A Fast and Robust Third-Order Multivariate Calibration Approach Coupled with Excitation–Emission Matrix Phosphorescence for the Quantification and Oxidation Kinetic Study of Fluorene in Wastewater Samples
Abstract
1. Introduction
2. Theory of Third-Order Multivariate Calibrations
2.1. The Quadrilinear Model
2.2. The Four-way PARAFAC Algorithm
2.3. The AQLD Algorithm
2.4. SWAQLD, the Proposed Algorithm
- (1)
- Estimation of chemical ranks for the considered four-way data set;
- (2)
- Random initialization of matrices A, B, and C;
- (3)
- Matrix D calculation applying Equation (24);
- (4)
- Matrix A calculation applying Equation (25) and then scaling it to be column-wise normalized;
- (5)
- Matrix B calculation applying Equation (26) and then scaling it to be column-wise normalized;
- (6)
- Matrix C calculation applying Equation (27) and then scaling it to be column-wise normalized;
- (7)
- Update the D matrix applying Equation (24);
- (8)
- Update matrices A, B, and C according to steps (4) to (7) until the stopping criteria are reached (Equation (28)):
3. Material and Methods
3.1. Generation of a Simulated Excitation (EX)–Emission (EM)–Kinetic Phosphorescence Data Array
3.2. Sample Preparation and EX–EM–Kinetic Data Arrays Acquisition
3.2.1. Reagents and Chemicals
3.2.2. Sample Preparation
3.2.3. Spectroscopic Acquisition
4. Results and Discussion
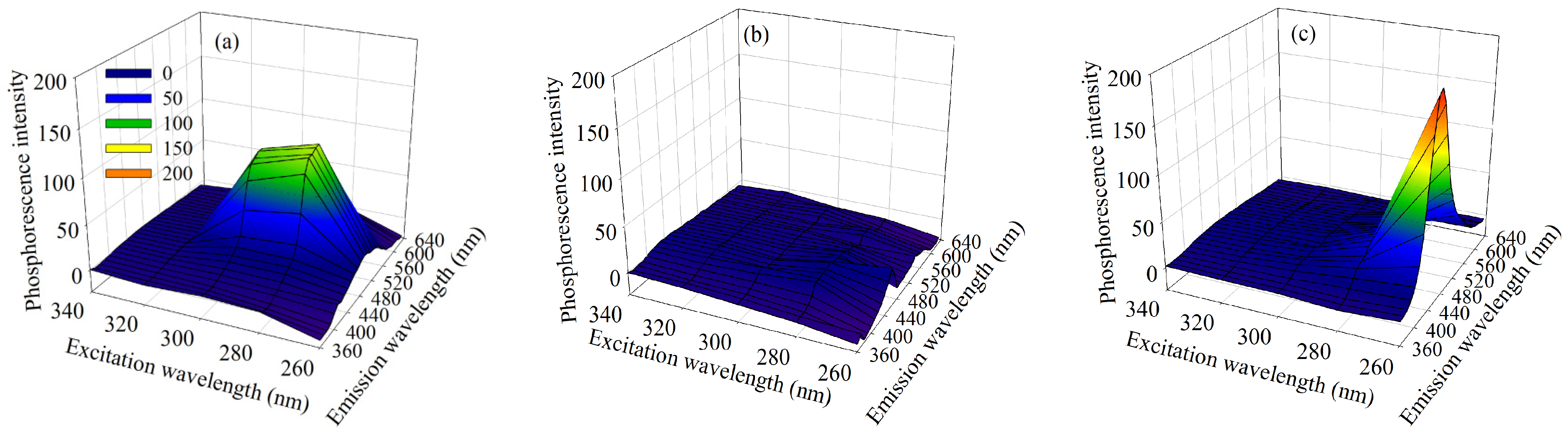
4.1. Analysis of the Simulated Data Sets
4.2. Data Analysis of Real Data Sets
4.2.1. Finding Optimal Experimental Conditions
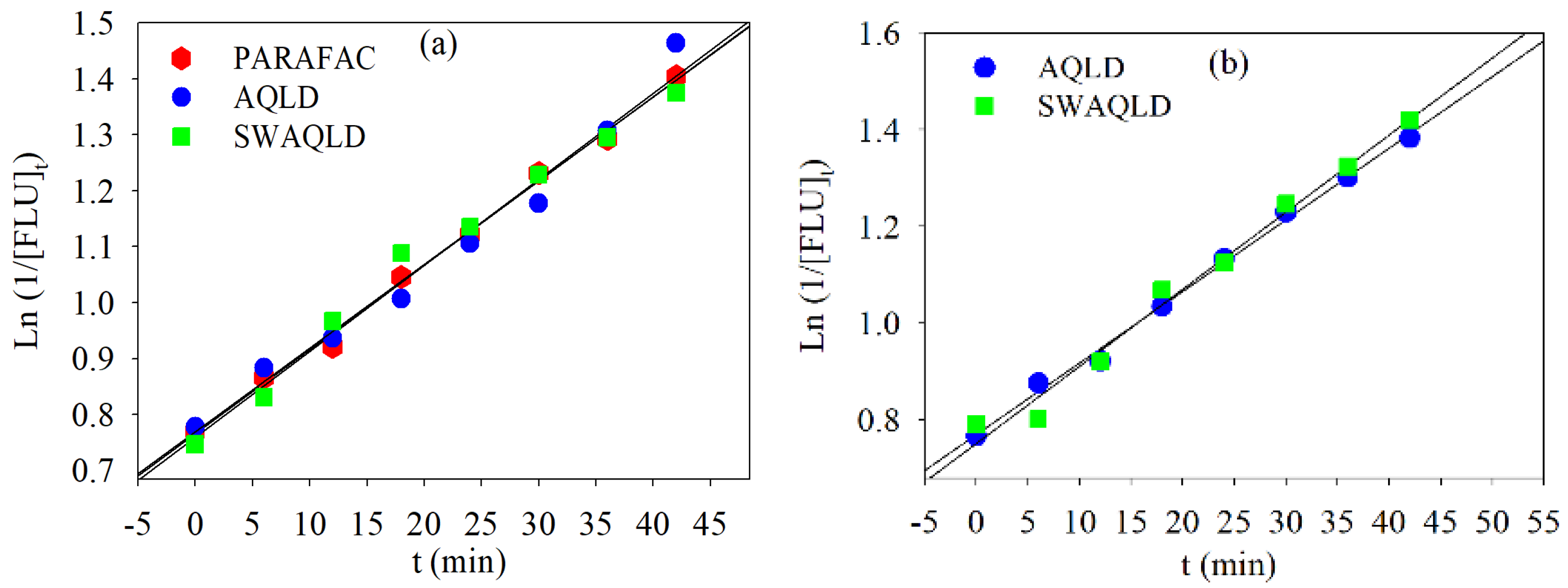
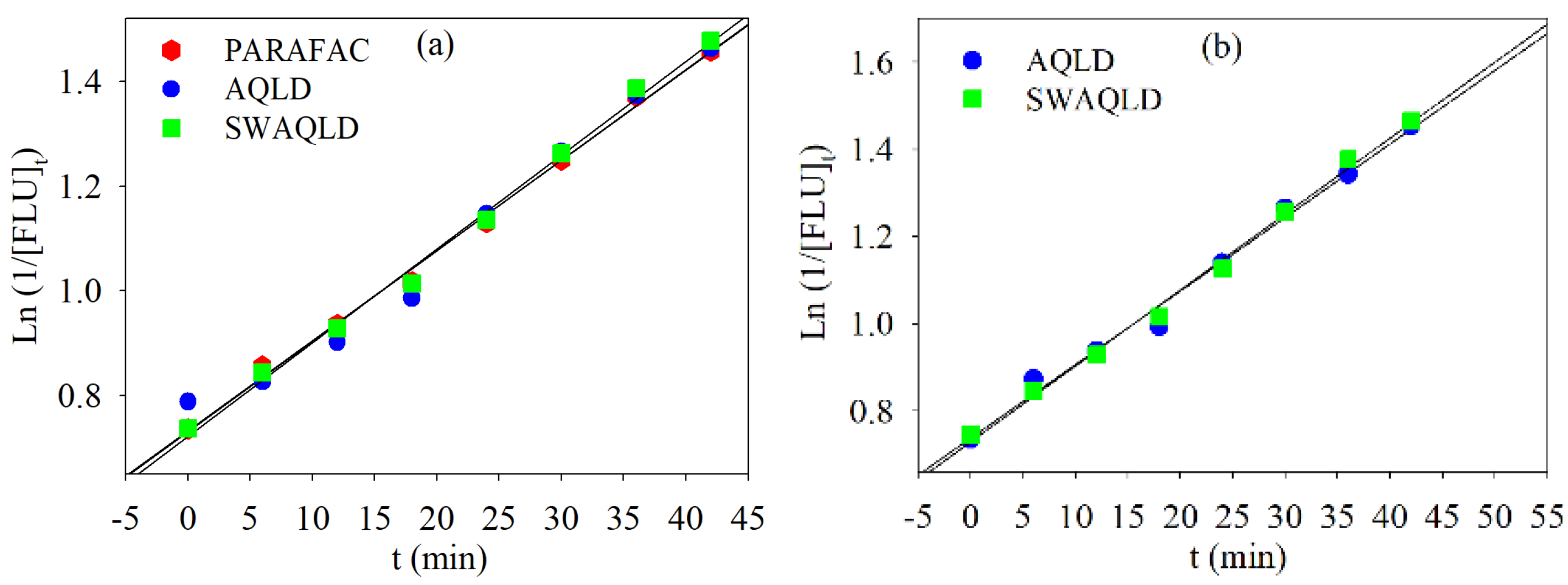
4.2.2. Global Analysis of the FLU Kinetic
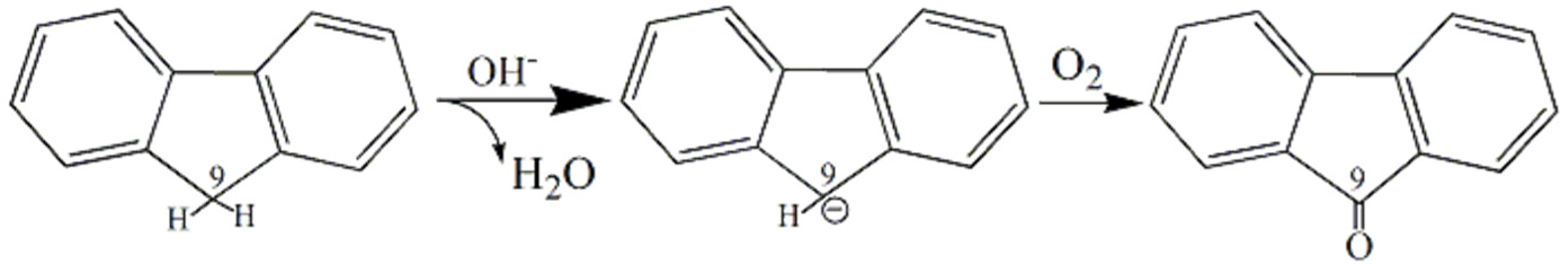
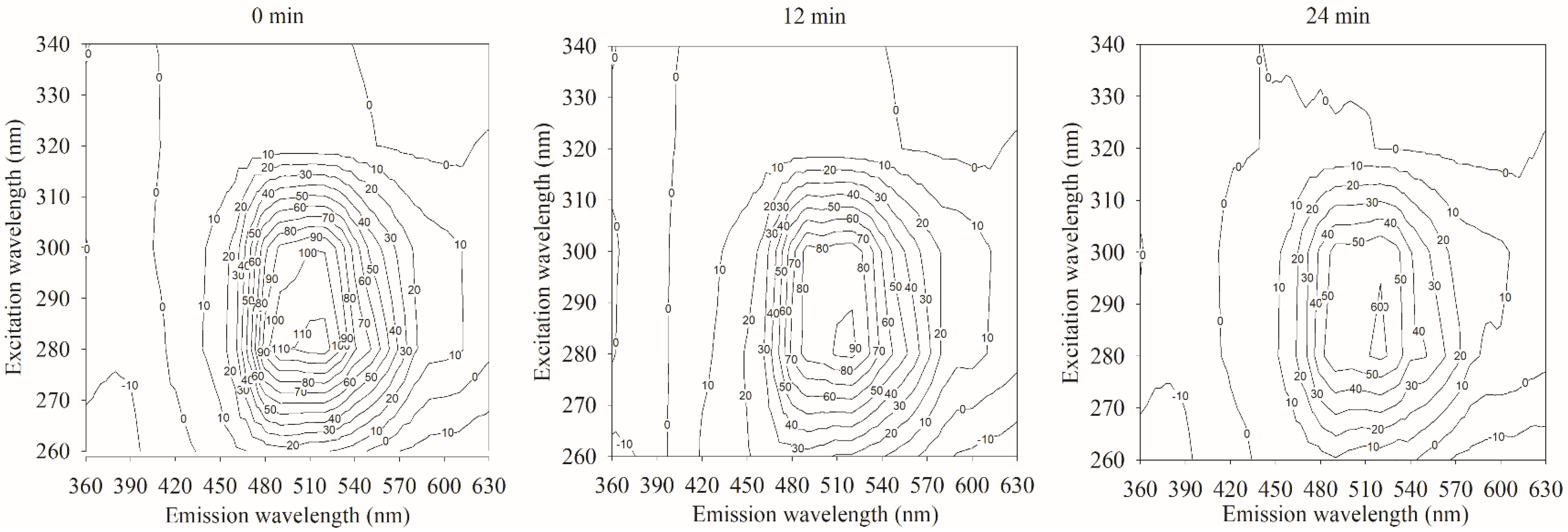
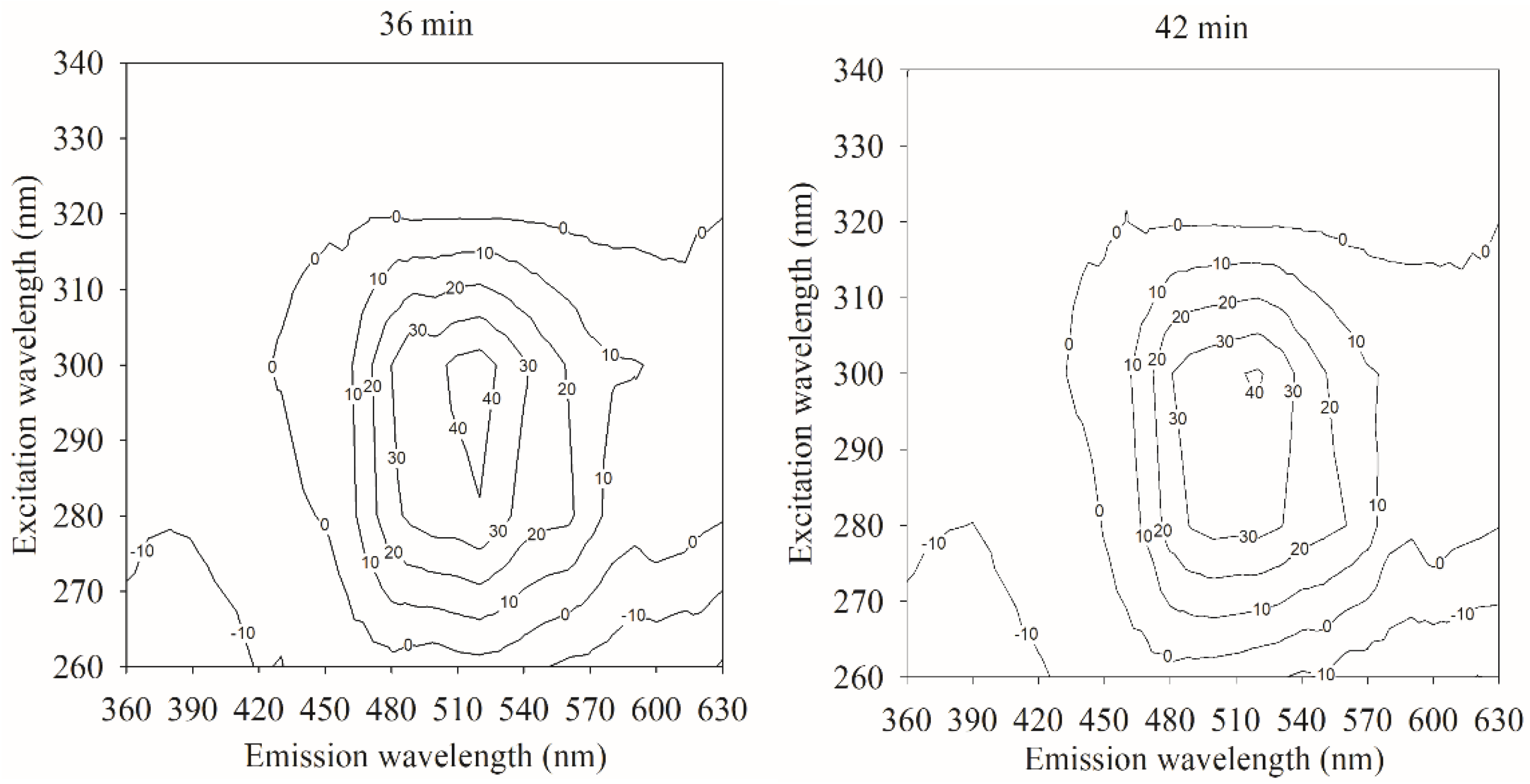
