Mo2C-Based Microfluidic Gas Sensor Detects SF6 Decomposition Components: A First-Principles Study
Abstract
:1. Introduction
2. Computational Details and Models
3. Results and Discussion
3.1. Adsorption Characteristic of Mo2C
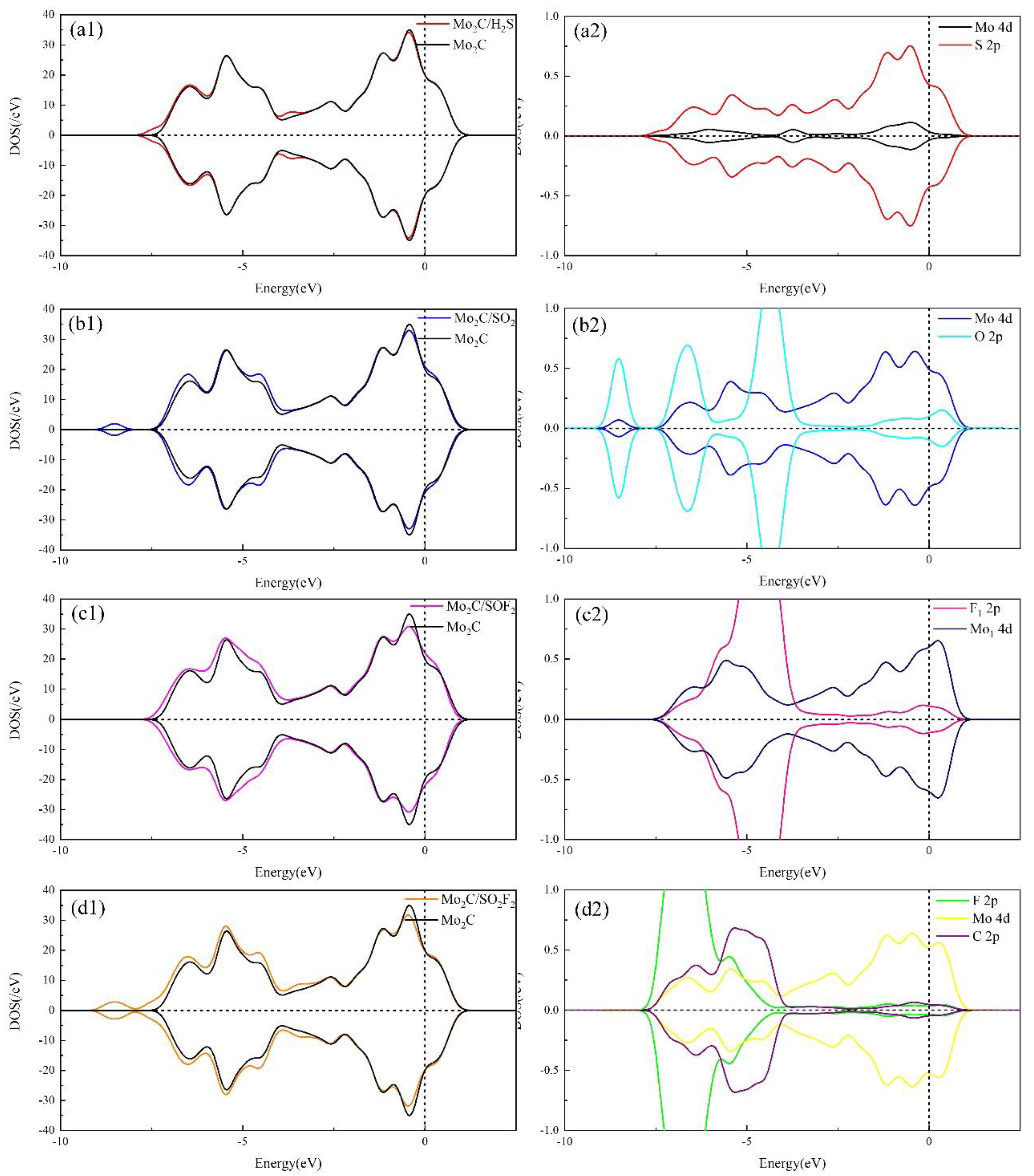
3.2. Electronic Behavior
3.3. Resistance-Type Sensor Exploration
3.4. Work Function (WF) Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, J.; Rao, X.; Zeng, F.; Cai, W.; Cheng, L.; Zhang, C. Influence Mechanisms of Trace H2O on the Generating Process of SF6 Spark Discharge Decomposition Components. Plasma Chem. Plasma Processing 2017, 37, 325–340. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, D.; Wang, Y.; Zhou, C.; Lu, H.; Zhang, X. Defect recognition and condition assessment of epoxy insulators in gas insulated switchgear based on multi-information fusion. Measurement 2022, 190, 110701. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, D.; Zhang, Y.; Yuan, Z.; Tian, S.; Zhang, X. Research on infrared spectrum characteristics and detection technology of environmental-friendly insulating medium C5F10O. Vib. Spectrosc. 2022, 118, 103336. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Wang, J.; Wang, Z. Detection of SF6 decomposition components by pristine and Cr-doped GaN based on the first-principles theory. Comput. Theor. Chem. 2021, 1205, 113431. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Liu, L.; Wang, Z. Adsorption of SF6 Decomposition Products by the S Vacancy Structure and Edge Structure of SnS2: A Density Functional Theory Study. ACS Omega 2021, 6, 28131–28139. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.; Zhou, J. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Meng, Q.; Zhang, X.; Tao, J. Partial discharge recognition through an analysis of SF6 decomposition products part 2: Feature extraction and decision tree-based pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, D.; Xiao, S.; Tang, J. Adsorption behavior of COF2 and CF4 gas on the MoS2 monolayer doped with Ni: A first-principles study. Appl. Surf. Sci. 2018, 443, 274–279. [Google Scholar] [CrossRef]
