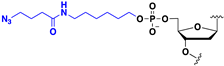
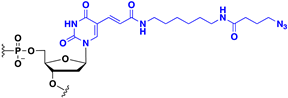
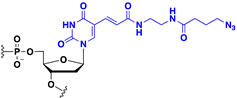
Abstract
Forced intercalation (FIT) probes have proven to be a reliable, rapid, inexpensive, and accurate method for the detection and visualization of specific nucleic acid sequences. The accommodation of a rationally designed chromone-based fluorogen within a double-stranded DNA structure was investigated by UV–Vis spectrophotometry and steady-state fluorescence spectroscopy under physiological pH conditions. After selective excitation matching with a 350 nm laser, the intrinsically negligible fluorescence of the tethered electroneutral label in a single-stranded context was increased 10-fold upon duplex formation. This fluorescence enhancement was also accompanied by a mega-Stokes shift (~100 nm) that placed the emission in the cyan color range; both features are appreciable for bio-imaging purposes. In sum, its fluorogenic behavior and its marginal impact on the double helix make this dye a prospective tool for selectively sensing sequences of interest with a remarkable ON/OFF contrast.
1. Introduction
Chromone dyes belong to the family of flavonoids—which are a group of plant secondary metabolites—found in vascular plants such as ferns and flowering plants [1]. 1-Benzopyran-4-one is the core fragment common to all flavones, which are considered a class of natural dyes (Scheme 1) [2]. Flavone derivatives with a hydroxyl group at the 3-position of the heterocycle are called 3-hydroxychromones (3HCs) [3]. These have been widely studied in the last decades because of their atypical photophysical properties [4,5]. Indeed, 3HCs are ratiometric fluorescent dyes presenting a well-resolved dual emission resulting from two excited species—the normal form (N*) and its tautomer (T*)—obtained after an excited-state intramolecular proton transfer (ESIPT) reaction [6,7]. It is noteworthy that the intensity ratio of these two-color fluorophores is extremely sensitive to environmental changes, and their N* band exhibits remarkable solvatofluorochromism over a wide range of polarities [8]. In our group, we have synthesized a library of 3HC derivatives over the past few years, extensively investigated their photophysics, and explored a variety of nucleic acid (NA)-related applications [9]. These advanced probes allowed us to overcome several bottlenecks encountered with currently used biosensors for NA labeling [10,11,12]. After a proof of concept of the 3HC efficiency for internal DNA labeling [13,14], this nucleobase replacement strategy was applied to preliminary mechanistic studies of DNA repair involving base excision [15,16]. The mechanism of DNA methylation involving the key step of 5mC eversion by the chaperone protein UHRF1 was also studied with this two-color reporter [17]. Both examples of this two-band fluorescence-based approach attested to the outstanding sensitivity of this nucleobase surrogate to small structural changes accompanying base flipping, while the structure of the duplex and its protein affinity were marginally affected, as subsequently confirmed [18,19]. Recently, we engineered an efficient synthetic route to DNA major groove labeling by electronically conjugating a 3HC fluorochrome to deoxyadenosine and uridine moieties [20,21,22,23,24,25].
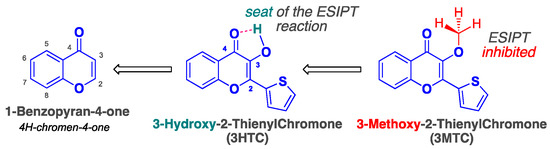
Scheme 1.
Numbering and general structures of the chromone scaffold involved in the present study.
Labeling NAs by means of emissive dyes to establish a fluorescent probe has been an active area of research in modern chemical biology for accurately sensing and localizing sequences of interest within cells [26,27]. Fluorescence is the technique of choice due to its non-invasive nature, exquisite sensitivity, and simplicity of implementation [28]. To advance this research field, a variety of probes have been developed, such as FRET-based binary probes and molecular beacons [29], reactive probes [30], ECHO probes [31,32,33], molecular rotors [34,35,36], and forced intercalation (FIT) probes [37,38]. Of these, fluorogenic FIT probes show a marked enhancement of fluorescence upon sequence-specific hybridization [39,40,41,42,43,44,45,46,47,48]. This turn-on is mainly due to steric changes in the vicinity of the reporter, which will prevent the free rotation of the scaffold around a strategic single bond and thus force the system to adopt a planar conformation capable of fluorescing [37]. Note that fluorogenic hybridization probes are also very useful for imaging NAs in living cells, where distinguishing between unbound and bound probes is almost impossible [49].
The literature data show that a planar aromatic moiety associated with a cationic group is required for efficient intercalation into the polyanion that is DNA [50]. Several classes of dyes, such as phenanthridine derivatives, thiazole orange (TO) analogs, and cyanines, have been developed on the basis of the above prerequisites and successfully applied to detect NAs in the cells, as well as in real-time polymerase chain reaction (PCR) [51,52,53]. Much less attention has been paid to the development of fluorescent covalent intercalators that are electrically neutral, sensitive to the local environment, and derived from natural small dyes [54]. In addition to the ability to substantially increase their fluorescence intensity upon intercalation, such size-controlled emitters could minimize duplex destabilization [55,56,57]. Herein, we report the synthesis and spectroscopic features of a novel 3HC-based FIT probe, which is small [58], electroneutral, and easy to chemically conjugate with functionalized oligodeoxynucleotides (ODNs). After screening different parameters, such as the nature of the linker and its location, a single-stranded FIT probe was eventually identified as promising upon hybridization, since it demonstrated a 10-fold fluorescence enhancement, accompanied by a mega-Stokes shift that placed the emission in the turquoise region (Figure 1).
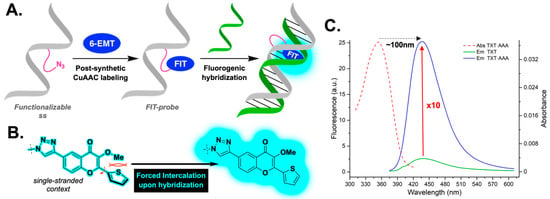
Figure 1.
(A) Schematic representation of the operating principle of the developed FIT probe to specifically sense a sequence by hybridization. (B) Zoomed in on the 6-EMT reporter clicked on a single strand to shed light on the forced intercalation at the molecular level. (C) Photophysical channels of information through which fluorogenic detection occurs.
