WO3-Nanocrystal-Modified Electrodes for Ultra-Sensitive and Selective Detection of Cadmium (Cd2+) Ions
Abstract
1. Introduction
2. Experiment
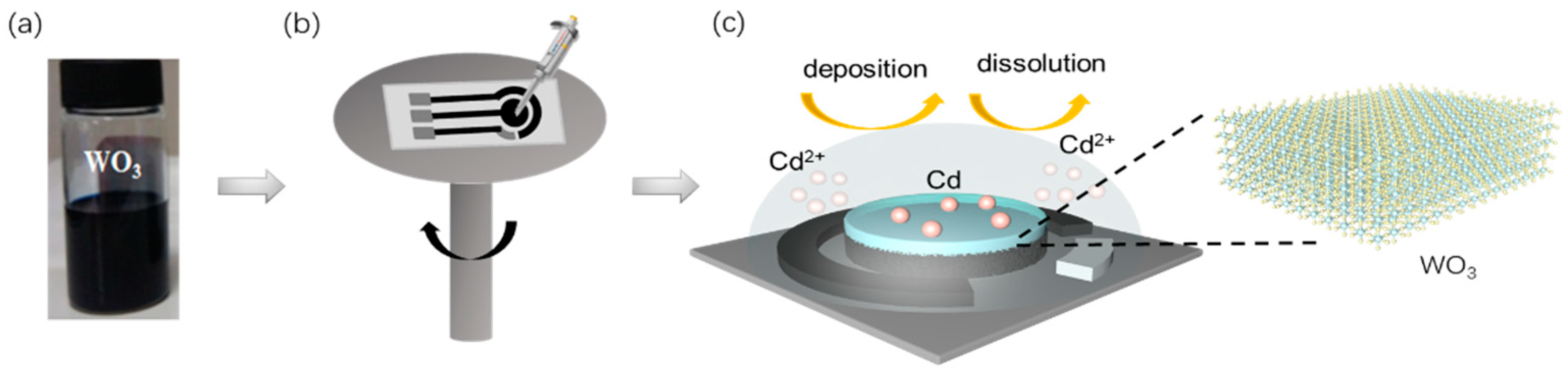
2.1. Synthesis of WO3 Nanocrystals
2.2. Preparation of WO3-Modified Electrodes
2.3. Characterization
3. Results and Discussion
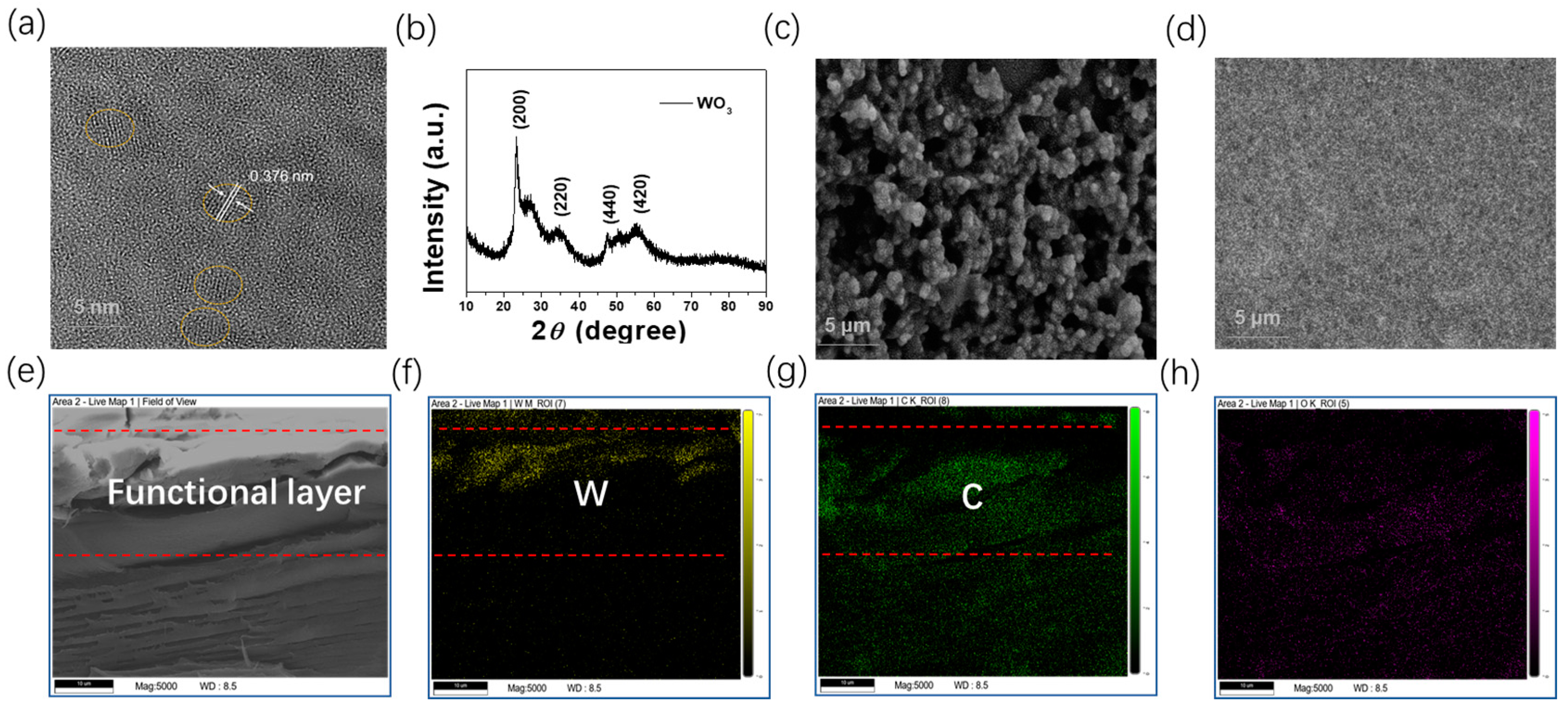
3.1. Structure and Morphology of the WO3 Nanocrystals and Modified Layers
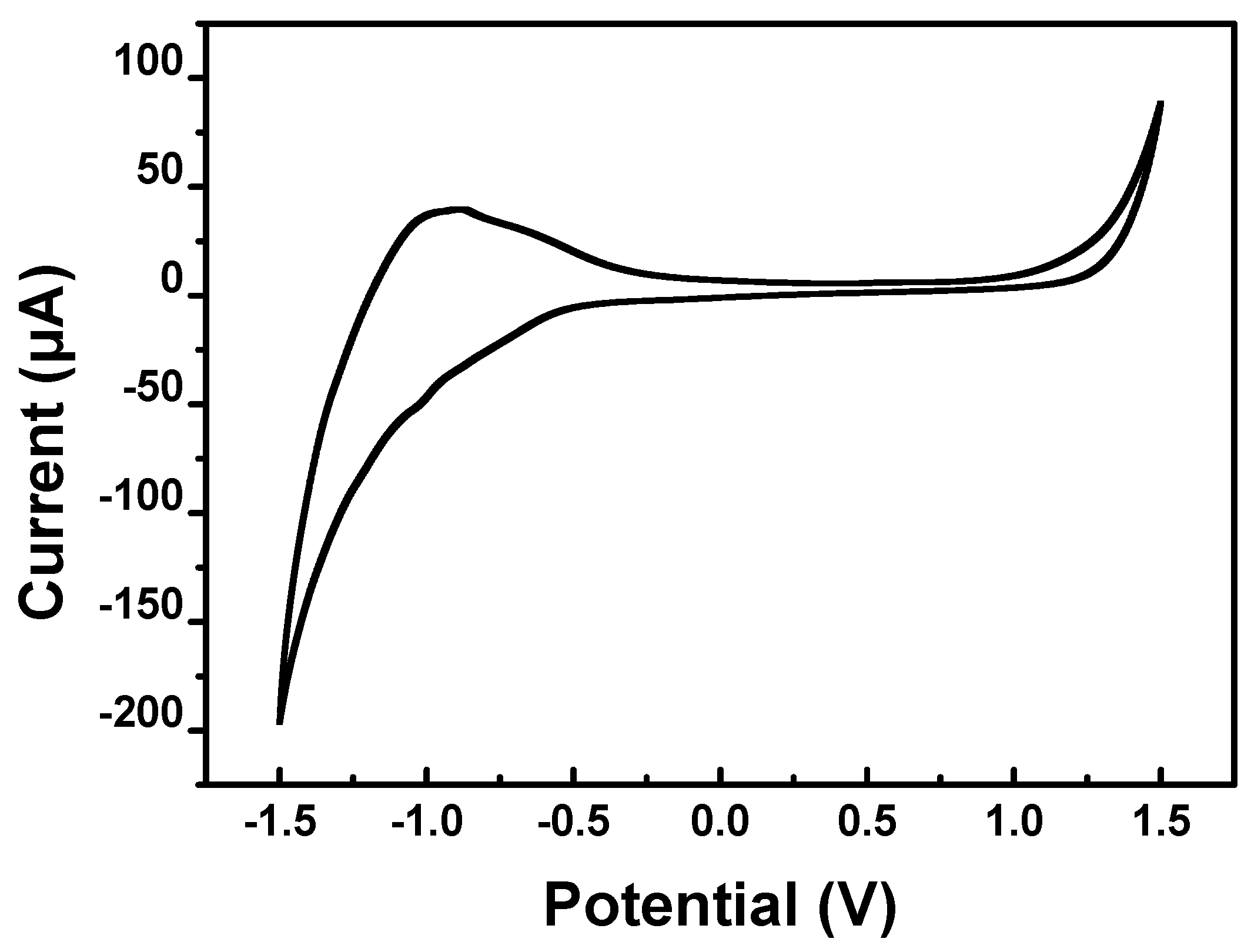
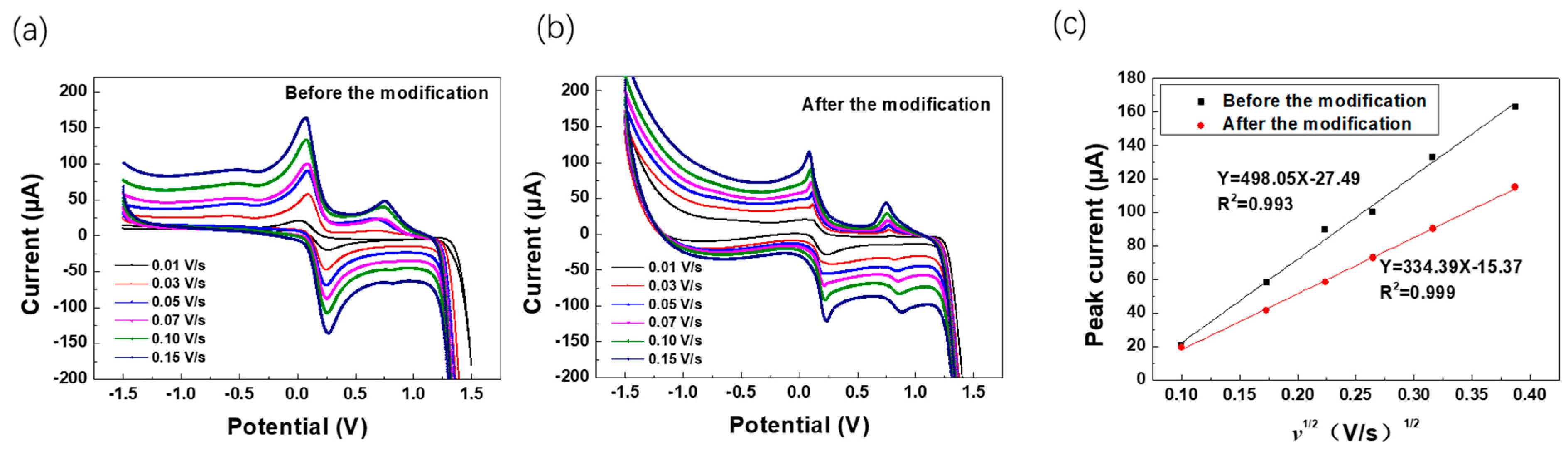
3.2. Performance Optimization of the WO3-Modified Electrodes
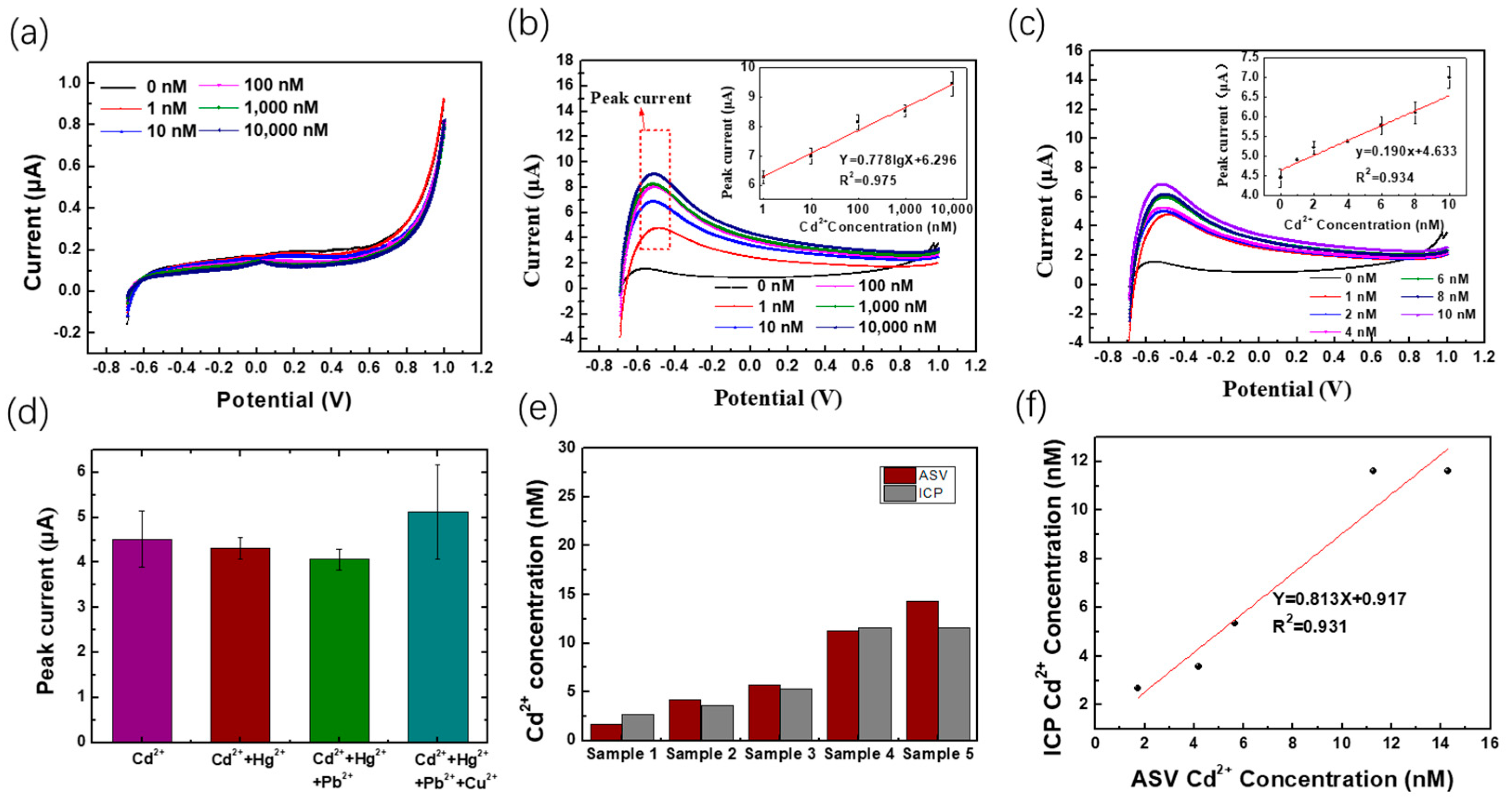
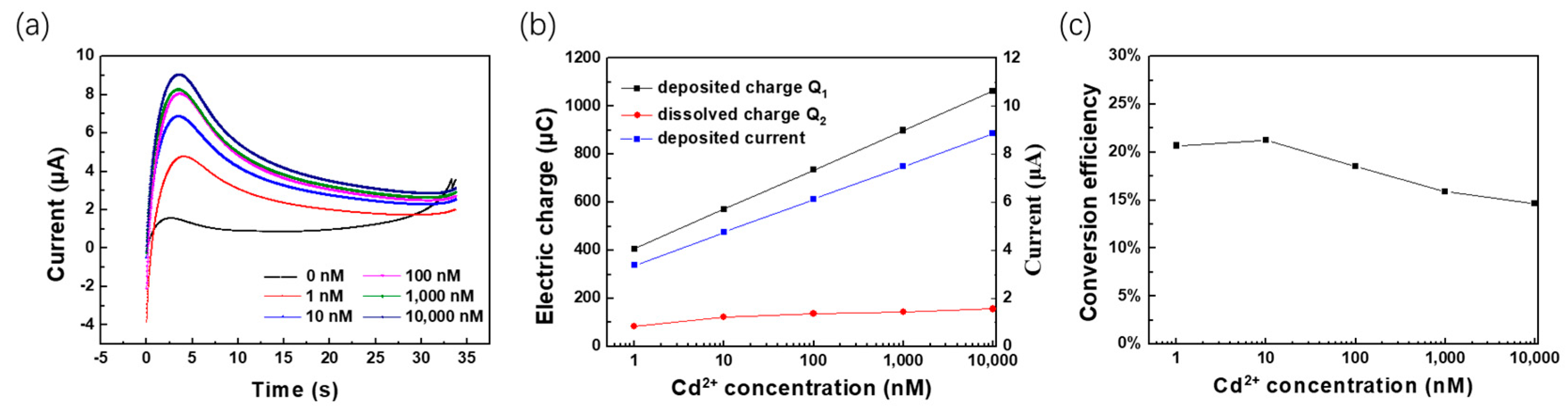
3.3. Electrochemical Performance of WO3-Nanocrystal-Modified Electrodes
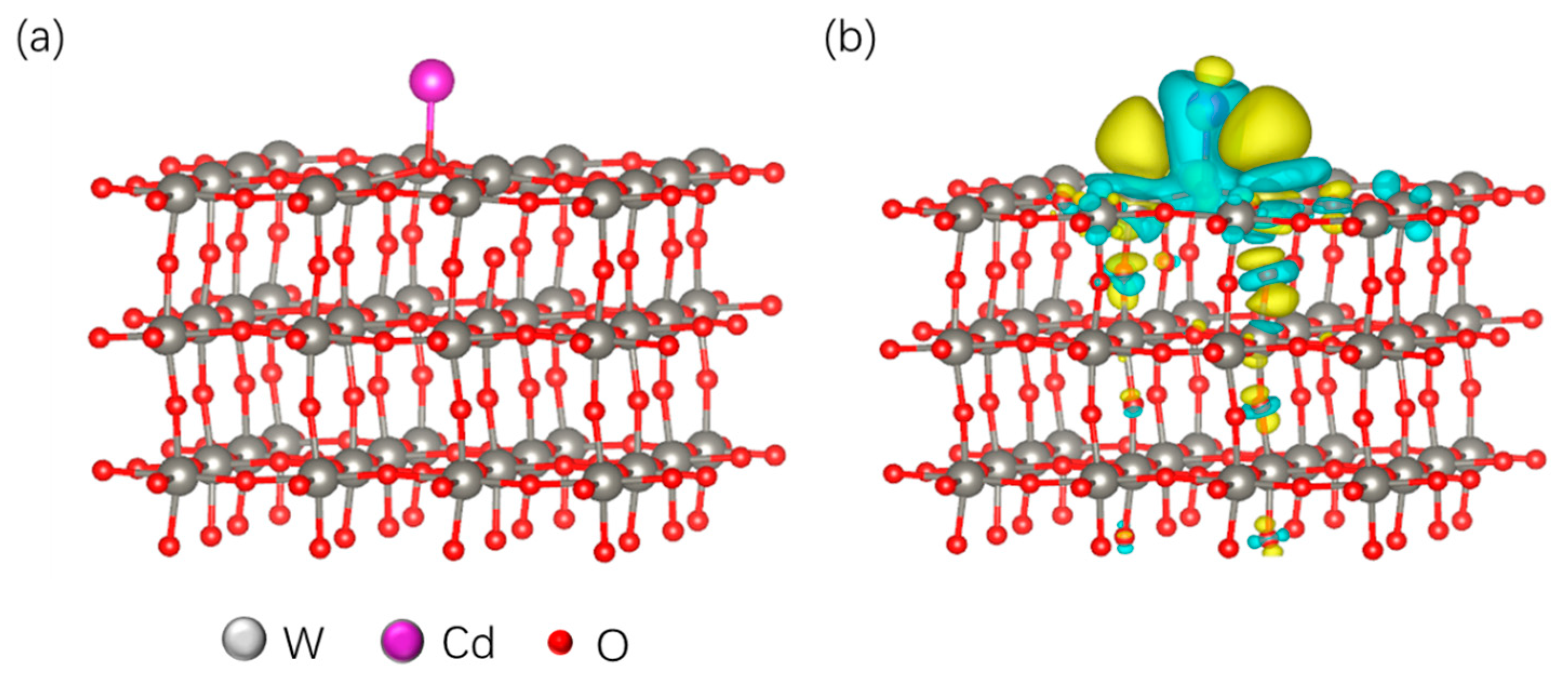
3.4. Analysis of the Sensitive Mechanism of WO3-Modified Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Saini, S.; Dhania, G. Cadmium as an Environmental Pollutant: Ecotoxicological Effects, Health Hazards, and Bioremediation Approaches for Its Detoxification from Contaminated Sites. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 357–387. [Google Scholar]
