Abstract
Surface-enhanced Raman scattering (SERS) spectroscopy has attracted increasing attention due to its high spectral reproducibility and unique selectivity to target molecules. Here, a facile approach is proposed to prepare Ag nanoparticles modified ZnO nanorod arrays (Ag/ZnO NR arrays). Ag nanoparticles were densely decorated on the surface of ZnO nanorods through silver mirror reaction and subsequent seed-assisted electrodeposition. The prepared Ag/ZnO NR arrays can be used as a sensitive, uniform, and repeatable SERS substrate for the rapid detection of organic dye molecules and biomolecules with concentrations higher than the corresponding limits of detection (LODs). The LODs for rhodamine 6G (R6G), 4-aminothiophenol (PATP) and adenine are calculated to be 1.0 × 10−13 M, 1.6 × 10−12 M and 3 × 10−11 M, respectively. The enhancement factor (EF) of the SERS substrate is estimated to be as high as ~2.7 × 108 when detecting 10−10 M R6G. Particularly, the as-synthesized substrate exhibits high selectivity to multiple components. In addition, the fabricated Ag/ZnO NR arrays can be recycled due to their superior self-cleaning ability and can realize photocatalytic degradation of R6G in water within 1 h driven by UV light, showing that the three-dimensional recyclable SERS substrates have wide applications in environmental pollution monitoring and biomedical analysis.
1. Introduction
Surface-enhanced Raman spectroscopy (SERS) as a powerful spectroscopy analytical technique for quick and nondestructive analysis [1,2] has attracted intensive attention and been widely applied in many fields, such as environment monitoring, biological sensing, chemical sensing, and healthcare inspection [3,4,5,6,7,8]. Obviously, an excellent SERS substrate is equipped with high sensitivity, good stability of performance, excellent spectral reproducibility, and so on. In recent years, lots of SERS substrates made of noble-metal (Au, Ag) nanomaterials with different structures and various sizes, such as nanoflowers, nanorods, nanomicrospheres, and gold–silver bimetallic systems [9,10,11,12]. The highly roughened nanostructured surfaces, sharp nano-tips, and nanoscale gaps around the nanostructures generate highly concentrated electromagnetic fields and produce prominent signal amplification [13]. Plasmonic nanoparticles or other particles modified with plasmonic nanoparticles were usually placed directly on a flat substrate for sensitive SERS detection [14,15,16]; if these SERS-active particles had been uniformly and firmly grown on the substrate, more robust and stable SERS substrate would be achieved for sensitive detection application.
Recently, metal oxide semiconductors with high SERS activity are attracting increasing interest and are used as effective SERS substrates for sensitive detection. Among different metal oxide semiconductors, ZnO can be employed for fabricating desirable SERS-active substrates. ZnO is regarded as a versatile material with a wide band gap, which can exhibit excellent photocatalyst performance to provide self-cleaning attributes and can self-assemble in different forms to form homogeneous arrays with various morphologies [17,18,19]. However, the application of ZnO nanomaterials as SERS substrates for rapid detection of trace organic molecules is still seriously impeded by their low electron density in conduction band (CB) and weak plasmonic effect [20,21]. Fortunately, the combination of ZnO and noble metals promotes the combination of the plasmonic effect of Au or Ag nanostructures with the charge transfer effect at the interface between noble metal and semiconductor nanomaterials, thus significantly improving the effectiveness of SERS detection, which undoubtedly offers another alternative pathway to fabricate highly sensitive SERS substrates [22,23,24,25,26]. Furthermore, the recombination process of electro-hole pairs in the hybrid structure is also significantly decelerated as a result of the Schottky barrier that forms in the noble metal-semiconductor interface, which is advantageous for increasing photocatalytic efficiency [27,28]. Among numerous noble metals, Ag nanostructures have the highest SERS enhancement [29]. Thus, a desirable SERS substrate can be achieved by combining ZnO with Ag, endowing it with potential application in the sensitive detection of ultra-trace organic pollutants.
In this paper, we propose a simple method to fabricate Ag nanoparticles modified ZnO nanorod (Ag/ZnO NR) arrays. As presented in Scheme 1, firstly, ZnO NRs were grown on an indium tin oxide (ITO) substrate by a hydrothermal method. Then, a layer of silver nuclei was deposited on the surface of ZnO nanorod by silver mirror reaction. Next, silver particles continued to grow to form larger Ag nanoparticles by electrodeposition under a constant current. The fabricated Ag/ZnO NR arrays can serve as SERS substrates and exhibit high sensitivity to rhodamine 6G (R6G), adenine, and aminothiophenol (PATP). The enhancement factor (EF) of such Ag/ZnO NR array is estimated to be to 2.7 × 108 when computed using 10−10 M R6G. Importantly, the Ag/ZnO NR arrays even show high SERS selectivity toward trace R6G in a mixed ternary solution containing R6G, PATP, and adenine. In addition, the Ag/ZnO-NR arrays not only exhibit high SERS sensitivity but also can be reused after photocatalytic degradation of the adsorbed analyte molecules by UV light irradiation.
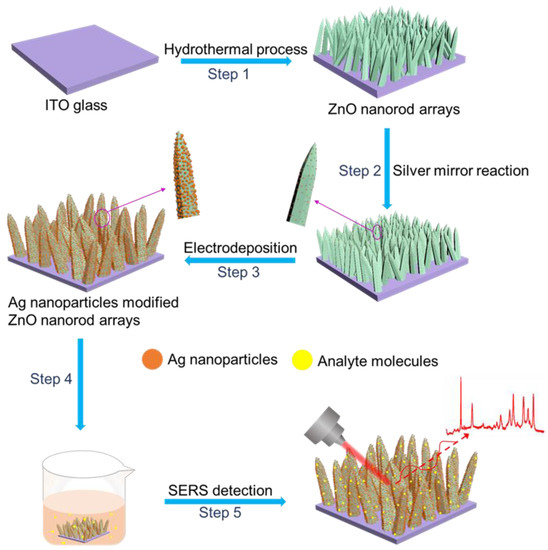
Scheme 1.
Schematic for fabricating Ag/ZnO-NR arrays. Step 1: ZnO nanorods are uniformly grown on an ITO glass by a hydrothermal method. Step 2: Ag nuclei are modified on the surface of ZnO nanorods by silver mirror reaction. Step 3: Ag nuclei on the surface of ZnO nanorods grow bigger with the help of electrodeposition. Step 4: Adsorption of analyte molecules has been achieved by immersing the substrate in the analyte solution. Step 5: Ag/ZnO-NR arrays are used as SERS substrates for sensitive detection of organic molecules.
2. Experimental
2.1. Materials
Zinc nitrate hexahydrate (Zn (NO3)2·6H2O, 99%), silver nitrate (AgNO3, 99%), ammonia (NH3·H2O, 26~28 wt%), and polyvinylpyrrolidone (PVP, K29-32) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Rhodamine 6G (C28H30N2O3, 95%), adenine (C5H5N5, 98%), and 4-aminothiophenol (PATP, 97%) were acquired from Aladdin Reagent Co., Ltd. (Shanghai, China). Indium tin oxide (ITO) glasses were bought from Huananxiangcheng Technology Co., Ltd. (Shenzhen, China).
