Abstract
The misuse of sulfadiazine (SFZ) has led to great hazard to the environment and human safety; therefore, a simple, rapid, and sensitive method to detect sulfadiazine is urgently needed. Herein, we report a simple fabrication method for rare earth vanadate samarium (SmV)-doped covalent organic framework COFTDBA-TTL nanocomposites (SmV/COFTDBA-TTL), which were used to construct a sulfadiazine (SFZ) electrochemical sensor. The synergistic effect arising from the combination of SmV and COFTDBA-TTL accelerates the charge transfer kinetics, along with the creation of more surface-active sites that benefit effective detection. Compared with other electrochemical sensors, this electrochemical sensor exhibits low detection limit (2.40 nM), wide linear range (7.32–12.0 μM), good reproducibility (RSD = 0.823%), and stability (RSD = 3.60%). It provides a novel method and theoretical basis for the application of rare earth COF-based electrochemical sensors to detect environmentally destructive pollutants.
1. Introduction
Sulfadiazine (SFZ) is a common sulfonamide antibacterial agent, which is mainly used to treat patients with infectious diseases, such as urinary tract infection and wound sepsis. In aquaculture, SFZ is widely used in the treatment of bacterial fish diseases, such as erythroderma and enteritis, to avoid economic losses [1]. The maximum allowable levels for SFZ residues in aquatic environments in China are 0.0140–0.280 μg/L [2]. The maximum allowable levels for sulfonamides residues in fish skin and flesh in China are 100 μg/kg [3]. However, with the rapid development of the medical industry and aquaculture, increasing SFZ inevitably enters aquatic environment and accumulates in the organism. Schar, D. et al. estimated that global antimicrobial consumption may rise to 13,600 tons in 2030, 14% of which are sulfonamides. This may lead to negative effects on all aspects of the environment [4]. For fish, Zhou et al. found that SFZ exposure to water bodies has effects on the growth, antioxidant capacity, and thyroid hormones of non-target aquatic organisms (rana nigromaculata tadpoles) [5]; Deng et al. found that SFZ enrichment in zebrafish was enantioselective, which would cause oxidative damage and induce enzyme activity in zebrafish (danio rerio) [6]; Jiang et al. showed that zebrafish embryo development, gene expression, and larval body weight were affected by SFZ at ambient concentrations [7]; Lei et al. have shown that SFZs disrupt lipid metabolism in zebrafish (danio rerio) larvae and 3T3-L1 preadipocytes [8]. Meanwhile, the risk of antibiotic resistance due to abuse cannot be ignored, which may produce new epizootics that are difficult to treat. Furthermore, long-term intake of fish with excessive exposure to SFZ can lead to human-specific liver failure, genetic damage, dysbacteriosis, and decreased immunity [9]. It will also alter the human normal intestinal flora indirectly and select for antibiotic-resistant bacteria, which may lead to the emergence of superbug. Indeed, sulfonamides have been reported in all fish samples from typical mariculture areas in southern China [10], which means that SFZ in fish has posed potential threats to the natural ecosystems. However, existing analytical methods for SFZ in fish muscle using ultra-high performance liquid chromatography are ambitious and complicated. Therefore, there is an urgent need to develop a reliable and efficient analytical method to monitor SFZ in fish muscles.
Compared with traditional detection techniques (such as capillary electrophoresis [11], UV-Vis spectroscopy [12], and high performance liquid chromatography [13]), electrochemical methods are simple, fast, and cost-effective [14,15]. In recent years, this method has been applied to the monitoring and analysis of organic and inorganic pollutants in the environment. For example, electrochemical sensing was constructed by Lu et al. to realize simultaneous detection of multiple metal ions in aqueous solution [16]. Yang et al. constructed an electrochemical immunosensor to realize ultra-sensitive detection of sulfadimethoxpyrimine [17]. Jiang et al. achieved the detection of multiple protein kinases by constructing magnetic biosensors [18]. Thus, electrochemical methods show great potential in the rapid and efficient detection of environmental pollutants in organisms. However, the development of electrochemistry is limited by matrix interference and poor reproducibility. In addition, the high cost and harsh storage conditions of immune sensors and enzyme sensors hinder their practical application. Although carbon materials [19], metal nanoparticles [20], nano-enzymes, and quantum dots [21] have been developed by scientists to address these issues, nanoparticles are prone to fall off the electrode surface without the protection of Nafion. In addition, the cost of precious metal nanoparticles is high, which may limit their further application.
Covalent organic framework materials (COFs) have been used as electrode materials for sensing analysts in recent years due to their unique physicochemical properties (controllable pore size and structure, large specific surface area, versatile functions, and stability) [22]. For instance, Pang et al. achieved the detection of bisphenol A and bisphenol S by synthesizing COF and CTpPa-2 [23]. Wu et al. achieved the detection of riboflavin by synthesizing COFTFPB-Thi [24]. COF/Pt/MWCNT-COOH nanocomposites were synthesized by Feng et al. to achieve sensitive detection of dopamine [25]. However, due to the poor insolubility and conductivity of porous polymers, it is difficult to connect them directly with electrodes, which greatly limits the practical application of COFs in electrochemistry. To effectively combine COF postmodification or modification on different substrates (GO [26], carbon nanotubes uses (CNT) [27], SiO2 [28]) have been attempted by scientists to overcome this defect. However, the selection of substrate and harsh growth conditions make COFs difficult to synthesize. In addition, the uniformity of COFs on the substrate is difficult to control. The nanostructure of rare earth mixed metal oxide samarium vanadate (SmV) has many advantages, such as valence states, abundant oxygen vacancies, excellent electrochemical activity, and electroactive sites [29,30]. For example, Gallis et al. prepared a rare earth metal–organic skeleton to realize acid gas adsorption and optical detection [31]; Ma et al. synthesized composite materials with luminescent response using rare earth metal–organic skeleton covalent linked microsphere resins [32]. Therefore, the inclusion of SmV in COFs is expected to solve the anneal problem, and there are few reports about it.
In this work, SmV/COFTDBA-TTL composites were prepared by doping SmV in COFTDBA-TTL, which achieved ultrafast detection of SFZ in fish muscles. The sensor has several advantages. First, abundant functional groups give COF a high carrier rate, and a large number of adsorption sites can selectively adsorb SFZ. Second, the introduction of rare earth SmV improves their electrocatalytic performance, further amplifying the detection signal of SFZ. Finally, the electrochemical sensor can be used to sensitively and aselectively detect SFZ in fish muscles, with a recovery of 89.8–110%. This work developed a rapid, reliable, and sensitive analytical method for antimicrobial investigation, and it provided a new direction for the research progress of electrochemical sensors based on rare earth COFs to detect environmentally destructive pollutants.
