Abstract
Valencian paella is a world-famous dish that is originally from the Valencia Spanish region, in which rice is the basic ingredient along with others such as extra virgin olive oil, vegetables, seafood and/or meat. During the cooking process, the paella rice suffers a loss of moisture and the socarrat is formed, being crunchy and brown in color. The objective of this work was to evaluate the aromas generated during the formation of socarrat in paella rice (P) by an electronic nose (E-nose), discriminating against the aromatic profile of white rice (WR), and validate it with sensory analysis and gas chromatography. The results of the sensory analysis showed a decrease in positive fruity and sweet aromas of some volatile compounds such as hexanal and nonanal, among others, and an increase in roasted aromas due to the appearance of furans and furanones compounds, which is probably associated with socarrat formation. The acrylamide content increased by 33.8–48.3% as the intensity of the thermal treatment rose. The higher value of acrylamide (179.5 ng g−1) was achieved in P. The E-nose was sensitive to changes in the aromatic profile, and the PCA analysis explained 85.7% and 91.6% of the variance for WR and P, respectively. Furthermore, a strong clustering in the thermal treatments was observed, which is related to the composition of volatile compounds.
1. Introduction
Paella is a typical Spanish dish of Valencian origin that became a national dish during the early years of the dictatorship in Spain, making it the culinary embodiment of the concept of Spanish nationality that persists to this day. Nowadays, all foreigners visiting Spain know that paella is the emblematic dish of Spain [1]. For the preparation of paella, a specific variety of rice, called bomba rice, is used due to its organoleptic and physical properties [2]. The main ingredients used in paella are extra virgin olive oil, peppers, crushed tomato, prawns, clams, mussels and crustaceans. Other ingredients such as chicken or rabbit can also be added, making it a mixed paella [1]. For a 100 g portion of paella, it contains 176 kcal, 7.7% protein and 6.2% lipids [3].
On the other hand, the surface of the paella pan is very important as it must allow the liquid in which the rice is cooked to evaporate. This therefore leads to the formation of socarrat. The socarrat is formed when the rice sticks to the bottom and the side of the pan. The socarrat has a brown color and a crispy quality [4]. Therefore, the heat provided to the rice must be sufficient to form a crust on the pan and it must be prevented from burning excessively [5].
Although there are studies on the effect of the roasting of rice kernels [6], there are no studies on the effect of socarrat on the aroma sensory properties of rice. According to Li et al. (2021) [7] the contaminants that are prominent in processed foods subjected to high temperatures are acrylamide, polycyclic aromatic hydrocarbons, heterocyclic aromatic amines, chloropropanol ester and N-nitroso compounds. Furthermore, several researchers have determined the concentration of various toxic compounds such as acrylamide, furfuryl alcohol, furan and 5-hydroxymethylfurfural in heat-induced coffee [8]. In paella rice, due to the high heat treatment used, acrylamide and furfural are found as major chemical contaminants.
Solid-phase extraction techniques and gas chromatography–mass spectrometry are used for the identification of different volatile organic compounds, some of which are toxic. Ramírez-Guízar et al. [9] used this method for the identification of nitrosamines, methanol and furfural. However, some researchers have used liquid chromatography with mass spectroscopy for acrylamide evaluation in food [10]. These traditional techniques require specialized technical staff and long analysis times [11].
Therefore, non-destructive techniques are available as an alternative. The most used non-destructive techniques for quality assessment are near-infrared spectroscopy, ultrasound measurement, machine vision, hyperspectral imaging, and electronic noses [12]. The electronic nose (E-nose) was used in several research works for determining the quality of table olive [13], virgin olive oil [14], carrots [15] and coffee [16]. This electronic device is powerful, useful and fast, and could be used to assist in gas chromatography and sensory evaluation. Thus, the purpose of this study was to evaluate the aromas during the formation of socarrat in Paella rice using an electronic nose to discriminate between the aromatic profile of white rice and paella, and validating the results with sensory analysis and gas chromatography with MS detection.
