Abstract
Lysozyme (Lyz) is found in animal and human bodily fluids, and is frequently utilized as a biomarker for various diseases. Even trace amounts of Lyz in food can potentially trigger adverse immune system reactions in sensitive individuals. Therefore, it is very important to monitor Lyz concentration in foods for safety. In this study, a simple and convenient electrochemical sensor for Lyz detection was prepared by modifying gold nanoparticles (AuNPs) and ferrocene dicarboxylic acid (Fc(COOH)2) on a glass carbon electrode (GCE), which was characterized fully by various electrochemical methods and field emission scanning electron microscope (FESEM). The proposed method utilized Fc(COOH)2 as a probe and AuNPs as an electron transfer medium to improve the sensor’s current response performance. Under optimal conditions, the sensor was used to detect Lyz with a linear range from 0.10~0.70 mmol·L−1 with a sensitivity of 50.55 μA·mM−1·cm−2, and a limit of detection (LOD) of 0.07 mmol·L−1. In the standard addition experiment of food samples (egg white), a total R.S.D. of less than 6.75% and an average recovery between 95.45% and 102.62% were obtained.
1. Introduction
Lysozyme (Lyz), also called muramidase or N-acetylmuramide glycanohydrlase, can hydrolyze mucopolysaccharides in pathogenic bacteria, combine with negatively charged viral protein, or directly form a complex with DNA, RNA, and apoprotein to inactivate the virus [1,2,3]. It widely exists in a variety of human tissues, egg white, tears, saliva, plasma, milk and microorganisms [4,5]. In addition, because of its unique properties and functions, Lyz is not only used as an antibacterial drug, but also used in baby foods and drink [6]. In presence of some pathological conditions, the concentration of Lyz may increase over a threshold in body fluids, tissues, etc. [7]. Therefore, Lyz is usually used as a biomarker molecule for various diseases, including leukemia, AIDS, tuberculosis, rheumatoid arthritis, and Crohn’s disease [8,9,10]. It is very important to monitor Lyz concentration in a pathological state, which is related to physical manifestations and treatment outcomes [8,9,10,11,12].
There are some common techniques for Lyz detection: enzyme-linked immunosorbent assay (ELISA) [13], high-performance liquid chromatography (HPLC) [14], mass spectrometry (MS) [15], fluorescence detection (FD) [16], and capillary gel electrophoresis (CGE) [17], etc. Many of them suffer from complex preparation processes or time-consuming immobilization of nanomaterials modifiers. In some other reports, aptasensors are developed for the detection of Lyz in foods [18], which is subject to the design of aptasequencesence and the immobilization process. In special, expensive instruments and complex operations are required for these methods above. Therefore, it is very necessary to develop a portable and rapid method for Lyz analysis.
In recent years, increased attention have be confined to the development of various electrochemical sensors due to their advantages of fast response, high sensitivity, low cost, simple analysis, and real-time [19,20,21,22,23]. They have performed an important role in food safety testing [24,25]. Most of the existing papers focus on the development of electrochemical aptasensors for Lyz detection [26], the few sensors are using no bioreceptors [18]. As a promising material, gold nanoparticles (AuNPs) have received extensive attention in recent years for their wide applications in electronics, catalysis, and biosensors [27,28,29], due to their large aspect ratio, good biocompatibility, and high electrical conductivity [30,31]. Moreover, ferrocene (Fc, known as dicyclopentadienyl iron) and its conjugates, such as ferrocene carboxylic acid (Fc(COOH)) and ferrocene dicarboxylic acid (Fc(COOH)2) [32,33,34], are widely used in the field of electrochemical sensors [35], acting as a redox mediator with stable structure, easy modification, good reactivity, and reversible single-electron redox pair [36,37].
In this work, a simple and portable electrochemical sensor for Lyz detection was fabricated with AuNPs and Fc(COOH)2 modified on a glassy carbon electrode (GCE), utilizing Fc(COOH)2 as a probe and AuNPs as an amplifier unit. The performance of different modified electrodes was observed by cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS), respectively. Then, they were further characterized by field emission scanning electron microscope (FESEM) via scanning electron microscopy (SEM) and energy dispersion spectroscopy (EDS). The results showed that the Fc(COOH)2/AuNPs/GCE sensor had excellent sensitivity, stability, and selectivity, and could be practically employed to detect Lyz in egg whites.