2. Experimental Section
2.1. Materials and Reagents
2,4,6-tris(4-aminophenyl)-1,3,5-triazine (TTL) and terphenyl dicarboxylic acid (TDBA) were purchased from Alfa Aesar Co., Ltd. (Shanghai, China). 1,4-dioxane (DOX) was purchased from Macklin Maclin Biochemical Technology Co., Ltd. (Shanghai, China). Tetrahydrofuran (THF) was purchased from Meryer Co., Ltd. (Shanghai, China). Mesitylene was purchased from Enerdy Chemical (Shanghai, China). Glacial acetic acid (Hac), sodium dihydrogen phosphate (NaH2PO4), disodium hydrogen phosphate (Na2HPO4), sulfadiazine (SFZ), NH4VO3, Sm(NO3)3, and other drugs were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Glassy carbon electrode (GCE), platinum wire (Pt) electrode, and saturated calomel electrode (SCE) were purchased from Shanghai Chenhua Instruments Co., Ltd. (Shanghai, China). The tilapia was purchased from Changsheng Market (Guangzhou, China). The purchased tilapia muscle was homogenized by a wall breaker, which was filtered through 0.2 μm filter paper to remove impurities. An amount of 5 mM phosphate buffer solution (PB, pH = 7.0) was prepared by mixing 5 mM NaH2PO4 and 5 mM Na2HPO4. Ultrapure water (ρ ≧ 18.2 M cm−1) obtained by Millipore-Q System was used during the whole experiment.
2.2. Preparation of COFTDBA-TTL
COFTDBA-TTL was synthesized according to the following procedures (Scheme 1). First, 35.0 mg of 2,4,6-tris(4-aminophenyl)-1,3,5-triazine (TTL), 41.0 mg of terphenyl dicarboxylic acid (TDBA), and 8.00 mL of 1,4-dioxane (DOX) were added to a 25.0 mL Schlenk tube and dissolved ultrasonically. Then, 0.600 mL glacial acetic acid (HAc) was added slowly and reacted at 120 °C. After two days, the product was collected and washed several times with DOX, THF, and ethanol. Finally, the light gray solid (COFTDBA-TTL) was collected and dried in an oven at 80.0 °C under vacuum.
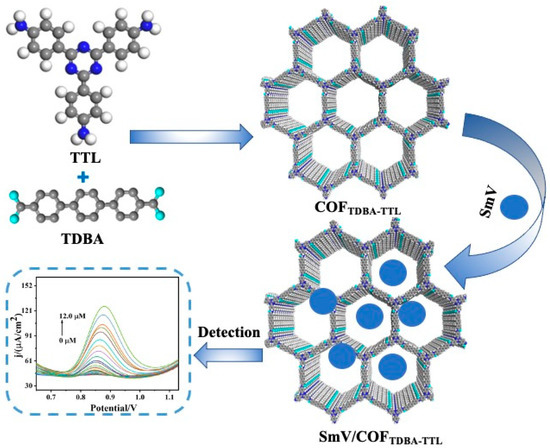
Scheme 1.
Schematic illustrating the preparation process of COFTDBA-TTL.
2.3. Preparation of SmV/COFTDBA-TTL
COFTDBA-TTL (20.0 mg), NH4VO3 (0.100 M, 0.116 g), Sm(NO3)3 (0.100 M, 0.444 g), and 10.0 mL of ultrapure water were dissolved and placed in a 50 mL reactor. Then, the mixture was heated at 180 °C for 12.0 h. The product was collected by centrifugation at 10,000 rpm until the supernatant was clarified and washed with ethanol and deionized with water several times. The solid (SmV/COFTDBA-TTL) at the bottom was collected and dried under vacuum at 80.0 °C.
2.4. Preparation of SmV/COFTDBA-TTL/GCE
Electrodes were prepared according to previous methods [33]. First, 4 mg SmV/COFTDBA-TTL was dissolved in 1.00 mL THF by sonication. Then, 5.00 μL of the dissolved solution was dropped on the surface of the treated glassy carbon electrode (GCE) and dried in air. Before use, the GCE was polished with 1.00 μM and 0.300 μM Al2O3, cleaned ultrasonically with ethanol and ultrapure water, respectively, and blow dried with N2.
2.5. Detection of Actual Samples
Tilapia were purchased from a retail store in the local aquatic product market in Guangzhou. The tilapia muscle was homogenized by a wall breaker filtered through 0.200 μm filter paper to remove impurities (considering that the filter paper may have a certain adsorption effect on SFZ, the initial 10.0 mL solution was not collected). The filtered sample and buffer solution were mixed a ratio of 1:4, and the content of SFZ was detected by SmV/COFTDBA-TTL/GCE. Furthermore, 5.00 μM SFZ was added to the annealed sample, and a spike recovery experiment was performed.
2.6. Instruments
All electrochemical experiments were performed on the CHI750E electrochemical station (CHI, Shanghai, China) at room temperature. A three-electrode system was adopted. The SmV/COFTDBA-TTL/GCE electrode was regarded as the working electrode, the platinum wire (Pt) electrode was the auxiliary electrode, and the saturated calomel electrode (Hg2Cl2) was the reference electrode. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were statically performed in a 0.100 M KCl solution containing 5.00 mM [Fe(CN)6]3−/4−, and the impedance frequency range was 0.0100 Hz to 10.0 KHz. CV had a potential range of −0.100 V–0.500 V. The square wave voltammetry (SWV) test was performed in a PB buffer solution (5.00 mM, pH = 7.0). A scanning electron microscope (SEM) was tested on a Hitachi SU8010 with an accelerating voltage of 15.0 kV. An amount of 1 mg of the target material was added to 0.500 mL ethanol, dispersed by ultrasound for half an hour, then 10.0 μL was dropped on clean silicon wafers and dried at room temperature. Then, a SEM test was conducted. Fourier transform infrared spectroscopy (FT-IR) was recorded on the EQUINOX 55.0 in Bruker, Germany. Mix the potassium bromide with the target material in a 20:1 ratio, grind for three minutes, and bake under a red light for five minutes. It is then compressed into a sheet, which is then scanned by infrared. The nitrogen adsorption and desorption experiments were carried out using JW-BK200C at a liquid nitrogen temperature of 77.0 K. All samples were pretreated at 100 °C for six hours. Thermogravimetric analysis (TGA) was carried out on a NETZSCH TG 209 F3 Tarsus in Germany, with N2 protection during the test, and the test temperature range was 35.0–800 °C, and the heating rate was 10.0 °C/min. X-ray photoelectron spectroscopy (XPS) was tested on ThermoFisher, ESCA. X-ray powder diffraction (PXRD) was tested on D8 ADVANCE, and theoretical simulation analysis was carried out with the help of Materials Studio 8.0. Water contact angle (CA) was tested on Kruss DS100, Germany. Dynamic light scattering particle size analysis (DLS) was carried out on EliteSizer. SmV/COFTDBA-TTL and COFTDBA-TTL were ultrasonically dispersed in water, and the concentration was maintained at 0.2 mg/L.
3. Results
3.1. Characterization of Composite Materials
In this work, COFs with -NH-CO- as building blocks were generated using a simple solvothermal synthesis method, utilizing the condensation of monomers TTL (-NH2) and TDBA (-COOH) (Figure S1, Support Information). The yields of COFTDBA-TTL and SmV/COFTDBA-TTL were 79.8% and 85.0%, respectively. The microstructure and surface morphology of the composites were characterized by scanning electron microscope (SEM, Figure 1). The synthesized COFTDBA-TTL was similar to sea urchins (4.61 μm diameter), and many well distributed nanowires grew from the surface of the sphere. An appropriate concentration was regarded as a necessary condition for the synthesis of SmV/COFTDBA-TTL [34,35]. Therefore, 0.0100 M, 0.0500 M, 0.100 M, 0.150 M, and 0.200 M SmV were added to the same amount of COFTDBA-TTL to determine the optimal concentration of SmV/COFTDBA-TTL. With the addition of SmV, the shape of the sea urchin gradually tends to be lantern-like. Excessive SmV would be attached to the surface of COFTDBA-TTL and would agglomerate. This may be detrimental to mass transfer, so the electrocatalytic activity of SFZ is affected. Therefore, the final concentration of SmV was determined to be 0.100 M. Then, a TEM image was used to characterize the microstructure of COFTDBA-TTL and SmV/COFTDBA-TTL. As shown in Figure 2, the surface of SmV/COFTDBA-TTL antenna is no longer smooth compared to the smooth sea urchin-like COFTDBA-TTL antenna, and many SmV particles are arranged on the surface of the sea urchin antenna, which further indicated that the target material has been synthesized successfully.