2. Materials and Methods
2.1. Elaboration of Paella
Commercial rice of the ‘bomba’ variety was selected to carry out this experiment. Two different experiments were carried out: (i) cooking white rice (WR) without the paella ingredients, and (ii) cooking paella (P) with its characteristic ingredients. Extra virgin olive oil was added to the pan over the heat, and when it was hot, 100 g of chopped squid was fried with salt and pepper. When it was cooked, the squid was placed on the plate. Then, two chopped garlic cloves were cooked in the pan for a few minutes along with two chopped red peppers. Next, 200 g of rice was added to the pan and it was fried for a few minutes, and 50 g of natural tomato, dusted saffron and salt was added. The entire mixture was gently mixed until 300 mL of commercial broth was added. It was cooked well for 10 min, and another 50 mL of broth was incorporated to ensure the rice cooked well and was then cooked for another 5 min. This is the time needed to make the socarrat. We raised the heat to the maximum in the center of the paella for 2 min, and then the fire was turned off and left to rest for 5 min. During socarrat elaboration, different samples (1, 2, 3 and 4) were taken. White rice was made in the same way without the addition of any type of ingredients. Three elaborations of each of the experiments were carried out.
2.2. Analysis
2.2.1. Humidity and Instrumental Color
The moisture content was obtained from 5 g of rice (WR and P) by drying in a force convection oven (Memmert, S.L.) at 100 °C for 24 h until a constant weight was reached. The color was measured according to the CIELab system using a Konica Minolta CM600 spectrophotometer. The parameters of brightness (L*), green to red (a*) and blue to yellow (b*) were obtained.
2.2.2. Acrylamide Quantification by HPLC-MS-QQQ
The determination of acrylamide (ng g−1) was carried out according to the methodology described by Fernández et al. [17]. MassHunter software B.07.00 (Agilent, Santa Clara, CA, USA) was used for data acquisition and management.
2.2.3. Sensory Analysis
Sensory analysis was accomplished by 9 tasters belonging to the Research Center of Extremadura (CICYTEX) and the University of Extremadura (UEx), who were trained for the descriptive analysis of products derived from rice. The evaluations were performed in a tasting room with standardized booths for the sensory analysis of food. Different tasting sessions were organized between groups of 3 tasters who evaluated the olfactory profile of the samples. White rice and paella rice were prepared in the kitchen of the tasting room, and at different moments of processing (WR1 to WR4 for the white rice, and P1 to P4 for the paella rice) attributes such as wet cereal, shellfish, roasted and burnt were evaluated using a non-structural scale of 10 cm (0: not detected to 10: maximum intensity). Medians of the values obtained in the sensory evaluation for each attribute with an acceptable coefficient of variation (CV) < 20% were obtained. To comply with the Ethics Committee when conducting research with human beings, the tasters were informed of the trial through a structured document and consented to participating by signing.
2.2.4. Volatile Organic Compound Analysis
These compounds were determined by the methods described by Sánchez et al. [13] and López-López et al. [18]. First, 10 g of the sample was homogenized, of which approximately 2 g was used in a glass vial of 15 mL with 7.0 mL of 30% (w/v) NaCl. The extraction was carried out with the help of a StableFlex fiber (PDMS/DVB) with a size of 65 μm (Supelco), and the extraction was carried out at 40 °C for 30 min. Analyses were performed on a triple-quadrupole gas chromatograph (Bruker Scion 456-GC, Markham, ON, Canada) with a DB WAXETR capillary column (60 m × 0.25 mm; ID: 0.25 mm), and mass spectra were used for identification with a NIST standard reference database.
2.2.5. E-Nose Analysis
Researchers from the School of Industrial Engineering at the University of Extremadura were the designers of the handmade electronic device (E-nose) used in this experiment. This device contains four electronic chips, in which there is a total of eleven signals from metal oxide (MOX) sensors. These belong to the latest generation of digital gas sensors. One or more gas sensors, other sensors (temperature, humidity and atmospheric pressure) alongside the signal processor, the controller of the whole system and the I2C communication module can be found in the same housing. Each of them emits a different signal regarding the aroma of the sample headspace, and thus a different fingerprint signal is obtained for different samples. The composition of the array of the sensors was optimized by performing previous measurements for typical compounds of food aromas. The designed E-nose also incorporates temperature and humidity sensors for ambient temperature and humidity monitoring and correction with compensation algorithms.