2. Experimental Section
2.1. Materials and Methods
Lysozyme (Lyz) and 5-hydroxytryptamine (5-HT) were purchased from Rhawn Reagent Co., Ltd. (Shanghai, China). Glucose (Glu), ascorbic acid (AA), trisodium citrate (Na3(C6H5O7)), Chloroauric acid (HAuCl4) was obtained from SANGON biological Co., Ltd., (Shanghai China). Bovine serum albumin (BSA) were obtained from Aladdin Reagent Co., Ltd., (Shanghai, China). L-cysteine (L-Cys) and L-lysine (Lys) were obtained from MacLin Biochemical Technology Co., Ltd. (Shanghai, China). Ferrocene (Fc) and Fc(COOH)2 were provided by Shanghai Changxi Biotechnology Co., Ltd. (Shanghai, China). Screen-printed carbon electrodes (SPCE) were purchased from Nanjing Yunyou Biotechnology Co. Ltd. (Nanjing, China). Eggs were purchased from a local supermarket (Guilin, Guangxi, China). All used water was Milli-Q water.
2.2. Instrumentation
A CHI660E electrochemical workstation (CH Instruments Co., Ltd., Shanghai, China) was used to carried out all electrochemical tests through a three-electrode system, which includes glass carbon electrode (GCE, working electrode), Ag/AgCl (3.0 mol·L−1 KCl, reference electrode), and platinum wire (Pt, counter electrode). Trisodium citrate solution was used as a supporting electrolyte when cyclic voltammetry (CV) and differential pulse voltammetry (DPV) technique was performed. For electrochemical impedance spectroscopy (EIS) measurements in the frequency of 0.1~100,000.0 Hz, which were performed in 5.0 mmol·L−1 K4[Fe(CN)6]/K3[Fe(CN)6] containing 0.1 mol·L−1 KCl solution as supporting electrolyte. The sensors were also characterized by field emission scanning electron microscope (FESEM, Hitachi SU8020, Tokyo, Japan) with an acceleration voltage of 0.5~30.0 kV and an X-ray energy resolution of 130.0 eV.
2.3. Preparation of Sensors and Electrochemical Detection of Lyz
First, all GCEs were polished by different specifications of alumina powder. Fc(COOH)2 was dissolved in phosphate−buffered solution (PBS, 0.2 mol·L−1) to prepare 10.0 mmol·L−1 stock solution. Lyz was ultrasonic dissolved in PBS supplemented with 0.02 mol·L−1 trisodium citrate. Then, the prepared solutions were put into a 4 °C environment for later use.
Scheme 1 shows the preparation of the developed electrochemical sensor Fc(COOH)2/AuNPs/GCE for Lyz detection. First, in chloroauric acid (1%), AuNPs were deposited on the GCE surface by amperometric i-t technique for 120.0 s with an initial potential of −0.5 V, after which the modified electrodes were cleaned and dried. Then, Fc(COOH)2 solution (5.0 μL) was evenly dripped on the AuNPs/GCE that were put back into the incubator (37 °C, 15 min). Finally, the modified electrodes immersed in Lyz solution were implored to measure the response current by DPV.
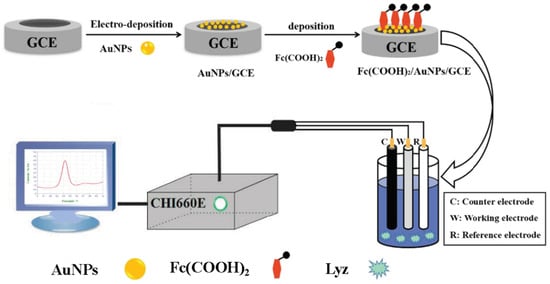
Scheme 1.
Preparation of Fc(COOH)2/AuNPs/GCE electrochemical sensor for Lyz detection by DPV technique.
3. Results and Discussion
3.1. Comparison of the Modified Materials on Electrode Surface
Nanomaterials are widely used in the field of electrochemistry due to their high conductivity, large specific surface area, and excellent electrocatalytic performance for analytes [38]. In this section, the electrochemical performance of different modified electrodes was explored by DPV, CV, and EIS techniques. As shown in Figure 1A, no obvious electrochemical signal is depicted while the bare GCE measured in the trisodium citrate solution. By contrast, three modified electrodes (the three kinds of modified substances with the same concentration are modified to the surface of the GCE electrode by dropping) showed three prominent and separate response peaks, holding an identical lower background current, indicating the feasibility of their utilization as an electrochemical probe. Amongst the three modified electrodes, Fc(COOH)2/GCE demonstrates the highest peak current (0.42 V, 4.51 µA), while the current of 5-HT/GCE (0.33 V, 2.53 µA) and Fc/GCE (0.21 V, 2.44 µA) were closed to each other. Therefore, Fc(COOH)2 was selected as the modifier GCE for further study.