- Petroczi, A.; Naughton, D. Mercury, cadmium and lead contamination in seafood: A comparative study to evaluate the usefulness of Target Hazard Quotients. Food Chem. Toxicol. 2009, 47, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bi, H.-M.; Han, X.-J. Research Progress of Electrochemical Detection of Heavy Metal Ions. Chin. J. Anal. Chem. 2021, 49, 330–340. [Google Scholar] [CrossRef]
- Zhao, G. Recent Advances in Chemically Modified Electrodes, Microfabricated Devices and Injection Systems for the Electrochemical Detection of Heavy Metals: A review. Int. J. Electrochem. Sci. 2017, 12, 8622–8641. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Fayazi, M.; Tarahomi, M.; Ghanei-Motlagh, R.; Maleki, B. A novel electrochemical sensor based on a glassy carbon electrode modified with dendrimer functionalized magnetic graphene oxide for simultaneous determination of trace Pb(II) and Cd(II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Dali, M.; Zinoubi, K.; Chrouda, A.; Abderrahmane, S.; Cherrad, S.; Jaffrezic-Renault, N. A biosensor based on fungal soil biomass for electrochemical detection of lead (II) and cadmium (II) by differential pulse anodic stripping voltammetry. J. Electroanal. Chem. 2018, 813, 9–19. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water-An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, H.; Fang, Y.; Guan, J.; Ma, S.; Pan, Y.; Zhu, H.; Liu, X.; Du, M. Detection of trace Cd2+, Pb2+ and Cu2+ ions via porous activated carbon supported palladium nanoparticles modified electrodes using SWASV. Mater. Chem. Phys. 2019, 225, 433–442. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Thinakaran, N. A novel voltammetric sensor for the simultaneous detection of Cd2+ and Pb2+ using graphene oxide/κ-carrageenan/l-cysteine nanocomposite. Carbohydr. Polym. 2018, 182, 199–206. [Google Scholar] [CrossRef]
- Ghenaatian, H.R.; Shakourian-Fard, M.; Moghadam, M.R.; Kamath, G.; Rahmanian, M. Tailoring of graphene quantum dots for toxic heavy metals detection. Appl. Phys. A 2019, 125, 754. [Google Scholar] [CrossRef]
