Preparation of Reduced Graphene Oxide Sheets with Large Surface Area and Porous Structure for High-Sensitivity Humidity Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
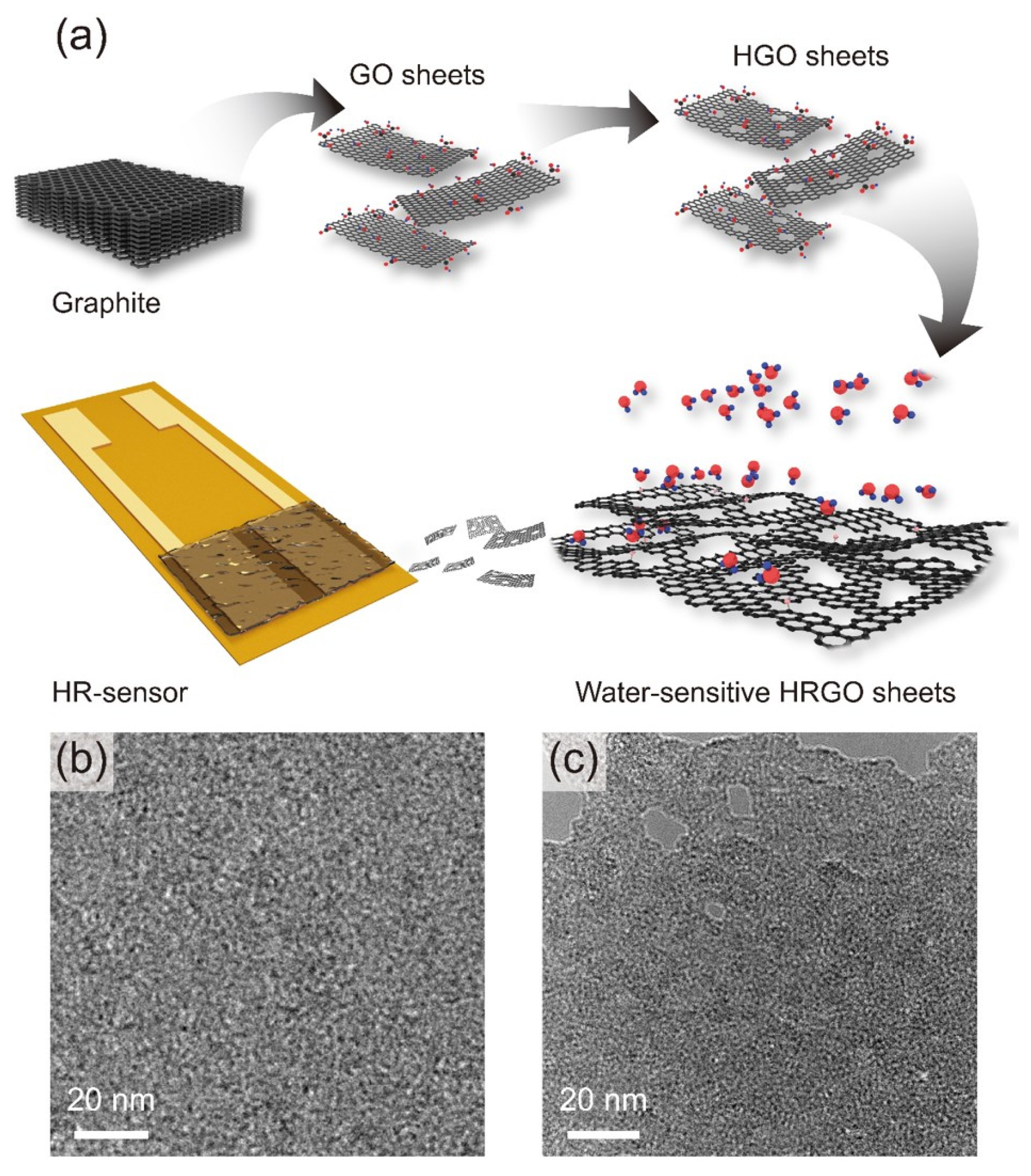
2.2. Preparation and Characterization of HRGO
2.3. Fabrication and Characterization of HR-Sensor
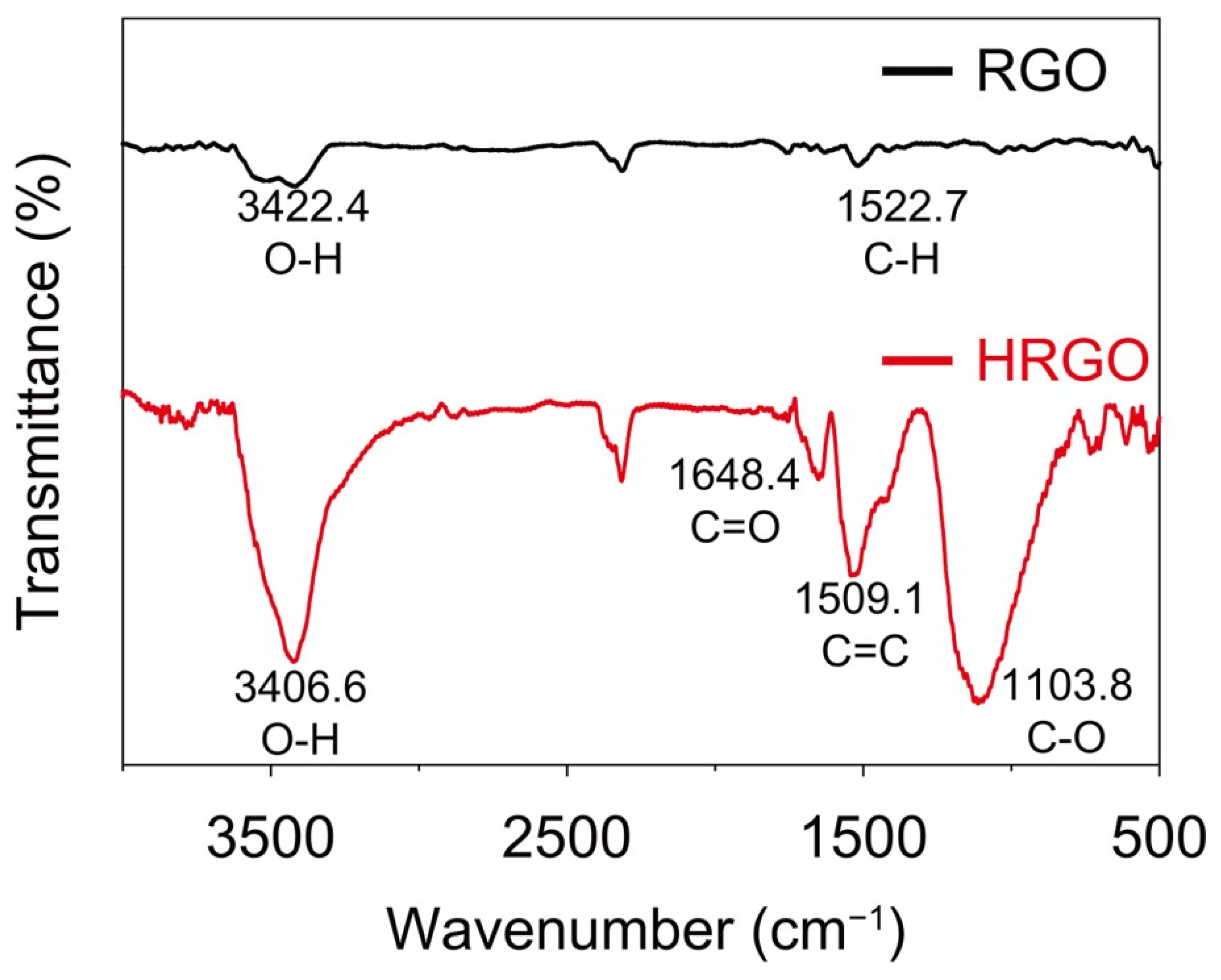
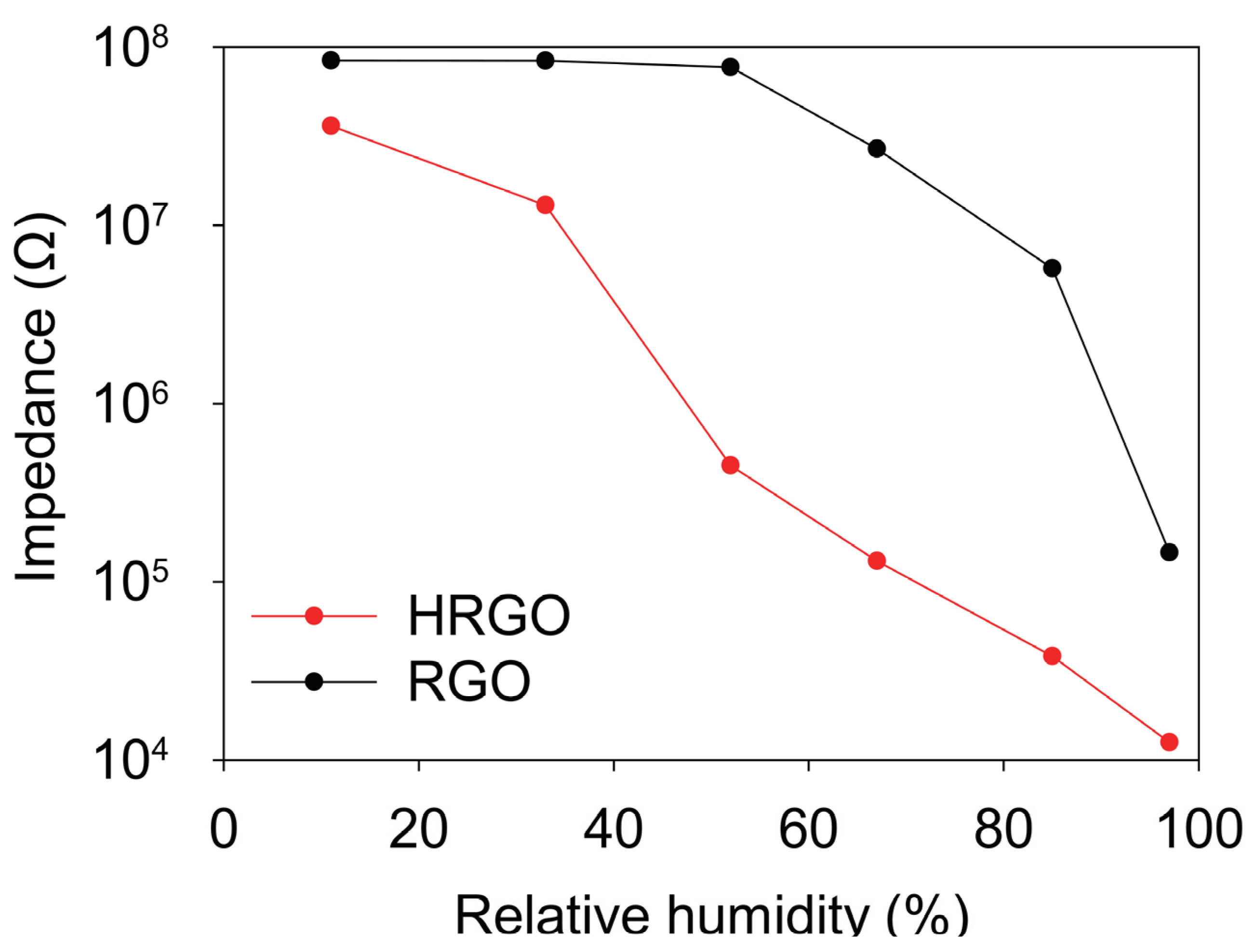
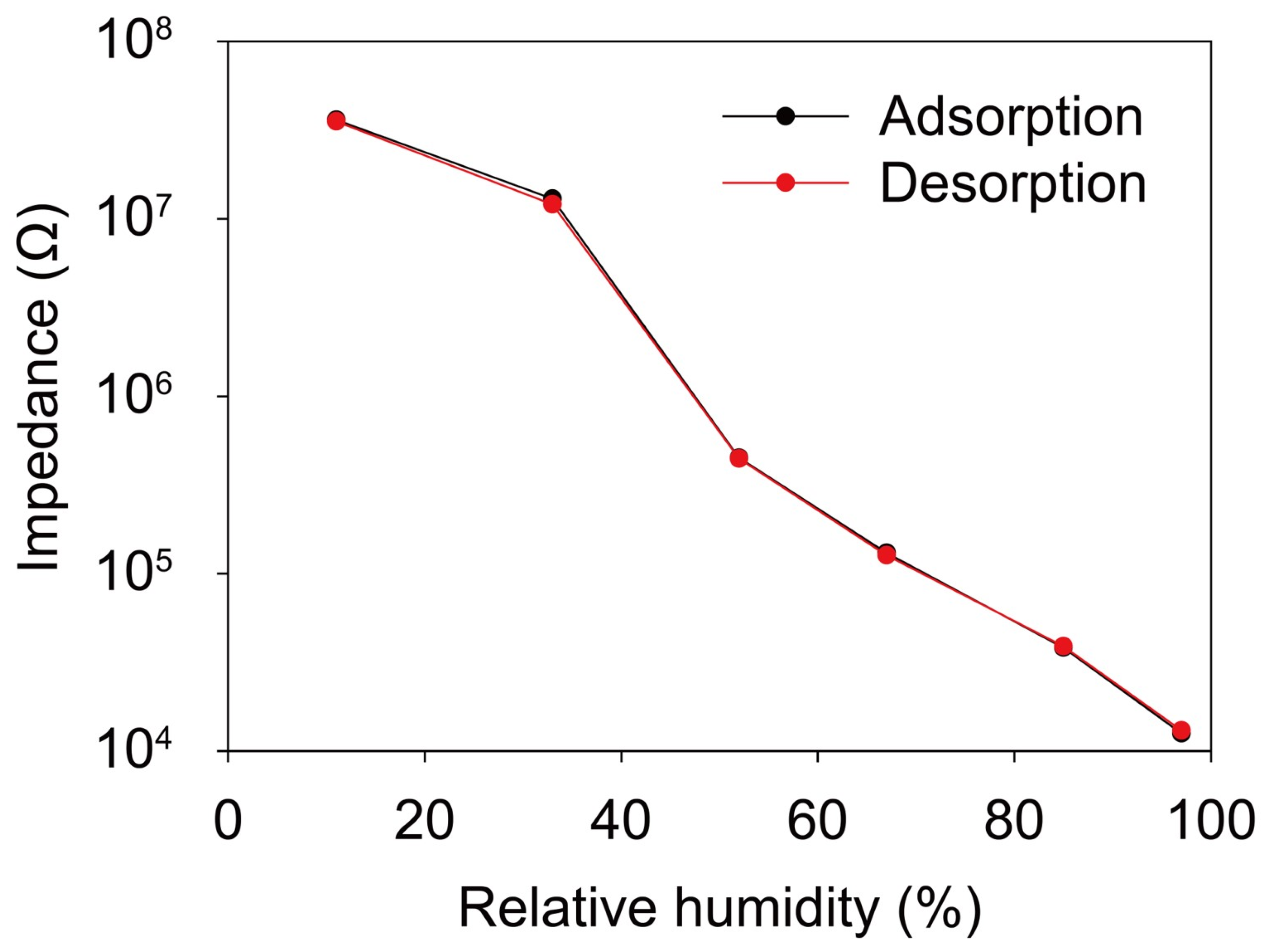
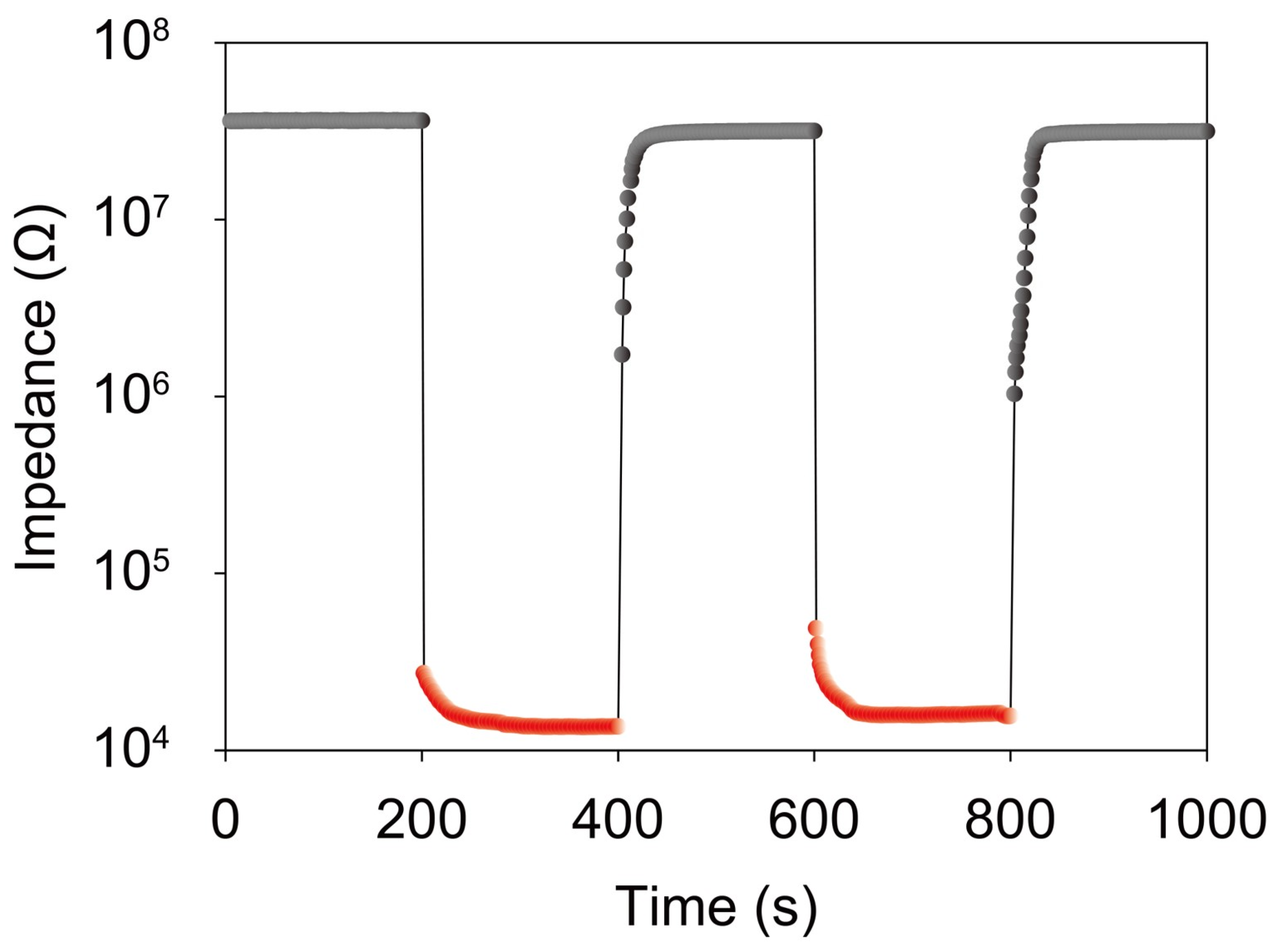
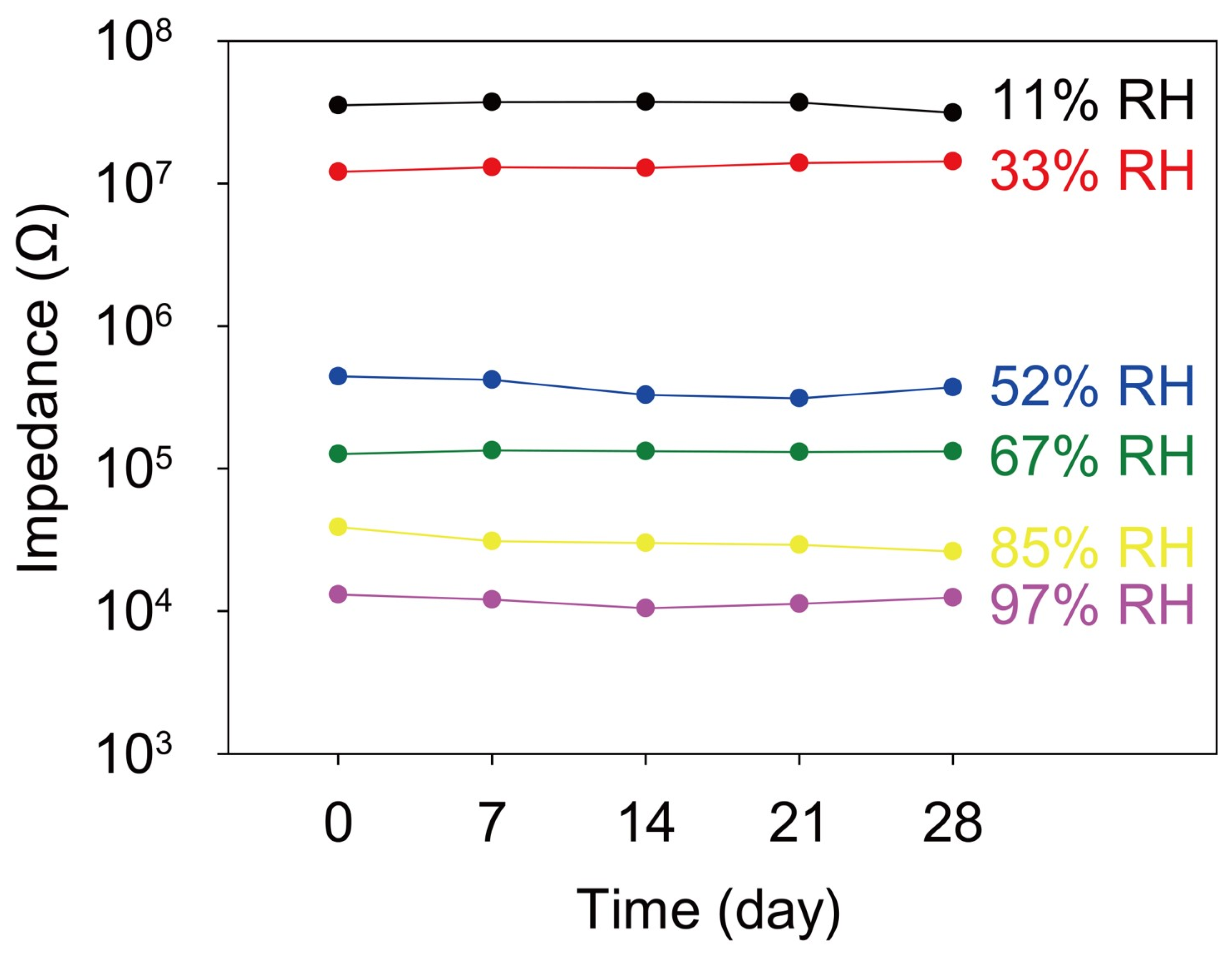
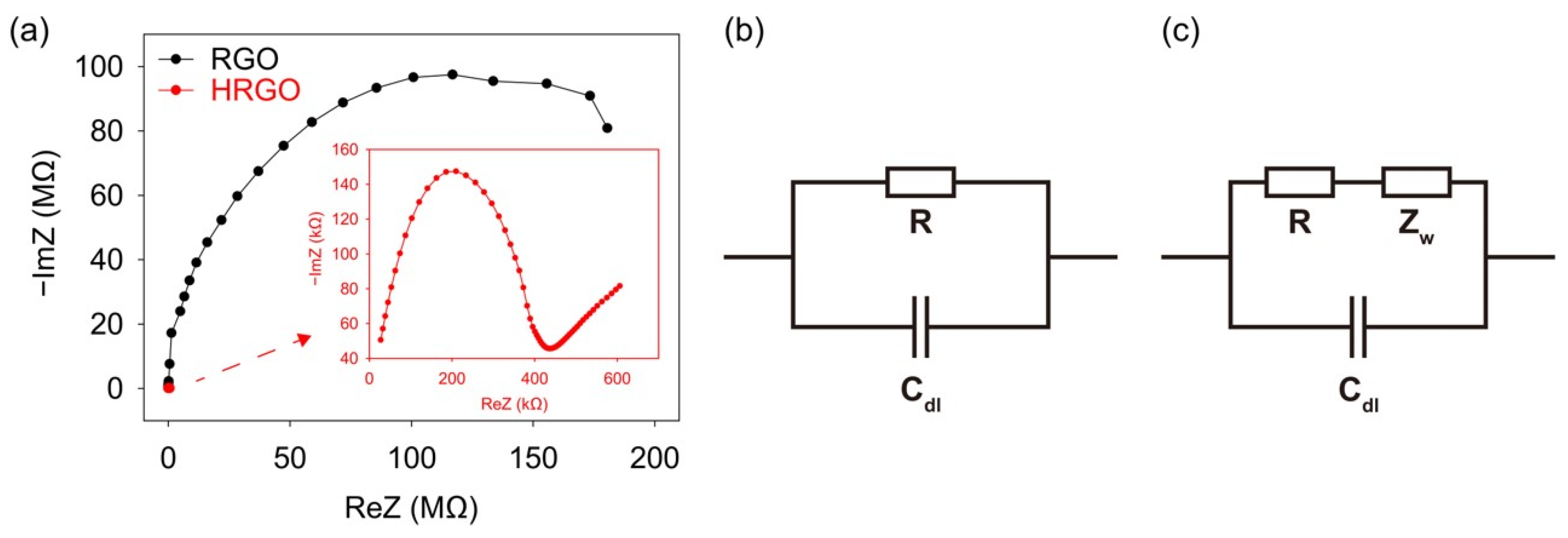
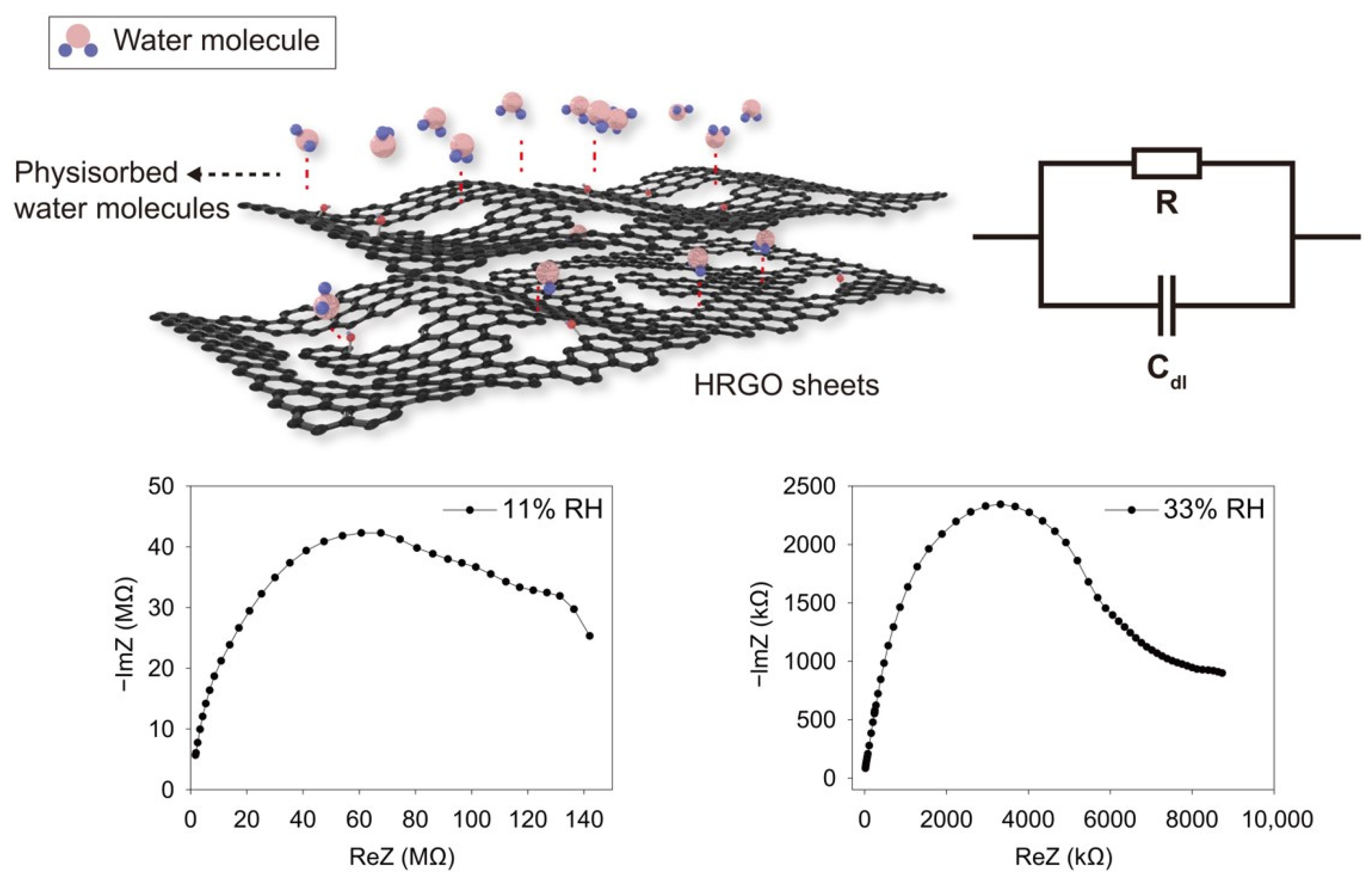
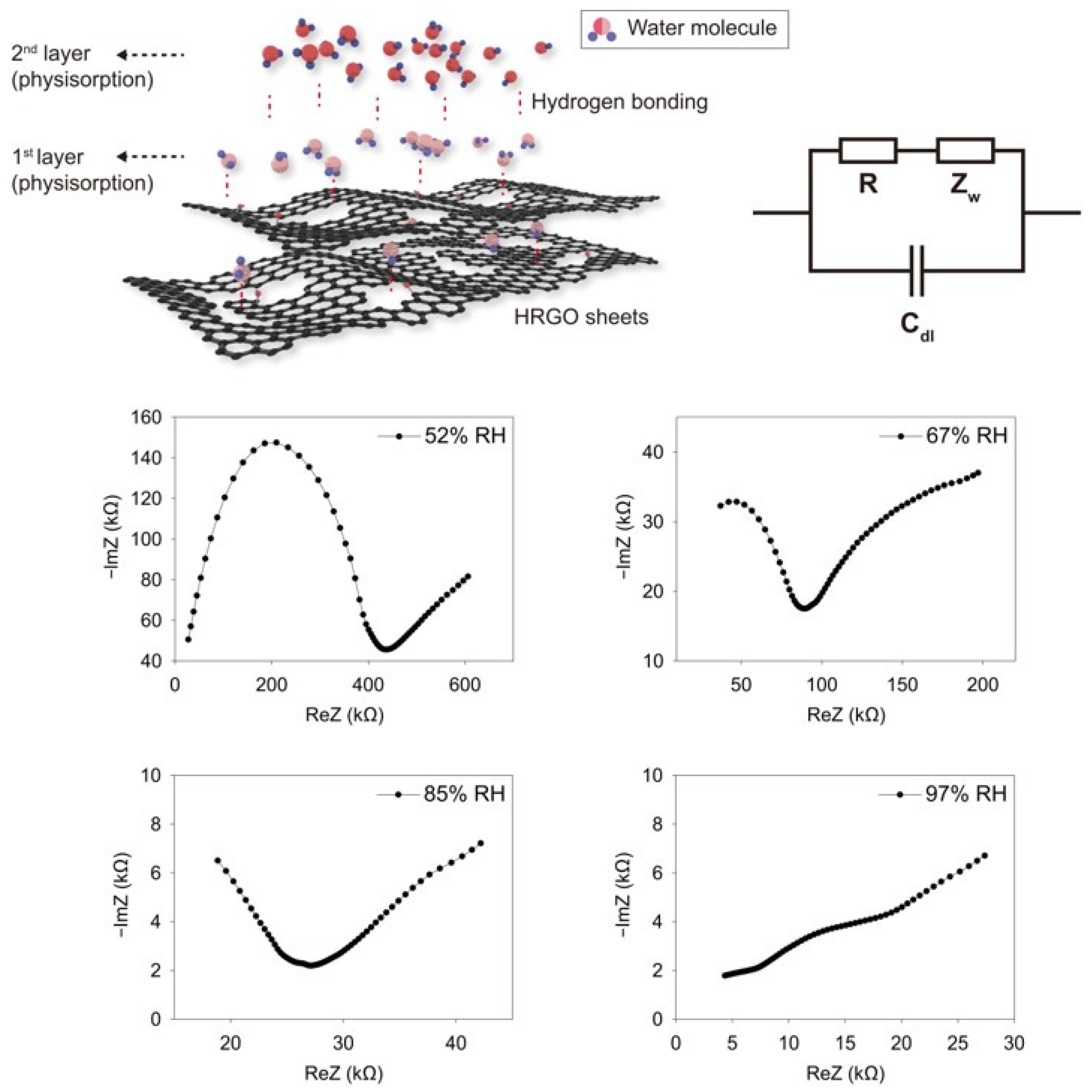
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic self-assembly enabled flexible paper-based humidity sensor with high sensitivity and superior durability. Chem. Eng. J. 2021, 404, 127105. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, G.; Shen, Y.; Yang, H.; Xu, K. Multifunctional Flexible Humidity Sensor Systems Towards Noncontact Wearable Electronics. Nano-Micro Lett. 2022, 14, 150. [Google Scholar] [CrossRef]
- Wu, J.; Yin, C.; Zhou, J.; Li, H.; Liu, Y.; Shen, Y.; Garner, S.; Fu, Y.; Duan, H. Ultra-thin Glass Based Flexible, Transparent, and Ultra-sensitive Surface Acoustic Wave Humidity Sensor with ZnO Nanowires and Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 39817–39825. [Google Scholar] [CrossRef]
- Li, W.; Huang, L.; Sun, H.; Wang, B.; Lu, Q.; Liang, X.; Liu, F.; Liu, F.; Sun, P.; Lu, G. Room temperature hydrogen sensor based on Nafion and Pd/CF sensing electrode. Sens. Actuator B Chem. 2022, 366, 132014. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Li, Y.; Yang, M. Single-sided and integrated polyaniline/poly(vinylidene fluoride) flexible membrane with micro/nanostructures as breathable, nontoxic and fast response wearable humidity sensor. J. Colloid Interface Sci. 2022, 607, 367–377. [Google Scholar] [CrossRef]
- Xu, L.; Zhai, H.; Chen, X.; Liu, Y.; Wang, M.; Liu, Z.; Umar, M.; Ji, C.; Chen, Z.; Jin, L.; et al. Coolmax/graphene-oxide functionalized textile humidity sensor with ultrafast response for human activities monitoring. Chem. Eng. J. 2021, 412, 128639. [Google Scholar] [CrossRef]