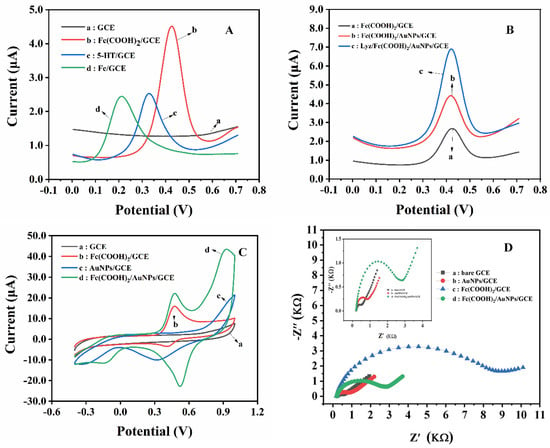
Figure 1.
(A) DPV of the different NPs modified on GCE in trisodium citrate solution (a. GCE, b. Fc(COOH)2/GCE, c. 5−HT/GCE, and d. Fc/GCE); (B) DPV of different sensors were detected in trisodium citrate solution (a. Fc(COOH)2/GCE and b. Fc(COOH)2/AuNPs/GCE), Fc(COOH)2/AuNPs/GCE was used in trisodium citrate solution of 0.5 mmol·L−1 Lyz (c. lyz/Fc(COOH)2/AuNPs/GCE); (C) CV of different sensors in trisodium citrate solution (a. GCE, b. Fc(COOH)2/GCE, c. AuNPs/GCE, and d. Fc(COOH)2/AuNPs/GCE; (D) EIS curves of different modified electrodes (a. bare GCE, b. AuNPs/GCE, c. Fc(COOH)2/GCE, and d. Fc(COOH)2/AuNPs/GCE), insert: EIS curves of an enlarged portion of (D) (a. bare GCE, b. AuNPs/GCE, and c. Fc(COOH)2/AuNPs/GCE), and supporting electrolyte solution is 5.0 mmol·L−1 [Fe(CN)6]3−/4− containing 0.1 mol·L−1 KCl.
As shown in Figure 1B, the Fc(COOH)2/AuNPs/GCE electrode (curve b) presents a higher response peak in trisodium citrate solution than the Fc(COOH)2/GCE electrode (curve a) does, which is attributed to the large surface area and excellent conductivity of AuNPs with a consideration of the simultaneous amplification of the Faraday and non-Faraday current [39,40]. In comparison with curve b, curve c represents a double increase in the peak current at the Fc(COOH)2/AuNPs/GCE electrode in Lyz solution (trisodium citrate as supporting electrolyte) while the peak potential did not shift, which confirms the unique existence of the synergy effect between Fc(COOH)2 and Lyz that is elucidated in Figure 1A.
As shown in Figure 1C, GCEs modified with different materials were researched in blank trisodium citrate solution by CV. The characteristic peaks of Fc(COOH)2 (0.48 V and 15.9 µA) and AuNPs can be clearly seen. Similarly, Figure 1C shows the amplified peak currents of the electrochemical probe Fc(COOH)2 by modifying with numerous AuNPs, which also improved the reversibility of the concerned redox pairs.
Next, various modified electrodes were investigated by EIS, as shown in Figure 1D, where all charge transfer impedances were fitted according to the existing semicircles [41]. The impedance results of EIS is obtained by EIS simulation based on Randles equivalent circuit [42]. The electrochemical independences of every surfaces are arranged in increasing order: Fc(COOH)2/GCE (8048.0 Ω) > Fc(COOH)2/AuNPs/GCE (2521.0 Ω) > AuNPs/GCE (748.0 Ω) > GCE (385.0 Ω). As expected, AuNPs decreased the electrochemical independence once deposited as an amplifier unit of the designed sensor. All these results proved the feasibility of the developed Fc(COOH)2/AuNPs/GCE for Lyz determination.
3.2. Characterization of the As-Prepared Electrodes
To characterize the proposed sensor, AuNPs and Fc(COOH)2 were deposited on SPCE (Φ = 0.3 cm). Then, the morphology and element distribution of the different modified electrodes were separately studied by SEM and EDS. As can be seen in Figure 2A,D, the rough surface of SPCE is mainly composed of C without any interfering substance attached. The presence of S is attributed to the activation of the SPCE by sulfuric acid before use Figure 2B,E show that dense spherical AuNPs of different sizes, such as the particle size of AuNPs: 94.44 nm, 144.00 nm, and 241.37 nm, were successfully electrodeposited on the electrode surface, which enlarged the surface available for adsorption of Fc(COOH)2, so that the detected current signal was increased greatly. Figure 2C shows that a 3D porous frame was formed by the polymerization of Fc(COOH)2 molecules, providing considerable active sites for binding. The corresponding EDS result shown in Figure 2F indicated the rising mass fraction of Fe.