2. Materials and Methods
2.1. General Methods and Instruments
All reactions requiring air- and water-sensitive conditions were conducted in oven-dried glassware with anhydrous solvents and under argon using Schlenk techniques with a double vacuum/argon manifold system. Synthetic intermediates were first coevaporated twice with toluene and dried under vacuum before use. All chemicals were obtained from commercial suppliers (Sigma-Aldrich (Saint-Louis, MO, USA), Acros (Geel, Belgium), Alfa Aesar (Heysham, Lancashire, UK)) and used as provided. Anhydrous solvents were prepared according to conventional procedures [59]. Reactions were controlled concomitantly by liquid chromatography–mass spectrometry (LC-MS) and thin-layer chromatography (TLC, silica gel 60 F254 plates). Intermediates and derivatives were visualized on TLC plates by both UV irradiation (254 and 365 nm) and spraying with a staining agent (ninhydrin, vanillin, or KMnO4), followed by warming with a heat gun [60]. Flash chromatography was performed on silica gel (40−63 µm) with the specified solvent system, in most cases employing gradients of increasing polarity [61]. All NMR spectra (1H, 13C, and 2D) were acquired on 200 or 400 MHz Avance™ NMR spectrometers (Bruker, Billerica, MA, USA). The 1H-NMR (200 and 400 MHz) and 13C{1H}-NMR (50 and 101 MHz, measured with full proton decoupling) spectra were recorded with samples dissolved in CDCl3, using the residual solvent signal as an internal reference: 7.26 ppm for CHCl3 for 1H-NMR experiments and 77.1 ppm for CDCl3 for 13C-NMR experiments [62]. Chemical shifts (δ) are expressed in ppm to the nearest 0.01 (1H) or 0.1 ppm (13C). Coupling constants (J) are reported in Hertz (Hz). Signals are indicated as follows: s = singlet, d = doublet, t = triplet, m = multiplet, and br = broad. The assignment of 1H- and 13C-NMR signals was established using D/H exchange, COSY, HMQC, HSQC, NOESY, and HMBC experiments (Figures S9–S11, Supplementary Materials). LC-MS spectra were collected on an Esquire™ 3000 Plus ion trap mass spectrometer (Bruker Daltonics, Billerica, MA, USA) coupled to an electrospray ionization (ESI) source in both positive and negative modes (ESI-ITMS). High-resolution mass spectrometry (HRMS) was carried out on a hybrid ion trap/Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany), which integrates quadrupole precursor selection with highly accurate mass detection in an Orbitrap using ESI techniques. Flavonoid nomenclature is used hereafter for the spectral assignment of each synthetic compound. Labelable and wild-type ODNs were purchased ready-to-use (Microsynth AG, Balgach, Switzerland). Clicked ODNs were pre-analyzed (0.5 mL/min) and then purified (2.0 mL/min) by RP-HPLC, which combines the Waters™ 600 Controller with the Waters™ 996 Photodiode Array Detector (Waters, Milford, MA, USA) as well as Clarity® Oligo-RP™ analytical and semi-preparative C18 columns with the following dimensions: 300 × 4.60 mm and 250 × 10 mm, 5 µm particle size, and 100 Å (Phenomenex, Torrance, CA, USA). The appropriate gradient system was used: 100% A (held for 2 min)—(10 min) → 40% A/60% B (held for 2 min)—(2 min) → 10% A/90% B (held for 6 min)—(5 min) → 100% A, with A = 0.9 TEAB buffer (100 mM, pH 7.8)/0.1 CH3CN and B = 0.9 CH3CN/0.1 TEAB (Figure S4, Supplementary Materials). For the preparation of 100 mM triethylamine bicarbonate (TEAB) buffer [(Et3NH)HCO3]: CO2 was bubbled into a 0.1 M Et3N deionized aq. solution until pH reached approximately 7.8, and the solution was then kept at 4 °C. All solvents used for absorption and fluorescence studies were of spectroscopic grade. Absorbance experiments were conducted on a Cary 100 Bio UV–Vis spectrophotometer (Varian/Agilent, Palo Alto, CA, USA) using 500 µL cuvettes (Hellma, Müllheim, Germany) in Suprasil® quartz (Heraeus, Hanau, Germany) with a 1 cm path length. Dye or ODN stock solutions were made from dimethylformamide or Milli-Q® water (Merck Millipore, Burlington, MA, USA). The fluorochrome sample used for spectroscopic measurements contained ≈0.2% (v/v) of the stock solution solvent. Fluorescence spectra were acquired on a FluoroMax 4.0 spectrofluorometer (Jobin Yvon, Horiba, Kyoto, Japan) in a temperature-controlled cell compartment at 20 ± 0.5 °C with slits open to 2 nm and were corrected for Raman scattering, lamp fluctuations, and wavelength-dependent instrumental bias. Emission recordings were obtained with an absorbance of approximately 0.05 at the excitation wavelength corresponding to the absorption maximum under consideration, unless otherwise stated. Quantum yields were corrected for variation in the refractive indexes of the different solvents. They were estimated by comparing the integrated area of the corrected emission spectrum of the sample with that of 4′-(N,N-dimethylamino)-3-hydroxyflavone (dMAF, λEx = 407 nm, Φ = 27%), which was taken as a reference [63], with a mean standard deviation of ±10%. The fluorophores 6-EMT and Mo-Thio were analyzed in duplicate at 10 and 2 µM, respectively, for UV–Vis and fluorescence studies. Labeled single-stranded (ss) and double-stranded (ds) ODNs were characterized in duplicate at 2 µM in phosphate-buffered saline, pH 7.4 ([P] = 12 mM and [Na] = 170 mM). For the preparation of a 2 µM ODN duplex solution: in a 500 µL quartz cuvette, samples were produced by mixing solutions of the modified ss-ODN (8 µM, 125 µL) and its wild-type complementary sequence (8 µM, 125 µL) with a PBS solution (250 µL containing 24 mM sodium phosphate and 300 mM NaCl). To guarantee the reproducibility of the hybridization and thus the determinations, ds-samples were initially denatured and then cooled to room temperature. Melting curves were measured in duplicate in a Peltier-thermostatted cell holder by following the temperature dependence of the absorbance changes at 260 nm of the sample (2 µM concentration of each strand). The temperature range for monitoring denaturation was 5–80 °C. The heating rate was 0.3 °C/min. Melting observables were converted to a plot of α versus temperature, where α represents the fraction of single strands in the duplex state. Melting temperatures (Tm) were extracted from these curves after differentiation as described in [64]. Photostability studies were run in a 100 µL fluorescence cell; excitation and emission slits were set at 8 nm, and sample concentrations were 2 µM. Circular dichroism (CD) experiments were performed at 20 °C with the help of a J-810 spectropolarimeter (JASCO, Tokyo, Japan). The wavelength range for CD measurements was 230–320 nm. Spectra were recorded from samples with a concentration of 2 µM for each strand. Reported values for the entire photophysical characterization are the average of at least two independent and reproducible measurements: ±1 nm for wavelengths and ±0.5 °C for Tm.
2.2. Synthesis Procedures
- 6-Ethynyl-3-hydroxy-2-(thiophen-2-yl)-4H-chromen-4-one (2, 6-EHT): To a stirred solution of 1 (252 mg, 0.65 mmol) [21] in CH2Cl2 (3.2 mL), a saturated solution of K2CO3 in MeOH (3.2 mL) was added. The reaction mixture was stirred at room temperature overnight before being quenched by the addition of a few drops of glacial acetic acid, and volatiles were concentrated under reduced pressure. The resulting residue was washed twice with cyclohexane to remove any impurities and then dried to give the crude product 2 (6-EHT) as a yellow solid (174 mg, 0.65 mmol, quant.), which was directly used in the next step. HRMS (ESI+): m/z calcd for C15H9O3S+: 269.0267 [M + H]+; found: 269.0251.