- Adib, M.R.; Lee, Y.; Kondalkar, V.V.; Kim, S.; Lee, K. A Highly Sensitive and Stable rGO:MoS2-Based Chemiresistive Humidity Sensor Directly Insertable to Transformer Insulating Oil Analyzed by Customized Electronic Sensor Interface. ACS Sens. 2021, 6, 1012–1021. [Google Scholar] [CrossRef]
- Guan, X.; Yu, Y.; Hou, Z.; Wu, K.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. A flexible humidity sensor based on self-supported polymer film. Sens. Actuators B Chem. 2022, 358, 131438. [Google Scholar] [CrossRef]
- Chani, M.T.S.; Karimov, K.S.; Khan, S.B.; Fatima, N.; Asiri, A.M. Impedimetric humidity and temperature sensing properties of chitosan-CuMn2O4 spinel nanocomposite. Ceram. Int. 2019, 45, 10565–10571. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Chen, M.; Xia, F. Research Advances in Microfiber Humidity Sensors. Small 2018, 14, 1800524. [Google Scholar] [CrossRef]
- Bao, W.; Chen, F.; Lai, H.; Liu, S.; Wang, Y. Wearable breath monitoring based on a flexible fiber-optic humidity sensor. Sens. Actuators B Chem. 2021, 349, 130794. [Google Scholar] [CrossRef]
- Le, X.; Liu, Y.; Peng, L.; Pang, J.; Xu, Z.; Gao, C.; Xie, J. Surface acoustic wave humidity sensors based on uniform and thickness controllable graphene oxide thin films formed by surface tension. Microsyst. Nanoeng. 2019, 5, 36. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic wave humidity sensor based on three-dimensional architecture graphene/PVA/SiO2 and its application for respiration monitoring. Sens. Actuators B Chem. 2020, 308, 127693. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Wang, D.; Li, T.; Song, X.; Kang, Z. A humidity sensing and respiratory monitoring system constructed from quartz crystal microbalance sensors based on a chitosan/polypyrrole composite film. J. Mater. Chem. A 2021, 9, 14524–14533. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, N.; Huang, X.; Yao, Y.; Jin, Y.; Pan, W.; Liu, D. Humidity-Sensing Properties of a BiOCl-Coated Quartz Crystal Microbalance. ACS Omega 2020, 5, 18818–18825. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Sun, X.; Wu, Y.; Wang, L.; Meng, G.; Kuang, D.; Guo, X.; Qu, W.; Du, B.; et al. An excellent impedance-type humidity sensor based on halide perovskite CsPbBr3 nanoparticles for human respiration monitoring. Sens. Actuators B Chem. 2021, 337, 129772. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, X.; Guo, X.; Ding, Y.; Ou, Y.; Yang, H.; Chen, Y.; Hu, Y.; Kuang, D.; Zhao, C.; et al. Development of a rGO-BiVO4 Heterojunction Humidity Sensor with Boosted Performance. ACS Appl. Mater. Interfaces 2021, 13, 27188–27199. [Google Scholar] [CrossRef]
