Variability Assessment of the Performance of MoS2-Based BioFETs
Abstract
1. Introduction
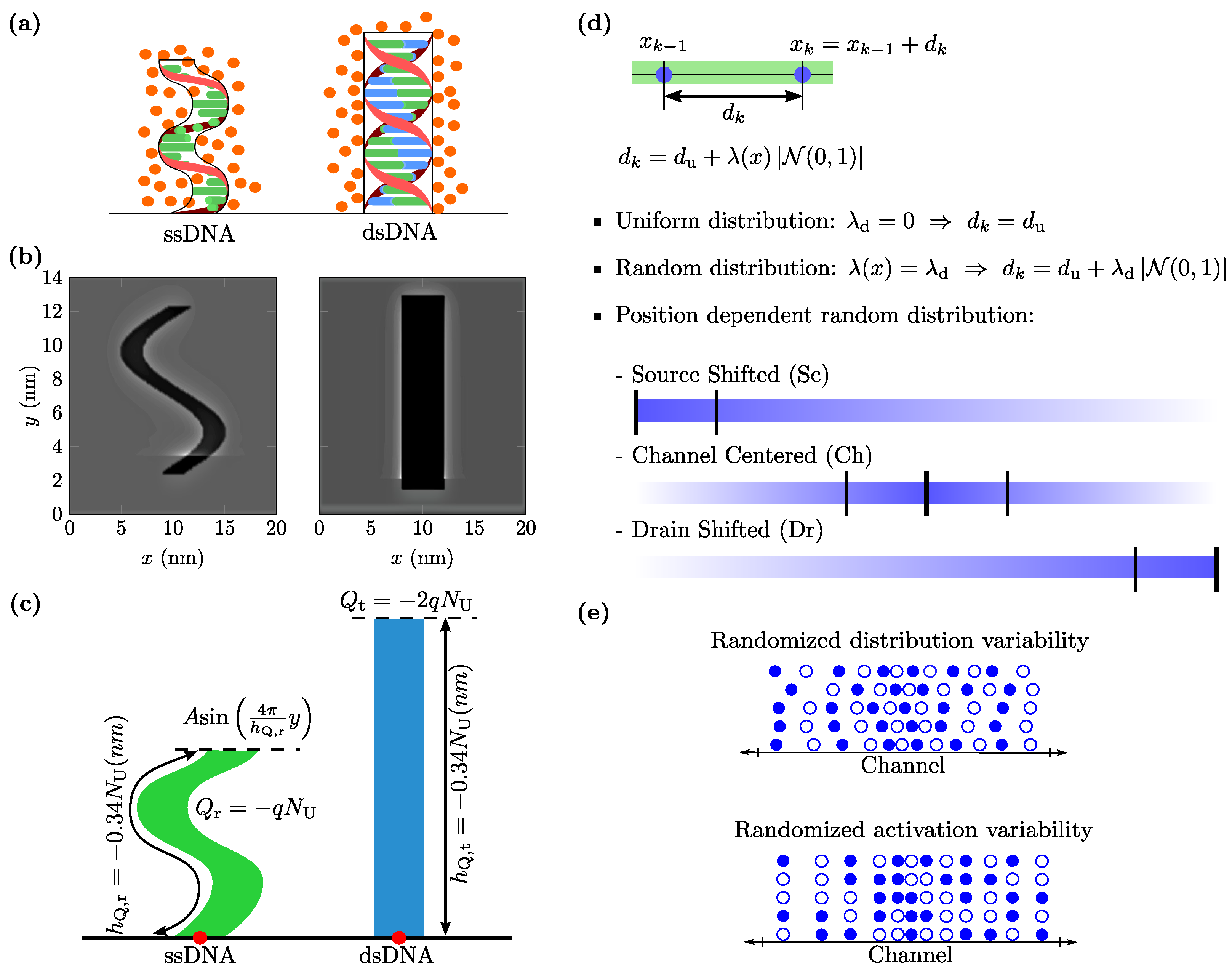
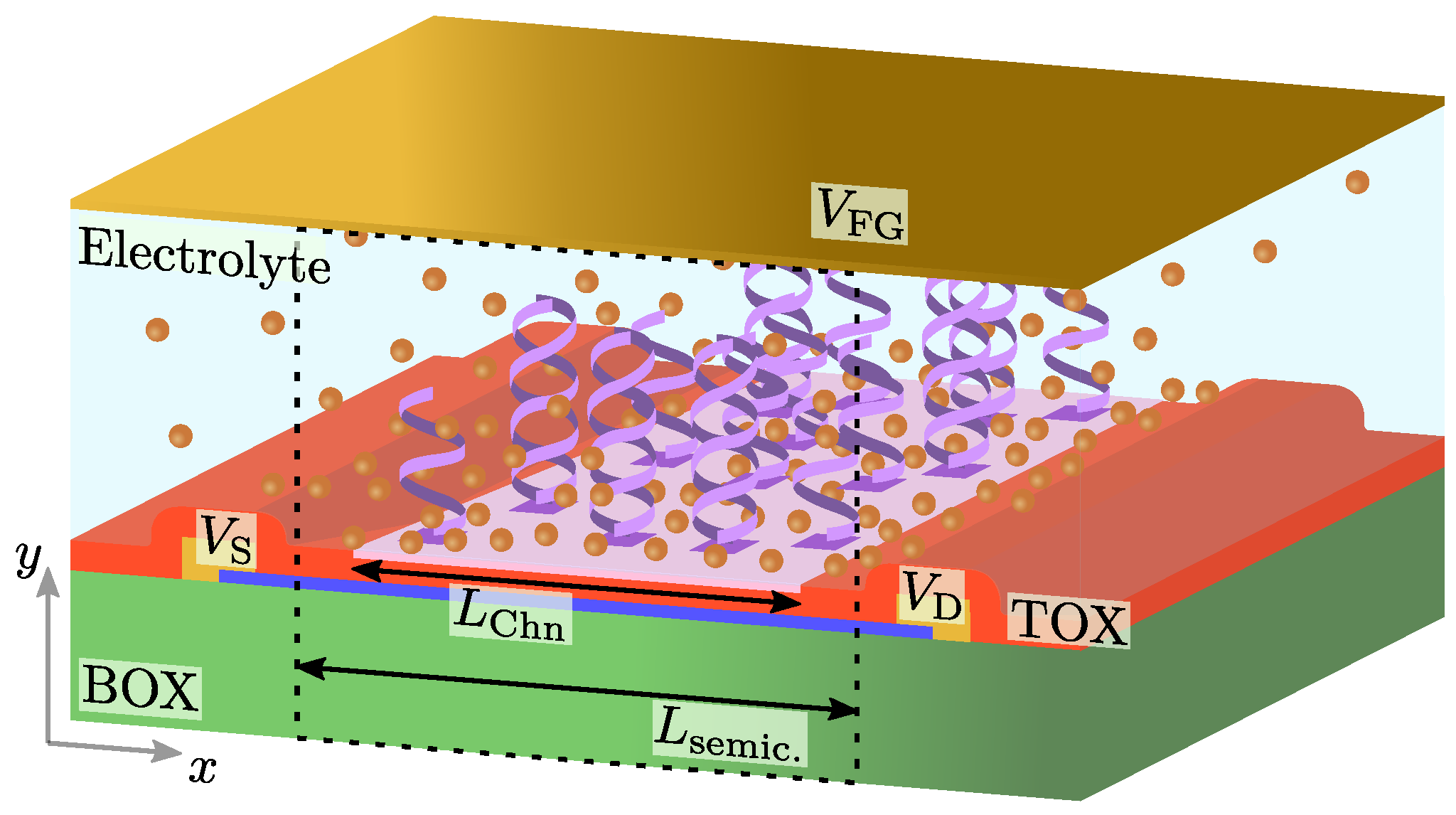
2. Materials and Methods
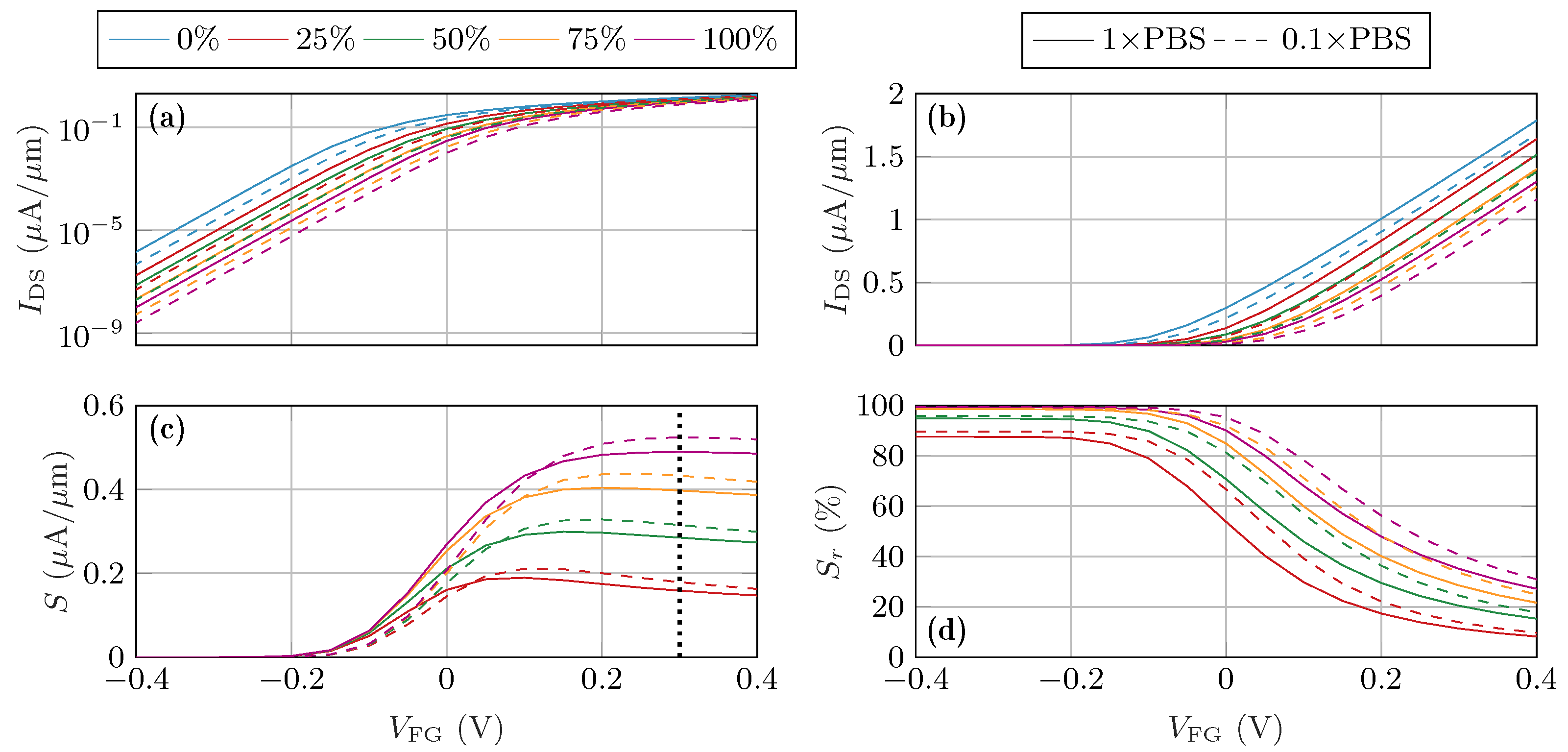
3. Results
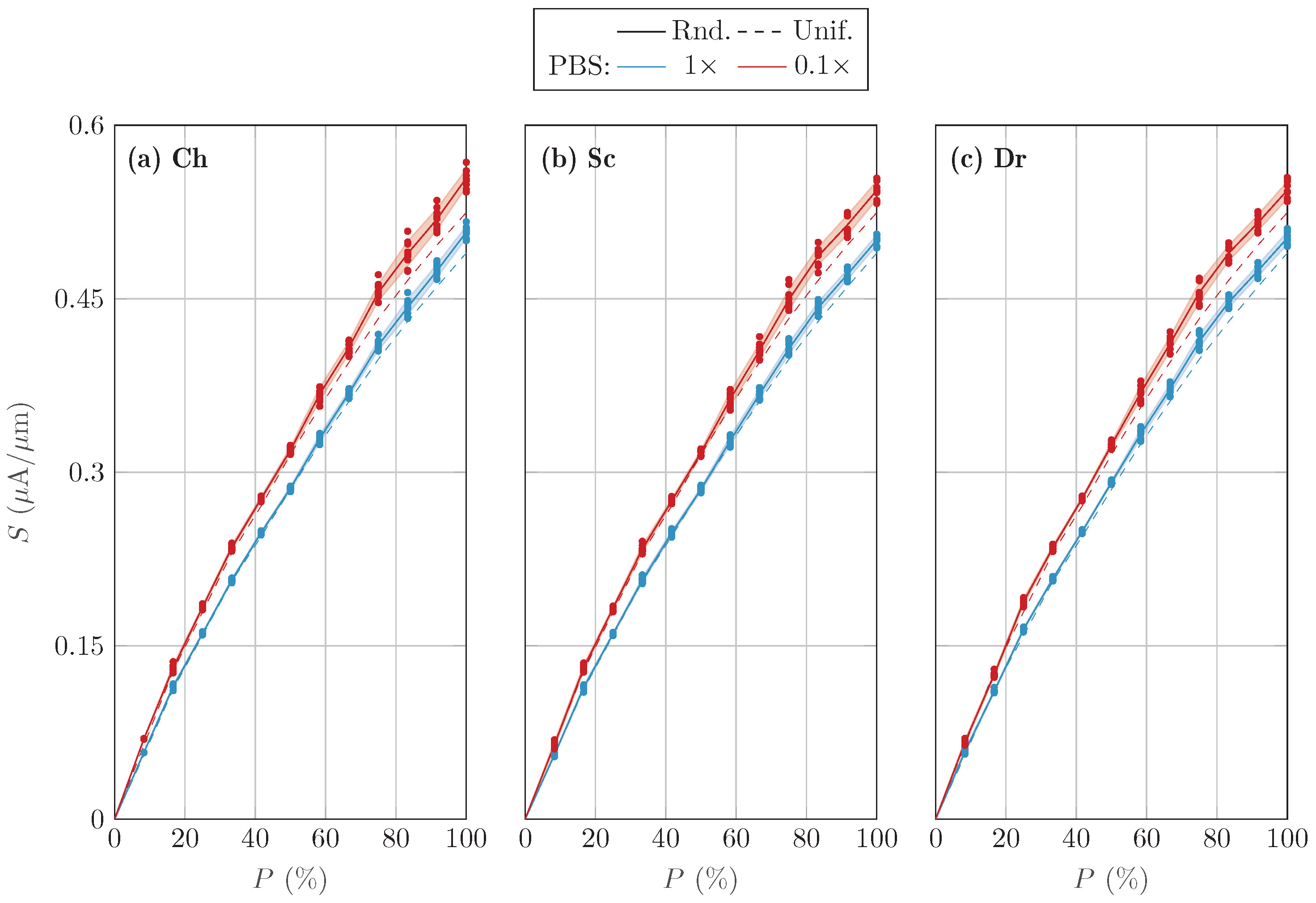
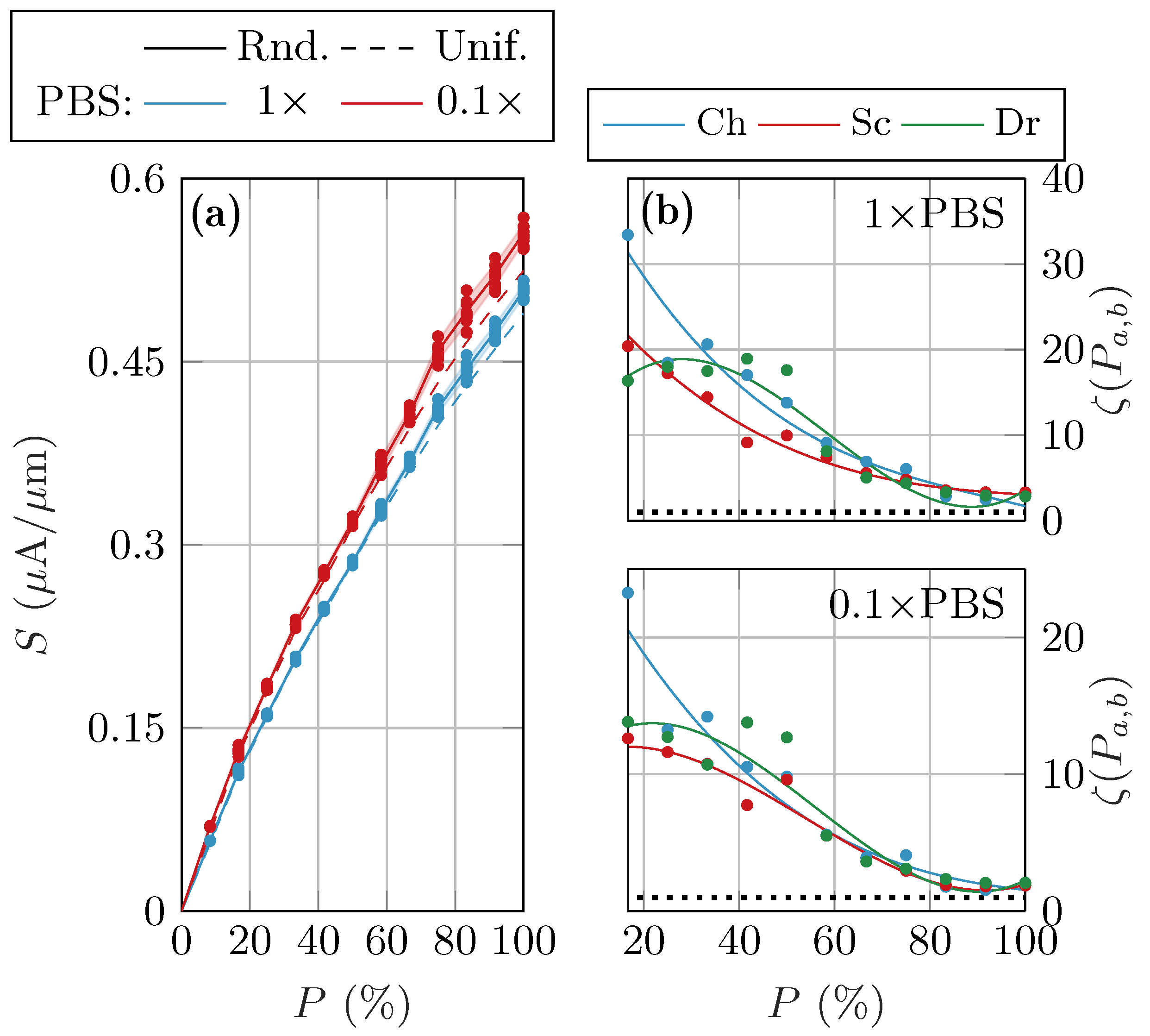
3.1. Randomized Distribution Variability
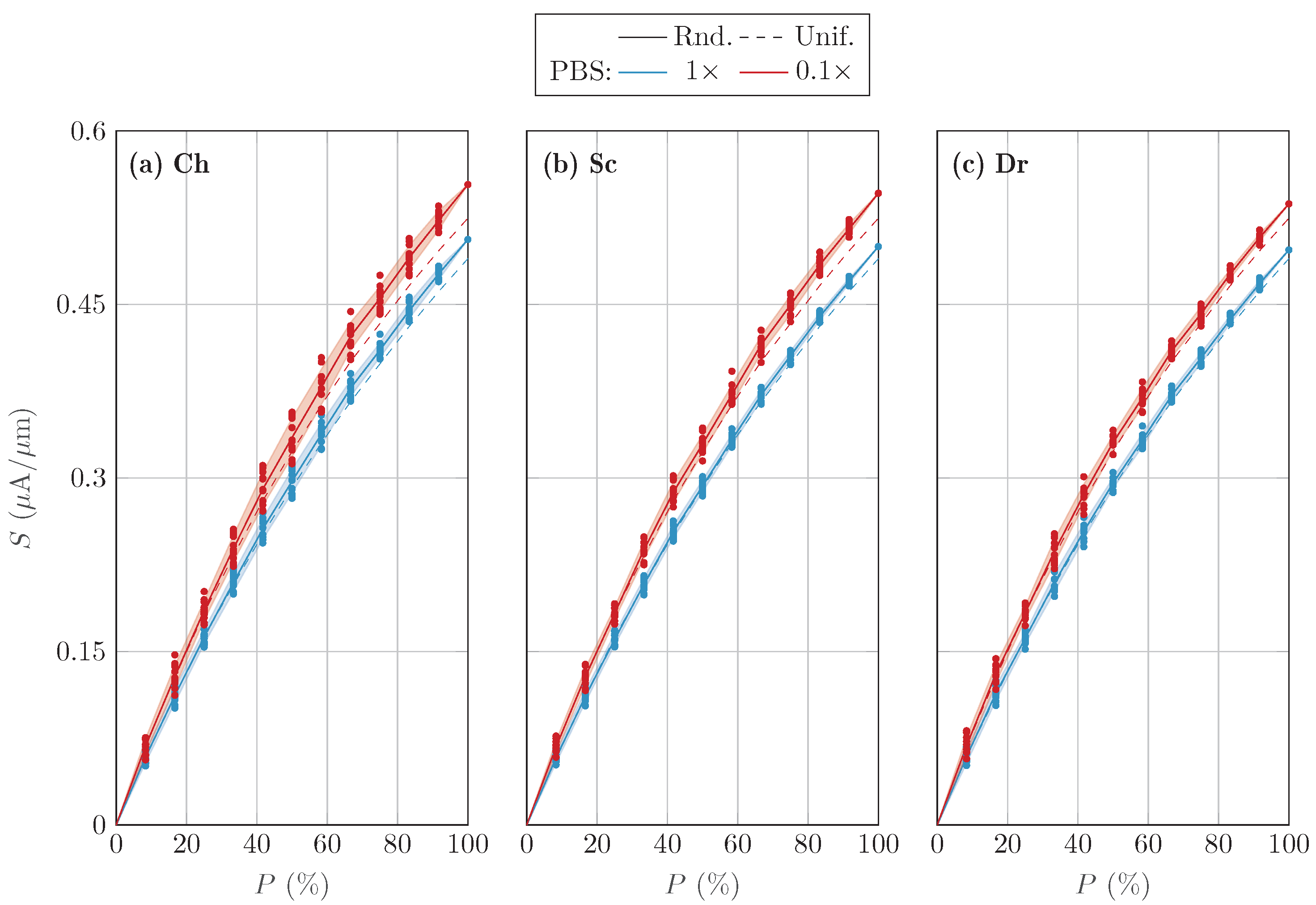
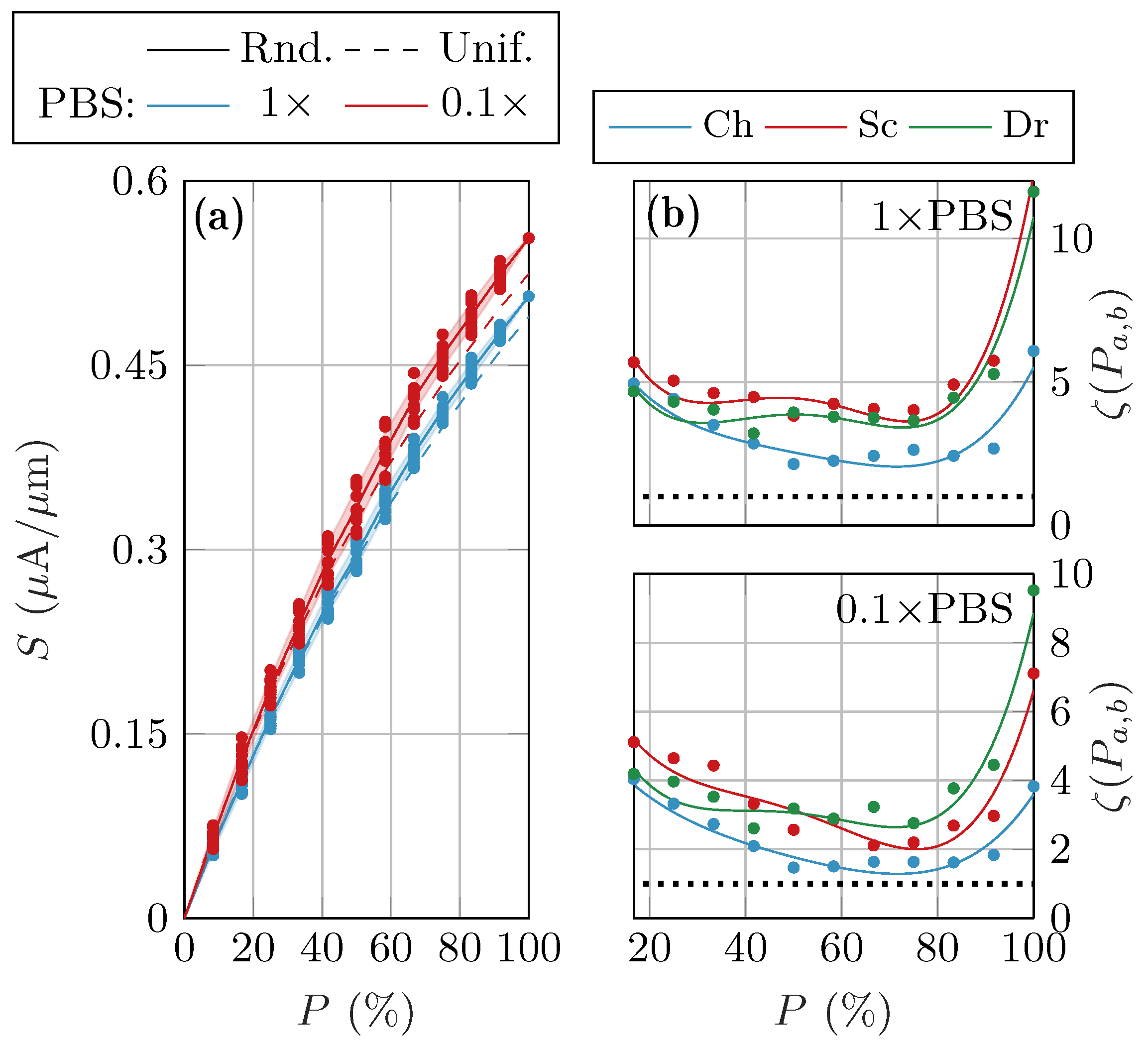
3.2. Randomized Activation Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2DM | Two-dimensional material |
| FET | Field-effect transistor |
| IoT | Internet of Things |
| BioFET | Biomolecular field-effect transistor |
| PBS | Phosphate Buffer Saline |
| ssDNA | Single-stranded DNA |
| dsDNA | Double-stranded DNA |
Appendix A
| PBS | ||||
|---|---|---|---|---|
| Ch 1×PBS | 4.87 | −12.25 | 1.18 | −4.25 |
| Ch 0.1×PBS | 3.09 | −7.19 | 0.59 | −1.69 |
| Sc 1×PBS | 3.27 | −7.74 | 0.68 | −2.11 |
| Sc 0.1×PBS | 1.02 | 2.26 | −0.80 | 4.98 |
| Dr 1×PBS | 45.29 | 11.46 | −2.69 | 1.53 |
| Dr 0.1×PBS | 91.48 | 4.56 | −1.29 | 7.71 |
| PBS | |||||
|---|---|---|---|---|---|