- Ding, K.; Lin, Y.; Huang, M. The enhancement of NO detection by doping strategies on monolayer MoS2. Vac. Technol. Appl. Ion Phys. Int. J. Abstr. Serv. Vac. Sci. Technol. 2016, 130, 146–153. [Google Scholar]
- Wang, J.; Zhang, X.; Liu, L.; Wang, Z. Dissolved gas analysis in transformer oil using Ni-Doped GaN monolayer: A DFT study. Superlattices Microstruct. 2021, 159, 107055. [Google Scholar] [CrossRef]
- Kadioglu, Y.; Ersan, F.; Kecik, D.; Aktürk, O.; Aktürk, E.; Ciraci, S. Chemical and substitutional doping, and anti-site and vacancy formation in monolayer AlN and GaN. Phys. Chem. Chem. Phys. 2018, 20, 16077–16091. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Qin, H.; Yang, D.; Zhang, G. First-Principles Investigation of the Adsorption Behaviors of CH2O on BN, AlN, GaN, InN, BP, and P Monolayers. Materials 2019, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Lu, X.; Ingebrandt, S.; Chen, X. Adsorption of Gas Molecules on Graphene-Like ZnO Nanosheets: The Roles of Gas Concentration, Layer Number, and Heterolayer. Adv. Mater. Interfaces 2017, 4, 1700647. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Haidry, A.A.; Saruhan-Brings, B.J.S. Nanotubular Cr-doped TiO2 for use as high-temperature NO2-selective gas sensor. Sens. Actuators B Chem. 2015, 217, 78–87. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, J.Y.; Kwon, O.; Kim, J.; Jung, H.-T. Molybdenum carbide chemical sensors with ultrahigh signal-to-noise ratios and ambient stability. J. Mater. Chem. A 2018, 6, 23408–23416. [Google Scholar] [CrossRef]
- Wang, T.; Tian, X.; Yang, Y.; Li, Y.-W.; Wang, J.; Beller, M.; Jiao, H. Coverage dependent adsorption and co-adsorption of CO and H2 on the CdI2-antitype metallic Mo2C(001) surface. Phys. Chem. Chem. Phys. 2014, 17, 1907–1917. [Google Scholar] [CrossRef]
- Hassan, A.; Ilyas, S.Z.; Ahmed, S.; Niaz, F.; Jalil, A.; Khan, Q. Ab-initio study of molybdenum carbide (Mo2C) as an adsorption-based filter. Phys. Lett. A 2021, 392, 127119. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuators B Chem. 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.E.; Li, P.; Zhang, Y. Facile fabrication of MoS2-modified SnO2 hybrid nanocomposite for ultrasensitive humidity sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef]