- Gu, R.; Zhao, Y.; Fu, H.; Yang, J.; Hu, Z.; Li, L.; Li, H.Y.; Huang, Q.; Chen, B.; Liu, H. Nanocrystalline SnO2-Modified Electrode for Ultra-Sensitive Mercury Ion Detection. IEEE Sens. J. 2022, 8, 7590–7598. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Kumar, S. Zinc Oxide Quantum Dots as Efficient Electron Mediator for Ultrasensitive and Selective Electrochemical Sensing of Mercury. Electrochim. Acta 2015, 178, 361–367. [Google Scholar] [CrossRef]
- Araujo, P. Key aspects of analytical method validation and linearity evaluation. J. Chromatogr. B 2009, 877, 2224–2234. [Google Scholar] [CrossRef]
- Labuda, J.; Vanǹičková, M.; Uhlemann, E.; Mickler, W. Applicability of chemically modified electrodes for determination of copper species in natural waters. Anal. Chim. Acta 1994, 284, 517–523. [Google Scholar] [CrossRef]
- Sajnóg, A.; Koko, E.; Paszyńska, K.; Barałkiewicz, D. Multielemental speciation analysis of Cd2+, Pb2+ and (CH3)3Pb+ in herb roots by HPLC/ICP-DRC-MS. Validation and application to real samples analysis. Talanta Open 2022, 5, 100119. [Google Scholar] [CrossRef]