| Ch 1 × PBS | 0.97 | −4.46 | 1.16 | −1.48 | 0.72 |
| Ch 0.1 × PBS | 0.68 | −2.56 | 58.60 | −0.73 | 0.37 |
| Sc 1 × PBS | 1.58 | −10.60 | 3.48 | −4.82 | 2.36 |
| Sc 0.1 × PBS | 1.05 | −5.30 | 1.62 | −2.29 | 1.16 |
| Dr 1 × PBS | 1.34 | −9.10 | 2.98 | −4.09 | 1.99 |
| Dr 0.1 × PBS | 1.06 | −6.55 | 2.09 | −2.89 | 1.43 |
References
- Karthick, G.S.; Pankajavalli, P.B. A Review on Human Healthcare Internet of Things: A Technical Perspective. SN Comput. Sci. 2020, 1, 1–19. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Li, X.; Kamins, T.I.; Nauka, K.; Williams, R.S. Sequence-Specific Label-Free DNA Sensors Based on Silicon Nanowires. Nano Lett. 2004, 4, 245–247. [Google Scholar] [CrossRef]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-dimensional layered MoS2 biosensors enable highly sensitive detection of biomolecules. Sci. Rep. 2014, 4, 7352. [Google Scholar] [CrossRef]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wong, J.I.; Palacios, T.; Kong, J.; Yang, H.Y. Functionalized MoS2 nanosheet-based field-effect biosensor for label-free sensitive detection of cancer marker proteins in solution. Small 2014, 10, 1101–1105. