Solvatochromic Behavior of 2,7-Disubstituted Sila- and Germafluorenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Theoretical Calculations
2.3. Spectroscopy
3. Results
3.1. Density Functional Theory Calculations
3.2. Spectroscopy
3.3. Solvatochromism and Dipole Moments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misra, R.; Bhattacharyya, S.P. Intramolecular Charge Transfer: Theory and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Noirbent, G.; Pigot, C.; Bui, T.-T.; Péralta, S.; Nechab, M.; Gigmes, D.; Dumur, F. Synthesis, optical and electrochemical properties of a series of push-pull dyes based on the 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene)malononitrile (TCF) acceptor. Dyes Pigments 2021, 184, 108807–108819. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Niko, Y.; Klymchenko, A.S. Emerging solvatochromic push–pull dyes for monitoring the lipid order of biomembranes in live cells. J. Biochem. 2021, 170, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Theodorakis, A.E. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S.; Kreder, R. Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef]
- Kucherak, O.A.; Oncul, S.; Darwich, Z.; Yushchenko, D.A.; Arntz, Y.; Didier, P.; Mély, Y.; Klymchenko, A.S. Switchable Nile Red-Based Probe for Cholesterol and Lipid Order at the Outer Leaflet of Biomembranes. J. Am. Chem. Soc. 2010, 132, 4907–4916. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Mély, Y.; Duportail, G.; Klymchenko, A.S. Monitoring Biophysical Properties of Lipid Membranes by Environment-Sensitive Fluorescent Probes. Biophys. J. 2009, 96, 3461–3470. [Google Scholar] [CrossRef]
- Loving, G.S.; Sainlos, M.; Imperiali, B. Monitoring protein interactions and dynamics with solvatochromic fluorophores. Trends Biotechnol. 