- Ma, L.; Wu, R.; Patil, A.; Zhu, S.; Meng, Z.; Meng, H.; Hou, C.; Zhang, Y.; Liu, Q.; Yu, R.; et al. Full-Textile Wireless Flexible Humidity Sensor for Human Physiological Monitoring. Adv. Funct. Mater. 2019, 29, 1904549. [Google Scholar] [CrossRef]
- Kuzubasoglu, B.A. Recent Studies on the Humidity Sensor: A Mini Review. ACS Appl. Electron. Mater. 2022, 4, 4797–4807. [Google Scholar] [CrossRef]
- Si, R.; Xie, X.; Li, T.; Zheng, J.; Cheng, C.; Huang, S.; Wang, C. TiO2/(K,Na)NbO3 Nanocomposite for Boosting Humidity-Sensing Performances. ACS Sens. 2020, 5, 1345–1353. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Li, P.; Yang, Z.; Mi, Q.; Yu, L. Electrospinning of Flexible Poly(vinyl alcohol)/MXene Nanofiber-Based Humidity Sensor Self-Powered by Monolayer Molybdenum Diselenide Piezoelectric Nanogenerator. Nano-Micro Lett. 2021, 13, 57. [Google Scholar] [CrossRef]
- Tahir, M.; Li, L.; He, L.; Xiang, Z.; Ma, Z.; Haider, W.A.; Liao, X.; Song, Y. Interdigital MnO2/PEDOT Alternating Stacked Microelectrodes for High-Performance On-Chip Microsupercapacitor and Humidity Sensing. Energy Environ. Mater. 2022, e12546. [Google Scholar] [CrossRef]
- Li, P.; Yu, S.; Zhang, H. Preparation and Performance Analysis of Ag/ZnO Humidity Sensor. Sensors 2021, 21, 857. [Google Scholar] [CrossRef]
- Gong, L.; Fu, H.; Liu, L.; Li, Z.; Guo, J.; Cao, Z.; Yao, J. Construction and Performance of a Nanocellulose–Graphene-Based Humidity Sensor with a Fast Response and Excellent Stability. ACS Appl. Polym. Mater. 2022, 4, 3656–3666. [Google Scholar] [CrossRef]
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A. Organic Thin-Film Capacitive and Resistive Humidity Sensors: A Focus Review. Adv. Mater. Interfaces 2018, 5, 1800969. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.; Ha, T. Wearable humidity sensors based on bar-printed poly(ionic liquid) for real-time humidity monitoring systems. Sens. Actuators B Chem. 2022, 354, 131248. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Zhao, Q.; Huang, Q.; Wang, S.; Zhang, Y.; Wu, Y.; Liu, B.; Zhen, Y.; Tai, H. Daily writing carbon ink: Novel application on humidity sensor with wide detection range, low detection limit and high detection resolution. Sens. Actuators B Chem. 2021, 339, 129884. [Google Scholar] [CrossRef]