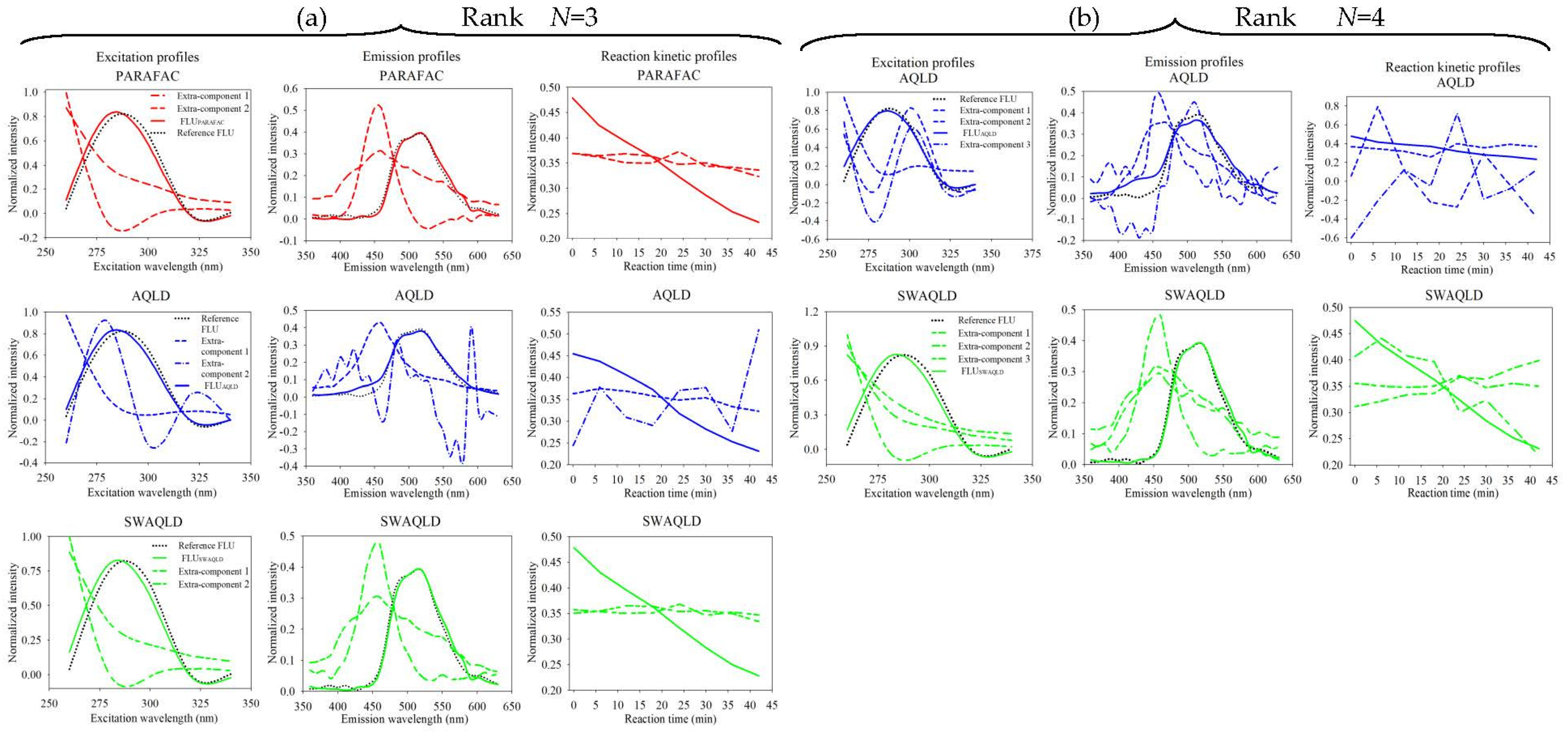
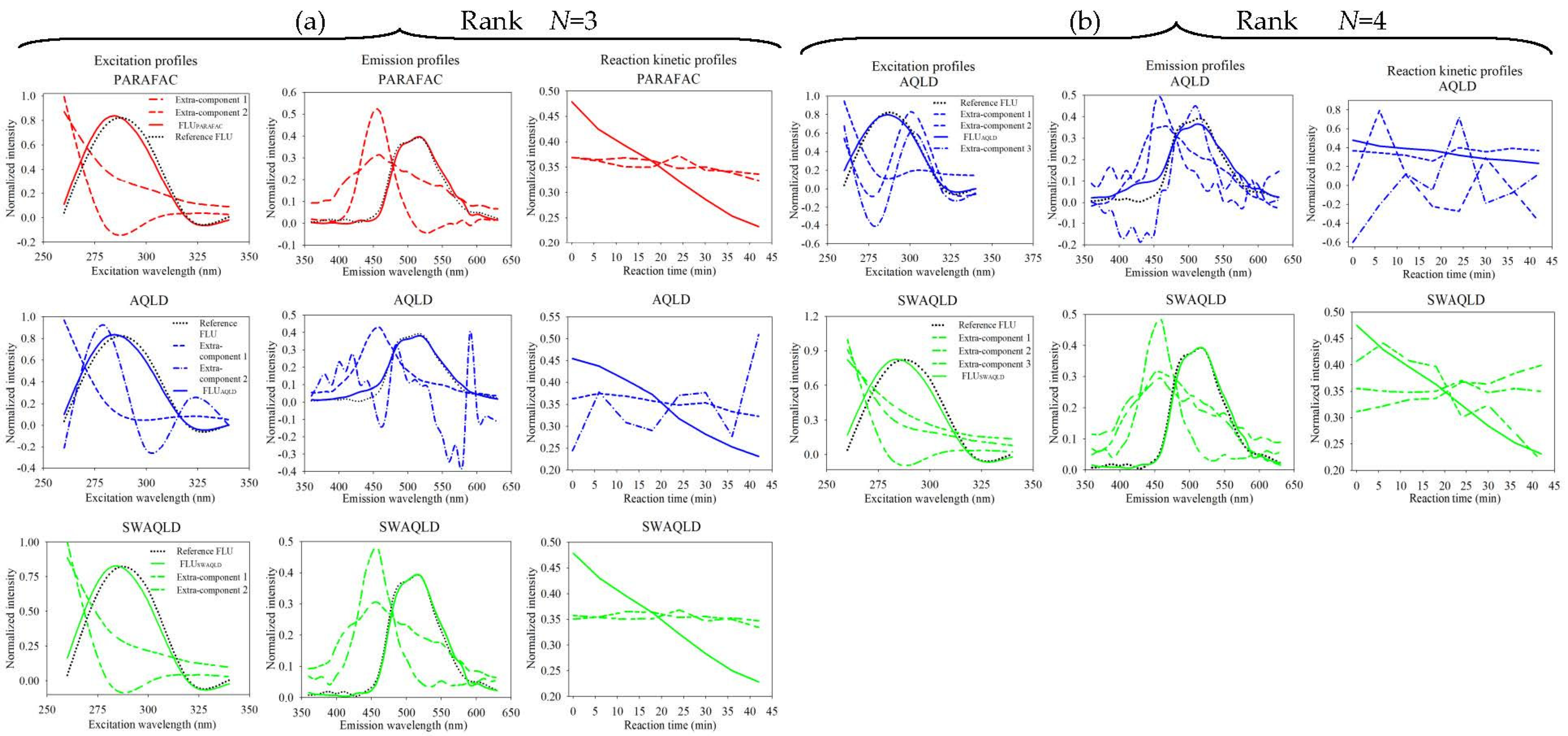
4.2.3. Spectral Characteristics of Samples
4.2.4. Analysis of FLU in Free-Interference Water Samples
4.2.5. Analysis of FLU in Wastewater Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escandar, G.M.; Muñoz de la Peña, A. Multi-way calibration for the quantification of polycyclic aromatic hydrocarbons in samples of environmental impact. Microchem. J. 2021, 164, 106016. [Google Scholar] [CrossRef]
- Shang, F.K.; Wang, Y.T.; Wang, J.Z.; Zhang, L.; Cheng, P.F.; Wang, S.T. Determination of three polycyclic aromatic hydrocarbons in tea using four-way fluorescence data coupled with third-order calibration method. Microchem. J. 2019, 146, 957–964. [Google Scholar] [CrossRef]
- Sánchez-Barragán, I.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A.; Salinas, A.; Segura, A.; Fernández-Gutiérrez, A.; Ballesteros, A.; González, J.M. Molecularly imprinted polymers based on iodinated monomers for selective room-temperature phosphorescence optosensing of fluoranthene in water. Anal. Chem. 2005, 77, 7005–7011. [Google Scholar] [CrossRef]
- Chen, J.C.; Wu, H.L.; Wang, T.; Dong, M.Y.; Chen, Y.; Yu, R.Q. High-Performance Liquid Chromatography–Diode Array Detection Combined with Chemometrics for Simultaneous Quantitative Analysis of Five Active Constituents in a Chinese Medicine Formula Wen-Qing-Yin. Chemosensors 2022, 10, 238. [Google Scholar] [CrossRef]
- Baroudi, F.; Al-Alam, J.; Chimjarn, S.; Delhomme, O.; Fajloun, Z.; Millet, M. Conifers as environmental biomonitors: A multi-residue method for the concomitant quantification of pesticides, polycyclic aromatic hydrocarbons and polychlorinated biphenyls by LC-MS/MS and GC–MS/MS. Microchem. J. 2020, 154, 104593. [Google Scholar] [CrossRef]