- 6-Ethynyl-3-methoxy-2-(thiophen-2-yl)-4H-chromen-4-one (3, 6-EMT): To a stirred solution of 6-EHT (174 mg, 0.65 mmol, 1 eq.) in CH2Cl2 (6.5 mL) were sequentially added 18-crown-6 (85 mg, 10 mol%), a KOH aq. solution (25% w/v, 0.9 mL, DCM/KOH 7:1), and dimethyl sulfate (323 µL, 3.25 mmol, 5 eq.). The resulting mixture was stirred overnight at room temperature. After quenching the reaction with the addition of H2O (13 mL), the organic layer was extracted with CH2Cl2 (3×). The combined organic phases were dried over MgSO4 and filtered, and the volatiles were removed in vacuo. The residue was purified by flash chromatography on silica gel eluted with cyclohexane/ethyl acetate (9:1 → 1:1, v/v) to afford the desired compound 3 (6-EMT) as a yellowish solid (111 mg, 0.39 mmol, 60% over 2 steps). Rf = 0.52 (Cyclohexane/EtOAc 3:1). 1H-NMR (CDCl3, 400 MHz): δ 3.07 (s, 1H, HC≡C), 4.00 (s, 3H, OCH3), 7.15 (dd, 3J = 5.0 Hz, 3J = 3.9 Hz, 1H, Hβ), 7.42 (d, 3J = 8.7 Hz, 1H, H8), 7.57 (dd, 3J = 5.0 Hz, 4J = 1.1 Hz, 1H, Hγ), 7.67 (dd, 3J = 8.7 Hz, 4J = 2.0 Hz, 1H, H7), 7.88 (dd, 3J = 3.9 Hz, 4J = 1.1 Hz, 1H, Hα), 8.30 (d, 4J = 2.0 Hz, 1H, H5). 13C-NMR (CDCl3, 101 MHz): δ 59.7 (OCH3). 78.2 (HC≡C), 82.0 (HC≡C), 118.2 (C8), 119.0 (C6), 124.2 (C10), 127.6 (Cβ), 129.8 (Cα), 129.9 (C5), 131.5 (C3), 131.9 (Cγ), 136.6 (C7), 138.7 (C11), 151.8 (C2), 154.5 (C9), 173.2 (C4). HRMS (ESI+): m/z calcd for C16H11O3S+: 283.0423 [M + H]+; found: 283.0442.
- 1-(Azidomethyl)-4-methoxybenzene (4): To a stirred solution of NaN3 (1.05 g, 16.15 mmol, 2.5 eq.) in DMSO was added PMBCl (1.0 g, 6.39 mmol, 1 eq.). The solution was stirred at 45 °C overnight until the starting material was consumed (monitored by GC-MS). The reaction mixture was cooled to room temperature and then quenched with H2O (30 mL). The organic layer was extracted with Et2O (3 × 20 mL). The combined organic phases were washed with H2O (2 × 30 mL) and brine (30 mL), dried over MgSO4, and filtered, and the volatiles were removed in vacuo to yield the desired product 4 as a yellowish oil (1.04 g, 6.39 mmol, quant.). Rf = 0.70 (Cyclohexane/EtOAc 3:1). 1H-NMR (CDCl3, 400 MHz): δ 3.84 (s, 3H), 4.30 (s, 2H), 6.95 (d, J = 8.7 Hz, 2H), 7.28 (d, J = 8.7 Hz, 2H). 13C-NMR (CDCl3, 101 MHz): δ 54.4 (N3CH2), 55.3 (OCH3), 114.2 (Cortho), 127.5 (Cpara), 129.8 (Cmeta), 159.7 (Ci). HRMS (ESI+): m/z calcd for C8H10N3O+: 164.0818 [M + H]+; found: 164.0833.
- 3-Methoxy-6-(1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)-2-(thiophen-2-yl)-4H-chromen-4-one (5, Mo-Thio): To a stirred solution of 6-EMT (41 mg, 0.15 mmol, 1 eq.) in DCE were sequentially added 4 (43 mg, 0.26 mmol, 1.8 eq.), DIPEA (306 µL, 1.74 mmol, 12 eq.), acetic acid (50 µL, 0.87 mmol, 6 eq.), and CuI (78 mg, 0.41 mmol, 2.8 eq.). The resulting solution was heated at 40 °C overnight under an argon atmosphere to give a homogeneous blue liquid. The reaction mixture was cooled to room temperature and then concentrated under reduced pressure. The crude was purified by flash chromatography on silica gel eluted with cyclohexane/ethyl acetate (9:1 → 3:2, v/v) to provide the desired product 5 (Mo-Thio) as a yellowish powder (37 mg, 0.08 mmol, 57%). Rf = 0.14 (Cyclohexane/EtOAc 3:1). 1H-NMR (CDCl3, 400 MHz): δ 3.77 (s, 3H, PhOCH3), 3.99 (s, 3H, OCH3), 5.46 (s, 2H, H12), 6.87 (d, 3J = 8.7 Hz, 2H, Hmeta), 7.16 (dd, 3J = 5.0 Hz, 3J = 3.9 Hz, 1H, Hβ), 7.24 (d, 3J = 8.7 Hz, 2H, Hortho), 7.54 (d, 3J = 8.8 Hz, 1H, H8), 7.57 (dd, 3J = 5.0 Hz, 4J = 1.2 Hz, 1H, Hγ), 7.69 (s, 1H, Hα’), 7.91 (dd, 3J = 3.9 Hz, 4J = 1.2 Hz, 1H, Hα), 8.28 (d, 4J = 2.2 Hz, 1H, H5), 8.33 (dd, 3J = 8.8 Hz, 4J = 2.2 Hz, 1H, H7). 13C-NMR (CDCl3, 400 MHz): δ 54.0 (C12), 55.4 (PhOCH3), 59.7 (OCH3), 114.7 (Cmeta), 118.6 (C8), 119.9 (Cα’), 122.0 (C5), 124.3 (C10), 126.2 (Ci), 127.6 (Cβ), 127.7 (C6), 129.8 (Cα), 129.9 (Cortho), 131.0 (C7), 131.7 (C3), 131.8 (Cγ), 138.7 (C11), 146.6 (Cβ’), 151.9 (C2), 154.8 (C9), 160.2 (Cpara), 173.9 (C4). HRMS (ESI+): m/z calcd for C24H20N3O4S+: 446.1169 [M + H]+; found: 446.1166.
Typical conjugation procedure for labeling ODNs with the 6-EMT dye. First, a 5 mM aq. solution of CuSO4·5H2O and the BTTES ligand [65] was freshly prepared. In a 200 µL vial were sequentially added the ODN sequence to be labeled (0.2 mM aq. solution, 50 µL, 10 nmol, 1 eq.), DMSO (20 µL), the 6-EMT label (5 mM in DMSO, 10 µL, 50 nmol, 5 eq.), sodium ascorbate (5 mM aq. solution, 10 µL, 50 nmol, 5 eq.), and finally the 5 mM CuSO4/BTTES aq. solution (10 µL, 50 nmol, 5 eq.). The reaction mixture was vortexed overnight at room temperature. The resulting solution was removed from the vial, which was then rinsed with minimal H2O and DMSO. The combined solution was passed through a 0.22 µm H-PTFE syringe filter before being purified by RP-HPLC (for conditions, vide supra and Figure S4, Supplementary Materials).
3. Results and Discussion
3.1. Molecular Design and Synthesis
Engineering a new fluorogenic probe with robust photophysical properties in aqueous media is a great challenge [66]. The most common approach—which has proven particularly successful so far—is to modify the structure of well-known dye families [67]. To produce a novel fluorescence turn-on FIT dye based on a 3HC platform to be used for labeling NAs, the fluorochrome must be able to be easily connected to ODNs through a linker of variable size and flexibility that can ultimately allow its adequate incorporation into the duplex. Moreover, the fluorophore must exhibit a strong change in emission intensity during hybridization.
Keeping these requirements in mind, the 6-EMT label was proposed, which is composed of a 3-methoxychromone (3MC) core unit branched by two moieties, namely, an ethynyl group at position 6 and a five-membered ring (thiophene) at the 2-position (Figure 1B and Scheme 2). Besides blocking the ESIPT reaction [68,69,70,71], the presence of the methyl group was previously shown to affect the planarity of the fluorochrome and thus quenches the fluorescence of 2-thienyl-3MC [25]. As the terminal alkyne has little impact on photophysics—because it is not electronically coupled with the acceptor group (4-ketone)—its role is exclusively related to the subsequent labeling of NAs by the post-synthetic click reaction.

Scheme 2.