- Cai, B.; Yin, H.; Huo, T.; Ma, J.; Di, Z.; Li, M.; Hu, N.; Yong, Z.; Zhang, Y.; Su, Y. Semiconducting single-walled carbon nanotube/graphene van der Waals junctions for highly sensitive all-carbon hybrid humidity sensors. J. Mater. Chem. C 2020, 8, 3386–3394. [Google Scholar] [CrossRef]
- He, Z.; Zhou, G.; Oh, Y.; Jung, B.; Um, M.; Lee, S.; Song, J.I.; Byun, J.; Chou, T. Ultrafast, highly sensitive, flexible textile-based humidity sensors made of nanocomposite filaments. Mater. Today Nano 2022, 18, 100214. [Google Scholar] [CrossRef]
- Rauf, S.; Vijjapu, M.T.; Andrés, M.A.; Gascón, I.; Roubeau, O.; Eddaoudi, M.; Salama, K.N. Highly Selective Metal–Organic Framework Textile Humidity Sensor. ACS Appl. Mater. Interfaces 2020, 12, 29999–30006. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, K.; Yang, M.; Tang, S.; Yang, T.; Fujita, Y.; Honda, S.; Arie, T.; Akita, S.; Chueh, Y.; et al. Highly stable Pd/HNb3O8-based flexible humidity sensor for perdurable wireless wearable applications. Nanoscale Horiz. 2021, 6, 260–270. [Google Scholar] [CrossRef]
- Kalsoom, U.; Waheed, S.; Paull, B. Fabrication of Humidity Sensor Using 3D Printable Polymer Composite Containing Boron-Doped Diamonds and LiCl. ACS Appl. Mater. Interfaces 2020, 12, 4962–4969. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Qi, D.; Zhao, C. High sensitive and fast response humidity sensor based on polymer composite nanofibers for breath monitoring and non-contact sensing. Sens. Actuators B Chem. 2021, 330, 129239. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon Nanocoil-Based Fast-Response and Flexible Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Ding, H.; Wei, Y.; Wu, Z.; Tao, K.; Ding, M.; Xie, X.; Wu, J. Recent Advances in Gas and Humidity Sensors Based on 3D Structured and Porous Graphene and Its Derivatives. ACS Materials Lett. 2020, 2, 1381–1411. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat Rev Mater 2017, 2, 17046. [Google Scholar] [CrossRef]