- Araújo, A.S.; Castro, J.P.; Sperança, M.A.; Andrade, D.F.; de Mello, M.L.; Pereira-Filho, E.R. Multiway calibration strategies in laser-induced breakdown spectroscopy: A proposal. Anal. Chem. 2021, 93, 6291–6300. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, T.; Yu, R.Q. Recent advances in chemical multi-way calibration with second-order or higher-order advantages: Multilinear models, algorithms, related issues and applications. Trends Anal. Chem. 2020, 130, 115954. [Google Scholar] [CrossRef]
- Anzardi, M.B.; Arancibia, J.A.; Olivieri, A.C. Processing multi-way chromatographic data for analytical calibration, classification and discrimination: A successful marriage between separation science and chemometrics. Trends Anal. Chem. 2021, 134, 116128. [Google Scholar] [CrossRef]
- Pérez-Cova, M.; Jaumot, J.; Tauler, R. Untangling comprehensive two-dimensional liquid chromatography data sets using regions of interest and multivariate curve resolution approaches. Trends Anal. Chem. 2021, 137, 116207. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Bortolato, S.A.; Olivieri, A.C. MVC3_GUI: A MATLAB graphical user interface for third-order multivariate calibration. An upgrade including new multi-way models. Chemom. Intel. Lab. Syst. 2018, 173, 21–29. [Google Scholar] [CrossRef]
- Yin, X.L.; Gu, H.W.; Liu, X.L.; Zhang, S.H.; Wu, H.L. Comparison of three-way and four-way calibration for the real-time quantitative analysis of drug hydrolysis in complex dynamic samples by excitation-emission matrix fluorescence. Spectrochim. Acta Part A 2018, 192, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.; Toledo-Neira, C.; Bravo, M.A. Critical evaluation of third-order advantage with highly overlapped spectral signals. Determination of fluoroquinolones in fish-farming