 × 10−2–1.0 × 103 | 3.4 × 10−3 | [47] |
| TiO2/FeVO4 | 0.1 M PBS | 1.0 × 10−4–5.0 × 10−1 | 6.9 × 10−5 | [48] |
| Au/GR doped CdS | 0.1 M Na2SO4 | 1.0 × 10−3–1.5 × 10−1 | 7.8 × 10−4 | [21] |
| Ni(OH)2 | 1.0 M PBS | 2.0 × 102–2.7 × 103 | 3.2 × 101 | [49] |
| CuCo2O4@CoO | 0.1 M Na2SO4 | 1.0 × 10−2–5.0 × 102 | 6.5 × 10−4 | [50] |
| Cu(OH)2 | 1.0 M PBS | 1.8 × 10−1–1.2 × 102 | 4.0 × 10−2 | [49] |
| BiVO4/rGO | 0.1 M Na2SO4 | 9.6 × 10−3–9.2 × 101 | 4.2 × 10−3 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruleviciene, M.; Savickaja, I.; Kovger-Jarosevic, J.; Skruodiene, M.; Juodkazyte, J.; Ramanavicius, S.; Ramanavicius, A. BiVO4-Based Photoelectrochemical Sensors for the Detection of Diclofenac: The Role of Doping, Electrolytes and Applied Potentials. Chemosensors 2024, 12, 249. https://doi.org/10.3390/chemosensors12120249
Petruleviciene M, Savickaja I, Kovger-Jarosevic J, Skruodiene M, Juodkazyte J, Ramanavicius S, Ramanavicius A. BiVO4-Based Photoelectrochemical Sensors for the Detection of Diclofenac: The Role of Doping, Electrolytes and Applied Potentials. Chemosensors. 2024; 12(12):249. https://doi.org/10.3390/chemosensors12120249
Chicago/Turabian StylePetruleviciene, Milda, Irena Savickaja, Jelena Kovger-Jarosevic, Monika Skruodiene, Jurga Juodkazyte, Simonas Ramanavicius, and Arunas Ramanavicius. 2024. "BiVO4-Based Photoelectrochemical Sensors for the Detection of Diclofenac: The Role of Doping, Electrolytes and Applied Potentials" Chemosensors 12, no. 12: 249. https://doi.org/10.3390/chemosensors12120249
APA StylePetruleviciene, M., Savickaja, I., Kovger-Jarosevic, J., Skruodiene, M., Juodkazyte, J., Ramanavicius, S., & Ramanavicius, A. (2024). BiVO4-Based Photoelectrochemical Sensors for the Detection of Diclofenac: The Role of Doping, Electrolytes and Applied Potentials. Chemosensors, 12(12), 249. https://doi.org/10.3390/chemosensors12120249