- Alonso, E.T.; Shin, D.; Rajan, G.; Neves, A.I.S.; Russo, S.; Craciun, M.F. Water-Based Solution Processing and Wafer-Scale Integration of All-Graphene Humidity Sensors. Adv. Sci. 2019, 6, 1802318. [Google Scholar] [CrossRef]
- Zhu, C.; Tao, L.; Wang, Y.; Zheng, K.; Yu, J.; Xiandong, L.; Chen, X.; Huang, Y. Graphene oxide humidity sensor with laser-induced graphene porous electrodes. Sens. Actuators B Chem. 2020, 325, 128790–128798. [Google Scholar] [CrossRef]
- Lan, L.; Le, X.; Dong, H.; Xie, J.; Ying, Y.; Ping, J. One-step and large-scale fabrication of flexible and wearable humidity sensor based on laser-induced graphene for real-time tracking of plant transpiration at bio-interface. Biosens. Bioelectron. 2020, 165, 112360–112366. [Google Scholar] [CrossRef]
- Han, D.; Zhang, Y.; Ma, J.; Liu, Y.; Mao, J.; Han, C.; Jiang, K.; Zhao, H.; Zhang, T.; Xu, H.; et al. Sunlight-reduced graphene oxides as sensitive moisture sensors for smart device design. Adv. Mater. Technol. 2017, 2, 1700045–1700053. [Google Scholar] [CrossRef]
- Su, P.; Chiou, C. Electrical and humidity-sensing properties of reduced graphene oxide thin film fabricated by layer-by-layer with covalent anchoring on flexible substrate. Sens. Actuators B Chem. 2014, 200, 9–18. [Google Scholar] [CrossRef]
- Strzelczyk, R.; Giusca, C.E.; Perrozzi, F.; Fioravanti, G.; Ottaviano, L.; Kazakova, O. Role of substrate on interaction of water molecules with graphene oxide and reduced graphene oxide. Carbon 2017, 122, 168–175. [Google Scholar] [CrossRef]
- Anichini, C.; Aliprandi, A.; Gali, S.M.; Liscio, F.; Morandi, V.; Minoia, A.; Beljonne, D.; Ciesielski, A.; Samorì, P. Ultrafast and highly sensitive chemically functionalized graphene oxide based humidity sensors: Harnessing the device performances via the supramolecular approach. ACS Appl. Mater. Interfaces 2020, 12, 44017–44025. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent Advances in Graphene-Based Humidity Sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Guo, F.; Bao, Y.; Mao, J.; Xie, Y.; Huang, J.; Hu, X.; Lai, Y. Carboxyl Graphene Oxide Functionalized Cotton Textile with Superwettable Patterns for Humidity Sensing. ACS Appl. Nano Mater. 2023, 6, 261–269. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Tai, H. Recent advances in humidity sensors for human body related humidity detection. J. Mater. Chem. C 2021, 9, 14963–14980. [Google Scholar] [CrossRef]