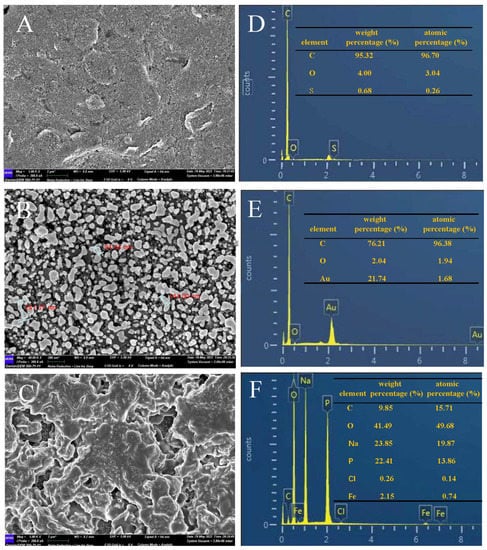
Figure 2.
Typical SEM and EDS images of different modified electrodes. (A,D): SPCE; (B,E): AuNPs/GCE; (C,F): Fc(COOH)2/AuNPs/SPCE.
3.3. Optimization Studies
Different modified electrodes were investigated by DPV performed in trisodium citrate solution of Lyz (0.5 mmol·L−1) for experimental optimization, including the modifier, the deposition potentials and time of AuNPs, the amounts of Fc(COOH)2, pH, and temperature. The DPV results obtained under different conditions are presented in Figure S1.
In Figure S1A, comparing the response current of four electrodes (GCE, Fc(COOH)2/GCE, Fc(COOH)2/AuNPs/GCE, and Fc(COOH)2@AuNPs/GCE) with each other. The highest response current was observed on Fc(COOH)2/AuNPs/GCE, which indicates that progressive deposition of AuNPs and Fc(COOH)2 is the best route for modification. The deposition voltage of AuNPs was set to −0.3, −0.4, −0.5, and −0.6 V for optimization. After comparison (Figure S1B), −0.5 V was chosen as the deposition time of AuNPs. Then, we explored the effect of deposition time (40.0 s, 60.0 s, 80.0 s, 100.0 s, and 140.0 s) of AuNPs. The results (Figure S1C) show that when the deposition time exceeds 60 s, the currents started to drop. The increase in current may be due to AuNPs enhancing the surface active site, while the decrease in current may be due to the over-thick gold nanoparticles on the surface. Thus, 60.0 s was chosen as the optimum deposition time. It can be seen that the response current increases as the deposition amount of Fc(COOH)2 increases in Figure S1D, and the response current changes little after deposition of 4.0 μL Fc(COOH)2. This may be due to more Fc(COOH)2 leading to more functional groups, such as methyl and carboxyl groups binding to Lyz leading to an increase in current until saturation. As a consequence, 4.0 μL was chosen as the deposition amount of Fc(COOH)2.
Afterward, the effect of trisodium citrate solution pH and temperature was explored. Figure S1E shows the current obtained under of different pH levels (4.0, 5.0, 6.0, 7.0, 8.0, and 9.0, respectively) of trisodium citrate solution. The highest current was observed when the pH was 7.0, which was similar to the pH value of the liquid in the living body. Moreover, the peak current decreases from 4 °C to 25 °C, but increases from 25 °C to 30 °C, then decreases from 30 °C to 37 °C, as shown in Figure S1F. High temperatures denature proteins and affect lysozyme activity, so subsequent experiments were performed at 30 °C, which is similar to the optimal activity temperature of lysozyme in other studies [3,43].
3.4. Lyz Assay, Selectivity, Reproducibility, and Stability
Under the optimal conditions, different concentrations of Lyz were measured with Fc(COOH)2/AuNPs/GCE by the DPV method, the results are shown in Figure 3A. A linear relationship between the concentration of Lyz (0.10~0.70 mmol·L−1) and the response current at Fc(COOH)2/AuNPs/GCE electrode was observed: I = 4.35592 + 3.57302c (R2 = 0.99486, c is the Lyz concentration), with a limit of detection (LOD) of 0.07 mmol·L−1 and a sensitivity of 50.55 μA·mM−1·cm−2. Moreover, the various performance of different modified electrochemical sensors was listed in Table S1 [19,44,45,46,47]. In fact, these sensors have a lower LOD than Fc(COOH)2/AuNPs/GCE. However, their preparation steps are relatively complex compared to the Fc(COOH)2/AuNPs/GCE sensor.