2010, 28, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tarai, A.; Huang, M.; Das, P.; Pan, W.; Zhang, J.; Gu, Z.; Yan, W.; Qu, J.; Yang, Z. ICT and AIE Characteristics Two Cyano-Functionalized Probes and Their Photophysical Properties, DFT Calculations, Cytotoxicity, and Cell Imaging Applications. Molecules 2020, 25, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Gou, G.; Zhang, Z.; Yuan, B.; Fan, T.; Li, L. Synthesis, photophysical properties and optical stabilities of dimethylthio modified dibenzosiloles: High quantum yield emitters. Dyes Pigments 2021, 194, 109642–109651. [Google Scholar] [CrossRef]
- Zhang, Z.; Gou, G.; Wan, J.; Li, H.; Wang, M.; Li, L. Synthesis, Structure, and Significant Energy Gap Modulation of Symmetrical Silafluorene-Cored Tetracyanobutadiene and Tetracyanoquinodimethane Derivatives. J. Org. Chem. 2022, 87, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Sıdır, Y.G.; Sıdır, I. Solvent effect on the absorption and fluorescence spectra of 7-acetoxy-6-(2,3-dibromopropyl)-4,8-dimethylcoumarin: Determination of ground and excited state dipole moments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Manjuladevi, V.; Gupta, R.K.; Kumar, S. Solvatochromism of a tricycloquinazoline based disk-shaped liquid crystal: A potential molecular probe for fluorescence imaging. RSC Adv. 2015, 5, 84592–84600. [Google Scholar] [CrossRef]
- Vequi-Suplicy, C.C.; Coutinho, K.; Lamy, M.T. Electric dipole moments of the fluorescent probes Prodan and Laurdan: Experimental and theoretical evaluations. Biophys. Rev. 2014, 6, 63–74. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chattopadhyay, A.; Samanta, A.; Soujanya, T. Dipole moment change of NBD group upon excitation studied using solvatochromic and quantum chemical approaches: Implications in membrane research. J. Phys. Chem. 1994, 98, 2809–2812. [Google Scholar] [CrossRef]
- Kawski, A.; Bojarski, P.; Kukliński, B. Estimation of ground- and excited-state dipole moments of Nile Red dye from solvatochromic effect on absorption and fluorescence spectra. Chem. Phys. Lett. 2008, 463, 410–412. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Dong, H.; Zhang, S.; Xu, W.; Wang, J.; Gao, P.; Peng, X. Fluorene-Derived Two-Photon Fluorescent Probes for Specific and Simultaneous Bioimaging of Endoplasmic Reticulum and Lysosomes: Group-Effect and Localization Hua. J. Mater. Chem. B 2013, 1, 5450–5455. [Google Scholar] [CrossRef]