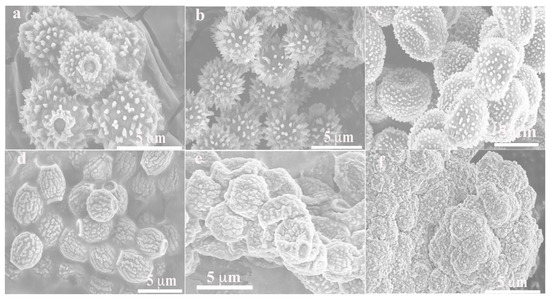
Figure 1.
(a) SEM image of COFTDBA-TTL; (b–f) SEM images of COFTDBA-TTL with different SmV concentrations: (b) 0.0100 M; (c) 0.0500 M; (d) 0.100 M; (e) 0.150 M; (f) 0.200 M.
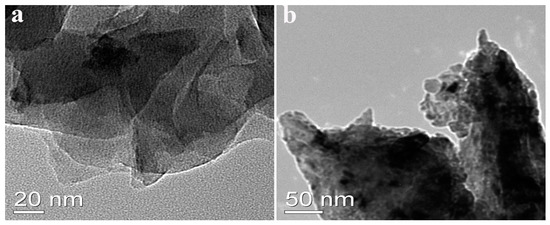
Figure 2.
(a) TEM image of COFTDBA-TTL; (b) TEM image of SmV/COFTDBA-TTL.
Subsequently, the particle size of the synthetic material was analyzed using the dynamic light scattering particle size analyzer (DLS). As shown in Figure 3, the average particle size of the synthesized COFTDBA-TTL was about 4.61 μm. When SmV was introduced, the particle size of the synthesized SmV/COFTDBA-TTL changed with the concentration of SmV. When the introduced concentration was 0.0100 M, 0.0500 M, and 0.100 M, the SmV were 4.65 μm, 4.67 μm, and 4.86 μm, respectively. However, high concentration of SmV would be covered on the surface of COFTDBA-TTL, resulting in aggregation of synthesized SmV/COFTDBA-TTL.
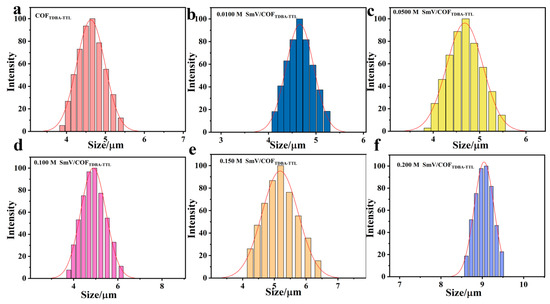
Figure 3.
(a) DLS image of COFTDBA-TTL; (b–f) DLS images of COFTDBA-TTL with different SmV concentrations: (b) 0.0100 M; (c) 0.0500 M; (d) 0.100 M; (e) 0.150 M; (f) 0.200 M.
The water contact angle (CA) of the COFTDBA-TTL surface was measured to be approximately 133.9°, indicating that the material was hydrophobic (Figure S2, Support Information). Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) were used to further prove the success of the synthesis of COFTDBA-TTL and SmV/COFTDBA-TTL. The FT-IR spectra of TTL, TDBA, and COFTDBA-TTL were measured, and it was found that, with the occurrence of the condensation reaction, the -NH (3320 cm−1) peak of monomeric TDBA disappeared. Among them, the C=O, C=N, and C=C peaks of COFTDBA-TTL appeared at 3200 cm−1, 1685 cm−1, and 1660 cm−1, respectively. In addition, the appearance of the characteristic peak of SmV at 803 cm−1 indicated that SmV was successfully introduced (Figure 4 and Figure S3, Support Information) [36]. XPS spectrum showed that COFTDBA-TTL contains only C (284.8 eV), N (399.61 eV), and O (531.8 eV) elements, while the characteristic peaks of Sm (Sm 3d and Sm 4d) and V elements appeared in SmV/COFTDBA-TTL (Figure S4, Support Information). The high-resolution XPS (COFTDBA-TTL) of C 1s showed three peaks at 284.78 eV, 285.55 eV, and 286.22 eV, which were assigned to C=C, C-C, and C-H groups, respectively (Figure 5a). The N 1s spectrum showed three peaks at 398.96 eV, 400.40 eV, and 402.39 eV, which were assigned to C-N, C=N, and N-H groups, respectively [37]. However, after SmV was modified, the XPS of SmV/COFTDBA-TTL shifted, and N1s appeared at 398.85 eV, 400.39 eV, and 402.23 eV (Figure 5b). This indicated that the combination of SmV and COF might be accompanied by chemical reaction [38]. On the other hand, two characteristic peaks at 1083.26 eV and 1110.61 eV of the fine map of Sm 3d corresponded to the Sm 3d5/2 and Sm 3d3/2 spin orbitals Sm3+, respectively. The peaks at 517.57 eV (V 2p3/2) and 524.77 eV (V 2p1/2) confirmed the presence of V5+ in SmV. Since the peaks of O and V were relatively close, the peak of O was also assigned in XPS (Figure 5c–f) [39]. It was found that the front of O 1s is also offset compared with that before modification (The O 1s characteristic peak of SmV/COFTDBA-TTL appeared at 530.37 eV, while that of COFTDBA-TTL appeared at 532.10 eV).
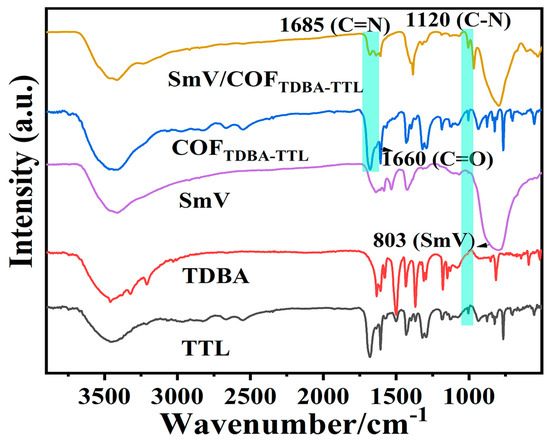
Figure 4.
The FT-IR spectra of TTL, TDBA, COFTDBA-TTL, and SmV/COFTDBA-TTL.
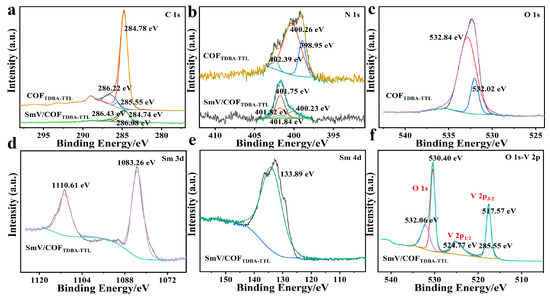
Figure 5.