Synthetic pathway of the fluorogenic tag 6-EMT and the derived model compound Mo-Thio.
Typically, 3HCs are dual emitters that fluoresce only when the aromatic ring at the 2-position remains coplanar with the rest of the core. The coplanarity of the entire conjugated π-system is therefore essential to achieving a light-on effect. In our case, we used this phenomenon in a reverse manner. We intentionally converted the 3-OH group to a methoxy group in order to increase steric hindrance and push the conjugated thiophene out of the plane, thus becoming a poor fluorophore. The idea behind this is that, once clicked to the single-stranded ODN, selective hybridization with a complementary sequence should force the fluorogen to flatten to allow for accommodation; in other words, the thienyl unit would become coplanar with the chromone part, which should result in a dramatic increase in fluorescence intensity (Figure 1A,B).
The synthesis strategy of 3 (6-EMT) was similar to that commonly used for our developed 3HC derivatives [21], namely, Claisen–Schmidt condensation in an alkaline medium providing the corresponding chalcone, which undergoes, in the same reaction pot, oxidative cyclization according to the Algar–Flynn–Oyamada mechanism [72,73,74], affording the 3HC scaffold. For subsequent click-type post-functionalizations [75,76,77], the incorporation of an alkyne moiety, with no significant effect on photophysics, is paramount [68]. The ethynyl unit was introduced at position 6 via a classical sequence composed of Sonogashira coupling in the presence of TMS-acetylene, followed by the cleavage of the silyl group with a fluoride source, leading to our starting point 1, the 3-protected chromone [21]. Ester removal under transesterification conditions yielded 2 (6-EHT).
The synthetic route to the 6-EMT label—consisting of the protection of the 3-OH group via an in-house methylation procedure using phase-transfer conditions [78]—is depicted in Scheme 2. The derived model compound 5 (Mo-Thio), on the other hand, is prepared using a standard Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) protocol from 4 [68]. Since this conjugate closely resembles the final fluorescent system once clicked onto the ODNs, the study of its photophysics should allow it to mimic that of the single-stranded probe, thus providing essential information.
3.2. Photophysical Properties of Mo-Thio
Our spectroscopic investigations first started with the clickable 6-EMT tag. The extinction coefficient, which is required to estimate the brightness of the marker, was determined in THF (ε = 23,200 L·mol−1·cm−1) and is in line with our expectations, considering similar reported 3HC derivatives [79].
Once covalently bound to the ODN, the clicked label will be in a more or less hydrated environment [80]. In order to better understand its optical behavior in water, the titration of Mo-Thio—the derived clicked tag—was performed by adding an incremental amount of water to a THF solution (Figure S1, Supplementary Materials). This experiment revealed a 20 nm bathochromic shift on the emission, which is typical of a dye with a moderate push–pull relationship (confers the electronic conjugation between the endocyclic oxygen and the 4-ketone, the donor and acceptor, respectively). As attested by the observed hypochromism and midband broadening, solubility issues occurred beyond 60% and led to the formation of H-aggregates [81] that do not reflect reality in an oligonucleotide context. The photostability of Mo-Thio was also checked before proceeding with the solvent spectroscopic study [82]. Due to the inhibition of its ESIPT reaction, the dye retains almost 95% of its initial emission intensity after one hour of continuous irradiation. This result is similar to those already reported for 3-methoxychromones [68,69] and compares favorably with Prodan [83]—the reference push–pull chromophore—and very clearly with dMAF, the methyl-group-free 3HC (Figure S2, Supplementary Materials).
Next, the photophysical properties (UV–Vis absorption and fluorescence) of Mo-Thio were investigated in detail in a set of eleven solvents varying in polarity. Four protic (hexafluoroisopropanol, methanol, ethanol, and n-butanol) and six aprotic (acetonitrile, DMF, dichloromethane, ethyl acetate, THF, and toluene) solvents were selected (Table 1) and ranked using the empirical Reichardt scale; this polarity index takes into account the solvent’s H-bonding ability.

Table 1.
Spectroscopic features of Mo-Thio.
The Mo-Thio conjugate shows a single absorption band centered at 333–352 nm (Figure 2). The position of the absorption maxima does not properly correlate with the solvent polarity but nevertheless exhibits slightly positive solvatochromism. Since this fluorophore is not a prominent push–pull dye with significant charge transfer, the solvatofluorochromism is expected to be weak. The absorptivity was found to be similar to that of the parent compound, as the new triazole connecting linker is not directly in conjugation with the push–pull relationship operating within the chromophore.
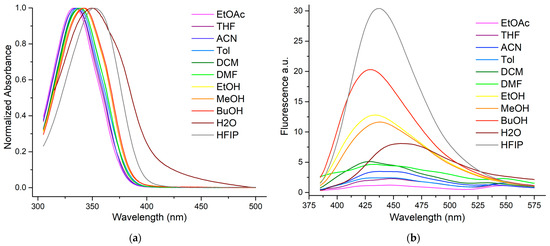
Figure 2.
Absorbance (a) and fluorescence (b) observables of Mo-Thio in a set of solvents varying in polarity.
As for the emission maxima, they oscillate in the turquoise range (432–457 nm, Figure 2), without displaying any notable solvatochromism, which attests to the poor push–pull character of this clicked dye. In protic media, the single emission band appears rather broad, and quantum yields are admittedly low (2.1–4.1%) but remain higher than in aprotic solvents (0.4–1.6%). Note that in the non-protogenic medium, two emission bands seem to emerge from the background noise; this could be due to the emission of different twisted excited forms.
Interestingly, the emission signal is highest (Φ = 4.1 %) in HFIP, which is the most viscous solvent, thus restraining molecular rotation. This hypothesis was further supported by carrying out a titration experiment with an increasing concentration of glycerol in a methanolic solution of the dye. When the amount of glycerol in the medium was increased from 10 to 50%, the quantum yield more than doubled (8 to 17%, Figure S3, Supplementary Materials). Additionally, as HFIP is an organic solvent that is more polar than water, it has the crucial advantage of perfectly solubilizing the fluorochrome, preventing its aggregation, and therefore constitutes an excellent observation model.
In summary, the fluorescence signal is markedly different in both form and intensity between protogenic and aprotic solvents. It seems that for protic media, H-bonds play a crucial role in stabilizing the excited state, which, after charge redistribution, is usually more polar than the ground state. This trend is less pronounced for HFIP, where its steric hindrance may affect H-bonding. Hydration studies were also performed, and their results are substantially similar to those of the parent core (Figure S1, Supplementary Materials). This is practically a tailor-made situation for our research project, as this dye exhibits the strongest emission in a more viscous environment than bulk water, i.e., its rotational movements are restricted there; an environment typical of what FIT probes encounter when accommodated in double-stranded DNA. Although this fluorophore does not display a large charge transfer, Stokes shifts vary over the range 83–100 nm (5400–6600 cm−1) in the presence of protic solvents and become “mega” (viz. >100 nm, 7500–7900 cm−1) in aprotic media. These results compare favorably with conventional dyes such as Alexa Fluor 405, which has a small Stokes shift (20 nm, 1200 cm−1). Fluorophores with large Stokes shifts are very convenient for DNA sensing, as they greatly reduce interference from the background emission of cellular pigments in biological samples.