Samples (~3 g) were placed in the tasting glasses in the same way as the tasting panel and the E-nose was put on the upper part of the cup. The standard glasses with the samples were put on a heating block at 25 °C. Each cycle of acquisition of the data of the sample consisted of two different parts after an initial period of sensor stabilization. In a first phase, known as the adsorption phase, the E-nose was left to adsorb the headspace of the sample for 60 s. Once this time elapsed, the electronic device was moved to another empty cup to carry out the desorption phase for 30 s to obtain the baseline. A relative resistance method was used for sensor calibration with respect to the baseline and for the feature extraction. Eight measurements (repetitions) were made for each rice sample. The analytical data were transferred from an electronic nose to an intelligent device (smartphone) via Bluetooth, and the multivariate analysis was completed [19].
2.3. Chemometric Analysis
MATLAB software and PLS_Toolbox were used for chemometric analysis. Principal component analysis (PCA) and partial least squares (PLS) were the methods used to reduce the dimensionality of the E-nose sensor data.
2.4. Statistical Analysis
For the statistical analyses, SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used to apply the one-way ANOVA with Tukey’s method (p < 0.05).
3. Results and Discussion
3.1. Evaluation of the Physicochemical Properties
The results obtained for moisture and color of the different samples of rice subjected to thermal treatment are shown in Table 1. In both rice samples, we observed a decrease in moisture content as the thermal treatment time increased (48.8–28.5 for white rice, and 55.7–38.8 for paella rice). Paella rice samples showed a higher moisture content, which may be due to the other added ingredients. Other researchers have determined the moisture distribution in rice grains during thermal treatment and obtained similar results where the moisture decreased with treatment time [20].

Table 1.
Moisture and CIELab color of white rice and elaborated paella. Results are expressed as mean ± standard deviation (SD) of three replicates. Different lowercase letters mean statistically significant differences between samples (p < 0.05). WR: white rice; P: paella.
Color is one of the most important sensory attributes for consumers. In this study, the color parameters (according to the CIELab color space) of the WR and P subjected to thermal treatment at different times were analyzed. Regarding the brightness parameter (L*), we observed higher values in WR compared to P (Table 1). This is in agreement with the CIELab space, in which more positive values of L* are associated with the color white. In both samples, L* decreases with increasing thermal treatment time. On the other hand, the a* parameter (green (−) to red (+)), showed values close to 0 for white rice, suggesting grayish-white tones. However, in paella rice, the a* parameter showed higher positive values for treatments P1 to P3 (10.9–11.8), but decreased in P4 (4.1). These values justify the change in color of paella rice from yellow-orange for the first sampling times, to toasted-burnt on the last sampling time. Regarding the parameter b* (from blue (−) to yellow (+)), higher values were obtained in paella rice due to the coloring added in its preparation. However, this color decreases with cooking time, appearing darker (brown), which coincides with a decrease in the values of the b* parameter. In white rice the b* parameter increases with the heat treatment, going from white to yellow tones. Other researchers have determined the color and other parameters to observe the temperature effect on the kinetics of the drying of cooked rice and its quality after rehydration [21], and they have also determined the color in samples of olives subjected to heat treatments, observing their evolution towards a brownish shade.
3.2. Sensory Analysis
A descriptive sensory analysis to evaluate attributes such as wet cereal, shellfish, roasted and burnt during the cooking process was carried out to verify the discrimination of the aromatic sensory characteristics of WR vs. P (Table 2). The attributes are related to the grain and cooking process (wet cereal), added ingredients (shellfish), and cooking time (roasted and burnt). These last attributes are associated with the formation of browning products, which is mainly due to the Maillard reaction (MR) [22]. They are often amenable for developments in the palatability of foods [23] through the development of desirable sensory attributes such as colors, aromas and flavors [24]. However, this processing at high temperatures gives rise to the production of unwanted compounds such as acrylamide [10] and 5-hydroxymethylfurfural that are undesirable food contaminants [25].

Table 2.