XPS fine map of COFTDBA-TTL and SmV/COFTDBA-TTL: (a) C 1s; (b) N 1s; (c) O 1s; (d) Sm 3d; (e) Sm 4d; (f) O 1s-V 2p.
The simulated PXRD of COFTDBA-TTL was obtained with Material Studio 8.0, which was in good agreement with the experimental results, with RWP and RP values of 5.89% and 12.7% respectively (Figure 6a). From the simulated PXRD, the symmetry space group was calculated to be P6, and the optimized unit cell parameters were a = b = 52.38 Å, c = 3.51 Å (interlayer distance), α = β = 90°, and γ = 120°. Comparing the simulated AA and AB models with the experimental model, the AA result is consistent with the XRD results (Figure 6b), and some twisted hexagonal structures were obtained by AA stacking structures (inset in Figure 6d) [40,41]. After SmV was added, the XRD crystal form of SmV/COFTDBA-TTL was still well preserved, and the characteristic peak of SmV appeared at 25.0 °C (Figure 6c). N2 adsorption and desorption showed that COFTDBA-TTL presented the H1 isotherm related type IV, and the BET of COFTDBA-TTL and SmV/COFTDBA-TTL were 102.4 m2/g and 73.8 m2/g, respectively (Figure 6e). The thermal stability of the material was investigated by using thermogravimetric analysis (TGA). As shown in Figure 6f, COFTDBA-TTL had only a small loss (<5%) when the temperature reached 350 °C, which can be attributed to the escape of adsorbed water/solvent in the pores. As temperatures continued to rise, the COFTDBA-TTL began to lose weight rapidly, possibly due to the collapse of the organic framework of the COFs. The TGA of SmV/COFTDBA-TTL was similar to that of COFTDBA-TTL, indicating that the stability of the materials was well maintained after the addition of SmV [42].
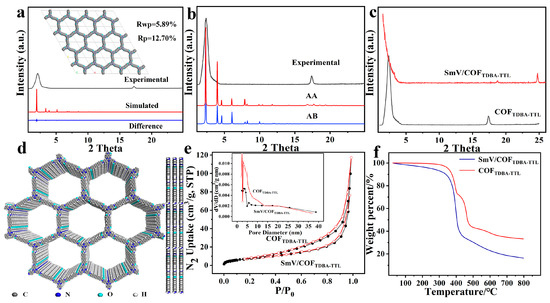
Figure 6.
(a) XRD pattern and AA-stacked model; (b) Experimental (black) XRD and simulated XRD patterns for AA structure (red) and AB structure (blue) of COFTDBA-TTL; (c) XRD comparison of COFTDBA-TTL and SmV/COFTDBA-TTL; (d) Eclipsed conformations and π–π accumulation distance between two adjacent layers of COFTDBA-TTL; (e) N2 adsorption and desorption curve of COFTDBA-TTL and SmV/COFTDBA-TTL, and inset was pore size distribution of COFTDBA-TTL and SmV/COFTDBA-TTL; (f) TGA images of COFTDBA-TTL and SmV/COFTDBA-TTL.
3.2. Electrochemical Behaviors of the SmV/COFTDBA-TTL/GCE Modified Electrode
With the rapid development of the medical industry, SFZ inevitably enters the environment, resulting in harm from all aspects of the environment. Therefore, it is necessary and urgent to develop a simple and sensitive electrochemical sensor for the detection of SFZ. Here, SmV/COFTDBA-TTL is used to construct an electrochemical sensor for sensing SFZ. Not only is the detection of SFZ important, but it can also be compared with other electrochemical sensors to evaluate the feasibility of SmV/COFTDBA-TTL in electrochemical sensors. According to the literature, the electrochemical mechanism of SFZ oxidation involves two-electron and two-proton irreversible transfer processes (Figure 7) [43,44].
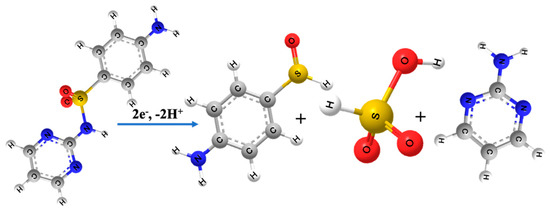
Figure 7.
Electrochemical mechanism of SFZ at SmV/COFTDBA-TTL/GCE modified electrode.
To enable the electrochemical performance of the modified electrode to be demonstrated, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) of different modified electrodes were tested in electrolytes containing 5.00 mM [Fe(CN)6]3−/4− and 0.100 M KCl, respectively (Figure 8a,b) [45,46]. It was found that the peak current of COFTDBA-TTL/GCE was reduced when compared to that of the GCE, but the redox peak still remained, with a slight increase in the peak-to-peak potential difference. EIS showed that the corresponding impedance also increased slightly, indicating that the COF was successfully modified on the GCE surface, leading to the delay of charge transfer. However, with the introduction of rare earth metals, the peak current of the SmV/COFTDBA-TTL/GCE modified electrode increased, and the corresponding impedance also decreased slightly. This indicated that the interfacial synergy formed by the combination of SmV/COFTDBA-TTL on the GCE simplified the charge transfer kinetics of the redox couple, causing the SmV/COFTDBA-TTL/GCE modified electrodes to have higher electrocatalytic activity and good electronic conductivity. In addition, the introduction of COF increased the specific surface area of the electrode, and abundant C=C and C=N functional groups offered a large number of active sites, which can further accelerate the electron transfer. Next, the CVs of the SmV/COFTDBA-TTL/GCE modified electrode were tested at different scan rates (50.0–250 mV/s) (Figure 8c) [47,48,49]. The redox peak of the SmV/COFTDBA-TTL/GCE modified electrode was positively correlated with the scan rate, which means that the redox reaction was a typical surface adsorption-controlled process (Figure 8d).
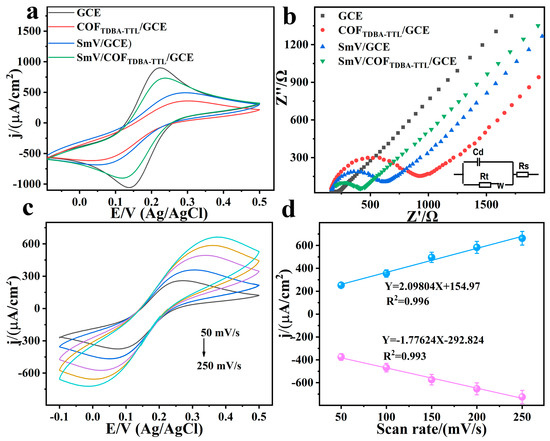
Figure 8.
(a) CVs and (b) EIS of different modified electrode (GCE, SmV/GCE, COFTDBA-TTL/GCE, SmV/COFTDBA-TTL/GCE modified electrode) in 0.100 M KCl solution containing 5.00 mM [Fe(CN)6]3−/4− at a scan rate was 50.0 mV/s; (c) CVs of SmV/COFTDBA-TTL/GCE modified electrode at different scan rates (50.0–250 mV/s); (d) the plot of peak current density versus scan rate.