3.3. Spectroscopic Features of Labeled ODNs
For the preparation of the single-stranded probes, 15-mer ODNs modified with azide-functionalized linkers (more or less long) were selected. The 6-EMT tag was then efficiently conjugated via a CuAAC post-synthetic labeling procedure described previously (Tables S1 and S2, Supplementary Materials) [68]. The composition of the sequences employed and the structures of the linkers are depicted in Table 2. The code is as follows: X and Xs represent the dye clicked onto a central uridine with a long or short linker, respectively; Y is a flexible spacer for 5’-conjugation. Thus, different locations (middle vs. terminal positions) were explored to evaluate the ability of the label to accommodate, intercalate, or stack with the neighboring base pairs in a ds-context. For that purpose, the three labeled sequences (TXT, TXsT, and YGCA) were selectively hybridized with their complementary strand, and the corresponding photophysics is reported in Table 3.

Table 2.
Azide-functionalized ODNs used for post-synthetic fluorescent labeling with the 6-EMT tag.

Table 3.
Photophysical features of labeled ss- and ds-ODNs.
The first observation that can be made is at the level of the absorption domain (Figure S5, Supplementary Materials). After its attachment to DNA, it seems that the fluorescent marker is located in a highly hydrated environment in an ss-context since its absorption range (λAbs ≃ 343–352 nm) is similar to those observed in MeOH and HFIP/water (Figure 2 and Figure S1, Supplementary Materials). It appears clear that in two of the three cases (TXT and TXsT), a red shift of the absorption maximum occurs after hybridization (∆λ = 4 and 11 nm, respectively). It is likely that the long and short linkers have the most appropriate size and positioning to allow the electroneutral and hydrophobic dye to intercalate into the double strand. Bathochromic shifts upon annealing are commonly reported for intercalating dyes [85,86]. This assumption is also substantiated by the position of the emission maxima, which are around 436 nm (Table 3), typical of a less hydrated environment (Figure S1, Supplementary Materials).
The single-stranded sequences were poorly emissive, with quantum yields ranging from only 1.1 to 1.9%. After duplex formation with the complementary sequence, QY increased significantly to ~11% for TXT (embedding the longer linker) and ~7% for TXsT (the shorter one), while Φ remained almost unchanged for the YGCA sequence, where the reporter is attached to the 5′-terminus (Figure 3). These differences can be rationally explained by the fact that when the linker is longer, the label has more flexibility to fold and thus properly intercalate into the DNA double helix. For the shorter linker, the intercalation was less efficient because of the degree of freedom of the side chain, which is less important. As for the 5′-labeling, it seems that there is no intercalation. π-Stacking with the last base plate cannot be ruled out; however, if this is the case, it would not be sufficient to constrain the fluorogen and cause the switch-on of its emission.
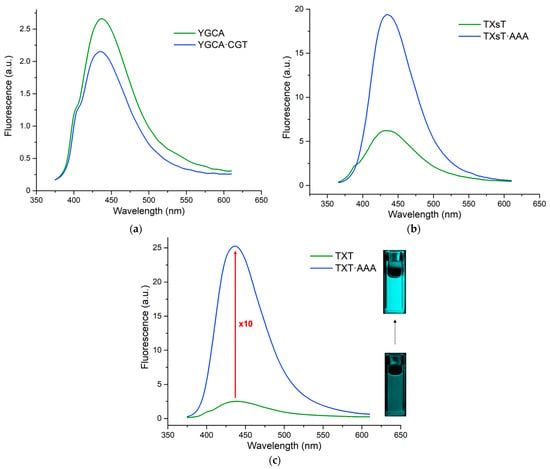
Figure 3.
Emission spectra of the 3 ss-probes and their corresponding ds-constructs.
To sum up, the duplex intercalation in the TXT context effectively screens the dye from bulk water and provides a much more constrained environment, where rotational motions are limited. Hence, it explains the observed fluorogenic character, viz., a 10-fold increase in emission intensity, as evidenced by the bright cyan light-on in the cuvette inserts (Figure 3). To confirm that the fluorescence enhancement was entirely hybridization-dependent, negative controls were performed by incubating the TXT single strand with either a GC- or AT-rich sequence or a completely random sequence (Figure S6, Supplementary Materials). The lack of a noticeable change in emission thereby corroborates that the observed turn-on is indeed due to strand pairing. Altogether, our results are fully consistent with experimental [13,19] and MD simulation data [87,88] of the parent thienyl chromone (3HTC, Scheme 1) when incorporated into DNA as a substitute for a natural base. In particular, the hybrid QM/MM approach demonstrated that, in a DNA duplex, the 3HTC dye was perfectly intercalated between neighboring base pairs and was flattened, which reduced torsional motion, promoted better electronic conjugation, and increased the oscillator strength, accounting for the red-shifted absorption and higher quantum yield.
It is well known in the literature that a nucleobase modification based on a flexible spacer arm will affect the stability of the duplex [9]. Thermal denaturation studies were then performed to evaluate the impact of the clicked fluorogen on duplex integrity. To this end, the labeled sequences were annealed with their complementary strand, and the duplex melting temperatures were compared to that of the corresponding wild-type duplex (Table 4 and Figure S7, Supplementary Materials). It is clear that the azido spacer itself has a 2–4 °C impact on the stability of the double strand [68]. Nevertheless, once the dye is conjugated to it, the influence is not the same, depending on the positioning of the reporter on the strand. When the probe is in the middle of the sequence, a moderate impact is observed (∆Tm = 5–7 °C), but when the aromatic sensor is at the 5′-end, stabilization takes place (+1 °C). This supports the idea that π-stacking occurs, and as reported for cyanines [89], its hydrophobic interaction with the terminal base pair stabilizes the system.

Table 4.
Thermal denaturation analyses of ds-constructs labeled with 6-EMT.
Further evidence, admittedly indirect but consistent with our previous statement on the capacity of the spacer arm to allow accommodation, is that when the flexible tether is longer, there is less destabilization. This corroborates in an indirect way that the folding and, consequently, the intercalation are better and destabilize the system less when the flexibility of the linker is greater.
Overall, we can conclude that the labeling effect is rather reasonable. Our DNA study systems are therefore perfectly matched at room temperature.
The maintenance of the B conformation of the resulting labeled duplexes was confirmed by circular dichroism. A spectral signature clearly overlaps with that of the wild-type ds-structure, evidencing that there is no disruption of the helical conformation by fluorogen accommodation and thus attesting to the integrity of the double helix (Figure S8, Supplementary Materials).
4. Conclusions
In sum, a novel chromone-based fluorogenic probe was engineered to detect DNA hybridization. Detailed spectroscopic studies were conducted on a model substrate to define and better understand the sensor photophysics using UV–Vis absorption and fluorescence spectroscopies. The poorly fluorescent 6-EMT tag was then employed to label ODNs functionalized with different azido-tethers using a post-synthetic click-type strategy. After screening, a single-stranded probe demonstrated a 10-fold increase in fluorescence intensity upon hybridization with its complementary sequence. The forced intercalation of this fluorogen within the DNA duplex is the trigger for this remarkable turn-on emission. Although not a traditional push–pull type fluorophore, this dye still exhibits a mega-Stokes shift, making it useful for bio-imaging purposes. This fluorochrome is also highly photostable and showed only marginal destabilization on the double helix. In brief, this chromone-based reporter allows the fluorogenic sensing of the double-stranded DNA structure and paves the way for the development of new diagnostic tools based on FIT probes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors11030161/s1. Figures S1–S3: Photophysical characterization of the model dye; Figures S4–S8 and Tables S1 and S2: Spectroscopic studies of ss- and ds-ODNs; Figures S9–S11: NMR spectra. References [63,64,65] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, B.Y.M. and A.B.; formal analysis, S.V., G.B. and H.-N.L.; funding acquisition, B.Y.M. and A.B.; investigation, S.V., G.B. and S.M.; methodology, S.V.; supervision, B.Y.M. and A.B.; writing—review and editing, S.V., S.M., A.B. and. B.Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the Agence Nationale de la Recherche (PFPImaging—ANR-18-CE09-0020-01, UCA JEDI project: ANR-15-IDEX-01) and the LIFE and SPECTRUM graduate schools (UCA). We thank the French Government for the PhD grants to S.V., H.-N.L., and G.B. We are grateful to the CNRS Emergence@INC2020 program for both the financial support and the postdoctoral fellowship of S.M.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Experimental data are available within this research article and in the related Supplementary Materials.