- Chen, R.-F.; Fan, Q.-L.; Liu, S.-J.; Zhu, R.; Pu, K.-Y.; Huang, W. Fluorene and silafluorene conjugated copolymer: A new blue light-emitting polymer. Synth. Met. 2006, 156, 1161–1167. [Google Scholar] [CrossRef]
- Shaya, J.; Collot, M.; Bénailly, F.; Mahmoud, N.; Mély, Y.; Michel, B.Y.; Klymchenko, A.S.; Burger, A. Turn-on Fluorene Push–Pull Probes with High Brightness and Photostability for Visualizing Lipid Order in Biomembranes. ACS Chem. Biol. 2017, 12, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zuo, Y.; Zhang, Y.; Gou, Z.; Wang, X.; Lin, W. Novel fluorene-based fluorescent probe with excellent stability for selective detection of SCN− and its applications in paper-based sensing and bioimaging. J. Mater. Chem. B 2019, 7, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Auvray, M.; Bolze, F.; Clavier, G.; Mahuteau-Betzer, F. Silafluorene as a promising core for cell-permeant, highly bright and two-photon excitable fluorescent probes for live-cell imaging. Dyes Pigments 2021, 187, 109083–109091. [Google Scholar] [CrossRef]
- Hammerstroem, D.W.; Braddock-Wilking, J.; Rath, N.P. Synthesis and characterization of luminescent 2,7-disubstituted silafluorenes. J. Organomet. Chem. 2016, 813, 110–118. [Google Scholar] [CrossRef]
- Hammerstroem, D.W.; Braddock-Wilking, J.; Rath, N.P. Luminescent 2,7-disubstituted germafluorenes. J. Organomet. Chem. 2017, 830, 196–202. [Google Scholar] [CrossRef]
- Germann, S.; Jarrett, S.J.; Dupureur, C.M.; Rath, N.P.; Gallaher, E.; Braddock-Wilking, J. Synthesis of Luminescent 2-7 Disubstituted Silafluorenes with alkynyl-carbazole, -phenanthrene, and -benzaldehyde substituents. J. Organomet. Chem. 2020, 927, 121514–121521. [Google Scholar] [CrossRef]
- Spikes, H.J.; Jarrett-Noland, S.J.; Germann, S.M.; Braddock-Wilking, J.; Dupureur, C.M. Group 14 Metallafluorenes as Sensitive Luminescent Probes of Surfactants in Aqueous Solution. J. Fluoresc. 2021, 31, 961–969. [Google Scholar] [CrossRef]