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Regonda, S.; Gao, J.; Liu, Y.; Hu, W. Ultrasensitive protein detection using lithographically defined Si multi-nanowire field effect transistors. Lab Chip 2011, 11, 1952–1961. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Chang, J.; Pu, H.; Lu, G.; He, Q.; Zhang, H.; Chen, J. Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 2017, 46, 6872–6904. [Google Scholar] [CrossRef]
- Hess, L.H.; Becker-Freyseng, C.; Wismer, M.S.; Blaschke, B.M.; Lottner, M.; Rolf, F.; Seifert, M.; Garrido, J.A. Electrical coupling between cells and graphene transistors. Small 2015, 11, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Li, D.; Wi, S.; Chen, M.; Ryu, B.; Liang, X. Nanoimprint-assisted shear exfoliation plus transfer printing for producing transition metal dichalcogenide heterostructures. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2016, 34, 1–6. [Google Scholar] [CrossRef]
- Quellmalz, A.; Wang, X.; Sawallich, S.; Uzlu, B.; Otto, M.; Wagner, S.; Wang, Z.; Prechtl, M.; Hartwig, O.; Luo, S.; et al. Large-area integration of two-dimensional materials and their heterostructures by wafer bonding. Nat. Commun. 2021, 12, 917. [Google Scholar] [CrossRef]
- Gibney, E. 2D or not 2D. Nature 2015, 522, 274–276. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.Y.; Jang, H.; Lee, J.; Moon, H.; Seo, S.M.; Kim, D.H. Simulation study on discrete charge effects of SiNW biosensors according to bound target position using a 3D TCAD simulator. Nanotechnology 2012, 23, 065202. [Google Scholar] [CrossRef] [PubMed]
- Bandiziol, A.; Palestri, P.; Pittino, F.; Esseni, D.; Selmi, L. A TCAD-Based Methodology to Model the Site-Binding Charge at ISFET/Electrolyte Interfaces. IEEE Trans. Electron Devices 2015, 62, 3379–3386. [Google Scholar] [CrossRef]
- Passeri, D.; Morozzi, A.; Kanxheri, K.; Scorzoni, A. Numerical simulation of ISFET structures for biosensing devices with TCAD tools. BioMed. Eng. 2015, 14, S3. [Google Scholar] [CrossRef]
- Nair, P.R.; Alam, M.A. Design considerations of silicon nanowire biosensors. IEEE Trans. Electron Devices 2007, 54, 3400–3408. [Google Scholar] [CrossRef]