Acknowledgments
Thanks to D. Dziuba for his seminal work on the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khadem, S.; Marles, R.J. Chromone and Flavonoid Alkaloids: Occurrence and Bioactivity. Molecules 2012, 17, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.M.; Pinto, D.C.G.A.; Silva, A.M.S. Chromones: A Promising Ring System for New Anti-inflammatory Drugs. ChemMedChem 2016, 11, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Mély, Y. Fluorescent Environment-Sensitive Dyes as Reporters of Biomolecular Interactions. In Progress in Molecular Biology and Translational Science; Morris, M.C., Ed.; Academic Press: Burlington, VT, USA, 2013; Volume 113, Chapter 2; pp. 35–58. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Klymchenko, A.S.; Pivovarenko, V.G.; Ercelen, S. Ratiometric Probes: Design and Applications. In Fluorescence Spectroscopy, Imaging and Probes; Springer Series on Fluorescence; Kraayenhof, R., Visser, A.J.W.G., Gerritsen, H.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 2, Chapter 5; pp. 101–110. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Lang, B.; Rosspeintner, A.; Vauthey, E. Ultrafast Elementary Photochemical Processes of Organic Molecules in Liquid Solution. Chem. Rev. 2017, 117, 10826–10939. [Google Scholar] [CrossRef]
- Tomin, V.I.; Demchenko, A.P.; Chou, P.-T. Thermodynamic vs. kinetic control of excited-state proton transfer reactions. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 1–18. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 46, 7105–7139. [Google Scholar] [CrossRef]
- Michel, B.Y.; Dziuba, D.; Benhida, R.; Demchenko, A.P.; Burger, A. Probing of Nucleic Acid Structures, Dynamics, and Interactions wiamoth Environment-Sensitive Fluorescent Labels. Front. Chem. 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, D.; Didier, P.; Ciaco, S.; Barth, A.; Seidel, C.A.M.; Mély, Y. Fundamental photophysics of isomorphic and expanded fluorescent nucleoside analogues. Chem. Soc. Rev. 2021, 50, 7062–7107. [Google Scholar] [CrossRef]
- Nakatani, K.; Tor, Y. (Eds.) Modified Nucleic Acids; Nucleic Acids and Molecular Biology Series; Springer: Cham, Switzerland, 2016; Volume 31, p. 276. [Google Scholar] [CrossRef]
- Demchenko, A.P. Introduction to Fluorescence Sensing, 3rd ed.; Springer: Cham, Switzerland, 2020; Volume 1, Materials and Devices; p. 657. [Google Scholar] [CrossRef]
- Dziuba, D.; Postupalenko, V.Y.; Spadafora, M.; Klymchenko, A.S.; Guérineau, V.; Mély, Y.; Benhida, R.; Burger, A. A Universal Nucleoside with Strong Two-Band Switchable Fluorescence and Sensitivity to the Environment for Investigating DNA Interactions. J. Am. Chem. Soc. 2012, 134, 10209–10213. [Google Scholar] [CrossRef]
- Spadafora, M.; Postupalenko, V.Y.; Shvadchak, V.V.; Klymchenko, A.S.; Mély, Y.; Burger, A.; Benhida, R. Efficient Synthesis of Ratiometric Fluorescent Nucleosides Featuring 3-Hydroxychromone Nucleobases. Tetrahedron 2009, 65, 7809–7816. [Google Scholar] [CrossRef]
- Kuznetsova, A.A.; Kuznetsov, N.A.; Vorobjev, Y.N.; Barthes, N.P.F.; Michel, B.Y.; Burger, A.; Fedorova, O.S. New environment-sensitive multichannel DNA fluorescent label for investigation of the protein-DNA interactions. PLoS ONE 2014, 9, e100007. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.A.; Kladova, O.A.; Barthes, N.P.F.; Michel, B.Y.; Burger, A.; Fedorova, O.S.; Kuznetsov, N.A. Comparative Analysis of Nucleotide Fluorescent Analogs for Registration of DNA Conformational Changes Induced by Interaction with Formamidopyrimidine-DNA Glycosylase Fpg. Russ. J. Bioorg. Chem. 2019, 45, 591–598. [Google Scholar] [CrossRef]
- Kilin, V.; Gavvala, K.; Barthes, N.P.F.; Michel, B.Y.; Shin, D.; Boudier, C.; Mauffret, O.; Yashchuk, V.; Mousli, M.; Ruff, M.; et al. Dynamics of Methylated Cytosine Flipping by UHRF1. J. Am. Chem. Soc. 2017, 139, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Sholokh, M.; Sharma, R.; Grytsyk, N.; Zaghzi, L.; Postupalenko, V.Y.; Dziuba, D.; Barthes, N.P.F.; Michel, B.Y.; Boudier, C.; Zaporozhets, O.A.; et al. Environmentally Sensitive Fluorescent Nucleoside Analogues for Surveying Dynamic Interconversions of Nucleic Acid Structures. Chem. Eur. J. 2018, 24, 13850–13861. [Google Scholar] [CrossRef] [PubMed]
- Zargarian, L.; Ben Imeddourene, A.; Gavvala, K.; Barthes, N.P.F.; Michel, B.Y.; Kenfack, C.A.; Morellet, N.; René, B.; Fossé, P.; Burger, A.; et al. Structural and Dynamical Impact of a Universal Fluorescent Nucleoside Analogue Inserted Into a DNA Duplex. J. Phys. Chem. B 2017, 121, 11249–11261. [Google Scholar] [CrossRef] [PubMed]
- Le, H.-N.; Brazard, J.; Barnoin, G.; Vincent, S.; Michel, B.Y.; Léonard, J.; Burger, A. Control of Intermolecular Photoinduced Electron Transfer in Deoxyadenosine-Based Fluorescent Probes. Chem. Eur. J. 2020, 26, 276–286. [Google Scholar] [CrossRef]
- Le, H.-N.; Zilio, C.; Barnoin, G.; Barthes, N.P.F.; Guigonis, J.-M.; Martinet, N.; Michel, B.Y.; Burger, A. Rational design, synthesis, and photophysics of dual-emissive deoxyadenosine analogs. Dyes Pigments 2019, 170, 107553. [Google Scholar] [CrossRef]
- Barthes, N.P.F.; Gavvala, K.; Dziuba, D.; Bonhomme, D.; Karpenko, I.A.; Dabert-Gay, A.S.; Debayle, D.; Demchenko, A.P.; Benhida, R.; Michel, B.Y.; et al. Dual emissive analogue of deoxyuridine as a sensitive hydration-reporting probe for discriminating mismatched from matched DNA and DNA/DNA from DNA/RNA duplexes. J. Mater. Chem. C 2016, 4, 3010–3017. [Google Scholar] [CrossRef]
- Gavvala, K.; Barthes, N.P.F.; Bonhomme, D.; Dabert-Gay, A.S.; Debayle, D.; Michel, B.Y.; Burger, A.; Mély, Y. A turn-on dual emissive nucleobase sensitive to mismatches and duplex conformational changes. RSC Adv. 2016, 6, 87142–87146. [Google Scholar] [CrossRef]
- Kladova, O.A.; Kuznetsova, A.A.; Barthes, N.P.F.; Michel, B.Y.; Burger, A.; Fedorova, O.S.; Kuznetsov, N.A. New Fluorescent Analogs of Nucleotides Based on 3-Hydroxychromone for Recording Conformational Changes of DNA. Russ. J. Bioorg. Chem. 2019, 45, 599–607. [Google Scholar] [CrossRef]