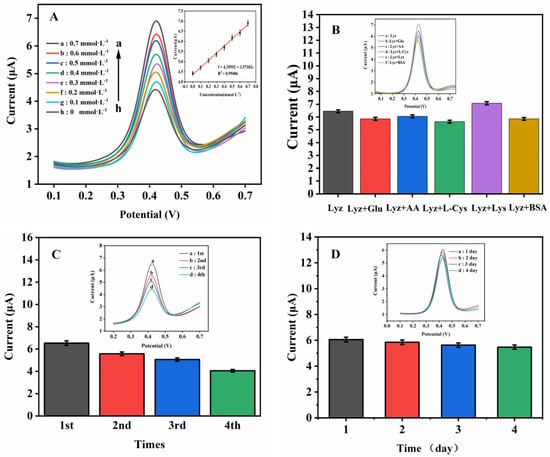
Figure 3.
(A) DPVs of Lyz at different concentrations (h–a: 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 mmol·L−1, respectively) under optimal conditions, insert: calibration plot in various concentrations of Lyz at Fc(COOH)2/AuNPs/GCE. The study of selectivity (B), repeatability (C), and stability (D) of the Fc(COOH)2/AuNPs/GCE for Lyz detection under optimal conditions.
Figure 3B–D display selectivity, repeatability, and stability of Fc(COOH)2/AuNPs/GCE sensors. The selectivity of Fc(COOH)2/AuNPs/GCE sensor was studied by DPV at the optimal conditions. Figure 3B shows that the peak current responses in Lyz (0.5 mmol·L−1) and the mixture of Lyz, and interferences in a 10:1 mixing ratio (Lyz, Lyz + Glu, Lyz + AA, Lyz + L-Cys, Lyz + Lys, and Lyz + BSA), a slight change in the current on the sensor was observed in different solutions with a total R.S.D. of 8.65%. The results verify an excellent selectivity of the sensor. To explore the repeatability of the sensor, a Fc(COOH)2/AuNPs/GCE sensor (after each measurement, the electrode was cleaned by Milli-Q water) was continuously tested four times by DPV in 0.5 mmol·L−1 Lyz solution. The peak current gradually decreases from first (6.53 μA) to fourth (4.05 μA), as shown in Figure 3C. This may be a result from the increase in the electrochemical reaction products at the interface and the decrease in electrodes surface-modifying species at the same time. For stability investigation, four Fc(COOH)2/AuNPs/GCE sensors were fabricated to detect 0.5 mmol·L−1 Lyz on different days. Figure 3D showed the sensor was stable within four days, which indicates Fc(COOH)2/AuNPs/GCE sensor has good stability.
3.5. Foods Sample Analysis
In the sample detection experiment, the egg white was diluted 10 times with trisodium citrate solution and stored in the refrigerator at 4 °C. The appropriate volume of the solution was tested three times using the standard addition method, and the results are shown in Table 1. It indicates the sensor had potential applications in detecting Lyz, the calculated value of Lyz concentration in undiluted blank egg white is 190 μmol·L−1, which is close to the value (50 μmol·L−1) reported in the literature [48], the average recovery of the Fc(COOH)2/AuNPs/GCE sensor for Lyz detection was from 95.45% to 102.62 %, and a total R.S.D. less than 6.75%, which proves that the sensor had the ability to be applied to practical applications.

Table 1.
Results of Lyz detection in egg white samples (n = 3).
4. Conclusions
In this study, we reported a simple and convenient electrochemical sensor, which was modified by AuNPs and Fc(COOH)2 for Lyz detection. AuNPs as a signal amplifying substance due to their large specific surface area and excellent electrical conductivity increases the electrochemical signal of the sensor. Lyz was captured by Fc(COOH)2 as a probe. The Fc(COOH)2/AuNPs/GCE sensor had a great linear range from 0.10 to 0.70 mmol·L−1 with the sensitivity of detection of 50.55 μA·mM−1·cm−2, and a limit of detection (LOD) of 0.07 mmol·L−1, which have excellent stability and anti-interference. Lyz was detected by Fc(COOH)2/AuNPs/GCE in egg white and the results showed a total R.S.D. less of than 6.75% and an average recovery between 95.45% to 102.62%. It indicated that the electrochemical sensor can be applied to detect Lyz in egg white.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors11040209/s1, Figure S1: Fc(COOH)2/AuNPs/GCE sensor current responses (The inserts are DPV curves) for 0.5 mmol·L−1 Lyz in trisodium citrate solution. A: different modified electrodes (a. GCE, b. Fc(COOH)2/GCE, c. Fc(COOH)2/AuNPs/GCE and d. Fc(COOH)2@AuNPs/GCE), Fc(COOH)2@AuNPs/GCE: AuNPs and Fc(COOH)2 mixed solution is deposited onto the GCE; B: different deposition potentials (−0.3, −0.4, −0.5, −0.6 V) of AuNPs; C: different deposition tine (40, 60, 80, 100, 140 s) of AuNPs; D: different amount (3, 4, 5, 6 μL) of Fc(COOH)2; E: different pH (4, 5, 6, 7, 8 and 9); F: different temperature (4, 25, 30, 37 °C); Table S1: Evaluation of Lyz various electrochemical sensors.