In order to prove the usability of SmV/COFTDBA-TTL/GCE modified electrode, the current responses of GCE, SmV/GCE, COFTDBA-TTL/GCE, and SmV/COFTDBA-TTL/GCE-modified electrodes were tested by adding 5.00 μM SFZ (in PB, 5.00 mM, pH = 7.0). It can be clearly seen that the current response of the COFTDBA-TTL/GCE-modified electrode was slightly higher than that of GCE (Figure S5, Support Information). The reason may be that the porous COFTDBA-TTL modified on the surface of GCE can increase the absorption sites of SFZ, which led to an increase in the current response. The current response of SmV/GCE modified electrode also increased slightly, which indicates that the SmV nanoparticles have good electrochemical activity of SFZ. Under the same conditions, the current response of the SmV/COFTDBA-TTL/GCE-modified electrode was much larger than that of the previously mentioned electrodes. This may be attributed to the combination of SmV and COFTDBA-TTL-generating surface active sites that can enhance the catalytic activity of SFZ and promote the reduction of SFZ groups (-SO2NH-) on the SmV/COFTDBA-TTL/GCE modified electrode. The above results preliminarily demonstrated that the SmV/COFTDBA-TTL/GCE modified electrode has stronger electrocatalytic activity.
The final sensing performance may be affected by different pH values, electrode modification amounts, SmV contents, etc. (Figure S6, Support Information). To determine the detection performance of the SmV/COFTDBA-TTL/GCE modified electrode, we optimized several other important parameters by square wave voltammetry (SWV). The effect of different COFTDBA-TTL synthesis times (one to three days) on the peak current of SFZ was investigated. As the number of reaction days increased, the electrocatalytic activity of the synthesized COFTDBA-TTL toward SFZ increased. Among them, the materials synthesized in two to three days effect little change on the current response of the SFZ. Considering the time cost, the synthesis of two days was selected as the final synthesis time (Figure 9a). The final performance of the electrode is also affected by the content of SmV. The current response of different modification amounts to SFZ was studied (Figure 9b). It can be seen that, as the concentration of SmV/COFTDBA-TTL increased, the peak current of SFZ increased. When the concentration of SmV/COFTDBA-TTL reached 3.00 mg/mL, the current response was the greatest and then began to decrease. This may be because the more SmV/COFTDBA-TTL nanoparticles there were assembled on the GCE surface, the more adsorption sites of SFZ were presented, resulting in an increase in the peak current. However, excessive SmV/COFTDBA-TTL accumulated on the surface of the GCE, which may hinder the electron transfer rate and result in a decrease in the peak current. Therefore, the optimal modification amount of SmV/COFTDBA-TTL on the surface of the GCE electrode was 3.00 mg/mL (Figure 9c). The electrochemical activity of the electrode is also affected by pH. As shown in Figure 9d, the peak current changed only slightly at pH values between 5.8–8.0, which may be due to the systematic error caused by changing the pH solution. Considering the neutrality of fish muscle, pH = 7.0 was finally chosen for the following studies.
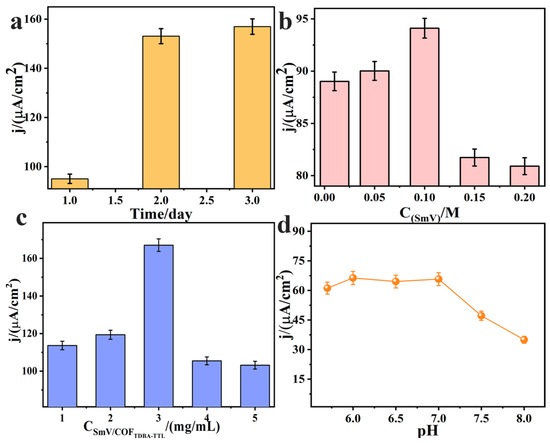
Figure 9.
(a) Comparison of electrochemical properties of COFTDBA-TT synthesized at different time (one day, two days, three days) to SFZ; (b) Optimization of different SmV (0.0100 M, 0.0500 M, 0.100 M, 0.150 M, 0.200 M) modification amount; (c) The optimization of different modification amount, adding 1 mg/mL, 2 mg/mL, 3 mg/mL, 4 mg/mL, 5 mg/mL SmV/COFTDBA-TTL, respectively; (d) The current response of the SmV/COFTDBA-TTL/GCE-modified electrode to SFZ was compared in the range of pH 5.8–8.0. The electrolyte was PB (5.00 mM, pH = 7.0), containing 5.00 μM SFZ. SWV was used to monitor the effects current response of SFZ.
3.3. SFZ Detection Based on the SmV/COFTDBA-TTL/GCE Modified Electrode
Under the optimal conditions we tested, SFZ (0–12.0 μM) was detected by using the SmV/COFTDBA-TTL/GCE modified electrode. The results showed that the peak current response increased linearly with increasing SFZ concentration (Figure 10). The linear regression equation was Y = 6.83X + 43.5, R2 = 0.9972. The sensitivity was as high as 6.83 μA/μM cm2, the detection limit was as low as 2.40 nM, and the linear range was as wide as 7.32 nM–12.0 μM. Compared with the published studies, it was found that the results of this work were significantly better than some previous studies, indicating that the sensor based on SmV/COFTDBA-TTL has good application prospects in the detection of SFZ (Table 1) [9,44,50,51,52,53,54,55].
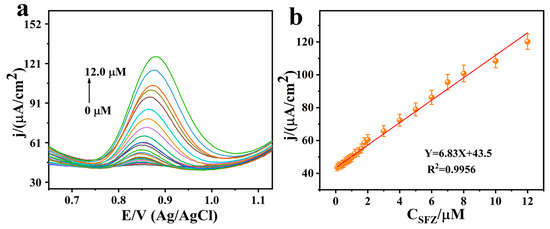
Figure 10.
(a) SWV of SmV/COFTDBA-TTL/GCE modified electrode with continuous addition of SFZ; (b) Linear relationship; the electrolyte was PB (5.00 mM, pH = 7.0), and the added SFZ concentration is 0–12.0 μM.

Table 1.
Analytical results of various electrochemical sensors.
3.4. Selectivity, Repeatability and Stability of the SmV/COFTDBA-TTL/GCE Modified Electrode
The repeatability and stability of the SmV/COFTDBA-TTL/GCE-modified electrode were very important. The electrode was left at ambient temperature for 30 days, and its current response to SFZ was found to be well maintained, which means that the electrochemical sensor constructed by SmV/COFTDBA-TTL/GCE has good stability (Figure 11a and Figure S7a, Support Information) [56,57]. To verify the reproducibility of the SmV/COFTDBA-TTL/GCE-modified electrode, we used five parallel electrodes under the same experimental conditions. After calculation, the RSD was 3.60%, indicating that the SmV/COFTDBA-TTL/GCE modified electrode presented good reproducibility (Figure 11b and Figure S7, Support Information). Furthermore, identifying the potential interferers is critical to determine sensor reliability (Figure 12). In PB solution (5.00 mM, pH = 7.0) containing 5.00 μM SFZ, we added metal ions (zinc ions, Zn2+), biomolecules ((ascorbic acid, AA), uric acid (UA), dopamine (DA), glucose (Glu)), and other antibiotic drugs (ibuprofen (Ibu), flumethazol (FMZ), and sulfisoxazole (SFA)) fifty times. The anti-interference ability showed that, after adding interfering substances, the relative standard deviation (RSD) of SmV/COFTDBA-TTL/GCE for SFZ detection ranged from 88.6% to 108%, indicating that it has high selectivity [58]. The excellent selectivity of the SmV/COFTDBA-TTL/GCE modified electrode for SFZ detection may be attributed to the synergistic effect produced by the combination of SmV and COFTDBA-TTL, which may lead to more active surface area, accelerate the charge transfer kinetics, and generate more surface active sites. In addition, the surface electron rate can be regulated by increasing the defect degree in the nanocomposite, and then the active edge sites are enriched, thereby improving the selectivity of the sensor.