- Yang, J.; He, J.; Liu, F.; Zhang, L.; Liu, F.; Zhang, X.; Chan, M. A compact model of silicon-based nanowire MOSFETs for circuit simulation and design. IEEE Trans. Electron Devices 2008, 55, 2898–2906. [Google Scholar] [CrossRef]
- Shadman, A.; Rahman, E.; Khosru, Q.D. Monolayer MoS2 and WSe2 Double Gate Field Effect Transistor as Super Nernst pH sensor and Nanobiosensor. Sens.-Bio-Sens. Res. 2016, 11, 45–51. [Google Scholar] [CrossRef]
- Datta, K.; Shadman, A.; Rahman, E.; Khosru, Q.D.M. Trilayer TMDC Heterostructures for MOSFETs and Nanobiosensors. J. Electron. Mater. 2016, 46, 1248–1260. [Google Scholar] [CrossRef]
- Yang, X.; Frensley, W.R.; Zhou, D.; Hu, W. Performance Analysis of Si Nanowire Biosensor by Numerical Modeling for Charge Sensing. IEEE Trans. Nanotechnol. 2012, 11, 501–512. [Google Scholar] [CrossRef]
- Toral-Lopez, A.; Marin, E.G.; Gonzalez-Medina, J.M.; Romero, F.J.; Ruiz, F.G.; Morales, D.P.; Rodriguez, N.; Godoy, A. Assessment of three electrolyte-molecule electrostatic interaction models for 2D material based BioFETs. Nanoscale Adv. 2019, 1, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Toral-Lopez, A.; Pasadas, F.; Marin, E.G.; Medina-Rull, A.; Gonzalez-Medina, J.M.; Ruiz, F.J.G.; Jiménez, D.; Godoy, A. Multi-scale analysis of radio-frequency performance of 2D-material based field-effect transistors. Nanoscale Adv. 2021, 3, 2377–2382. [Google Scholar] [CrossRef]
- Toral-Lopez, A.; Marin, E.G.; Medina, A.; Ruiz, F.G.; Rodriguez, N.; Godoy, A. GFET Asymmetric Transfer Response Analysis through Access Region Resistances. Nanomaterials 2019, 9, 1027. [Google Scholar] [CrossRef]
- Toral-Lopez, A.; Kokh, D.B.; Marin, E.G.; Wade, R.C.; Godoy, A. Graphene BioFET sensors for SARS-CoV-2 detection: A multiscale simulation approach. Nanoscale Adv. 2022, 4, 3065–3072. [Google Scholar] [CrossRef]
- Nishio, Y.; Uno, S.; Nakazato, K. Three-Dimensional Simulation of DNA Sensing by Ion-Sensitive Field-Effect Transistor: Optimization of DNA Position and Orientation. Jpn. J. Appl. Phys. 2013, 52, 04CL01. [Google Scholar] [CrossRef]
- Poghossian, A.; Cherstvy, A.; Ingebrandt, S.; Offenhäusser, A.; Schöning, M.J. Possibilities and limitations of label-free detection of DNA hybridization with field-effect-based devices. Sens. Actuators B Chem. 2005, 111–112, 470–480. [Google Scholar] [CrossRef]