- Yang, Q.-L.; Ban, Y.-L.; Lian, J.-W.; Yu, Z.-F.; Wu, B. SIW Butler Matrix with Modified Hybrid Coupler for Slot Antenna Array. IEEE Access 2016, 4, 9561–9569. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, K.; Tao, L.; Yu, J.; Zhu, X.; Li, X.; Chen, X. Monolayer Tellurene-Based Gas Sensor to Detect SF6 Decompositions: A First-Principles Study. IEEE Electron Device Lett. 2019, 40, 1522–1525. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Han, J.; Yang, Y.; Guo, Y.; Bai, Z.; Cheng, J.; Chu, P.K.; Pang, H.; Luo, Y. Porous Mo2C-Mo3N2 heterostructure/rGO with synergistic functions as polysulfides regulator for high-performance lithium sulfur batteries. Chem. Eng. J. 2021, 433, 133629. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Wang, X.; Huang, J.; Pan, W.; Zhang, J.; Meteku, B.E.; Zeng, J. UV illumination-enhanced ultrasensitive ammonia gas sensor based on (001)TiO2/MXene heterostructure for food spoilage detection. J. Hazard. Mater. 2022, 423, 127160. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Han, L.-F.; Xiao, Y.-H.; Jia, D.-Z.; Guo, Z.-H.; Li, F. Understanding dopant and defect effect on H2S sensing performances of graphene: A first-principles study. Comput. Mater. Sci. 2013, 69, 222–228. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Mi, H.; Wang, J.; Zeng, W. Gas-sensing mechanism of Cr doped SnP3 monolayer to SF6 partial discharge decomposition components. Appl. Surf. Sci. 2021, 546, 149084. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Chen, D.; Liu, L. The adsorption performance of harmful gas on Cu doped WS2: A first-principle study. Mater. Today Commun. 2021, 28, 102488. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Meng, R.; Tan, C.; Liang, Q.; Jiang, J.; Ye, H.; Chen, X. Adsorption of gas molecules on graphene-like InN monolayer: A first-principle study. Appl. Surf. Sci. 2017, 404, 291–299. [Google Scholar] [CrossRef]