- Mc Eleney, C.; Alves, S.; Mc Crudden, D. Novel determination of Cd and Zn in soil extract by sequential application of bismuth and gallium thin films at a modified screen-printed carbon electrode. Anal. Chim. Acta 2020, 1137, 94–102. [Google Scholar] [CrossRef]
- Hai, T.; Hung, L.; Phuong, T.; Ha, B.; Nguyen, B.-S.; Hai, T.; Nguyen, V.H. Multiwall carbon nanotube modified by antimony oxide (Sb2O3/MWCNTs) paste electrode for the simultaneous electrochemical detection of cadmium and lead ions. Microchem. J. 2020, 153, 104456. [Google Scholar] [CrossRef]
- Palisoc, S.T.; Estioko, L.C.D.; Natividad, M.T. Voltammetric determination of lead and cadmium in vegetables by graphene paste electrode modified with activated carbon from coconut husk. Mater. Res. Express 2018, 5, 085035. [Google Scholar] [CrossRef]
- Rojas-Romo, C.; Aliaga, M.E.; Arancibia, V.; Gomez, M. Determination of Pb(II) and Cd(II) via anodic stripping voltammetry using an in-situ bismuth film electrode. Increasing the sensitivity of the method by the presence of Alizarin Red S. Microchem. J. 2020, 159, 105373. [Google Scholar] [CrossRef]