2.2. Fabrication of ZnO NR Arrays
An ITO glass (0.5 cm × 4 cm) was ultrasonically cleaned for 10 min with water and ethanol, and then treated with plasma for 300 s to improve the surface hydrophilicity. Next, the ITO glass was placed horizontally in a zinc ammonia solution (0.1 M) to grow ZnO nanorod arrays on its surface in a water bath at 80 °C for 1 h. After that, the fabricated ZnO nanorod arrays on ITO surface were rinsed by deionized water, and then were dried using Ar gas.
2.3. Deposition of Ag Nanoparticles on ZnO NR Arrays
Firstly, a silver ammonia solution was prepared by incrementally adding NH3·H2O (10 wt%) solution into an aqueous solution of AgNO3 (20 mM, 100 mL) until the mixture turned transparent. Then, glucose (50 mM) was poured into the as-prepared silver ammonia solution that was under constant stirring. The ITO glass covered with ZnO NR arrays was then vertically submerged in the silver ammonia solution for 20 min, which was heated to 50 °C in a water bath to modify Ag particles upon ZnO NRs. After modifying with Ag seeds, the substrate was thoroughly rinsed using deionized water, and then blow-dried by argon gas. Subsequently, an electrolyte was made by dissolving PVP (0.5 g) in a silver ammonia solution under stirring for 10 min. Utilizing the ITO glass covered with Ag seeds decorated ZnO-NR arrays as a cathode, a graphite plate as an anode, dense Ag nanoparticles were grown on the ZnO NRs under a current of 150 μA for 1 h. After that, the sample was taken out, washed using purified water, and blow-dried by argon.
2.4. Characterization
The fabricated Ag/ZnO NR arrays were observed by a scanning electron microscopy (SEM, Hitachi Regulus 8230), a transmission electron microscope (TEM, JEOL JEM-2100F), and an X-ray diffractometer (XRD, Rigaku SmartLab). Optical absorption spectrum was measured by an ultraviolet, visible and near-infrared (UV–Vis–NIR) spectrophotometer (Hitachi, U-4100). X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi) was employed to analyze surface compositions. A Raman system (Renishaw, in Via-Reflex) with a 532 nm laser was used for SERS measurement. The laser beam was vertically focused onto the SERS substrate, resulting in a laser spot diameter of about 5 μm. The integration duration was 10 s, and the power of laser was about 0.5 mW.
2.5. Preparation of SERS Sample
For the sake of assessing SERS performance of the prepared Ag/ZnO NR arrays, R6G, adenine and PATP were used as probe molecules. ITO glasses (0.5 cm × 0.5 cm) covered with Ag/ZnO NR arrays were placed into aqueous solutions (5 mL) of R6G or adenine, or an ethanol solution (5 mL) of PATP with various concentrations for 3 h. Then, the SERS substrates adsorbed analyte molecules were taken out and blow-dried with Ar gas. After realization of SERS measurement, UV light (365 nm, 220 mW cm−2) was used to remove organic molecules adsorbed on the SERS substrate through photocatalytic degradation to achieve reuse of SERS substrate.
3. Results and Discussion
3.1. Characterization of 3D Ag/ZnO NR Arrays
The morphologies of the prepared nanostructured samples were first characterized by scanning electron microscope (SEM). Vertically aligned ZnO nanorod arrays were grown over a large area on an ITO substrate as shown in Figure 1a. The ZnO nanorods with an average diameter of 200 nm at the top, 300 nm at the root, and an average length ~2 μm were uniformly and densely distributed on the flat surface of ITO substrate (Figure 1a,b). Apparently, the fabricated ZnO nanorods featured a rough surface, a hexagonally tapered shape, and stripes that could be seen visibly on the apexes (Figure 1b), in good agreement with the transmission electron microscope (TEM) observations (Figure S1). The prepared ZnO nanorod arrays provide a three-dimensional framework for the construction of SERS substrate, which has the advantage of a large surface area available for loading Ag nanoparticles and forming a high-density “hot spot”. Then, plenty of tiny silver seeds were successfully deposited uniformly on the surface of ZnO nanorods through a silver mirror reaction (Figure 1c,d). The low density and the larger nanogaps between adjacent silver seeds are not conducive to the generation of high-density of hot spots and lead to low SERS activity. Therefore, the ZnO nanorod arrays modified with silver seeds were subjected to constant current electrodeposition in a silver ammonia solution, where the silver crystal nuclei on the ZnO surface continued to grow in situ. It was found that after electrodeposition irregular Ag nanoparticles with a mean size of ~50 nm covered on surfaces of ZnO NR arrays and linked with each other (Figure 1e,f and Figure S2). There are a large number of nanogaps with sub-10 nm widths (Figure S3) between the mutual Ag nanoparticles, forming three-dimensionally distributed high-density hot spots, which generate a highly enhanced localized electromagnetic field capable of correspondingly amplifying the Raman signal [30]. The energy dispersive X-ray spectrometry (EDS) mapping results further disclosed the existence and even spatial distribution of Zn, O, and Ag elements in Ag/ZnO NR arrays (Figure S4). To further confirm accurately the structure of the Ag/ZnO NR arrays, X-ray diffraction (XRD) was conducted. In Figure S5, the distinct diffraction peaks could be assigned to the (100), (002), (101), (102) and (103) planes of hexagonal wurtzite ZnO. After the decoration of Ag, apart from the diffraction peaks of ZnO, the new peaks around 38.1°, 44.2°, and 77.3° originating from the (111), (200), and (311) planes of face-centered cubic silver, could be easily distinguished. The absorption spectrum of the Ag/ZnO NR composite structure revealed a wide absorption band from 370 nm to 550 nm, and the strong optical absorption of the fabricated Ag/ZnO NR arrays was induced by the localized surface plasmon resonance (LSPR) absorption of Ag nanoparticles (Figure S6). A comparison of SERS spectra of R6G excited by laser beams with different wavelengths (532 nm, 785 nm, and 325 nm) is shown in Figure S7. It was found that the strongest SERS signal was obtained when using the 532 nm laser. Therefore, the excitation laser beam with wavelength of 532 nm was chosen for SERS measurement.
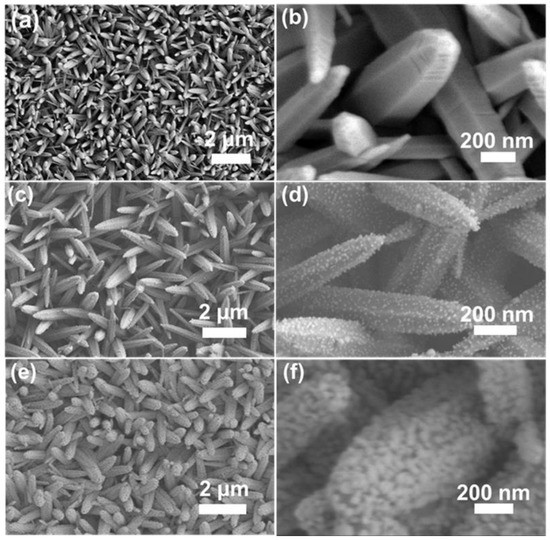
Figure 1.