- Ma, S.; Li, D.; Rao, X.; Xia, X.; Su, Y.; Lu, Y. Pd-doped h-BN monolayer: A promising gas scavenger for SF6 insulation devices. Adsorption 2020, 26, 619–626. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Gui, Y.; Shi, J.; Yang, P.; Li, T.; Tang, C.; Xu, L. Platinum modified MoS2 monolayer for adsorption and gas sensing of SF6 decomposition products: A DFT study. High Volt. 2020, 5, 454–462. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Liu, X.; Ni, Y.X.; Wang, H.Y.; Wang, H. Tuning structural, electronic, and magnetic properties of black-AsP monolayer by adatom adsorptions: A first principles study. Chin. J. Chem. Phys. 2020, 33, 311–318. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Chen, D.; Tang, J. Pt & Pd decorated CNT as a workable media for SOF2 sensing: A DFT study. Appl. Surf. Sci. 2018, 471, 335–341. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, Y.; Xu, L.; Chen, X. Adsorption and sensing performances of transition metal (Ag, Pd, Pt, Rh, and Ru) modified WSe2 monolayer upon SF6 decomposition gases (SOF2 and SO2F2). Appl. Surf. Sci. 2021, 581, 152365. [Google Scholar] [CrossRef]
- Zhu, H.; Cui, H.; He, D.; Cui, Z.; Wang, X. Rh-doped MoTe2 Monolayer as a Promising Candidate for Sensing and Scavenging SF6 Decomposed Species: A DFT Study. Nanoscale Res. Lett. 2020, 15, 129. [Google Scholar] [CrossRef]
- Sun, H.; Tao, L.-Q.; Li, T.; Gao, X.; Wang, G.; Peng, Z.; Zhu, C.; Zou, S.; Gui, Y.; Xia, S.-Y.; et al. Sensing Characteristics of Toxic C₄F₇N Decomposition Products on Metallic- Nanoparticle Co-Doped BN Monolayer: A First Principles Study. IEEE Sens. J. 2021, 21, 13082–13089. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, F.; Qiu, J.; Wu, L.; Zhu, B.; Chen, X.; Yu, J. PbSnS₂-Based Gas Sensor to Detect SF₆ Decompositions: DFT and NEGF Calculations. IEEE Trans. Electron Devices 2021, 68, 5322–5325. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as a toxic gas scavenger: A first-principles study. Nanoscale Adv. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, X.; Song, Y.; Zhang, X.; Tian, S.; Liu, L. Adsorption behaviour of CF4 and COF2 gas on the GaN monolayer doped with Pt catalytic: A first-principles study. Surf. Sci. 2022, 719, 122032. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Wang, Z.T.; Zhang, X.X. Theoretical screening into Ru-doped MoS2 monolayer as a promising gas sensor upon SO2 and SOF2 in SF6 insulation devices. Mol. Phys. 2022, 120, 8. [Google Scholar] [CrossRef]
- Kou, L.; Tang, C.; Zhang, Y.; Heine, T.; Chen, C.; Frauenheim, T. Tuning Magnetism and Electronic Phase Transitions by Strain and Electric Field in Zigzag MoS2 Nanoribbons. J. Phys. Chem. Lett. 2012, 3, 2934–2941. [Google Scholar] [CrossRef] [PubMed]

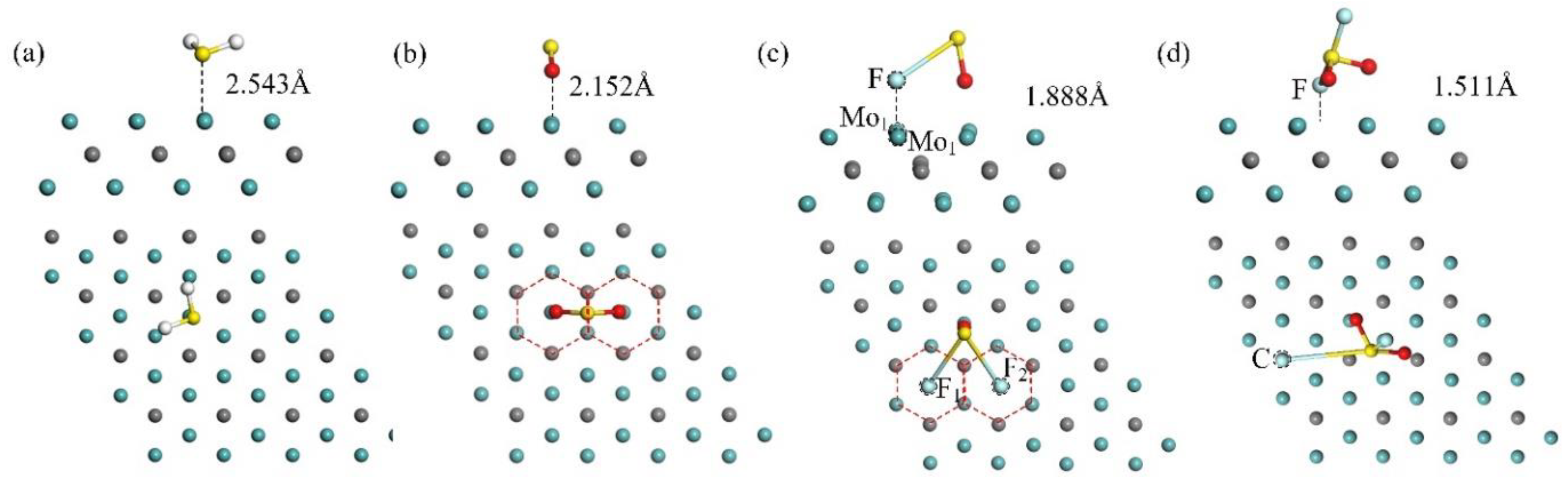
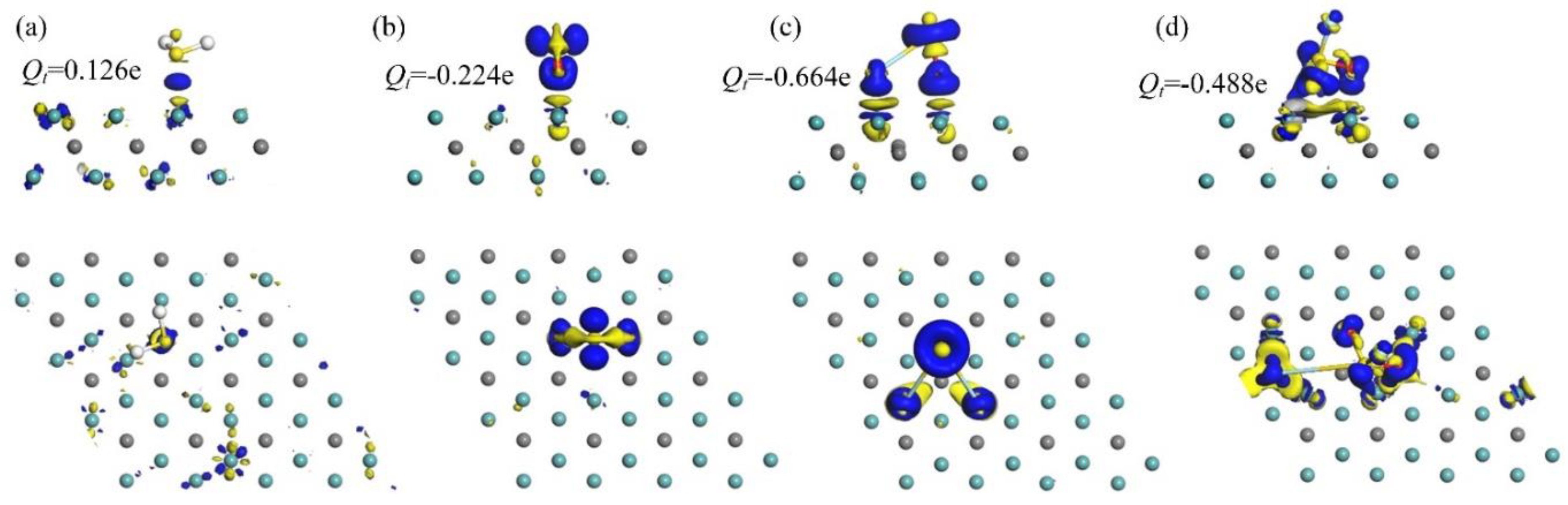


| Molecule | Ead (eV) | D (Å) | Q (e) |
|---|---|---|---|
| −1.560 | 2.543 | 0.125 | |
| −2.538 | 2.152 | −0.224 | |
| −3.458 | 1.888 | −0.643 | |
| −4.944 | 1.511 | −0.488 |
| Molecule | HOMO (eV) | LUMO (eV) | Energy Gap |
|---|---|---|---|
| −0.226 | −0.069 | 0.157 | |
| −0.301 | −0.180 | 0.121 | |
| −0.319 | −0.121 | 0.198 | |
| −0.342 | −0.108 | 0.234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, G.; Wang, Z.; Yuan, J.; Tan, S.; Li, Y. Mo2C-Based Microfluidic Gas Sensor Detects SF6 Decomposition Components: A First-Principles Study. Chemosensors 2022, 10, 368. https://doi.org/10.3390/chemosensors10090368
Liu L, Zhang G, Wang Z, Yuan J, Tan S, Li Y. Mo2C-Based Microfluidic Gas Sensor Detects SF6 Decomposition Components: A First-Principles Study. Chemosensors. 2022; 10(9):368. https://doi.org/10.3390/chemosensors10090368
Chicago/Turabian StyleLiu, Li, Guozhi Zhang, Zengting Wang, Jiawei Yuan, Senyuan Tan, and Yi Li. 2022. "Mo2C-Based Microfluidic Gas Sensor Detects SF6 Decomposition Components: A First-Principles Study" Chemosensors 10, no. 9: 368. https://doi.org/10.3390/chemosensors10090368
APA StyleLiu, L., Zhang, G., Wang, Z., Yuan, J., Tan, S., & Li, Y. (2022). Mo2C-Based Microfluidic Gas Sensor Detects SF6 Decomposition Components: A First-Principles Study. Chemosensors, 10(9), 368. https://doi.org/10.3390/chemosensors10090368