Author Contributions
Investigation, writing—original draft, methodology, conceptualization, J.W.; project administration, supervision, and conceptualization, G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We appreciate the financial support from the National Natural Science Foundation of China (No.61661014, 81873913, 61861010, and 61627807), the Nature Science Foundation of Guangxi Province (No. 2018GXNSFAA281198, 2018GXNSFBA281135), Guangxi One Thousand Young and Middle-aged College, and University Backbone Teachers Cultivation Program, and Guangxi Colleges and Universities Key Laboratory of Biomedical Sensors and Intelligent Instruments. Fundings from the Natural Science and Engineering Research Council of Canada and the University of Toronto are appreciated. We also appreciate the following people for their contribution: Xiao-Zhen Feng, Jin-Guang Liu, Tao Zhan, Mingyue Xue, Zhencheng Chen, and Heinz-Bernhard Kraatz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Siddiqi, R.A. Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 2018, 240, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Menacho-Melgar, R.; Moreb, E.A.; Efromson, J.P.; Yang, T.; Hennigan, J.N.; Wang, R.; Lynch, M.D. Improved two-stage protein expression and purification via autoinduction of both autolysis and auto DNA/RNA hydrolysis conferred by phage lysozyme and DNA/RNA endonuclease. Biotechnol. Bioeng. 2020, 117, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-L.; Ding, B.-X.; Lan, X.-M.; Guo, S.-B.; Xie, Y.-Y.; Wang, C.-B. The toxicity study on marine low-temperature lysozyme. Food Chem. Toxicol. 2008, 46, 604–609. [Google Scholar] [CrossRef]
- Konstan, M.W.; Chen, P.W.; Sherman, J.M.; Thomassen, M.J.; Wood, R.E.; Boat, T.F. Human lung lysozyme: Sources and properties. Am. Rev. Respir. Dis. 1981, 123, 120–124. [Google Scholar] [PubMed]
- Porstmann, B.; Jung, K.; Schmechta, H.; Evers, U.; Pergande, M.; Porstmann, T.; Kramm, H.J.; Krause, H. Measurement of lysozyme in human body fluids: Comparison of various enzyme immunoassay techniques and their diagnostic application. Clin. Biochem. 1989, 22, 349–355. [Google Scholar] [CrossRef]
- Huang, J.M.; Nandi, S.; Wu, L.Y.; Yalda, D.; Bartley, G.; Rodriguez, R.; Lonnerdal, B.; Huang, N. Expression of natural antimicrobial human lysozyme in rice grains. Mol. Breed. 2002, 10, 83–94. [Google Scholar] [CrossRef]
- Ishii, H.; Iwata, A.; Oka, H.; Sakamoto, N.; Ishimatsu, Y.; Kadota, J.-i. Elevated serum levels of lysozyme in desquamative interstitial pneumonia. Intern. Med. 2010, 49, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Venge, P.; Foucard, T.; Henriksen, J.; Hakansson, L.; Kreuger, A. Serum-levels of lactoferrin, lysozyme and myeloperoxidase in normal, infection-prone and leukemic children. Clin. Chim. Acta 1984, 136, 121–130. [Google Scholar] [CrossRef]
- Grieco, M.H.; Reddy, M.M.; Kothari, H.B.; Lange, M.; Buimovici-Klein, E.; William, D. Elevated beta 2-microglobulin and lysozyme levels in patients with acquired immune deficiency syndrome. Clin. Immunol. Immunopathol. 1984, 32, 174–184. [Google Scholar] [CrossRef]
- Perillie, P.E.; Khan, K.; Finch, S.C. Serum lysozyme in pulmonary tuberculosis. Am. J. Med. Sci. 1973, 265, 297–302. [Google Scholar] [CrossRef]
- Falchuk, K.R.; Perrotto, J.L.; Isselbacher, K.J. Serum lysozyme in Crohn’s: A useful index of disease activity. Gastroenterology 1975, 69, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Ueda, K.; Maeda, H.; Kambara, T. Determination of lysozyme activity by fluorescence polarization in rheumatoid synovial fluids and release of lysozyme from polymorphonuclear leukocytes by chemotactic factors. J. Immunol. Methods 1987, 103, 221–227. [Google Scholar] [CrossRef]