Morphology characterization. (a) SEM image of ZnO NR arrays. (b) The enlarged view of (a). (c) SEM image of ZnO NR arrays decorated with Ag seeds. (d) The enlarged image of (c). (e) SEM image of Ag/ZnO NR arrays. (f) The enlarged view of (e).
Then, the influences of experimental parameters on the resultant Ag nanoparticles were investigated. First, the parameters for silver mirror reaction were tuned. The size of Ag particles on the surface of ZnO NR arrays increased by changing the concentrations of AgNO3 from 10 to 50 mM and keeping the reaction duration unchanged (20 min) (Figure S8a–c). When the concentration of AgNO3 was too low (e.g., 10 mM), only a small number of Ag nanoparticles were sparsely formed on ZnO NRs (Figure S8a). When the concentration of AgNO3 reached 20 mM, plenty of tiny silver seeds uniformly distributed on the surface of ZnO NRs, which is suitable for the subsequent electrodeposition of Ag nanoparticles. If the concentration of AgNO3 was further raised to a high value (e.g., 50 mM), abundant Ag covered ZnO NR arrays, leading to the formation of big Ag nanoparticles and Ag nanotubes wrapped on ZnO NR arrays. Then, the duration of silver mirror reaction was adjusted to obtain a suitable value. When prolonging the silver mirror reaction duration from 10 to 50 min (Figure S8d–f) and keeping the concentration of AgNO3 solution (20 mM) constant, the size of Ag nanoparticles became bigger and the gaps between adjacent Ag nanoparticles decreased accordingly. The short duration of silver mirror reaction such as 10 min induced Ag particles with very small size that cannot be readily observed in the SEM image (Figure S8d). Under a silver mirror reaction duration of 20 min, plenty of Ag particles were uniformly grown on ZnO NR arrays (Figure S8b,e). When the reaction time was extended to 50 min, lots of much bigger Ag nanoparticles were formed, and a large number of gaps between Ag nanoparticles disappeared (Figure S8f), which would decrease the number of hot spots and weaken the SERS activity. It was found that the size, distribution, and density of Ag nanoparticles could not be easily tuned for high SERS activity only through the parameters of silver mirror reaction. Therefore, using the small Ag particles as seeds, subsequent electrodeposition was performed to achieve vertically aligned ZnO NR arrays decorated with dense Ag nanoparticles which possessed a high density of hot spots for high SERS sensitivity. The Ag particles modified ZnO NR arrays fabricated using 20 mM AgNO3 and under a reaction duration of 20 min during silver mirror reaction were employed for the subsequent electrodeposition. Furthermore, we investigated the growth trend of silver nanoparticles when gradually prolonging electrodeposition durations. When the electrodeposition lasted for 0.5 h or even 1 h, plenty of silver nanoparticles continued to grow bigger and began to connect with each other (Figure S8g,h). The distribution of silver nanoparticles adhered to the surface of ZnO NR arrays was relatively uniform under the electrodeposition duration of 1 h, which can generate dense “hot spots” and highly enhance Raman signals. When the electrodeposition duration was extended to 2 h, a large number of Ag NPs started to agglomerate together, probably inducing the disappearance of some hot spots and remarkably reducing SERS activity (Figure S8i). In order to verify this conjecture, the SERS sensitivity of the substrates achieved under different electrodeposition durations was studied (Figure S9). The intensity of Raman signals increased when the electrodeposition duration was prolonged from 0.5 h to 1 h and then dramatically dropped with further extending electrodeposition duration after reaching a maximum value at an electrodeposition duration of 1 h. Consequently, the Ag/ZnO NR arrays composite prepared via electrodeposition reaction for 1 h was employed as the optimal SERS platforms for subsequent studies.
3.2. SERS Performance of Ag/ZnO NR Arrays
To measure the SERS activity of Ag/ZnO arrays, R6G was employed as a target molecule. A series of distinct R6G peaks can be explicitly discovered in the SERS spectra even if the concentration of an aqueous R6G solution is lowered to 10−12 M (Figure 2a and Figure S10). There are four characteristic peaks around 612, 774, 1185, and 1651 cm−1, and these vibrations correspond to the C-C-C ring in-plane bending, C-H out-of-plane bending, C-H in-plane bending, and the aromatic C-C stretching vibrations, respectively [31,32]. The logarithmic concentration and logarithmic intensity of 612 cm−1 peak showed a strong linear correlation (Figure 2b), as shown below.
where I is the intensity of characteristic peak, and C is R6G concentration. According to the linear fitting result, the R2 value is 0.99. Limit of detection (LOD) is a concentration where signal-to-noise ratio is 3 [33,34,35,36]. The LOD value can be calculated by a linear fit. The LOD of aqueous R6G solutions was evaluated to be 1.0 × 10−13 M, demonstrating the high sensitivity of the fabricated Ag/ZnO NR arrays to R6G [37,38]. The enhancement factor (EF) of the fabricated Ag/ZnO NR arrays is calculated to be as high as 2.7 × 108 (Figure S11, details can be found in Part S3 of the Supporting Information), demonstrating that this SERS substrate has high SERS activity. It was also found that a smaller EF was obtained when estimated using a higher concentration of R6G solution (Figure S12) probably due to that lots of R6G molecules did not locate on the hot spots in the higher concentration situation. It is worth mentioning that the Raman spectrum of polyvinyl chloride film shows no prominent Raman peaks (Figure S13), and that the background signal of R6G SERS spectra detected on the polyvinyl chloride film is removed (Figure S14) for accurate EF estimation. Compared with other SERS substrates made of Ag or Au nanoparticles-modified ZnO nanostructures, our Ag/ZnO NR arrays exhibit comparable or superior sensitivity (Table S1) [39,40,41,42,43,44,45,46]. The high EF of the fabricated SERS substrate is attributed to the three dimensionally distributed hot spots formed in the sub-10 nm gaps between Ag nanoparticles.
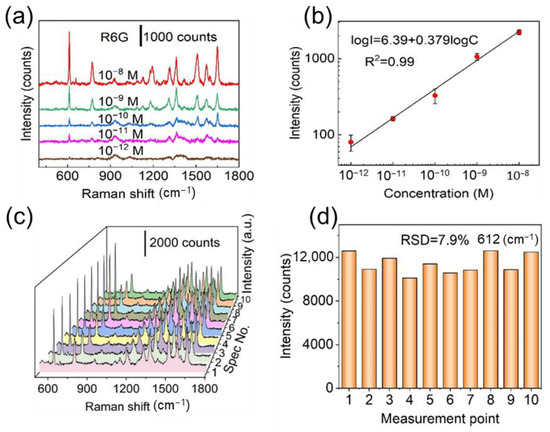
Figure 2.