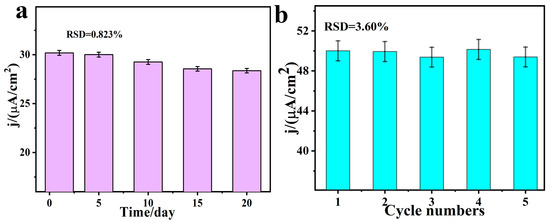
Figure 11.
(a) Stability of the SmV/COFTDBA-TTL/GCE-modified electrode. (b) Reproducibility of SmV/COFTDBA-TTL/GCE modified electrode. The electrolyte was PB (5.00 mM, pH = 7.0), and 5.00 μM SFZ was added. SWV was used to monitor the effects of current response of SFZ.
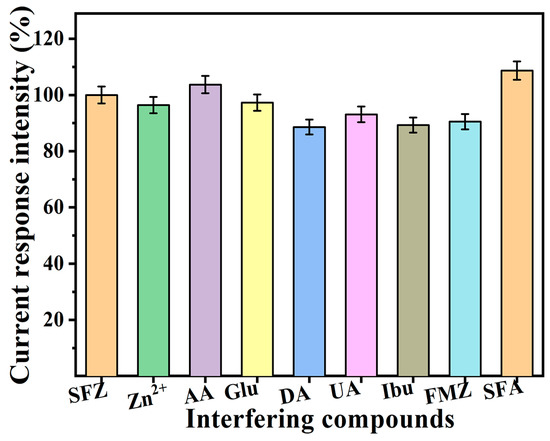
Figure 12.
The current response intensity of the SmV/COFTDBA-TTL/GCE modified electrode to SFZ after the interference substance is added: In PB, containing 5.00 μM SFZ (5.00 mM, pH = 7.0), 50 times metal ions (zinc ions, Zn2+), biological molecules (ascorbic acid, AA), uric acid (UA), dopamine (DA), glucose (Glu)), and other antibiotic drugs (ibuprofen (Ibu), fluomethyl azole (FMZ) and sulfamoxazole (SFA)) were added, respectively, and SWVwas used to monitor changes in the current response of SFZ.
3.5. Analysis of Real Samples
To evaluate the practical electrochemical sensing feasibility for SFZ, tilapia was purchased from the local aquatic product market retail store in Guangzhou as the actual detection sample. The tilapia muscle was homogenized by a wall breaker filtered through 0.2 μm filter paper to remove impurities (considering that the filter paper may have a certain adsorption effect on SFZ, the initial 10.0 mL solution was not collected). The filtered sample and buffer solution were mixed a ratio of 1:4, and the content of SFZ was detected by SmV/COFTDBA-TTL/GCE modified electrode. Furthermore, 5.00 μM SFZ was added to the annealed sample, and a spike recovery experiment was performed. For the SmV/COFTDBA-TTL/GCE modified electrode, the SWV results showed that no SFZ existed, indicating that the detected samples lacked the target analyte or that the concentration was too low. The standard addition method was used to explore the recovery rate range, and the recovery rate of the spiked samples was between 89.8% and 110%, demonstrating that electrochemical sensing has potential application value in the detection of actual samples (Table 2).

Table 2.
Analytical results of the SmV/COFTDBA-TTL/GCE-modified electrode in fish muscles.
4. Conclusions
In this work, a novel covalent organic framework COFTDBA-TTL was synthesized and doped with rare earth vanadate to prepare SmV/COFTDBA-TTL. By virtue of the synergistic effect generated from the structure of the nanomaterial and the specific feature of vanadate, this electrochemical sensing can realize ultrafast detection (10.0 s) of SFZ in fish muscle. The mesoporous structure of COFs provided a large space for the adsorption of SFZ. Abundant functional groups (C=C, C=N) offered a large number of active sites for the bonding of SFZ. In addition, metal SmV adulteration can reduce the electrode resistance, so that the electrocatalytic activity is increased and further enhances the electronic conductivity of this electrode. The proposed sensor showed excellent sensitivity, selectivity, and low detection limits. It paved the way and supplied the theoretical basis for the application of COF-based sensors to detect environmentally damaging pollutants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11050277/s1, Figure S1: Schematic illustrating preparation of COFTDBA-TTL and the structure of COFTDBA-TTL; Figure S2: (a) The water contact angle (CA) of COFTDBA-TTL. The deviation of measurement of CA is between 133.9 ± 0.002°.; Figure S3: The FT-IR spectra of TTL, TDBA, COFTDBA-TTL, SmV/COFTDBA-TTL; Figure S4: The XPS spectra of COFTDBA-TTL and SmV/COFTDBA-TTL; Figure S5: (a) SWV of GCE, COFTDBA-TTL/GCE, SmV/GCE and SmV/COFTDBA-TTL/GCE modified electrode. The electrolyte was PB (5.00 mM, pH = 7.0) containing 5.00 μM SFZ; Figure S6: (a) Comparison of electrochemical properties of COFTDBA-TTL synthesized at different time (1 day, 2 days, 3 days) to SFZ; (b) Optimization of different SmV (0.0100 M, 0.0500 M, 0.100 M, 0.150 M, 0.200 M) modification amount; (c) The optimization of different modification amount, adding 0 mg/mL, 1 mg/mL, 2 mg/mL, 3 mg/mL, 4 mg/mL, 5 mg/mL SmV/COFTDBA-TTL, respectively; (d) The current response of the SmV/COFTDBA-TTL/GCE modified electrode to SFZ was compared in the range of pH 5.8–8.0. The electrolyte was PB (5.00 mM, pH = 7.0) containing 5.00 μM SFZ. SWV was used to monitor the effects current response of SFZ; Figure S7: (a) Stability of SmV/COFTDBA-TTL/GCE modified electrode; (b) Reproducibility of SmV/COFTDBA-TTL/GCE modified electrode. The electrolyte was PB (5.00 mM, pH = 7.0), and 5.00 μM SFZ was added. SWV was used to monitor the effects current response of SFZ.