waters by fluorescence spectroscopy coupled to multivariate calibration. Talanta 2019, 204, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Carabajal, M.D.; Arancibia, J.A.; Escandar, G.M. Excitation-emission fluorescence-kinetic third-order/four-way data: Determination of bisphenol A and nonylphenol in food-contact plastics. Talanta 2019, 197, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, H.C.; Yu, S.; Moore, A.F.T.; Campiglia, A.D. Four-way modeling of 4.2K time-resolved excitation emission fluorescence data for the quantitation of polycyclic aromatic hydrocarbons in soil samples. Talanta 2012, 101, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Lenardon Vinciguerra, L.; Carla Böck, F.; Pires Schneider, M.; Alejandra Pisoni Canedo Reis, N.; Flores Silva, L.; Christina Mendes de Souza, K.; Crivellaro Guerra, C.; de Araújo Gomes, A.; Maria Bergold, A.; Flôres Ferrão, M. Geographical origin authentication of southern Brazilian red wines by means of EEM-pH four-way data modelling coupled with one class classification approach. Food Chem. 2021, 362, 130087. [Google Scholar] [CrossRef]
- Fu, H.Y.; Wu, H.L.; Yu, Y.J.; Yu, L.L.; Zhang, S.R.; Nie, J.F.; Li, S.F.; Yu, R.Q. A new third-order calibration method with application for analysis of four-way data arrays. J. Chemom. 2011, 25, 408–429. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Valdés-Sánchez, E.; Muñoz de la Peña, A.; Durán-Merás, I. Combination of fluorescence excitation emission matrices in polar and non-polar solvents to obtain three- and four-way arrays for classification of Tempranillo grapes according to maturation stage and hydric status. Talanta 2019, 199, 652–661. [Google Scholar] [CrossRef]