- Falk, M.; Miller, A.G. Infrared spectrum of carbon dioxide in aqueous solution. Vib. Spectrosc. 1992, 4, 105–108. [Google Scholar] [CrossRef]
- Naghani, M.E.; Neghabi, M.; Zadsar, M.; Ahangar, H.A. Synthesis and characterization of linear/nonlinear optical properties of graphene oxide and reduced graphene oxide-based zinc oxide nanocomposite. Sci. Rep. 2023, 13, 1496. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Victoria, S.N.; Ramanathan, S. Effect of potential drifts and ac amplitude on the electrochemical impedance spectra. Electrochim. Acta 2011, 56, 2606–2615. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, C.; Chen, B.; Sun, H. Enhanced humidity sensing of functionalized reduced graphene oxide with 4-chloro-3-sulfophenylazo groups. Sens. Actuators B Chem. 2019, 287, 258–266. [Google Scholar] [CrossRef]
- Zou, S.; Tao, L.; Wang, G.; Zhu, C.; Peng, Z.; Sun, H.; Li, Y.; Wei, Y.; Ren, T. Humidity-Based Human–Machine Interaction System for Healthcare Applications. ACS Appl. Mater. Interfaces 2022, 14, 12606–12616. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Z.; Wang, Q.; Liu, R.; Xu, Y.; Wang, K.; Shi, X.; Ye, S. Surface Engineering on Polyimide–Silver Films in Low-Cost, Flexible Humidity Sensors. ACS Appl. Mater. Interfaces 2022, 14, 16621–16630. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Lee, S.Y.; Sohn, W.; Lee, J.E.; Kim, D.H.; Shim, Y.; Kwon, K.C.; Choi, K.S.; Yoo, H.J.; et al. Highly selective and sensitive chemoresistive humidity sensors based on rGO/MoS2 van der Waals composites. J. Mater. Chem. A 2018, 6, 5016–5024. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Ding, X.; Chen, X.; Yu, X.; Zhao, X. High-sensitivity and low-hysteresis humidity sensor based on hydrothermally reduced graphene oxide/nanodiamond. Sens. Actuators B Chem. 2019, 283, 761–768. [Google Scholar] [CrossRef]
- Li, C.; Chong, M.; Zhang, L.; Tang, B.; Bie, L. All-inorganic lead-free halide perovskite Cs2TeBr6 enables real-time touchless human breath and finger related humidity monitoring. Sens. Actuators B Chem. 2023, 379, 133240. [Google Scholar] [CrossRef]