- Daus, A.; Vaziri, S.; Chen, V.; Köroğlu, Ç.; Grady, R.W.; Bailey, C.S.; Lee, H.R.; Schauble, K.; Brenner, K.; Pop, E. High-performance flexible nanoscale transistors based on transition metal dichalcogenides. Nat. Electron. 2021, 4, 495–501. [Google Scholar] [CrossRef]
- Kumar, A.; Schauble, K.; Neilson, K.M.; Tang, A.; Ramesh, P.; Wong, H.S.; Pop, E.; Saraswat, K. Sub-200 Ω·μm Alloyed Contacts to Synthetic Monolayer MoS2. In Proceedings of the 2021 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 11–16 December 2021. [Google Scholar] [CrossRef]
- Yan, X.; Gu, Y.; Li, C.; Tang, L.; Zheng, B.; Li, Y.; Zhang, Z.; Yang, M. Synergetic catalysis based on the proline tailed metalloporphyrin with graphene sheet as efficient mimetic enzyme for ultrasensitive electrochemical detection of dopamine. Biosens. Bioelectron. 2016, 77, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Lu, N.; Wang, Y.; Dai, P.; Li, T.; Gao, X.; Wang, Y.; Fan, C. Enhanced sensing of nucleic acids with silicon nanowire field effect transistor biosensors. Nano Lett. 2012, 12, 5262–5268. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta-Lopez, J.; Toral-Lopez, A.; Marin, E.G.; Ruiz, F.G.; Pasadas, F.; Medina-Rull, A.; Godoy, A. Variability Assessment of the Performance of MoS2-Based BioFETs. Chemosensors 2023, 11, 57. https://doi.org/10.3390/chemosensors11010057
Cuesta-Lopez J, Toral-Lopez A, Marin EG, Ruiz FG, Pasadas F, Medina-Rull A, Godoy A. Variability Assessment of the Performance of MoS2-Based BioFETs. Chemosensors. 2023; 11(1):57. https://doi.org/10.3390/chemosensors11010057
Chicago/Turabian StyleCuesta-Lopez, Juan, Alejandro Toral-Lopez, Enrique G. Marin, Francisco G. Ruiz, Francisco Pasadas, Alberto Medina-Rull, and Andres Godoy. 2023. "Variability Assessment of the Performance of MoS2-Based BioFETs" Chemosensors 11, no. 1: 57. https://doi.org/10.3390/chemosensors11010057
APA StyleCuesta-Lopez, J., Toral-Lopez, A., Marin, E. G., Ruiz, F. G., Pasadas, F., Medina-Rull, A., & Godoy, A. (2023). Variability Assessment of the Performance of MoS2-Based BioFETs. Chemosensors, 11(1), 57. https://doi.org/10.3390/chemosensors11010057