- Spikes, H.J.; Jarrett-Noland, S.J.; Germann, S.M.; Olivas, W.; Braddock-Wilking, J.; Dupureur, C.M. Group 14 Metallafluorenes for Lipid Structure Detection and Cellular Imaging. Chem. Proc. 2021, 5, 83–88. [Google Scholar] [CrossRef]
- Vazquez, M.E.; Blanco, J.B.; Imperiali, B. Photophysics and biological applications of the environment-sensitive fluorophore 6-N,N-dimethylamino-2,3-naphthalimide. J. Am. Chem. Soc. 2005, 127, 1300–1306. [Google Scholar] [CrossRef]
- Shimizu, M.; Mochida, K.; Katoh, M.; Hiyama, T. Synthesis and Photophysical Properties of 2-Donor-7-acceptor-9-silafluorenes: Remarkable Fluorescence Solvatochromism and Highly Efficient Fluorescence in Doped Polymer Films. J. Phys. Chem. C 2010, 114, 10004–10014. [Google Scholar] [CrossRef]
- Kedenburg, S.; Vieweg, M.; Gissibl, T.; Giessen, H. Linear refractive index and absorption measurements of nonlinear optical liquids in the visible and near-infrared spectral region. Opt. Mater. Express 2012, 2, 1588–1611. [Google Scholar] [CrossRef]
- Patil, O.; Ingalgondi, P.; Mathapati, G.; Gounalli, S.; Sankarappa, T.; Hanagodimath, S. Ground and Excited State Dipole Moments of a Dye. J. Appl. Phys. 2016, 8, 55–59. [Google Scholar]
- Renuka, C.G.; Shivashankar, K.; Boregowda, P.; Bellad, S.S.; Muregendrappa, M.V.; Nadaf, Y.F. An Experimental and Computational Study of 2-(3-Oxo-3H-benzo[f] chromen-1-ylmethoxy)-Benzoic Acid Methyl Ester. J. Solut. Chem. 2017, 46, 1535–1555. [Google Scholar] [CrossRef]
- Yablon, D.G.; Schilowitz, A.M. Solvatochromism of Nile Red in Nonpolar Solvents. Appl. Spectrosc. 2004, 58, 843–847. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How polar are choline chloride-based deep eutectic solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Chen, Y.; Zheng, J.; Zhang, J.; Li, R.; Wan, H.; Yin, J.; Yuan, Z.; Chen, H. A family of push-pull bio-probes for tracking lipid droplets in living cells with the detection of heterogeneity and polarity. Anal. Chim. Acta 2020, 1096, 166–173. [Google Scholar] [CrossRef]