Descriptive sensory olfactory evaluation of white rice and paella aroma evaluated by the tasting panel. Results are expressed as mean ± standard deviation (SD) of three replicates. Different lowercase letters mean statistically significant differences between samples (p < 0.05). WR: white rice; P: paella; n.d.: not detected.
The wet cereal aroma was only detected in WR, being more intense in the second sampling time (WR2), which is probably due to a longer cooking process time that favors the formation of the aroma. From the third sampling time (WR3), its perception begins to decrease due to the presence of other roasted and burnt aromas. For the last sampling time (WR4), the wet cereal aroma was not detected and only a burnt aroma appeared. In paella rice, the wet cereal aroma was not detected at any sampling time due to the presence of other aromas related to the added ingredients such as shellfish. This aroma in paella rice was detected with greater intensity in the first sampling times (P1 and P2), but decreased significantly from the third time (P3) (p < 0.05), at which time the roasted and burnt aromas began to appear. We can highlight the difference in roasted and burnt aromas observed in the last sampling times (WR4 and P4). Thus, in WR the tasters only perceived a burnt aroma, and a roasted aroma was not detected at the last sampling time (WR4). However, in P, the roasted aroma was perceived with greater intensity compared to the burnt aroma. This suggests the formation of the socarrat forming a crust in the pan that prevents the rice from burning.
3.3. Evolution of Acrylamide
The acrylamide content of the elaborated WR and P is shown in Figure 1. It should be noted that in all phases of rice cooking, the formation of acrylamide is shown. The acrylamide content increased significantly with the increase in the heat treatment time (p < 0.05), being higher in the last sampling for WR and P. In addition, P had a higher acrylamide content compared to WR for all sampling times (p < 0.05). The addition of ingredients in P could favor the Maillard reaction, increasing the acrylamide content in P. Furthermore, in the last step of socarrat formation, the amount of this toxic substance is significantly higher. These results are similar to those obtained by other researchers who found a higher acrylamide content when the rice was accompanied by other ingredients (risotto rice) [26]. It is interesting to determine the acrylamide content since the synthesis of this compound is related to processing at high temperatures [27].
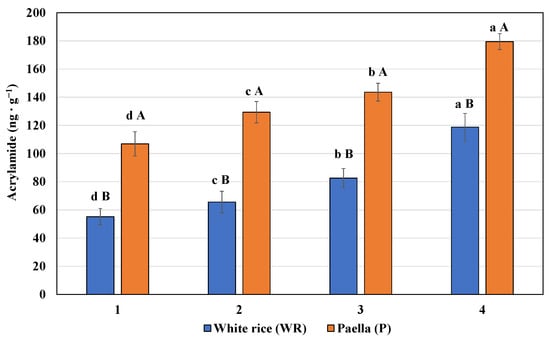
Figure 1.
Acrylamide content (expressed in ng g−1) of white rice (WR) and paella (P) submitted to different thermal treatments (1–4) during the cooking process. Results are expressed as mean ± SD of five sample replicates. Different lowercase letters mean a significant difference between each elaboration process (WR and P) submitted to different thermal treatments. Different uppercase letters mean a significant difference between white rice and paella in each thermal treatment.