- Dziuba, D.; Karpenko, I.A.; Barthes, N.P.F.; Michel, B.Y.; Klymchenko, A.S.; Benhida, R.; Demchenko, A.P.; Mély, Y.; Burger, A. Rational design of a solvatochromic fluorescent uracil analogue with a dual-band ratiometric response based on 3-hydroxychromone. Chem. Eur. J. 2014, 20, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, L.M.; Tor, Y. (Eds.) Fluorescent Analogues of Biomolecular Building Blocks: Design and Applications; Wiley-VCH: Hoboken, NJ, USA, 2016; p. 448. [Google Scholar] [CrossRef]
- Herdewijn, P. (Ed.) Modified Nucleosides: In Biochemistry, Biotechnology and Medicine; Wiley-VCH: Weinheim, Germany, 2008; p. 658. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; p. 954. [Google Scholar] [CrossRef]
- Medintz, I.; Hildebrandt, N. (Eds.) FRET—Förster Resonance Energy Transfer: From Theory to Applications; Wiley-VCH: Weinheim, Germany, 2013; p. 791. [Google Scholar] [CrossRef]
- Shi, W.; Ma, H. Spectroscopic probes with changeable π-conjugated systems. Chem. Commun. 2012, 48, 8732–8744. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A. Next-generation fluorescent nucleic acids probes for microscopic analysis of intracellular nucleic acids. Appl. Microsc. 2019, 49, 14. [Google Scholar] [CrossRef]
- Hayashi, G.; Okamoto, A. Probe Design for the Effective Fluorescence Imaging of Intracellular RNA. Chem. Rec. 2013, 13, 209–217. [Google Scholar] [CrossRef]
- Okamoto, A. ECHO probes: A concept of fluorescence control for practical nucleic acid sensing. Chem. Soc. Rev. 2011, 40, 5815–5828. [Google Scholar] [CrossRef]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Teoh, C.L.; Wang, L.; Liu, X.; Chang, Y.-T. Motion-induced change in emission (MICE) for developing fluorescent probes. Chem. Soc. Rev. 2017, 46, 4833–4844. [Google Scholar] [CrossRef]
- Dal Molin, M.; Verolet, Q.; Soleimanpour, S.; Matile, S. Mechanosensitive Membrane Probes. Chem. Eur. J. 2015, 21, 6012–6021. [Google Scholar] [CrossRef]
- Hövelmann, F.; Seitz, O. DNA Stains as Surrogate Nucleobases in Fluorogenic Hybridization Probes. Acc. Chem. Res. 2016, 49, 714–723. [Google Scholar] [CrossRef]
- Köhler, O.; Jarikote, D.V.; Singh, I.; Parmar, V.S.; Weinhold, E.; Seitz, O. Forced intercalation as a tool in gene diagnostics and in studying DNA-protein interactions. Pure Appl. Chem. 2005, 77, 327–338. [Google Scholar] [CrossRef]
- Gebhard, J.; Hirsch, L.; Schwechheimer, C.; Wagenknecht, H.-A. Hybridization-Sensitive Fluorescent Probes for DNA and RNA by a Modular “click” Approach. Bioconjugate Chem. 2022, 33, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Chamiolo, J.; Fang, G.-M.; Hövelmann, F.; Friedrich, D.; Knoll, A.; Loewer, A.; Seitz, O. Comparing Agent-Based Delivery of DNA and PNA Forced Intercalation (FIT) Probes for Multicolor mRNA Imaging. ChemBioChem 2019, 20, 595–604. [Google Scholar] [CrossRef]
- Gaspar, I.; Hövelmann, F.; Chamiolo, J.; Ephrussi, A.; Seitz, O. Quantitative mRNA Imaging with Dual Channel qFIT Probes to Monitor Distribution and Degree of Hybridization. ACS Chem. Biol. 2018, 13, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Hövelmann, F.; Gaspar, I.; Chamiolo, J.; Kasper, M.; Steffen, J.; Ephrussi, A.; Seitz, O. LNA-enhanced DNA FIT-probes for multicolour RNA imaging. Chem. Sci. 2016, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hövelmann, F.; Gaspar, I.; Loibl, S.; Ermilov, E.A.; Röder, B.; Wengel, J.; Ephrussi, A.; Seitz, O. Brightness through Local Constraint—LNA-Enhanced FIT Hybridization Probes for in Vivo Ribonucleotide Particle Tracking. Angew. Chem. Int. Ed. 2014, 53, 11370–11375. [Google Scholar] [CrossRef]
- Hövelmann, F.; Gaspar, I.; Ephrussi, A.; Seitz, O. Brightness Enhanced DNA FIT-Probes for Wash-Free RNA Imaging in Tissue. J. Am. Chem. Soc. 2013, 135, 19025–19032. [Google Scholar] [CrossRef]
- Hövelmann, F.; Bethge, L.; Seitz, O. Single Labeled DNA FIT Probes for Avoiding False-Positive Signaling in the Detection of DNA/RNA in qPCR or Cell Media. ChemBioChem 2012, 13, 2072–2081. [Google Scholar] [CrossRef]
- Kummer, S.; Knoll, A.; Socher, E.; Bethge, L.; Herrmann, A.; Seitz, O. Fluorescence Imaging of Influenza H1N1 mRNA in Living Infected Cells Using Single-Chromophore FIT-PNA. Angew. Chem. Int. Ed. 2011, 50, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Socher, E.; Bethge, L.; Knoll, A.; Jungnick, N.; Herrmann, A.; Seitz, O. Low-Noise Stemless PNA Beacons for Sensitive DNA and RNA Detection. Angew. Chem. Int. Ed. 2008, 47, 9555–9559. [Google Scholar] [CrossRef]
- Socher, E.; Jarikote, D.V.; Knoll, A.; Röglin, L.; Burmeister, J.; Seitz, O. FIT probes: Peptide nucleic acid probes with a fluorescent base surrogate enable real-time DNA quantification and single nucleotide polymorphism discovery. Anal. Biochem. 2008, 375, 318–330. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.R.; Pearson, C.E.; Potaman, V.N.; Ussery, D.W. DNA: Structure and function. In Genes and Genomes; Advances in Genome Biology; Verma, R.S., Ed.; Elsevier: Stamford, CT, USA, 1998; Volume 5, pp. 1–141. [Google Scholar] [CrossRef]
- Braselmann, E.; Rathbun, C.; Richards, E.M.; Palmer, A.E. Illuminating RNA Biology: Tools for Imaging RNA in Live Mammalian Cells. Cell Chem. Biol. 2020, 27, 891–903. [Google Scholar] [CrossRef]
- Tomoike, F.; Abe, H. RNA imaging by chemical probes. Adv. Drug Deliv. Rev. 2019, 147, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Socher, E.; Seitz, O. FIT-Probes in Real-Time PCR. In Molecular Beacons: Signalling Nucleic Acid Probes, Methods, and Protocols; Methods in Molecular, Biology; Marx, A., Seitz, O., Eds.; Humana Press: Totowa, NJ, USA, 2008; Volume 429, Chapter 13; pp. 187–197. [Google Scholar] [CrossRef]
- Suseela, Y.V.; Narayanaswamy, N.; Pratihar, S.; Govindaraju, T. Far-red fluorescent probes for canonical and non-canonical nucleic acid structures: Current progress and future implications. Chem. Soc. Rev. 2018, 47, 1098–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Wu, T.; Zhou, X.; Shao, Y. Triggered Excited-State Intramolecular Proton Transfer Fluorescence for Selective Triplex DNA Recognition. Anal. Chem. 2015, 87, 11620–11624. [Google Scholar] [CrossRef]
- Janjua, N.K.; Shaheen, A.; Yaqub, A.; Perveen, F.; Sabahat, S.; Mumtaz, M.; Jacob, C.; Ba, L.A.; Mohammed, H.A. Flavonoid-DNA binding studies and thermodynamic parameters. Spectrochim. Acta A 2011, 79, 1600–1604. [Google Scholar] [CrossRef]