- Ns, A.; Ashoka, S.; Malingappa, P. Nano zinc ferrite modified electrode as a novel electrochemical sensing platform in simultaneous measurement of trace level lead and cadmium. J. Environ. Chem. Eng. 2018, 6, 6939–6946. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, W.; Yan, Y.; Wang, Y.; Zhang, J. Core-Shell Self-Doped Polyaniline Coated Metal-Organic-Framework (SPAN@UIO-66-NH2) Screen Printed Electrochemical Sensor for Cd2+ Ions. J. Electrochem. Soc. 2019, 166, B873–B880. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar diffusion to macro disc electrodes—What electrode size is required for the Cottrell and Randles-Sevcik equations to apply quantitatively. J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]

| Modification Material | Method | Detection Range | Detection Limit | Reference |
|---|---|---|---|---|
| RGO/g-C3N4/BiTF | SW-ASV | 180–4050 nM | 90 nM | [18] |
| Graphene Oxide/k-carrageenan/L-cysteine nanocomposite | SW-ASV | 550 nM | 0.58 nM | [10] |
| Pd@PAC/GCE | SW-ASV | 25,500 nM | 13.3 nM | [11] |
| Sb2O3/MWCNTs | LASV | 715,625 nM | 100 nM | [19] |
| AC/MGPE | ASV | 452,678 nM | 500 nM | [20] |
| Bismuth/GCE | DP-ASV | 9–135 nM | 0.81 nM | [21] |
| zinc ferrite/GCE | DP-ASV | 89–1160 nM | 22.5 nM | [22] |
| WO3 nanocrystals | ASV | 1–10,000 nM | 0.029 nM | This work |
| Sites | Items | O | W-O | W | C-C | C |
|---|---|---|---|---|---|---|
| WO3 surface | Eads (eV) | −1.373 | −0.671 | −0.394 | —— | —— |
| Length (Å) | 2.861 | 2.713 | 3.295 | —— | —— | |
| WO3–C interface | Eads (eV) | −2.054 | −1.663 | −0.617 | —— | —— |
| Length (Å) | 2.651 | 2.741 | 3.013 | —— | —— | |
| C surface | Eads (eV) | —— | —— | —— | −0.855 | −1.066 |
| Length (Å) | —— | —— | —— | 3.269 | 3.054 |
| Sites | Items | Cd | Pb | Hg | Cu |
|---|---|---|---|---|---|
| O | Eads (eV) | −2.054 | −1.371 | −0.751 | −0.573 |
| Length (Å) | 2.651 | 2.763 | 2.968 | 2.967 | |
| W-O | Eads (eV) | −1.663 | −0.811 | −0.423 | −0.217 |
| Length (Å) | 2.741 | 2.861 | 3.154 | 3.051 | |
| W | Eads (eV) | −0.617 | −0.185 | −0.159 | −0.413 |