(a) Raman signal of R6G with concentrations from 10−8 M to 10−12 M. (b) A linear relationship between the logarithmic concentrations and logarithmic intensities at 612 cm−1. (c) SERS spectra of 10−7 M R6G gathered from 10 different points on the fabricated Ag/ZnO NR arrays. (d) Distribution of relative signal intensities at 612 cm−1 peak.
Spectral uniformity and reproducibility are other vital indicators for the practical application of a SERS substrate. As shown in Figure 2c, a series of SERS spectra of 10−7 M R6G randomly collected from 10 different measuring points on Ag/ZnO NR arrays were highly consistent. The relative standard deviation (RSD) of the signal intensity variations at 612 cm−1 was calculated to be 7.9% (Figure 2d). In order to further investigate the uniformity of the fabricated SERS substrate, SERS intensity mapping over an arbitrarily chosen 20 × 20 μm2 region with 220 measurement points was performed (Figure S15). The RSD of intensities of such 220 measurement points is computed to be ~8.5%, indicating the good spectral uniformity. Additionally, the average RSD value of the 612 cm−1 peak from five different substrates is 3.7% (Figure S16), further confirming that such SERS substrate possesses good spectral reproducibility.
In addition to dye molecules such as R6G, which has a much large Raman cross-section, the Ag/ZnO NR arrays also possess high SERS sensitivity to small molecules such as adenine or 4-aminothiophenl (PATP) [47]. Base pairs are the chemical structures that form DNA and RNA monomers. as well as encode genetic information. Abnormal changes in DNA base concentrations are indicative of possible HIV infection, blood disorders, and cancer in the body. As one of the four nucleotide bases that make up DNA and RNA molecules, adenine plays an important role in maintaining the metabolic functions of the organism. Therefore, the detection of adenine is of great importance for clinical medical analysis and diagnosis. The characteristic Raman bands at 733 cm−1 and 1331 cm−1 could be ascribed to the C-H ring breathing mode and N-C vibrational of adenine, respectively (Figure 3a and Figure S17) [48,49]. The fabricated Ag/ZnO arrays can detect adenine at a concentration as low as 10−8 M (Figure S18). The LOD was calculated to be 3 × 10−11 M for adenine and the R2 value was 0.99 (Figure 3b), showing promising potential for sensitive detection of adenine. Likewise, the Ag/ZnO NR arrays can be applied to identify PATP that is used as a synthetic intermediate of between a dye and pesticide and is harmful to human health and pollutes environment. The characteristic bands of PATP (10−10 M) at 1075 cm−1 (C-S stretching mode), 1142 cm−1 (the C-H bending modes), and 1390 cm−1 and 1435 cm−1 (a combination of the C-C stretching mode and C-H bending mode) can be observed remarkably in Figure 3c and Figure S19 [50]. A linear relationship, with R2 = 0.99, was established between the logarithmic concentration and the logarithmic intensity of 1435 cm−1 peak for PATP (Figure 3d). The LOD for PATP was computed to be 1.6 × 10−12, further demonstrating that the fabricated Ag/ZnO NR arrays have high sensitivity to small molecules.
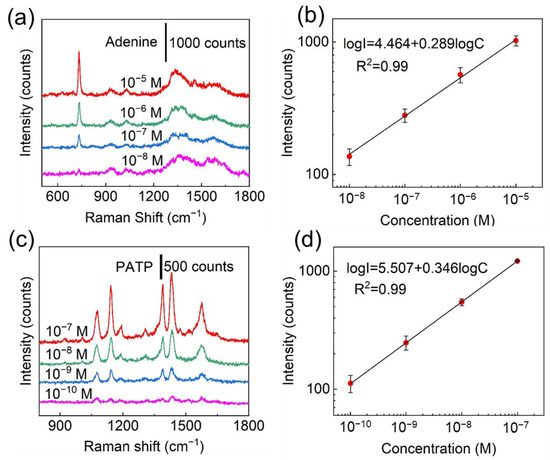
Figure 3.
(a) Raman spectra of adenine at concentrations from 10−5 M to 10−8 M. (b) Linear relationship between logarithmic concentrations of adenine and logarithmic SERS intensities of 733 cm−1 peak. (c) SERS spectra of PATP at concentrations ranging from 10−7 M to 10−10 M. (d) Linear relationship between logarithmic intensities at 1435 cm−1 and logarithmic concentration of PATP.
From the viewpoint of practical applications, the prepared Ag/ZnO NR arrays should possess multicomponent detection capability or the ability to resist interference. A complex system consisting of R6G, PATP, and adenine was employed to demonstrate the multicomponent detection ability. It could be found that the composite SERS spectrum of the multicomponent sample contained their respective characteristic peaks (Figure 4), suggesting that the Ag/ZnO NR arrays have capability to sensitively detect complex components. The Ag/ZnO NR arrays can also be employed to detect R6G in spiked lake water (Figure S20). Apparently, even if the concentration of R6G is under a very low level, its primary characteristic peaks can still be ascertained. The lowest detectable concentration for R6G in spiked lake water is 10−11 M. Therefore, the constructed Ag/ZnO NR arrays could be utilized to rapidly detect some pollutants in aquatic environments.
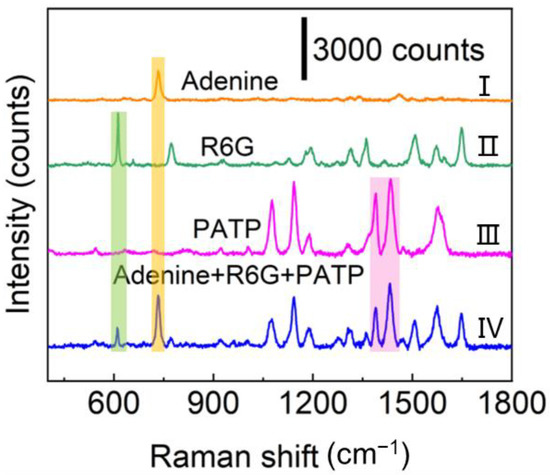
Figure 4.
Raman spectra of (I) 10−4 M adenine, (II) 10−6 M R6G, (III) 10−4 M PATP, and (IV) the mixed ternary analytes containing 10−6 M R6G, 10−4 M adenine and 10−4 M PATP.
The feature of long-term stability is certainly important for the fabricated SERS substrate. As reported previously, the oxidation of silver may lead to a reduction in the activity of silver-based SERS substrate [51,52,53]. An X-ray photoelectron spectrometer (XPS) was employed to analyze the freshly fabricated substrates as well as those being stored for six months in order to precisely prove the oxidation of silver nanomaterials on ZnO NRs (Figure S21a,b). Ag 3d XPS spectra showed that there were Ag+ peaks after the sample being stored for 6 months, proving that the Ag atoms on the surface of the Ag nanoparticles had been indeed oxidized. Therefore, to evaluate the SERS stability of the substrate, the Ag/ZnO NR arrays stored for 6 months was immersed in an aqueous solution of R6G (10−7 M) for subsequent SERS measurement. As displayed in Figure S21c,d, comparing the SERS spectrum from the newly prepared substrate with that from the substrate stored for 6 months, intensities of the peaks at 612 cm−1, 772 cm−1, and 1361 cm−1 declined slightly, demonstrating the good long-term stability of such SERS substrate.