- Ragazzon, P.A.; Bradshaw, T.; Matthews, C.; Iley, J.; Missailidis, S. The characterisation of flavone-DNA isoform interactions as a basis for anticancer drug development. Anticancer Res. 2009, 29, 2273–2283. [Google Scholar] [PubMed]
- Benson, S.; de Moliner, F.; Tipping, W.; Vendrell, M. Miniaturized Chemical Tags for Optical Imaging. Angew. Chem. Int. Ed. 2022, 61, e202204788. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2017; p. 1024. [Google Scholar] [CrossRef]
- Jork, H.; Funk, W.; Fischer, W.R.; Wimmer, H. Thin-Layer Chromatography: Reagents and Detection Methods; Physical and Chemical Detection Methods: Fundamentals, Reagents I; VCH: Weinheim, Germany, 1990; Volume 1a, p. 464. [Google Scholar]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Ormson, S.M.; Brown, R.G.; Vollmer, F.; Rettig, W. Switching between charge-and proton-transfer emission in the excited state of a substituted 3-hydroxyflavone. J. Photochem. Photobiol. A Chem. 1994, 81, 65–72. [Google Scholar] [CrossRef]
- Breslauer, K.J. [10] Extracting thermodynamic data from equilibrium melting curves for oligonucleotide order-disorder transitions. In Energetics of Biological Macromolecules; Methods in Enzymology; Johnson, M.L., Ackers, G.K., Eds.; Academic Press: San Diego, CA, USA, 1995; Volume 259, pp. 221–242. [Google Scholar] [CrossRef]
- Ivancová, I.; Leone, D.-L.; Hocek, M. Reactive modifications of DNA nucleobases for labelling, bioconjugations, and cross-linking. Curr. Opin. Chem. Biol. 2019, 52, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Kele, P. Fluorogenic probes for super-resolution microscopy. Org. Biomol. Chem. 2019, 17, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Jullien, L.; Gautier, A. Fluorogen-based reporters for fluorescence imaging: A review. Methods Appl. Fluoresc. 2015, 3, 042007. [Google Scholar] [CrossRef]
- Vincent, S.; Mallick, S.; Barnoin, G.; Le, H.-N.; Michel, B.Y.; Burger, A. An Expeditious Approach towards the Synthesis and Application of Water-Soluble and Photostable Fluorogenic Chromones for DNA Detection. Molecules 2022, 27, 2267. [Google Scholar] [CrossRef]
- Barthes, N.P.F.; Gavvala, K.; Bonhomme, D.; Dabert-Gay, A.S.; Debayle, D.; Mély, Y.; Michel, B.Y.; Burger, A. Design and Development of a Two-Color Emissive FRET Pair Based on a Photostable Fluorescent Deoxyuridine Donor Presenting a Mega-Stokes Shift. J. Org. Chem. 2016, 81, 10733–10741. [Google Scholar] [CrossRef]
- Kreder, R.; Oncul, S.; Kucherak, O.A.; Pyrshev, K.A.; Real, E.; Mély, Y.; Klymchenko, A.S. Blue fluorogenic probes for cell plasma membranes fill the gap in multicolour imaging. RSC Adv. 2015, 5, 22899–22905. [Google Scholar] [CrossRef]
- Kucherak, O.A.; Richert, L.; Mély, Y.; Klymchenko, A.S. Dipolar 3-methoxychromones as bright and highly solvatochromic fluorescent dyes. Phys. Chem. Chem. Phys. 2012, 14, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Algar-Flynn-Oyamada (AFO) Reaction. In Comprehensive Organic Name Reactions and Reagents; Wiley-VCH: Weinheim, Germany, 2010; Chapter 13; pp. 52–56. [Google Scholar] [CrossRef]
- Sharma, A.; Singh Tuli, H.; Sharma, A.K. Chemistry and Synthetic Overview of Flavonoids. In Current Aspects of Flavonoids: Their Role in Cancer Treatment; Singh Tuli, H., Ed.; Springer: Singapore, 2019; pp. 23–38. [Google Scholar] [CrossRef]
- Dean, F.M.; Podimuang, V. 737. The course of the Algar–Flynn–Oyamada (A.F.O.) reaction. J. Chem. Soc. Perkin Trans. 1965, 3978–3987. [Google Scholar] [CrossRef]
- Fantoni, N.Z.; El-Sagheer, A.H.; Brown, T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(l)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Dziuba, D.; Benhida, R.; Burger, A. A Mild and Efficient Protocol for the Protection of 3-Hydroxychromones under Phase-Transfer Catalysis. Synthesis 2011, 47, 2159–2164. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Pivovarenko, V.G.; Demchenko, A.P. Perturbation of planarity as the possible mechanism of solvent-dependent variations of fluorescence quantum yield in 2-aryl-3-hydroxychromones. Spectrochim. Acta A 2003, 59, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.J.; Lynch, G.C.; Pettitt, B.M. Ion and solvent density distributions around canonical B-DNA from integral equations. J. Phys. Chem. B 2011, 115, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yuan, W.; Jia, Z.; Liu, G. H- and J-Aggregation of Fluorene-Based Chromophores. J. Phys. Chem. B 2014, 118, 14536–14545. [Google Scholar] [CrossRef]
- Demchenko, A.P. Photobleaching of organic fluorophores: Quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 2020, 8, 022001. [Google Scholar] [CrossRef]
- Niko, Y.; Didier, P.; Mély, Y.; Konishi, G.-I.; Klymchenko, A.S. Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes. Sci. Rep. 2016, 6, 18870. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Hainke, S.; Seitz, O. Binaphthyl-DNA: Stacking and Fluorescence of a Nonplanar Aromatic Base Surrogate in DNA. Angew. Chem. Int. Ed. 2009, 48, 8250–8253. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Hecker, W.; Prasad, A.K.; Parmar, V.S.; Seitz, O. Local disruption of DNA-base stacking by bulky base surrogates. Chem. Commun. 2002, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Sougnabé, A.; Lissouck, D.; Fontaine-Vive, F.; Nsangou, M.; Mély, Y.; Burger, A.; Kenfack, C.A. Electronic transitions and ESIPT kinetics of the thienyl-3-hydroxychromone nucleobase surrogate in DNA duplexes: A DFT/MD-TDDFT study. RSC Adv. 2020, 10, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Kenfack, C.A.; Klymchenko, A.S.; Duportail, G.; Burger, A.; Mély, Y. Ab initio study of the solvent H-bonding effect on ESIPT reaction and electronic transitions of 3-hydroxychromone derivatives. Phys. Chem. Chem. Phys. 2012, 14, 8910–8918. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.G.; You, Y.; Owczarzy, R. Cy3 and Cy5 dyes attached to oligonucleotide terminus stabilize DNA duplexes: Predictive thermodynamic model. Biophys. Chem. 2015, 198, 36–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).