| Length (Å) | 3.013 | 3.261 | 3.369 | 2.975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, R.; Zhao, Y.; Fu, H.; Huang, Q.; Li, L.; Hu, Z.; Zhou, L.; Chen, B.; Liu, H. WO3-Nanocrystal-Modified Electrodes for Ultra-Sensitive and Selective Detection of Cadmium (Cd2+) Ions. Chemosensors 2023, 11, 54. https://doi.org/10.3390/chemosensors11010054
Gu R, Zhao Y, Fu H, Huang Q, Li L, Hu Z, Zhou L, Chen B, Liu H. WO3-Nanocrystal-Modified Electrodes for Ultra-Sensitive and Selective Detection of Cadmium (Cd2+) Ions. Chemosensors. 2023; 11(1):54. https://doi.org/10.3390/chemosensors11010054
Chicago/Turabian StyleGu, Ruiqin, Yunong Zhao, Huibing Fu, Qing Huang, Long Li, Zhixiang Hu, Licheng Zhou, Bingbing Chen, and Huan Liu. 2023. "WO3-Nanocrystal-Modified Electrodes for Ultra-Sensitive and Selective Detection of Cadmium (Cd2+) Ions" Chemosensors 11, no. 1: 54. https://doi.org/10.3390/chemosensors11010054
APA StyleGu, R., Zhao, Y., Fu, H., Huang, Q., Li, L., Hu, Z., Zhou, L., Chen, B., & Liu, H. (2023). WO3-Nanocrystal-Modified Electrodes for Ultra-Sensitive and Selective Detection of Cadmium (Cd2+) Ions. Chemosensors, 11(1), 54. https://doi.org/10.3390/chemosensors11010054