3.3. Recyclable Properties of the Ag/ZnO NR Arrays
The self-cleaning capacity plays a significant role in SERS detection application [54,55,56]. For the photocatalytic degradation of organic molecules under UV light irradiation, ZnO nanomaterial functions as a high-performance photocatalyst [57]. The intensity of R6G peak at different UV light treatment durations is shown in Figure 5a. It was found that the intensity of R6G SERS signals gradually dropped with the extension of UV light treatment duration. The results indicate that the characteristic SERS peaks of R6G were hard to be measured after UV light treatment for 60 min, demonstrating that R6G molecules have been entirely decomposed and removed due to the presented outstanding photocatalytic degradation performance of Ag/ZnO NR hybrid arrays. Our substrate’s photocatalytic performance is comparable to or surpasses that reported in the previous papers (Table S2) [18,44,45,46,58,59,60,61,62]. The photocatalytic degradation mechanism of the Ag/ZnO hybrid NR arrays is portrayed in Figure 5b. The UV light irradiation induces electron transition from the valence band (VB) of ZnO to its conduction band (CB), forms VB holes and generates electron–hole pairs. The active holes could oxidize H2O to form active hydroxyl radicals, and at the same time, CB electrons are captured by O2 to generate superoxide radical anions. The superoxide radical anions and oxidative hydroxyl radicals produced can decompose R6G into CO2 and H2O [28,58,62,63] The self- cleaning cycles were accomplished via adsorbing 10−7 M R6G for 3 h and then illuminating the Ag/ZnO substrate by UV light for 1 h in the water. The SERS spectra collected from the Ag/ZnO NR arrays before UV irradiation exhibit all the characteristic peaks of R6G (Figure 5c). The prominent SERS peaks of R6G could still be clearly observed after several photocatalytic degradation cycles. Notably, after the photocatalytic degradation process being repeated for four times, the SERS spectra of R6G on the self-cleaning Ag/ZnO NR substrate were comparable to those collected from the pristine substrate. Figure 5d clearly reflects the SERS intensity change at the 612 cm−1 peak of R6G collected from the substrate over four cycles. There was no noticeable decline in the SERS intensity of R6G as the cycle number increased, that is to say, several repeated Raman measurements and photocatalytic degradation had no significant impact on the SERS activity of Ag/ZnO NR arrays. The recyclability and reusability of the Ag/ZnO substrate are also suitable for some other organic molecules such as PATP and adenine (Figure S22), which reflects the high photocatalytic activity and recyclability of the fabricated Ag/ZnO NR arrays for practical SERS detection applications.
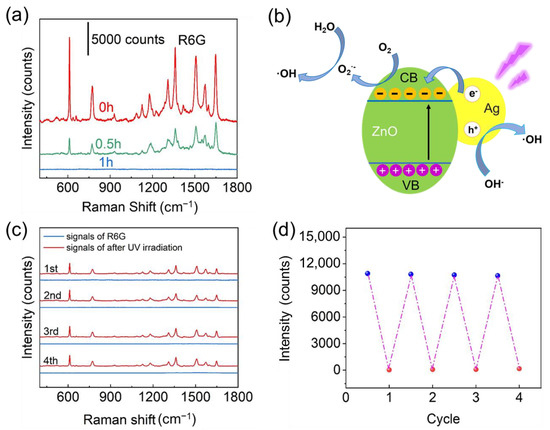
Figure 5.
(a) SERS spectra of R6G (10−7 M) obtained from the Ag/ZnO NR arrays after adsorbing R6G molecules and subsequent exposure to UV light for 0, 0.5 h, and 1 h. (b) Mechanistic diagram of photocatalytic degradation for the Ag/ZnO hybrid nanomaterial. (c) SERS spectra collected from a 10−7 M R6G loaded SERS substrate before and after subsequent photocatalytic degradation. (d) Intensity of 612 cm−1 peak (10−7 M R6G) in 4 cycles.
4. Conclusions
In summary, vertically aligned ZnO nanorods decorated with dense Ag nanoparticles were synthesized by a simple method. ZnO nanorods were uniformly and densely grown on a flat conductive substrate via a hydrothermal approach. Then, the Ag nanoparticles were densely decorated on the surface of ZnO nanorods through silver mirror reaction and seed-assisted electrodeposition. The fabricated Ag nanoparticles decorated ZnO nanorod arrays can serve as three-dimensional SERS substrates with high-density hot spots for sensitive detection. Such Ag/ZnO nanostructure hybrid SERS substrate showed high SERS activity to rhodamine 6G (EF~2.7 × 108), good spectral uniformity (RSD~7.9%), and reproducibility (RSD~3.7%). In addition, the Ag nanoparticles decorated ZnO nanorod arrays displayed high photocatalytic efficiency for complete degradation of R6G molecules within 60 min via UV irradiation in water, allowing its reuse at least for 4 cycles without any loss of SERS activity. Furthermore, the prepared Ag nanoparticles decorated ZnO nanorod arrays also can be employed for SERS detection of small molecules and achieved LODs down to 3 × 10−11 M and 1.6 × 10−12 M for adenine and 4-aminothiophenl, respectively. Additionally, the fabricated Ag nanoparticles decorated ZnO nanorod arrays also showed the capability of sensitive detection of mixtures of different organics. Therefore, the fabricated reusable three-dimensional SERS substrate exhibited potential applications for the sensitive detection of chemical molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11040210/s1, Figure S1: TEM image of ZnO nanorods; Figure S2: TEM image of a single ZnO nanorod decorated with Ag nanoparticles; Figure S3: SEM image of Ag/ZnO marked with gap distances between neighboring Ag nanoparticles; Figure S4: Energy dispersive spectrometry (EDS) mapping images of Ag/ZnO NR; Figure S5: XRD patterns of ITO glass and Ag/ZnO NR arrays; Figure S6: UV-vis absorption spectrum of ZnO NR and Ag/ZnO NR arrays and Ag nanoparticles; Figure S7: Raman spectra of R6G detected using different excitation wavelengths. Figure S8: SEM images of the Ag/ZnO NR arrays after silver mirror reaction under different concentrations and durations and the subsequent electrodepositions; Figure S9: SERS spectra of R6G (10−7 M) collected from Ag/ZnO NR arrays fabricated by electrodeposited for 0.5, 1 and 2 h, respectively; Figure S10: SERS spectrum of 10−12 M R6G; Figure S11: Normal Raman spectrum of R6G (10−2 M) on a polyvinyl chloride film, and SERS spectrum of 10−10 M R6G absorbed on the fabricated Ag/ZnO NR arrays; Figure S12: EFs calculated by R6G with different concentrations of 10–8 M, 10–9 M and 10–10 M absorbed on the fabricated Ag/ZnO NR arrays; Figure S13: Raman spectrum of a polyvinyl chloride film; Figure S14: Normal Raman spectrum of R6G (10−2 M) on a polyvinyl chloride film after removal of background signal; Figure S15: SERS mapping image of R6G (10−6 M, 612 cm−1 peak intensity) on Ag/ZnO arrays with an intensity RSD of ~8.5%; Figure S16: R6G (612 cm–1) peak intensity distribution of five different samples. The RSD value is 3.7%; Figure S17: SERS spectrum of 10–3 M adenine; Figure S18: SERS spectrum of 10–8 M adenine; Figure S19: SERS spectrum of 10–10 M PATP; Figure S20: SERS spectra of R6G in spiked lake water under different concentrations; Figure S21: (a) Ag 3d XPS spectrum of a freshly prepared substrate. (b) Ag 3d XPS spectrum of a substrate stored for 6 months. (c) SERS spectra of R6G (10−7 M) collected from the freshly prepared substrate and the one kept for 6 months. (d) The intensity of R6G peaks at 612 cm−1, 772 cm−1 and 1361 cm−1 from the freshly prepared substrate and the one being stored for 6 months; Figure S22: (a) SERS spectra of PATP (10−5 M) collected from Ag/ZnO NR arrays after UV irradiation for 0, 30 min, and 60 min. (b) SERS spectra of adenine (10−3 M) collected from Ag/ZnO NR arrays after UV irradiation for 0, 30 min, and 45 min. (c) SERS spectra of 10−5 M PATP in 3 self-cleaning cycles. (d) SERS spectra of 10−3 M adenine in 3 self-cleaning cycles. Table S1: Comparison of detection limits/LOD and enhancement factors for R6G; Table S2: Comparison of the time for R6G degradation on different SERS substrates [18,44,45,46,51,54,55,56,57,58,59,60,61,62,63,64].