- Sauer, M.; Hofkens, J.; Enderlein, J. Handbook of Fluorescence Spectroscopy and Imaging; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Kumari, R.; George, L.; Varghese, A. Estimation of Ground-State and Singlet Excited-State Dipole Moments of Substituted Schiff Bases Containing Oxazolidin-2-one Moiety through Solvatochromic Methods. J. Fluoresc. 2017, 27, 151–165. [Google Scholar] [CrossRef]
- Thipperudrappa, J. Analaysis of solvatochromism of a biologically active ketocyanine dye using different solvent polarity scales and estimation of dipole moments. Int. J. Life Sci. Pharm. Res. 2014, 4, 1–11. [Google Scholar]
- Aaron, J.-J.; Buna, M.; Parkanyi, C.; Antonious, M.S.; Tine, A.; Cisse, L. Quantitative treatment of the effect of solvent on the electronic absorption and fluorescence spectra of substituted coumarins: Evaluation of the first excited singlet-state dipole moments. J. Fluoresc. 1995, 5, 337–347. [Google Scholar] [CrossRef]
- Ogunsipe, A. Solvent Effects on the Spectral Properties of Rhodamine 6G: Estimation of Ground and Excited State Dipole Moments. J. Solution Chem. 2018, 47, 203–219. [Google Scholar] [CrossRef]
- Desai, V.R.; Sidarai, A.; Hunagund, S.M.; Basanagouda, M.; Melavanki, R.; Fattepur, R.; Kadadevarmath, J. Steady state absorption and fluorescence study: Estimation of ground and excited state dipole moments of newly synthesized pyridazin-3(2H)-one derivatives. J. Mol. Liq. 2016, 223, 141–149. [Google Scholar] [CrossRef]
- Dong, X.; Wan, W.; Zeng, L.; Jin, W.; Huang, Y.; Shen, D.; Bai, Y.; Zhao, Q.; Zhang, L.; Liu, Y.; et al. Regulation of Fluorescence Solvatochromism to Resolve Cellular Polarity upon Protein Aggregation. Anal. Chem. 2021, 93, 16447–16455. [Google Scholar] [CrossRef] [PubMed]


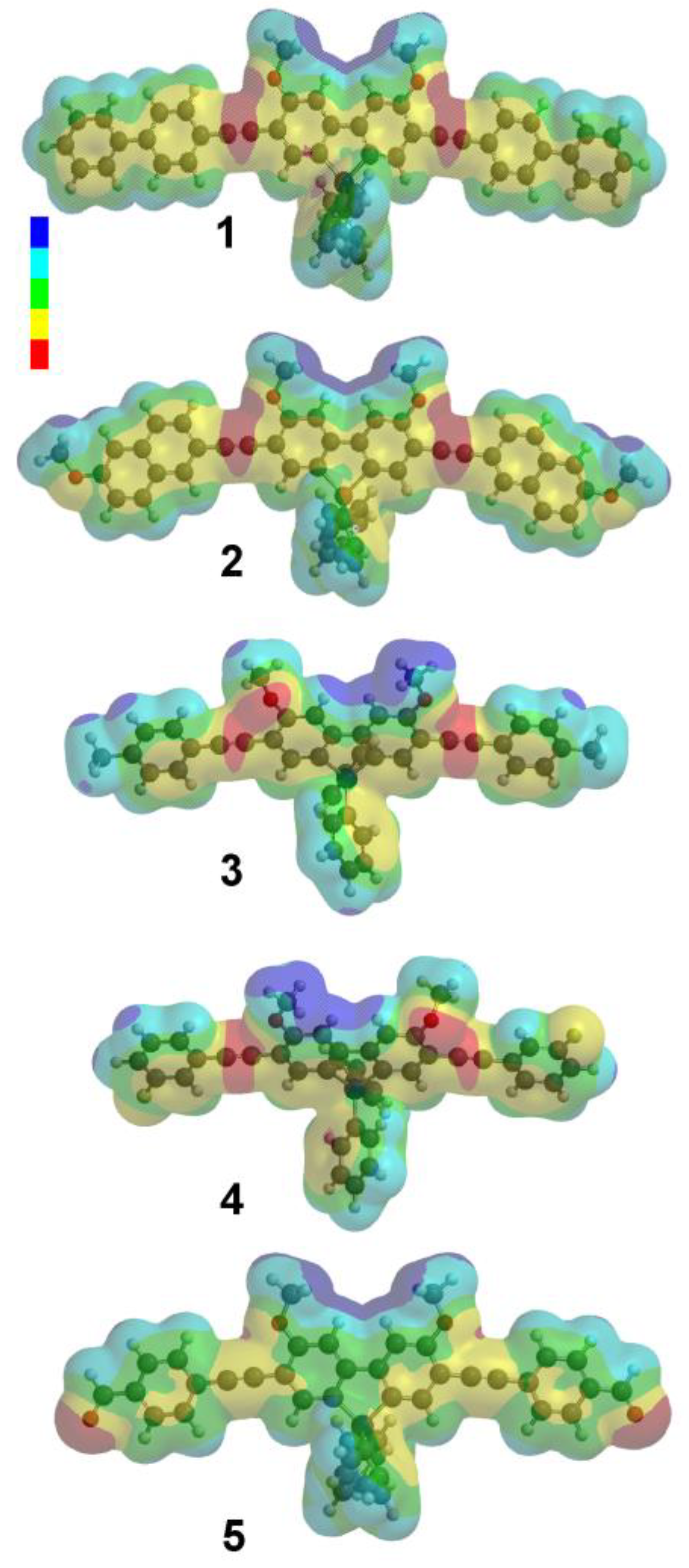
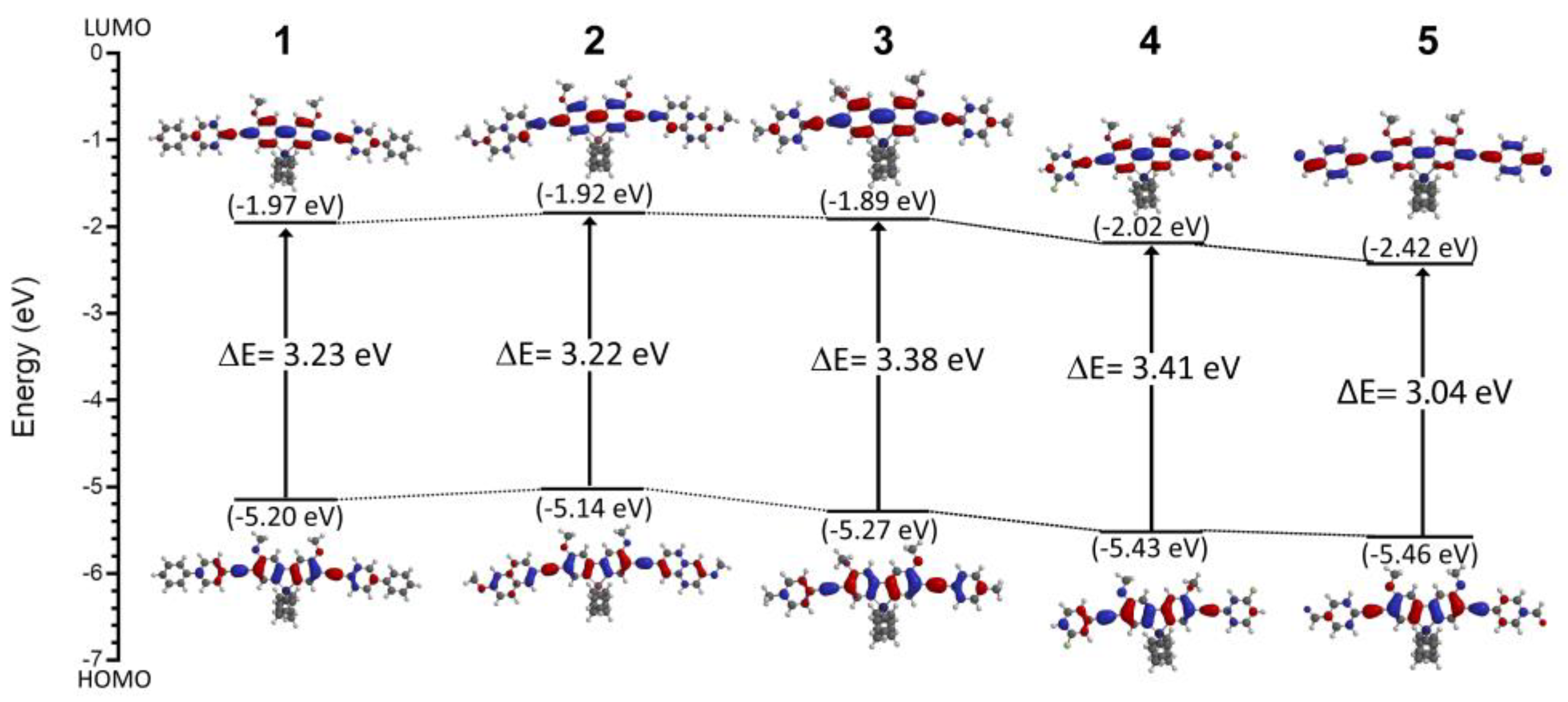
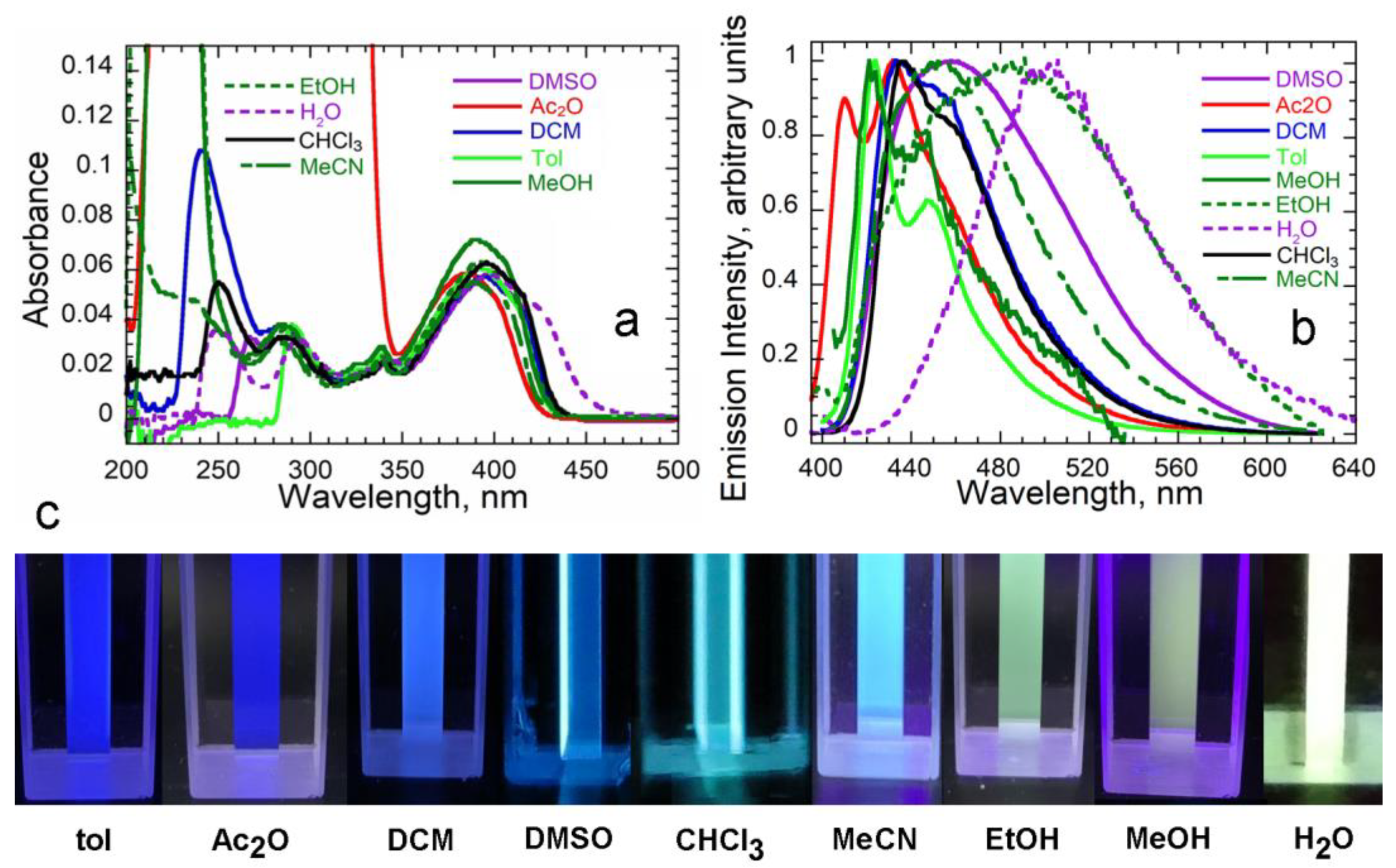

| Compound | HOMO (eV) | LUMO (eV) | Energy Gap (eV) | Dipole Moment (DMF GS), (D) |
|---|---|---|---|---|