- Thonar, E.J.; Feist, S.B.; Fassbender, K.; Lenz, M.E.; Matijevitch, B.L.; Kuettner, K.E. Quantification of hen egg white lysozyme in cartilage by an enzyme-linked immunosorbent assay. Connect. Tissue Res. 1988, 17, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Jaeser, M.; Moeckel, U.; Weigel, K.; Henle, T. Natural association of lysozyme and casein micelles in human milk. J. Agric. Food Chem. 2022, 70, 1652–1658. [Google Scholar] [CrossRef]
- Haselberg, R.; Harmsen, S.; Dolman, M.E.M.; de Jong, G.J.; Kok, R.J.; Somsen, G.W. Characterization of drug-lysozyme conjugates by sheathless capillary electrophoresis-time-of-flight mass spectrometry. Anal. Chim. Acta 2011, 698, 77–83. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.-Y.; Chen, H.; Wang, X.-H.; Chen, Q.; He, P.-G. Sensitive fluorescence detection of lysozyme using a tris(bipyridine)ruthenium(II) complex containing multiple cyclodextrins. Chem. Commun. 2015, 51, 6613–6616. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, W.; Ruan, Y.; Huang, L.; Wu, Z.; Cai, Y.; Fu, F. Ultra-sensitive quantification of lysozyme based on element chelate labeling and capillary electrophoresis inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2014, 812, 12–17. [Google Scholar] [CrossRef]
- Melinte, G.; Selvolini, G.; Cristea, C.; Marrazza, G. Aptasensors for lysozyme detection: Recent advances. Talanta 2021, 226, 122169. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Q.; Tang, G.; Liu, S.; Xu, S.; Zhang, X. A facile electrochemical aptasensor for lysozyme detection based on target-induced turn-off of photosensitization. Biosens. Bioelectron. 2019, 126, 412–417. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-beta-Estradiol determination. Sen. Actuators B Chem. 2017, 241, 698–705. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, X.; Kong, B.; Wang, Y.; Wei, W. A sensitive nonenzymatic hydrogen peroxide sensor based on DNA-Cu2+ complex electrodeposition onto glassy carbon electrode. Sen. Actuators B Chem. 2008, 133, 381–386. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Jiang, R.; Sun, L.; Pang, S.; Luo, A. Fluorescent molecularly imprinted membranes as biosensor for the detection of target protein. Sen. Actuators B Chem. 2018, 254, 1078–1086. [Google Scholar] [CrossRef]
- Liu, J.G.; Wan, J.Z.; Lin, Q.M.; Han, G.C.; Feng, X.Z.; Chen, Z. Convenient heme nanorod modified electrode for quercetin sensing by two common electrochemical methods. Micromachines 2021, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- An, Q.Q.; Feng, X.Z.; Zhou, Z.F.; Zhan, T.; Lian, S.F.; Zhu, J.; Han, G.C.; Chen, Z.; Kraatz, H.B. One step construction of an electrochemical sensor for melamine detection in milk towards an integrated portable system. Food Chem. 2022, 383, 132403. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Z.; Ferranco, A.; Su, X.R.; Chen, Z.C.; Jiang, Z.L.; Han, G.C. A facile electrochemical sensor labeled by ferrocenoyl cysteine conjugate for the detection of nitrite in pickle juice. Sensors 2019, 19, 268. [Google Scholar] [CrossRef]
- Li, L.-D.; Chen, Z.-B.; Zhao, H.-T.; Guo, L.; Mu, X. An aptamer-based biosensor for the detection of lysozyme with gold nanoparticles amplification. Sens. Actuators B Chem. 2010, 149, 110–115. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; de la Zerda, A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef]
- Guo, S.J.; Wang, E.K. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Kannan, P.; John, S.A. Determination of nanomolar uric and ascorbic acids using enlarged gold nanoparticles modified electrode. Anal. Biochem. 2009, 386, 65–72. [Google Scholar] [CrossRef]
- Chen, Z.; Li, L.; Zhao, H.; Guo, L.; Mu, X. Electrochemical impedance spectroscopy detection of lysozyme based on electrodeposited gold nanoparticles. Talanta 2011, 83, 1501–1506. [Google Scholar] [CrossRef]