Author Contributions
Conceptualization, N.L. and C.Z.; methodology, N.L. and C.Z.; validation, N.L. and C.Z.; formal analysis, N.L. and C.Z.; investigation, N.L.; resources, C.Z., Y.Y. and G.X.; data curation, N.L.; writing—original draft preparation, N.L.; writing—review and editing, C.Z., Y.Y., M.Y., B.C. and G.X.; supervision, C.Z.; project administration, C.Z.; funding acquisition, C.Z. and B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 22175002) and Natural Science Foundation of Anhui Province (1808085J12 and 2108085MB40).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, X.; Hu, Z.; Yang, D.; Xie, S.; Jiang, Z.; Niessner, R.; Haisch, C.; Zhou, H.; Sun, P. Bacteria Detection: From Powerful SERS to Its Advanced Compatible Techniques. Adv. Sci. 2020, 7, 2001739. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Zhang, Q.; Sun, P. Application of Surface-Enhanced Raman Spectroscopy in Fast Detection of Toxic and Harmful Substances in Food. Biosens. Bioelectron. 2020, 167, 112480. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Wang, Y.; DuChene, J.; Huo, F.; Wei, W. An in situ Approach for Facile Fabrication of Robust and Scalable SERS Substrates. Nanoscale 2014, 6, 7232. [Google Scholar] [CrossRef] [PubMed]
- Cialla, D.; Marz, A.; Bohme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-Enhanced Raman Spectroscopy (SERS): Progress and Trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.-H.; Kim, G.; Noh, M.S.; Kang, H.; Kim, Y.-K.; Cho, M.-H.; Jeong, D.H.; Lee, Y.-S. Surface-Enhanced Raman Scattering-Active Nanostructures and Strategies for Bioassays. Nanomed. 2011, 6, 1463–1480. [Google Scholar] [CrossRef]
- Guo, L.; Tang, H.; Wang, X.; Yuan, Y.; Zhu, C. Nanoporous Ag-Decorated Ag7O8NO3 Micro-Pyramids for Sensitive Surface-Enhanced Raman Scattering Detection. Chemosensors 2022, 10, 539. [Google Scholar] [CrossRef]
- Zhai, H.; Zhu, C.; Wang, X.; Yuan, Y.; Tang, H. Arrays of Ag-Nanoparticles Decorated TiO2 Nanotubes as Reusable Three-Dimensional Surface-Enhanced Raman Scattering Substrates for Molecule Detection. Front. Chem. 2022, 10, 992236. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Li, H.; Luo, Y.; Yu, R.; Zhang, L.; Yang, Y.; Song, Q. Au Nanoflower–Ag Nanoparticle Assembled SERS-Active Substrates for Sensitive MC-LR Detection. Chem. Commun. 2015, 51, 16908–16911. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.M.; Zhu, J.; Li, H.; Chen, Q. Functionalized Hollow Au@Ag Nanoflower SERS Matrix for Pesticide Sensing in Food. Sens. Actuators B Chem. 2020, 324, 128718. [Google Scholar] [CrossRef]
- Gambucci, M.; Cambiotti, E.; Sassi, P.; Latterini, L. Multilayer Gold-Silver Bimetallic Nanostructures to Enhance SERS Detection of Drugs. Mol. Basel Switz. 2020, 25, 3405. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, Z.Y.; Dluhy, R.A.; Zhao, Y.P. The SERS Response of Semiordered Ag Nanorod Arrays Fabricated by Template Oblique Angle Deposition. Raman Spectrosc. 2010, 41, 1112–1118. [Google Scholar] [CrossRef]
- Wu, C.; Hu, Q.; Benison, M.; Faulds, K.; Graham, D. Modulation of Interparticle Gap for Enhanced SERS Sensitivity in Chemically Stable Ag@Au Hetero-Architectures. New J. Chem. 2020, 44, 13843–13851. [Google Scholar] [CrossRef]
- Wang, D.; Hui, B.; Zhang, X.; Zhu, J.; Gong, Z.; Fan, M. Facile Preparation of Ag-NP-Deposited HRGB-SERS Substrate for Detection of Polycyclic Aromatic Hydrocarbons in Water. Chemosensors 2022, 10, 406. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Wang, Y.; Mei, R.; Fu, L.; Li, J.; Wang, X.; Chen, L. Chiral Molecular Imprinting-Based SERS Detection Strategy for Absolute Enantiomeric Discrimination. Nat. Commun. 2022, 13, 5757. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, Z.; Wang, W.; Wu, Y.; Xu, H. Highly Surface-Roughened “Flower-like” Silver Nanoparticles for Extremely Sensitive Substrates of Surface-Enhanced Raman Scattering. Adv. Mater. 2009, 21, 4614–4618. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Ma, Y.; Li, S.; Wei, Y.; Huang, Z.; Long, N.V. Fabrication of Semiconductor ZnO Nanostructures for Versatile SERS Application. Nanomaterials 2017, 7, 398. [Google Scholar] [CrossRef]
- Kandjani, A.E.; Mohammadtaheri, M.; Thakkar, A.; Bhargava, S.K.; Bansal, V. Zinc Oxide/Silver Nanoarrays as Reusable SERS Substrates with Controllable ‘Hot-Spots’ for Highly Reproducible Molecular Sensing. J. Colloid Interface Sci. 2014, 436, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, S.; Yang, X.; Yuan, R.; Chai, Y. A SERS Biosensor Constructed by Calcined ZnO Substrate with High-Efficiency Charge Transfer for Sensitive Detection of Pb2+. Sens. Actuators B Chem. 2021, 343, 130142. [Google Scholar] [CrossRef]
- Zheng, Z.; Cong, S.; Gong, W.; Xuan, J.; Li, G.; Lu, W.; Geng, F.; Zhao, Z. Semiconductor SERS Enhancement Enabled by Oxygen Incorporation. Nat Commun. 2017, 8, 1993. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, X.; Fu, Q. TiO2 Thickness-Dependent Charge Transfer in an Ordered Ag/TiO2/Ni Nanopillar Arrays Based on Surface-Enhanced Raman Scattering. Mater. Basel Switz. 2022, 15, 3716. [Google Scholar] [CrossRef]