3.4. Evaluation of Volatile Organic Compounds
The volatile profiles of the WR and P samples grouped into compound families are shown in Table 3. In both processed rice samples, the largest group was aldehyde compounds. This group decreased with cooking time (from 72.6 to 36.9% for WR, and from 36.4 to 13.8% for P), which can be related to a decrease in the wet cereal aroma of cooked rice, since most aldehydes are associated with positive aromas. Buttery et al. [28] indicated that octanal and nonanal aldehydes, among others, were the principal volatile compounds that give cooked rice its aroma. Furthermore, Hu et al. [29] showed that aldehydes such as hexanal, octanal, nonanal, etc., probably contribute the most to the overall flavor because of the low odor threshold. Hydrocarbon compounds, which were the second largest group, are also related to positive aromas [30], and their content decreased with treatment time (from 22.4 to 2.9% for WR and from 5.4 to 0.5% for P). In both cases of aldehyde and hydrocarbon compounds, higher contents were obtained for WR compared to P. This is due to the influence of other aromas from the ingredients added in P, such as vegetables, garlic and shellfish related to sulfurous compounds [31], which were not found in WR. These sulfurous compounds appear in greater proportion in P1 (52.5%), and decrease with the processing time up to P4 (15.8%). They decrease due to the appearance of other aromas related to socarrat formation. Furan compounds, related to negative aromas such as roasted and woody [31], appear with greater intensity in the last sampling times for WR and P. Similarly, pyrazine compounds are related to roasted aromas [32], and high-fired aromas [33] and their contents increase with processing time. Regarding the alcohol group, it can be related to different aromas such as toasted [34], shellfish [35] and floral sweets [36]. In general, the content of alcohol compounds increases with processing time, which is mainly due to the appearance of toasted aromas. In paella rice this increase is more significant (from 4.5 to 23.3% for P1 to P4) than white rice (from 5.0 to 12.3 for WR1 to WR4), which is possibly due to the socarrat formation that is associated with the formation these aromas. Finally, ketone and furanone compounds appear in appreciable amounts for the last processing time. This is in accordance with the roasted and pungent aromas [37] provided by ketones, as well as bitter-caramel aromas [38].

Table 3.
Relative contents of volatile compounds (mean % (n = 3)) obtained from white rice (WR) and paella (P) during the elaboration process. n.d.: not detected.
A total of 33 volatile compounds were analyzed (Table 3). In the alcohol group, heptane and o-xylene, which are related to sweet [39] and petroleum-like [40] aromas, appeared in WR but decreased with the heat treatment time. However, they did not appear in P, probably due to other ingredients that mask these aromas. D-limonene, which is related to a sweet orange aroma [41], appeared in both rice samples. Regarding the ketone group, 1-hydroxy-2-propanone, and 1-(2-furanyl)-ethanone, which are related to negative attributes such as roasted [37] and spicy [31], respectively, appeared only at the last sampling time (WR4 and P4). In the alcohol group, 3-cyclohexen-1-ol was found only in paella rice, increasing with processing time because it is related to the shellfish aroma [35]. On the contrary, 2-ethyl-1-hexanol, which is related to sweet and slightly floral-pink aromas [36], appeared only in white rice (WR1 and WR2). 2-Furanmethanol, which is associated with roasted aromas [34], was found at the last sampling time for both rice samples. Regarding the aldehyde group, benzaldehyde compounds were the main aldehydes found in white rice, and their quantity decreased with the processing time. These compounds are related to almond aromas [42]. Hexanal, octanal and nonanal, which are related to positive aromas such as fruity [31], citrus [43] and sweet [44] odors, were present in both rice samples, but decreased at the end of the processing time. 5-Methyl-2-furancarboxaldehyde, which is associated with smoky aromas, was found at a high intensity at the end of the thermal treatment, being higher for white rice. This result can be related to the sensory analysis (Table 2), where the burnt aroma was higher in white rice compared to paella rice (for the last sampling time), which was probably due to the formation of the socarrat that delays or prevents rice burning. In the pyrazine group, it is worth highlighting methyl-pyrazine, 2,6-dimethyl-pyrazine and trimethyl-pyrazine, which appeared during thermal treatment and which are associated with varnish and roasted aromas [45]. Sulfur compounds were only found in paella rice due to the added ingredients and their transformation during processing. 2-propenyl trisulfide has been found in garlic, onion and green aromas [31], and also decreased significantly with the processing time. Diallyl disulfide and diallyl sulfide, which are associated with spicy and green aromas [31], respectively, increased slightly with thermal treatment, probably due to the cooking of the ingredients. Furan and furanone compounds, which are related to roasted, bitter and caramel aromas [30,31,32,38], were found at the end of the processing time in WR and P. Furfural was the one that was found with the highest intensity in WR, which coincides with the previous analysis of 5-methyl-2-furancarboxaldehyde and the sensory analysis results that found a greater burnt aroma at the end of the processing time in WR compared to P. In general, these furans and furanone compounds can be formed by the Maillard reaction by heating carbohydrates [29].