- Trachioti, M.G.; Prodromidis, M.I. Humidity impedimetric sensor based on vanadium pentoxide xerogel modified screen−printed graphite electrochemical cell. Talanta 2020, 216, 121003. [Google Scholar] [CrossRef]
- Choi, S.; Yu, H.; Jang, J.; Kim, M.; Kim, S.; Jeong, H.S.; Kim, I. Nitrogen-doped single graphene fiber with platinum water dissociation catalyst for wearable humidity sensor. Small 2018, 14, 1703934–1703942. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuators B Chem. 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.H.; Park, S.; Kang, J.; Kim, S.; Choi, Y.; Shin, U. Carbon nanotubes immobilized on gold electrode as an electrochemical humidity sensor. Sens. Actuators B Chem. 2019, 300, 127049. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, Z.; Sun, P.; Chen, H. High-Sensitivity Wearable and Flexible Humidity Sensor Based on Graphene Oxide/Non-Woven Fabric for Respiration Monitoring. Langmuir 2020, 36, 9443–9448. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, N.; Yin, Y.; Xu, B.; Zhang, W.; Wang, C. High-Sensitivity and Low-Hysteresis GO-NH2/Mesoporous SiO2 Nanosphere-Fabric-Based Humidity Sensor for Respiratory Monitoring and Noncontact Sensing. Adv. Mater. Interfaces 2020, 9, 2101498. [Google Scholar] [CrossRef]
- Jlassi, K.; Mallick, S.; Eribi, A.; Chehimi, M.M.; Ahmad, Z.; Touati, F.; Krupa, I. Facile preparation of N-S co-doped graphene quantum dots (GQDs) from graphite waste for efficient humidity sensing. Sens. Actuators B Chem. 2021, 328, 129058. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Liu, X.; Yang, Y.; Wang, X.; Xue, Q. Self-powered multifunctional monitoring and analysis system based on dual-triboelectric nanogenerator and chitosan/activated carbon film humidity sensor. Nano Energy 2022, 94, 106881. [Google Scholar] [CrossRef]
- Jia, G.; Zheng, A.; Wang, X.; Zhang, L.; Li, L.; Li, C.; Zhang, Y.; Cao, L. Flexible, biocompatible and highly conductive MXene-graphene oxide film for smart actuator and humidity sensor. Sens. Actuators B Chem. 2021, 346, 130507. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef]
- Jiang, K.; Zhao, H.; Dai, J.; Kuang, D.; Fei, T.; Zhang, T. Excellent humidity sensor based on LiCl loaded hierarchically porous polymeric microspheres. ACS Appl. Mater. Interfaces. 2016, 38, 25529–25534. [Google Scholar] [CrossRef]