- Marica, I.; Nekvapil, F.; Ștefan, M.; Farcău, C.; Falamaș, A. Zinc Oxide Nanostructures for Fluorescence and Raman Signal Enhancement: A Review. Beilstein J. Nanotechnol. 2022, 13, 472–490. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Peng, J.; Huang, B.; Deng, W.; Xie, W.; Luo, Z. Preparation of CuO Nanowires/Ag Composite Substrate and Study on SERS Activity. Plasmonics 2021, 16, 1059–1070. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, W.; Yang, J.; Xu, Q.; Hu, X.-Y. Improved SERS Performance of a Silver Triangular Nanoparticle/TiO2 Nanoarray Heterostructure and Its Application for Food Additive Detection. New J. Chem. 2022, 46, 7070–7077. [Google Scholar] [CrossRef]
- Xue, X.; Chen, L.; Wang, L.; Wang, C.; Qiao, Y.; Zhao, C.; Wang, H.; Nie, P.; Shi, J.; Chang, L. Facile Fabrication of PS/Cu2S/Ag Sandwich Structure as SERS Substrate for Ultra-Sensitive Detection. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2022, 265, 120370. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jin, B.; Liu, H.; Li, X.; Zhang, Q.; Chu, S.; Peng, R.; Chu, S. Controllable Synthesis of Flower-like MoSe2 3D Microspheres for Highly Efficient Visible-Light Photocatalytic Degradation of Nitro-Aromatic Explosives. J. Mater. Chem. A 2018, 6, 11424–11434. [Google Scholar] [CrossRef]
- Mendonça, C.D.; Khan, S.U.; Rahemi, V.; Verbruggen, S.W.; Machado, S.A.S.; De Wael, K. Surface Plasmon Resonance-Induced Visible Light Photocatalytic TiO2 Modified with AuNPs for the Quantification of Hydroquinone. Electrochimica Acta 2021, 389, 138734. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A Highly Efficient Ag-ZnO Photocatalyst: Synthesis, Properties, and Mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, C.; Meng, G.; Wu, N. Review—Surface-Enhanced Raman Scattering Sensors for Food Safety and Environmental Monitoring. J. Electrochem. Soc. 2018, 165, B3098–B3118. [Google Scholar] [CrossRef]
- Zhao, W.; Xiao, S.; Zhang, Y.; Pan, D.; Wen, J.; Qian, X.; Wang, D.; Cao, H.; He, W.; Quan, M.; et al. Binary “Island” Shaped Arrays with High-Density Hot Spots for Surface-Enhanced Raman Scattering Substrates. Nanoscale 2018, 10, 14220–14229. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsu, T.-C.; Liu, Y.-C.; Yang, K.-H. Surface-Enhanced Raman Scattering-Active Silver Substrates Electrochemically Prepared in Solutions Containing Bielectrolytes. J. Mater. Chem. 2011, 21, 6660–6667. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yu, C.-C.; Sheu, S.-F. Improved Surface-Enhanced Raman Scattering on Optimum Electrochemically Roughened Silver Substrates. Anal. Chim. Acta 2006, 577, 271–275. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, S.; Huo, Y.; Ning, T.; Liu, A.; Zhang, C.; He, Y.; Wang, M.; Li, C.; Man, B. 3D Silver Nanoparticles with Multilayer Graphene Oxide as a Spacer for Surface Enhanced Raman Spectroscopy Analysis. Nanoscale 2018, 10, 5897–5905. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection a Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-Inspired 3D SERS Substrate for Multiple Mycotoxins Detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, Y.; Gao, Z.; Zhou, H.; Zhang, Q.; Xu, R.; Zhang, C.; Yao, H.; Liu, M. High-Performance Surface-Enhanced Raman Scattering Substrates Based on the ZnO/Ag Core-Satellite Nanostructures. Nanomaterials 2022, 12, 1286. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Khanna, S.; Utsav; Paneliya, S.; Takhar, V.; Banerjee, R.; Mukhopadhyay, I. Fabrication of Silver Nanodome Embedded Zinc Oxide Nanorods for Enhanced Raman Spectroscopy. Colloids Surf. Physicochem. Eng. Asp. 2022, 639, 128336. [Google Scholar] [CrossRef]
- Zhang, G.; Deng, C.; Shi, H.; Zou, B.; Li, Y.; Liu, T.; Wang, W. ZnO/Ag Composite Nanoflowers as Substrates for Surface-Enhanced Raman Scattering. Appl. Surf. Sci. 2017, 402, 154–160. [Google Scholar] [CrossRef]
- Sakir, M.; Salem, S.; Sanduvac, S.T.; Sahmetlioglu, E.; Sarp, G.; Onses, M.S.; Yilmaz, E. Photocatalytic Green Fabrication of Au Nanoparticles on ZnO Nanorods Modified Membrane as Flexible and Photocatalytic Active Reusable SERS Substrates. Colloids Surf. Physicochem. Eng. Asp. 2020, 585, 124088. [Google Scholar]
- He, X.; Yue, C.; Zang, Y.; Yin, J.; Sun, S.; Li, J.; Kang, J. Multi-Hot Spot Configuration on Urchin-like Ag Nanoparticle/ZnO Hollow Nanosphere Arrays for Highly Sensitive SERS. J. Mater. Chem. A 2013, 1, 15010–15015. [Google Scholar] [CrossRef]
- Tiwari, M.; Singh, A.; Dureja, S.; Basu, S.; Pattanayek, S.K. Au Nanoparticles Decorated ZnO/ZnFe2O4 Composite SERS-Active Substrate for Melamine Detection. Talanta 2022, 236, 122819. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, Y.; Cao, Y.; Fu, C.; Huang, Z. Synthesis of Wheatear-like ZnO Nanoarrays Decorated with Ag Nanoparticles and Its Improved SERS Performance through Hydrogenation. Nanotechnology 2016, 27, 145502. [Google Scholar] [CrossRef]
- Korkmaz, I.; Sakir, M.; Sarp, G.; Salem, S.; Torun, I.; Volodkin, D.; Yavuz, E.; Onses, M.S.; Yilmaz, E. Fabrication of Superhydrophobic Ag@ZnO@Bi2WO6 Membrane Disc as Flexible and Photocatalytic Active Reusable SERS Substrate. J. Mol. Struct. 2021, 1223, 129258. [Google Scholar] [CrossRef]