3.5. Discrimination of Samples by Using the E-Nose
Electronic nose data for each type of treatment for WR and P were processed individually using the principal component analysis (PCA) (Figure 2). The PCA results for WR explained 85.7% of the total variance of the data, of which more than 55% of the total variance of the data was explained by the principal component PC1. For the P treatment, 91.6% of the total variance was explained, which was somewhat higher than for the WR treatment, of which more than 80% of the data variance was mainly represented with the main component PC1. In the WR treatments, a strong grouping between WR1 and WR2 is observed for PC2, but it is very different from WR3 and WR4. In WR3 a negative correlation with the second principal component is observed. These differences are related to the composition of volatile compounds, since as the temperature applied to each of the treatments increased, the difference between the concentration of volatile compounds became greater. These results are supported by the tasting panel of the sensory analysis, where the treatments WR3 and WR4 present a higher score for burnt odor and WR1 and WR2 present a higher score for wet cereal.
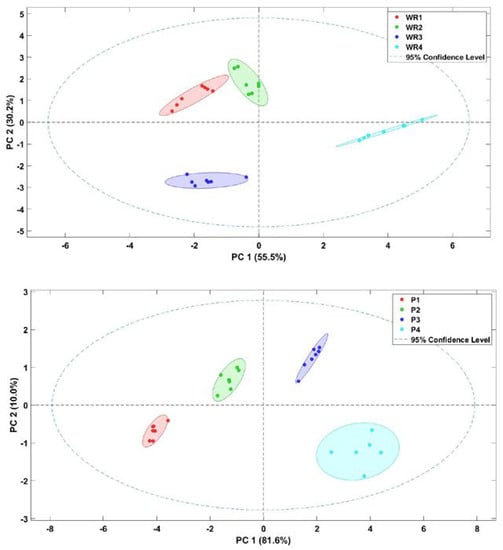
Figure 2.
Score plot of the principal component analysis (PCA) for white rice (WR) and paella (P) aroma evaluated by E-nose.
For the four paella treatments, the groupings between differentiated treatments can also clearly be observed, where P1 and P2 present negative values in PC1 and P3 and P4 present positive ones in PC1. These results are supported by those obtained by the tasting panel for P. Therefore, the electronic equipment was able to discriminate the samples treated at different cooking intensities for the two different types of processing. This is because the sensors of the electronic equipment were able to react to the aroma emitted by the volatile compounds in the samples.
4. Conclusions
During the cooking process, paella suffers a loss of moisture and acquires a characteristic color; thus, the more intense the heat treatment, the darker the color will be. This causes the sensory characteristics of this product to evolve until it acquires a more burnt smell when the cooking becomes more intense. In fact, the synthesis of toxic substances, such as acrylamide, increases more when the thermal intensity is higher. The profile of volatile organic compounds also changes, showing a decrease in those that contribute positive aromas and an increase in others that contribute toasted aromas. Finally, it was found that the electronic device can discriminate these aromas of both preparations since the sensors are sensitive to changes in the aromatic profile of the samples.
Author Contributions
Formal analysis, J.D.B.-R., J.P.S., J.L. and D.M.-V.; investigation, J.P.S., J.L. and D.M.-V.; supervision, M.J.R. and I.M.-F., writing—original draft, J.D.B.-R., M.J.R., I.M.-F. and D.M.-V.; writing—review and editing, J.P.S., J.L. and D.M.-V.; funding acquisition, D.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the regional government of Extremadura and the European Regional Development Fund (FEDER) (ref. GR21121 and GR21045 projects).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and the raw data that support the findings are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Navarro, G.; Medina, F.X. Stamps, tourism and gastronomy: The role of gastronomy in promoting tourism in Spain through the postage stamp. Medina 2018, FX, 15–29. [Google Scholar]
- Saba-Mayoral, A.; Bassie, L.; Christou, P.; Capell, T. Development of a facile genetic transformation system for the Spanish elite rice paella genotype Bomba. Transgenic Res. 2022, 31, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Barrera, A.P.F.; de la Rosa, P.C. Paella. Boletín Científico de las Cienc. Económico Adm. del ICEA 2017, 6. [Google Scholar] [CrossRef]