- Zhan, T.; Feng, X.-Z.; Cheng, Y.-Y.; Han, G.-C.; Chen, Z.; Kraatz, H.-B. Synergistic electrochemical amplification of ferrocene carboxylic acid nanoflowers and Cu nanoparticles for folic acid sensing. J. Electrochem. Soc. 2022, 169, 007510. [Google Scholar] [CrossRef]
- Feng, X.Z.; Su, X.R.; Ferranco, A.; Chen, Z.C.; Han, G.C.; Jiang, Z.L.; Kraatz, H.B. Real-time electrochemical detection of uric acid, dopamine and ascorbic acid by heme directly modified carbon electrode. J. Biomed. Nanotechnol. 2020, 16, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Han, G.C.; Su, X.R.; Hou, J.T.; Ferranco, A.; Feng, X.Z.; Zeng, R.S.; Chen, Z.C.; Kraatz, H.B. Disposable electrochemical sensors for hemoglobin detection based on ferrocenoyl cysteine conjugates modified electrode. Sen. Actuators B Chem. 2019, 282, 130–136. [Google Scholar] [CrossRef]
- Kondzior, M.; Grabowska, I. Antibody-electroactive probe conjugates based electrochemical immunosensors. Sensors 2020, 20, 2014. [Google Scholar] [CrossRef] [PubMed]
- Han, G.C.; Hou, J.T.; Huang, Z.L.; Feng, X.Z.; Chen, Z.C.; Xiao, W.X.; Li, S. Electrochemical behaviors of ferrocene dicarboxylate and its application for heme detection. Int. J. Electrochem. Sci. 2017, 12, 6245–6254. [Google Scholar] [CrossRef]
- Han, G.C.; Su, X.R.; Hou, J.T.; Feng, X.Z.; Chen, Z.C. Interaction study of Fc(COOH)(2) and BSA by UV-Vis spectroscopy. Spectrosc. Spect. Anal. 2018, 38, 3958–3962. [Google Scholar]
- Cheng, Y.Y.; Zhan, T.; Feng, X.Z.; Han, G.C. A synergistic effect of gold nanoparticles and melamine with signal amplifification for C-reactive protein sensing. J. Electroanal. Chem. 2021, 895, 115417. [Google Scholar] [CrossRef]
- Sano, S.; Kato, K.; Ikada, Y. Introduction of functional-groups onto the surface of polyethylene for protein immobilization. Biomaterials 1993, 14, 817–822. [Google Scholar] [CrossRef]
- Bezuneh, T.T.; Fereja, T.H.; Kitte, S.A.; Li, H.; Jin, Y. Gold nanoparticle-based signal amplified electrochemiluminescence for biosensing applications. Talanta 2022, 248, 123611. [Google Scholar] [CrossRef]
- Ates, M.; Chebil, A. Supercapacitor and battery performances of multi-component nanocomposites: Real circuit and equivalent circuit model analysis br. J. Energy Storage 2022, 53, 105093. [Google Scholar] [CrossRef]
- Gaberscek, M. Impedance spectroscopy of battery cells: Theory versus experiment. Curr. Opin. Electrochem. 2022, 32, 100917. [Google Scholar] [CrossRef]
- Sarkar, D.; Kang, P.; Nielsen, S.O.; Qin, Z. Non-arrhenius reaction-diffusion kinetics for protein inactivation over a large temperature range. ACS Nano 2019, 13, 8669–8679. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Aguayo, D.; del Valle, M. Label-free aptasensor for lysozyme detection using electrochemical impedance spectroscopy. Sensors 2018, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Titoiu, A.M.; Porumb, R.; Fanjul-Bolado, P.; Epure, P.; Zamfir, M.; Vasilescu, A. Detection of allergenic lysozyme during winemaking with an electrochemical aptasensor. Electroanal 2019, 31, 2262–2273. [Google Scholar] [CrossRef]
- Cao, X.; Xia, J.; Liu, H.; Zhang, F.; Wang, Z.; Lu, L. A new dual-signalling electrochemical aptasensor with the integration of “signal on/off” and “labeling/label-free” strategies. Sen. Actuators B Chem. 2017, 239, 166–171. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Li, J.; Guo, L. Tetrahexahedral Au nanocrystals/aptamer based ultrasensitive electrochemical biosensor. RSC Adv. 2013, 3, 14385–14389. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Salimi, A. Novel voltammetric and impedimetric sensor for femtomolar determination of lysozyme based on metal-chelate affinity immobilized onto gold nanoparticles. Biosens. Bielectron 2015, 74, 270–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).