- Xiao, C.; Xiao, B.; Wang, Y.; Zhang, J.; Wang, S.; Wang, P.; Yang, T.; Zhao, R.; Yu, H.; Li, Z.; et al. Synthesis of ZnO Nanosheets Decorated with Au Nanoparticles and Its Application in Recyclable 3D Surface-Enhanced Raman Scattering Substrates. RSC Adv. 2015, 5, 17945–17952. [Google Scholar] [CrossRef]
- Tieu, D.T.; Quynh Trang, T.N.; Tuan Hung, L.V.; Hanh Thu, V.T. Assembly Engineering of Ag@ZnO Hierarchical Nanorod Arrays as a Pathway for Highly Reproducible Surface-Enhanced Raman Spectroscopy Applications. J. Alloys Compd. 2019, 808, 151735. [Google Scholar] [CrossRef]
- Pal, A.K.; Chandra, G.K.; Umapathy, S.; Mohan, D.B. Ultra-Sensitive, Reusable, and Superhydrophobic Ag/ZnO/Ag 3D Hybrid Surface Enhanced Raman Scattering Substrate for Hemoglobin Detection. J. Appl. Phys. 2020, 127, 164501. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Fateixa, S.; Nogueira, H.I.S.; Trindade, T. SERS Study on Adenine Using a Ag/Poly(t-Butylacrylate) Nanocomposite. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2013, 101, 36–39. [Google Scholar] [CrossRef]
- Tegegne, W.A.; Su, W.-N.; Beyene, A.B.; Huang, W.-H.; Tsai, M.-C.; Hwang, B.-J. Flexible Hydrophobic Filter Paper-Based SERS Substrate Using Silver Nanocubes for Sensitive and Rapid Detection of Adenine. Microchem. J. 2021, 168, 106349. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, S.; Guang, S.; Ge, F.; Wang, J. Ag-Coated Nylon Fabrics as Flexible Substrates for Surface-Enhanced Raman Scattering Swabbing Applications. J. Mater. Res. 2020, 35, 1271–1278. [Google Scholar] [CrossRef]
- Erol, M.; Han, Y.; Stanley, S.K.; Stafford, C.M.; Du, H.; Sukhishvili, S. SERS Not to Be Taken for Granted in the Presence of Oxygen. J. Am. Chem. Soc. 2009, 131, 7480–7481. [Google Scholar] [CrossRef]
- Gao, N.; Yang, T.; Liu, T.; Zou, Y.; Jiang, J. Graphene Oxide Wrapped Individual Silver Nanocomposites with Improved Stability for Surface-Enhanced Raman Scattering. RSC Adv. 2015, 5, 55801–55807. [Google Scholar] [CrossRef]
- Han, Y.; Lupitskyy, R.; Chou, T.-M.; Stafford, C.M.; Du, H.; Sukhishvili, S. Effect of Oxidation on Surface-Enhanced Raman Scattering Activity of Silver Nanoparticles: A Quantitative Correlation. Anal. Chem. 2011, 83, 5873–5880. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, W.; Yao, L.; Wang, J.; Han, H.; Zhu, T.; Liang, Y.; Fu, J.; Wang, Y. 3D Ag/ZnO Microsphere SERS Substrate with Ultra-Sensitive, Recyclable and Self-Cleaning Performances: Application for Rapid in Site Monitoring Catalytic Dye Degradation and Insight into the Mechanism. Colloids Surf. Physicochem. Eng. Asp. 2020, 607, 125507. [Google Scholar] [CrossRef]
- Wu, J.Y.; Hsieh, C.-H.; Feria, D.N.; Shen, J.-L. PAA/ZnO Raspberry-Shaped Composite Microspheres Decorated with Ag Nanoparticles as Cleanable SERS Substrates. ACS Omega 2020, 5, 29795–29800. [Google Scholar] [CrossRef]
- Zhao, K.; Lin, J.; Guo, L. ZnO/Ag Porous Nanosheets Used as Substrate for Surface-Enhanced Raman Scattering to Detect Organic Pollutant. RSC Adv. 2015, 5, 53524–53528. [Google Scholar] [CrossRef]
- Su, G.; Dang, L.; Liu, G.; Feng, T.; Wang, W.; Wang, C.; Wei, H. MOF-Derived Hierarchical Porous 3D ZnO/Ag Nanostructure as a Reproducible SERS Substrate for Ultrasensitive Detection of Multiple Environmental Pollutants. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2022, 270, 120818. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Ma, Z.; Wang, M.; Zhao, R.; Zou, Y.; Fan, Y. Ag Nanoparticles Decorated ZnO Nanorods as Multifunctional SERS Substrates for Ultrasensitive Detection and Catalytic Degradation of Rhodamine B. Nanomaterials 2022, 12, 2394. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, M.; Li, Z.; Du, Z.; Chen, B.; He, X.; Zhao, S. Hexagonally Arranged Arrays of Urchin-like Ag-Nanoparticle Decorated ZnO-Nanorods Grafted on PAN-Nanopillars as Surface-Enhanced Raman Scattering Substrates. CrystEngComm 2018, 20, 3550–3558. [Google Scholar] [CrossRef]
- Ye, F.; Ju, S.; Liu, Y.; Jiang, Y.; Chen, H.; Ge, L.; Yan, C.; Yuan, A. Ag-CuO Nanocomposites: Surface-Enhanced Raman Scattering Substrate and Photocatalytic Performance. Cryst. Res. Technol. 2019, 54, 1800257. [Google Scholar] [CrossRef]
- Singh, N.; Prakash, J.; Misra, M.; Sharma, A.; Gupta, R.K. Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds. ACS Appl. Mater. Interfaces 2017, 9, 28495–28507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, L.; Xi, M.; Feng, Q.; Jiang, C.; Fong, H. Electrospun TiO2 Nanofelt Surface-Decorated with Ag Nanoparticles as Sensitive and UV-Cleanable Substrate for Surface Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2014, 6, 5759–5767. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, A.; Wang, J.; Ge, F.; Zhu, Q. Recyclable SERS Substrate with Coral-like Nano Ag/ZnO Structure Based on Cotton Fabric Used for In-Situ Detection of Pesticides. Fibers Polym. 2022, 23, 636–643. [Google Scholar] [CrossRef]
- Pal, A.K.; Pagal, S.; Prashanth, K.; Chandra, G.K.; Umapathy, S.; Mohan, D.B. Ag/ZnO/Au 3D Hybrid Structured Reusable SERS Substrate as Highly Sensitive Platform for DNA Detection. Sens. Actuators B Chem. 2019, 279, 157–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).