| 1 | −5.20 | −1.97 | 3.23 | 2.96 |
| 2 | −5.14 | −1.92 | 3.22 | 3.24 |
| 3 | −5.27 | −1.89 | 3.38 | 1.98 |
| 4 | −5.43 | −2.02 | 3.41 | 2.23 |
| 5 | −5.46 | −2.42 | 3.04 | 8.82 |
| Solvent | λEM max nm | Stokes nm | Shift cm−1 | |||
|---|---|---|---|---|---|---|
| Toluene | 2.38 | 1.497 | 0.012 | 424 | 31 | 1798 |
| Chloroform | 4.81 | 1.442 | 0.147 | 436 | 41 | 2324 |
| Dichloromethane | 8.93 | 1.424 | 0.217 | 433 | 39 | 2221 |
| Dimethyl sulfoxide | 46.7 | 1.483 | 0.263 | 458 | 60 | 3261 |
| Acetone | 21.1 | 1.359 | 0.289 | 432 | 49 | 2728 |
| Ethanol | 24.5 | 1.357 | 0.289 | 485 | 92 | 4675 |
| Acetonitrile | 36.6 | 1.344 | 0.305 | 454 | 62 | 3355 |
| Methanol b | 33.7 | 1.329 | 0.309 | 422 | 31 | 1798 |
| Water (10 mM Tris) c | 80.1 | 1.333 | 0.320 | 500 c | 102 | 5080 |
| Compound | Onsager Radius, | a (D) | Slope LM b | c (D) |
|---|---|---|---|---|
| 1 | 8.331 | 3.05 | −300 | 4.10 |
| 2 | 8.285 | 4.94 | 160 | 2.98 |
| 3 | 6.608 | 2.06 | −120 | 1.89 |
| 4 | 6.166 | 2.30 | −250 | 2.43 |
| 5 | 6.733 | 8.82 | 6500 | 14.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrett-Noland, S.J.; McConnell, W.; Braddock-Wilking, J.; Dupureur, C.M. Solvatochromic Behavior of 2,7-Disubstituted Sila- and Germafluorenes. Chemosensors 2023, 11, 160. https://doi.org/10.3390/chemosensors11030160
Jarrett-Noland SJ, McConnell W, Braddock-Wilking J, Dupureur CM. Solvatochromic Behavior of 2,7-Disubstituted Sila- and Germafluorenes. Chemosensors. 2023; 11(3):160. https://doi.org/10.3390/chemosensors11030160
Chicago/Turabian StyleJarrett-Noland, Shelby J., William McConnell, Janet Braddock-Wilking, and Cynthia M. Dupureur. 2023. "Solvatochromic Behavior of 2,7-Disubstituted Sila- and Germafluorenes" Chemosensors 11, no. 3: 160. https://doi.org/10.3390/chemosensors11030160
APA StyleJarrett-Noland, S. J., McConnell, W., Braddock-Wilking, J., & Dupureur, C. M. (2023). Solvatochromic Behavior of 2,7-Disubstituted Sila- and Germafluorenes. Chemosensors, 11(3), 160. https://doi.org/10.3390/chemosensors11030160