- Richardson, P. Late Dinner: Discovering the Food of Spain; Simon and Schuster: New York, NY, USA, 2007. [Google Scholar]
- Paesani, C.; Gómez, M. Effects of the pre-frying process on the cooking quality of rice. LWT Food Sci. Technol. 2021, 140, 11074. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Yu, H.; Cheng, Y.; Xie, Y.; Yao, W.; Qian, H. Chemical food contaminants during food processing: Sources and control. Crit. Rev. Food Sci. Nutr. 2021, 61, 1545–1555. [Google Scholar] [CrossRef]
- Lachenmeier, D.; Schwarz, S.; Teipel, J.; Hegmanns, M.; Kuballa, T.; Walch, S.; Breitling-Utzmann, C. Potential Antagonistic Effects of Acrylamide Mitigation during Coffee Roasting on Furfuryl Alcohol, Furan and 5-Hydroxymethylfurfural. Toxics 2019, 7, 1. [Google Scholar] [CrossRef]
- Ramírez-Guízar, S.; González-Alatorre, G.; Pérez-Pérez, M.C.I.; Piñeiro-García, A.; Lona-Ramírez, F.J. Identification and quantification of volatile toxic compounds in tequila. J. Food Meas. Charact. 2020, 14, 2059–2066. [Google Scholar] [CrossRef]
- Castle, L.; Eriksson, S. Analytical methods used to measure acrylamide concentrations in foods. J. AOAC Int. 2005, 88, 274–284. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Barea-Ramos, J.D.; Lozano, J.; Durán-Merás, I.; Martín-Vertedor, D. E-Nose Quality Evaluation of Extra Virgin Olive Oil Stored in Different Containers. Chemosensors 2023, 11, 85. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Mao, H.; Abomohra, A.E.F. Applications of non-destructive technologies for agricultural and food products quality inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose discrimination of abnormal fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Elec-tronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Barea-Ramos, J.D.; Cascos, G.; Mesías, M.; Lozano, J.; Martín-Vertedor, D. Evaluation of the Olfactory Quality of Roasted Coffee Beans Using a Digital Nose. Sensors 2022, 22, 8654. [Google Scholar] [CrossRef]
- Fernández, A.; Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020, 44, e14384. [Google Scholar] [CrossRef]
- López-López, A.; Cortés-Delgado, A.; de Castro, A.; Sánchez, A.H.; Montaño, A. Changes in volatile composition during the processing and storage of black ripe olives. Food Res. Int. 2019, 125, 108568. [Google Scholar] [CrossRef]
- Sánchez, R.; Boselli, E.; Fernández, A.; Arroyo, P.; Lozano, J.; Martín-Vertedor, D. Determination of the Masking Effect of the ‘Zapateria’ Defect in Flavoured Stuffed Olives Using E-Nose. Molecules 2022, 27, 4300. [Google Scholar] [CrossRef]
- Arns, B.; Bartz, J.; Radunz, M.; do Evangelho, J.A.; Pinto, V.Z.; da Rosa Zavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment on rice starch, applied directly in grain paddy rice or in isolated starch. LWT-Food Sci. Technol. 2015, 60, 708–713. [Google Scholar] [CrossRef]
- Luangmalawat, P.; Prachayawarakorn, S.; Nathakaranakule, A.; Soponronnarit, S. Effect of temperature on drying characteristics and quality of cooked rice. LWT-Food Sci. Technol. 2008, 41, 716–723. [Google Scholar] [CrossRef]
- Hardy, J.; Parmentier, M.; Fanni, J. Functionality of nutrients and thermal treatments of food. Proc. Nutr. Soc. 1999, 58, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.M. Applications of the Maillard reaction in the food industry. Food Chem. 1998, 62, 431–439. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Mai, T.V.T.; Huynh, L.K. Detailed kinetic mechanism for CH3OO + NO reaction—An ab initio study. Comput. Theor. Chem. 2017, 1113, 14–23. [Google Scholar] [CrossRef]
- Tateo, F.; Bononi, M.; Andreoli, G. Acrylamide levels in cooked rice, tomato sauces and some fast food on the Italian market. J. Food Compos. Anal. 2007, 20, 232–235. [Google Scholar] [CrossRef]