Author Contributions
J.H.: Investigation, Data curation and Writing—original draft, Visualization, Investigation, Data curation; Z.L.: Writing—original draft and Data curation; G.C.: Investigation formal analysis, Writing—review and editing, Project administration; J.Q.: Investigation formal analysis, Writing—review and editing, Project administration; F.Z.: Supervision conceptualization, Methodology, Writing—review and editing, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by projects of Guangdong Provincial Key R&D Programme (2020B1111350002), Guangzhou Science and Technology Planning Project (202206010074), the National Nature Science Foundation of China (21737006), the National Nature Science Foundation of China (22076222), the National Nature Science Foundation of China (22036003), and the Nature Science Foundation of Guangdong Province (S2013030013474).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the haihe river basin, china. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Shi, J.; Zhang, X.; Qin, L.; Jiang, Z.; Wang, J.; Li, Y.; Liu, B. Occurrence and bioaccumulation of sulfonamide antibiotics in different fish species from Hangbu-Fengle River, Southeast China. Environ. Sci. Pollut. Res. Int. 2021, 28, 44111–44123. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Deng, Y.; Wang, R.; Wang, F.; Cui, H.H.; Hu, D.Y.; Lu, P. Toxic effects of imidacloprid and sulfoxaflor on Rana nigromaculata tadpoles: Growth, antioxidant indices and thyroid hormone-related endocrine system. Arab. J. Chem. 2023, 6, 104723. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, R.; Song, B.; Yang, Y.; Hu, D.; Xiao, X.; Chen, X.; Lu, P. Enantioselective bioaccumulation and toxicity of rac-sulfoxaflor in zebrafish (Danio rerio). Sci. Total Environ. 2022, 817, 153007. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, L.; Zhang, C.; Zhao, X. Health risks of sulfentrazone exposure during zebrafish embryo-larvae development at environmental concentration. Chemosphere 2022, 288, 132632. [Google Scholar] [CrossRef]
- Lei, Y.; Li, F.; Mortimer, M.; Li, Z.; Peng, B.X.; Li, M.; Guo, L.H.; Zhuang, G. Antibiotics disrupt lipid metabolism in zebrafish (Danio rerio) larvae and 3T3-L1 preadipocytes. Sci. Total Environ. 2023, 858, 159755. [Google Scholar] [CrossRef]
- Velmurugan, S.; Yang, T.C.K.; Chen, S.W.; Chen, J.N. Metal-organic frameworks derived ZnO-Co3O4 pn heterojunction photocatalyst for the photoelectrochemical detection of sulfadiazine. J. Environ. Chem. Eng. 2021, 9, 106169. [Google Scholar] [CrossRef]
- Palmblad, M.; van Eck, N.J.; Bergquist, J. Capillary electrophoresis—A bibliometric analysis. TrAC Trends Anal. Chem. 2023, 159, 116899. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Azcarate, S.M. How chemometrics revives the UV-Vis spectroscopy applications as an analytical sensor for spectralprint (nontargeted) analysis. Chemosensors 2023, 11, 8. [Google Scholar] [CrossRef]
- Krstic, D.D.; Ristivojevic, P.M.; Gasic, U.M.; Lazovic, M.; Aksic, M.M.F.; Milivojevic, J.; Morlock, G.E.; Milojkovic-Opsenica, D.M.; Trifkovic, J.D. Authenticity assessment of cultivated berries via phenolic profiles of seeds. Food Chem. 2023, 402, 134184. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, S.; Ranallo, S.; Ricci, F. Electrochemical cell-free biosensors for antibody detection. Angew. Chem. Int. Ed. Engl. 2023, 62, 134184. [Google Scholar]
- Tsuneishi, C.; Koizumi, Y.; Sueto, R.; Nishiyama, H.; Yasuhara, K.; Yamagishi, T.A.; Ogoshi, T.; Tomita, I.; Inagi, S. The controlled synthesis of pillar 6 arene-based hexagonal cylindrical structures on an electrode surface via electrochemical oxidation. Chem. Commun. 2017, 53, 7454–7456. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, J.; Kang, S.; Sun, H.; Li, M. Hierarchical manipulation of uniform multi-nanoparticles by electrochemical coupling assembly. J. Mater. Chem. C 2015, 3, 5214–5219. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.W.; Wang, J. Graphene aerogel-metal-organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.; Hu, X.; Wang, K.; Zhang, C.; Gyimah, E.; Yakubu, S.; Zhang, Z. Electrochemical immunosensor based on Ag+-dependent CTAB-AuNPs for ultrasensitive detection of sulfamethazine. Biosens. Bioelectron. 2019, 144, 11643. [Google Scholar] [CrossRef]
- Jiang, S.; Geng, Y.X.; Liu, W.J.; Wang, Z.Y.; Zhang, C.Y. Construction of a phos-tag-directed self-assembled fluorescent magnetobiosensor for the simultaneous detection of multiple protein kinases. J. Mater. Chem. B 2022, 10, 9992–10000. [Google Scholar] [CrossRef]
- Sarabaegi, M.; Roushani, M.; Hosseini, H. Hollow carbon nanocapsules-based nitrogen-doped carbon nanofibers with rosary-like structure as a high surface substrate for impedimetric detection of Pseudomonas aeruginosa. Talanta 2021, 223, 121700. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Zhao, G.; Wu, C. In situ growth of FeOOH nanoparticles on physically-exfoliated graphene nanosheets as high performance H2O2 electrochemical sensor. Sens. Actuators B 2020, 313, 128038. [Google Scholar] [CrossRef]
- Rydzek, G.; Toulemon, D.; Garofalo, A.; Leuvrey, C.; Dayen, J.F.; Felder-Flesch, D.; Schaaf, P.; Jierry, L.; Begin-Colin, S.; Pichon, B.P.; et al. Selective nanotrench filling by one-pot electroclick self-constructed nanoparticle films. Small 2015, 11, 4638–4642. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-San-Miguel, D.; Montoro, C.; Zamora, F. Covalent organic framework nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2020, 49, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.H.; Huang, Y.Y.; Shen, X.F.; Wang, Y.Y. Electro-enhanced solid-phase microextraction with covalent organic framework modified stainless steel fiber for efficient adsorption of bisphenol A. Anal. Chim. Acta 2021, 1142, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, L.; Xie, Y.; Du, Y.; Song, Y.; Wang, L. Double signal ratiometric electrochemical riboflavin sensor based on macroporous carbon/electroactive thionine-contained covalent organic framework. J. Colloid Interface Sci. 2021, 162, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. An electrochemical sensor for sensitive detection of dopamine based on a COF/Pt/MWCNT-COOH nanocomposite. Chem. Commun. 2022, 58, 6092–6095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Liu, C.; Zhang, C.; Cao, L.; Kong, D.; Wang, W.; Chen, S. Effect of covalent organic framework modified graphene oxide on anticorrosion and self-healing properties of epoxy resin coatings. J. Colloid Interface Sci. 2022, 608, 1025–1039. [Google Scholar] [CrossRef]
- Yang, X.; Lin, C.; Han, D.; Li, G.; Huang, C.; Liu, J.; Wu, X.; Zhai, L.; Mi, L. In situ construction of redox-active covalent organic frameworks/carbon nanotube composites as anodes for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 3989–3995. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, L.; Wang, Q.; Zhang, L.; Zhu, P.; Yu, J.; Zhang, Y. Porphyrin-based covalent organic framework thin films as cathodic materials for “on-off-on” photoelectrochemical sensing of lead ions. ACS Appl. Mater. Interfaces 2021, 13, 20397–20404. [Google Scholar] [CrossRef]