- Lozano, V.A.; Muñoz de la Peña, A.; Durán-Merás, I.; Espinosa Mansilla, A.; Escandar, G.M. Four-way multivariate calibration using ultra-fast high-performance liquid chromatography with fluorescence excitation–emission detection. Application to the direct analysis of chlorophylls a and b and pheophytins a and b in olive oils. Chemom. Intel. Lab. Syst. 2013, 125, 121–131. [Google Scholar] [CrossRef]
- Bailey, H.P.; Rutan, S.C. Comparison of chemometric methods for the screening of comprehensive two-dimensional liquid chromatographic analysis of wine. Anal. Chim. Acta 2013, 770, 18–28. [Google Scholar] [CrossRef]
- Arancibia, J.A.; Damiani, P.C.; Escandar, G.M.; Ibañez, G.A.; Olivieri, A.C. A review on second- and third-order multivariate calibration applied to chromatographic data. J.Chromatogr. B 2012, 910, 22–30. [Google Scholar] [CrossRef]
- Gao, Z.W.; Mou, L.; Xue, S.F.; Tao, Z.; Zeng, X. Cucurbit[8] urils-induced room temperature phosphorescence of phenanthrene and fluorene. Spectrosc. Spectral Anal. 2010, 30, 1026–1029. [Google Scholar] [CrossRef]
- Guo, J.; Yang, C.; Zhao, Y. Long-lived organic room-temperature phosphorescence from amorphous polymer systems. Accounts Chem. Res. 2022, 94, 5190–5195. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Al-Tameemi, M.; Wilson, W.B.; Wise, S.A.; Barbosa, F.; Campiglia, A.D. Low-temperature time-resolved phosphorescence excitation emission matrices for the analysis of phenanthro-thiophenes in chromatographic fractions of complex environmental extracts. Talanta 2020, 212, 120805. [Google Scholar] [CrossRef] [PubMed]
- Pulgarin, J.A.M.; Molina, A.A.; Sanchez-Ferrer, I. Determination of propranolol and naproxen in urine by using excitation-emission matrix phosphorescence coupled with multivariate calibration algorithms. Curr. Pharm. Anal. 2012, 8, 83–92. [Google Scholar] [CrossRef]