- Pérez-Nevado, F.; Cabrera-Bañegil, M.; Repilado, E.; Martillanes, S.; Martín-Vertedor, D. Effect of different baking treatments on the acrylamide formation and phenolic compounds in Californian-style black olives. Food Control 2018, 94, 22–29. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Mon, T.R. Quantitative analysis of 2-acetyl-1pyrroline in rice. J. Agric. Food Chem. 1986, 34, 112–114. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Cascos, G.; Barea-Ramos, J.D.; Montero-Fernández, I.; Ruiz-Canales, A.; Lozano, J.; Martín-Vertedor, D. Burn Defect and Phenol Prediction for Flavoured Californian-Style Black Olives Using Digital Sensors. Foods 2023, 12, 1377. [Google Scholar] [CrossRef]
- Sukchum, N.; Surasereewong, S.; Chaethong, K. Volatile compounds and physicochemical characteristics of Thai roasted chilli seasoning. Food Res. 2022, 6, 309–318. [Google Scholar] [CrossRef]
- Lee, J.; Cho, J.J.; Hong, S.J.; Kim, D.S.; Boo, C.G.; Shin, E.C. Platycodon grandiflorum roots: A comprehensive study on odor/aroma and chemical properties during roasting. J. Food Biochem. 2020, 44, e13344. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Y.; Qu, F.; Wang, P.; Gao, J.; Zhang, X.; Hu, J. Characterization of the Key Aroma Compounds of Shandong Matcha Using HS-SPME-GC/MS and SAFE-GC/MS. Foods 2022, 11, 2964. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Reineccius, G. Identification of aroma compounds in Parmigiano-Reggiano cheese by gas chromatography/olfactometry. J. Dairy Sci. 2002, 85, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Fratini, G.; Lois, S.; Pazos, M.; Parisi, G.; Medina, I. Volatile profile of Atlantic shellfish species by HS-SPME GC/MS. Food Res. Int. 2012, 48, 856–865. [Google Scholar] [CrossRef]
- McGinty, D.; Scognamiglio, J.; Letizia, C.S.; Api, A.M. Fragrance material review on 2-ethyl-1-hexanol. Food Chem. Toxicol. 2010, 48, S115–S129. [Google Scholar] [CrossRef]
- Cardinal, M.; Chaussy, M.; Donnay-Moreno, C.; Cornet, J.; Rannou, C.; Fillonneau, C.; Courcoux, P. Use of random forest methodology to link aroma profiles to volatile compounds: Application to enzymatic hydrolysis of Atlantic salmon (Salmo salar) by-products combined with Maillard reactions. Food Res. Int. 2020, 134, 109254. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Mori, K.; Oda, Y.; Miyazawa, M. Volatile Oil Compounds from Corms and Flowers of Crocus vernus L. Hill and Corms of C. sativus L. Libyan Agric. Res. Cent. J. Int. 2010, 1, 244–249. [Google Scholar]
- Kandyala, R.; Raghavendra, S.P.C.; Rajasekharan, S.T. Xylene: An overview of its health hazards and preventive measures. J. Oral. Maxillofac. Pathol. 2010, 14, 1. [Google Scholar] [CrossRef]
- Palmquist, E.; Claeson, A.S. Odor perception and symptoms during acrolein exposure in individuals with and without building-related symptoms. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Peris, J.E.; Redondo, A.M.; Shimada, T.; Costell, E.; Carbonell, I.; Peña, L. Impact of D-limonene synthase up-or down-regulation on sweet orange fruit and juice odor perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef]
- Kulkarni, P.; Stolberg, T.; Sullivanjr, J.M.; Ferris, C.F. Imaging evolutionarily conserved neural networks: Preferential activation of the olfactory system by food-related odor. Behav. Brain Res. 2012, 230, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Lee, K.S.; Jeong, O.Y.; Kim, K.J.; Kays, S.J. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2008, 56, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Motooka, R.; Usami, A.; Nakahashi, H.; Koutari, S.; Nakaya, S.; Shimizu, R.; Miyazawa, M. Characteristic odor components of essential oils from Eurya japonica. J. Oleo Sci. 2015, 64, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiago, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).