- Adijanto, L.; Padmanabhan, V.B.; Holmes, K.J.; Gorte, R.J.; Vohs, J.M. Physical and electrochemical properties of alkaline earth doped, rare earth vanadates. J. Solid State Chem. 2012, 190, 12–17. [Google Scholar] [CrossRef]
- Alkorbi, A.S.; Kumar, K.Y.; Prashanth, M.K.; Parashuram, L.; Abate, A.; Alharti, F.A.; Jeon, B.H.; Raghu, M.S. Samarium vanadate affixed sulfur self doped g-C3N4 heterojunction; photocatalytic, photoelectrocatalytic hydrogen evolution and dye degradation. Int. J. Hydrogen Energy 2022, 47, 12988–13003. [Google Scholar] [CrossRef]
- Li, Q.; Zuo, W.; Li, F. Chelating ligand-mediated hydrothermal synthesis of samarium orthovanadate with decavanadate as vanadium source. Sci. World J. 2013, 2013, 127816. [Google Scholar] [CrossRef] [PubMed]
- Gallis, D.F.S.; Vogel, D.J.; Vincent, G.A.; Rimsza, J.M.; Nenoff, T.M. NOx adsorption and optical detection in rare earth metal-organic frameworks. ACS Appl. Mater. Interfaces 2019, 11, 43270–43277. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, L.M.; Jin, C.Y.; Yan, B. Luminescence responsive composites of rare earth metal-organic frameworks covalently linking microsphere resin. Dyes Pigments 2020, 173, 107883. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Lima Moura, S.; Ali, F.H.M.; Moselhy, W.A.; Taboada Sotomayor, M.d.P.; Isabel Pividori, M. Electrochemical sensing of methyl parathion on magnetic molecular. Biosens. Bioelectron. 2018, 118, 181–187. [Google Scholar] [CrossRef]
- Babulal, S.M.; Koventhan, C.; Chen, S.M.; Hung, W. Construction of sphere like samarium vanadate nanoparticles anchored graphene nanosheets for enhanced electrochemical detection of nitrofurantoin in biological fluids. Compos. Part B 2022, 237, 109847. [Google Scholar] [CrossRef]
- Nataraj, N.; Chen, S.M. Samarium vanadate nanospheres integrated carbon nanofiber composite as an efficient electrocatalyst for antituberculosis drug detection in real samples. Colloids Surf. 2021, 617, 126385. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Tian, J.; Bu, F.; Zhao, T.; Liu, M.; Lin, R.; Zhang, F.; Lee, M.; Zhao, D.; et al. Imparting multi-functionality to covalent organic framework nanoparticles by the dual-ligand assistant encapsulation strategy. Nat. Commun. 2021, 12, 4556. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Royuela, S.; Mar Ramos, M. Post-synthetic modification of covalent organic frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef]
- Li, T.; Zhao, L.; He, Y.; Cai, J.; Luo, M.; Lin, J. Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation. Appl. Catal. B 2013, 129, 255–263. [Google Scholar] [CrossRef]
- Wang, J.; Qu, X.; Zhao, L.; Yan, B. Fabricating nanosheets and ratiometric detection of 5-fluorouracil by covalent organic framework hybrid material. Anal. Chem. 2021, 93, 4308–4316. [Google Scholar] [CrossRef]
- Liang, H.; Wang, L.; Yang, Y.; Song, Y.; Wang, L. A novel biosensor based on multienzyme microcapsules constructed from covalent-organic framework. Biosens. Bioelectron. 2021, 193, 113553. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, Z.; Liao, J.; Li, G. Chemical bonding approach for fabrication of hybrid magnetic metal-organic framework-5: High efficient adsorbents for magnetic enrichment of trace analytes. Anal. Chem. 2013, 85, 6885–6893. [Google Scholar] [CrossRef] [PubMed]
- Mutharani, B.; Chen, T.W.; Chen, S.M.; Liu, X. Reversibly switchable ruthenium hybrid thermo-responsive electrocatalyst-based voltammetric sensor for sensitive detection of sulfamethazine in milk samples. Sens. Actuators B 2020, 316, 128103. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Almutairi, G.; Chen, S.M.; Chen, T.W.; Ahmed, F.; Arshi, N.; AlOtaibi, B. Design and in situ synthesis of titanium carbide/boron nitride nanocomposite: Investigation of electrocatalytic activity for the sulfadiazine senso. ACS Sustain. Chem. Eng. 2021, 9, 2784–2794. [Google Scholar] [CrossRef]
- Karazan, Z.M.; Roushani, M. A new method for electrochemical determination of Hippuric acid based on molecularly imprinted copolymer. Talanta 2022, 246, 123491. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical sensors and their applications: A review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Dascalescu, D.; Apetrei, C. Nanomaterials based electrochemical sensors for serotonin detection: A review. Chemosensors 2021, 9, 14. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, S. Electrochemical sensors for antibiotic susceptibility testing: Strategies and applications. Chemosensors 2022, 10, 53. [Google Scholar] [CrossRef]
- Nehru, R.; Dong, C.D.; Chen, C.W. Cobalt-doped Fe3O4 nanospheres deposited on graphene oxide as electrode materials for electrochemical sensing of the antibiotic drug. ACS Appl. Nano Mater. 2021, 4, 6768–6777. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Waterhouse, G.I.N.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B 2019, 300, 126993. [Google Scholar] [CrossRef]
- Baby, J.N.; Sriram, B.; Wang, S.F.; George, M. Integration of samarium vanadate/carbon nanofiber through synergy: An electrochemical tool for sulfadiazine analysis. J. Hazard. Mater. 2021, 408, 124940. [Google Scholar] [CrossRef] [PubMed]
- Afsharipour, R.; Shabani, A.M.H.; Dadfarnia, S.; Kazemi, E. Selective fluorometric determination of sulfadiazine based on the growth of silver nanoparticles on graphene quantum dots. Microchim. Acta 2020, 187, 54. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, A.K.; Muthukutty, B.; Chen, S.M.; Sivakumar, M.; Chen, S.H. Intermetallic ompound Cu2Sb nanoparticles for effective electrocatalytic oxidation of an antibiotic drug: Sulphadiazine. ACS Sustain. Chem. Eng. 2020, 8, 17718–17726. [Google Scholar] [CrossRef]
- You, H.; Bai, L.; Yuan, Y.; Zhou, J.; Bai, Y.; Mu, Z. An amperometric aptasensor for ultrasensitive detection of sulfadimethoxine based on exonuclease-assisted target recycling and new signal tracer for amplification. Biosens. Bioelectron. 2018, 117, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; Hu, H.; Qu, K.; Cui, Z. A low-cost electrochemical method for the determination of sulfadiazine in aquaculture wastewater. Int. J. Environ. Res. Public Health 2022, 19, 16945. [Google Scholar] [CrossRef]
- Srinithi, S.; Arumugam, B.; Chen, S.M.; Annamalai, S.; Ramaraj, S.K. Synthesis and characterization of pyrochlore-type lanthanum cerate nanoparticles: Electrochemical determination of antibiotic drug sulfadiazine in biological and environmental samples. Mater. Chem. Phys. 2023, 296, 127244. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, G.; Lu, N.; Yuan, X.; Li, B. A miniaturized electrochemical toxicity biosensor based on graphene oxide quantum dots/carboxylated carbon nanotubes for assessment of priority pollutants. J. Hazard. Mater. 2017, 324, 272–280. [Google Scholar] [CrossRef]
- Shi, S.; Reisberg, S.; Anquetin, G.; Noel, V.; Pham, M.C.; Piro, B. General approach for electrochemical detection of persistent pharmaceutical micropollutants: Application to acetaminophen. Biosens. Bioelectron. 2015, 72, 205–210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).