- Muñoz de la Peña, A.; Mora Diez, N.; Bohoyo Gil, D.; Cano Carranza, E. Second-order data obtained by time-resolved room temperature phosphorescence. A new approach for PARAFAC multicomponent analysis. J. Fluoresc. 2009, 19, 345–352. [Google Scholar] [CrossRef]
- Arancibia, J.A.; Escandar, G.M. Room-temperature excitation–emission phosphorescence matrices and second-order multivariate calibration for the simultaneous determination of pyrene and benzo[a]pyrene. Anal. Chim. Acta 2007, 584, 287–294. [Google Scholar] [CrossRef]
- Arancibia, J.A.; Boschetti, C.E.; Olivieri, A.C.; Escandar, G.M. Screening of oil samples on the basis of excitation−emission room-temperature phosphorescence data and multiway chemometric techniques. Introducing the second-order advantage in a classification study. Anal. Chem. 2008, 80, 2789–2798. [Google Scholar] [CrossRef]
- Goicoechea, H.C.; Yu, S.; Olivieri, A.C.; Campiglia, A.D. Four-way data coupled to parallel factor model applied to environmental analysis: Determination of 2,3,7,8-tetrachloro-dibenzo-para-dioxin in highly contaminated waters by solid−liquid extraction laser-excited time-resolved Shpol’skii spectroscopy. Anal. Chem. 2005, 77, 2608–2616. [Google Scholar] [CrossRef]
- Qing, X.D.; Wu, H.L.; Yan, X.F.; Li, Y.; Ouyang, L.Q.; Nie, C.C.; Yu, R.Q. Development of a novel alternating quadrilinear decomposition algorithm for the kinetic analysis of four-way room-temperature phosphorescence data. Chemom. Intel. Lab. Syst. 2014, 132, 8–17. [Google Scholar] [CrossRef]
- Harshman, R.A. Foundation of the PARAFAC procedure: Models and conditions for “explanatory” multimodal factor analysis. UCLA Work. Pap. Phon. 1970, 16, 1–84. Available online: https://www.psychology.uwo.ca/faculty/harshman/wpppfac0.pdf (accessed on 1 December 2022).