| Sensing Material | Type | Working Range (% RH) | Sensitivity | Hysteresis | Response/Recovery Time (s) | Ref. |
|---|---|---|---|---|---|---|
| GO/LIG | C | 11–97 | 9.15 nF/%RH | 3.3% | 2/49 | [38] |
| GO | C | 10–90 | 3.25 nF/%RH | - | -/15.8 | [39] |
| RGO | I | 11–95 | - | 9% | 16/47 | [40] |
| RGO | I | 30–90 | 0.0423 log Z/%RH | 2.5% | 28/48 | [41] |
| nRGO | R | 6.1–66 | 4.51% | - | - | [59] |
| PDDA/RGO | R | 11–97 | 8.69–37.43% | - | 94/108 | [60] |
| rGO:MoS2 | R | 30–80 | 0.973 kΩ/% RH | Less than 1% | 240/900 | [7] |
| rGO-BiVO4 | I | 11–95 | – | 0.47% | 3.6/18 | [17] |
| rGO-SC | I | 11–95 | – | 4.56% | 1.3/23.5 | [52] |
| CS-MWCNT | R | 30–100 | 10.35 Ω/% RH | 0.30 ± 0.001% | 30/40 | [61] |
| GO/NWF | R | 44–91 | – | – | 8.9/11.76 | [62] |
| rCMGO | R | 0–100 | 0.33 MΩ/% RH | 1.1% | 0.025/0.125 | [43] |
| GO-NH2/mSiO2 | R | 23–97 | 14.6 MΩ/% RH | 2.71% | 12.6/58.5 | [63] |
| GQDs prepared from waste | I | 40–90 | – | 2.2% | 15/55 | [64] |
| CS/AC (Chitosan/Activated carbon) | V | 0–97 | – | – | 22/21 | [65] |
| MXene-GO | R | 3–80 | –0.52x + 50.91 | 5.5% | 5/19 | [66] |
| HRGO | I | 11–97 | −0.04317 log Z/%RH | 2.57% | 3/29 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Park, H.J.; Yoon, E.S.; Choi, B.G. Preparation of Reduced Graphene Oxide Sheets with Large Surface Area and Porous Structure for High-Sensitivity Humidity Sensor. Chemosensors 2023, 11, 276. https://doi.org/10.3390/chemosensors11050276
Kim SJ, Park HJ, Yoon ES, Choi BG. Preparation of Reduced Graphene Oxide Sheets with Large Surface Area and Porous Structure for High-Sensitivity Humidity Sensor. Chemosensors. 2023; 11(5):276. https://doi.org/10.3390/chemosensors11050276
Chicago/Turabian StyleKim, Seo Jin, Hong Jun Park, Eun Seop Yoon, and Bong Gill Choi. 2023. "Preparation of Reduced Graphene Oxide Sheets with Large Surface Area and Porous Structure for High-Sensitivity Humidity Sensor" Chemosensors 11, no. 5: 276. https://doi.org/10.3390/chemosensors11050276
APA StyleKim, S. J., Park, H. J., Yoon, E. S., & Choi, B. G. (2023). Preparation of Reduced Graphene Oxide Sheets with Large Surface Area and Porous Structure for High-Sensitivity Humidity Sensor. Chemosensors, 11(5), 276. https://doi.org/10.3390/chemosensors11050276