- Wu, H.L.; Shibukawa, M.; Oguma, K. An alternating trilinear decomposition algorithm with application to calibration of HPLC–DAD for simultaneous determination of overlapped chlorinated aromatic hydrocarbons. J. Chemom. 1998, 12, 1–26. [Google Scholar] [CrossRef]
- Chen, Z.P.; Wu, H.L.; Jiang, J.H.; Li, Y.; Yu, R.Q. A novel trilinear decomposition algorithm for second-order linear calibration. Chemom. Intel. Lab. Syst. 2000, 52, 75–86. [Google Scholar] [CrossRef]
- Bro, R.; Kiers, H.A.L. A new efficient method for determining the number of components in PARAFAC models. J. Chemom. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Qing, X.D.; Li, Y.; Wen, J.; Shen, X.Z.; Li, C.Y.; Liu, X.L.; Xie, J. A new method to determine the number of chemical components of four-way data from mixtures. Microchem. J. 2017, 135, 114–121. [Google Scholar] [CrossRef]
- Wang, X.M.; Dong, M.J.; Li, Z.J.; Wang, Z.P.; Liang, F.S. Recent advances of room temperature phosphorescence and long persistent luminescence by doping system of purely organic molecules. Dyes Pigments 2022, 204, 2–12. [Google Scholar] [CrossRef]
- Machicote, R.G.; Bruzzone, L. Simultaneous determination of carbaryl and 1-naphthol by first-derivative synchronous non-protected room temperature phosphorescence. Anal. Sci. 2009, 25, 623–626. [Google Scholar] [CrossRef]
- Liu, L.L.; Yang, B.; Zhang, H.Y.; Tang, S.; Xie, Z.Q.; Wang, H.P.; Wang, Z.M.; Lu, P.; Ma, Y.G. Role of Tetrakis(triphenylphosphine)palladium(0) in the degradation and optical properties of fluorene-based compounds. J. Phys. Chem. C 2008, 112, 10273–10278. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, J.; Tian, Y.; Fang, M.M.; Liao, Q.Y.; Wang, L.W.; Hu, W.P.; Tang, B.Z.; Li, Z. Persistent organic room temperature phosphorescence: What is the role of molecular dimers? Chem. Sci. 2020, 11, 833–838. [Google Scholar] [CrossRef]
- Liu, L.L.; Tang, S.; Liu, M.R.; Xie, Z.Q.; Zhang, W.; Lu, P.; Hanif, M.; Ma, Y.G. Photodegradation of polyfluorene and fluorene oligomers with alkyl and aromatic disubstitutions. J. Phys. Chem. B 2006, 110, 13734–13740. [Google Scholar] [CrossRef]
| Sample No. | Reference Concentration (μg.mL−1) | Recovery (%) | ||||
|---|---|---|---|---|---|---|
| PARAFAC | AQLD | SWAQLD | ||||
| N = 3 | N = 3 | N = 4 | N = 3 | N = 4 | ||
| V01 | 1.71 | 117.8 | 117.3 | 115.8 | 111.2 | 113.2 |
| V02 | 2.09 | 101.8 | 91.3 | 93.6 | 95.5 | 96.3 |
| V03 | 2.47 | 97.7 | 88.5 | 89.6 | 96.6 | 96.2 |
| V04 | 2.85 | 100.4 | 102.1 | 100.4 | 99.5 | 101.7 |
| V05 | 3.23 | 100.0 | 112.7 | 111.4 | 100.4 | 105.6 |
| V06 | 3.61 | 94.1 | 95.9 | 98.6 | 93.6 | 97.3 |
| AR a (%) SDR b (%) | 101.9 5.3 | 101.3 9.4 | 101.6 8.0 | 99.5 4.2 | 101.7 5.1 | |
| RMSEP c (μg.mL−1) | 0.17 | 0.28 | 0.24 | 0.15 | 0.15 | |
| Sample No. | Reference Concentration (μg mL−1) | Recovery (%) | ||||
|---|---|---|---|---|---|---|
| PARAFAC | AQLD | SWAQLD | ||||
| N = 3 | N = 3 | N = 4 | N = 3 | N = 4 | ||
| W01 | 2.09 | 121.4 | 122.7 | 108.1 | 113.6 | 118.8 |
| W02 | 2.47 | 86.5 | 91.7 | 97.8 | 92.9 | 93.9 |
| W03 | 2.85 | 93.8 | 96.9 | 84.8 | 92.8 | 94.6 |
| W04 | 3.23 | 89.5 | 94.9 | 84.1 | 90.0 | 88.4 |
| W05 | 3.61 | 103.0 | 105.5 | 91.6 | 93.6 | 97.3 |
| AR | 98.8 | 102.3 | 93.3 | 96.6 | 98.6 | |
| SDR | 10.7 | 9.4 | 7.7 | 6.8 | 8.1 | |
| RMSEP (μg mL−1) | 0.34 | 0.29 | 0.38 | 0.28 | 0.30 | |
| SEN (mL μg−1) a | 12.98 | 41.33 | 8.03 | 18.71 | 1.98 | |
| LOD (μg mL−1) b | 0.21 | 0.13 | 0.04 | 0.10 | 0.11 | |
| LOQ (μg mL−1) c | 0.64 | 0.40 | 0.11 | 0.29 | 0.34 | |
| Noisehomo (%) | Iteration Number (Computation Time (s)) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PARAFAC | AQLD | SWAQLD | |||||||
| Min | Max | Average a | Min | Max | Average | Min | Max | Average | |
| 0.02 | 162 (250.9) | 1951 (2756.9) | 281 (443.8) | 5 (0.4) | 10 (1.0) | 6 (0.6) | 52 (4.3) | 183 (13.3) | 80 (6.6) |
| 0.2 | 118 (191.4) | 1921 (3113.2) | 235 (375.9) | 5 (0.4) | 10 (0.9) | 6 (0.6) | 24 (1.8) | 168 (11.6) | 66 (4.6) |
| 2 | 135 (165.7) | 2132 (4230.5) | 756 (1156.6) | 5 (0.4) | 13 (1.1) | 7 (0.7) | 29 (1.8) | 227 (16.0) | 58 (3.9) |
| 20 | 91 (123.1) | 882 (1473.3) | 252 (412.6) | 8 (0.8) | 68 (5.0) | 13 (1.1) | 29 (2.0) | 256 (15.4) | 47 (3.1) |
| Mode | PARAFAC | AQLD | SWAQLD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.02% | 0.2% | 2% | 20% | 0.02% | 0.2% | 2% | 20% | 0.02% | 0.2% | 2% | 20% | ||
| A | a1 | 1.000 a | 1.0000 | 1.0000 | 0.9998 | 1.0000 | 1.0000 | 1.0000 | 0.9988 | 1.0000 | 1.0000 | 1.0000 | 0.9997 |
| a2 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 0.9998 | 1.0000 | 1.0000 | 1.0000 | 0.9998 | |
| a3 | 1.0000 | 1.0000 | 1.0000 | 0.9998 | 1.0000 | 1.0000 | 1.0000 | 0.9976 | 1.0000 | 1.0000 | 1.0000 | 0.9998 | |
| B | b1 | 1.0000 | 1.0000 | 1.0000 | 0.9994 | 1.0000 | 1.0000 | 1.0000 | 0.9986 | 1.0000 | 1.0000 | 1.0000 | 0.9994 |
| b2 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 0.9984 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | |
| b3 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 0.9952 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | |
| C | c1 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9991 | 1.0000 | 1.0000 | 1.0000 | 0.9997 |
| c2 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9997 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | |
| c3 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9996 | 1.0000 | 1.0000 | 1.0000 | 0.9997 | |
| D | d1 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9920 | 1.0000 | 1.0000 | 1.0000 | 0.9999 |
| d2 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9997 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| d3 | 1.0000 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 0.9902 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| RMSEP | Analyte 1 | 0.0000 | 0.0002 | 0.0005 | 0.0026 | 0.0000 | 0.0001 | 0.0007 | 0.0546 c | 0.0000 | 0.0003 | 0.0006 | 0.0049 |
| Analyte 2 | 0.0000 | 0.0001 | 0.0006 | 0.0032 | 0.0000 | 0.0001 | 0.0010 | 0.0093 | 0.0000 | 0.0002 | 0.0003 | 0.0023 | |
| Predicted k b | 0.0999 | 0.0992 | 0.1005 | 0.0941 | 0.0999 | 0.0996 | 0.0940 | 0.0367 | 0.0999 | 0.0992 | 0.1000 | 0.0997 | |
| Mode | PARAFAC | AQLD | SWAQLD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 3 | N = 4 | N = 10 | N = 3 | N = 4 | N = 10 | N = 3 | N = 4 | N = 10 | ||
| A | a1 | 1.0000 | 1.0000 | 0.9964 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| a2 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| a3 | 1.0000 | 1.0000 | 0.9919 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| B | b1 | 1.0000 | 1.0000 | 0.9122 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| b2 | 1.0000 | 1.0000 | 0.9638 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| b3 | 1.0000 | 1.0000 | 0.9397 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| C | c1 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| c2 | 1.0000 | 1.0000 | 0.9995 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| c3 | 1.0000 | 1.0000 | 0.9813 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| D | d1 | 1.0000 | 1.0000 | 0.9891 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| d2 | 1.0000 | 1.0000 | 0.9196 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| d3 | 1.0000 | 1.0000 | 0.9967 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| RMSEP | Analyte 1 | 0.0000 | 0.0000 | 0.0679 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 |
| Analyte 2 | 0.0000 | 0.0014 | 0.2455 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Predicted k | 0.0999 | 0.1000 | 0.0640 | 0.0999 | 0.0999 | 0.1000 | 0.0999 | 0.1000 | 0.1001 | |
| Regression Equation | Correlation Coefficient | Rate Constant (min−1) | Half Life (min) | |
|---|---|---|---|---|
| PARAFAC (N = 3) | y = 0.0151x + 0.7655 | 0.9962 | 0.0151 | 45.9 |
| AQLD (N = 3) | y = 0.0154x + 0.7600 | 0.9889 | 0.0154 | 45.0 |
| AQLD (N = 4) | y = 0.0148x + 0.7698 | 0.9980 | 0.0148 | 46.8 |
| SWAQLD (N = 3) | y = 0.0150x + 0.7687 | 0.9928 | 0.0150 | 46.2 |
| SWAQLD (N = 4) | y = 0.0159x + 0.7526 | 0.9931 | 0.0159 | 43.6 |
| Regression Equation | Correlation Coefficient | Rate Constant (min−1) | Half Life (min) | |
|---|---|---|---|---|
| PARAFAC (N = 3) | y = 0.0172x + 0.7321 | 0.9981 | 0.0172 | 40.3 |
| AQLD (N = 3) | y = 0.0173x + 0.7310 | 0.9908 | 0.0173 | 40.1 |
| AQLD (N = 4) | y = 0.0169x + 0.7374 | 0.9954 | 0.0169 | 41.0 |
| SWAQLD (N = 3) | y = 0.0179x + 0.7219 | 0.9978 | 0.0179 | 38.7 |
| SWAQLD (N = 4) | y = 0.0174x + 0.7288 | 0.9977 | 0.0174 | 39.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qing, X.-D.; Zhang, X.-H.; An, R.; Zhang, J.; Xu, L.; Duponchel, L. A Fast and Robust Third-Order Multivariate Calibration Approach Coupled with Excitation–Emission Matrix Phosphorescence for the Quantification and Oxidation Kinetic Study of Fluorene in Wastewater Samples. Chemosensors 2023, 11, 53. https://doi.org/10.3390/chemosensors11010053
Qing X-D, Zhang X-H, An R, Zhang J, Xu L, Duponchel L. A Fast and Robust Third-Order Multivariate Calibration Approach Coupled with Excitation–Emission Matrix Phosphorescence for the Quantification and Oxidation Kinetic Study of Fluorene in Wastewater Samples. Chemosensors. 2023; 11(1):53. https://doi.org/10.3390/chemosensors11010053
Chicago/Turabian StyleQing, Xiang-Dong, Xiao-Hua Zhang, Rong An, Jin Zhang, Ling Xu, and Ludovic Duponchel. 2023. "A Fast and Robust Third-Order Multivariate Calibration Approach Coupled with Excitation–Emission Matrix Phosphorescence for the Quantification and Oxidation Kinetic Study of Fluorene in Wastewater Samples" Chemosensors 11, no. 1: 53. https://doi.org/10.3390/chemosensors11010053
APA StyleQing, X.-D., Zhang, X.-H., An, R., Zhang, J., Xu, L., & Duponchel, L. (2023). A Fast and Robust Third-Order Multivariate Calibration Approach Coupled with Excitation–Emission Matrix Phosphorescence for the Quantification and Oxidation Kinetic Study of Fluorene in Wastewater Samples. Chemosensors, 11(1), 53. https://doi.org/10.3390/chemosensors11010053







